Abstract
Aims
To test the effect of %0.4 stannous fluoride (SnF2) glycerin based gels on the bacterial ecology in both a clinical observational study and in vitro polymicobial biofilm model.
Methods and Results
The influence of stannous fluoride (0.4% SnF2) gels on bacteria was tested in both an observational study in children 6-12 years of age (n=20) and an in vitro biofilm model system. The plaque derived multi-species bacterial biofilm model was based on clinical bacterial strains derived directly from the clinical study. Potential changes in the plaque ecology were determined through the Human Oral Microbial Identification Microarray-HOMIM (n=10). The semiquantitative data resulting from this system were analyzed with cumulative logit models for each bacterial strain and Bonferroni adjustments were employed to correct for multiple hypothesis testing. Both hierarchical biclustering and principal components analysis were used to graphically assess reproducibility within subjects over time. Mixed effects models were used to examine changes in plaque scores and numbers of bacterial strains found in the various conditions.
Conclusions
Both the observational clinical study and the biofilm model showed that short-term use of 0.4% SnF2 gel has little effect on the bacterial plaque ecology. The amount of plaque accumulation on a subject's teeth, which was measured by plaque index scores failed to show statistical significant changes over the two baselines or after treatment (p=0.9928). The in vitro results were similar when examining the effect of 0.4% SnF2 gels on biofilm adherence through a crystal violet assay (p= 0.1157).
Significance and Impact of the Study
The bacteria within the dental biofilms showed resilience in maintaining the overall community diversity after exposure to 0.4% Stannous Fluoride Gels. The study supports that the immediate benefits of using these gels each night to manage caries in children may be strictly from fluoride ions inhibiting tooth demineralization.
Keywords: Dental Plaque, Biofilm, Stannous Fluoride, HOMIM, Microbiota
Introduction
Novel antimicrobials have the potential to be compounded with available topical therapies to substantially reduce dental plaque levels and possibly change the plaque's bacterial composition. One thorough method for examining changes to the oral bacterial taxa during a period of topical therapies is through 16s rRNA based metagenomics analyses[1]. 16s rRNA based methods like reverse-capture RNA probes in microarrays such as Human Oral Microbial Identification Microarray (HOMIM, Forsyth Institute, Boston, MA) or sequence-based analysis of the 16s rRNA gene allow for assessing cultivatable and uncultivable bacteria[2–4]. Before examining novel compounds and their effect on plaque ecology, there is a need to reexamine traditional therapies to understand the effects on bacterial composition of the plaque. Although this bacterial ecology assessment is possible, the actual importance and function of many bacterial taxa in the plaque, especially those difficult to culture, remains unclear[5]. For this reason, clinical examination needs to be complemented with in vitro biofilm models that further examine clinical strains in a plaque derived multi-species bacterial model. For available fluoride based topical therapies, it remains important to understand how these compounds affect the plaque ecology in both clinical and biofilm models before adding novel compounds.
Our study examined the nightly use of stannous fluoride (0.4% SnF2, Just for Kids, 3M) gels in children. The main reason for choosing to re-study this type of therapy is that gel based therapies are meant to augment the contact time of tin(II) and fluoride. Contact time, which is related to the substantivity of these ions, is increased by instructing the patient to spit, refrain from rinsing with water, and avoiding any drink or food for 30 minutes after use. The effect of these nightly gel treatments remain understudied especially in the plaque ecology of younger children. Gel based stannous fluorides are one of the few supplemental caries management options for children in the United States. FDA approved topical prevention options for children in the United States are extremely limited. For example, even 0.12% chlorhexidine rinses, which are now available in alcohol-free formulations, are not approved for use in children under 12. It is also a frustrating reminder that 5% sodium fluoride varnish in the United States is an off-label (non-FDA approved) use when applied by dental professionals for caries prevention. 0.4% stannous fluorides have a long history in dentistry and had traditionally been out of favor by clinicians due to the excessive exogenous tooth staining[6–8]. More recent formulations have stabilized the tin ion more effectively to address the incidence of staining. However, questions remain if the anti-plaque and antimicrobial effects of the tin ion (‘stannous’) within these gels are changed by any reduction in the staining potential of these products.
In this study, the influence of stannous fluoride (0.4% SnF2) gels on bacteria was tested in both an observational study in children 6-12 years of age and an in vitro biofilm model system. The plaque derived multi-species biofilm model was based on clinical bacterial strains derived directly from the clinical study. Potential changes in the plaque ecology were determined through the HOMIM system. Although Next Generation Sequencing will soon replace the HOMIM system (e.g. HOMING, Forsyth Institute, Boston MA) the ability to evaluate specific oral taxa was invaluable with HOMIM for our study. The HOMIM system uses reverse-capture ribosomal RNA probes to detect and evaluate the relative abundance of over 379 distinct oral taxa[9].
The specific goal of this project was to investigate the effect of short term use of a 0.4% SnF2 gel on the bacterial plaque ecology in children. The bacterial ecology was measured by the HOMIM system in both the clinical and in vitro biofilm model systems. The main purpose was to see if short-term exposure to stannous fluoride differentially affected certain oral taxa. In addition, the study examined if the bacterial species that were common between the clinical and in vitro studies shared a similar response to the 0.4% SnF2 gels.
Materials and Method
Human Subjects
Institutional Review Board approval was obtained from the Human Subjects Research Protection Program at the University of Minnesota and informed consent was obtained from parents, and assent from children age eight and older. Our initial power analysis (power= 0. 78, see below) for our main clinical objective was ten subjects who were followed longitudinally (three visits) to analyze distinct changes to their oral flora using HOMIM. In total, twenty three subjects were initially recruited to enter the study. Three subjects did not complete all aspects of the study based on cooperation. Surprisingly, only a single patient did not complete the study. That single subject made it through the first appointment but due to cooperation issues did not return. The two other subjects were not able to have their initial plaque index scored. A higher attrition rate was expected. The twelve additional subjects that were recruited were used as additional subjects for the twenty subject plaque scoring assessment comparison.
Subjects were identified in two ways; most subjects were active patients treated at the University of Minnesota Pediatric Dental Residency Program, other subjects were recruited from a list including past research participates who expressed interest in future research opportunities. Patients were further identified based on exclusion and inclusion criteria as follows. Inclusion criteria: ages 6-12 years old, current dental radiographic films within standard of care practices, high caries risk, active caries at time of initial exam. Exclusion criteria: antibiotics use within the past 3 months; significant past or current medical problem history, especially conditions that may affect salivary flow, dietary intake patterns, or routine oral hygiene; dental prophylaxis within the last 30 days; inability to spit and use regular type of toothpaste; no more than 1 prematurely lost tooth; no cross arch space maintainers. After the patients were screened, they were scheduled for the initial exam. A written consent and assent (for patients over the age of 8) was obtained prior to study enrollment. Subjects were instructed to not brush their teeth on the day of each visit.
