Abstract
Facial pain is a complex disease with a number of possible etiologies. Trigeminal neuropathic pain (TNP) is defined as pain caused by a lesion or disease of the trigeminal branch of the peripheral nervous system resulting in chronic facial pain over the distribution of the injured nerve. First line treatment of TNP includes management with anticonvulsant medication (carbamazepine, phenytoin, gabapentin, etc.), baclofen, and analgesics. TNP, however, can be a condition difficult to adequately treat with medical management alone.
Patients with TNP can suffer from significant morbidity as a result of inadequate treatment or the side effects of pharmacologic therapy. TNP refractory to medical management can be considered for treatment with a growing number of invasive procedures. Peripheral nerve stimulation (PNS) is a minimally invasive option that has been shown to effectively treat medically intractable TNP.
We present a case series of common causes of TNP successfully treated with PNS with up to a 2 year follow-up. Only one patient required implantation of new electrode leads secondary to electrode migration. The patients in this case series continue to have significant symptomatic relief, demonstrating PNS as an effective treatment option for intractable TNP.
Though there are no randomized trials, peripheral neuromodulation has been shown to be an effective means of treating TNP refractory to medical management in a growing number of case series. PNS is a safe procedure that can be performed even on patients that are not optimal surgical candidates and should be considered for patients suffering from TNP that have failed medical management.
Keywords: Trigeminal neuropathic pain, peripheral nerve stimulation, neuromodulation, intractable pain, facial trauma, postherpetic neuralgia
The fifth cranial nerve carries tactile, proprioceptive, and nociceptive afferents of the face, mouth, and portions of the meninges. Injury or disease of this nerve can lead to trigeminal neuropathic pain (TNP), described as a constant, burning facial pain often in an area of partial sensory deficit, which is frequently disabling (1–3). TNP is caused by injury to the nerve from events such as infection, trauma, surgery, or dental procedures to the face or cranium. Unfortunately, TNP can be difficult to manage and refractory to conventional treatment. Opioids are most commonly used to treat this chronic pain syndrome, often in conjunction with other medications such as anticonvulsants as well as interventional pain procedures. Longstanding use of opioids, however, is associated with tolerance and dependence with a required opioid dose escalation over time to maintain analgesic effects. Chronic opioid use has even been associated with abuse, overuse, addiction, opioid-induced pain sensitivity, and lack of proven efficacy (3–14). New efficacious forms of treatment for TNP are needed to adequately manage this debilitating disease.
A logical treatment strategy for TNP is to deliver targeted relief to the precise area of pain over the distribution the trigeminal nerve. The use of peripheral nerve stimulation (PNS) for various neuropathic pain syndromes have been well documented including occipital neuralgia (15–20), postherpetic neuralgia (2), post-traumatic neuropathic pain (2,25–27), and complex regional pain syndrome (28–30). PNS as a treatment for TNP is a promising treatment modality for this otherwise difficult chronic pain syndrome that does not have the same concerns of tolerance and dependence as opioid therapy. We present a case report of 3 patients who have undergone successful PNS of the ophthalmic V1 and/or maxillary V2 branches of the trigeminal nerve for TNP secondary to multiple etiologies including facial surgery, trauma, and herpetic infection.
