Abstract
Purpose
To test whether altered radiation fractionation schemes improved local-regional control (LRC) rates for patients with squamous cell cancers (SCC) of the head and neck when compared to standard fractionation (SFX) of 70 Gy.
Patients and Methods
Patients with stage III or IV (or stage II base of tongue) SCC were randomized to 4 treatment arms: (1) SFX-70 Gy/35 daily fxns/7 week; (2) HFX-81.6 Gy/68 BID fxns/7 weeks; (3) AFX-S 67.2 Gy/42 fxns/6 weeks with a 2 week rest after 38.4 Gy; and (4) AFX-C 72 Gy/42 fxns/6 weeks. n=1,076. The 3 experimental arms were to be compared to SFX.
Results
With patients censored for LRC at 5 years, only the comparison of HFX with SFX was significantly different (HFX: Hazard Ratio-0.79(0.62–1.00), p=0.05; AFX-C 0.82 (0.65–1.05), p=0.11). Censored at 5 years, HFX improved Overall Survival (OS) (Hazard Ratio 0.81, p=0.05). Prevalence of any Grade 3, 4, or 5 toxicity at 5 years, any feeding tube use after 180 days, or feeding tube use at 1 year did not differ significantly when the experimental arms were compared to SFX. When 7 week treatments were compared to 6 week treatments, accelerated fractionation appeared to increase Grade 3, 4 or 5 toxicity at 5 years (p=0.06). Looking at the worst toxicity per patient by treatment only the AFX-C arm seemed to trend worse than the SFX arm when comparing Grade 0–2 vs. 3–5 toxicity(p=0.09).
Conclusions
At 5 years, only HFX improved LRC & OS for patients with locally advanced SCC without increasing late toxicity.
Introduction
The Radiation Therapy Oncology Group (RTOG) 9003 phase III trial was the largest randomized study of fractionation ever conducted[1]. It was designed to answer the question of whether each of three experimental altered fractionation arms would improve local-regional control (LRC) for patients with locally advanced squamous cell cancer of the head and neck. During the study period, patients with unresectable head and neck squamous cell cancer were relegated to definitive radiation without systemic therapy and lack of local-regional control (LRC) was the primary pattern of failure[1].
Hyperfractionation was not a new concept, and Coutard described using hyperfractionation with curative intent in 1931[2]. The European Organization’s (EORTC) final report of protocol 22791 concluded that for patients with oropharyngeal cancers(excluding base of tongue) who had T3N0 or T3N1 disease, 80.5 Gy delivered in 70 BID fractions of 1.15 Gy produced local control rates that were significantly higher than 70 Gy in 35–40 fractions[3]. The tolerability of doses up to 81.6 Gy using BID irradiation was confirmed by the RTOG[4] prior to embarking on this study.
The three experimental altered fractionation arms of RTOG 9003 were based on the radiation fractionation schedules developed at three leading academic institutions in the United States, each with encouraging Phase II data. Believing that hyperfractionation allowed for higher tolerable total doses, University of Florida had championed hyperfractionation since 1978 (1.2 Gy BID, 5 days per week, to a total dose of 74.4–81.6 Gy) [5]. The two accelerated fractionation regimens sought to deliver the total tumoricidal dose over 6 as opposed to the standard 7 weeks. At the Massachusetts General Hospital, Wang used a split-course hyperfractionation schedule[6]. At M.D. Anderson Cancer Center, an accelerated fractionation with concomitant boost schedule[7] where a second fraction of 1.5 Gy was delivered in the afternoon of the last 12 treatment days was designed with two additional premises: 1) the boost dose would be delivered to a smaller volume, making it more tolerable, 2) accelerated repopulation after initial radiation[8] could best be overcome by treatment intensification when the tumor was growing at its fastest rate, which was at the end of the treatment course[9]. The primary study question was whether each of the experimental arms had a different effect on two-year local-regional failure rate compared to the control arm.
Head and neck cancer patients are at considerable risk for the development of a second primary cancer[10]. Some authorities believe that any squamous cell cancer developing within the head and neck after a disease-free interval of 3–5 years is by definition a new primary cancer, and any “local failure” after 5 years may be miscategorized and confound the analysis. The original RTOG 9003 protocol did not specify whether a subsequent cancer developed in the region of the original cancer would be declared a second primary, rather than a recurrence. For this report local regional failure was analyzed at two-year maximum follow-up, at 5 years, as well as at last follow-up.
Patients and Methods
Patient Selection and Stratification
Patient selection criteria, demographics, planned interventions and acute toxicities have been previously described[1]. Patients with previously untreated, locally advanced, squamous cell cancers of the oral cavity, oropharynx, larynx, and hypopharynx were eligible. Eligible patients had AJCC Stage III or IV disease, or if they had base of tongue cancer, T2N0 disease. Patients were stratified by primary site(oral cavity vs. oropharynx vs. larynx/hypopharynx), Karnofsky Performance Status(60–80 vs. 90–100), and nodal status(N0 vs. N1–3). The study was approved by the local Internal Review Board at each institution.
Interventions
Patients were centrally randomized to 1 of 4 different treatment arms with treatments delivered 5 days a week for all arms and all BID treatments executed with a minimum interfraction interval of 6 hours. The study was designed to compare the Standard Fractionation Arm (SFX) with three experimental arms. SFX was delivered with 2 Gy/fraction/day to 70 Gy in 35 fractions over 7 weeks. Hyperfractionation (HFX) was delivered at 1.2 Gy/fraction, twice daily, to 81.6 Gy over 7 weeks. Accelerated fractionation with a Split (AFX-S) was delivered at 1.6 Gy/fraction, BID to 67.2 Gy over 6 weeks, with a 2 week break after 38.4 Gy. For accelerated fractionation-continuous (AFX-C), also known as accelerated fractionation with concomitant boost, a total dose of 72 Gy was delivered over 6 weeks, using 1.8 Gy daily fractions, and for the last 12 treatment days, a 1.5 Gy treatment was added to a boost field in the afternoon. Thus, SFX and HFX were to be delivered over 7 weeks and the two accelerated treatments were to be delivered over 6 weeks.