Clinical Study
Visit 1
The caregiver/parent and child were interviewed and screened to derive a detailed caries risk assessment (CAMBRA). We utilized the MyCAMBRA program (Firsthand Technologies, Seattle, WA) to record and calculate caries risk assessment. This mobile application program was used to remove the subjective assessment in traditional CAMBRA interviews. All subjects enrolled were identified via caries risk assessment as high risk. A thorough medical history along with clinical and radiographic examination was completed. The American Dental Association radiographic protocol was followed according to frequency of radiographs. After the initial screening, approximately 5 ml of saliva was collected from the subject's mouth using a large straw to funnel the saliva. The saliva was kept under ice during collection. The saliva was frozen for the biofilm model system. A sterile sickle scaler was used to collect a supragingival plaque sample from the lingual interproximal region of teeth #24 and 25. The plaque was then placed in a vial of 0.5ml of 10 % (1:9 v/v) glycerol/Gibbon's buffer solution and kept on ice during the remainder of the appointment. Both the saliva and plaque were later frozen (-80°C) for the biofilm model system (see below). The details of the DNA extraction and 16S rRNA based metagenomics analysis are to follow. Two examiners calibrated their assessment for the first two subjects. Any plaque scores that differed between the two examiners were reassessed by both examiners. A plaque index was collected by a single examiner per visit using a modified mixed dentition Silness-Löe plaque index score. This involved recording a plaque score of 0-3 for four surfaces on 6 teeth in the dental arches for a total of 24 surfaces. Details of the o-3 plaque index score per surface are found elsewhere[10]. The teeth recorded were as follows: upper right first permanent molar, upper right permanent or primary lateral incisor, upper left first premolar or first primary molar, lower left first permanent molar, lower left permanent or primary lateral incisor and lower right first permanent premolar or first primary molar. Recording of scores of 2 and 3 was completed first. These values represent plaque accumulation that could be seen with the naked eye. Plaque disclosing solution was then utilized and all 6 teeth were evaluated again. Any site not previously recorded for plaque accumulation was then given a score of 0 or 1. 0 represented no plaque and 1 represented plaque that could only be seen via disclosing solution. After completing the plaque index, a rubber cup (without prophy paste) or toothbrush prophylaxis was used to remove the dental plaque and disclosing solution. Patients were compensated for their time and scheduled to return in approximately 14-21 days for the second visit.
Visit 2
An update of medical and dental history was obtained. The same protocol as described during visit 1 was utilized at visit 2 for collection of saliva, plaque collection for HOMIM analysis and plaque index. At the end of visit 2, parents and research subjects were given the manufacturer's instructions for the 0.4% Stannous Fluoride gel (Just for Kids, 3M,) application. Parents and research subjects were given specific instructions for use. Instructions were to use a pea-size amount once a day after brushing with toothpaste. To standardize application of the gel, subjects measured out ∼0.2ml of gel into a 1ml syringe and then applied to a toothbrush. Subjects were instructed to apply the gel over all surfaces on the teeth and brush-on thoroughly. The gel was to remain on the teeth for an additional 1 minute and then the child was instructed to spit out and not rinse with water after application. The parent and child were warned to not swallow the gel but to spit until the gel was mostly gone from the mouth. The child was instructed to refrain from eating or drinking for 30 minutes after brushing. The bottle of stannous fluoride was weighed before dispensing. Patients were compensated for their time and scheduled to return in 14-21 days for the final visit.
Visit 3
An update of the subject's medical and dental history was obtained. The same protocol, as described during visit 1, was utilized at visit 3 for collection of saliva, plaque collection for HOMIM analysis and plaque index. At the end of the study visit, a dental prophylaxis was performed with prophy paste. Patients returned the bottle of stannous fluoride gel at visit 3. The bottle of stannous fluoride was weighed. Patients were compensated for their time involved with completing the third visit.
In vitro Biofilm Study
The effect of 0.4% stannous fluoride on multispecies biofilms was investigated to determine if similar effects can be predicted in vitro. The biofilms were developed from dental plaque inoculums, which were derived from the plaque sample taken from visit 2. This approach has been used in various studies [11, 12] including an affiliated study that used plaque inoculums to develop multispecies/strains biofilms[13]. The goal of using this approach was to preserve the clinical strain attributes for a biofilm model examining the effect of 0.4 % stannous fluoride gels.
Biofilms were grown in a modified drip flow reactor system(DFR) [14], which is available from BioSurface Technologies, Bozeman, MT, USA. Adding hydroxyapatite discs custom mounted into slides that can be placed in the drip flow was the main modification. Plaque samples (Figure 1, V2) were defrosted and dispersed by sonification, and a portion was retained for DNA extraction and HOMIM analysis. The remainder was used an inoculum for the DFR. 10 μl solution of the frozen plaque was re-suspended in a Gibbon's buffer was incubated overnight anaerobically in 15 ml conical tube of 100% in basal mucin medium (BMM). BMM was used as the complex oral saliva analog growth medium [15].
Figure 1.
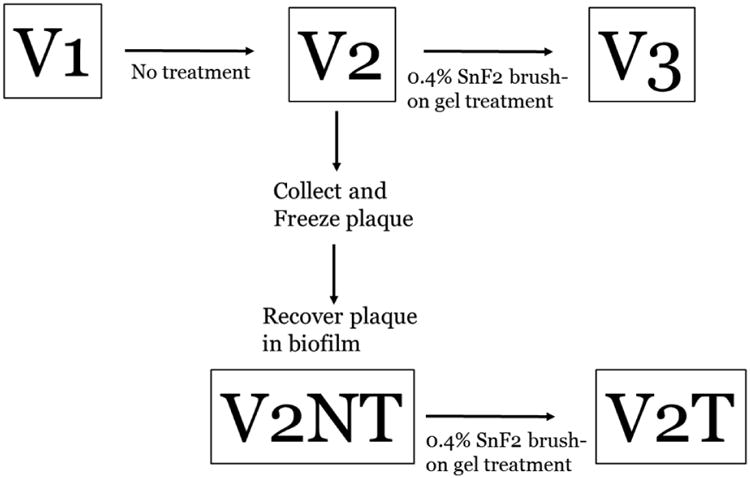
Experimental Design. At Visit 1 (V1), a baseline exam was performed including plaque collection and plaque index scoring. Since a caries indicator dye was used for the plaque index score, a rubber cup without prophy paste was used to remove the dye stained plaque. The research subject was not given any additional oral hygiene instruction. After three weeks, the research subject underwent a second baseline appointment (V2) that repeated the plaque sampling and scoring. The plaque collected at this visit was frozen at -80 degrees Celsius in 20% glycerol. This sample was later thawed and used as an inoculum for the drip flow reactor system. After two days of growth in the drip flow reactor system, biofilms were collected (V2NT). Stannous fluoride gel treatment was performed after V2 for the clinical study and V2NT. Between V2 and V3, the subject used the 0.4% SnF2 brush-on gel treatment nightly for three weeks. Between V2NT and V2T, saliva coated hydroxyapatite discs were treated 0.4% SnF2 brush-on gel prior to a 2 day growth in the drip flow reactor system.
Hydroxyapatite discs (5 mm diameter, HA; Clarkson Chromatography, South Williamsport, PA, USA), were initially coated with 0.2 μm filtered saliva [13]. The filtering preserved the salivary pellicle portion of the saliva and removed the bacterial component. This pre-wetting was done prior to the application of the placebo gel, which was custom formulated without stannous fluoride to the same relative viscosity and pH as the 0.4% stannous fluoride gel (Just for Kids, 3M,). This preparation was formulated by 3M gratis by request from the PI (RSJ). In total 6 HA discs were mounted on slides for each chamber. 0.2 ml of placebo gel was coated evenly across the 6 discs and across the entire slide. The incubated 15 ml conical tube of media (without discs) was adjusted [to 0.2 OD (600nm)] with 25 ml of new BMM media to inoculate the HA discs, anaerobically, overnight. Some initial colonization on the HA discs was encouraged through this incubation step. Samples were placed again in the anaerobic chamber shaking at 15 rpm. On the third day, the HA discs on the slides were placed in the drip flow reactor, which was housed in a 37°C incubator (Figure 2). The peristaltic pump system was set to pump a 20% concentration of BMM at 39ml/min for 10s, and then off for 50s, to equal an average a rate of 6.5ml/min/channel. One channel was run with media-only and was not inoculated with plaque. This chamber served as our contamination control. For the first several runs of our drip flow experiments, contamination was detected despite autoclaving. If the slide within our contamination control experienced any growth, the additional 5 chambers were discarded. After exploring several options, 2-hour bleaching of the whole tubing system prior to autoclaving produced the ideal sterilization. Additionally, both the placebo and stannous fluoride gels were autoclaved prior to application to standardize any residual exogenous bacterial contamination from repeated use of the container.