Case Reports
Case 1
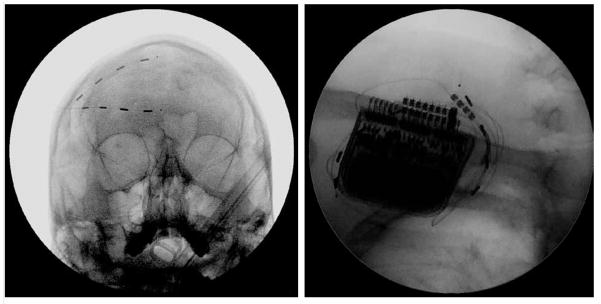
Case 1 is a 71-year-old man who presented with 11 years of severe left-sided facial pain. This patient was involved in a work-related accident, and sustained minor fractures along the left superior and lateral orbital wall, as well as severe damage to the eye. Surgical intervention at that time included enucleation of the left eye and a replacement with a prosthetic eye. Subsequently, the patient developed chronic pain over his left face. He underwent 7 operations on the eye with no significant relief in pain. On presentation to our clinic he reported 10/10 pain on a visual analog scale (VAS), which was described as sharp and shooting in the V1 and V2 nerve distribution. His medications included fentanyl patch, oxycodone, gabapentin, and oxcarbazepine, which only provided minimal symptomatic relief. He had also tried alcohol injections, which provided no benefit. After a 10 day PNS trial of the supraorbital and infraorbital nerves, the patient returned to the clinic and reported significant improvement in pain relief. He was subsequently implanted with 2 percutaneous linear quadripolar electrodes in the left V1 and V2 regions that were connected to a RestoreUltra pulse generator (Medtronic, Minneapolis, MN) which was implanted in the left infraclavicular subcutaneous space (Fig. 1). The implanted stimulator was programmed with a pulse width of 390 microseconds and a rate of 40 Hz. The electrode polarities were set to positive in the 0 and 3 positions and negative in the 1 and 2 positions. The patient obtained excellent pain control (0/10 on VAS). The only noticeable side effect reported was occasional headaches that developed when the stimulator was left on in the absence of facial pain. The patient discontinued all previous pain medications except for ibuprofen. Overall, the patient was very satisfied with the results of the nerve stimulator.
Fig. 1.
Anteroposterior fluoroscopic radiograph of Case 1 showing placement of 2 quadripolar electrodes in the left supraorbital and infraorbital positions.
Case 2
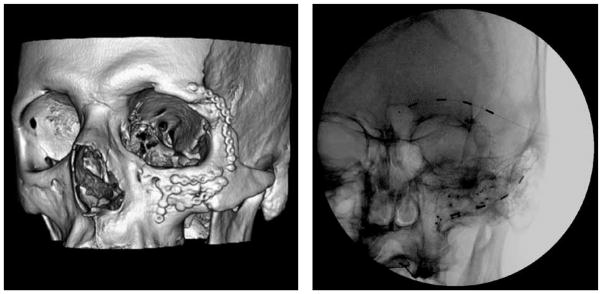
Case 2 is a 52-year-old man who was involved in an all-terrain vehicle accident 2 years prior to presentation. At the time of the accident, the patient was not wearing a helmet and sustained significant left facial trauma. A zygomaticomaxillary tripod fracture was stabilized with titanium plating by the plastic surgery service. The patient later complained of left-sided V2 distribution neuralgia and was taken back to the operating room 8 months later by the plastic surgery service to remove a titanium plate that was in proximity to the infraorbital nerve outlet and to decompress the infraorbital nerve (Fig. 2A). However, the patient continued to have left facial pain in the V1–V2 distribution rated as 8/10 that was poorly managed medically. On presentation to our clinic he reported an 8/10 (VAS), severe throbbing, and aching pain that had been gradually increasing since the time of the surgeries. The pain was primarily in the infraorbital region with less pain in the supraorbital region. On exam he had slight allodynia and hyperpathia of the inferior bony orbit. He was taking gabapentin, carbamazepine, hydrocodone, and ibuprofen at his initial visit. First, the patient underwent supraorbital and infraorbital nerve blocks with a total of 6.5 mL of 0.2% ropivacaine and 2 mL of triamcinolone (20 mg/mL), which resulted in rapid pain relief lasting for about 24 hours. Next, a 7-day PNS trial of the supraorbital and infraorbital nerves provided 100% pain relief. The patient was implanted with 2 percutaneous linear quadripolar electrodes in the left V1 and V2 regions that were connected to a RestoreUltra pulse generator which was implanted in the left infraclavicular subcutaneous space (Fig. 2B). The implanted stimulator was programmed with a pulse width of 300 microseconds and a rate of 40 Hz. The electrode polarities were set to positive in the 0 and 3 positions and negative in the 1 and 2 positions. The patient discontinued all previous pain medications, and reported complete pain relief (0/10 on VAS) 6 months post implant.
Fig. 2.
(A) Post-surgical CT 3D reconstruction of Case 2 showing significant left facial fractures that have been fixated with titanium plates. Of note, a titanium plate is partially obstructing the left inferior orbital foramen and was later removed. (B) Anteroposterior fluoroscopic radiograph of Case 2 showing placement of 2 quadripolar electrodes in the left supraorbital and infra-orbital positions.
