Abstract
STUDY HYPOTHESIS
Is it possible to immunologically activate human cervical epithelial cells to produce antiviral factors that inhibit herpes simplex virus type 2 (HSV-2) replication?
STUDY FINDING
Our results indicate that human cervical epithelial cells possess a functional TLR3/RIG-I signaling system, the activation of which can mount an Interferon-λ (IFN-λ)-mediated anti-HSV-2 response.
WHAT IS KNOWN ALREADY
There is limited information about the role of cervical epithelial cells in genital innate immunity against HSV-2 infection.
STUDY DESIGN, SAMPLES/MATERIALS, METHODS
We examined the expression of toll-like receptors (TLRs) and retinoic acid-inducible I (RIG-I) in End1/E6E7 cells by real-time PCR. The IFN-λ induced by TLR3 and RIG-I activation of End1/E6E7 cells was also examined by real-time PCR and ELISA. HSV-2 infection of End1/E6E7 cells was evaluated by the real-time PCR detection of HSV-2 gD expression. The antibody to IL-10Rβ was used to determine whether IFN-λ contributes to TLR3/RIG-I mediated HSV-2 inhibition. Expression of interferon regulatory factor 3 (IRF3), IRF7, IFN-stimulated gene 56 (ISG56), 2′-5′-oligoadenylate synthetase I (OAS-1) and myxovirus resistance A (MxA) were determined by the real-time PCR and western blot. End1/E6E7 cells were transfected with shRNA to knockdown the IRF3, IRF7 or RIG-I expression. Student's t-test and post Newman–Keuls test were used to analyze stabilized differences in the immunological parameters above between TLR3/RIG-I-activated cells and control cells.
MAIN RESULTS AND THE ROLE OF CHANCE
Human cervical epithelial cells expressed functional TLR3 and RIG-I, which could be activated by poly I:C and 5′ppp double-strand RNAs (5′ppp dsRNA), resulting in the induction of endogenous interferon lambda (IFN-λ). The induced IFN-λ contributed to TLR3/RIG-I-mediated inhibition of HSV-2 replication in human cervical epithelial cells, as an antibody to IL-10Rβ, an IFN-λ receptor subunit, could compromise TLR3/RIG-I-mediated inhibition of HSV-2. Further studies showed that TLR3/RIG-I signaling in the cervical epithelial cells by dsRNA induced the expression of the IFN-stimulated genes (ISGs), ISG56, 2′-5′-oligoadenylate synthetase I (OAS-1) and myxovirus resistance A (MxA), the key antiviral elements in the IFN signaling pathway. In addition, we observed that the topical treatment of genital mucosa with poly I:C could protect mice from genital HSV-2 infection.
LIMITATIONS, REASONS FOR CAUTION
Future prospective studies with primary cells and suitable animal models are needed in order to confirm these outcomes.
WIDER IMPLICATIONS OF THE FINDINGS
The findings provide direct and compelling evidence that there is intracellular expression and regulation of IFN-λ in human cervical epithelial cells, which may have a key role in the innate genital protection against viral infections.
LARGE SCALE DATA
Not applicable.
STUDY FUNDING AND COMPETING INTEREST(S)
This work was supported by the National Natural Science Foundation of China (81301428 to L.Z. and 81271334 to W.-Z.H.), the Fundamental Research Funds for the Central Universities (2042015kf0188 to L.Z.), the China Postdoctoral Science Foundation (2013M531745 to L.Z.), the Development Program of China (‘973’, 2012CB518900 to W.-Z.H.) from the Ministry of Science and Technology of the People's Republic of China, grants (DA12815 and DA022177 to W.-Z.H.) from the National Institute on Drug Abuse (NIDA) and the open project of Hubei Key Laboratory of Wudang Local Chinese Medicine Research (WDCM005 to M.S.). The authors declare no competing financial interests.
Keywords: herpes simplex virus type 2, human cervical epithelial cells, interferon-stimulated genes, interferon-λ, Toll-like receptor 3, retinoic acid-inducible gene I
Introduction
Herpes simplex virus type 2 (HSV-2) is an enveloped, nuclear replicating, double-stranded DNA virus, that belongs to the subgroup of alphaherpesvirinae. HSV-2 is among the most common human infectious viral pathogens, causing lifelong diseases with clinical manifestations including cold sores, genital lesions, corneal blindness and encephalitis (Fife et al., 2008; Sperling et al., 2008; Zhang et al., 2009, 2012; Dasgupta and BenMohamed, 2011). Although diseases caused by HSV-2 are generally self-limiting, the virus can cause devastating dissimilated infections and encephalitis in immunocompromised individuals, such as newborns, persons with transplantation, and AIDS-patients (Zhang et al., 2015). Furthermore, HSV-2 infection promotes HIV infection and transmission (Shao et al., 2015; Wen et al., 2015). To date, there is no vaccine available to prevent genital HSV-2 infection (Lin et al., 2015). In addition, current treatment options are limited to the control of virus reactivation and do not eliminate latent virus (Chao et al., 2015; Chiang et al., 2015). Engagement of toll-like receptors (TLRs) activates signaling cascades that culminate in inflammatory and immune defense responses. Among the 10 identified human TLRs, TLR3 has been recognized as one of the major sensors in virus-mediated innate immune response (Barton and Medzhitov, 2002). TLR3 activation induces type I interferon (IFN) expression (Katze et al., 2002; Borden et al., 2007), which is crucial for protection against HSV infection of the target cells in both the genital tract (Gill et al., 2006) and central nervous system (CNS) (Zhou et al., 2009b). In addition, TLR3 activation could induce the expression of type III IFN (IFN-λ), a new member of IFN family (Ank and Paludan, 2009; Li et al., 2012). IFN-λ can be produced by a number of cell types, including non-immune cells (Ank et al., 2006), although the pattern of its expression has not been elucidated. In addition to TLR3, retinoic acid-inducible gene I (RIG-I) is also an important mediator of antiviral immunity, as it has the ability to recognize viral RNAs in cytoplasm and trigger IFN-mediated immune protection against viral infections (Fujita et al., 2007). HSV-2 infection occurs at, and emanates, from mucosal surfaces (Roizman and Taddeo, 2007). The mucosal immunity in the genital tract is considered as an initial and primary innate defense mechanism against HSV-2 infection (Chan et al., 2011). Epithelial cells at the surface of the female reproductive tract participate in the mucosal innate immunity against viral infections. Epithelial cells have been reported to selectively express TLR1, TLR2, TLR3, TLR5 and TLR6 (Fichorova et al., 2002; Hertz et al., 2003; Smith et al., 2003; Schaefer et al., 2004; Funami et al., 2007). However, it remains to be determined whether cervical epithelial cells can contribute to TLR-mediated innate immunity against HSV-2 infection. In the present study, we examined whether human cervical epithelial cells have the ability to mount a TLR3/RIG-I-mediated innate immunity that is effective in the control of HSV-2 infection.
Materials and Methods
Reagents
All culture plasticware was obtained from Corning (Corning, NY, USA). Mouse anti-IL-10Rβ antibody was purchased from R&D system Inc. (Minneapolis, MH, USA) and mouse IgG (control IgG) was from Molecular Probes (Eugene, OR, USA).
Cell culture
Vero cell line (African green monkey kidney epithelial cells) was cultured at 37°C with 5% CO2 in Dulbecco's modified Eagle's culture medium (DMEM, Gibco, Grand Island, NY, USA) containing 10% heat-inactivated fetal bovine serum (FBS, Gibco), 2 mM l-glutamine, 50 units/ml penicillin and 50 μg/ml streptomycin (Gibco). Human End1/E6E7 cell line is a well differentiated endocervical epithelial cell line immortalized by HPV 16 E6/E7 and derived from normal endocervical epithelium (Fichorova et al., 1997), which can be grown as a polarized monolayer (Sathe and Reddy, 2014). The morphological and immunocytochemical characteristics of this immortalized cell line closely resemble the tissue of origin and primary cultures, and differ significantly from the Hela cells (Fichorova et al., 1997). End1/E6E7 cells have been extensively used as a human endocervical epithelial model for in vitro studies (Verhoog et al., 2011; Govender et al., 2014; Sathe and Reddy, 2014; Hijazi et al., 2015). The cells respond well to the TLRs and RIG-I ligands even after polarization (Sathe and Reddy, 2014). The cells were cultured in keratinocyte growth medium (Gibco) supplemented with the provided 50 μg/ml bovine pituitary extract, 0.1 ng/ml recombinant epidermal growth factor, 50 units/ml penicillin and 50 μg/ml streptomycin (Gibco).