Acute versus Late Toxicities
Toxicities occurring ≤ 180 days from the start of radiation were considered acute, and those occurring later were considered as late effects.
Follow-Up
Assessment of toxicities and disease recurrence was performed weekly during radiotherapy, 4 months after treatment completion, then every 3 months for 1.5 years, every 4 months between 1.5 and 3 years, every 6 months in years 3–5, then annually until death.
Statistics
The primary endpoint was local-regional control (LRC), where failure was defined as persistent or recurrent local and/or regional disease. Death without local-regional failure was considered a competing risk. Distant metastases and second primary cancers were considered separately, so if the patient developed either a second primary cancer or distant metastases they were still considered at risk for development of local-regional failure.
Initially the trial was designed to detect an absolute improvement in 2-year LRC from 25% to 45%. Based on results from RTOG 8527, which showed a two-year LRC of 40% for SFX, the study was subsequently revised in 1995 to have an 80% power to detect a 15% difference in LRC, from 40% to 55% (2-sided p value of 0.05). After allowing for 10% of patients to die within 2 years without local-regional failure and an additional 10% of patients to be ineligible, the revised sample size was 1080, or 270 patients per arm.
After adjusting for the stratification factors discussed above, the primary statistical test specified by the protocol was a multivariate Cox model analysis [11]. Exploratory results from the log-rank test[12] and Gray’s test [13] are also reported. The log-rank test is less powerful with longer follow-up when more failures occur early. To adjust for this, exploratory results with Gehan’s test are also reported. Hazard ratios (HR) were defined so that <1 indicated a lower risk for the experimental arm.
Toxicity and feeding tube rates were compared by Fisher’s exact test. Cumulative incidence estimates[14] were provided for local-regional failure, distant metastases, and second primary tumors. To allow comparisons to the initial report, 2-year Kaplan-Meier estimates were also provided for local-regional control. Disease-free and overall survival rates were estimated by the Kaplan-Meier method [15]. First occurrence of local-regional failure, distant metastasis, second primary, or death was considered an event for disease-free survival.
Results
Between September 1991 and August 1997 the study accrued 1,113 patients. Thirty-seven patients were excluded from this analysis (1-withdrawn consent, 33-ineligible, 3-no follow-up), leaving 1,076 patients for analysis. At the time of data analysis (October 1, 2012), the median follow-up for surviving patients was 14.1 years.
Local-Regional Control
When initially published, 537 patients had experienced local-regional failure[1]. Both HFX and AFX-C were statistically superior to SFX (p=0.045 and p=0.05, respectively) but AFX-S was not (p=0.55). At this update (Figure 1a), 568 patients had experienced local-regional failure, of which 553 (97.4%) were within the first 5 years. Two-year LRC rates for the initial report and this update were 46.0%/45.7%, 54.4%/53.3%, 47.5%/47.8%, and 54.5%/53.8%, for the SFX, HFX, AFX-S, and AFX-C arms, respectively.
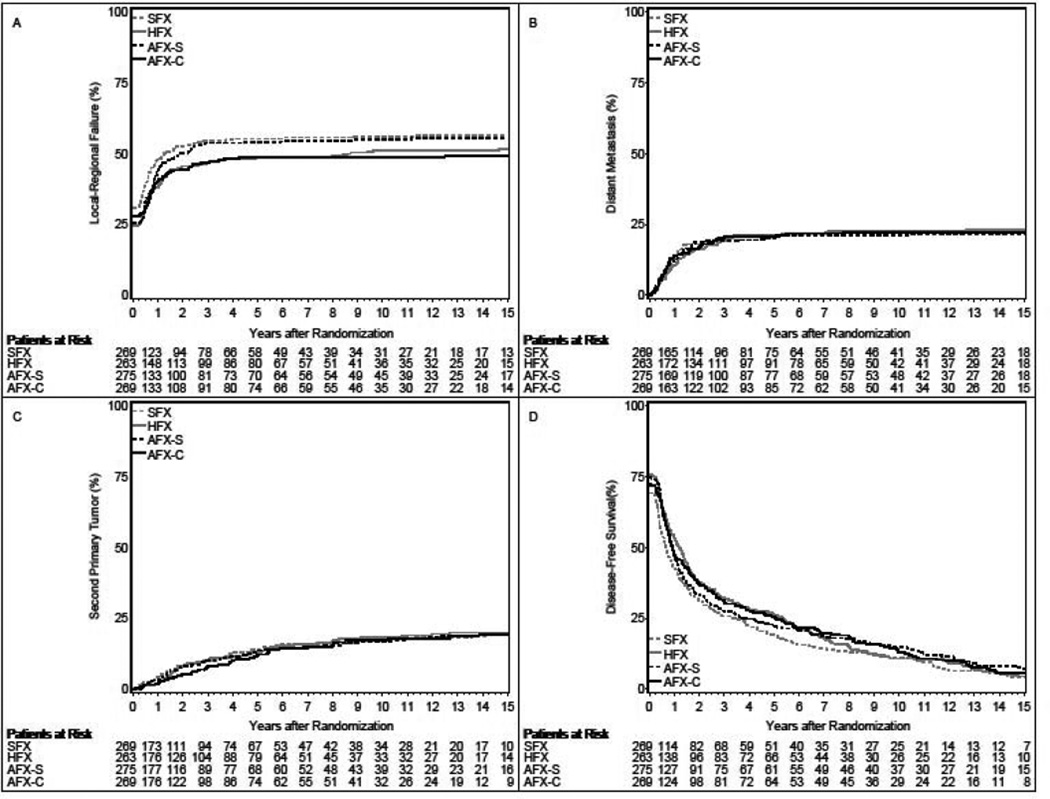
Figure 1.
