Abstract
Nociceptors and neurons in the central nervous system (CNS) that receive nociceptive input show remarkable plasticity in response to injury. This plasticity is thought to underlie the development of chronic pain states. Hence, further understanding of the molecular mechanisms driving and maintaining this plasticity has the potential to lead to novel therapeutic approaches for the treatment of chronic pain states. An important concept in pain plasticity is the presence and persistence of “hyperalgesic priming.” This priming arises from an initial injury and results in a remarkable susceptibility to normally subthreshold noxious inputs causing a prolonged pain state in primed animals. Here we describe our current understanding of how this priming is manifested through changes in signaling in the primary nociceptor as well as through memory like alterations at CNS synapses. Moreover, we discuss how commonly utilized analgesics, such as opioids, enhance priming therefore potentially contributing to the development of persistent pain states. Finally we highlight where these priming models draw parallels to common human chronic pain conditions. Collectively, these advances in our understanding of pain plasticity reveal a variety of targets for therapeutic intervention with the potential to reverse rather than palliate chronic pain states.
Keywords: Atypical PKC, AMPA, NMDA, mTORC1, PKC, Epac, Hyperalgesic priming, Prostaglandins, NGF, Interleukin 6
1 Introduction
A fundamental principle underlying our current understanding of pathological pain states is plasticity in the nociceptive system. While research into pathological pain states has long recognized this idea, it is only relatively recently that we have started to gain insight into mechanisms that cause this plasticity. On the most general level, plasticity in the pain system occurs at two locations, at the primary afferent nociceptor and at synapses receiving nociceptive input throughout the central nervous system (CNS). Preclinical models of acute and chronic inflammatory pain as well as models of neuropathic pain have revealed a plethora of molecular targets that have developed our understanding of how chronic pain develops as well as revealing important potential therapeutic intervention points. In the late 1990s and early 2000s, Jon Levine and colleagues developed “hyperalgesic priming” models (for review see Reichling and Levine 2009). These models provide unique insight into plasticity in the nociceptive system because they allow for molecular dissection of pain states in two distinct phases. These models involve a priming stimulus, aimed at causing an acute sensitization of peripheral nociceptors and their central inputs, albeit with some notable exceptions which will be discussed later. Next, in opposition to most other preclinical models, the initial sensitization is allowed to resolve and a second, normally subthreshold, stimulus is delivered. Importantly, this second stimulus, which has only a transient effect in naïve animals, leads to a prolonged state of pain hypersensitivity that allows for investigation of molecular mechanisms that define the primed nociceptor and/or the primed nociceptive system. Here we will argue that models of hyperalgesic priming have led to unique insight into how relatively brief pain states lead to reorganization of molecular machinery throughout the pain system rendering animals, and potentially humans, susceptible to prolonged pain states provoked by insults that would have little effect in unprimed individuals. This primed state, therefore, represents a kind of “pain memory” that, if reversed, has the potential to permanently remove the presence of a chronic pain state. Hence, our goals in this chapter will be to highlight (1) mechanisms underlying the priming in peripheral nociceptors, (2) mechanisms controlling priming in the CNS, and (3) potential therapeutic interventions elucidated by these findings with a view toward future pharmacological means to reverse chronic pain states.
2 Why Use Hyperalgesic Priming Models?
In order to fully grasp the importance of the research findings discussed herein, it is critical to first reflect on the utility of using hyperalgesic priming models to study pain plasticity. First, the experimental framework of the hyperalgesic priming model provides important insight into clinical chronic pain because it captures the recurrent nature of some of the most common pathological pain conditions (Reichling and Levine 2009). In 1921, Wilfred Harris described his clinical experience treating patients with presumed injuries to peripheral nerves. He described pain in these patients as episodic with pain episodes provoked by acute exacerbation (Harris 1921). Hence, from some of the earliest descriptions of pain as a disease, the notion of priming followed by subthreshold provocation of long-lived pain episodes has been apparent.
Population-based studies in several prevalent chronic pain conditions have directly demonstrated the episodic yet progressive nature of these disease states. Perhaps the best-known episodic pain condition is headache and, in the case of migraine, frequency of attacks is the best predictor of a transition to chronic migraine (Lipton 2009). In fact, the vast majority of migraineurs move from a low-frequency episodic headache stage to a high-frequency stage and eventually into chronic migraine (Bigal and Lipton 2008), highlighting the progressive worsening of this disorder. Moreover, migraines can frequently be provoked by what are often called migraine triggers. These are, by definition, subthreshold stimuli because they fail to provoke migraines in the non-migraineur population. This situation is not unique to migraine. Acute episodes of low back pain generally resolve (Bartleson 2001; Cassidy et al. 2005), but recurrence rates over 5 years are as high as 70 % (Von Korff and Saunders 1996; Carey et al. 1999; Cassidy et al. 2005; Kolb et al. 2011) and lifetime recurrence is estimated at 85 % (Andersson 1999; Tamcan et al. 2010). Moreover, the probability of low back pain recurrence increases with previous episodes of low back pain (Kolb et al. 2011). A similar clinical picture has been found for chronic neck pain (Croft et al. 2001; Nolet et al. 2010). Finally, in the case of surgery and chronic postsurgical pain, there is evidence that preexisting pain is a major risk factor for chronic post-incision pain suggesting that the preexisting pain can act as a priming stimulus causing a very long-lasting pain state induced by incision (Althaus et al. 2012; Pinto et al. 2013). Hence, we take the viewpoint, which is shared by others (Reichling and Levine 2009; Reichling et al. 2013), that the “priming” event in the hyperalgesic priming model may be viewed as an induction of the transition to chronic pain with important clinical implications for understanding molecular mechanisms involved in maintaining this disease state.
3 Mechanisms of Priming in the Periphery: A Model for Sustained Nociceptor Plasticity
Tissue injury, inflammation, and nerve injury are all examples where primary sensory nociceptors become sensitized. This sensitization leads to prolonged hyperalgesia, which sometimes outlasts or is disproportionate to the initial stimulus. A variety of chronic pain conditions have readily identified pathologies (e.g., rheumatoid arthritis, postherpetic neuropathic pain); however, some chronic pain conditions are defined by intermittent yet progressive periods of pain (e.g., low back pain, migraine, fibromyalgia) sometimes with no readily identified injury. However, one crucial question remains. What are the mechanisms responsible for pain chronification? In other words, how does acute pain ultimately transition to chronic pain?
