Abstract
Chronic pain represents an immense clinical problem. With tens of millions of people in the United States alone suffering from the burden of debilitating chronic pain, there is a moral obligation to reduce this burden by improving the understanding of pain and treatment mechanisms, developing new therapies, optimizing and testing existing therapies, and improving access to evidence-based pain care. Here, we present a goal-oriented research agenda describing the American Pain Society’s vision for pain research aimed at tackling the most pressing issues in the field.
Perspective
This article presents the American Pain Society’s view of some of the most important research questions that need to be addressed to advance pain science and to improve care of patients with chronic pain.
Keywords: Chronic pain, pain research, pain treatment, research funding, pain education
The Disease of Chronic Pain and the Desperate Status of Pain Research in America
Chronic pain refers to multiple clinical conditions defined by longstanding pain that adversely impact quality of life. Common chronic pain conditions include, but are not limited to, low back pain, headache conditions (eg, migraine and tension-type headache), osteoarthritis, temporomandibular disorders, irritable bowel syndrome, chronic widespread pain, and neuropathic pain conditions (eg, diabetic neuropathy, postherpetic neuralgia). Although historically conceptualized as distinct disorders whose only common feature was persistent pain, increasing evidence suggests that many chronic pain conditions may share some underlying pathophysiologic mechanisms. Specifically, altered neurologic function resulting in aberrant processing of somatosensory information and leading to comorbid symptoms and syndromes has been documented in numerous chronic pain conditions, including alterations in peripheral nociceptor activity as well as brain structure and function.22,84 Thus, a recent Institute of Medicine (IOM) report stated, “Chronic pain can be a disease in itself. Chronic pain has a distinct pathology, causing changes throughout the nervous system that often worsen over time. It has significant psychological and cognitive correlates and can constitute a serious, separate disease entity” (p. 3).50 Moreover, chronic pain represents the most prevalent, disabling, and expensive public health condition in the United States, affecting more than 100 million people in the United States, with annual costs to society estimated at $635 billion.38,50 This exceeds the combined costs of cancer, heart disease, and diabetes38 (Table 1). Further, low back pain is the leading cause of years lived with disability, and chronic pain conditions comprise 3 of the top 5 conditions producing the greatest years lived with disability in the United States.94
Table 1.
Pain Impact Statistics
| Pain Impact | Total Number or Cost |
|---|---|
| Number of affected Americans | >100,000,000 people |
| Annual societal costs | $635,000,000,000 |
| Annual government expenditures | $99,000,000,000 |
IOM Report: Relieving Pain in America, 2011.50
Importantly, although chronic pain affects individuals of all ages, races, and genders, it disproportionately impacts members of vulnerable population groups. Indeed, women are at substantially greater risk for multiple chronic pain disorders compared to men,31,68 and the prevalence and impact of chronic pain is greater in older adults.37,44 Racial and ethnic disparities in chronic pain have also been reported, with members of minority groups at increased risk for more severe pain and disability as well as for undertreatment of their pain.3,41 Lastly, historically pain in young children has often gone undertreated, which can cause altered neurologic function, placing individuals at potentially increased risk of chronic pain later in life.7,97
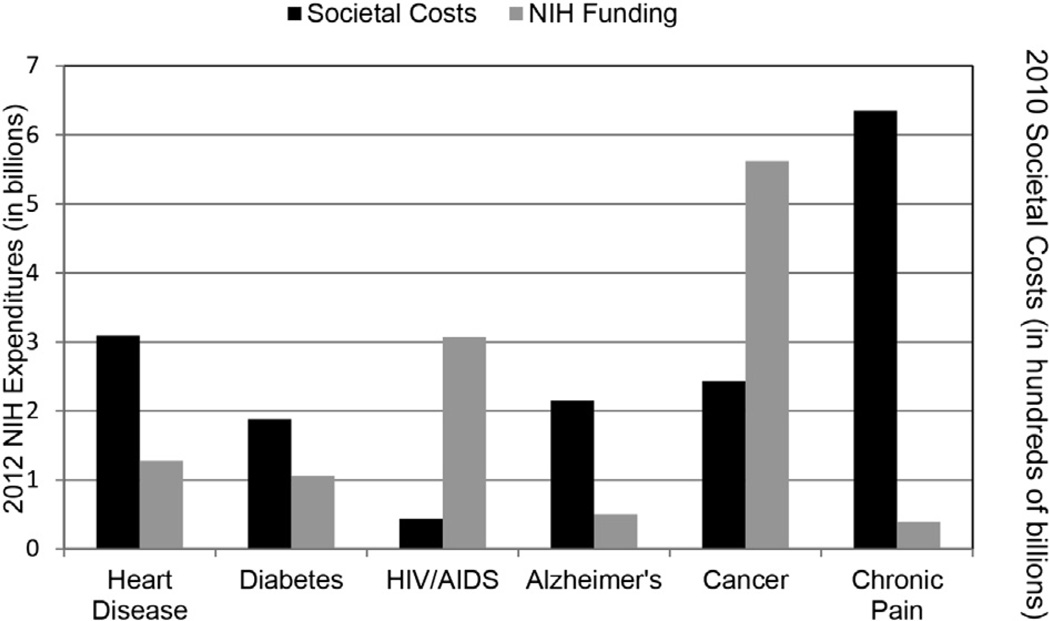
Despite its enormous societal impact, we have not seen advancements in treatment of chronic pain that reduce its burden in the population.63,64 Although there are multiple explanations for this limited progress, the clearest and most direct path to achieving dramatic advances in pain treatment is through substantially increased investment in pain research and education funding, which would enable the pursuit of an aggressive translational pain research agenda (described below). Although the National Institutes of Health (NIH) reports that pain research expenditures have increased in recent years,72 independent analyses of NIH data showed decrements in funding for pain research from 2003 to 2007.10 Even the most optimistic estimates indicate that pain research is woefully underfunded relative to its public health impact. Based on information provided by the NIH,72 pain research expenditures in 2012 totaled $396 million, approximately 1.2% of the NIH budget. To put this into perspective, NIH expenditures for several health conditions, relative to their societal costs and population prevalence in the United States, are depicted in Table 2 and Fig 1. Moreover, chronic pain represents a major health concern among members of the active duty military and veterans. However, the Department of Defense ($21 million) and the Department of Veterans Affairs ($13.4 million) also allocate a very small percentage of their research budgets to pain research.51 These data show that compared to other disease conditions, chronic pain is substantially underfunded relative to its prevalence, disease burden, and economic toll.
Table 2.
NIH Expenditures per Affected Person in the United States for 6 Major Chronic Conditions
| Chronic Condition | Dollars Per Affected Person* |
|---|---|
| Heart disease | 48 |
| Diabetes | 41 |
| HIV/AIDS | 2,562 |
| Alzheimer’s disease | 97 |
| Cancer | 431 |
| Chronic pain | 4 |
Abbreviations: HIV, human immunodeficiency virus; AIDS, acquired immune deficiency syndrome; NIH, National Institutes of Health; SEER, Surveillance, Epidemiology, and End Results Program.
Results are based on the following data. NIH expenditures are 2012 data from the NIH website.72 Prevalence data for each condition were derived from the following sources: heart disease, 26.5 million89; diabetes, 25.8 million87; HIV/ AIDS, 1.2 million45,46; cancer, 13 million81; and chronic pain, 100 million.50 Figures rounded to nearest dollar.
Figure 1.
Expenditures for 6 major chronic conditions. Societal cost data (black bars) were derived from the following sources: chronic pain,38 heart disease,38 cancer,38 diabetes,38 HIV/AIDS,49 and Alzheimer’s disease.48 All societal costs were converted to 2010 dollars. NIH expenditures (gray bars) are from the NIH categorical spending data providedonnih.gov for 2012,72 the most recent year available.
The limited funding allocated for pain research has hampered clinicians’ ability to provide optimal, evidence-based care to individuals suffering from chronic pain. In addition to the relative lack of newly developed pain treatments, the inadequacy of available information regarding the long-term safety and effectiveness of existing treatments has hampered evidence-based pain treatment. Indeed, the dramatic increase in the prescription of opioids over the last 2 decades emerged in the context of limited availability of alternative treatments and insufficient data regarding the long-term safety and efficacy of long-term opioid therapy for chronic pain.