Figure 2.
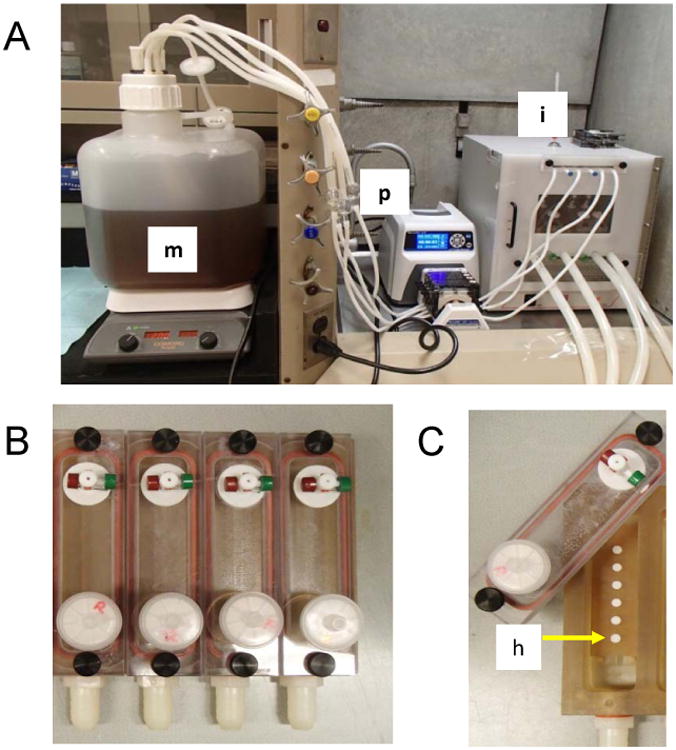
A). Drip Flow Biofilm Reactor System. BMM Media (m) was continuously pumped (p) for 10 seconds each minute. The rate of flow was to 39ml/min on 10s, off 50s to average a rate of 6.5ml/min/channel with the reactor kept at 15 degree incline to produce a light sheer stress against the biofilm. The drip flow was reactor was contained in a 37 degree C incubator (i). B) This drip flow had 6 multiple chambers (4 shown). C) Hydroxyapatite (h) discs where placed in a serial chain along the drip flow media path to the efflux tubing that drained into a waste container.
After 48 hours, biofilms were recovered from the HA discs treated with the placebo gel. These biofilms were referred to as V2NT. This denoted that these biofilms were recovered from HA discs coated with the non-treatment placebo gel and the original inoculum was the plaque from the visit 2 clinical sample. For some experiments, a single disc within each chamber was used for a crystal violet assay (see below) to determine the degree of biomass accumulation. V2NT biofilms from each research subject were handled individually. Biofilms from each V2NT subject were collected and frozen (-20°C) for temporary use as a stock biofilm and for HOMIM analysis within a short time period(see below). Longer storage for stock biofilms were kept at -80°C
For the next step of the in vitro biofilm model, we examined the effect of 0.4% stannous fluoride gels on the V2NT samples. Although V2NT biofilms would be expected to have a reduced number of species from the original plaque sample, the goal of the next step of the experiment was to determine if 0.4% stannous fluoride gel had any effect on the isolated bacteria within the simplified model. 10 μl solutions of the frozen V2NT biofilms re-suspended in Gibbon's buffer were used as the inoculum for discs coated the 0.4% Stannous Fluoride gel (Just for Kids, 3M,). The entire drip flow reactor experiment was repeated for these discs treated with 0.4 % stannous fluoride gel. The recovered biofilms (V2T) were analyzed by crystal violet, collected and frozen for later HOMIM analysis.
To reiterate, for each research subject, visit 2 plaque (V2) was used as an inoculum for biofilms that were first exposed to the placebo gel. Recovered biofilms (V2NT) were then frozen. Then the frozen biofilms were used as an inoculum for a second drip flow reactor experiment with HA discs coated with 0.4% Stannous Fluoride gel (Figure 1).
In vitro Biofilm Accumulation
For this crystal violet assay, fresh biofilm coated discs were retained from the drip flow reactor for samples treated with the placebo (V2NT, n=6) and 0.4% stannous fluoride gel (V2T, n=6). These discs were transferred to 96 well plates. The supernatant was removed, and 0.5 ml of 0.1% crystal violet solution was added to each tube. The samples were incubated with shaking at 125 rpm for 15 min at room temperature. Samples were again centrifuged for 5 minutes, and rinsed twice with water. 1.0 ml of 30% acetic acid was added to each stained disc and the dye was allowed to solubilize during 15 minutes of incubation. The optical density then was measured at 600 nm (Synergy HT, Biotek).
HOMIM analysis
From each subject, at each clinical visit (V1, V2, V3) and the in vitro biofilms (V2NT, V2T), DNA was extracted from all the biofilm samples, using the recommended HOMIM protocol. The protocol was developed by Forsyth to extract DNA from hard-to-lyse bacteria and utilized a protocol that used Ready-Lyse™ Lysozyme Solution (Epicentre, Madison, WI) for overnight incubation and MasterPure DNA Purification Kit (Epicentre, Madison, WI). DNA extracts were stored at -80°C and shipped all together for HOMIM analysis at the Forsyth Dental Center (Boston, MA, USA). A comprehensive description of the HOMIM protocol including PCR primers, thermal cycling conditions, labelling, hybridization and normalization has been published previously[16]. A condensed overview of the process is described below.
HOMIM used the bacterial 16S rRNA gene as a means of genotyping. DNA extracts were amplified in two separate polymerase chain reactions that used primers (forward and reverse) to gain overlapping coverage of the bacterial 16S rRNA gene. The amplified DNA from each of these reactions were pooled, purified and then labelled by incorporating Cy3-deoxycytidine triphosphate during a third round of amplification.
The purified labelled PCR products were hybridized to the HOMIM arrays, which is described in more detail elsewhere[16].
The microarray component of the whole genotyping process consisted of aldehyde-coated glass slides printed with oligonucleotide reverse-capture probes directed towards species and Human Oral Taxons (HOT) specific regions of the 16S rRNA. This work was carried out in February 2014. Universal regions of bacterial 16S rRNA acted as positive control probes. There were five arrays on each slide. Each of the arrays included four (8 × 15) duplicate sub-arrays[16]. The HOMIM probes were organized phylogenetically on each sub-array. After the PCR products were hybridized to the microarray, the slides were then scanned to determine probe fluorescence intensity. Since each array has duplicate probes, the intensities of these replicates were averaged (after background correction) to obtain probe specific fluorescence intensity. The intensity was related to the average intensity of the universal (positive control) 16S rRNA probe intensity. Full details of this normalization process to obtain the relative average intensity value that ranged from o to 5 is described in more detail elsewhere[17]. The process has a minimal detection threshold, which is related in part to non-specific binding of probes that produce a background ‘noise’ of fluorescence. This limit has been reported to be ≥ 104 bacterial cells[16]. The HOMIM results were sent to our group in the form of a large numerical array (0-5) that semi-quantitatively measured the overall abundance of a particular HOMIM probe ID. Further analysis by our group is described below.