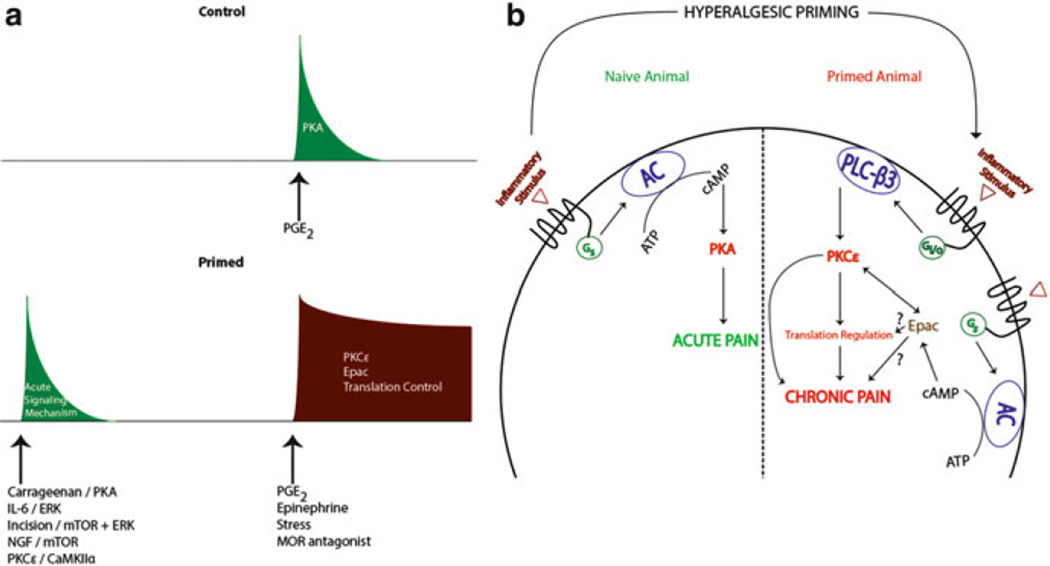
The careful delineation of the mechanisms underlying the transition from acute to chronic pain can be difficult to examine, in part, due to the lack of a reliable animal model that could capture the complexity of this phenomenon. Although animal models of persistent, or “chronic” pain, exist (e.g., hindpaw CFA injection, spinal nerve ligation), it is not always straightforward to assess how an initial injury leads to persistent changes in signaling following that initial injury or stimulus. To address this, Jon Levine and colleagues developed the hyperalgesic priming model to isolate the mechanisms responsible for the persistence of the primed state, analogous to chronic pain. In this model, a hyperalgesic state is first evoked by a hindpaw injection of stimuli (e.g., carrageenan) that have traditionally been utilized to study acute pain in rodents. The subsequent hyperalgesia is transient and resolves within 4 days (Reichling and Levine 2009). After the resolution of the original hyperalgesia, a second stimulus is given to the same hindpaw. In naïve rodents, this stimulus (e.g., prostaglandin E2; PGE2) either fails to evoke hyperalgesia or is transient. In primed animals, this second stimulus causes a hyperalgesic state that lasts for at least 24 h and can persist for weeks (Fig. 1a) (Reichling and Levine 2009). Therefore, the model allows for the clear dissociation between the initiation phase, or priming, and the maintenance phase, which lacks any signs of hyperalgesia until another administration of an inflammatory mediator reestablishes hypersensitivity.
Fig. 1.
Hyperalgesic priming induces a switch in nociceptor second messenger signaling pathways. In a naïve animal, administration (hindpaw injection) of an inflammatory stimulus (e.g., PGE2) results in transient Gs/PKA-mediated acute pain (green curve in (a) and left side in (b)). In the primed animal, the same inflammatory stimulus recruits an additional Gi/o/PKCε and/or an Epac/PKCε-mediated pathway, which contributes to prolonged hyperalgesia (red curve in (a) and right side in (b)) in previously primed animals. Adapted from Reichling and Levine (2009)
3.1 PKCε as a Crucial Mechanism of Nociceptor Priming
How then is this exaggerated response to PGE2, and other mediators like serotonin or A2 adenosine receptor agonists (Aley et al. 2000), which can also precipitate hyperalgesia in primed animals, generated? Extensive studies have demonstrated that this priming effect is dependent on switches in signaling mechanisms in nociceptors. In naïve animals, the hyperalgesic effects of PGE2 injection are mediated by adenylyl cyclase (AC) activation downstream of PGE2 receptors causing protein kinase A (PKA) activation (Fig. 1b) (Aley and Levine 1999). This effect can be attenuated via injection of PKA antagonists (Aley and Levine 1999). Although the second messenger pathway underlying the early phase of PGE2-induced hyperalgesia is PKA mediated, even in primed animals, the same cannot be said for the pathway responsible for the long-lasting hyperalgesia that is uniquely present in primed rodents. While PGE2-induced hyperalgesia in primed animals is still cyclic AMP (cAMP) dependent, it now bypasses PKA to activate exchange proteins directly activated by cAMP (Epac) which can activate protein kinase Cε (PKCε, Fig. 1b, Hucho et al. 2005). Importantly, inflammatory stimulation of nociceptors leads to a decrease in G-protein receptor kinase 2 (GRK2) which results in enhanced Epac activity (Eijkelkamp et al. 2010; Wang et al. 2013). These changes occur in IB4-positive nociceptors, and decreases in GRK2 and increases in Epac expression are correlated with the persistence of priming (Wang et al. 2013). Moreover, in primed animals, PGE2 results in an activation of pertussis toxin-sensitive G-protein αi subunits (Dina et al. 2009) and phospholipase Cβ (PLCβ) leading to a downstream engagement of PKCε (Joseph et al. 2007); hence, multiple pathways for PKCε engagement may exist in primed nociceptors (Fig. 1b). Critically, in primed animals, the long-lasting hyperalgesia arising from exposure to compounds that can precipitate priming is blocked by selective inhibition of PKCε (Aley et al. 2000) and by intrathecal delivery of antisense oligonucleotides knocking down PKCε expression (Parada et al. 2003). Additionally, injection of a PKCε agonist alone results in a prolonged hyperalgesic state and hyperalgesic priming, pointing to a key role for nociceptor PKCε in hyperalgesic priming (Reichling and Levine 2009). Importantly, this PKCε-dependent primed state does not require an initial bout of hyperalgesia as subthreshold doses of PKCε activators (Parada et al. 2003), previous exposure to unpredictable sound stress (Khasar et al. 2008), and even repeated administration of opioid agonists into the paw (Joseph et al. 2010) are capable of causing an emergence of a primed state (Fig. 1a).
Is there a distinct subset of nociceptors required for PKCε-dependent priming? To elucidate the population of nociceptors involved in hyperalgesic priming, Joseph and colleagues lesioned IB4(+) nociceptors via intrathecal administration of IB4-saporin (Joseph and Levine 2010). In these animals, the PKCε activator, ψεRACK, produces an initial hyperalgesia; however, the PGE2-induced prolonged hyperalgesia is absent in animals treated with toxin suggesting that priming is localized to IB4(+) neurons. Furthermore, select agents known to act on peptidergic vs. IB4(+) cells stimulate an initial hyperalgesic state in IB4-lesioned animals but fail to establish priming indicating that a switch in signaling to PKCε selectively occurs in IB4(+) nociceptors (Joseph and Levine 2010). As mentioned above, this may be linked to a pronounced loss of GRK2 expression and an increase in Epac activity in IB4(+) nociceptors of primed animals (Wang et al. 2013).