The American Pain Society (APS) continues to advocate for increased pain research funding, and an important complement to these advocacy efforts is a vision for where pain research should go in the future—a pain research agenda for the 21st century, which we present in this article. The idea for this APS pain research agenda germinated with the release of the IOM report on chronic pain some 3 years ago. Because the APS mission focuses on interdisciplinary research and evidence-based pain care, the APS President and Board of Directors decided that development and publication of the APS Research Agenda was both important and timely given the ongoing activities related to pain research at the national level (ie, the work of the Interagency Pain Research Coordinating Committee). Authors were chosen from the APS Board of Directors and the APS membership, and the authorship team was selected to ensure representation from multiple professional disciplines and included expertise in clinical, translational, and basic science research as well as educational and health policy research. Rather than a topical or method-based approach, we propose a goal-oriented research agenda, which emphasizes important outcomes that need to be achieved in order to advance pain treatment. The proposed goals include a mix of intermediate (Goals 3–5) and long-term (Goals 1 and 2) initiatives, attainment of which has the potential to transform pain management. The focus of this research agenda is on chronic rather than acute pain, although many of the proposed goals are of relevance to both. Specifically, the APS Pain Research Agenda identifies 5 broad goals:
Develop novel pain treatments that enhance clinically meaningful pain relief and functional improvement with acceptable adverse effects.
Expedite progress toward the prevention, diagnosis, and management of chronic pain conditions.
Optimize the use of and access to currently available treatments that are known to be effective.
Understand the impact of health policies and systems on pain treatment.
Improve pain management through education research.
Within each goal, several lines of research that would help fulfill the goal are discussed. However, this is not meant to be a comprehensive overview of important research topics. Instead, the intended focus of the research agenda is on achieving the proposed goals through outstanding science that covers the entire translational spectrum. The article concludes by discussing potential barriers to transformative pain research and discusses potential solutions.
A Goal-Oriented Agenda for Pain Research
Goal 1: Develop Novel Pain Treatments That Enhance Clinically Meaningful Pain Relief and Functional Improvement With Acceptable Adverse Effects
Although clinical implementation of novel treatments represents a long-term enterprise, short-term and intermediate-term progress toward this goal is attainable in the form of discovery of potential new targets and therapies and translating these discoveries into humans. In the past 3 decades, physiologic and pharmacologic pain research has produced enormous advances in our understanding of 1) the basic transduction mechanisms that activate pain-sensing neurons (nociceptors) and propagate this information centrally, 2) the molecular mechanisms that drive plasticity in the nervous system promoting the development and maintenance of chronic pain states, and 3) the pharmacology of endogenous circuits that negatively and positively regulate pain. This research has led to 3 paths of therapeutic development for pain: interventions targeted at blocking the transduction of pain signals at their peripheral source or along the nociceptor, therapeutics that disrupt or reverse molecular mechanisms of plasticity that are thought to underlie pain plasticity, and therapeutics that modulate or mimic endogenous pain control mechanisms. All 3 of these approaches have been successful in bringing new pain therapeutics either to market or at least to clinical trials. Examples include TRPV1 antagonists (transduction blocker), which have been verified to block thermal hyperalgesia in humans15; nerve growth factor–sequestering treatments (plasticity modulator), which have shown remarkable efficacy in a variety of chronic pain conditions11,54,57,59; and norepinephrine reuptake inhibitors (endogenous pain modulation enhancer), which have been particularly successful for neuropathic pain.75,100 Although some of these treatments may not gain regulatory approval because of adverse side effects,40,54 these represent powerful examples of the utility of physiologic and pharmacologic pain research for bringing new, mechanism-based approaches forward for clinical development for the treatment of clinical pain disorders.
Recent advances have also occurred in nonpharmacologic treatments that activate endogenous pain modulatory systems, with minimal adverse effects. For example, transcranial magnetic stimulation and direct current stimulation provide noninvasive methods for altering central pain processing, and some evidence suggests potential efficacy in ameliorating acute and chronic pain.71,73 Further, emerging psychological approaches such as neurofeedback offer the possibility of training patients to directly control their own pain modulatory processes.14,52
Despite these successes, research investment in basic pain research in academia is falling, and biotechnology and pharmaceutical industrial support for pain research has decreased dramatically in terms of dollars and jobs since the financial collapse of 2007.82,88 Why has this happened? There are fiscal and governmental policy issues that unquestionably have played an important role; however, a negative outlook on the therapeutic development for chronic pain has undoubtedly taken hold in some circles8,90 (for an opposing view, see69). Despite this, a recent survey of neurologists found that the most transformative drug of the past decade was the triptan class because of its profound impact on the treatment of the world’s most common neurologic disorder, migraine headache.55 It should be noted that this family of drugs was identified via rationally designed preclinical studies searching for compounds with a specific mechanism and represents a clear success in translating hypotheses into humans. We argue that there is every reason for an optimistic outlook in terms of future therapeutic development for the pain and analgesia area. However, this opportunity cannot be seized without investment from both the public and private sectors and innovation from within the pain research community. The problem of chronic pain is accelerating in developed countries as their populations age and the burden of chronic disease continues to grow.50 We propose the following priorities for continuing to build on the dramatic growth in knowledge of basic mechanisms of pain and pain plasticity to find novel therapeutics that will alleviate suffering brought about by chronic pain.
Discovery Research Is the Foundation for Development of Novel Pain Therapies
Physiologic and pharmacologic research into pain transduction and plasticity has led to the elucidation of countless targets for potential therapeutic development.101 For instance, the pain gene database contains at least 390 distinct genes encompassing transgenic mice that have a nociceptive phenotype.58 The large number of receptors, channels, signaling molecules, and other proteins identified as potential pain therapeutic targets is not surprising given what we know from clinical experience: chronic pain is a tremendously complex set of conditions, and the many forms of chronic pain do not all involve the same underlying mechanism(s). Thus, physiologic and pharmacologic discovery research—the identification of potential targets for the development of novel analgesics—must continue. The large number of potential pain therapeutic targets may seem overwhelming, but we are only now beginning to gain the technical capacity to look at how these targets integrate to form complex physiology and how basic studies in model organisms can be combined with rapid advances in human genetics and imaging to develop and validate targets.
The foundation for therapeutic interventions can be laid by basic science advances that elucidate the fundamental biology of pain and its development into the chronic pain disease state. This includes basic science research conducted in animals and in humans and can range from discovery of neurotransmitters, receptors, and underlying mechanisms, to genetic discovery studies, to brain imaging research designed to further elucidate the central processing of acute and chronic pain. We must increase efforts to identify new targets for the development of novel pain therapies, and further we must increase our focus on conducting proof-of-concept studies in humans. Importantly, novel targets (as referred to throughout this article) include not only molecular mechanisms but also peripheral tissues, central nervous system structures and pathways, and behavioral and psychosocial processes. This will necessitate financial support for such studies.
Augmenting and/or Optimizing Existing Treatments Can Lead to Development of New Therapeutics
Innovation in pain research need not rely solely on novel target development. Multimodal analgesia is commonly employed in primary care and in specialized pain clinics throughout the world, and the rapid proliferation of medical devices has opened up new horizons in pain therapeutics. Basic pharmacology has long been a stronghold of research in the pain area, and some of the most highly cited studies in the area involve the elucidation of synergistic drug interactions (eg, spinal α2-adrenergic receptors and mu-opioid receptors76). The discovery of a new target for pain does not have to mean the instigation of a decade-long drug discovery program. In some cases, clinically available drugs may already exist that are utilized in completely different disease areas. The pain research community should prioritize trials to identify and validate new pharmacotherapies.