Statistical Analysis
To test for differences attributable to the treatment, a mixed effect cumulative logit model was fit to all of the HOMIM data. These models had the semiquantitative measures of bacterial abundance produced by the HOMIM arrays as the response variable (so there is a model fit for each probe on the HOMIM array) and explanatory variables that encoded what type of sample the measurements were from (e.g. baseline clinic or after freezing). The data was such that the model was estimable for only 93 probes, thus we conducted a Bonferroni adjustment using this value. These models included random effects for subjects and hypothesis tests of differences between patient groups were conducted by comparison of observed test statistics to the permutation distribution of these test statistics since the sample size may not be large enough for the usual asymptotic distribution of the test statistics to be valid (up to 10,000 permutations were considered for each probe to allow for a Bonferroni correction). We supplemented these analyses with fitting normal theory mixed effects models (again with subject specific random effects), and these largely agreed with the findings of the more appropriate cumulative link models. These analyses were supplemented with hierarchical cluster analysis at all-time points to graphically investigate potential differences in the correlation structure among probes upon exposure to 0.4% stannous fluoride gel treatment. Principal components analysis (PCA) of the clinical and biofilm data was performed and visualized using the ggplot2 package for R.
To test for differences in the plaque scores over time, mixed effects models were fit to the plaque scores. These models had random subject specific effects and fixed effects for the treatment group. To test for differences between the crystal violet assay a paired t-test was used and a confidence interval for the difference was computed. To test for differences in the overall number of species observed, the total number of positive probe sets was computed for each sample and a generalized linear mixed effects model was fit to these counts for all time points and subjects. Due to overdispersion of these counts the negative binomial distribution was used to model the counts as they depend on subject specific random effects and fixed effects describing the sample types. The parameters in this model were fit using penalized quasilikelihood with the glmmPQL routine in the MASS package for R.
To investigate the power of the proposed design we used data obtained from our affiliate study, where longitudinal sampling was done 1-2 months apart on adult subjects, to estimate the standard deviation in bacterial quantities within a subject over time [13]. This was necessary as this power calculation was conducted prior to data collection to ensure an adequate sample size. Since some probes had a variance of zero across subjects (typically because the probe was zero for all subjects as the corresponding species was absent) we excluded those probes leaving 144 probes using the plaque samples (this was 120 for the saliva samples). As different probes exhibited different standard deviations we used the third quartile of the remaining standard deviations, which for the plaque samples was 1.46 (it was 1.00 for the saliva samples). To account for multiple comparisons we used the Bonferroni method for the 144 probes. As a test we use the normal approximation to the 1 sample t-statistic for the differences from the first to last visit. We then computed the power for testing for a difference of 2 as this corresponds to a large change in terms of the range of the probe values (0 to 5) but is not unusual in terms of differences observed across other experimental conditions. A sample size of ten subjects would give a power of 0.78 to test for chances in plaque ecology across the three time points.
The HOMIM data probes were also cross-referenced through the Human Oral Microbiome Database to reference the appropriate National Center for Biotechnology Information Taxonomy ID[18].
Results
Among our ten subjects, there were 22 HOMIM probes that produced a positive signal in at least 70% of the research subjects at visit 2 prior to the 0.4% SnF2 home therapy (Table 1). Those probes detected oral taxa common in several oral genera. This includes gram positive taxa from Streptococcus and the facultative anaerobes of Propionibacterium. Also present were specific taxa from gram negative bacteria that are either microaerophilic or facultative anaerobes such as Actinomyces, Capnocytophaga, Campylobacter, Haemophilus, and Porphyromonas [19]. General probes targeting clusters of species belonging to the genus of Fusobacterium and Neisseria were also detected. Our study found high frequency of Bergeyella sp., Haemophilus parainfluenzae, and Lautropia mirabilis and these have been associated with periodontal health in children[20]. The HOMIM data performed and reported directly to us in excel format by the Forsyth Institute can be found in the supplemental file (additional_file_1).
Table 1.
The HOMIM probes that were prevalent from the DNA extracts from the clinical plaque samples on visit 2. National Center for Biotechnology Information Taxonomy ID are listed (NA=not applicable at time of publication, ‘-‘ represents cluster probes that are not associated with a specific taxa).
| HOMIM probe ID | Associated NCBI # |
|---|---|
| Positive in 70% or more of the DNA extracts. Asterisk (*) denotes positive in all ten samples | |
| *Actinomyces georgiae HOT-617_AG01 | 52768 |
| Bacteroidetes[G-3] spp. HOT-281,365_AG17 | NA |
| *Bergeyella sp. HOT-322_AD84 | NA |
| Campylobacter showae HOT-763_X35, HOT-763_W36 | 204 |
| Campylobacter curvus HOT-580 / Campylobacter rectus HOT-748 | 200, 203 |
| Campylobacter concisus HOT-575 / Campylobacter rectus HOT-748_T86 | 199, 203 |
| Campylobacter concisus HOT-575 / Campylobacter rectus HOT-748_X36 | 199,203 |
| Capnocytophaga granulosa HOT-325_AG23 | 45242 |
| Capnocytophaga leadbetteri HOT-329_O08 | NA |
| Capnocytophaga ochracea HOT-700 / Capnocytophaga spp. HOT-323,326,864_Y83 | 1018,NA,NA, 742458 |
| Capnocytophaga sputigena HOT-775_AC15 | 1019 |
| Cardiobacterium hominis HOT-633_O97 | 2718 |
| Fusobacterium Cluster_AE01 | |
| *Haemophilus parainfluenzae HOT-718_W79 | 729 |
| Neisseria Cluster_O45 | |
| Porphyromonas sp. HOT-279_Q93 | NA |
| *Propionibacterium propionicum HOT-739_AB72 | 1750 |
| Streptococcus constellatus HOT-576 / Streptococcus intermedius HOT-644_F48 | 76860, 1338 |
| Streptococcus mitis bv2 HOT-398_AH36 | NA |
| Streptococcus mitis bv2 HOT-398 / Streptococcus sp. HOT-069_Q64 | NA |
| *Streptococcus oralis HOT-707/ Streptococcus sp. HOT-064_F46 | 1303 |
| Eubacterium[11][G-7] yurii HOT-377 / Peptostreptococcaceae[11][G-7] sp.HOT-106_W84 | 39498, NA |
| Lautropia mirabilis HOT-022_X44 | 47671 |
| Corynebacterium matruchotii HOT-666_AG29 | 43768 |
| Fusobacterium periodonticum HOT-201_R20 | 860 |
| Rothia dentocariosa HOT-587 / Rothia mucilaginosa HOT-681_E52 | 2047, 43675 |
| Gemella haemolysans HOT-626_AG51 | 1379 |
| Streptococcus australis HOT-073_AH32 | 113107 |
| Campylobacter concisus HOT-575_X33 | 199 |
| Dialister invisus HOT-118_AG33 | 218538 |
| Mycoplasma salivarium HOT-754_AD10 | 2124 |
| Capnocytophaga gingivalis HOT-337_X23 | 1017 |
Not surprisingly our biofilm model seemed to select for mainly taxa that were oxygen tolerant species given the conditions of the Drip Flow Reactor (DFR) system. We had expected, with an initial inoculation step done anaerobically, that certain anaerobes would be able to survive within the biofilm during the aerobic conditions of the DFR. However, the final two day cycle of the DFR is done aerobically and does not fully immerse the biofilm in media continuously. Oral taxa that were associated with either fastidious or facultative anaerobes were not expressed in the biofilm community (Table 2). The main members isolated were clinical human oral taxa associated with the genera of Streptococcus. 6 of the 9 most common taxa isolated in the biofilm model were expressed in similar counts as the plaque sample. The clinical and biofilm samples were normalized by the same process, but the biofilm model had significantly less species within the community (p<0.0001 from the generalized linear mixed effect model for the comparison of any clinic visit to either biofilm sample). This indicates that the majority taxa Streptococcus oralis HOT-707/Streptococcus sp. HOT-064_F46 that was found at the maximum relative number (5) in every biofilm (V2NT) may have been an over-represented dominant species.