TLRs and RIG-I activation
Because of the lack of the uptake of poly I:C by the cells, the direct addition of poly I:C to the cell cultures failed to induce IFN-λ induction (Supplementary Fig. S3). Thus, we used a transfection reagent Lyovec (Invivogen, San Diego, CA, USA) for TLR3/RIG-I activation experiments throughout the study. The cells were stimulated with poly I:C (TLR3 ligand), imiquimod (TLR7 ligand), ssRNA 40 (TLR8 ligand), ODN2006 (TLR9 ligand), 5′ppp dsRNA (RIG-I ligand), or 5′ppp dsRNA control using Lyovec transfection reagent (Invivogen). Lyovec-treated cells were used as a vehicle control. The cell culture medium with transfection reagent was replaced with fresh keratinocyte growth medium 12 h post-transfection. Cells were collected for RNA extraction at indicated time points. For disruption of TLR3 function, cells were treated with 100 nM of TLR3/dsRNA Complex Inhibitor (abbreviated as TCI; EMD Millipore, Inc., Billerica, MA, USA) for 1 h prior to Poly I:C transfection (Cheng et al., 2011).
Cell viability assay
End1/E6E7 cells seeded in 96-well plate (104/well) were treated with poly I:C or 5′ppp dsRNA for 72 h. Cells were then exposed to 3-(4, 5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (20 μl/well). The formation of soluble formazan from MTT was measured by spectrophotometric determination of absorption at 570 nm using a plate reader (SpectraMax i3, Molecular Devices, Sunnyvale, CA, USA).
Virus propagation and infection
HSV-2 G strain was obtained as a gift from Dr Qinxue Hu (State Key Laboratory of Virology, Wuhan Institute of Virology, Chinese Academy of Sciences, China). Viruses were propagated and purified from Vero cells by the standard sucrose gradient procedure and used for infection studies at a multiplicity of infection of 0.001. After adding the viruses, plates were incubated at 37°C for 90 min followed by washing with DMEM. The cell cultures were then maintained in fresh keratinocyte growth medium for up to 72 h. Cellular DNA was extracted from HSV-2-infected cells with DNA lysis buffer containing 100 mM KCl, 20 mM Tris, pH 8.4, 500 μg/ml proteinase K (Sigma-Aldrich, St. Louis, MO, USA), and 0.2% (v/v) NP-40 (BDH, Poole, UK). Lysates were incubated at 60°C for 2 h followed by 100°C for 15 min. HSV-2 gD was analyzed by real-time PCR using the specific primers 5′-TCTCCGTCCAGTCGTTTAT-3′ (sense) and 5′-ATCCGAACGCAGCCCCGC-3′ (antisense), and was quantified by using serial dilutions of HSV-2 gD standards with known copy numbers.
HSV-2 genital infection of mice
The protocol for the animal experiments was approved by the Institutional Animal Care and Use Committee at the Center for Animal Experiment, Wuhan University. In order to reduce experimental variations due to menstruation difference of female mice, 6-week-old WT Balb/c female mice were pretreated by subcutaneous injection (s.c.) of 2 mg Depo-Provera (Pfizer, Morris Plains, NJ, USA), the female hormone progesterone that prevents pregnancy by stopping the oocyte release from ovaries. Five days later, mice were inoculated intravaginally (i.vag.) with 30 μl of a half lethal dose (LD50, 3000 pfu) of HSV-2 G strain in DMEM. The mice were placed on their back and maintained for 10 min.
Animals were assessed daily for disease signs (genital pathology) for 15 days after HSV-2 infection (p.i.). The survival rate of infected animals was observed for 21 days p.i. (Herbst-Kralovetz and Pyles, 2006). Genital pathology was scored daily using a 5-point scale (Kaushic et al., 2003): 0, no pathology; 1, mild vulvar erythema; 2, moderate vulvar erythema and swelling; 3, severe vulvar erythema and swelling and perineal fur loss; 4, perineal ulceration; 5, extension of perineal ulceration and fur loss to surrounding tissue, or death. Mice were euthanized when they reached score 5. To quantify the protection provided by poly I:C treatment, 16 h before or 4 h after intravaginal inoculation of HSV-2, mice were given i.vag. poly I:C (25 μg) suspended in 30 μl of Lyovec transfection reagent.
RNA extraction and real-time RT–PCR
Total cellular RNA was extracted from cells using TRI-Reagent (Molecular Research Center, Cincinnati, OH, USA) as described previously (Zhou et al., 2009a, b; Li et al., 2012). Total RNA was subjected to the reverse transcription using reagents obtained from Promega (Madison, WI, USA). The real-time PCR for the quantification of GAPDH, IFN-λ1, IFN-λ2/3, TLR1-10, RIG-I, IRF3, IRF7, ISG56, OAS-1, MxA and PKR mRNA (Table I) was performed with IQ SYBR Green supermix (Bio-Rad Laboratories, Hercules, CA, USA) as described previously (Li et al., 2012). The levels of GAPDH mRNA were used as an endogenous reference to normalize the quantities of target mRNA. The sequences of oligonucleotide primers used are listed in Table I and were synthesized by Invitrogen Inc.
Table 1.
Primer sets for real-time RT–PCR.
| Primer | Accession no. | Orientation | Sequences | Product (bp) |
|---|---|---|---|---|
| GAPDH | NM_002046 | Sense | 5′-GGTGGTCTCCTCTGACTTCAACA-3′ | 127 |
| Antisense | 5′-GTTGCTGTAGCCAAATTCGTTGT-3′ | |||
| IFN-λ1 | NM_172140 | Sense | 5′-CTTCCAAGCCCACCCCAACT-3′ | 142 |
| Antisense | 5′-GGCCTCCAGGACCTTCAGC-3′ | |||
| IFN-λ2/3 | NM_172138/ | Sense | 5′-TTTAAGAGGGCCAAAGATGC-3′ | 267 |
| NM_172139 | Antisense | 5′-TGGGGCTGAGGCTGGATACAG-3′ | ||
| IRF3 | NM_001571 | Sense | 5′-ACCAGCCGTGGACCAAGAG-3′ | 60 |
| Antisense | 5′-TACCAAGGCCCTGAGGCAC-3′ | |||
| IRF7 | NM_001572 | Sense | 5′-TGGTCCTGGTGAAGCTGGAA-3′ | 134 |
| Antisense | 5′-GATGTCGTCATAGAGGCTGTTGG-3′ | |||
| TLR1 | NM_003263 | Sense | 5′-GCCTATATGCAAAGAGTTTGGC-3′ | 134 |
| Antisense | 5′-CTCTCCTAAGACCAGCAAGACC-3′ | |||
| TLR2 | NM_003264 | Sense | 5′-GGCTTCTCTGTCTTGTGACC-3′ | 292 |
| Antisense | 5′-GGGCTTGAACCAGGAAGACG-3′ | |||
| TLR3 | NM_003265 | Sense | 5′-AGCCACCTGAAGTTGACTCAGG-3′ | 268 |
| Antisense | 5′-AGCCACCTGAAGTTGACTCAGG-3′ | |||
| TLR4 | NM_138554 | Sense | 5′-CAGAGTTTCCTGCAATGGATCA-3′ | 85 |
| Antisense | 5′-GCTTATCTGAAGGTGTTGCACAT-3′ | |||
| TLR5 | NM_003268 | Sense | 5′-AGCCATCTGACTGCATTAAGG-3′ | 336 |
| Antisense | 5′-GACTTCCTCTTCATCACAACC-3′ | |||
| TLR6 | NM_006068 | Sense | 5′-ATTGAAAGCATTCGTGAAGAAG-3′ | 123 |
| Antisense | 5′-ACGGTGTACAAAGCTGTCTGTG-3′ | |||
| TLR7 | NM_016562 | Sense | 5′-AAAATGGTGTTTCCAATGTGG-3′ | 107 |
| Antisense | 5′-GGCAGAGTTTTAGGAAACCATC-3′ | |||
| TLR8 | NM_138636 | Sense | 5′-TTATGTGTTCCAGGAACTCAGAGAA-3′ | 83 |
| Antisense | 5′-TAATACCCAAGTTGATAGTCGATAAGTTTG-3′ | |||
| TLR9 | NM_017442 | Sense | 5′-TACCAACATCCTGATGCTAGACTC-3′ | 231 |
| Antisense | 5′-TACCAACATCCTGATGCTAGACTC-3′ | |||
| TLR10 | NM_001017388 | Sense | 5′-GGCCAGAAACTGTGGTCAAT-3′ | 205 |
| Antisense | 5′-AAATGACTGCATCCAGGGAG-3′ | |||
| RIG-I | AF038963 | Sense | 5′-CTTGGCATGTTACACAGCTGAC-3′ | 104 |
| Antisense | 5′-GCTTGGGATGTGGTCTACTCA-3′ | |||
| ISG56 | NM_001270930 | Sense | 5′-TTCGGAGAAAGGCATTAGA-3′ | 85 |
| Antisense | 5′-TCCAGGGCTTCATTCATAT-3′ | |||
| OAS-1 | NM_001032409 | Sense | 5′-AGAAGGCAGCTCACGAAACC-3′ | 71 |
| Antisense | 5′-CCACCACCCAAGTTTCCTGTA-3′ | |||
| MxA | NM_001178046 | Sense | 5′-GCCGGCTGTGGATATGCTA-3′ | 69 |
| Antisense | 5′-TTTATCGAAACATCTGTGAAAGCAA-3′ | |||
| PKR | NM_001139518 | Sense | 5′-AGAGTAACCGTTGGTGACATAACCT-3′ | 80 |
| Antisense | 5′-GCAGCCTCTGCAGCTCTATGTT-3′ |
Knockdown of RIG-I, IRF3 or IRF7
Lentiviral plasmids with sequence-verified shRNA for gene silencing of RIG-I was obtained from Dr. Shi Liu (Wuhan University, China) and plasmids for gene silencing of IRF3 and IRF7 were gifts from Dr. Rongtuan Lin (McGill University, Canada). End1/E6E7 cells were plated in a 48-well plates at 2 × 104 per well 24 h before transfection. Cells were then transfected with the plasmids using Lipofectamine 2000 (Invitrogen) at a ratio of 1:2 (μg:μl) prepared in keratinocyte growth medium. After 48 h, cells were stimulated with poly I:C. shRNA control vector pLKO.1 (SHC002; Sigma) was included as a nontarget knockdown control in the experiments.