Patients are often managed not only with multiple pharmaceutical agents but also with multiple nonpharmacologic therapies. Research into medical device utilization, such as spinal cord and peripheral nerve stimulators and/or transcranial magnetic stimulation, is demonstrating that important pain targets can be engaged without drug administration. Research into physical activity, exercise, and cognitive therapies shows that these therapies can be as effective as pharmaceutical therapies and can modify pain processing. Similarly, complementary and alternative medicine therapies are used by a large proportion of individuals with pain, and the quantity and quality of research on effectiveness and mechanisms of complementary and alternative medicine have grown exponentially in recent years. As our knowledge continues to grow in this area, opportunities for combining nonpharmacologic and pharmacologic therapies are sure to emerge. Thus, discovering interactions between a variety of treatments, positive and negative, and predictors of what makes treatments successful or unsuccessful is important in advancing and optimizing existing treatments.
Bidirectional Translational Research Will Improve Development of Models and Targets
Much has been made in recent years of the degree to which animal models are or are not predictive of the ability of a given therapy to effectively treat pain in humans.8,69,90 Despite notable failures in translation (which occur in all fields of biomedicine),20,29,47 there have indeed been successes.54,85 Translational research should be bidirectional, with basic science informing clinical pain research concerning mechanisms of treatment effects, and clinical practice and research providing the clinical questions and definitive determinations of patient benefit. Thus, basic science and clinical research on pain must move to align preclinical and clinical study design by exploiting “translational bridges,” which represent opportunities to ask similar questions in clinical and preclinical studies. For example, preclinical studies typically assess measures of sensitization that are easily accessible in animal models (hypersensitivity to touch, heat, cold), whereas clinical studies ask patients how they feel. Notable recent efforts have been made to develop novel animal model outcome measures toward accessing the “degree of pain an animal feels” or the impact of pain on function (as opposed to sensitization).19,56,60 Similarly, the use of quantitative sensory testing (similar to hypersensitivity measures in animals) in clinical studies is still relatively rare, but incorporating quantitative sensory testing into clinical studies may facilitate reverse translation of findings from clinical studies to preclinical research, particularly when animal studies employ more sophisticated measures that do not rely exclusively on reflexes.
Translational pain research must incorporate a direct examination of the similarities and differences in the anatomy and physiology of the animals being used in preclinical studies relative to that of humans. The concept of using animal models of pain is predicated on the assumption that there is congruency in mechanisms and the models are predictive of treatment effectiveness between animal models and humans. Unfortunately, these assumptions are not commonly tested directly, although there are some innovative examples.2,21,95 Determining cross-species similarity in the expression and function of pain-related biopsychosocial processes should be a major goal of preclinical pain research.
Another key area for bidirectional translation is genetics. Advances in human genetic profiling have led to the identification and validation of numerous targets for pain,25,33,102 with some of the most rapid advancements occurring in the migraine and headache fields.4 Similarly, the rapid development of brain imaging approaches is elucidating the central nervous system pathways and networks involved in the experience of chronic pain and its successful treatment, and imaging represents another opportunity for bidirectional translation.61 These developments, however, cannot reach their full potential without translation back into preclinical models for elucidation of underlying mechanisms. Moving ahead, increasing bidirectional translational research has the potential to catalyze new target development and the refinement of pain models for both preclinical and clinical research.
Clinical Trials Should Be Fast Tracked to Advance or Eliminate Targets
The proliferation of potential targets to treat pain demands a concerted effort to test the hypotheses posed by these targets in humans. The pain research community needs to work collaboratively in the preclinical and clinical arenas to foster a culture promoting rapid translation of promising discoveries into clinical trials. Although this is not a call for compromises on patient safety, stakeholders must recognize that chronic intractable pain has a profound negative impact on quality of life and is one of the most frequent causes of disability and of proliferation of other severe health problems.50 Hence, there is a strong rationale for moving rapidly and decisively toward the testing of new mechanisms and therapies in humans either through repurposing existing pharmacologic and non-pharmacologic therapeutics or through efforts to develop novel treatments. As a community, we must also advocate for widespread dissemination of clinical trial results, including negative results,78 to ensure that clinical management of pain is based on the available evidence. Finally, the pain research community must be willing to abandon certain targets when efforts in that area do not lead to clinically meaningful endpoints in humans. Demanding the resources for research that the chronic pain problem requires must be accompanied by a spirit of agile innovation in the pain research community to make advances for the patients we ultimately serve.
Goal 2: Expedite Progress Toward the Prevention, Diagnosis, and Management of Chronic Pain Conditions
Over the last several decades, both basic and clinical pain researchers have significantly advanced our understanding of the underlying biopsychosocial processes and risk determinants that contribute to chronic pain conditions. Importantly, the conceptual model that best incorporates existing evidence regarding risk for developing common complex persistent pain conditions is the biopsychosocial model,28,39 which posits that a mosaic of biological, psychological, and social factors represent the pathways of vulnerability to a variety of chronic pain conditions. The identification of the biological, psychological, social, and associated molecular pathways that contribute to the onset and persistence of chronic pain conditions is needed if advances in the prevention, diagnosis, and management of chronic pain conditions are to occur. Our understanding of the peripheral, spinal, and supraspinal processes that contribute to chronic pain conditions has been enabled by advances in the quantitative and qualitative clinical phenotyping of chronic pain patients using validated methods that assess putative psychosocial and neurobiological risk determinants for chronic pain conditions. In parallel, substantial advances in genomics, proteomics, and bioinformatics now permit the pain research community to identify molecular pathways associated with the complex biopsychosocial phenotypes commonly observed across multiple most chronic pain conditions. These mechanistic studies need to be guided by large population-based epidemiologic studies that identify the biological, psychological, and social risk factors for the onset and maintenance of chronic pain conditions. Further, these epidemiologic approaches can enhance clinical trial design by enabling stratification of patients into subpopulations based on biopsychosocial characteristics, moving the field closer to personalized pain treatment (see12). This will increase the availability and efficacy of current and new therapies for chronic pain conditions.24 Despite the formidable challenges that lie ahead, personalized pain management (ie, tailoring treatment based on biopsychosocial mechanisms or characteristics identified in individual patients within diagnostic groups) represents an important objective. Although no large-scale studies have yet adopted this approach, several smaller studies have shown that stratification based on sensory profiles may help predict treatment responses among patients with neuropathic pain.5 Thus, there is a need to conduct proof-of-concept clinical trials that use novel stratification procedures with the goal of validating individual selection criteria to choose therapeutic (pharmacologic and nonpharmacologic) interventions that are tailored to treat the individual chronic pain patient.
The accomplishment of Goal 2 will require substantial resources from both public and private sectors. A national effort that enables the development of public and private consortia, cooperative agreements, databases, and registries is needed. Large population-based initiatives are required that identify risk determinants and molecular pathways associated with chronic pain conditions. At present, only a few public or private initiatives show promise in advancing the accomplishment of Goal 2. Two large federally funded programs—Orofacial Pain Prospective Evaluation and Risk Assessment (OPPERA) and Multi-Disciplinary Approach to the Study of Chronic Pelvic Pain (MAPP)—are the first large population studies that seek to identify risk factors and pathways associated with complex persistent pain conditions.32,62,65,83 These 2 programs represent a blueprint for the development of consortia, cooperative agreements, databases, and registries that will permit the successful accomplishment of Goal 2. A national effort involving both public (ie, U.S. Department of Health and Human Services) and private (industry) contributions is required to accomplish Goal 2 in a manner that impacts our ability to diagnose and manage patients suffering from the silent epidemic of chronic pain in America.
Goal 3: Optimize the Use of and Access to Currently Available Treatments That Are Known to Be Effective
A number of pharmacologic and nonpharmacologic treatments are effective for a variety of chronic pain conditions. Despite the increased availability of evidence-based guidelines to guide management of clinical pain,16–18,43,86 translation of effective treatments to the clinic is lacking. Interdisciplinary approaches represent among the most effective treatments for chronic pain.35 However, access to interdisciplinary programs is limited because of lack of reimbursement.92 Therefore, research that investigates how to not only implement individual treatments proven efficacious for chronic pain (ie, physical therapy, psychology) but also promote access to interdisciplinary pain treatment programs is essential to improve quality of life for the person with pain. This will require studies aimed at identifying the key barriers to implementation of therapies that have been shown to be effective but are not commonly prescribed. In addition, research is needed to determine effective methods to promote active engagement of the individual with pain in their own treatment through exercise and other self-management approaches.