Table 2.
The HOMIM probes that were prevalence in the DNA extracts from the pretreatment biofilm sample (V2NT). National Center for Biotechnology Information Taxonomy ID are listed (NA=not applicable at time of publication, ‘-‘ represents cluster probes that are not associated with a specific taxa).
| HOMIM probe ID | Associated NCBI # |
|---|---|
| Positive in 70% or more of the DNA extracts. Asterisk (*) denotes positive in all ten samples | |
| 1. *Haemophilus parainfluenzae HOT-718_W79 | 729 |
| 2. *Streptococcus oralis HOT-707/ Streptococcus sp. HOT-064_F46 | 1303 |
| 3. *Streptococcus australis HOT-073_AH32 | 113107 |
| 4. Streptococcus anginosus HOT-543 / Streptococcus gordonii HOT-622_F49 | 1328, 1302 |
| 5. *Streptococcus constellatus HOT-576 / Streptococcus intermedius HOT-644_F48 | 76860, 1338 |
| 6. Granulicatella adiacens HOT-534_AB30 | 46124 |
| 7. *Granulicatella adiacens HOT-534 / Granulicatella elegans HOT-596_W81 | 46124, 137732 |
| 8. Gemella haemolysans HOT-626_A651 | |
| 9. Gemella Haemolysans HOT-626 / Gemella sanguinis HOT-757_K63 |
Interestingly, HOMIM probes identifying taxa associated with the genus of Granulicatella spp. were isolated within the biofilm model. It was detected in biofilm samples as one of the main taxa that grew within the biofilm system with Streptococcus spp. Granulicatella adiacens requires key nutrients from other bacterial organisms and grows well as satellite colonies[21]. Granulicatella adiacens was first characterized as a nutritional variant streptococci [22] with its pleomorphic gram-positive cocci forming chains but recently re-categorized through 16S rRNA gene sequencing [23]. This illustrates how our biofilm model fosters species that are incapable of growth in a single species model under similar environmental conditions but can thrive within a plaque derived multi-species model. This is quite different from plaque derived multi-species models that are a mixture of species that can be grown and isolated independently. Our biofilm model ecology also had a substantial amount of Streptococcus australis, which is a newly designated Streptococcus species closely related to the more well-known Streptococcus infantis [24]. This is a common bacteria found in children.
All parents reported that the subjects refrained from rinsing and eating/drinking for 30 minutes and that the gels were used each night, following instructions, with possible one day exception. As a measure of compliance, each individual bottle was weighed before and after treatment. The weight differences indicated that children used the gel throughout the treatment period. The final weight difference was divided by the treatment time and show that children used an average of at least 0.257 mg per day, but this assumes that the dosing was even throughout the treatment period. This weight confirmation should be seen as supporting our parent interview instead of providing an exact estimate of daily dosage intake.
For both the clinical and biofilm intervention, 0.4% SnF2 gel did not change the overall number of HOMIM probes (Figure 3) or any particular HOMIM probe. Principal components analysis plots (Figure 4) reveal that samples from the same subject tend to co-localize, and this is also event in heat map graphical representation (Figure 5 and 6). This indicates that there was considerable reproducibility over time in the same subject. The amount of plaque accumulation of subject's teeth, which was measured by Plaque index scores did not show statistical significant changes (Figure 7A, n=20 for all 3 clinic visits) over the two baselines or after treatment (p=0.9928 with a 95% confidence interval for the difference attributable to the treatment of (-0.112, 0.111)). The in vitro results were similar when examining the crystal violet data in the samples we examined (Figure 7B, n=9 at 2 time points). The test for no difference yielded a p-value of 0.1157 with a 95% confidence interval of (-0.016, 0.119). In short, there was no difference found between the biomass accumulation on discs treated with the placebo or 0.4% stannous fluoride gel, and plaque scores failed to demonstrate a statistically significant difference attributable to the gel treatment.
Figure 3.
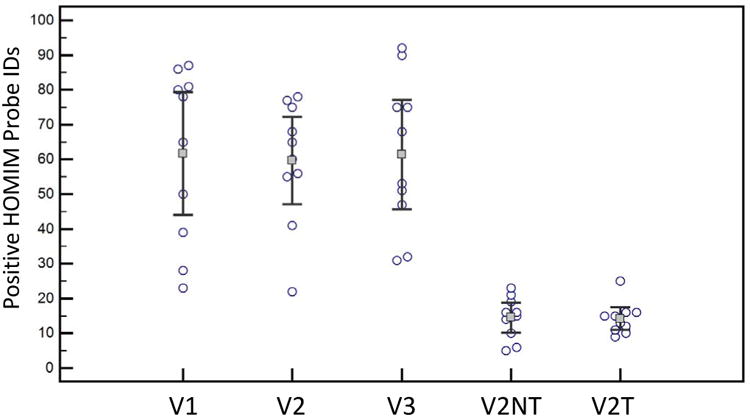
Relative bacterial oral taxa counts for each of the ten subjects for each clinical visit and the two biofilm conditions. Visits 1(V1) and visit 2 (V2) were baseline visits before stannous fluoride treatment with treatment between V2 and visit 3 (V3). There was not a significant change in the number of positive HOMIM probes between the V1, V2, and V3. Plaque samples were taken at V2 for the biofilm drip flow reactor (DFR) with no treatment placebo gel (V2NT). V2NT biofilm was then used as the inoculum for discs pre-treated with 0.4% stannous fluoride. V2T represented the recovered biofilm after stannous fluoride exposure and two days of exposure in the DFR. There were no significant change in the overall number of positive HOMIM probes between V2NT and V2T. The DFR (V2NT and V2T) recovered a limited fraction of the total HOMIM probes in the clinical samples.
Figure 4.
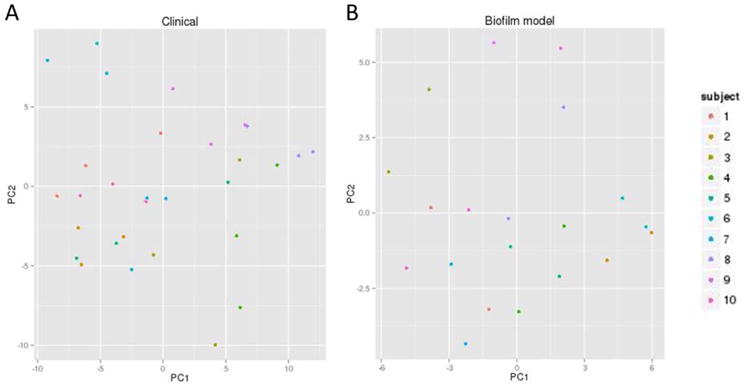
Principal Component Analysis plots of the 16S rRNA HOMIM probes across A) the clinical time points (V1,V2, V3) and B) the two biofilm time points (V2NT and V2T). Samples from the same subject tend to co-localize in the plot, which indicated that there was some reproducibility of the plaque ecology over time in the same subject.