Collectively these findings support a key role for PKCε in the initiation and maintenance of nociceptor priming therefore making this PKC isoform a key therapeutic target. However, PKCε-dependent forms of priming show a marked sexual dimorphism in rats with female rats failing to show priming, an effect that is apparently due to a protective effect of estrogen (Joseph et al. 2003). Importantly, this does not appear to be the case with mice where both carrageenan and PKC-ε-activating peptides cause robust priming to subsequent PGE2 injection in females (Wang et al. 2013). At this point, it remains unclear as to whether there is sexual dimorphism in PKCε-mediated effects in humans. Despite these potential discrepancies, it is clear that one mechanism of priming is shared across species and sexes, a requirement for changes in gene expression at the level of protein synthesis.
3.2 Local Translation Is a Key Mediator of Nociceptor Priming
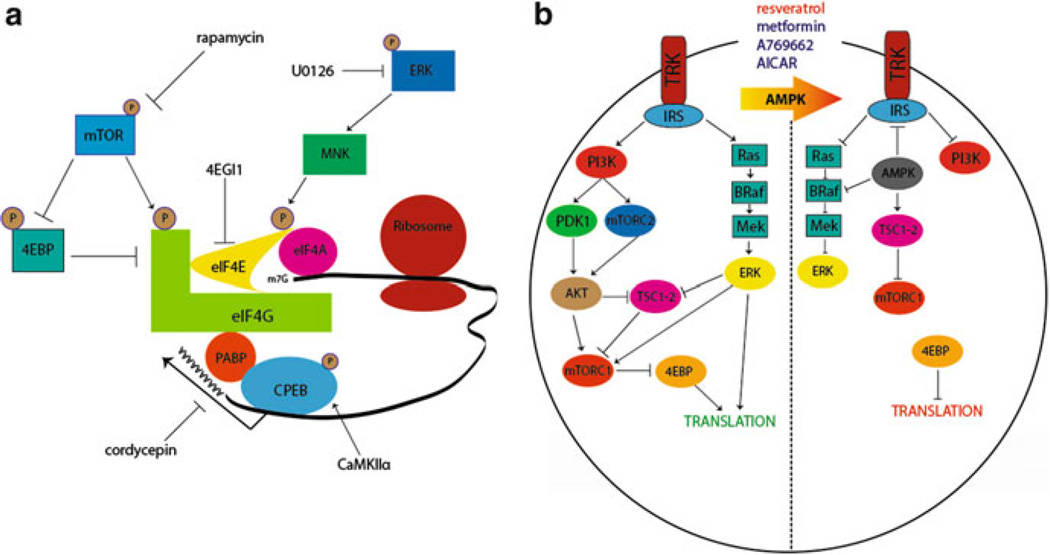
Due to the continuous turnover of cellular proteins, a simple switch in G-protein coupling is likely not sufficient to explain such a long-lasting maladaptive change in peripheral nociceptors (Bogen et al. 2012). It is likely that this effect is coupled to changes in gene expression and a possible solution to this problem is a localized change occurring at the level of protein translation. Translation can be controlled by extracellular factors signaling via kinase cascades offering a rapid, locally mediated control of gene expression. Two important kinases for translation control are the mechanistic target of rapamycin complex 1 (mTORC1) and extracellular signal-regulated kinase (ERK) (Fig. 2a). Both of these kinases signal to proteins that bind to the 5′ cap structure of mRNAs. mTORC1 phosphorylates 4E-binding proteins (4EBPs) leading to a release of inhibition on eukaryotic initiation factor (eIF) proteins allowing for the eIF4F complex (composed of eIF4E, the 5′ cap-binding protein; eIF4G, a scaffolding protein; and eIF4A, a helicase that unwinds secondary structure in mRNAs) to form (Fig. 2a) (Sonenberg and Hinnebusch 2009). ERK, on the other hand, activates the mitogen-activated kinase-interacting kinases (MNKs), which then phosphorylate eIF4E (Fig. 2a) (Wang et al. 1998). Phosphorylation of eIF4E, which is mediated specifically by MNK1/2, is thought to enhance eIF4F complex formation leading to an increase in translation (Ueda et al. 2010; Herdy et al. 2012). In sensory neurons, nerve growth factor (NGF) and interleukin 6 (IL6), two factors known to induce priming, induce an increase in ERK and mTORC1 signaling leading to a local, axonal increase in protein synthesis (Melemedjian et al. 2010, 2014). Blockade of these kinases, or blockade of eIF4F complex formation with the eIF4F inhibitor compound 4EGI1 (Fig. 2a), inhibits mechanical hypersensitivity induced by these factors and abrogates priming to subsequent PGE2 exposure (Melemedjian et al. 2010; Asiedu et al. 2011). Hence, local translation is required for the induction of priming downstream of NGF and IL6 signaling.
Fig. 2.
Translational control pathways involved in hyperalgesic priming. (a) mTORC1 phosphorylates 4EBPs, negative regulators of eIF4F formation. This results in its dissociation from eIF4E, allowing the binding of eIF4E to eIF4G. Phosphorylation of eIF4E (via ERK/MNK1/2) or eIF4G (via mTORC1) enhances the formation of the eIF4F complex, promoting translation. Phosphorylation of CPEB by CamKIIα enhances translation efficiency by increasing the length of the poly A tail in mRNAs containing a CPE sequence. Taken together, eIF4F cap complex formation enhances cap-dependent translation, which is necessary for the induction of priming via translational control of gene expression in sensory afferents. (b) Pharmacological activation of AMPK results in the inhibition of IRS-1, dampening Trk-mediated signaling. AMPK activation also results in TSC2 phosphorylation, thereby inhibiting mTORC1. Moreover, AMPK phosphorylates BRaf leading to inhibition of ERK signaling. Taken together, AMPK activation decreases activity-dependent translation by turning off both the ERK and mTORC1 pathways, presenting a novel opportunity to prevent or reverse hyperalgesic priming. Adapted from Melemedjian et al. (2010) and Price and Dussor (2013)
An alternative mechanism to decrease ERK and mTORC1 signaling is via stimulation of adenosine monophosphate-activated protein kinase (AMPK) (Fig. 2b). AMPK is a ubiquitously expressed energy sensing kinase well known to inhibit mTORC1 signaling through multiple phosphorylation events (Carling et al. 2012). AMPK also abrogates ERK signaling in many cell types, and this effect has recently been linked to negative regulation of the upstream ERK activator BRaf (Fig. 2b) (Shen et al. 2013). In sensory neurons, AMPK activation with pharmacological stimulators (for review see Price and Dussor 2013) leads to decreased ERK and mTORC1 activity (Melemedjian et al. 2011; Tillu et al. 2012), decreased eIF4F complex formation (Melemedjian et al. 2011; Tillu et al. 2012), and inhibition of axonal protein synthesis as measured by enhanced processing body (P body) formation (Melemedjian et al. 2014). AMPK activators decrease peripheral nerve injury- and inflammation-induced mechanical hyperalgesia (Melemedjian et al. 2011; Russe et al. 2013) suggesting an important role for this kinase in pain plasticity. In the context of hyperalgesic priming, AMPK activation with the natural product resveratrol (Fig. 2b) decreases mechanical hypersensitivity caused by incision and completely blocks the development of priming when given locally around the time of incision (Tillu et al. 2012). These findings further suggest a role for local translation in the initiation of plasticity leading to priming of nociceptors.