Western blot
Total cell lysates of End1/E6E7 cells treated with poly I:C for 24 h were prepared by using the cell extraction buffer (Invitrogen, Shanghai, China) with 1% protease inhibitor cocktail (Sigma, MO, USA) according to the manufacturer's instructions. Equal amounts of protein lysates (20 μg) were separated on 4–12% sodium dodecyl sulfate polyacrylamide gel electrophoresis precast gels and transfected to an polyvinylidene difluoride membranes (Millipore, Germany). The blots were incubated with primary antibodies in 2% BSA in phosphate-buffered saline with 0.05% Tween 20 (PBST) overnight at 4°C (IRF3, 1:2000; IRF7, 1:2000; ISG56, 1:2000; OAS-1, 1:1000; MxA, 1:1000; GAPDH, 1:5000). The blots were then washed with PBST and further incubated with horseradish peroxidase-conjugated appropriate second antibodies diluted at 1:5000 to 1:8000 in 2% nonfat milk PBST. Blots were developed with SuperSignal West Pico Chemiluminescent Substrated (Thermo Fisher Scientific, Waltham, MA, USA).
ELISA
IFN-λ protein levels in End1/E6E7 culture supernatant were measured with ELISA (Human IL-29/ IFN-λ1: eBioscience, San Diego, CA; Human IL-28A/IFN-λ2: R&D system Inc., Minneapolis, MH, USA). Assays were performed according to the manufacturer's instructions.
Data analysis
Where appropriate, data were expressed as mean ± Standard deviation (SD) from at least three independent experiments. For comparison of the mean of two groups, statistical significance was measured by Student's t-test. To compare the difference between multiple groups, statistical significance was analyzed using a one-way analysis of variance followed by post Newman–Keul's test. Calculations were performed with Graphpad Prism Statistical Software (GraphPad Software Inc., San Diego, CA, USA). Statistical significance was defined as P < 0.05 or P < 0.01.
Results
TLR3 activation induces IFNs
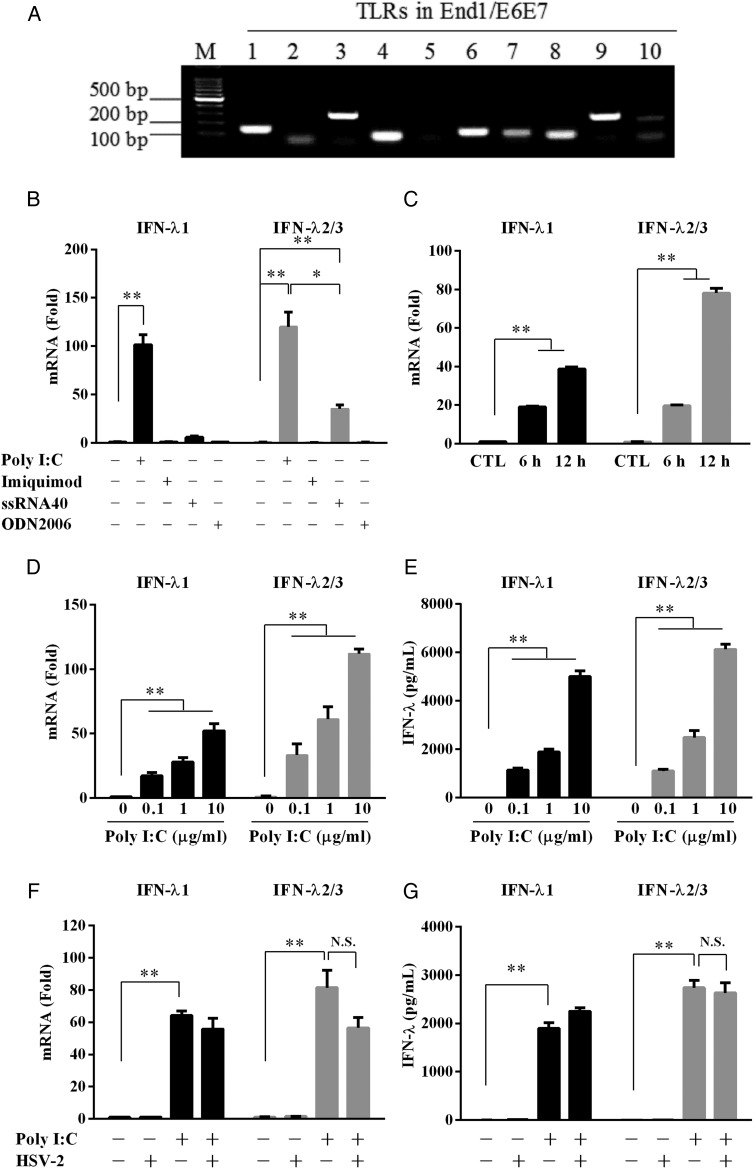
Activation of TLRs triggers intracellular IFN-mediated innate immunity against virus infections, including HSV-2. Thus, we first examined the expression of TLRs in human cervical epithelial cells. As shown in Fig. 1A, End1/E6E7 cells express mRNA for all TLRs except TLR5, however upon HSV-2 infection, only TLR3, 7, 8 and 9, the key sensors for viral infection, were significantly up-regulated (Supplementary Fig. S1). To exam whether these antiviral TLRs are biologically functional in the epithelial cells, we transfected the cells with the ligands (Poly I:C, imiquimod, ssRNA40 and ODN2006) of these TLRs. As shown in Fig. 1B, only poly I:C could significantly induce IFN-λ expression. This poly I:C-mediated induction of IFN-λ was time- and dose-dependent (Fig. 1C–E). In contract, poly I:C had little effect on the induction of IFN-α (Supplementary Fig. S2A). Although poly I:C could induce IFN-β expression in a time- and dose-dependent manner (Supplementary Fig. S2A and B), the degree of the induction was much less than that for IFN-α. HSV-2 infection inhibited poly I:C-mediated IFN-β expression in the cervical epithelial cells (Supplementary Fig. S2C). However, HSV-2 infection had little effect on the induction of IFN-λs (Fig. 1F and G). To determine the uptake efficiency of the cells by different routes of poly I:C delivery, cells were treated with fluorescein labeled poly I:C (FITC-poly I:C; TLR3 ligand) either by directly adding the reagent to the cell culture or by transfection with Lyovec. Interestingly, we found that only the transfection successfully delivered poly I:C into cells (Supplementary Fig. S3).
Figure 1.
Effect of TLRs activation on IFN-λ expression. (A) Expression of TLRs in End1/E6E7 cells. Total cellular RNA extracted from End1/E6E7 cells was subjected to the RT–PCR with the primers specific for toll-like receptors (TLRs) 1–10. Amplified PCR products were displayed on 2% agarose gel. (B) End1/E6E7 cells were transfected with poly I:C (10 μg/ml), imiquimod (10 μg/ml), ssRNA40 (10 μg/ml) or ODN2006 (5 μM) for 12 h. (C) End1/E6E7 cells were transfected with or without poly I:C (1 μg/ml) for the indicated times. Total cellular RNA was subjected to the real-time RT–PCR for the mRNA levels of IFN-λ1 and IFN-λ2/3 as indicated. (D and E) End1/E6E7 cells were transfected with or without poly I:C at indicated concentrations (1, 10 μg/ml) for mRNA (D) or protein (E) detection. (F and G) End1/E6E7 cells infected with or without HSV-2 (MOI = 0.001) for 24 h were then transfected with or without poly I:C (1 μg/ml) for mRNA (F) or for protein (G) detection. The results are the mean ± SD of triplicate cultures, representative of three experiments (*P < 0.05, **P < 0.01, N.S. as no significance difference).