Figure 5.
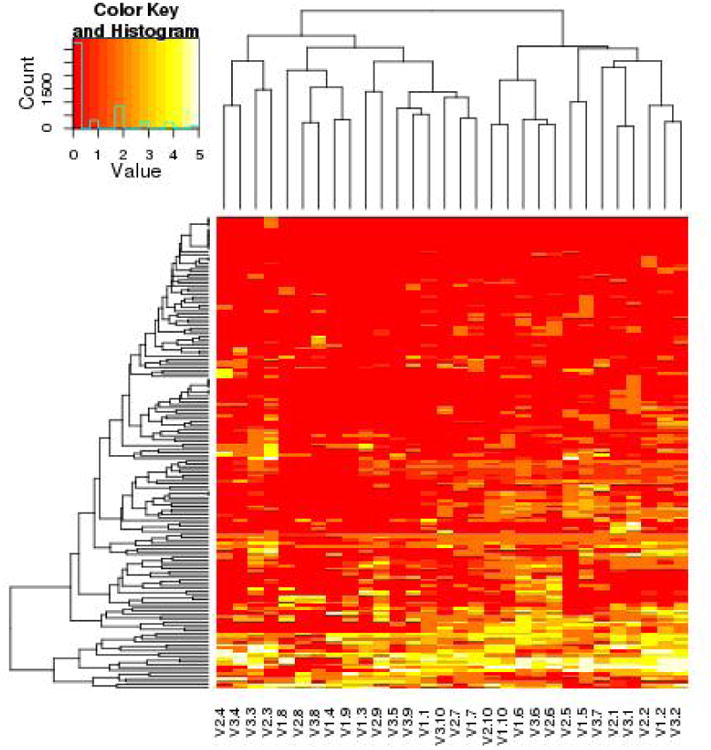
Clinical HOMIM Data for visit 1, visit 2, and visit 3 represented by a heat map graphical representation. The vertical cluster analysis examined the individual taxa or species. The horizontal Hierarchical cluster analyses using average-linkage compared individual subjects. Nomenclature is V2.3 is visit 2 for the third pediatric subject enrolled in the study. Human Oral Taxa differences were more broadly seen across different subjects. The ecology of the oral taxa were more likely to cluster within individual subjects across the three visits. This indicated the resilience of the oral plaque ecology during the stannous fluoride gel therapy.
Figure 6.
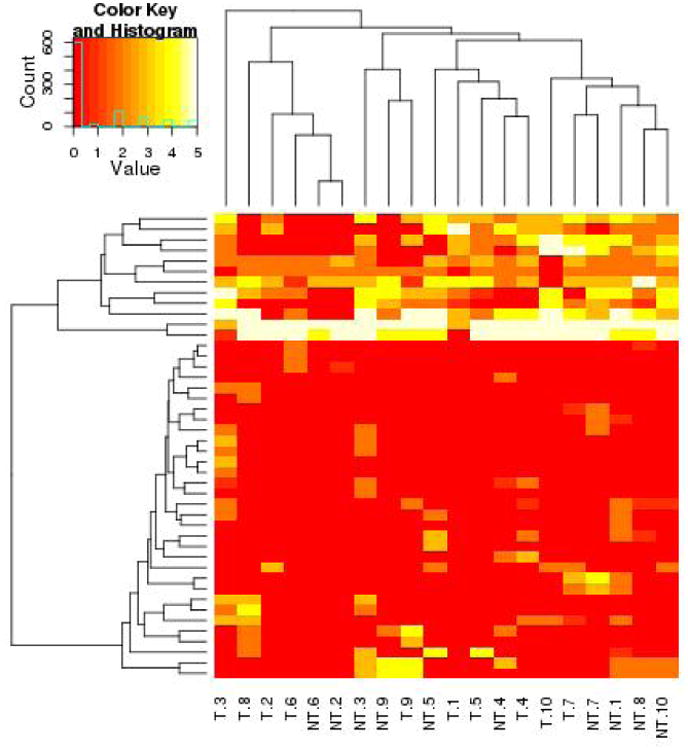
Biofilm system HOMIM Data for Visit 1,Visit 2, and Visit 3 represented by a heat map graphical representation.
Figure 7.
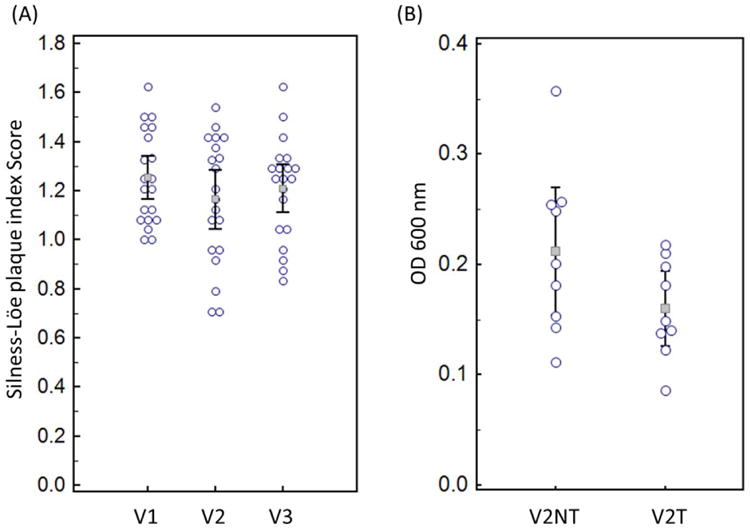
A) Comparison of the plaque index scores (Silness-Löe) between the three clinical visits. No statistical significance was found between the three time points. B) Crystal Violet Assay examining the adherent biomass on discs treated with the placebo gel (V2NT) and the 0.4% Stannous Fluoride Gel (V2T).
Discussion
The observational clinical study in this paper showed that short-term nightly use of a 0.4% SnF2 gel has little effect on the bacterial plaque ecology in children. This is important for clinicians considering these gels for managing high caries risk children. The results of this study do not imply that these gels have little therapeutic benefit. Rather, the study suggests that the immediate benefits of using the gel each night to manage caries in children may be strictly from fluoride ions[25]. Since stannous fluoride glycerin gels lack the mineral abrasives, pediatric patients are able to refrain from rinsing, drinking, and eating afterwards. Our study showed the high compliance of these pediatric subjects. The expected therapeutic benefits would be higher residual intraoral fluoride levels inhibiting tooth demineralization. Based on 0.4% SnF2 composition, there is 1,000 ppm of fluoride in these gels and without rinsing with water there may be residual benefits especially if used nightly; however, the stannous (Sn) ion levels (based on stoichiometry) are 3 times the concentration of fluoride with Sn at 3,000 ppm. In the glycerin gel, SnF2 is relatively stable, but once applied into the oral cavity or in the biofilm model, SnF2 may react with water and precipitate from solution to form Sn(OH)2 or oxidize with oxygen and form Sn4+ [26]. This stannous ion has traditionally been thought to have an antimicrobial role [26]. However, it is difficult to estimate the biologically active concentration in ppm of Sn4+, since in addition to the two main reactions described other reactions exist, especially in the presence of phosphate and calcium[27].