Panel A: Cumulative Incidence Estimates of Local-Regional Failure; Panel B: Cumulative Incidence Estimates of Distant Metastasis; Panel C: Cumulative Incidence Estimates of Second Primary Tumor; Panel D: Kaplan-Meier Estimates of Disease-Free Survival; SFX-Standard Fractionation, HFX-Hyperfractionation, AFX-S –Accelerated Fractionation with Split, AFX-C Accelerated Fractionation, Continuous
With patients censored for local-regional failure at 5 years, only the comparison of HFX with SFX was significantly different (HFX: hazard ratio-0.79(0.62–1.00), p=0.05; AFX-C 0.82 (0.65–1.05), p=0.11) as shown in Table 1. Log-rank, Gray’s, and Gehan’s tests yield similar results: HFX p=0.06, 0.09, and 0.04; AFX-C p=0.09, 0.09, and 0.10.
Table 1.
Local-Regional Control Overall and Censored at 5 Years, Relative to Standard Fractionation (SFX)
| HFX vs. SFX | AFX-S vs. SFX | AFX-C vs. SFX | ||
|---|---|---|---|---|
| Univariate [1] | Overall | 0.81 (0.65–1.03); p=0.10 | 0.92 (0.73–1.15); p=0.53 | 0.81 (0.64–1.02); p=0.07 |
| Censored at 5 years | 0.79 (0.62–1.00); p=0.06 | 0.92 (0.73–1.15); p=0.52 | 0.82 (0.65–1.04); p=0.09 | |
| Multivariate [2] | Overall | 0.81 (0.64–1.02); p=0.08 | 0.90 (0.72–1.13); p=0.36 | 0.81 (0.64–1.03); p=0.08 |
| Censored at 5 years | 0.79 (0.62–1.00); p=0.05 | 0.90 (0.71–1.13); p=0.35 | 0.82 (0.65–1.05); p=0.11 |
P-value from 2-sided failure specific log-rank test
P-value from multivariate Cox model including stratifying variables
HFX-Hyperfractionation, AFX-S –Accelerated Fractionation with Split, AFX-C Accelerated Fractionation, Continuous
Using all available follow-up information, there was a 19% reduction in local-regional failure for both the HFX and AFX-C arms relative to SFX (hazard ratio 0.81, p=0.08 for both). There was an estimated 10% reduction in local-regional failure for the AFX-S arm relative to SFX (hazard ratio 0.90, p=0.36). Log-rank test and Gray’s test show all comparisons were not significantly different (HFX vs. SFX: p=0.10 and p=0.15; AFX-S vs. SFX: p=0.53 and p=0.58; AFX-C vs. SFX: p=0.07 and p=0.07). But using Gehan’s test, the HFX arm’s statistical superiority persists. P-values for Gehan’s test were 0.05, 0.22, and 0.09 for the HFX, AFX-S, and AFX-C comparisons with SFX, respectively. The 5-year reduction in cumulative local-regional failure was an absolute 6.5% for HFX, 6.6% for AFX-C, and 1.1% for AFX-S. The 5-year cumulative incidences of death without local-regional failure are 22.6%, 19.8%, 20.2%, and 22.3% for the SFX, HFX, AFX-S, and AFX-C arms, respectively.
Distant Disease
There were no significant differences between SFX and the experimental arms in the spread of distant disease (Figure 1B, Supplemental Table 1). Two-hundred twenty-two of 236 failures (94.1%) were within the first 5 years. Rates of distant metastases fell with longer follow-up
Second Malignancies
There were no significant differences between the SFX and the experimental arms in rates of second malignancies (Figure 1C, Supplemental Table 2). Overall, a second primary was reported for 200 patients, of which 50% occurred within the first 3 years, and 75% within the first 5.5 years. Thereafter, events were infrequent, with incidences of 1% or less per year.
DFS, Survival and Causes of Death
Disease-free survival was improved for all 3 experimental arms relative to SFX (hazard ratio 0.83, p=0.04 for HFX; hazard ratio 0.84, p=0.05 for AFX-S; and hazard ratio 0.84, p=0.05 for AFX-C) (Figure 1D and Supplemental Table 3). With patients censored at 5 years, both HFX and AFX-C, but not AFX-S, showed improvement over SFX (hazard ratio 0.78, p=0.01 for HFX; hazard ratio 0.86, p=0.12 for AFX-S; and hazard ratio 0.82, p=0.05 for AFX-C).