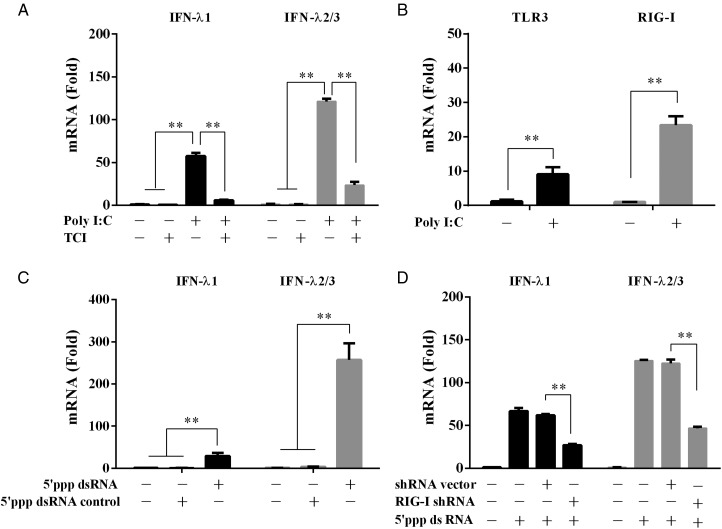
TLR3 and RIG-I are involved in poly I:C-induced IFN-λ expression
To determine the involvement of TLR3 signaling in poly I:C-mediated induction of IFN-λ, we pretreated End1/E6E7 cells with TLR3/dsRNA complex inhibitor (TCI) that blocks the binding of dsRNA to TLR3. As shown in Fig. 2A, poly I:C-induced expression of IFN-λ was significantly inhibited in TCI-pretreated cells (Fig. 2A). Since poly I:C could induce the expression of TLR3 and RIG-I, which is a key intracellular dsRNA sensor (Fig. 2B), we then examined whether RIG-I activation can induce IFN-λ in human cervical epithelial cells. As shown in Fig. 2C, treatment of End1/E6E7 cells with the 5′ppp dsRNA (RIG-I ligand) up-regulated IFN-λ expression. This 5′ppp dsRNA-induced IFN-λ expression could be compromised by RIG-I shRNA, while the control shRNA had little effect (Fig. 2D).
Figure 2.
Roles of TLR3 and RIG-I in poly I:C-mediated IFN-λ induction. (A) Effect of TLR3/dsRNA complex inhibitor (TCI) on the induction of IFN-λ by poly I:C. End1/E6E7 cells were pretreated with TCI (100 nM) for 1 h prior to poly I:C (1 μg/ml) transfection for 12 h. (B) End1/E6E7 cells were stimulated with 1 μg/ml poly I:C for 12 h. (C) End1/E6E7 cells were stimulated with 1 μg/ml 5′ppp dsRNA or 5′ppp dsRNA control for 12 h. (D) End1/E6E7 cells were pretransfected with RIG-I shRNA or control shRNA for 48 h prior to stimulation with 5′ppp dsRNA (1 μg/ml). Total cellular RNA was subjected to the real-time RT–PCR for the mRNA levels of IFN-λ1 and IFN-λ2/3 (A, C and D), TLR3 and RIG-I (B). The data are expressed as mRNA levels relative (-fold) to control (without poly I:C or 5′ppp dsRNA defined as 1). The results are mean ± SD of triplicate cultures, representative of three experiments (*P < 0.05, **P < 0.01).
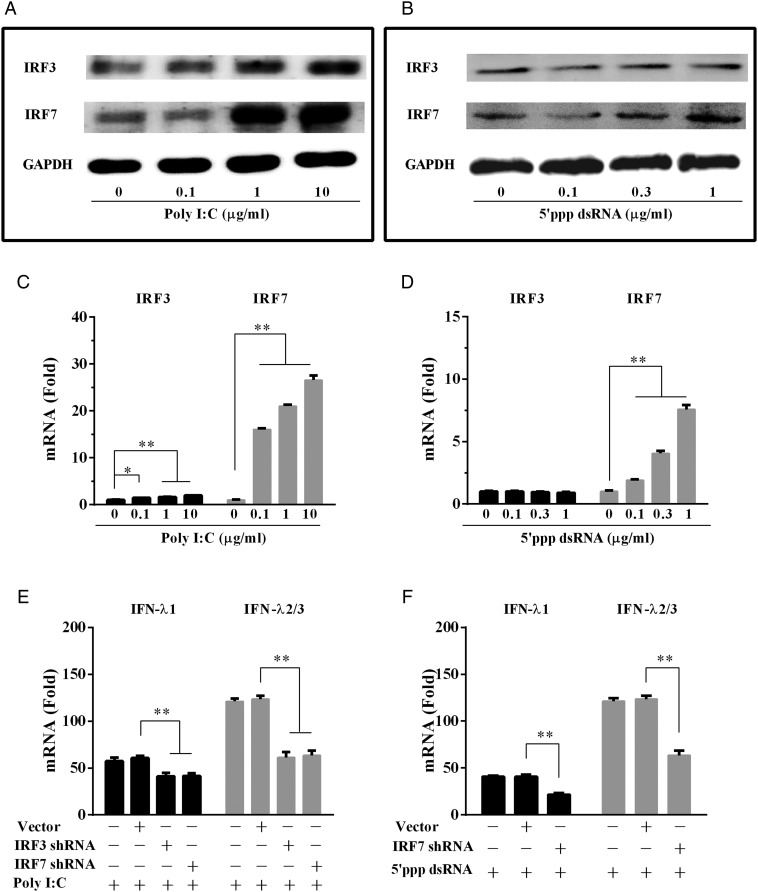
TLR3/RIG-I activation induces IRF3 and/or IRF7
The IFN regulatory factors (IRFs), particularly IRF3 and IRF7, have been implicated in the control of IFN-λ expression (Li et al., 2012). We thus examined the effect of poly I:C or 5′ppp dsRNA on the expression of IRF3 and IRF7 in the human cervical cells. As shown in Fig. 3A and C, poly I:C in a dose-dependent fashion, induced the expression of IRF3 and IRF7. While 5′ppp dsRNA could also induce the expression of IRF7 (Fig. 3B and D), but it had little effect on IRF3 expression (Fig. 3B and D). To determine the role of IRF3 and/or IRF7 in dsRNA-stimulated IFN-λ induction, we examined whether the knockdown of IRF3 or IRF7 could compromise the induction of IFN-λ. As shown in Fig. 3E, knockdown of IRF3 or IRF7 reduced poly I:C-induced expression of both IFN-λ1 and IFN-λ2/3. In addition, knockdown of IRF7 compromised 5′ppp dsRNA-induced IFN-λ1 and IFN-λ2/3 expression (Fig. 3F).
Figure 3.
Effect of TLR3/RIG-I activation on IFN regulatory factors (IRFs). (A and B) End1/E6E7 cells, transfected with or without poly I:C (A) or 5′ppp dsRNA (B) at indicated concentrations for 24 h, were subjected to western blot assay using antibodies to IRF3, IRF7 or GAPDH. GAPDH was used as the loading control. (C and D) End1/E6E7 cells were transfected with or without poly I:C (C) or 5′ppp dsRNA (D) at indicated concentrations for 12 h. (E and F) shRNA-mediated knockdown of IRF3 and/or IRF7 impaired IFN-λ expression. End1/E6E7 cells were transfected with or without control vector, IRF3 shRNA or IRF7 shRNA for 48 h. Cells were then transfected with or without poly I:C (E) or 5′ppp dsRNA (F) for additional 12 h. Total cellular RNA extracted from End1/E6E7 cells was subjected to the real-time RT–PCR detection for the mRNA levels of IRF3, IRF7 and IFN-λ. The data are expressed as mRNA levels relative (-fold) to control (without poly I:C or 5′ppp dsRNA stimulation, which is defined as 1). The results are mean ± SD of triplicate cultures, representative of three experiments (*P < 0.05, **P < 0.01).