Although randomized controlled clinical trials represent the gold standard of clinical evidence, their substantial limitations have been cogently presented.9,77,79 Indeed, it is widely accepted that even efficacious pain therapies provide clinical benefit to only a minority of patients. Enhancements in the methodology and execution of clinical trials for pain treatments are needed, including improvement in pain measurement, patient selection, study design, and analytic approaches.27,30 Additional research is needed to identify patient characteristics that predict responsiveness to specific treatments, which would allow better matching between patients and treatments. Further, it has become increasingly clear that the use of comparative effectiveness research examining evidence for effectiveness, benefits, and harms and the cost of a variety of treatment options can provide a more informed decision-making process from both the clinician and patient perspective. For example, people with chronic pain conditions often take drugs not just for months but for years, yet few clinical trials test for the effects of long-term usage over years. This is particularly true for opioids, a class of analgesics that are clearly effective for acute pain but whose benefits and adverse effects with chronic use have been the topic of considerable discussion.6,12,96 It is critically important to test pain therapies not only for efficacy but also for both therapeutic and potential side effects with long-term usage, because such effects may vary over time. Although funding of long-term clinical studies is challenging, public-private partnerships could be exploited to support such research (see the Barriers section). Moreover, large-scale observational studies that exploit existing clinical data sets can provide valuable information regarding long-term outcomes. Finally, research is needed to determine whether combinations of therapies can produce additive or synergistic effects. As noted above, drug combinations may be useful; however, the effectiveness of providing nonpharmacologic treatment concurrent with pharmacologic or other medical therapies has received scant empirical attention.
Paramount in optimizing the use of and access to effective pain treatments is identifying which of the available treatments are effective in the management of chronic pain. Unfortunately, the effectiveness and relative costs of various treatments for treating different types of patients are often unclear. Randomized trials of therapies, which maximize the internal validity (ie, reduced bias) of treatment comparisons, need to be balanced with real-world observational studies of therapies as used in clinical practice, which maximize the external validity (ie, generalizability) of treatment comparisons. Recently, efforts have begun to put in place large-scale open source pain registries to help to address these issues. Development and implementation of these pain registries should expand, with the goal of broad application and sufficient granularity to enable outcomes and comparative effectiveness research on a large scale. Providing this information will enable informed decision making on care paths for patients suffering from chronic pain. This work is well aligned with the goals of the Patient-Centered Outcomes Research Institute (PCORI), which is authorized by Congress to “fund and disseminate research that will provide information about the best available evidence to help patients and their health care providers make more informed decisions. PCORI’s research is intended to give patients a better understanding of the prevention, treatment and care options available, and the science that supports those options.”74 Thus, PCORI represents a significant opportunity for funding this type of work, but this effort needs wide support by federal and private funders, as all have a vested interest in the success of this work.
A related issue is that most of the evidence for efficacy of various treatments is based on studies of adult patients, with very little data available on efficacy among pediatric patients. This lack of information regarding treatment effects among children is at best a barrier to pain care in the pediatric population and at worst may put these patients at risk.36,42,98 Much work is therefore needed to test the safety and efficacy of current and emerging therapies for treating pain in children.
Goal 4: Understand the Influence of Health Policies and Systems on Pain Treatment
Health policy and systems research is an emerging interdisciplinary field devoted to understanding how health systems respond and adapt to health policies, how health policies can shape and be shaped by health systems, and how both health policies and health systems shape health outcomes.13 Health policy and health systems research can help determine optimal contextual factors for implementation of existing treatments and the effectiveness of different treatments and implementation strategies. Implementation of the Affordable Care Act (ACA) in the United States affords opportunities to study the impact of new health care policies and systems of care on pain treatment delivery and outcomes. Several ACA initiatives could impact pain care. For example, the ACA designates chronic disease management, ostensibly including chronic pain, as a priority with potentially increased support for care coordination and self-management training. Also, the ACA mandates parity of mental health and substance care with other medical care in expanded Medicaid and state exchanges. Mental health comorbidities such as depression and anxiety are more prevalent in chronic pain and are predictors of poor outcome to treatment. Further, the ACA incentivizes evidence-based treatment and supports collaborative, outcomes-based care, which could promote implementation of existing guidelines and clinical trial results into chronic pain management. Lastly, the ACA supports expanded use of technology including electronic health records and telemedicine,66,80 which should increase access to high quality care for chronic pain patients, particularly in more rural parts of the country where pain specialists are not readily available. These ACA features create a need for research aimed at determining the effects of these health policy and systems changes not only on pain but also on functional status and quality of life. Heath policy and systems research often requires fairly large populations to provide the statistical power to test the most meaningful outcomes. Because chronic pain treatment and chronic pain–related disability are among the largest expenses encountered by workers’ compensation, state and federal employee health systems, Medicaid, and private insurers, these systems should be motivated to support the study of innovative pain treatment strategies and system changes.
The following research questions should be addressed in the context of health policy and systems research. First, what are the most cost-effective models of pain care for specific chronic pain conditions? These models could include interdisciplinary treatment centers, self-management strategies in clinics, or primary care specialty care collaborations.23,26,53,93 Second, what factors influence implementation of evidence-based pain treatment? For example, how do factors such as availability of pain specialists, third-party reimbursement practices, and adoption of systems-wide clinical policies affect delivery and outcomes of pain treatment?91 Finally, the impact of technological advances on the availability and outcomes of pain treatment needs to be investigated.
Goal 5: Improve Pain Management Through Education Research
In 2012, the International Association for the Study of Pain revised and coordinated curriculum guidelines in pain education for multiple disciplines including medicine, nursing, pharmacy, psychology, physical therapy, and occupational therapy. Core competencies for prelicensure health professional education were recently established34 by consensus of an interprofessional group and aligned around the IASP guidelines. The need for investment in pain education is substantial as highlighted by the IOM report, which observed that preprofessional pain education is quite limited, and a large proportion of primary care providers feel unprepared in pain management. A study of Canadian health professions found that pain education was low in medical, dental, and pharmacy programs, averaging 15, 16, and 13 hours, respectively, across the entire curriculum; moderate in nursing, occupational, and physical therapy curricula, averaging 31, 28, and 41 hours, respectively; and high in veterinary schools, averaging 87 hours in all.99 A detailed study of U.S. and Canadian medical schools concluded that U.S. medical schools taught a median of 9 hours across the curriculum, whereas Canadian schools provided 19 hours by comparison.67 Thus, it is clear that pain education is inadequate in U.S. health profession training programs. Beyond this, a multitude of questions remains to be addressed in this field, as addressed below.
One of the first pain education research initiatives should be a formal needs assessment and task assessment at the level of prelicensure training across all health professions. There should be an evidence-based appraisal of all domains essential to pain management. Because of the complex nature of pain, the learning tasks of pain-focused education will be complex as well. Very little is known about how to most effectively change behaviors and to foster more effective clinical outcomes in pain through education. There is tension between the current trend away from specifying curricular content and toward competency-based approaches to educational planning, and the trend emphasizing professional, personal, and affective development—for example, empathy and compassion—in the effective delivery of pain care. Well-developed pain education curricula are needed to prepare health care providers who are competent to practice pain management in both the primary care setting and advanced tertiary care settings. This will require our institutions of higher education to modify curricula and to employ more basic and clinical scientists with backgrounds and training in pain research and pain management. Importantly, the outcomes of such educational interventions need to be empirically evaluated.
Pain education research needs to be considered within the broader context of health profession education in general. Emerging trends in health profession education that are of particular relevance to pain education include practice change and methods to produce and measure improvements in clinically meaningful outcomes; enhancing knowledge transfer and maintaining current and accurate content; and the implementation of patient-centered care practices. Moreover, interprofessional and “uni-professional” education must be coordinated lest essential profession-specific knowledge and competencies be lost in a focus on interprofessional practices. Pain education should take advantage of ongoing developments in health profession teaching methodologies, including optimal use of high- versus low-fidelity simulations, and innovations in educational assessment (eg, script concordance testing, normalized gains analysis, and case-oriented assessment approaches).