Case 3
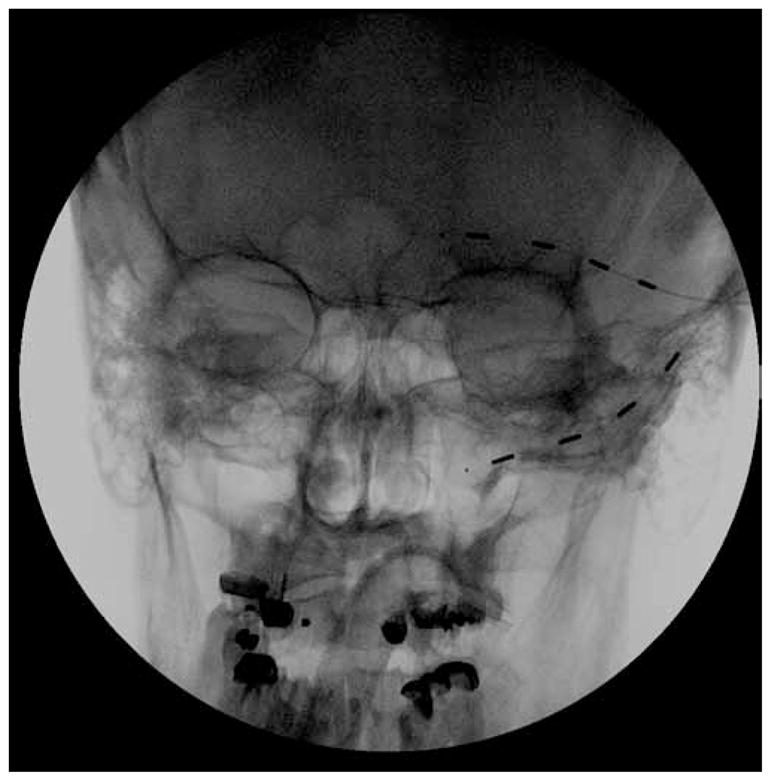
Case 3 is a 44-year-old man who presented with 18 months of severe right-sided facial pain. The patient was involved in a rollover motor vehicle collision about 2 weeks prior to a bout of shingles on the right side of his face. He did not have any trauma to the face, but the stress of the event might have contributed to the herpes zoster outbreak. On presentation to our clinic the patient reported a VAS of 10/10; and constant throbbing and shooting pain on the right side of his face, including his right eye and right ear. He had tried opiates with poor pain control. Although 2 other pain physicians recommended injections, the patient wished to seek additional options. The patient underwent a 7-day PNS trial in the right supraorbital distribution and experienced significant pain relief, with a decrease in pain to 4/10 (VAS). He was implanted with 2 percutaneous linear quadripolar electrodes both in the right V1 region that were connected to a RestoreUltra pulse generator which was implanted in the right infraclavicular fossa (Fig. 3A). The implanted stimulator was programmed with a pulse width of 450 microseconds and a rate of 40 Hz, creating cross stimulation between both leads. After the implant, the patient initially reported a 60% decrease in pain. A short time later, however, he reported no pain relief from the stimulator. A fluoroscopic radiograph of the head demonstrated that both electrode leads had migrated from the implanted position along the V1 distribution to the implanted pulse generator in the infraclavicular fossa where both leads were wrapped around the generator (Fig. 3B). New leads were re-implanted along the same V1 distribution and secured with an anchor. The new leads were attached to the original generator in the infraclavicular fossa and the patient reported a 60% decrease in pain.
Fig. 3.
(A) Anteroposterior fluoroscopic radiograph of Case 3 showing placement of 2 quadripolar electrodes in the right supraorbital position. The patient reported a 60% reduction of pain for his postherpetic neuropathy. (B) Anteroposterior fluoroscopic radiograph of the right infraclavicular fossa demonstrating migration of the leads from the forehead region to a coiled position around the implanted pulse generator. New electrodes were later implanted in the same V1 position and attached to the same implanted pulse generator. These new electrodes were secured with plastic anchors to prevent future lead migration.