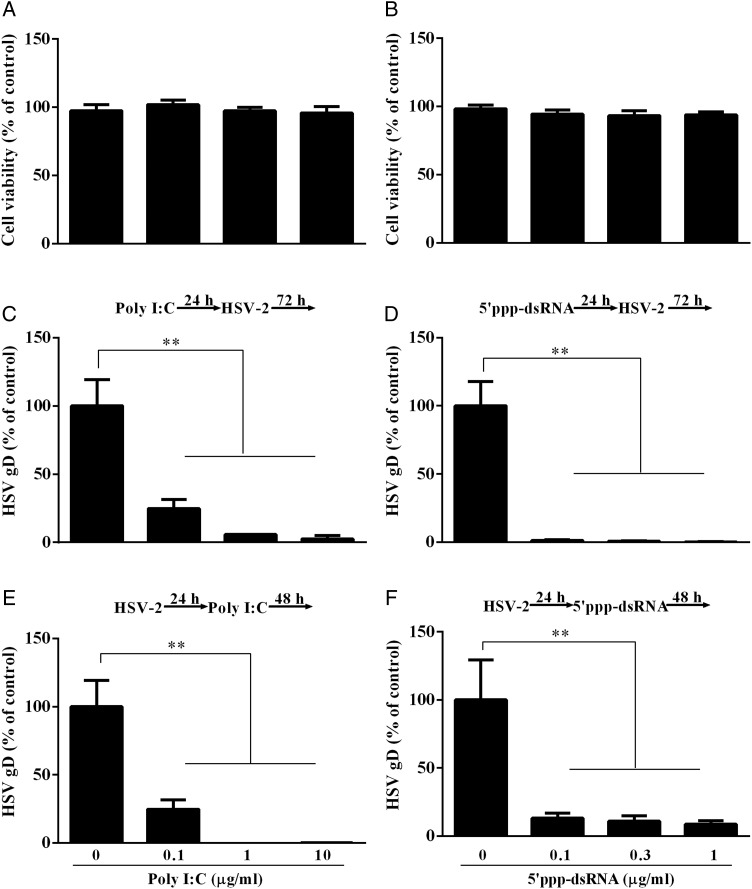
TLR3/RIG-I activation inhibits HSV-2 replication
To determine whether TLR3/RIG-I signaling of the cervical epithelial cells can inhibit HSV-2 replication, we first examined the cytotoxic effect of poly I:C or 5′ppp dsRNA on the cervical epithelial cells (End1/E6E7 cells). No cytotoxic effect was observed in the cells stimulated with either poly I:C (Fig. 4A) or 5′ppp dsRNA (Fig. 4B). We then examined the impact of poly I:C or 5′ppp dsRNA on HSV-2 infection/replication. As demonstrated in Fig. 4C–F, either poly I:C or 5′ppp dsRNA could dose-dependently inhibit HSV-2 replication in the cervical epithelial cells.
Figure 4.
Effect of TLR3, RIG-I activation on HSV-2 replication. (A and B) MTT assay of End1/E6E7 cells transfected with poly I:C or 5′ppp dsRNA. End1/E6E7 cells were transfected with poly I:C (A) or 5′ppp dsRNA (B) at the concentrations indicated at the bottom of the figure. Cells viability was assessed by MTT assay 72 h post transfection. The results are mean ± SD of triplicate cultures, representative of three experiments. (C and E) End1/E6E7 cells were transfected with poly I:C at the concentrations indicated at the bottom of the figure for 24 h prior to HSV-2 infection (MOI = 0.001) (C) or transfected with poly I:C at 24 h post-infection (E). (D and F) End1/E6E7 cells were transfected with 5′ppp dsRNA at concentrations indicated at the bottom of the figure for 24 h prior to HSV-2 infection (MOI = 0.001) (D) or transfected with 5′ppp dsRNA at 24 h post-infection (F). Genomic DNA extracted from the cell cultures at 72 h post-infection was subjected to the real-time PCR for HSV-2 quantification. The data are expressed as HSV-2 gD gene levels relative (%) to the control (without poly I:C treatment, which is defined as 100). The results are mean ± SD of triplicate cultures, representing three independent experiments (**P < 0.01).
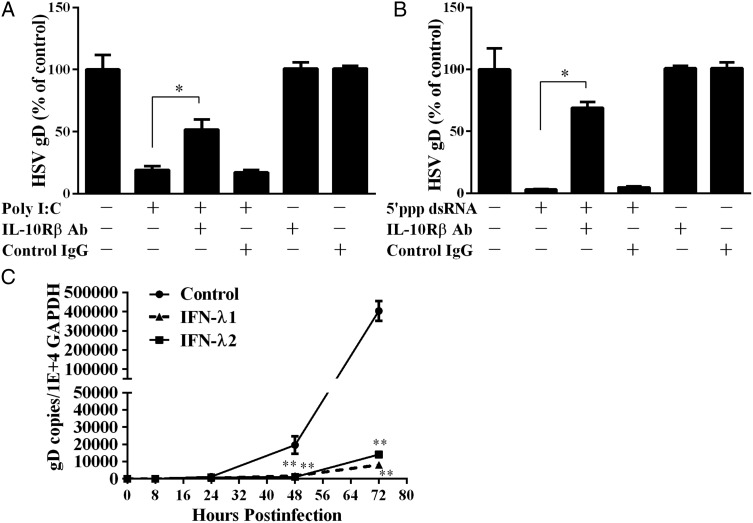
IFN-λ contributes to TLR3/RIG-I-mediated anti-HSV-2 activation
To determine whether TLR3/RIG-I activation-induced IFN-λ is responsible for HSV-2 inhibition in epithelial cells, the cells were treated with or without the antibody to IFN-λ receptor (IL-10Rβ) in the presence of poly I:C or 5′ppp dsRNA. As shown in Fig. 5A and B, the anti-HSV-2 effects of poly I:C or 5′ppp dsRNA were partially reversed in the cells treated with the antibody to IL-10Rβ. The anti-HSV-2 effect of endogenous IFN-λ was also confirmed in experiments using recombinant IFN-λ1 or IFN-λ2 (Fig. 5C).
Figure 5.
The role of IFN-λ in TLR3/RIG-I activation-mediated HSV-2 inhibition. (A and B) End1/E6E7 cells were treated with poly I:C (1 μg/ml) (A) or 5′ppp dsRNA (B) and anti-IL-10Rβ antibody or control IgG at the concentration of 5 μg/ml for 24 h prior to HSV-2 infection. HSV-2 replication was quantified 72 h post-infection. (C) End1/E6E7 cells were treated with recombinant IFN-λ1 or IFN-λ2 (100 ng/ml) for 24 h and then infected with HSV-2 (MOI = 0.001). Genomic DNA extracted from the cell cultures at the indicated time points post-infection was subjected to the real-time PCR for HSV-2 quantification by using serial dilutions of HSV-2 gD standards with known copy numbers. The results are mean ± SD of triplicate cultures, representative of three experiments (*P < 0.05, **P < 0.01).
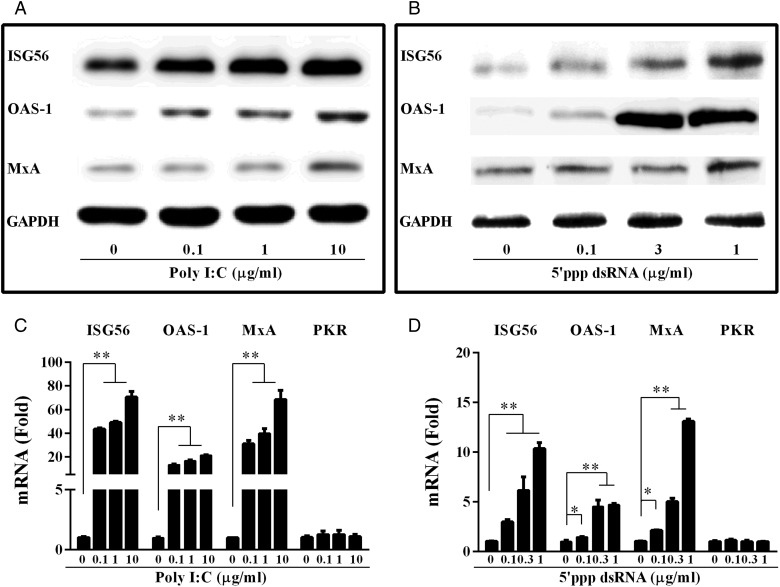
TLR3/RIG-I activation induces ISGs
It is known that IFN activation triggers an antiviral state, which is characterized by the induction of multiple IFN-stimulated genes (ISGs) (Katze et al., 2002). We thus examined the effect of poly I:C or 5′ppp dsRNA on the expression of several key antiviral ISGs (ISG56, OAS-1, MxA and PKR) in epithelial cells. As shown in Fig. 6, poly I:C or 5′ppp dsRNA dose-dependently induced the expression of ISG56, OAS-1 and MxA, but not PKR, in the cells.
Figure 6.