Barriers to Transformative Pain Research
The above discussion highlights 5 major research priorities identified by the APS. Substantive progress in each of these areas could have tremendous public health impact and could produce dramatic improvements in quality of life for tens of millions of chronic pain sufferers. This is an aggressive and ambitious research agenda encompassing both intermediate and long-term goals. Given the finite resources available to support pain research, identifying priorities and timelines to achieve these goals represents an important next step in developing the research agenda, but this will require further discussion and input from multiple stakeholders. There are several additional factors that serve as barriers to progress.
Inadequate Research Funding
It is difficult to overstate the societal impact of pain, yet pain research remains woefully underfunded in both public and private sectors. A major investment is needed to improve the lives of the 100 million Americans suffering from chronic pain. Although efforts are being made to improve federal funding for pain research, these incremental measures are inadequate for the magnitude of the problem. Moreover, the current environment in Washington generates little optimism that the situation will improve in the near future. A paradigm-shifting and transformative realignment of funding priorities and a broader-based commitment to fund these priorities in pain research is needed if real progress is to be made. This should involve a shift away from classical mortality- and morbidity-based measures of disease burden to more modern measures appropriate for chronic disease such as disability-adjusted life-years and healthy life expectancy.70 This is not simply a need for tweaking funding at the NIH. The U.S. Food and Drug Administration, insurers, industry, and private foundations are all needed as partners. This will require greatly expanded advocacy efforts, including partnerships between professional organizations and patient advocacy groups, in order to educate legislators and policy makers about the societal impact of chronic pain.
Lack of Public Awareness
Despite the fact that chronic pain represents the most prevalent public health condition in the United States, there is an alarming lack of public awareness about the magnitude of the problem. In a recent poll of U.S. adults commissioned by Research America,1 only 18% of respondents identified chronic pain as a major public health problem, whereas far less prevalent conditions were much more frequently described as major public health conditions (diabetes [52%], drug addiction [47%], alcoholism [37%], and Alzheimer’s disease [34%]). This lack of public awareness of chronic pain as a major public health issue not only contributes to the continued lack of federal research funding but also helps to explain the lack of private and philanthropic support for pain research and treatment. Thus, efforts are sorely needed to raise public awareness of both the societal impact of chronic pain and the personal suffering of individuals with chronic pain.
Another barrier to enacting the proposed research agenda is the lack of enabling infrastructure to support large-scale and transformative pain science. Examples include the need for the development of coordinated clinical trial networks and practice-based research networks. We will need to support an expansion of interdisciplinary pain research and treatment centers and networks (translational medicine centers focused on pain) and, importantly, to provide career development infrastructure to expand the number of highly trained pain scientists and pain management clinicians who can create novel discoveries and translate them into safe and effective new therapies.
Cultural transformation is needed if we are to move forward with the proposed research agenda and overcome the identified barriers. As the IOM report stated, “addressing the nation’s enormous burden of pain will require a cultural transformation in the way pain is understood, assessed, and treated” (p. 1).50 That is, we need a fundamental change in the way physicians, legislators, and society view the problem of pain. Chronic pain must become a national priority, being recognized as a disease in its own right that produces enormous suffering and must be treated aggressively. Effecting this societal change in beliefs and attitudes will require significant efforts in education for health care providers, patients, legislators, policy makers, and the general public.
Changes in attitude, a refocusing of our efforts and refinement of approaches, improved education, the breaking down of barriers, and major investment are needed. These are daunting goals, which in the current economic climate might appear unachievable. But this is within our reach. Much larger investments in areas of identified urgent need have been made, and the results have been nothing short of transformative. The top– down decisions to send humans to the moon, declare a war on cancer, decode the human genome, and find a way to halt the acquired immune deficiency syndrome epidemic serve as excellent examples. Is the daily suffering of 100 million Americans less important? The APS will support implementation of this research agenda through increased advocacy efforts; continuing and increasing APS funding of pain research; educating policy makers, providers, and the public regarding the societal burden of chronic pain; and forging partnerships and collaborations with other organizations and institutions to further promote the agenda. As so poignantly stated by an individual with chronic pain, “I wouldn’t wish this on anyone, but if researchers [and policy makers] could go through just one day of life as I live it, maybe they would understand how devastating this is… There is no hope for people with R.S.D. (reflex sympathetic dystrophy)… Please help us” (p. 238; bracketed text added).50 Discovery and application of knowledge foster hope, which people with chronic pain deserve. We cannot ignore this problem any longer.
Acknowledgments
W.M. has had relationships with the following companies within the past 12 months: Algynomics, Inc, through royalties, receipt of intellectual property rights/patent holder and ownership interest via stocks, stock options, or other ownership interest excluding diversified mutual funds; Orthogen through consulting fees. M.D.S. has received a grant from the REMS-RPC to support continuing medical education concerning opioid treatment of chronic pain.
Footnotes
All other authors declare no relevant conflicts of interest.
References
- 1.Americans talk about pain. Available at: https://www.researchamerica.org/uploads/poll2003pain.pdf.
- 2.Anand U, Facer P, Yiangou Y, Sinisi M, Fox M, McCarthy T, Bountra C, Korchev YE, Anand P. Angiotensin II type 2 receptor (AT2 R) localization and antagonist-mediated inhibition of capsaicin responses and neurite outgrowth in human and rat sensory neurons. Eur J Pain. 2013;17:1012–1026. doi: 10.1002/j.1532-2149.2012.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson KO, Green CR, Payne R. Racial and ethnic disparities in pain: Causes and consequences of unequal care. J Pain. 2009;10:1187–1204. doi: 10.1016/j.jpain.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Anttila V, Winsvold BS, Gormley P, Kurth T, Bettella F, McMahon G, Kallela M, Malik R, de Vries B, Terwindt G, Medland SE, Todt U, McArdle WL, Quaye L, Koiranen M, Ikram MA, Lehtimaki T, Stam AH, Ligthart L, Wedenoja J, Dunham I, Neale BM, Palta P, Hamalainen E, Schurks M, Rose LM, Buring JE, Ridker PM, Steinberg S, Stefansson H, Jakobsson F, Lawlor DA, Evans DM, Ring SM, Farkkila M, Artto V, Kaunisto MA, Freilinger T, Schoenen J, Frants RR, Pelzer N, Weller CM, Zielman R, Heath AC, Madden PA, Montgomery GW, Martin NG, Borck G, Gobel H, Heinze A, Heinze-Kuhn K, Williams FM, Hartikainen AL, Pouta A, van den Ende J, Uitterlinden AG, Hofman A, Amin N, Hottenga JJ, Vink JM, Heikkila K, Alexander M, Muller-Myhsok B, Schreiber S, Meitinger T, Wichmann HE, Aromaa A, Eriksson JG, Traynor BJ, Trabzuni D, North American Brain Expression Consortium, UK Brain Expression Consortium. Rossin E, Lage K, Jacobs SB, Gibbs JR, Birney E, Kaprio J, Penninx BW, Boomsma DI, van Duijn C, Raitakari O, Jarvelin MR, Zwart JA, Cherkas L, Strachan DP, Kubisch C, Ferrari MD, van den Maagdenberg AM, Dichgans M, Wessman M, Smith GD, Stefansson K, Daly MJ, Nyholt DR, Chasman DI, Palotie A North American Brain Expresssion Consortium; UK Brain Expression Consortium. International Headache Genetics Consortium: Genome-wide meta-analysis identifies new susceptibility loci for migraine. Nat Genet. 2013;45:912–917. doi: 10.1038/ng.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baron R, Forster M, Binder A. Subgrouping of patients with neuropathic pain according to pain-related sensory abnormalities: A first step to a stratified treatment approach. Lancet Neurol. 2012;11:999–1005. doi: 10.1016/S1474-4422(12)70189-8. [DOI] [PubMed] [Google Scholar]
- 6.Becker WC, Fraenkel L, Kerns RD, Fiellin DA. A research agenda for enhancing appropriate opioid prescribing in primary care. J Gen Intern Med. 2013;28:1364–1367. doi: 10.1007/s11606-013-2422-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berde CB, Sethna NF. Analgesics for the treatment of pain in children. N Engl J Med. 2002;347:1094–1103. doi: 10.1056/NEJMra012626. [DOI] [PubMed] [Google Scholar]