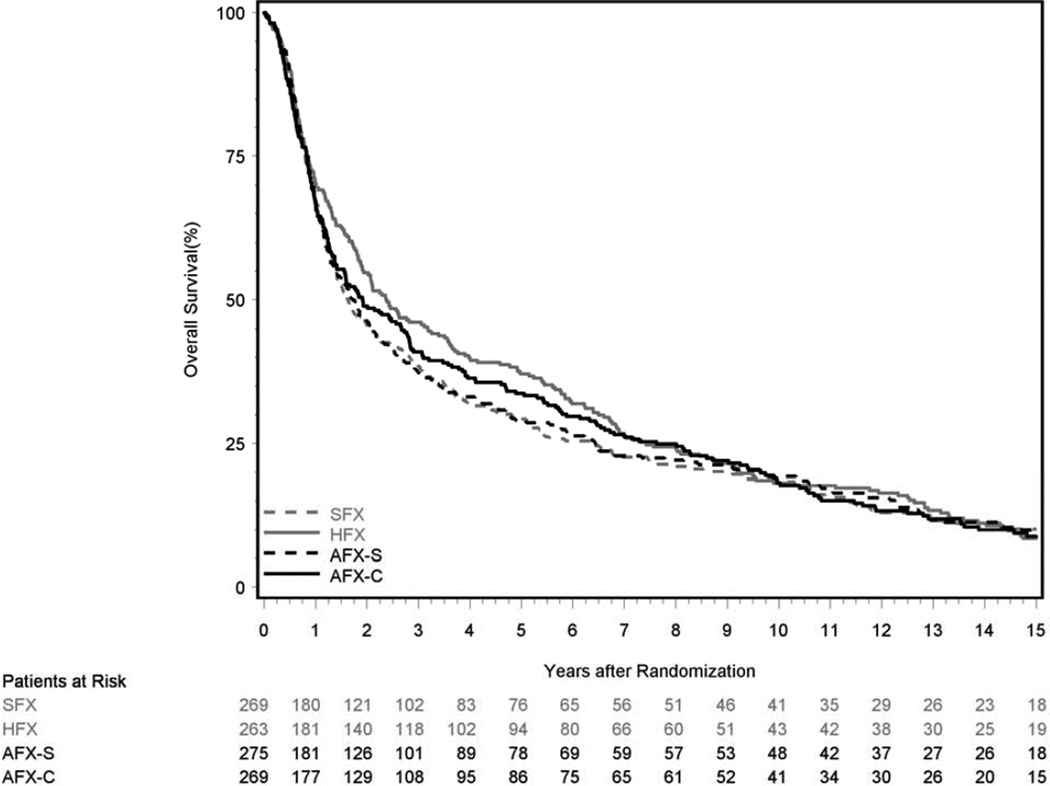
HFX improved OS (hazard ratio 0.81, p=0.05) when patients were censored at 5 years. But with longer follow-up the difference in OS did not retain significance (Figure 2 and Supplemental Table 4). Across all 4 arms, in the first 5 years 65.4% of deaths were due to the original cancer, but after 5 years only 15.9% of deaths were from the original cancer (Table 2).
Figure 2.
Kaplan-Meier Estimates of Overall Survival; SFX-Standard Fractionation, HFX-Hyperfractionation, AFX-S –Accelerated Fractionation with Split, AFX-C Accelerated Fractionation, Continuous
Table 2.
Causes of Death
| Cause of Death | Years 1–5 (n=719) |
Years 6+ (n=239) |
Overall (n=958) |
|---|---|---|---|
| Study Cancer | 65.4% | 15.9% | 53.0% |
| Second Malignancy | 7.7% | 16.7% | 9.9% |
| Complications of Treatment* | 4.3% | 3.3% | 2.9% |
| Unrelated to Cancer or Treatment | 18.6% | 36.4% | 23.1% |
| Unknown | 5.6% | 27.6% | 11.1% |
RTOG 9003 protocol treatment and/or non-protocol treatment.
Late Toxicities
At 5 years the prevalence of any Grade 3, 4, or 5 toxicity, any feeding tube use after 180 days, or feeding tube use at 1 year did not differ significantly when the experimental arm was compared to SFX when including all patients(Table 3). In disease-free patients (DFP), the 1- and 5-year feeding tube rates were 12.1% and 7.8%, compared to 27.0% and 9.1% for all patients, suggesting disease may have increased dysphagia. Limited to DFP, there was evidence that the experimental regimens had higher feeding tube rates at 1 year. At 5 years, among DFP, only 4.8% of HFX patients had feeding tubes versus 13.0% of AFX-C patients.
Table 3.
Late Toxicities and Feeding Tube Use
| SFX (7 weeks) |
HFX (7 weeks) | AFX-S (6 weeks) | AFX-C (6 weeks) | |
|---|---|---|---|---|
| Late grade 3–5 toxicity at 5 years | 8.5% (6/71) | 9.5% (8/84); p=1.00 |
17.1% (12/70); p=0.14 |
16.3% (13/80); p=0.22 |
| Feeding tube > 180 days | 47.0% (110/234) | 48.3% (112/232); p=0.85 |
46.4% (111/239); p=0.93 |
47.4% (110/232); p=1.00 |
| Feeding tube >180 days [1] | 35.1% (52/148) | 45.0% (77/171); p=0.09 |
42.0% (73/174); p=0.25 |
42.5% (74/174); p=0.21 |
| Feeding tube at 1 year | 25.0% (47/188) | 29.6% (55/186); p=0.35 |
42.5% (74/174); p=0.21 |
30.3% (57/188); p=0.30 |
| Feeding tube at 1 year [1] | 4.6% (5/110) | 17.3% (23/133); p<0.01 |
12.0% (15/125); p=0.06 |
13.4% (16/119); p=0.02 |
| Feeding tube at 5 years | 5.0% (3/58) | 12.5% (8/64); p=0.21 |
6.8% (4/59); p=1.00 |
11.3% (7/62); p=0.33 |
| Feeding tube at 5 years [1] | 8.3% (3/36) | 4.8% (2/42); p=0.66 |
4.8% (2/42); p=0.66 |
13.0% (6/46); p=0.72 |
All times measured from start of RT
P-values are relative to SFX
SFX-Standard Fractionation, HFX-Hyperfractionation, AFX-S –Accelerated Fractionation with Split, AFX-C Accelerated Fractionation, Continuous
Limited to patients disease-free
A comparison of 7 weeks (SFX and HFX) versus 6 weeks (AFX-S and AFX-C) yields rates of 9.0% (14/155) and 16.7% (25/150) with p=0.06.