Effect of poly I:C and 5′ppp dsRNA on the expression of IFN-stimulated genes (ISGs). (A and B) Total proteins extracted from End1/E6E7 cells transfected with or without poly I:C (A) or 5′ppp dsRNA (B) at indicated concentrations for 24 h were subjected to western blot assay using antibodies to ISG56, MxA, OAS-1 or GAPDH. (C and D) End1/E6E7 cells were transfected with or without poly I:C (C) or 5′ ppp dsRNA (D) at indicated concentrations for 12 h. Total cellular RNA extracted from End1/E6E7 cells was subjected to the real-time RT–PCR detection for the mRNA levels of ISG56, OAS-1 MxA and PKR. The data are expressed as mRNA levels relative (-fold) to control (without treatment, defined as 1). The results are mean ± SD of triplicate cultures, representative of three experiments (*P < 0.05, **P < 0.01).
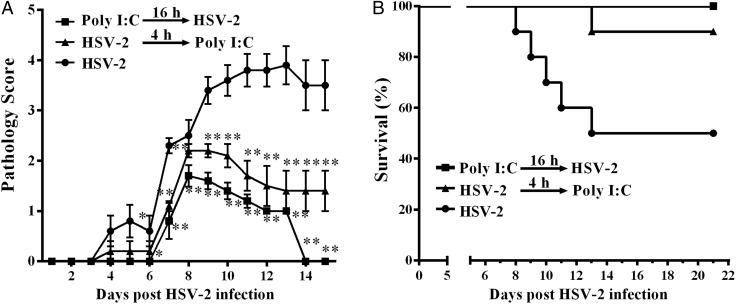
Poly I:C treatment reduces genital HSV-2 infection of mice
To determine whether the in vitro anti-HSV-2 activity mediated by TLR3/RIG-I signaling of the cervical epithelial cells has in vivo significance, we investigated the preventive effect of poly I:C topical treatment on genital mucosa of mice. Prophylactic poly I:C treatment (16 h prior to HSV-2 infection) significantly reduced HSV-2 infection-mediated pathological damage in genital mucosa and completely protected the mice from death due to HSV-2 infection (Fig. 7 and Table II). The treatment of mice with poly I:C 4 h after HSV-2 infection also reduced the pathological damage and death by HSV-2 infection (Fig. 7 and Table II).
Figure 7.
Effect of disease progression in HSV-2 infected mice. Poly I:C 25 μg suspended in 30 μl Lyovec transfection reagent was delivered to each mice prophylactically (n = 10) or therapeutically (n = 10), 16 h before or 4 h after, intravaginal inoculation of HSV-2 (3000 pfu/mouse). Mice were then monitored daily for genital pathology (A) and survival rate (B). Groups were compared for statistical significance relative to HSV-2 infection group (n = 10) by analysis of variance (*P < 0.05, **P < 0.01).
Table II.
Poly I:C treatment reduced HSV-2 infection-mediated pathological damage in genital mucosa.
| Treatmenta | Incidenceb (%) | Time to symptomsc (days) | Survival timec (days) | Survivald (%) |
|---|---|---|---|---|
| Poly I:C/Lyovec, 16 h prior | 60 | 7.6 ± 0.5 | 21 | 100 |
| Poly I:C/Lyovec, 4 h after | 80 | 6.7 ± 0.9 | 20.1 ± 2.7 | 90 |
| PBS alone | 100 | 5.9 ± 1.4 | 16.4 ± 4.9 | 50 |
aIntravaginal application of Poly I:C/Lyovec was delivered prophylactically (n = 10, 16 h prior to challenge) or therapeutically (n = 10, 4 h after challenge) relative to viral challenge with 3000 pfu.
bIncidence of disease is measured by the development of hair loss and erythema and is expressed as the number of mice that develop symptoms/number of the animals in the group.
cMean for mice with disease signs with 15 days p.i. or mean survival time within 21 days p.i.
dSurvival is presented as number of mice alive 21 days p.i./number of animals in the group.
Discussion
As the first line of cells in contact with invading pathogens, cervical epithelial cells play an essential role in mucosal innate immunity against viral infections (Wira et al., 2005; Drannik et al., 2013; Cannella et al., 2014). Human cervical epithelial cells express functional TLR3 as well as other TLRs (Ashkar et al., 2004; Sathe and Reddy, 2014). The ligands for TLRs 3, 5, or 9 can protect genital epithelial cells from HSV-2 genital infection (Janeway and Medzhitov, 2002; Herbst-Kralovetz and Pyles, 2006; Nazli et al., 2009). In addition, the mucosal delivery of TLR3 or TLR9 ligands can protect mice from HSV-2 genital infection (Ashkar et al., 2004; Gill et al., 2006). Compared with TLR5 or TLR9 ligand, the TLR3 ligand poly I:C has selective advantages, including less local inflammation in the genital mucosa (Ashkar et al., 2004) and no splenomegaly (Alexopoulou et al., 2001; Azulay-Debby et al., 2007; Gill et al., 2008). These advantages are clinically significant and relevant. Therefore, the present study focused on poly I:C-mediated anti-HSV-2 effects in genital epithelial cells. We demonstrated that the activation of TLR3 by poly I:C significantly enhanced the expression of all three members of the IFN-λ family (Fig. 1B), which provided a sound explanation for the poly I:C-mediated anti-HSV-2 effect. Interestingly, TLR7 or TLR9 stimulation with their ligands had little impact on IFN-λ induction (Fig. 1B), suggesting that TLR3 is a key player in the induction of IFN-λ in human cervical epithelial cells. An early study showed that there was differential induction of innate antiviral responses by TLR ligands against HSV-2 infection in primary genital epithelium of women (Nazli et al., 2009).
To determine whether the TLR3 signaling pathway plays a major role in poly I:C-mediated IFN-λ induction in human cervical epithelial cells, we used TCI, an inhibitor of the TLR3 signaling pathway, to treat End1/E6E7 cells prior to poly I:C stimulation. The observation that TCI treatment could largely block the action of poly I:C on the induction of IFN-λ (Fig. 2A) supports the key role of TLR3 activation in IFN-λ induction. In addition to TLR3 signaling, the RIG-I signaling of human cervical epithelial cells also induced the expression of IFN-λ by 5′ppp dsRNA (Fig. 2C). Our further investigation of the mechanisms for the induction of IFN-λ by human cervical epithelial cells showed that the induction of IRF3 and IRF7 by poly I:C or 5′ppp dsRNA (Fig. 3A–D). The contributing role of IRF3 or IRF7 in TLR3 or RIG-I-mediated IFN-λ induction was confirmed by the knockdown experiments (Fig. 3E and F).
IFN-λ has been shown to have antiviral activities against a number of viruses (Ank et al., 2006; Doyle et al., 2006; Hou et al., 2009; Cannella et al., 2014), including HSV-2. IFN-λ can be induced by the low-risk human papillomavirus (HPV) infection, in human cervical epithelial cells (Cannella et al., 2014), although the mechanism(s) of the induction of IFN-λ remain to be determined. The induction of IFN-λ expression in the cervical epithelial cells is at least partially responsible for the TLR3/RIG-I activation-mediated anti-HSV-2 activity, as an antibody to IL-10Rβ (one of subunits of IFN-λ receptor) could at least partially reverse the inhibitory effect of poly I:C (Fig. 5A) or 5′ppp dsRNA (Fig. 5B) on HSV-2 replication in the cervical epithelial cells. Due to the lack of a commercial antibody to IL-28Rα (a subunit of IFN-λ receptor), we were unable to demonstrate whether IFN-λ is a sole contributor of TLR3/RIG-I activation-mediated HSV-2 inhibition. Nevertheless, we did not expect a complete blockage of the poly I:C effect on HSV-2 by the antibody to IFN-λ receptor, as TLR3 activation also induced IFN-β expression, that could also contribute to the suppression of HSV-2 (Nazli et al., 2009).
Like many viruses that can establish persistent infections (Finlay and McFadden, 2006), HSV-2 has evolved several mechanisms to subvert and repress the type I IFN-mediated antiviral immunity (Harle et al., 2002; Kotenko et al., 2003; Murphy et al., 2003; Pletnev et al., 2003; Duerst and Morrison, 2004; Melroe et al., 2004). We demonstrated that the ability of poly I:C to induce IFN-β was compromised in HSV-2-infected human cervical epithelial cells (Supplementary Fig. 2C). This finding is in the line with studies by others showing that HSV-2 inhibits intracellular IFN-β expression in human embryonic kidney cells and human vaginal epithelial cells (Yao and Rosenthal, 2011; Xing et al., 2013). Interestingly, HSV-2 infection had little effect on poly I:C-mediated induction of IFN-λ at both mRNA and protein levels (Fig. 1F and G). This finding suggests that IFN-λ-mediated antiviral immunity is critical in the protection of cervical epithelial cells from HSV-2 infection, as it is not impaired by HSV-2 infection. Therefore, the induction of IFN-λ and its associated antiviral factors by TLR3/RIG-I activation in cervical epithelial cells is therapeutically important for people infected with HSV-2. This statement is supported by our in vitro as well as in vivo studies. We demonstrated that TLR3/RIG-I activation in cervical epithelial cells inhibited HSV-2 infection/replication (Fig. 4). The protective effect of poly I:C or 5′ppp dsRNA on human cervical epithelial cells was observed even after HSV-2 infection had taken place (Fig. 4E and F). These findings are supported by the previous reports (Herbst-Kralovetz and Pyles, 2006; Zhou et al., 2009b; Li et al., 2012) showing that TLR3 activation results in the inhibition of HSV-2 infection of both murine and human cervical epithelial cells. In addition, we showed that the topical treatment of genital mucosa with poly I:C could protect the study mice from genital HSV-2 infection (Fig. 7). Because 5′ppp dsRNA failed to induce type I IFNs in a mouse macrophage line (data not shown), we did not examine the in vivo impact of 5′ppp dsRNA on the prevention of HSV-2 infection.