- 8.Berge OG. Predictive validity of behavioural animal models for chronic pain. Br J Pharmacol. 2011;164:1195–1206. doi: 10.1111/j.1476-5381.2011.01300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Black N. Why we need observational studies to evaluate the effectiveness of health care. BMJ. 1996;312:1215–1218. doi: 10.1136/bmj.312.7040.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradshaw DH, Empy C, Davis P, Lipschitz D, Dalton P, Nakamura Y, Chapman CR. Trends in funding for research on pain: A report on the National Institutes of Health grant awards over the years 2003 to 2007. J Pain. 2008;9:1077–1087. 1087.e1-.e8. doi: 10.1016/j.jpain.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Brown MT, Murphy FT, Radin DM, Davignon I, Smith MD, West CR. Tanezumab reduces osteoarthritic knee pain: Results of a randomized, double-blind, placebo-controlled phase III trial. J Pain. 2012;13:790–798. doi: 10.1016/j.jpain.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Bruehl S, Apkarian AV, Ballantyne JC, Berger A, Borsook D, Chen WG, Farrar JT, Haythornthwaite JA, Horn SD, Iadarola MJ, Inturrisi CE, Lao L, Mackey S, Mao J, Sawczuk A, Uhl GR, Witter J, Woolf CJ, Zubieta JK, Lin Y. Personalized medicine and opioid analgesic prescribing for chronic pain: Opportunities and challenges. J Pain. 2013;14:103–113. doi: 10.1016/j.jpain.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cape Town statement on advancing implementation research and delivery science. Available at: http://www.who.int/alliance-hpsr/en/
- 14.Chapin H, Bagarinao E, Mackey S. Real-time fMRI applied to pain management. Neurosci Lett. 2012;520:174–181. doi: 10.1016/j.neulet.2012.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chizh BA, O’Donnell MB, Napolitano A, Wang J, Brooke AC, Aylott MC, Bullman JN, Gray EJ, Lai RY, Williams PM, Appleby JM. The effects of the TRPV1 antagonist SB-705498 on TRPV1 receptor-mediated activity and inflammatory hyperalgesia in humans. Pain. 2007;132:132–141. doi: 10.1016/j.pain.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Chou R, Huffman L. American Pain Society, American College of Physicians: Medications for acute and chronic low back pain: A review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:505–514. doi: 10.7326/0003-4819-147-7-200710020-00008. [DOI] [PubMed] [Google Scholar]
- 17.Chou R, Huffman LH. American Pain Society, American College of Physicians: Nonpharmacologic therapies for acute and chronic low back pain: A review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:492–504. doi: 10.7326/0003-4819-147-7-200710020-00007. [DOI] [PubMed] [Google Scholar]
- 18.Chou R, Qaseem A, Snow V, Casey D, Cross JT, Jr, Shekelle P, Owens DK. Clinical Efficacy Assessment Subcommittee of the American College of Physicians, American College of Physicians, American Pain Society Low Back Pain Guidelines Panel: Diagnosis and treatment of low back pain: A joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147:478–491. doi: 10.7326/0003-4819-147-7-200710020-00006. [DOI] [PubMed] [Google Scholar]
- 19.Cobos EJ, Ghasemlou N, Araldi D, Segal D, Duong K, Woolf CJ. Inflammation-induced decrease in voluntary wheel running in mice: A nonreflexive test for evaluating inflammatory pain and analgesia. Pain. 2012;153:876–884. doi: 10.1016/j.pain.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Contopoulos-Ioannidis DG, Ntzani E, Ioannidis JP. Translation of highly promising basic science research into clinical applications. Am J Med. 2003;114:477–484. doi: 10.1016/s0002-9343(03)00013-5. [DOI] [PubMed] [Google Scholar]
- 21.Davidson S, Copits BA, Zhang J, Page G, Ghetti A, Gereau RW. Human sensory neurons: Membrane properties and sensitization by inflammatory mediators. Pain. 2014;155:1861–1870. doi: 10.1016/j.pain.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis KD, Moayedi M. Central mechanisms of pain revealed through functional and structural MRI. J Neuroimmune Pharmacol. 2013;8:518–534. doi: 10.1007/s11481-012-9386-8. [DOI] [PubMed] [Google Scholar]
- 23.de Heer EW, Dekker J, van Eck van der Sluijs JF, Beekman AT, van Marwijk HW, Holwerda TJ, Bet PM, Roth J, Hakkaart-Van Roijen L, Ringoir L, Kat F, van der Feltz-Cornelis CM. Effectiveness and cost-effectiveness of transmural collaborative care with consultation letter (TCCCL) and duloxetine for major depressive disorder (MDD) and (sub)chronic pain in collaboration with primary care: A randomized placebo-controlled multi-Centre trial. TCC: PAINDIP. BMC Psychiatry. 2013;13:147. doi: 10.1186/1471-244X-13-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diatchenko L, Fillingim RB, Smith SB, Maixner W. The phenotypic and genetic signatures of common musculoskeletal pain conditions. Nat Rev Rheumatol. 2013;9:340–350. doi: 10.1038/nrrheum.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diatchenko L, Nackley AG, Tchivileva IE, Shabalina SA, Maixner W. Genetic architecture of human pain perception. Trends Genet. 2007;23:605–613. doi: 10.1016/j.tig.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Dickinson KC, Sharma R, Duckart JP, Corson K, Gerrity MS, Dobscha SK. VA healthcare costs of a collaborative intervention for chronic pain in primary care. Med Care. 2010;48:38–44. doi: 10.1097/MLR.0b013e3181bd49e2. [DOI] [PubMed] [Google Scholar]
- 27.Dworkin RH, Turk DC, Peirce-Sandner S, Burke LB, Farrar JT, Gilron I, Jensen MP, Katz NP, Raja SN, Rappaport BA, Rowbotham MC, Backonja MM, Baron R, Bellamy N, Bhagwagar Z, Costello A, Cowan P, Fang WC, Hertz S, Jay GW, Junor R, Kerns RD, Kerwin R, Kopecky EA, Lissin D, Malamut R, Markman JD, McDermott MP, Munera C, Porter L, Rauschkolb C, Rice AS, Sampaio C, Skljarevski V, Sommerville K, Stacey BR, Steigerwald I, Tobias J, Trentacosti AM, Wasan AD, Wells GA, Williams J, Witter J, Ziegler D. Considerations for improving assay sensitivity in chronic pain clinical trials: IMMPACT recommendations. Pain. 2012;153:1148–1158. doi: 10.1016/j.pain.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Engel GL. The need for a new medical model: A challenge for biomedicine. Science. 1977;196:129–136. doi: 10.1126/science.847460. [DOI] [PubMed] [Google Scholar]
- 29.Ergorul C, Levin LA. Solving the lost in translation problem: Improving the effectiveness of translational research. Curr Opin Pharmacol. 2013;13:108–114. doi: 10.1016/j.coph.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farrar JT, Troxel AB, Haynes K, Gilron I, Kerns RD, Katz NP, Rappaport BA, Rowbotham MC, Tierney AM, Turk DC, Dworkin RH. Effect of variability in the 7-day baseline pain diary on the assay sensitivity of neuropathic pain randomized clinical trials: An ACTTION study. Pain. 2014;155:1622–1631. doi: 10.1016/j.pain.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 31.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL3rd. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10:447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fillingim RB, Slade GD, Diatchenko L, Dubner R, Greenspan JD, Knott C, Ohrbach R, Maixner W. Summary of findings from the OPPERA baseline case-control study: Implications and future directions. J Pain. 2011;12:T102–T107. doi: 10.1016/j.jpain.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fillingim RB, Wallace MR, Herbstman DM, Ribeiro-Dasilva M, Staud R. Genetic contributions to pain: A review of findings in humans. Oral Dis. 2008;14:673–682. doi: 10.1111/j.1601-0825.2008.01458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fishman SM, Young HM, Lucas Arwood E, Chou R, Herr K, Murinson BB, Watt-Watson J, Carr DB, Gordon DB, Stevens BJ, Bakerjian D, Ballantyne JC, Courtenay M, Djukic M, Koebner IJ, Mongoven JM, Paice JA, Prasad R, Singh N, Sluka KA, St Marie B, Strassels SA. Core competencies for pain management: results of an interprofessional consensus summit. Pain Med. 2013;14:971–981. doi: 10.1111/pme.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flor H, Fydrich T, Turk DC. Efficacy of multidisciplinary pain treatment centers: A meta-analytic review. Pain. 1992;49:221–230. doi: 10.1016/0304-3959(92)90145-2. [DOI] [PubMed] [Google Scholar]
- 36.Friedrichsdorf SJ, Nugent AP. Management of neuropathic pain in children with cancer. Curr Opin Support Palliat Care. 2013;7:131–138. doi: 10.1097/SPC.0b013e3283615ebe. [DOI] [PubMed] [Google Scholar]
- 37.Gagliese L. Pain and aging: The emergence of a new subfield of pain research. J Pain. 2009;10:343–353. doi: 10.1016/j.jpain.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 38.Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13:715–724. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 39.Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: Scientific advances and future directions. Psychol Bull. 2007;133:581–624. doi: 10.1037/0033-2909.133.4.581. [DOI] [PubMed] [Google Scholar]
- 40.Gavva NR. Body-temperature maintenance as the predominant function of the vanilloid receptor TRPV1. Trends Pharmacol Sci. 2008;29:550–557. doi: 10.1016/j.tips.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 41.Green CR, Anderson KO, Baker TA, Campbell LC, Decker S, Fillingim RB, Kalauokalani DA, Lasch KE, Myers C, Tait RC, Todd KH, Vallerand AH. The unequal burden of pain: Confronting racial and ethnic disparities in pain. Pa Med. 2003;4:277–294. doi: 10.1046/j.1526-4637.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- 42.Gregoire MC, Finley GA. Drugs for chronic pain in children: A commentary on clinical practice and the absence of evidence. Pain Res Manag. 2013;18:47–50. doi: 10.1155/2013/402863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hauser W, Thieme K, Turk DC. Guidelines on the management of fibromyalgia syndrome—A systematic review. Eur J Pain. 2010;14:5–10. doi: 10.1016/j.ejpain.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 44.Helme RD, Gibson SJ. The epidemiology of pain in elderly people. Clin Geriatr Med. 2001;17:417–431. doi: 10.1016/s0749-0690(05)70078-1. [DOI] [PubMed] [Google Scholar]
- 45. [Accessed July, 2013];HIV in the United States: at a glance. Available at: http://www.cdc.gov/hiv/statistics/basics/ataglance.html.