In an ad hoc analysis, when the treatments delivered over 7 weeks were compared to the treatments delivered over 6 weeks, it appeared that accelerated fractionation might have increased any Grade 3, 4 or 5 toxicity at 5 years (16.7% vs. 9.0%, p=0.06). Looking at the worst toxicity per patient by treatment arm(Table 4) only the AFX-C arm seemed to trend worse than the SFX arm comparing Grade 0–2 vs. 3–5 (p=0.09).
Table 4.
Worst Grade Late Toxicity per Patient
| Grade | SFX (n=234) |
HFX (n=232) |
AFX-S (n=239) |
AFX-C (n=232) |
|---|---|---|---|---|
| 1 | 33 (14.1%) | 20 (8.6%) | 34 (14.2%) | 20 (8.6%) |
| 2 | 97 (41.5%) | 117 (50.4%) | 102 (42.7%) | 95 (40.9%) |
| 3 | 44 (18.8%) | 40 (17.2%) | 45 (18.8%) | 61 (26.3%) |
| 4 | 22 (9.4%) | 24 (10.3%) | 22 (9.2%) | 23 (9.9%) |
| 5 | 2 (0.9%) | 1 (0.4%) | 2 (0.8%) | 1 (0.4%) |
| Grade 3–5 vs. 0–2 (experimental vs. control) p-value (2-sided Fisher's exact test) |
0.84 | 1.00 | 0.09 | |
Toxicities did not vary between SFX and the experimental arms when they were examined by anatomical site of the primary disease (Supplemental Tables 5–9). The only trend we observed was that for patients with an oral cavity primary site, the AFX-C arm, when compared to the SFX showed a p-value of 0.059 for more Grade 3–5 toxicities. Looking at whether Grade 3–5 toxicities varied between the 7 and 6 week courses by primary site, we found no statistically significant differences; Oral Cavity: 10/44 vs. 16/47; p=0.25] [Oropharynx: 85/283 vs. 92/279; p=0.47] [Hypopharynx: 13/50 vs. 19/72; p=1.00] [Larynx: 25/89 vs. 27/73; p=0.24]. There were 6 Grade 5 toxicities, and there was no obvious pattern to these deaths (Supplemental Table 10)
Discussion
One important observation from the long-term follow up of this study is the low incidence of local-regional and distant failures after 5 years. With 15 total local-regional failures beyond 5 years versus 25 new head and neck primaries occurring after 5 years, there was a risk of misclassifying new primary cancers in the same region as recurrences of the index cancer. Regardless of how they are classified, the total number of local-regional failures and new primary head and neck cancers was small, justifying the practice of censoring the local-regional failure endpoint at 5 years. We believe that for our trial, and future head and neck trials, the best estimate of the true local-regional failure would be achieved by censoring the localregional failure endpoint at 5 years. With 97% of the failures occurring within the first 5 years, there would be little loss of statistical power.
After 5.5 years, the rate of new primary cancers decreased unexpectedly to < 1% per year. Understanding that the endpoints of this study were measured from time at randomization, whereas most physicians use time since completion of treatment as the starting point, there were few local-regional recurrences beyond 3 years and even fewer beyond 5 years. Only 6% of patients suffered a distant failure beyond 5 years, and that 6% figure may have inadvertently included some new primary cancers. The cost-effectiveness of surveillance for local-regional, distant metastases or new primaries beyond 3–5.5 years is expensive and its utility might be challenged.
Treatment benefit in LRC for HFX and AFX-C compared with SFX was relatively stable over time and this study was not designed to assess the differences in effectiveness between experimental arms. In terms of cumulative incidence of local-regional failure, HFX has an absolute 7% benefit over SFX at 2 years, and a 6.5% benefit at 5 years. AFX-C had a 2- and 5-year absolute benefit of 8.1% and 6.6%, respectively. The differences in statistical significance compared to the initial report of RTOG 9003 should not be interpreted as a diminution of LRC benefit over time. Although the initial complete response rate was higher in the HFX arm compared with the AFX-C arm, the cumulative incidences of local-regional failure were very similar between these 2 experimental arms and the multivariate hazard ratios (0.81 for both) were consistent with the MARCH meta-analysis which estimated a hazard ratio of 0.79 (i.e., 21% reduction of local-regional failure) for accelerated fractionation without total dose reduction[16].
The reasons that AFX-C rather than HFX was chosen for further investigations were logistical and financial. Within the RTOG, based on the early findings, it was felt that if there were no differences in efficacy or toxicity, it would be more convenient as well as less expensive to deliver 42 rather than 68 fractions. New data from this analysis showed that the 6 week schedules produced an increased frequency of any Grade 3–5 late toxicity(p=0.06). Specifically, the AFX-C arm seemed to trend worse than the SFX arm comparing Grade 0–2 vs. 3–5 late toxicity (p=0.09).
With 14 years of follow-up, we have also demonstrated that toxicities from radiation therapy alone over time, tended to slightly decrease or remain stable.
Due to the survival advantage of concurrent chemotherapy and standard fraction radiation over standard fraction radiation alone, we are in the era of concurrent chemoradiation for many locally advanced head and neck cancers[16]. In the United States, cisplatin is the systemic agent of choice. Concurrent carboplatin and flourouracil added to AFX-C significantly improved locoregional control as well as overall survival, but the benefit was restricted to patients with oropharyngeal cancers[17,18].