Our IRB approved study was constrained by the recommendations of the manufacturer, which focused on nightly use; most past studies of 0.4% SnF2 toothpaste or gels have focused on twice daily applications [28–30]. Our results indicate that the gel may need to be used more often (twice daily) or used for a longer time period if a practitioners is utilizing the gel for its antimicrobial effects. But as a caries management option in children, it should be pointed out that the rate of fluorosis is rising[31]. Twice daily gel application, in addition, to brushing with fluoride toothpaste, may not be desirably in terms of fluorosis risk. In addition, the gels ideally should be considered in a caries management regimen focusing on short-term (secondary prevention) not long term use to address acute risk factors in caries management.
This study used two time point baseline visits prior to the treatment intervention to establish the natural bacterial ecology variation in time. By taking this approach, this study was able to take into account both individual and temporal variations. This study was powered (0.78) to examine bacterial taxa that would show a two-fold relative difference measured by HOMIM. More subtle difference may exist in our data set. But the results showed that there were not any oral taxa that were particularly sensitive to the stannous fluoride gels. When considering the lower diversity plaque derived multi-species biofilm model, none of the oral taxa showed sensitivity to the SnF2 gel. Previous studies that have examined the anti-microbial component of SnF2 gels have focused on Streptococcus mutans [30, 32, 33]. Most of our research subjects did not have Streptococcus mutans that was detectable with HOMIM. Subjects may have had Streptococcus mutans levels that were below the detection limit of the HOMIM (∼104 bacterial cells is the detection threshold). Also it should be noted that the universal primers used in the HOMIM system may not be effective for all strains of Streptococcus mutans (unpublished data). But in either case, it is important to note that the single patient that had Streptococcus mutans within their dental plaque at both visit 1 and visit 2 did not have a measurable change in Streptococcus mutans after SnF2 treatment.
Short term nightly use of a 0.4% SnF2 gel also had little effect on the plaque index scores of our pediatric subjects. Previous studies have brought up potential issues with plaque scores and SnF2 gels [26]. The issue is SnF2 may increase the salivary (non-bacterial) pellicle collection on the tooth. This could confound the in vivo results, but the concerns of this non-pellicle collection on the tooth remains speculative. In addition, the in vitro experiments of our study did not find a difference in the biomass accumulation, which was measured by the crystal violet assay, between the placebo (without stannous fluoride) gel and the 0.4% SnF2 gel. The plaque derived multi-species bacterial biofilm model supports the observations of the clinical intervention.
Our affiliate studies have shown a higher percentage of bacteria recovered than the methods used in this study [13]. In our previous affiliated studies, we were able to immediately incubate fresh plaque as an inoculum but this was not feasible in our three visit study [13]. We were unable to have research subjects come Monday or Tuesday each week, since the biofilm model requires multiple days from incubation to analysis So in our study, we had to freeze and then later thaw our plaque samples on a Monday morning. This may have been the chief reason we saw a reduction in the oral taxa especially the fastidious anaerobes. Our study also used the Drip flow system that did not fully immerse our samples. We choose this model because we did not want to rinse away the gel by fully immersing the HA discs with media in an immersed bioreactor [13]. Nonetheless, although this model was a simplified version of the oral taxa derived from our plaque samples, the bacteria recovered represented species that were found in great abundance in the original plaque sample. The biofilm model showed similar bacterial ecology stability in terms of before and after the SnF2 gels. For future studies, this work provides a framework to examine novel antimicrobials added to stannous fluoride gels where improved antimicrobial effects are needed.[34]
Supplementary Material
Additional File 1. Human Oral Microbial Identification Microarray (HOMIM) performed by Forsyth Dental Center (Boston, MA, USA) for the clinical visits (v1,v2,v3) and biofilm model. Data is listed as under de-identified study subject numbers.
Acknowledgments
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We also thank Dr. Joel D. Rudney and Ms. Ruoqiong Chen for their work on recovering the initial high diversity biofilm. We thank Mr. Michael Weston for his technical assistance with the drip-flow bioreactor.
Footnotes
Conflict of Interest: For the laboratory study, the authors received an unrestricted donation of the stannous fluoride and placebo gels from 3M (Saint Paul, MN). The authors contacted 3M because manufacturing a placebo gel was necessary for the laboratory study design. This gel could not be used in a clinical study based on manufacturing regulations. 3M was not involved in any aspect of the study besides the unrestricted donation. The authors themselves declare that they have no competing financial interests.
References
- 1.Söderling E, Elsalhy M, Honkala E, Fontana M, Flannagan S, Eckert G, Kokaras A, Paster B, Tolvanen M, Honkala S. Effects of short-term xylitol gum chewing on the oral microbiome. Clin Oral Investig. 2014 doi: 10.1007/s00784-014-1229-y. [DOI] [PubMed] [Google Scholar]
- 2.Liu B, Faller LL, Klitgord N, Mazumdar V, Ghodsi M, Sommer DD, Gibbons TR, Treangen TJ, Chang YC, Li S, Stine OC, Hasturk H, Kasif S, Segrè D, Pop M, Amar S. Deep sequencing of the oral microbiome reveals signatures of periodontal disease. PLoS One. 2012;7:e37919. doi: 10.1371/journal.pone.0037919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanner ACR, Mathney JMJ, Kent RL, Chalmers NI, Hughes CV, Loo CY, Pradhan N, Kanasi E, Hwang J, Dahlan MA, Papadopolou E, Dewhirst FE. Cultivable anaerobic microbiota of severe early childhood caries. J Clin Microbiol. 2011;49:1464–74. doi: 10.1128/JCM.02427-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gross EL, Beall CJ, Kutsch SR, Firestone ND, Leys EJ, Griffen AL. Beyond Streptococcus mutans: dental caries onset linked to multiple species by 16S rRNA community analysis. PLoS One. 2012;7:e47722. doi: 10.1371/journal.pone.0047722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomar-Vercher S, Cabrera-Rubio R, Mira A, Montiel-Company JM, Almerich-Silla JM. Relationship of children's salivary microbiota with their caries status: a pyrosequencing study. Clin Oral Investig. 2014 doi: 10.1007/s00784-014-1200-y. [DOI] [PubMed] [Google Scholar]
- 6.Wade W, Addy M, Hughes J, Milsom S, Doherty F. Studies on stannous fluoride toothpaste and gel (1). Antimicrobial properties and staining potential in vitro. J Clin Periodontol. 1997;24:81–85. doi: 10.1111/j.1600-051x.1997.tb00471.x. [DOI] [PubMed] [Google Scholar]
- 7.Leverett DH, McHugh WD, Jensen OE. Dental caries and staining after twenty-eight months of rinsing with stannous fluoride or sodium fluoride. J Dent Res. 1986;65:424–7. doi: 10.1177/00220345860650031001. [DOI] [PubMed] [Google Scholar]