Discussion
Electrical stimulation for the treatment of TNP is an increasingly common indication for PNS. Although first reported in the 1960s (31), PNS had not been a widely used treatment for intractable facial neuropathy until the last decade. Initially, placement of peripheral electrodes was performed by first dissecting and directly visualizing the target nerve before a large flat electrode or a wraparound electrode was placed next to the nerve. This technique resulted in poor pain control and reports emerged of nerve injury and epineural fibrosis (32,33). Not until the first description of a percutaneous technique of electrode placement without open dissection of the nerve in 1999 did PNS become more popular as a means of treating neuropathic pain (33,34).
Although there is a paucity of literature on facial PNS, the published results have been uniformly positive, particularly in regards to posttraumatic or postsurgical neuropathic trigeminal pain (2,16,17,25,33,35). Amin et al (36) reported favorable results in a 10 patient study of supraorbital PNS for intractable supraorbital neuralgia, reporting decreased headache scores and halving opioid consumption for a duration of 30 months. In another study of 10 patients, Johnson and Burchiel (2) found that PNS provided at least 50% pain relief in 70% of patients with TNP following facial trauma or herpes zoster infection. While there were no treatment failures (considered less than 50% pain relief) in the post-traumatic group, 2 of the 4 postherpetic patients had failed trials (2). However, multiple other case reports addressing the use of PNS for postherpetic neuralgia have been encouraging (21–24).
This case series demonstrates successful PNS of supraorbital and infraorbital nerves in TNP after facial surgery, trauma, and herpes zoster infection. The postsurgical and posttraumatic patients had complete resolution of their pain, while the postherpetic patient had a smaller degree of pain relief (Table 1). Though he described pain over both the V1 and V2 distribution, the postherpetic patient only elected to have one linear electrode array placed in the supraorbital region and thus this may account for his incomplete pain relief.
Table 1.
Summary of Cases
| Case | Age/Sex | Pre-Op Diagnosis | Duration of Symptoms | Trigeminal Branch | Pain Relief† | Follow-up Duration |
|---|---|---|---|---|---|---|
| 1 | 71/M | TNP secondary to enucleation | 11 years | V1 and V2 | 100% | 27 months |
| 2 | 52/M | TNP secondary to zygomaticomaxillary fracture | 18 months | V1 and V2 | 100% | 23 months |
| 3 | 44/M | Postherpetic neuralgia | 18 months | V1 | 60% | 6 months |
Pain was assessed using the visual analogue scale (VAS)
The overall complication rate is very low in facial PNS. The majority of complications are wound breakdown or skin erosion with hardware exposure, electrode fractures, component disconnections or displacement, and focal infections (33). In addition, persistent hardware pain can occur, and is estimated to occur in 5% of cases (35). Case 3 illustrates electrode migration that required repositioning of new leads. When compared to spinal cord stimulation, PNS has a better safety profile (35). By avoiding the spinal canal, the risk of epidural hematoma or epidural abscess resulting in possible neurologic deficit is essentially eliminated. The infection risk for PNS is minimal and estimated to be between 3–5% (35).
Though the efficacy of PNS has been shown for a growing number of indications, the exact mechanism of action for PNS as in spinal cord stimulation (SCS) remains poorly understood. The gate control theory proposed by Melzack and Wall in 1965 (37) states that rapidly conducting, large afferents into the spinal cord and medulla not only synapse with second order neurons sending ascending projections to the brain, but also form excitatory synapses with inhibitory intermediate neurons in the substantia gelatinosa. In this scenario, stimulation of large diameter A-fibers can lead to an increase in inhibitory tone in the substantia gelatinosa leading to a decrease in the efficacy of synaptic transmission imparted by incoming C-fiber discharge. The net effect is that ascending pain signals can be diminished at the level of the spinal cord or medulla with constant large fiber activation. This antinociceptive effect of peripheral nerve stimulation was later demonstrated in healthy volunteers (38).
Further understanding of the neurobiology of pain and the mechanism of PNS will help to develop newer technology and indications for PNS. TPN. Patients with TNP should be carefully selected for PNS, similar to any patient with neuropathic pain. Candidates’ pain should be chronic in nature, cause significant social or vocational dysfunction, and fail to improve with conservative treatments including physical therapies, management with oral analgesics, and minor interventions such as nerve block steroid injections. All potential candidates should undergo a neuropsychological evaluation prior to a PNS trial. An outpatient PNS trial is considered successful if the patient receives at least 50% pain relief (33). Contraindications to PNS are minimal and include coagulopathies, active infections, psychiatric problems, and failed PNS trial.