Epac signaling, which was described above as an important mediator of priming induced by inflammatory mediators (Wang et al. 2013), may also play an important role in regulating translation in sensory neurons. Decreased GRK2 leads to enhanced activation of Epac causing an increase in ERK activity (Eijkelkamp et al. 2010). While this signaling event has not been linked to translation control, based on findings involving IL6-induced priming and its dependence of ERK/eIF4E-mediated changes in translation control, it is conceivable that this is an important mediator for priming where ERK activity is increased. Furthermore, Epac activation causes an increase in mTORC1 activity in some transformed cells (Misra and Pizzo 2009) suggesting that enhanced Epac signaling in primed nociceptors may lead to convergent signaling onto the eIF4F complex in a manner analogous to that observed with NGF and IL6 (Melemedjian et al. 2010). It remains to be seen if these Epac-mediated events occur in sensory neurons and their axons, but this may be a fruitful area for further work and therapeutic intervention.
The regulation of translation via 5′ cap-binding proteins and their upstream kinases clearly comprise an important mechanism for the priming of nociceptors. However, translation is likewise regulated by RNA-binding proteins that bind to either 5′ or 3′ untranslated regions (UTRs). The fragile X mental retardation protein (FMRP) is a key RNA-binding protein regulating plasticity in the PNS and CNS (Bassell and Warren 2008). Mice lacking FMRP fail to sensitize in several preclinical pain models (Price et al. 2007; Price and Melemedjian 2012), and these mice also have deficits in priming induced by NGF and IL6 (Asiedu et al. 2011). Another important RNA-binding protein in hyperalgesic priming is the cytoplasmic polyadenylation element-binding protein (CPEB). CPEB binds preferentially to mRNAs containing a CPE sequence in their 3′ UTR near the polyadenylation sequence. These mRNAs contain short poly A tails, and CPEB, upon phosphorylation, can enhance the poly A tail length leading to enhanced translation efficiency (Fig. 2a) (Richter 2007). This process is linked to long-term potentiation (LTP) in the CNS (Udagawa et al. 2012) and has recently been shown to play an important role in nociceptor priming (Bogen et al. 2012; Ferrari et al. 2012, 2013a). CPEB is phosphorylated by the aurora family kinases (Mendez et al. 2000) and, likely more importantly for neurons, by Ca2+/calmodulin-activated protein kinase IIα (Fig. 2a) (CaMKIIα, Atkins et al. 2005). In the PNS, CPEB is primarily expressed by IB4(+) nociceptors (Bogen et al. 2012). Knockdown of CPEB with intrathecally injected antisense oligonucleotides leads to inhibition of the initiation of priming induced by PKCε activators linking translation events to PKCε-dependent priming (Bogen et al. 2012). Importantly, in priming induced by peripheral inflammation, CPEB may act downstream of PKCε and CaMKIIα to initiate and maintain a primed state (Ferrari et al. 2013a). Since CPEB is thought to have prion-like properties that are crucial for its role in memory maintenance (Si et al. 2003a, b), these findings point to a potential role for CPEB in creating a permanently primed state in peripheral nociceptors.
While it is clear that translation regulation is required to initiate a primed state in the periphery, a crucial question is whether disruption of local translation is capable of interrupting priming once it has been fully established. This is an important question because it gives insight into devising therapeutic strategies to reverse priming in the clinical state. While there are certain situations (e.g., surgery) where inhibition of priming mechanisms at the time of injury is a viable strategy, the majority of clinical situations are likely to require intervention following the full establishment of priming. An experimental paradigm to test this translation dependency is to induce priming with a locally administered stimulus and then allow the initial hyperalgesia to resolve. Then, prior to injection of the stimulus to precipitate hyperalgesia in primed animals, translation inhibitors can be administered locally to test whether continuous translation is required to maintain a primed state (Asiedu et al. 2011; Ferrari et al. 2013a). In this regard, following injection of IL6 and resolution of mechanical hyperalgesia in mice, injection of anisomycin or rapamycin (at doses that block the initiation of priming) fail to reverse a primed state (Asiedu et al. 2011). In contrast, in rats, injection of carrageenan causes priming that is disrupted both at the time of carrageenan injection and during the maintenance phase by either the mTORC1 inhibitor rapamycin or the polyadenylation inhibitor cordycepin (Ferrari et al. 2013b). Interestingly, in addition to cordycepin action on polyadenylation, this compound has recently been identified as an AMPK activator (Wu et al. 2014). Similar effects with rapamycin and cordycepin are observed in rats primed with paw injection of CaMKIIα. Since CaMKIIα phosphorylates CPEB and CPEB regulates CaMKIIα translation, this raises the intriguing possibility that CaMKIIα/CPEB signaling could represent a positive feedback mechanism to maintain pain memory in the peripheral nociceptor (Ferrari et al. 2013a). Importantly, in rats where PKCε-induced priming is sexually dimorphic, priming that is dependent on local translation occurs both in male and female rats (Ferrari et al. 2013a). Hence, while there are conflicting results in different models, it is formally possible that brief disruption of local translation in primed nociceptors is capable of resolving a primed state. Further work is clearly needed to further interrogate mechanisms involved in these effects; however, they point to the tantalizing possibility that therapeutics targeting translation regulation might have disease-modifying effects in chronic pain conditions. This hypothesis is consistent with the observation that AMPK activators, which have a strong effect on translation regulation pathways, have disease-modifying effects in neuropathic pain models (Melemedjian et al. 2011, 2013b).