If chemotherapy could be added to altered fractionation to improve results, did altered fractionation in place of standard fractionation improve chemoradiation results? If the altered fractionation was AFX-C the answer would seem to be “no”. GORTEC 99-02 used carboplatin-flourouracil in place of cisplatin to test the concept [19]. There were three arms, 70 Gy in 7 weeks with 3 cycles of 4 days of concurrent carboplatin-flourouracil, 70 Gy in 6 weeks(1.5 Gy BID after 40 Gy) plus 2 cycles of 5 days of concurrent carboplatin-flourouracil and very accelerated radiation without chemotherapy. There were no significant differences between the two arms that received carboplatin-flourouracil. RTOG 0129 compared the standard of care with 70 Gy in 35 fractions over 7 weeks plus 3 cycles of cisplatin at a dose of 100 mg/m2 delivered on days 1, 22, and 43 with an experimental arm that included AFX-C plus only 2 cycles of cisplatin[20]. AFX-C was combined with less chemotherapy and despite 721 eligible patients and a mean follow-up of 7.9 years, there was no demonstration of improved outcome for any endpoint[21]. AFX-C might have balanced out decreased cisplatin, or alternatively, neither change was significant. An unanswered question is whether a more effective fractionation schedule can be added to standard chemotherapy to improve results. Many ongoing RTOG studies have adopted the results from DAHANCA 6&7[22] and the IAEA-ACC[23] randomized trials to incorporate 6 fractions per week into the concurrent chemoradiation environment.
From the late toxicity and long-term efficacy endpoints, RTOG 90-03 suggested that HFX yielded the optimal results. In addition to improving LRC, HFX did not increase the non-cancer or non-treatment related death rate beyond 5 years when compared to SFX(See Supplemental Table 11). There may be a reason to consider HFX in place of concurrent, 100 mg/m2, q 3 week, Cisplatin therapy. Evidence suggesting that there is an increased risk of death beyond 5 years for patients treated with concurrent radiation and cisplatin compared to either radiation or sequential therapy exists. RTOG 9111 compared radiation alone, induction chemotherapy followed by radiation and concurrent chemoradiation in the treatment of patients with laryngeal cancers[24]. At 5 years the survival rate was 54% for radiation alone, 55% for concomitant therapy, and 58% for induction therapy, all relatively similar. However after 4.5 years the survival curves diverged and the 10-year survival was 32% for radiation only, 28% for concomitant radiation and chemotherapy, and 39% for induction chemotherapy followed by radiation. Our present study demonstrated that both local-regional and distant failure beyond 5 years were rare in head and neck cancer. Yet in RTOG 9111, the survival rate for concomitant therapy went from 1% better than radiation alone at 5 years to 4% worse at 10 years, a relative difference of −5%. This was not true for the induction chemotherapy arm where the difference in survival rate from the radiation alone arm increased from 4% at 5 years to 7% at 10 years, a relative difference of 3%. These results suggest that there may be an increased death risk from the addition of concurrent cisplatin to radiation beyond 5 years.
Data on treatment outcome of combined modality therapy beyond 5 years are scarce but, Cooper et al. recently published the ten-year results of Intergroup/RTOG 9501[25] and reported a similar trend. This Intergroup study compared post-operative radiation therapy alone versus concurrent cisplatin (100 mg/m2 q 3 weeks × 3) and radiation. At 10 years concurrent cisplatin and radiation still significantly improved LRC for patients with positive margins or extracapsular extension by 12%(p=0.02). Ten-year survival for this subgroup improved from 19.6% to 27.1%(p=0.07). Looking at deaths not attributable to the study cancer, there were 22 excess deaths in the concurrent arm than in the radiation therapy alone arm (72 versus 50 deaths). Ignoring the fact that concurrent cisplatin improved LRC, improved causespecific survival(p=0.01) and possibly OS by 7.5% in those 130 patients with positive margins or extracapsular disease[26], one could hypothesize that the risk of cisplatin causing an excess death at 10 years was 11%(22 excess, non-cancer deaths divided by 202 patients randomized to concurrent cisplatin and radiation). Published data for survival of the patients without extracapsular extension or positive margins are not available.
If concurrent cisplatin at 100 mg/m2 q 3 weeks × 3 and standard fractionated radiotherapy has a 5–11% excess long-term mortality not attributable to the study cancer that begins at 4.5–5 years, but hyperfractionated radiotherapy does not, then hyperfractionated radiotherapy may be a better option for some patients.
In the IMRT years, differential dosing is common but the use of HFX for purposes of dose escalation has not been fully explored. Using the lessons learned here, and employing IMRT, improved imaging(CT, fused MRI & PET), and IGRT to “dose paint” gross disease, HFX could allow a very high dose to be administered to gross disease with a tighter margin while taking advantage of lower doses per fraction to areas of subclinical disease where we are necessarily irradiating more normal tissue. As real-time treatment planning becomes plausible, the volume reductions can become more frequent and further dose escalation becomes practical. For both HFX and AFX-C, the more the treatment is tailored to the response of the tumor the better tolerated the treatment should be. Hopefully, as the volumes decrease, the increased effectiveness due to the higher doses would be retained. The smaller fraction sizes delivered to the normal tissue using HFX when compared to AFX-C would argue for HFX rather than AFX-C in the IMRT years. Though IMRT will become less expensive, with the present economic climate hyperfractionated IMRT’s benefit and the utility of multiple re-simulations would need to be demonstrable. This study, as well as others,[16] suggests its potential. While we have argued against abandoning concurrent cisplatin and radiation without Phase III studies[27], the recent literature warns us of potential increased late mortality following concurrent cisplatin and radiation. Chemotherapy has been a terrific radiosensitizer which has brought welcome improvements in local control, but as new agents are introduced, their effects may be more systemic rather than local-regional. All prior trials trying to incorporate altered fractionation into concurrent chemoradiation treatments have been performed with 2D radiotherapy. While it seems unlikely that we are ready to compare IMRT based HFX(or AFX-C) with conventional chemoradiation, improvements in radiation techniques as well as new biological agents should force us to reconsider all therapeutic combinations. These questions need to be addressed in future clinical trials.