The surgical technique for peripheral stimulating electrode implantation has become more refined since the first applications of PNS. Stimulating electrodes were implanted in this case series using a similar previously described subcutaneous technique (25,34). A small stab incision was first made in the lateral frontal scalp region anterior to the superficial temporal artery. A gentle bend was made in a standard 14-gauge Tuohy needle to conform to the convexity of the forehead and the needle was advanced medially through the incision within the epifascial plane below the skin perpendicular to the nerve of interest. A quadripolar cylindrical electrode (Medtronic, Minneapolis, MN) was then passed into the needle after removing the stylet and the needle was then carefully backed out over the electrode. The position of the electrode was confirmed in all cases with fluoroscopic imaging to ensure that the electrode remained perpendicular to the location of the symptomatic nerve using anatomic landmarks and that electrode contacts were both medial and lateral to the nerve. The remaining distal end of the electrode was then tunneled to the infraclavicular fossa through a counter incision in the retroauricular area where a stain-relief loop was made. The electrode was connected to a pulse generator (Medtronic, Minneapolis, MN) implanted in the infraclavicular fossa. Anchors were not routinely used in this case series because of a concern of creating a large, noticeable profile below the scalp, but was used in Case 3 after the initially implanted electrodes migrated into the battery pocket. The gluteal region has also been reported as a site for the implanted pulse generator (33,39).
Conclusion
Some patients with TNP have significant morbidity and pain that is difficult to manage with conservative treatment alone. This small observational study describes a series of 3 patients with TNP in the V1 and V2 distribution that were successfully treated with PNS after failing conservative care. Patients with TNP that are referred for PNS have failed medical management and thus efficacy of PNS cannot be directly compared to medical management alone (40), though the benefit of PNS therapy is evident for select patients. There was an overall 87% reduction of pain in this study for patients with common causes of TNP refractory to medical management. Further studies are warranted for PNS in the treatment of TNP.
Footnotes
Disclaimer: Emil Annabi, MD is a Consultant for Medtronic.
Conflict of interest: None.
References
- 1.Dworkin RH. An overview of neuropathic pain: Syndromes, symptoms, signs, and several mechanisms. Clin J Pain. 2002;18:343–349. doi: 10.1097/00002508-200211000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Johnson MD, Burchiel KJ. Peripheral stimulation for treatment of trigeminal postherpetic neuralgia and trigeminal posttraumatic neuropathic pain: A pilot study. Neurosurgery. 2004;55:135–141. discussion 141–142. [PubMed] [Google Scholar]
- 3.Boogaard S, Heymans MW, Patijn J, de Vet HCW, Faber CG, Peters ML, Loer SA, Zuurmond WWA, Perez R. Predictors for persistent neuropathic pain - A Delphi Study. Pain Physician. 2011;14:559–568. [PubMed] [Google Scholar]
- 4.Mao J. Opioid-induced abnormal pain sensitivity: Implications in clinical opioid therapy. Pain. 2002;100:213–217. doi: 10.1016/S0304-3959(02)00422-0. [DOI] [PubMed] [Google Scholar]
- 5.Vallejo R, Barkin RL, Wang VC. Pharmacology of opioids in the treatment of chronic pain syndromes. Pain Physician. 2011;14:E343–E360. [PubMed] [Google Scholar]
- 6.Solanki DR, Koyyalagunta D, Shah RV, Silverman SM, Manchikanti L. Monitoring opioid adherence in chronic pain patients: Assessment of risk of substance abuse. Pain Physician. 2011;14:E119–E131. [PubMed] [Google Scholar]