- 46. [Accessed July, 2013];How many people have HIV/AIDS? Available at: http://www.nichd.nih.gov/health/topics/hiv/conditioninfo/Pages/how-many.aspx.
- 47.Hug A, Weidner N. From bench to beside to cure spinal cord injury: Lost in translation? Int Rev Neurobiol. 2012;106:173–196. doi: 10.1016/B978-0-12-407178-0.00008-9. [DOI] [PubMed] [Google Scholar]
- 48.Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013;368:1326–1334. doi: 10.1056/NEJMsa1204629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hutchinson AB, Farnham PG, Dean HD, Ekwueme DU, del Rio C, Kamimoto L, Kellerman SE. The economic burden of HIV in the United States in the era of highly active antiretroviral therapy: Evidence of continuing racial and ethnic differences. J Acquir Immune Defic Syndr. 2006;43:451–457. doi: 10.1097/01.qai.0000243090.32866.4e. [DOI] [PubMed] [Google Scholar]
- 50.Institute of Medicine: Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington (DC): The National Academies Collection: Reports funded by National Institutes of Health; 2011. [PubMed] [Google Scholar]
- 51.IPRCC: Overview of the 2011 Federal Pain Research Portfolio Analysis. Available at: http://iprcc.nih.gov/docs/102212_mtg_presentations/IPRCC_prelim_portfolio_analysis_508comp.pdf.
- 52.Jensen MP, Gertz KJ, Kupper AE, Braden AL, Howe JD, Hakimian S, Sherlin LH. Steps toward developing an EEG biofeedback treatment for chronic pain. Appl Psychophysiol Biofeedback. 2013;38:101–108. doi: 10.1007/s10484-013-9214-9. [DOI] [PubMed] [Google Scholar]
- 53.Katon W, Russo J, Lin EH, Schmittdiel J, Ciechanowski P, Ludman E, Peterson D, Young B, Von Korff M. Cost-effectiveness of a multicondition collaborative care intervention: A randomized controlled trial. Arch Gen Psychiatry. 2012;69:506–514. doi: 10.1001/archgenpsychiatry.2011.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Katz N, Borenstein DG, Birbara C, Bramson C, Nemeth MA, Smith MD, Brown MT. Efficacy and safety of tanezumab in the treatment of chronic low back pain. Pain. 2011;152:2248–2258. doi: 10.1016/j.pain.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 55.Kesselheim AS, Avorn J. The most transformative drugs of the past 25 years: A survey of physicians. Nat Rev Drug Discov. 2013;12:425–431. doi: 10.1038/nrd3977. [DOI] [PubMed] [Google Scholar]
- 56.King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields HL, Porreca F. Unmasking the tonicaversive state in neuropathic pain. Nat Neurosci. 2009;12:1364–1366. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kivitz AJ, Gimbel JS, Bramson C, Nemeth MA, Keller DS, Brown MT, West CR, Verburg KM. Efficacy and safety of tanezumab versus naproxen in the treatment of chronic low back pain. Pain. 2013;154:1009–1021. doi: 10.1016/j.pain.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 58.Lacroix-Fralish ML, Ledoux JB, Mogil JS. The Pain Genes Database: An interactive web browser of pain-related transgenic knockout studies. Pain. 2007;131:3.e1–3.e4. doi: 10.1016/j.pain.2007.04.041. [DOI] [PubMed] [Google Scholar]
- 59.Lane NE, Schnitzer TJ, Birbara CA, Mokhtarani M, Shelton DL, Smith MD, Brown MT. Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med. 2010;363:1521–1531. doi: 10.1056/NEJMoa0901510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, Lacroix-Fralish ML, Matsumiya L, Sorge RE, Sotocinal SG, Tabaka JM, Wong D, van den Maagdenberg AM, Ferrari MD, Craig KD, Mogil JS. Coding of facial expressions of pain in the laboratory mouse. Nat Methods. 2010;7:447–449. doi: 10.1038/nmeth.1455. [DOI] [PubMed] [Google Scholar]
- 61.Lee MC, Tracey I. Imaging pain: A potent means for investigating pain mechanisms in patients. Br J Anaesth. 2013;111:64–72. doi: 10.1093/bja/aet174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maixner W, Diatchenko L, Dubner R, Fillingim RB, Greenspan JD, Knott C, Ohrbach R, Weir B, Slade GD. Orofacial Pain Prospective Evaluation and Risk Assessment study— The OPPERA study. J Pain. 2011;12:T4.e11-12–T11.e11-12. doi: 10.1016/j.jpain.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mao J. Translational pain research: Achievements and challenges. J Pain. 2009;10:1001–1011. doi: 10.1016/j.jpain.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mao J. Current challenges in translational pain research. Trends Pharmacol Sci. 2012;33:568–573. doi: 10.1016/j.tips.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.MAPP research network home page. Available at: http://www.mappnetwork.org/
- 66.Meghani SH, Polomano RC, Tait RC, Vallerand AH, Anderson KO, Gallagher RM. Advancing a national agenda to eliminate disparities in pain care: Directions for health policy, education, practice, and research. Pa Med. 2012;13:5–28. doi: 10.1111/j.1526-4637.2011.01289.x. [DOI] [PubMed] [Google Scholar]
- 67.Mezei L, Murinson BB. Johns Hopkins Pain Curriculum Development Team: Pain education in North American medical schools. J Pain. 2011;12:1199–1208. doi: 10.1016/j.jpain.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 68.Mogil JS. Sex differences in pain and pain inhibition: Multiple explanations of a controversial phenomenon. Nat Rev Neurosci. 2012;13:859–866. doi: 10.1038/nrn3360. [DOI] [PubMed] [Google Scholar]
- 69.Mogil JS, Davis KD, Derbyshire SW. The necessity of animal models in pain research. Pain. 2010;151:12–17. doi: 10.1016/j.pain.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 70.Murray CJ, Lopez AD. Measuring the global burden of disease. N Engl J Med. 2013;369:448–457. doi: 10.1056/NEJMra1201534. [DOI] [PubMed] [Google Scholar]
- 71.Mylius V, Borckardt JJ, Lefaucheur JP. Noninvasive cortical modulation of experimental pain. Pain. 2012;153:1350–1363. doi: 10.1016/j.pain.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 72. [Accessed July, 2013];NIH analysis of spending on Pain Conditions - Chronic. Available at: http://report.nih.gov/categorical_spending.aspx.