Conclusions
HFX and AFX-C decreased 5-year local-regional failure by 19% when compared to SFX for patients with locally advanced head and neck squamous cell cancer treated with radiotherapy alone. HFX, unlike AFX-C(p=0.09) or accelerated therapy(p=0.06), did so without increasing late toxicities. At 5 years HFX improved OS. Limited to DFP, there was evidence that the experimental regimens had higher feeding tube rates at 1 year. At 5 years, among DFP, only 4.8% of HFX patients had feeding tubes versus 13.0% of AFX-C patients. The increase in total dose tolerated through HFX has not been explored in the IMRT era. For future head and neck trials, it may be prudent to censor local-regional failure at 5 years, and clinically, surveillance beyond 5 years may be questioned.
Supplementary Material
Summary.
1,076 eligible patients with Stage III/IV SCC were randomized to 4 treatment arms: SFX-70 Gy/35 fxns/7 weeks, HFX-81.6 Gy/68fxns/7weeks; (3) AFX-S 67.2 Gy/42 fxns/6 weeks −2 week rest after 38.4 Gy (4) AFX-C 72 Gy/42 fxns/6 weeks. At 5 years, compared to SFX, HFX, but not AFX-S, or AFX-C improved LRC & OS for patients with LASCC without increasing long-term toxicity.
Acknowledgements
This trial was conducted by the Radiation Therapy Oncology Group (RTOG), and was supported by RTOG grant U10 CA21661 and CCOP grant U10 CA37422 from the National Cancer Institute (NCI). This manuscript’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Dr. Beitler receives support as a Georgia Research Alliance Cancer Scientist
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fu KK, Pajak TF, Trotti A, Jones CU, Spencer SA, Phillips TL, Garden AS, Ridge JA, Cooper JS, Ang KK. A radiation therapy oncology group (rtog) phase iii randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: First report of rtog 9003. Int J Radiat Oncol Biol Phys. 2000;48:7–16. doi: 10.1016/s0360-3016(00)00663-5. [DOI] [PubMed] [Google Scholar]
- 2.Coutard H. Principles of x ray therapy of malignant diseases. Lancet. 1934;2:1–8. [Google Scholar]
- 3.Horiot JC, Le Fur R, N'Guyen T, Chenal C, Schraub S, Alfonsi S, Gardani G, Van Den Bogaert W, Danczak S, Bolla M, et al. Hyperfractionation versus conventional fractionation in oropharyngeal carcinoma: Final analysis of a randomized trial of the eortc cooperative group of radiotherapy. Radiother Oncol. 1992;25:231–241. doi: 10.1016/0167-8140(92)90242-m. [DOI] [PubMed] [Google Scholar]
- 4.Fu KK, Pajak TF, Marcial VA, Ortiz HG, Rotman M, Asbell SO, Coia LR, Vora NL, Byhardt R, Rubin P, Sorgen SD, Cox JD, Stetz J. Late effects of hyperfractionated radiotherapy for advanced head and neck-cancer - long-term follow-up results of rtog-83–13. Int J Radiat Oncol. 1995;32:577–588. doi: 10.1016/0360-3016(95)00080-I. [DOI] [PubMed] [Google Scholar]
- 5.Parsons JT, Mendenhall WM, Stringer SP, Cassisi NJ, Million RR. Twice-a-day radiotherapy for squamous cell carcinoma of the head and neck: The university of florida experience. Head Neck. 1993;15:87–96. doi: 10.1002/hed.2880150202. [DOI] [PubMed] [Google Scholar]
- 6.Wang CC, Blitzer PH, Suit HD. Twice-a-day radiation therapy for cancer of the head and neck. Cancer. 1985;55:2100–2104. doi: 10.1002/1097-0142(19850501)55:9+<2100::aid-cncr2820551411>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 7.Ang KK, Peters LJ, Weber RS, Maor MH, Morrison WH, Wendt CD, Brown BW. Concomitant boost radiotherapy schedules in the treatment of carcinoma of the oropharynx and nasopharynx. Int J Radiat Oncol Biol Phys. 1990;19:1339–1345. doi: 10.1016/0360-3016(90)90341-g. [DOI] [PubMed] [Google Scholar]
- 8.Dutreix J, Tubiana M, Wambersie A, Malaise E. The influence of cell proliferation in tumours and normal tissues during fractionated radiotherapy. European journal of cancer. 1971;7:205–213. doi: 10.1016/0014-2964(71)90018-1. [DOI] [PubMed] [Google Scholar]
- 9.Peters LJ, Ang KK, Thames HD., Jr Accelerated fractionation in the radiation treatment of head and neck cancer. A critical comparison of different strategies. Acta Oncol. 1988;27:185–194. doi: 10.3109/02841868809090339. [DOI] [PubMed] [Google Scholar]
- 10.Brown LM, McCarron P, Freedman DM. Buccal cavity and pharynx. In: Curtis RE, Freedman DM, Ron E, Ries LA, Hacker DG, Edwards BK, Tucker MA, Fraumeni JF Jr, editors. New malignancies among cancer survivors: Seer cancer registries, 1973–2000. Bethesda, MD: National Cancer Institute; 2006. [Google Scholar]
- 11.Cox DR. Regression models and life-tables. Journal of the Royal Statistical Society Series B (Methodological) 1972:187–220. [Google Scholar]
- 12.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 13.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. The Annals of statistics. 1988:1141–1154. [Google Scholar]