4 CNS Regulation of Hyperalgesic Priming
4.1 Atypical PKCs and Brain-Derived Neurotropic Factor
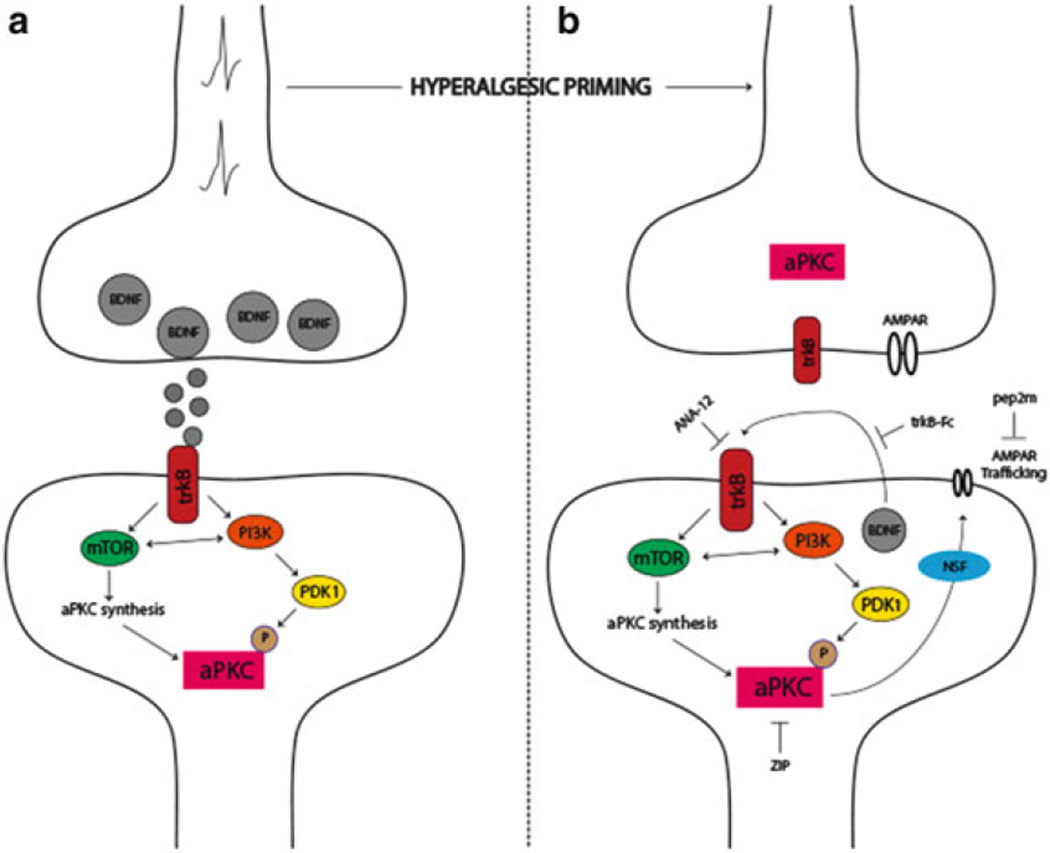
While there is strong evidence for memory-like mechanisms controlling hyperalgesic priming at the level of the peripheral nociceptor, plasticity in the CNS, especially in the dorsal horn of the spinal cord, also plays a central role in hyperalgesic priming. Here, analogies to mechanisms of memory formation and maintenance in other CNS regions, such as the hippocampus and cortex, have played a central role in directing research in the area. LTP, long recognized as a neurophysiological correlate of learning and memory at central synapses, can be divided into an early and late phase. Early LTP requires the activation of CaMKIIα, PKA, and conventional PKC leading to the phosphorylation of AMPA receptors (Huganir and Nicoll 2013). Early LTP also leads to changes in gene expression which occur both on the level of transcription and translation. These changes in gene expression are needed for the consolidation of early LTP into late LTP (Abraham and Williams 2008). Mechanisms involved in the maintenance of late LTP have been more difficult to clearly elucidate but are thought to involve brain-derived neurotropic factor (BDNF) and an atypical PKC (aPKC) isoform called PKMζ (Fig. 3) (Sacktor 2011). While LTP has been extensively studied in the cortex and hippocampus, LTP can also be induced at central synapses in the dorsal horn of the spinal cord, and many mechanisms elucidated in other CNS regions are shared at these spinal synapses (Sandkuhler 2007). Spinal LTP is a potential mechanism for primary hyperalgesia (Sandkuhler 2007), and heterosynaptic LTP may explain other aspects of pain plasticity that occur in chronic pain disorders (Klein et al. 2008).
Fig. 3.
The role of aPKCs in hyperalgesic priming initiation and maintenance. (a) Nociceptor activation leads to spinal BDNF release and a postsynaptic mTORC1-dependent translation of aPKC protein following trkB activation. These newly synthesized aPKCs are then phosphorylated by PDK1. Increased levels and phosphorylation of aPKCs are thought to be involved in initiating priming. (b) Once priming is established, increased aPKC protein and phosphorylation leads to a constitutive increase in AMPAR trafficking to the postsynaptic membrane. This appears to be regulated by BDNF signaling via trkB with BDNF potentially being released from postsynaptic dendrites in the maintenance stage of priming. Presynaptic trkB may also be activated by increased BDNF action in primed animals. Once established, hyperalgesic priming can be permanently reversed by inhibition of aPKCs with ZIP, by disruption of AMPAR trafficking with pep2M, or via inhibition of trkB/BDNF signaling with ANA-12 or trkB-Fc, respectively. Adapted from Price and Ghosh (2013)
Does spinal LTP explain features of the maintenance of hyperalgesic priming? While no direct measurements of spinal LTP as a correlate of hyperalgesic priming have been made, pharmacological similarities abound. Inhibition of translation and/or CaMKIIα during LTP induction blocks consolidation of late LTP (Nicoll and Roche 2013). Likewise, intrathecal injection of translation inhibitors or CaMKIIα inhibitors at the time of priming induction in mice inhibits initial hyperalgesia and prevents priming. In contrast, intrathecal injection of these compounds following the resolution of initial hyperalgesia fails to resolve priming consistent with a lack of effect of these mechanisms on the maintenance of late LTP (Asiedu et al. 2011; Melemedjian et al. 2013a). Late LTP can be reversed by inhibition of aPKCs with a pseudo substrate inhibitor called ZIP (Pastalkova et al. 2006). Intrathecal injection of ZIP either at the time of priming induction or following the resolution of the initial hyperalgesia leads to a complete reversal of hyperalgesic priming (Asiedu et al. 2011; Melemedjian et al. 2013a). ZIP also reverses established pain states that have become dependent on central plasticity following sustained afferent input (Laferriere et al. 2011). These findings are consistent with a role for PKMζ in the maintenance of late LTP, memory retention, and the maintenance of a chronic pain state. However, recent experiments using genetic methods to dissect the role of PKMζ in late LTP and memory maintenance have called the specificity of ZIP and the role of PKMζ in these effects into question (Lee et al. 2013; Volk et al. 2013). It remains to be seen if PKMζ plays a specific role in the maintenance of hyperalgesic priming in the dorsal horn of the spinal cord (for review on this topic see Price and Ghosh 2013).