- 7.Koyyalagunta D, Burton AW, Toro MP, Driver L, Novy DM. Opioid abuse in cancer pain: Report of two cases and presentation of an algorithm of multidisciplinary care. Pain Physician. 2011;14:E361–E371. [PubMed] [Google Scholar]
- 8.Sehgal N, Smith HS, Manchikanti L. Peripherally acting opioids and clinical implications for pain control. Pain Physician. 2011;14:249–258. [PubMed] [Google Scholar]
- 9.Manchikanti L, Malla Y, Wargo BW. Comparative evaluation of the accuracy of immunoassay with liquid chromatography tandem mass spectrometry (LC/ MS/MS) of urine drug testing (UDT) opioids and illicit drugs in chronic pain patients. Pain Physician. 2011;14:175–187. [PubMed] [Google Scholar]
- 10.Manchikanti L, Vallejo R, Manchikanti KN, Benyamin RM, Datta S, Christo PJ. Effectiveness of long-term opioid therapy for chronic non-cancer pain. Pain Physician. 2011;134:E133–E156. [PubMed] [Google Scholar]
- 11.Christo PJ, Manchikanti L, Ruan X, Bottros M, Hansen H, Solanki DR, Jordan AE, Colson J. Urine drug testing in chronic pain. Pain Physician. 2011;14:123–143. [PubMed] [Google Scholar]
- 12.Manchikanti L, Singh V, Caraway DL, Benyamin RM. Breakthrough pain in chronic non-cancer pain: Fact, fiction, or abuse? Pain Physician. 2011;14:E103–E117. [PubMed] [Google Scholar]
- 13.Manchikanti L, Ailinani H, Koyyalagunta D, Datta S, Singh V, Eriator I, Sehgal N, Shah RV, Benyamin RM, Vallejo R, Fellows B, Christo PJ. A systematic review of randomized trials of long-term opioid management for chronic non-cancer pain. Pain Physician. 2011;14:91–121. [PubMed] [Google Scholar]
- 14.Manchikanti L, Fellows B, Ailinani H, Pampatil V. Therapeutic use, abuse, and nonmedical use of opioids: A ten-year perspective. Pain Physician. 2010;13:401–435. [PubMed] [Google Scholar]
- 15.Jasper JF, Hayek SM. Implanted occipital nerve stimulators. Pain Physician. 2008;11:187–200. [PubMed] [Google Scholar]
- 16.Slavin KV, Nersesyan H, Wess C. Peripheral neurostimulation for treatment of intractable occipital neuralgia. Neurosurgery. 2006;58:112–119. doi: 10.1227/01.neu.0000192163.55428.62. [DOI] [PubMed] [Google Scholar]
- 17.Slavin KV, Colpan ME, Munawar N, Wess C, Nersesyan H. Trigeminal and occipital peripheral nerve stimulation for craniofacial pain: A single-institution experience and review of the literature. Neurosurg Focus. 2006;21:E5. doi: 10.3171/foc.2006.21.6.8. [DOI] [PubMed] [Google Scholar]
- 18.Kapural L, Mekhail N, Hayek SM, Stan-ton-Hicks M, Malak O. Occipital nerve electrical stimulation via the midline approach and subcutaneous surgical leads for treatment of severe occipital neuralgia: A pilot study. Anesth Analg. 2005;101:171–174. doi: 10.1213/01.ANE.0000156207.73396.8E. [DOI] [PubMed] [Google Scholar]
- 19.Strand NH, Trentman TL, Vargas BB, Dodick DW. Occipital nerve stimulation with the Bion microstimulator for the treatment of medically refractory chronic cluster headache. Pain Physician. 2011;14:435–440. [PubMed] [Google Scholar]
- 20.Deshpande KK, Wininger Kl. Feasibility of combined epicranial temporal and occipital neurostimulation: Treatment of a challenging case of headache. Pain Physician. 2011;14:37–44. [PubMed] [Google Scholar]
- 21.Dunteman E. Peripheral nerve stimulation for unremitting ophthalmic postherpetic neuralgia. Neuromodulation. 2002;5:32–37. doi: 10.1046/j.1525-1403.2002._2006.x. [DOI] [PubMed] [Google Scholar]
- 22.Yakovlev AE, Peterson AT. Peripheral nerve stimulation in treatment of intractable postherpetic neuralgia. Neuromodulation. 2007;10:373–375. doi: 10.1111/j.1525-1403.2007.00126.x. [DOI] [PubMed] [Google Scholar]
- 23.Kouroukli I, Neofytos D, Panaretou V, Zompolas V, Papastergiou D, Sanidas G, Papavassilopoulou T, Georgiou L. Peripheral subcutaneous stimulation for the treatment of intractable postherpetic neuralgia: Two case reports and literature review. Pain Pract. 2009;9:225–229. doi: 10.1111/j.1533-2500.2009.00263.x. [DOI] [PubMed] [Google Scholar]