- 8.Artopoulou II, Powers JM, Chambers MS. In vitro staining effects of stannous fluoride and sodium fluoride on ceramic material. J Prosthet Dent. 2010;103:163–9. doi: 10.1016/S0022-3913(10)60023-6. [DOI] [PubMed] [Google Scholar]
- 9.Colombo APV, Boches SK, Cotton SL, Goodson JM, Kent R, Haffajee AD, Socransky SS, Hasturk H, Van Dyke TE, Dewhirst F, Paster BJ. Comparisons of subgingival microbial profiles of refractory periodontitis, severe periodontitis, and periodontal health using the human oral microbe identification microarray. J Periodontol. 2009;80:1421–32. doi: 10.1902/jop.2009.090185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oral Health Database. MALMÖ UNIVERSITY; Silness-Löe Index. http://www.mah.se/CAPP/Methods-and-Indices/Oral-Hygiene-Indices/Silness-Loe-Index/ [Google Scholar]
- 11.Ledder RG, Sreenivasan PK, DeVizio W, McBain AJ. Evaluation of the specificity and effectiveness of selected oral hygiene actives in salivary biofilm microcosms. J Med Microbiol. 2010;59(Pt 12):1462–8. doi: 10.1099/jmm.0.024372-0. [DOI] [PubMed] [Google Scholar]
- 12.Wong L, Sissons C, Sissions CH. A comparison of human dental plaque microcosm biofilms grown in an undefined medium and a chemically defined artificial saliva. Arch Oral Biol. 2001;46:477–86. doi: 10.1016/s0003-9969(01)00016-4. [DOI] [PubMed] [Google Scholar]
- 13.Rudney JD, Chen R, Lenton P, Li J, Li Y, Jones RS, Reilly C, Fok aS, Aparicio C. A reproducible oral microcosm biofilm model for testing dental materials. J Appl Microbiol. 2012;113:1540–53. doi: 10.1111/j.1365-2672.2012.05439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu KD, Stewart PS, Xia F, Huang CT, McFeters GA. Spatial physiological heterogeneity in Pseudomonas aeruginosa biofilm is determined by oxygen availability. Appl Environ Microbiol. 1998;64:4035–9. doi: 10.1128/aem.64.10.4035-4039.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sissons CH, Anderson SA, Wong L, Coleman MJ, White DC. Microbiota of plaque microcosm biofilms: effect of three times daily sucrose pulses in different simulated oral environments. Caries Res. 2007;41:413–22. doi: 10.1159/000104801. [DOI] [PubMed] [Google Scholar]
- 16.Colombo AP, Boches SK, Cotton SL, Goodson JM, Kent R, Haffajee AD, Socransky SS, Hasturk H, Van Dyke TE, Dewhirst F, Paster BJ. Comparisons of subgingival microbial profiles of refractory periodontitis, severe periodontitis, and periodontal health using the human oral microbe identification microarray. J Periodontol. 2009;80:1421–1432. doi: 10.1902/jop.2009.090185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.No Title [http://bioinformatics.forsyth.org/]
- 18.Chen T, Yu WH, Izard J, Baranova OV, Lakshmanan A, Dewhirst FE. The Human Oral Microbiome Database: a web accessible resource for investigating oral microbe taxonomic and genomic information. Database (Oxford) 2010;2010:baq013. doi: 10.1093/database/baq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson JL, Moore LV, Kaneko B, Moore WE. Actinomyces georgiae sp. nov., Actinomyces gerencseriae sp. nov., designation of two genospecies of Actinomyces naeslundii, and inclusion of A. naeslundii serotypes II and III and Actinomyces viscosus serotype II in A. naeslundii genospecies 2. Int J Syst Bacteriol. 1990;40:273–86. doi: 10.1099/00207713-40-3-273. [DOI] [PubMed] [Google Scholar]
- 20.Shaddox LM, Huang H, Lin T, Hou W, Harrison PL, Aukhil I, Walker CB, Klepac-Ceraj V, Paster BJ. Microbiological characterization in children with aggressive periodontitis. J Dent Res. 2012;91:927–33. doi: 10.1177/0022034512456039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koh YR, Yi J, Kim HH, Chang CL, Kim SY. Discrepant satellitism for identification of Granulicatella adiacens isolates. Ann Lab Med. 2014;34:174–6. doi: 10.3343/alm.2014.34.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouvet A, Grimont F, Grimont PAD. Streptococcus defectivus sp. nov. and Streptococcus adjacens sp. nov., Nutritionally Variant Streptococci from Human Clinical Specimens. Int J Syst Bacteriol. 1989;39:290–294. [Google Scholar]
- 23.Collins MD, Lawson PA. The genus Abiotrophia (Kawamura et al.) is not monophyletic: proposal of Granulicatella gen. nov., Granulicatella adiacens comb. nov., Granulicatella elegans comb. nov. and Granulicatella balaenopterae comb. nov. Int J Syst Evol Microbiol. 2000;50:365–369. doi: 10.1099/00207713-50-1-365. [DOI] [PubMed] [Google Scholar]
- 24.Willcox MD, Zhu H, Knox KW. Streptococcus australis sp. nov., a novel oral streptococcus. Int J Syst Evol Microbiol. 2001;51(Pt 4):1277–81. doi: 10.1099/00207713-51-4-1277. [DOI] [PubMed] [Google Scholar]
- 25.Faller RV, Eversole SL, Saunders-Burkhardt K. Protective benefits of a stabilised stannous-containing fluoride dentifrice against erosive acid damage. Int Dent J. 2014;64 Suppl 1:29–34. doi: 10.1111/idj.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tinanoff N. Progress regarding the use of stannous fluoride in clinical dentistry. J Clin Dent. 1995;6 Spec No:37–40. [PubMed] [Google Scholar]
- 27.Jordan TH, Wei SH, Bromberger SH, King JC. Sn3-F3-PO4: the product of the reaction between stannous fluoride and hydroxyapatite. Arch Oral Biol. 1971;16:241–6. doi: 10.1016/0003-9969(71)90017-3. [DOI] [PubMed] [Google Scholar]
- 28.Wolff LF, Pihlstrom BL, Bakdash MB, Aeppli DM, Bandt CL. Effect of toothbrushing with 0.4% stannous fluoride and 0.22% sodium fluoride gel on gingivitis for 18 months. J Am Dent Assoc. 1989;119:283–9. doi: 10.14219/jada.archive.1989.0209. [DOI] [PubMed] [Google Scholar]
- 29.Boyd RL, Leggott PJ, Robertson PB. Effects on gingivitis of two different 0.4% SnF2 gels. J Dent Res. 1988;67:503–7. doi: 10.1177/00220345880670021501. [DOI] [PubMed] [Google Scholar]
- 30.Ota K, Beierle JW. Stannous fluoride and its effects adhesive properties in vitro on oral microbial. 1989;11:21–25. [PubMed] [Google Scholar]
- 31.Beltrán-Aguilar ED, Barker LDB. NCHS data brief, no 53. Hyattsville, MD: Natl Cent Heal Stat; 2010. Prevalence and severity of dental fluorosis in the United States, 1999-2004. [PubMed] [Google Scholar]
- 32.Brêtas SM, Macari S, Elias AM, Ito IY, Matsumoto MAN. Effect of 0.4% stannous fluoride gel on Streptococci mutans in relation to elastomeric rings and steel ligatures in orthodontic patients. Am J Orthod Dentofacial Orthop. 2005;127:428–33. doi: 10.1016/j.ajodo.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 33.Vierrou AM, Manwell MA, Zamek RL, Sachdeva RC, Tinanoff N. Control of Streptococcus mutans with topical fluoride in patients undergoing orthodontic treatment. J Am Dent Assoc. 1986;113:644–6. doi: 10.14219/jada.archive.1986.0233. [DOI] [PubMed] [Google Scholar]
- 34.Van Loveren C, Gerardu VAM, Sissons CH, van Bekkum M, ten Cate JM. Effect of various rinsing protocols after use of amine fluoride/stannous fluoride toothpaste on the bacterial composition of dental plaque. Caries Res. 2009;43:462–7. doi: 10.1159/000264683. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional File 1. Human Oral Microbial Identification Microarray (HOMIM) performed by Forsyth Dental Center (Boston, MA, USA) for the clinical visits (v1,v2,v3) and biofilm model. Data is listed as under de-identified study subject numbers.