- 14.Kalbfleisch J, Prentice R. Book The statistical analysis of failure time data. New York: Wiley; 1980. The statistical analysis of failure time data. Editor, editor^editors. [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American statistical association. 1958;53:457–481. [Google Scholar]
- 16.Bourhis J, Overgaard J, Audry H, Ang KK, Saunders M, Bernier J, Horiot JC, Le Maitre A, Pajak TF, Poulsen MG, O'Sullivan B, Dobrowsky W, Hliniak A, Skladowski K, Hay JH, Pinto LH, Fallai C, Fu KK, Sylvester R, Pignon JP. Meta-Analysis of Radiotherapy in Carcinomas of H, neck Collaborative G. Hyperfractionated or accelerated radiotherapy in head and neck cancer: A meta-analysis. Lancet. 2006;368:843–854. doi: 10.1016/S0140-6736(06)69121-6. [DOI] [PubMed] [Google Scholar]
- 17.Semrau R, Mueller RP, Stuetzer H, Staar S, Schroeder U, Guntinas-Lichius O, Kocher M, Eich HT, Dietz A, Flentje M, Rudat V, Volling P, Schroeder M, Eckel HE. Efficacy of intensified hyperfractionated and accelerated radiotherapy and concurrent chemotherapy with carboplatin and 5-fluorouracil: Updated results of a randomized multicentric trial in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2006;64:1308–1316. doi: 10.1016/j.ijrobp.2005.10.039. [DOI] [PubMed] [Google Scholar]
- 18.Staar S, Rudat V, Stuetzer H, Dietz A, Volling P, Schroeder M, Flentje M, Eckel HE, Mueller RP. Intensified hyperfractionated accelerated radiotherapy limits the additional benefit of simultaneous chemotherapy--results of a multicentric randomized german trial in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;50:1161–1171. doi: 10.1016/s0360-3016(01)01544-9. [DOI] [PubMed] [Google Scholar]
- 19.Bourhis J, Sire C, Graff P, Gregoire V, Maingon P, Calais G, Gery B, Martin L, Alfonsi M, Desprez P, Pignon T, Bardet E, Rives M, Geoffrois L, Daly-Schveitzer N, Sen S, Tuchais C, Dupuis O, Guerif S, Lapeyre M, Favrel V, Hamoir M, Lusinchi A, Temam S, Pinna A, Tao YG, Blanchard P, Auperin A. Concomitant chemoradiotherapy versus acceleration of radiotherapy with or without concomitant chemotherapy in locally advanced head and neck carcinoma (gortec 99-02): An open-label phase 3 randomised trial. The lancet oncology. 2012;13:145–153. doi: 10.1016/S1470-2045(11)70346-1. [DOI] [PubMed] [Google Scholar]
- 20.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, Westra WH, Chung CH, Jordan RC, Lu C, Kim H, Axelrod R, Silverman CC, Redmond KP, Gillison ML. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010 doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen-Tan P, Zhang E, Wheeler RH, Weber RS, Rosenthal DI, Vigneault E, Kim H, Silverman C, Raben A, Ang KK. A phase 3 trial to test accelerated versus standard fractionation in combination with concurrent cisplatin for head and neck carcinomas (rtog 0129): Long-term report of efficacy and toxicity. International journal of radiation oncology, biology, physics. 2013;87:S56–S57. [Google Scholar]
- 22.Overgaard J, Hansen HS, Specht L, Overgaard M, Grau C, Andersen E, Bentzen J, Bastholt L, Hansen O, Johansen J, Andersen L, Evensen JF. Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck: Dahanca 6 and 7 randomised controlled trial. Lancet. 2003;362:933–940. doi: 10.1016/s0140-6736(03)14361-9. [DOI] [PubMed] [Google Scholar]
- 23.Overgaard J, Mohanti BK, Begum N, Ali R, Agarwal JP, Kuddu M, Bhasker S, Tatsuzaki H, Grau C. Five versus six fractions of radiotherapy per week for squamous-cell carcinoma of the head and neck (iaea-acc study): A randomised, multicentre trial. The lancet oncology. 2010;11:553–560. doi: 10.1016/S1470-2045(10)70072-3. [DOI] [PubMed] [Google Scholar]
- 24.Forastiere AA, Zhang Q, Weber RS, Maor MH, Goepfert H, Pajak TF, Morrison W, Glisson B, Trotti A, Ridge JA, Thorstad W, Wagner H, Ensley JF, Cooper JS. Long-term results of rtog 91-11: A comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol. 2013;31:845–852. doi: 10.1200/JCO.2012.43.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper JS, Zhang Q, Pajak TF, Forastiere AA, Jacobs J, Saxman SB, Kish JA, Kim HE, Cmelak AJ, Rotman M, Lustig R, Ensley JF, Thorstad W, Schultz CJ, Yom SS, Ang KK. Long-term follow-up of the rtog 9501/intergroup phase iii trial: Postoperative concurrent radiation therapy and chemotherapy in high-risk squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2012;84:1198–1205. doi: 10.1016/j.ijrobp.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernier J, Cooper JS, Pajak TF, van Glabbeke M, Bourhis J, Forastiere A, Ozsahin EM, Jacobs JR, Jassem J, Ang KK, Lefebvre JL. Defining risk levels in locally advanced head and neck cancers: A comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the eortc (#22931) and rtog (# 9501) Head Neck. 2005;27:843–850. doi: 10.1002/hed.20279. [DOI] [PubMed] [Google Scholar]
- 27.Beitler JJ, Cooper JS. Seduction by induction? J Clin Oncol. 2009;27:9–10. doi: 10.1200/JCO.2008.19.5875. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.