Taken together, we have, for the first time, provided the experimental evidence that human cervical epithelial cells possess a functional TLR3/RIG-I signaling system, the activation of which can mount an IFN-λ-mediated anti-HSV-2 response. The ability to mount an effective innate immune response to HSV-2 infection by cervical epithelial cells is clinically significant as it has potential in clinical use for genital protection from HSV-2 infection. However, due to the limitation of using the cervical epithelial cell line in this study, more studies with primary cells and suitable animal models are necessary to determine whether the TLR3/RIG-I signaling in cervical epithelial cells is clinically beneficial for anti-HSV-2 mucosal immunity in the female reproductive tract. These studies will be critical for the design and development of TLR3/RIG-I signaling-based intervention and treatment strategies for the control of HSV-2 transmission and infection.
Supplementary data
Supplementary data are available at http://molehr.oxfordjournals.org/.
Authors' roles
L.Z. and W.-Z.H. conceived and designed the experiments; L.Z., J.-L.L. and Y.Z. performed the experiments; L.Z., K.Z., M.S. and W.-Z.H. analyzed the data; J.-B.L. S.L., J.-G.W. and J.-F.G. contributed to the reagents, materials and analysis tools; L.Z., J.-L.L. and W.-Z.H. wrote the paper.
Funding
This work was supported by the National Natural Science Foundation of China (81301428 to L.Z. and 81271334 to W.-Z.H.), the Fundamental Research Funds for the Central Universities (2042015kf0188 to L.Z.), the China Postdoctoral Science Foundation (2013M531745 to L.Z.), the Development Program of China (‘973’, 2012CB518900 to W.-Z.H.) from the Ministry of Science and Technology of the People's Republic of China, grants (DA12815 and DA022177 to W.-Z.H.) from the National Institute on Drug Abuse (NIDA) and the open project of Hubei Key Laboratory of Wudang Local Chinese Medicine Research (WDCM005 to M.S.).
Conflict of interest
The authors declare no competing financial interests.
Supplementary Material
Acknowledgements
We thank Dr Qinxue Hu (State Key Laboratory of Virology, Wuhan Institute of Virology, Chinese Academy of Sciences) for providing the HSV-2 G strain, and Dr Rongtuan Ling (McGill University) for the lentiviral plasmids encoding IRF3 and IRF7 shRNA.
References
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 2001;413:732–738. [DOI] [PubMed] [Google Scholar]
- Ank N, Paludan SR. Type III IFNs: new layers of complexity in innate antiviral immunity. Biofactors 2009;35:82–87. [DOI] [PubMed] [Google Scholar]
- Ank N, West H, Bartholdy C, Eriksson K, Thomsen AR, Paludan SR. Lambda interferon (IFN-lambda), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. J Virol 2006;80:4501–4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkar AA, Yao XD, Gill N, Sajic D, Patrick AJ, Rosenthal KL. Toll-like receptor (TLR)-3, but not TLR4, agonist protects against genital herpes infection in the absence of inflammation seen with CpG DNA. J Infect Dis 2004;190:1841–1849. [DOI] [PubMed] [Google Scholar]
- Azulay-Debby H, Edry E, Melamed D. CpG DNA stimulates autoreactive immature B cells in the bone marrow. Eur J Immunol 2007;37:1463–1475. [DOI] [PubMed] [Google Scholar]
- Barton GM, Medzhitov R. Toll-like receptors and their ligands. Curr Top Microbiol Immunol 2002;270:81–92. [DOI] [PubMed] [Google Scholar]
- Borden EC, Sen GC, Uze G, Silverman RH, Ransohoff RM, Foster GR, Stark GR. Interferons at age 50: past, current and future impact on biomedicine. Nat Rev Drug Discov 2007;6:975–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannella F, Scagnolari C, Selvaggi C, Stentella P, Recine N, Antonelli G, Pierangeli A. Interferon lambda 1 expression in cervical cells differs between low-risk and high-risk human papillomavirus-positive women. Med Microbiol Immunol 2014;203:177–184. [DOI] [PubMed] [Google Scholar]
- Chan T, Barra NG, Lee AJ, Ashkar AA. Innate and adaptive immunity against herpes simplex virus type 2 in the genital mucosa. J Reprod Immunol 2011;88:210–218. [DOI] [PubMed] [Google Scholar]
- Chao CT, Chen YC, Chiang CK, Huang JW, Hu FC, Fang CC, Chang CC, Yen CJ. Sequence variants of peroxisome proliferator-activated receptor-gamma gene and the clinical courses of patients with end-stage renal disease. Dis Markers 2015;2015:763459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K, Wang X, Yin H. Small-molecule inhibitors of the TLR3/dsRNA complex. J Am Chem Soc 2011;133:3764–3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C, Beljanski V, Yin K, Olagnier D, Ben Yebdri F, Steel C, Goulet ML, DeFilippis VR, Streblow DN, Haddad EK et al. . Sequence-specific modifications enhance the broad spectrum antiviral response activated by RIG-I agonists. J Virol 2015;89:8011–8025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta G, BenMohamed L. Of mice and not humans: how reliable are animal models for evaluation of herpes CD8(+)-T cell-epitopes-based immunotherapeutic vaccine candidates? Vaccine 2011;29:5824–5836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle SE, Schreckhise H, Khuu-Duong K, Henderson K, Rosler R, Storey H, Yao L, Liu H, Barahmand-pour F, Sivakumar P et al. . Interleukin-29 uses a type 1 interferon-like program to promote antiviral responses in human hepatocytes. Hepatology 2006;44:896–906. [DOI] [PubMed] [Google Scholar]
- Drannik AG, Nag K, Sallenave JM, Rosenthal KL. Antiviral activity of trappin-2 and elafin in vitro and in vivo against genital herpes. J Virol 2013;87:7526–7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerst RJ, Morrison LA. Herpes simplex virus 2 virion host shutoff protein interferes with type I interferon production and responsiveness. Virology 2004;322:158–167. [DOI] [PubMed] [Google Scholar]
- Fichorova RN, Rheinwald JG, Anderson DJ. Generation of papillomavirus-immortalized cell lines from normal human ectocervical, endocervical, and vaginal epithelium that maintain expression of tissue-specific differentiation proteins. Biol Reprod 1997;57:847–855. [DOI] [PubMed] [Google Scholar]
- Fichorova RN, Cronin AO, Lien E, Anderson DJ, Ingalls RR. Response to Neisseria gonorrhoeae by cervicovaginal epithelial cells occurs in the absence of toll-like receptor 4-mediated signaling. J Immunol 2002;168:2424–2432. [DOI] [PubMed] [Google Scholar]
- Fife KH, Warren TJ, Justus SE, Heitman CK. An international, randomized, double-blind, placebo-controlled, study of valacyclovir for the suppression of herpes simplex virus type 2 genital herpes in newly diagnosed patients. Sex Transm Dis 2008;35:668–673. [DOI] [PubMed] [Google Scholar]
- Finlay BB, McFadden G. Anti-immunology: evasion of the host immune system by bacterial and viral pathogens. Cell 2006;124:767–782. [DOI] [PubMed] [Google Scholar]
- Fujita T, Onoguchi K, Onomoto K, Hirai R, Yoneyama M. Triggering antiviral response by RIG-I-related RNA helicases. Biochimie 2007;89:754–760. [DOI] [PubMed] [Google Scholar]
- Funami K, Sasai M, Ohba Y, Oshiumi H, Seya T, Matsumoto M. Spatiotemporal mobilization of Toll/IL-1 receptor domain-containing adaptor molecule-1 in response to dsRNA. J Immunol 2007;179:6867–6872. [DOI] [PubMed] [Google Scholar]
- Gill N, Deacon PM, Lichty B, Mossman KL, Ashkar AA. Induction of innate immunity against herpes simplex virus type 2 infection via local delivery of Toll-like receptor ligands correlates with beta interferon production. J Virol 2006;80:9943–9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill N, Davies EJ, Ashkar AA. The role of toll-like receptor ligands/agonists in protection against genital HSV-2 infection. Am J Reprod Immunol 2008;59:35–43. [DOI] [PubMed] [Google Scholar]