An important component of the proposed role of PKMζ in LTP and memory is the trafficking of AMPA receptors to synaptic sites leading to a persistent augmentation of postsynaptic glutamate-mediated signaling (Fig. 3) (Sacktor 2011). This trafficking can be disrupted with a peptide called pep2m (Migues et al. 2010). Similar to experiments in other CNS regions, intrathecal injection of pep2m disrupts the maintenance of hyperalgesic priming (Asiedu et al. 2011) suggesting that aPKC-mediated regulation of AMPA receptor trafficking may play a central role in chronic pain states (Fig. 3). This is consistent with a wide variety of experimental findings indicating that AMPA receptor trafficking plays a central role in mediating pain plasticity induced by peripheral injury (Tao 2012).
As mentioned above, while it is clear that ZIP is capable of permanently reversing a primed state in a variety of experimental models (Asiedu et al. 2011; Laferriere et al. 2011; Melemedjian et al. 2013a), the target of ZIP is less clear based on recent evidence from transgenic mice (Lee et al. 2013; Volk et al. 2013). One possibility is that aPKC isoforms play a redundant role in synaptic plasticity, and therefore PKCλ may be involved in maintenance mechanisms of hyperalgesic priming (Price and Ghosh 2013). Since this isoform is also inhibited by ZIP (Melemedjian et al. 2013a; Volk et al. 2013), this is a parsimonious explanation for the discrepancy between pharmacological effects of ZIP and findings from mice lacking aPKCs derived from the Prckz locus (PKMζ and PKCζ). If this were the case, upstream mechanisms that regulate all aPKCs isoforms, either via phosphorylation or through their translation at synapses, would represent potential alternative targets to reverse hyperalgesic priming. A candidate molecule fitting this description is BDNF.
BDNF has long been recognized as an important mediator of pain plasticity. BDNF is expressed by DRG neurons and released in the spinal dorsal horn (Balkowiec and Katz 2000), where it can act on pre- and postsynaptic trkB receptors to regulate plasticity of presynaptic afferent fibers (Matayoshi et al. 2005) and postsynaptic dorsal horn neurons (Kerr et al. 1999; Pezet et al. 2002; Garraway et al. 2003). BDNF expression shows considerable plasticity following peripheral injury (Mannion et al. 1999) and nociceptor-specific knockout of BDNF leads to abrogation of many forms of injury-induced pain plasticity (Zhao et al. 2006). BDNF is also a key factor in LTP. In the hippocampus, BDNF is required for the induction of LTP, and during late LTP, dendritic-expressed BDNF appears to play an autocrine role in the maintenance of late-phase LTP (Fig. 3) (Lu et al. 2008). Likewise, BDNF is capable of inducing LTP in dorsal horn neurons (Zhou et al. 2008) linking BDNF-induced pain plasticity to memory-like mechanisms that may be involved in the maintenance of hyperalgesic priming. Indeed, intrathecal injection of a BDNF scavenging agent, trkB-fc, or systemic injection of a trkB antagonist, ANA-12 (Cazorla et al. 2011), blocks hyperalgesia induced by priming agents and prevents the precipitation of a primed state by subsequent PGE2 injection. Significantly, interruption of BDNF/trkB signaling with either trkB-fc or ANA-12 after the establishment of a primed state leads to a resolution of priming precipitated by PGE2 injection (Melemedjian et al. 2013a). This suggests a key role of BDNF/trkB signaling in the maintenance of a primed state. At spinal synapses, BDNF induces phosphorylation and translation of the two major aPKC isoforms found in the CNS, PKMζ and PKCλ (Fig. 3), suggesting a potential link between BDNF/trkB and aPKCs in the regulation of the maintenance of hyperalgesic priming (Melemedjian et al. 2013a). Because precise targets of ZIP remain unknown, these findings point to BDNF/trkB signaling as a therapeutic target for the reversal of established chronic pain states. Importantly, the precise location of BDNF action and trkB signaling in the maintenance of hyperalgesic priming has not been established. While it is tempting to speculate that an autocrine role for BDNF release from dendrites of dorsal horn neurons in the primed state plays a role in maintaining priming, especially based on the role of this mechanism in the maintenance of late LTP in other CNS structures (Lu et al. 2008), this hypothesis requires further experimental exploration.
4.2 Endogenous Opioids, μ-Opioid Receptor Constitutive Activity, and Hyperalgesic Priming
An intriguing question in the neurobiology of hyperalgesic priming is why does the initial hyperalgesia resolve if a primed state can persist for many weeks to months after the initial injury? This is especially interesting when one considers the strong pharmacological parallels between the maintenance of hyperalgesic priming and the maintenance of late LTP (e.g., aPKCs, AMPA receptor trafficking and BDNF/trkB signaling). In other words, if LTP persists and consolidates into late LTP to maintain priming, why does the initial hyperalgesia resolve? One possibility is that endogenous analgesic mechanisms mask the hyperalgesic state. In such a scenario, the precipitation of hyperalgesia in primed animals would be able to override this endogenous mechanism leading to a very long-lasting hyperalgesic state to a normally subthreshold stimulus. As described above, this is precisely what occurs in hyperalgesic priming models. One candidate for endogenous analgesia overriding hyperalgesia is the endogenous opioid system. This system is robust in the dorsal horn with interneurons capable of releasing peptides that act on μ-opioid receptors (MORs) expressed throughout the dorsal horn (Ribeiro-da-Silva et al. 1992; Ma et al. 1997), including on presynaptic nociceptor nerve endings (Schroeder et al. 1991; Schroeder and McCleskey 1993). Exciting new evidence suggests that this mechanism may be at play in hyperalgesic priming.