- 24.Rodrigo-Royo MD, Azcona JM, Quero J, Lorente MC, Acín P, Azcona J. Peripheral neurostimulation in the management of cervicogenic headache: Four case reports. Neuromodulation. 2005;8:241–248. doi: 10.1111/j.1525-1403.2005.00032.x. [DOI] [PubMed] [Google Scholar]
- 25.Slavin KV, Wess C. Trigeminal branch stimulation for intractable neuropathic pain: Technical note. Neuromodulation. 2005;8:7–13. doi: 10.1111/j.1094-7159.2005.05215.x. [DOI] [PubMed] [Google Scholar]
- 26.Hegarty D, Goroszeniuk T. Peripheral nerve stimulation of the thoracic paravertebral plexus for chronic neuropathic pain. Pain Physician. 2011;14:295–300. [PubMed] [Google Scholar]
- 27.Graybill J, Conermann T, Kabazie AJ, Chandy S. Spinal cord stimulation for treatment of pain in a patient with post thoracotomy pain syndrome. Pain Physician. 2011;14:441–445. [PubMed] [Google Scholar]
- 28.Hassenbusch SJ, Stanton-Hicks M, Schoppa D, Walsh JG, Covington EC. Long-term results of peripheral nerve stimulation for reflex sympathetic dystrophy. J Neurosurg. 1996;84:415–423. doi: 10.3171/jns.1996.84.3.0415. [DOI] [PubMed] [Google Scholar]
- 29.Kemler MA, Barendse GA, van Kleef M, de Vet HC, Rijks CP, Furnée CA, van den Wildenberg FA. Spinal cord stimulation in patients with chronic reflex sympathetic dystrophy. N Engl J Med. 2000;343:618–624. doi: 10.1056/NEJM200008313430904. [DOI] [PubMed] [Google Scholar]
- 30.Kemler MA, De Vet HC, Barendse GA, Van Den Wildenberg FA, Van Kleef MV. The effect of spinal cord stimulation in patients with chronic reflex sympathetic dystrophy: Two years’ follow-up of the randomized control trial. Ann Neurol. 2004;55:13–18. doi: 10.1002/ana.10996. [DOI] [PubMed] [Google Scholar]
- 31.White JC, Sweet WH. Pain and the neuro-surgeon, a fourty-year experience. Charles C. Thomas; Springfield, IL: 1969. [Google Scholar]
- 32.Nielson KD, Watts C, Clark WK. Peripheral nerve injury from implantation of chronic stimulating electrodes for pain control. Surg Neurol. 1976;5:51–53. [PubMed] [Google Scholar]
- 33.Slavin KV. Peripheral nerve stimulation for neuropathic pain. Neurotherapeutics. 2008;5:100–106. doi: 10.1016/j.nurt.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiner RL, Reed KL. Peripheral neuro-stimulation for control of intractable occipital neuralgia. Neuromodulation. 1999;2:217–221. doi: 10.1046/j.1525-1403.1999.00217.x. [DOI] [PubMed] [Google Scholar]
- 35.de Leon-Casasola OA. Spinal cord and peripheral nerve stimulation techniques for neuropathic pain. J Pain Symptom Manage. 2009;38:S28–38. doi: 10.1016/j.jpainsymman.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Amin S, Buvanendran A, Park KS, Kroin JS, Moric M. Peripheral nerve stimulator for the treatment of supraorbital neuralgia: A retrospective case series. Cephalalgia. 2008;28:355–359. doi: 10.1111/j.1468-2982.2008.01535.x. [DOI] [PubMed] [Google Scholar]
- 37.Melzack R, Wall PD. Pain mechanisms: A new theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 38.Ellrich J, Lamp S. Peripheral nerve stimulation inhibits nociceptive processing: An electrophysiological study in healthy volunteers. Neuromodulation. 2005;8:225–232. doi: 10.1111/j.1525-1403.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- 39.Royster EI, Crumbley K. Initial experience with implanted peripheral nerve stimulation for the treatment of refractory cephalgia. Ochsner J. 2011;11:147–150. [PMC free article] [PubMed] [Google Scholar]
- 40.Mobbs R, Nair S, Blum P. Peripheral nerve stimulation for the treatment of chronic pain. J Clin Neurosci. 2007;14:216–221.1. doi: 10.1016/j.jocn.2005.11.007. [DOI] [PubMed] [Google Scholar]