- Govender Y, Avenant C, Verhoog NJ, Ray RM, Grantham NJ, Africander D, Hapgood JP. The injectable-only contraceptive medroxyprogesterone acetate, unlike norethisterone acetate and progesterone, regulates inflammatory genes in endocervical cells via the glucocorticoid receptor. PLoS One 2014;9:e96497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harle P, Sainz B Jr, Carr DJ, Halford WP. The immediate-early protein, ICP0, is essential for the resistance of herpes simplex virus to interferon-alpha/beta. Virology 2002;293:295–304. [DOI] [PubMed] [Google Scholar]
- Herbst-Kralovetz MM, Pyles RB. Quantification of poly(I:C)-mediated protection against genital herpes simplex virus type 2 infection. J Virol 2006;80:9988–9997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz CJ, Wu Q, Porter EM, Zhang YJ, Weismuller KH, Godowski PJ, Ganz T, Randell SH, Modlin RL. Activation of Toll-like receptor 2 on human tracheobronchial epithelial cells induces the antimicrobial peptide human beta defensin-2. J Immunol 2003;171:6820–6826. [DOI] [PubMed] [Google Scholar]
- Hijazi K, Cuppone AM, Smith K, Stincarelli MA, Ekeruche-Makinde J, De Falco G, Hold GL, Shattock R, Kelly CG, Pozzi G et al. . Expression of genes for drug transporters in the human female genital tract and modulatory effect of antiretroviral drugs. PLoS One 2015;10:e0131405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou W, Wang X, Ye L, Zhou L, Yang ZQ, Riedel E, Ho WZ. Lambda interferon inhibits human immunodeficiency virus type 1 infection of macrophages. J Virol 2009;83:3834–3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway CA Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol 2002;20:197–216. [DOI] [PubMed] [Google Scholar]
- Katze MG, He Y, Gale M Jr. Viruses and interferon: a fight for supremacy. Nat Rev Immunol 2002;2:675–687. [DOI] [PubMed] [Google Scholar]
- Kaushic C, Ashkar AA, Reid LA, Rosenthal KL. Progesterone increases susceptibility and decreases immune responses to genital herpes infection. J Virol 2003;77:4558–4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, Langer JA, Sheikh F, Dickensheets H, Donnelly RP. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol 2003;4:69–77. [DOI] [PubMed] [Google Scholar]
- Li J, Ye L, Wang X, Hu S, Ho W. Induction of interferon-Lambda contributes to Toll-like receptor 3-mediated herpes simplex virus type 1 inhibition in astrocytes. J Neurosci Res 2012;90:399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HD, Lin FJ, Chiang TY, Lee TW. The complete mitochondrial genome sequence of Metzia formosae (Cypriniformes, Cyprinidae). Mitochondrial DNA 2015;26:257–258. [DOI] [PubMed] [Google Scholar]
- Melroe GT, DeLuca NA, Knipe DM. Herpes simplex virus 1 has multiple mechanisms for blocking virus-induced interferon production. J Virol 2004;78:8411–8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JA, Duerst RJ, Smith TJ, Morrison LA. Herpes simplex virus type 2 virion host shutoff protein regulates alpha/beta interferon but not adaptive immune responses during primary infection in vivo. J Virol 2003;77:9337–9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazli A, Yao XD, Smieja M, Rosenthal KL, Ashkar AA, Kaushic C. Differential induction of innate anti-viral responses by TLR ligands against Herpes simplex virus, type 2, infection in primary genital epithelium of women. Antiviral Res 2009;81:103–112. [DOI] [PubMed] [Google Scholar]
- Pletnev S, Magracheva E, Kozlov S, Tobin G, Kotenko SV, Wlodawer A, Zdanov A. Characterization of the recombinant extracellular domains of human interleukin-20 receptors and their complexes with interleukin-19 and interleukin-20. Biochemistry 2003;42:12617–12624. [DOI] [PubMed] [Google Scholar]
- Roizman B, Taddeo B. The strategy of herpes simplex virus replication and takeover of the host cell. In Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, Yamanishi K (eds). Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge: Cambridge University Press, 2007. [PubMed] [Google Scholar]
- Sathe A, Reddy KV. TLR9 and RIG-I signaling in human endocervical epithelial cells modulates inflammatory responses of macrophages and dendritic cells in vitro. PLoS One 2014;9:e83882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer TM, Desouza K, Fahey JV, Beagley KW, Wira CR. Toll-like receptor (TLR) expression and TLR-mediated cytokine/chemokine production by human uterine epithelial cells. Immunology 2004;112:428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Q, Xu W, Guo Q, Yan L, Rui L, Liu J, Zhao Y, Li Z. RIG-I from waterfowl and mammals differ in their abilities to induce antiviral responses against influenza A viruses. J Gen Virol 2015;96:277–287. [DOI] [PubMed] [Google Scholar]
- Smith MF Jr, Mitchell A, Li G, Ding S, Fitzmaurice AM, Ryan K, Crowe S, Goldberg JB. Toll-like receptor (TLR) 2 and TLR5, but not TLR4, are required for Helicobacter pylori-induced NF-kappa B activation and chemokine expression by epithelial cells. J Biol Chem 2003;278:32552–32560. [DOI] [PubMed] [Google Scholar]
- Sperling RS, Fife KH, Warren TJ, Dix LP, Brennan CA. The effect of daily valacyclovir suppression on herpes simplex virus type 2 viral shedding in HSV-2 seropositive subjects without a history of genital herpes. Sex Transm Dis 2008;35:286–290. [DOI] [PubMed] [Google Scholar]
- Verhoog NJ, Du Toit A, Avenant C, Hapgood JP. Glucocorticoid-independent repression of tumor necrosis factor (TNF) alpha-stimulated interleukin (IL)-6 expression by the glucocorticoid receptor: a potential mechanism for protection against an excessive inflammatory response. J Biol Chem 2011;286:19297–19310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen G, Chen C, Guo J, Zhang Z, Shang Y, Shao H, Luo Q, Yang J, Wang H, Wang H et al. . Development of a novel thermostable Newcastle disease virus vaccine vector for expression of a heterologous gene. J Gen Virol 2015;96:1219–1228. [DOI] [PubMed] [Google Scholar]
- Wira CR, Grant-Tschudy KS, Crane-Godreau MA. Epithelial cells in the female reproductive tract: a central role as sentinels of immune protection. Am J Reprod Immunol 2005;53:65–76. [DOI] [PubMed] [Google Scholar]
- Xing J, Ni L, Wang S, Wang K, Lin R, Zheng C. Herpes simplex virus 1-encoded tegument protein VP16 abrogates the production of beta interferon (IFN) by inhibiting NF-kappaB activation and blocking IFN regulatory factor 3 to recruit its coactivator CBP. J Virol 2013;87:9788–9801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao XD, Rosenthal KL. Herpes simplex virus type 2 virion host shutoff protein suppresses innate dsRNA antiviral pathways in human vaginal epithelial cells. J Gen Virol 2011;92:1981–1993. [DOI] [PubMed] [Google Scholar]
- Zhang X, Chentoufi AA, Dasgupta G, Nesburn AB, Wu M, Zhu X, Carpenter D, Wechsler SL, You S, BenMohamed L. A genital tract peptide epitope vaccine targeting TLR-2 efficiently induces local and systemic CD8+ T cells and protects against herpes simplex virus type 2 challenge. Mucosal Immunol 2009;2:129–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Dervillez X, Chentoufi AA, Badakhshan T, Bettahi I, Benmohamed L. Targeting the genital tract mucosa with a lipopeptide/recombinant adenovirus prime/boost vaccine induces potent and long-lasting CD8+ T cell immunity against herpes: importance of MyD88. J Immunol 2012;189:4496–4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Liang Z, Yin X, Shao X. Proteomic analysis of the occlusion derived virus of Clostera anachoreta granulovirus. J Gen Virol 2015;96:2394–2404. [DOI] [PubMed] [Google Scholar]
- Zhou L, Wang X, Wang YJ, Zhou Y, Hu S, Ye L, Hou W, Li H, Ho WZ. Activation of toll-like receptor-3 induces interferon-lambda expression in human neuronal cells. Neuroscience 2009a;159:629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Ye L, Wan Q, Zhou L, Wang X, Li J, Hu S, Zhou D, Ho W. Activation of Toll-like receptors inhibits herpes simplex virus-1 infection of human neuronal cells. J Neurosci Res 2009b;87:2916–2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.