- 73.O’Connell NE, Wand BM, Marston L, Spencer S, Desouza LH. Non-invasive brain stimulation techniques for chronic pain. A report of a Cochrane systematic review and meta-analysis. Eur J Phys Rehabil Med. 2011;47:309–326. [PubMed] [Google Scholar]
- 74.Patient centered outcomes research institute. Available at: http://www.pcori.org/
- 75.Raskin J, Pritchett YL, Wang F, D’Souza DN, Waninger AL, Iyengar S, Wernicke JF. A double-blind, randomized multi-center trial comparing duloxetine with placebo in the management of diabetic peripheral neuropathic pain. Pa Med. 2005;6:346–356. doi: 10.1111/j.1526-4637.2005.00061.x. [DOI] [PubMed] [Google Scholar]
- 76.Roerig SC, Lei S, Kitto K, Hylden JK, Wilcox GL. Spinal interactions between opioid and noradrenergic agonists in mice: Multiplicativity involves delta and alpha-2 receptors. J Pharmacol Exp Ther. 1992;262:365–374. [PubMed] [Google Scholar]
- 77.Rothwell PM. Treating individuals, Part 2: Subgroup analysis in randomised controlled trials: importance, indications, and interpretation. Lancet. 2005;365:176–186. doi: 10.1016/S0140-6736(05)17709-5. [DOI] [PubMed] [Google Scholar]
- 78.Rowbotham MC. The case for publishing “negative” clinical trials. Pain. 2009;146:225–226. doi: 10.1016/j.pain.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 79.Sanson-Fisher RW, Bonevski B, Green LW, D’Este C. Limitations of the randomized controlled trial in evaluating population-based health interventions. Am J Prev Med. 2007;33:155–161. doi: 10.1016/j.amepre.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 80.Savage SR. Affordable Care Act Offers Opportunity to Combat Pain and Drug Abuse. Available at: http://www.rollcall.com/news/savage_affordable_care_act_offers_opportunity_to_combat_pain_and_drug_abuse-223628-1.html.
- 81. [Accessed July, 2013];SEER Cancer Statistics Review, 1975–2010. Available at: http://seer.cancer.gov/csr/1975_2011/.
- 82.Semeniuk I, Thompson H. Cuts loom for US science. Nature. 2012;487:414–415. doi: 10.1038/487414a. [DOI] [PubMed] [Google Scholar]
- 83.Slade GD, Fillingim RB, Sanders AE, Bair E, Greenspan JD, Ohrbach R, Dubner R, Diatchenko L, Smith SB, Knott C, Maixner W. Summary of findings from the OPPERA prospective cohort study of incidence of first-onset temporomandibular disorder: Implications and future directions. J Pain. 2013;14:T116–T124. doi: 10.1016/j.jpain.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Smallwood RF, Laird AR, Ramage AE, Parkinson AL, Lewis J, Clauw DJ, Williams DA, Schmidt-Wilcke T, Farrell MJ, Eickhoff SB, Robin DA. Structural brain anomalies and chronic pain: A quantitative meta-analysis of gray matter volume. J Pain. 2013;14:663–675. doi: 10.1016/j.jpain.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Staats PS, Yearwood T, Charapata SG, Presley RW, Wallace MS, Byas-Smith M, Fisher R, Bryce DA, Mangieri EA, Luther RR, Mayo M, McGuire D, Ellis D. Intrathecal ziconotide in the treatment of refractory pain in patients with cancer or AIDS: A randomized controlled trial. J Am Med Assoc. 2004;291:63–70. doi: 10.1001/jama.291.1.63. [DOI] [PubMed] [Google Scholar]
- 86.Stapelkamp C, Carter B, Gordon J, Watts C. Assessment of acute pain in children: Development of evidence-based guidelines. Int J Evid Based Healthc. 2011;9:39–50. doi: 10.1111/j.1744-1609.2010.00199.x. [DOI] [PubMed] [Google Scholar]
- 87. [Accessed July, 2013];Statistics about diabetes. Available at: http://www.diabetes.org/diabetes-basics/statistics/?loc=db-slabnav.
- 88.Stovall S. R & D cuts curb brain-drug pipeline. Wall St J. 2011 [Google Scholar]
- 89. [Accessed July, 2013];Summary health statistics for U.S. adults: National Health Interview Survey. Available at: http://www.cdc.gov/nchs/data/series/sr_10/sr10_256.pdf.
- 90.Taneja A, Di Iorio VL, Danhof M, Della Pasqua O. Translation of drug effects from experimental models of neuropathic pain and analgesia to humans. Drug Discov Today. 2012;17:837–849. doi: 10.1016/j.drudis.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 91.Trescott CE, Beck RM, Seelig MD, Von Korff MR. Group Health’s initiative to avert opioid misuse and overdose among patients with chronic noncancer pain. Health Aff. 2011;30:1420–1424. doi: 10.1377/hlthaff.2011.0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Turk DC, Stanos SP, Palermo TM, Paice JA, Jaminson RN, Gordon DR, Cowan P, Convington EC, Clark ME. Interdisciplinary pain management. Available at: http://www.americanpainsociety.org/uploads/pdfs/2010%20Interdisciplinary%20White%20Paper-FINAL.pdf.
- 93.Unutzer J, Katon WJ, Fan MY, Schoenbaum MC, Lin EH, Della Penna RD, Powers D. Long-term cost effects of collaborative care for late-life depression. Am J Manag Care. 2008;14:95–100. [PMC free article] [PubMed] [Google Scholar]
- 94.US Burden of Disease Collaborators: The state of US health, 1990–2010: Burden of diseases, injuries, and risk factors. J Am Med Assoc. 2013;310:591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Valeyev AY, Hackman JC, Wood PM, Davidoff RA. Pharmacologically novel GABA receptor in human dorsal root ganglion neurons. J Neurophysiol. 1996;76:3555–3558. doi: 10.1152/jn.1996.76.5.3555. [DOI] [PubMed] [Google Scholar]
- 96.Von Korff MR. Long-term use of opioids for complex chronic pain. Best Pract Res Clin Rheumatol. 2013;27:663–672. doi: 10.1016/j.berh.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Walco GA, Cassidy RC, Schechter NL. Pain, hurt, and harm. The ethics of pain control in infants and children. N Engl J Med. 1994;331:541–544. doi: 10.1056/NEJM199408253310812. [DOI] [PubMed] [Google Scholar]
- 98.Walker SM. Neonatal pain. Paediatr Anaesth. 2014;24:39–48. doi: 10.1111/pan.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Watt-Watson J, Siddall PJ, Carr E. Interprofessional pain education: The road to successful pain management outcomes. Pain Manag. 2012;2:417–420. doi: 10.2217/pmt.12.46. [DOI] [PubMed] [Google Scholar]
- 100.Wernicke JF, Pritchett YL, D’Souza DN, Waninger A, Tran P, Iyengar S, Raskin J. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology. 2006;67:1411–1420. doi: 10.1212/01.wnl.0000240225.04000.1a. [DOI] [PubMed] [Google Scholar]
- 101.Woodcock J, Witter J, Dionne RA. Stimulating the development of mechanism-based, individualized pain therapies. Nat Rev Drug Discov. 2007;6:703–710. doi: 10.1038/nrd2335. [DOI] [PubMed] [Google Scholar]
- 102.Young EE, Lariviere WR, Belfer I. Genetic basis of pain variability: Recent advances. J Med Genet. 2012;49:1–9. doi: 10.1136/jmedgenet-2011-100386. [DOI] [PMC free article] [PubMed] [Google Scholar]