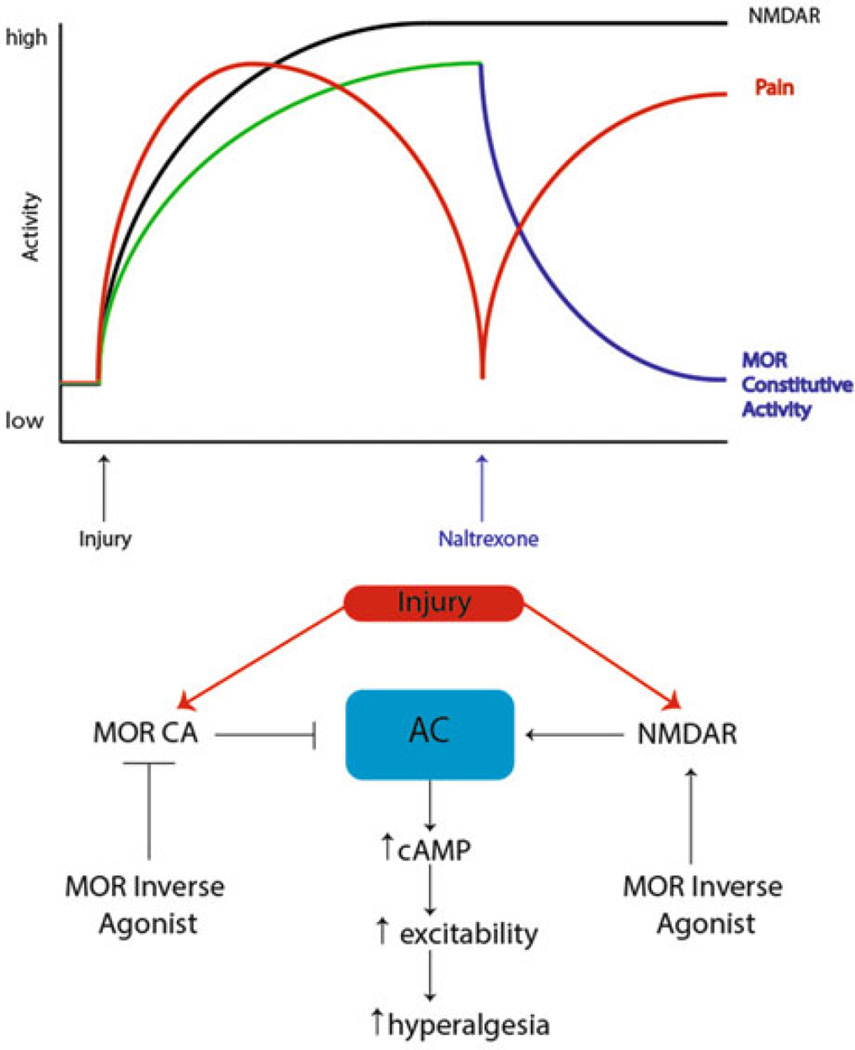
Animals exposed to a strong inflammatory stimulus demonstrate hyperalgesia that normally resolves within 10–14 days; however, when these same animals are infused with MOR inverse agonists, the initial hyperalgesia is prolonged for the duration of inverse agonist infusion (Corder et al. 2013). Importantly, even when the initial hyperalgesia is allowed to resolve, infusion of MOR inverse agonists immediately precipitates a reinstatement of hyperalgesia, an effect that is absent in sham animals and an effect that is analogous to precipitation of hyperalgesia in primed animals with a subthreshold peripheral stimulus. What governs this effect? Peripheral inflammation, and presumably other nociceptive stimuli, induces a change in spinal MORs such that they now acquire constitutive activity (signaling through G-proteins in the absence of agonist). This MOR constitutive activity causes a tonic inhibition of pain signaling presumably masking a hyperalgesic state that would otherwise persist following the initial insult. When MOR inverse agonists remove this acquired MOR constitutive activity, a cAMP overshoot occurs (a classical cellular sign of opioid dependence) in a Ca2+-dependent adenylyl cyclase 1 (AC1)-dependent fashion that also involves the engagement of NMDA receptors (Fig. 4) (Corder et al. 2013). This leads to the reinstatement of pain in animals that have a previous history of strong nociceptive input (Fig. 4).
Fig. 4.
Signaling pathways regulating endogenous opioid control of hyperalgesic priming. Prior to injury, MORs do not have constitutive activity and nociceptive input to the CNS is absent. However, after injury, nociceptor firing induces activity in CNS circuits leading to release of endogenous opioid peptides in the dorsal horn and the eventual development of MOR constitutive activity resulting in agonist-independent modulation of pain responses. Upon infusion of the MOR inverse agonist naltrexone, AC1 is disinhibited, likely in an NMDA receptor-dependent fashion, allowing for superactivation of cAMP and the reinstatement of hyperalgesia. Adapted from Corder et al. (2013)
These findings have several important implications for understanding central mechanisms governing hyperalgesic priming. First, they provide an elegant solution to the question of why initial hyperalgesia resolves despite the persistence of a primed state. This occurs, at least in part, because tonic opioid signaling, including MOR constitutive activity, masks the presence of mechanisms that would otherwise drive hyperalgesia (Corder et al. 2013). Second, this study provides some possible links to priming and late LTP maintenance that potentially solve questions stated above. Opioid-dependent mechanisms play an important role in governing spinal LTP. While there is evidence that high-dose opioids can stimulate LTP at certain synapses after their abrupt removal (Drdla et al. 2009), there is likewise evidence that MOR activation can resolve even late LTP at spinal synapses (Drdla-Schutting et al. 2012). Therefore, it is formally possible that the initial priming stimulus leads to late LTP consolidation, but this is subsequently resolved by endogenous opioid-mediated mechanisms. A key question then is: does the previous establishment of late LTP at central synapses lead to a drop in threshold for establishment of subsequent LTP? If the mechanisms governing the MOR-dependent reversal of spinal late LTP are constitutively expressed, as appears to be the case (Corder et al. 2013), then this may lead to a tonic reversal of late LTP with underlying mechanisms (e.g., aPKC and BDNF/trkB signaling) still in place. While this idea obviously requires extensive experimental work, it could represent an important mechanism linking changes in peripheral sensitivity to CNS plasticity responsible for the maintenance of priming. Reversing these mechanisms could lead to revolutionary new therapeutics with disease-modifying effects on chronic pain.
4.3 Surgery as a Priming Stimulus and the Effects of Opioids
Opioids have been the first line of therapy for moderate to severe acute pain for decades, if not centuries. However, there is abundant preclinical and some clinical evidence suggesting that opioid administration, designed to alleviate acute pain, paradoxically primes the patient and renders them more susceptible to a transition to chronic pain. Studies using rodents have demonstrated a steady reduction in withdrawal thresholds with intrathecal morphine administration, fentanyl boluses, and repeated systemic morphine (Vanderah et al. 2001; Gardell et al. 2002, 2006) or heroin administration (Mao et al. 1995; Celerier et al. 2000, 2001), generating a sensitized state that is presumably independent of noxious stimulation from the periphery. These preclinical studies highlight the neuroplastic changes induced by opioids and provide another mechanism for the induction of a sensitized and/or primed state in animals.
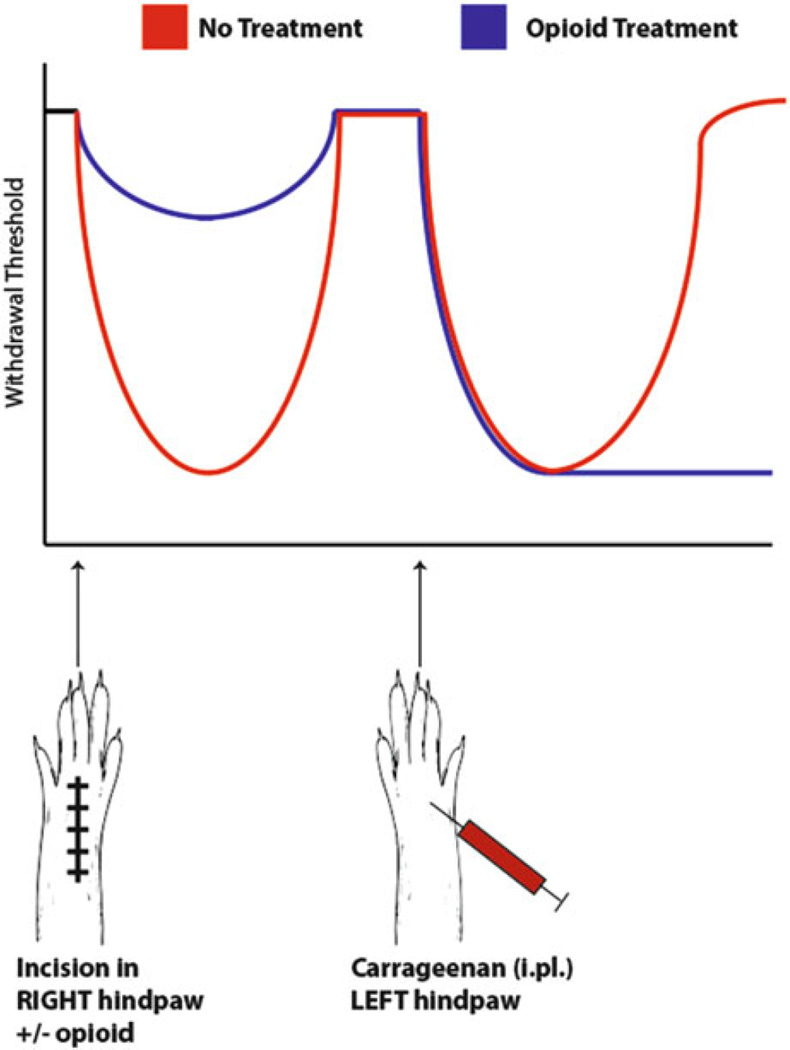
First, it is clear that surgical incision can produce priming in rodents and that these mechanisms are largely shared with other models involving the use of inflammatory mediators (Asiedu et al. 2011). Do the use of common postsurgical pain analgesics alleviate this priming? Here the answer appears to be no as opioid administration in the perioperative period can exacerbate a primed state revealed up to weeks later by injection of an inflammatory mediator (Fig. 5, Rivat et al. 2007). Specifically, in mouse models of postsurgical pain, remifentanil administration leads to the enhancement of the initial hyperalgesic state and the development of enhanced and prolonged response upon the precipitation of a second phase of hyperalgesia in animals primed with incision + opioids (Cabanero et al. 2009a, b). Additionally, in rodents that received incision and were simultaneously treated with opioids, these animals were primed to develop a long-lasting hyperalgesic state in response to subsequent contralateral inflammation, environmental stress, or subsequent opioid administration (Fig. 5, Rivat et al. 2002, 2007; Cabanero et al. 2009a). Thus, opioid administration as an analgesic for postsurgery pain might be viewed as a catalyst that contributes to pain chronification after incision providing a model system to study the effect of opioids on exacerbation of the transition from acute to chronic pain. Significantly, this process appears to involve spinal NMDA receptors because the effect of opioids on enhancement of priming is prevented, in most cases, by concomitant treatment with intrathecal NMDA receptor antagonists (Rivat et al. 2013). Hence, despite their obviously beneficial post-surgical analgesic effect, opioids induce long-lasting sensitization after initial analgesia at least in animal models. Therefore, it is formally possible that high rates of pain chronification following surgery (Macrae 2001) may be at least partially due to the nearly universal use of opioids as postsurgical pain medications. Hence, continued work into mechanisms that might mitigate these effects (e.g., NMDA receptor blockers) or non-opioid analgesics is warranted.
Fig. 5.
Opioids given as postsurgical analgesics can exacerbate priming in animal models. Upon incision of the hindpaw, withdrawal thresholds in animals not receiving analgesics drop significantly compared to animals treated with opioids at the time of incision. Following resolution of the initial hyperalgesia, when carrageenan is subsequently injected into the contralateral hindpaw, withdrawal thresholds drop in both groups; however, animals previously treated with opioids demonstrate an exaggerated hyperalgesia to the same stimulus, suggesting that opioids, despite their analgesic effect, exacerbate nociceptive priming induced by injury
5 Therapeutic Opportunities and Conclusions
We have made the case above for hyperalgesic priming as a model system to understand plasticity in the PNS and CNS underlying the maintenance of chronic pain states. While human experimental pain models have, as of yet, not given clear evidence of priming in our species, the chronic intermittent nature of many chronic pain states makes a strong case for priming as a key feature of clinical pain disorders. What then are the key opportunities for therapeutic intervention?
Targeting the peripheral nervous system is advantageous because it provides an opportunity to avoid CNS side effects. In our view, key targets here are PKCε, Epac, and translation regulation including AMPK. PKCε inhibitors have been the focus of intense research for some time now and may eventually enter pain clinical trials (Reichling and Levine 2009). Because PKCε is involved in both the initiation and maintenance of priming, this mechanism might be engaged for the reversal of a primed state; however, there is now evidence that several other key mechanisms are downstream of this kinase making them more attractive targets (Ferrari et al. 2013a; Wang et al. 2013). One of these is Epac. As noted above, Epac signaling may play a key role in signaling switches occurring exclusively in primed nociceptors (Hucho et al. 2005; Eijkelkamp et al. 2010; Ferrari et al. 2012; Wang et al. 2013). Hence, inhibition of Epac activity may likewise provide an opportunity for interruption of priming. Having said this, there is strong evidence that translation control is a final common denominator in all of these signaling mechanisms indicating that targeting gene expression at the level of local translation in the primed nociceptor may provide the greatest therapeutic opportunity. Additional preclinical work is needed to identify key signaling mechanisms involved in regulating translation regulation in primed nociceptors, but the currently available evidence points strongly to mTORC1, ERK (Melemedjian et al. 2010, 2013a; Tillu et al. 2012), and CPEB (Bogen et al. 2012; Ferrari et al. 2013a) as targets. It is currently not clear if CPEB can be targeted pharmacologically and its upstream kinases in priming have not been elucidated although CaMKIIα is a strong candidate. Targeting mTORC1 and ERK is likely a more viable approach because compounds that target these pathways are already in clinical use. While rapamycin clearly inhibits mTORC1, this approach also leads to feedback activation of ERK over the longer term, an action that may counteract rapamycin efficacy (Melemedjian et al. 2013b). Having said that, as detailed above, AMPK activation inhibits both mTORC1 and ERK pathways, and AMPK activators have already been shown to block the initiation of priming. Here, the common antidiabetic drug metformin is in wide clinical use for other indications and acts via AMPK activation (Shaw et al. 2005). While further work is needed to investigate preclinical effects of AMPK activators in hyperalgesic priming maintenance, clinical trials can be designed to test the hypothesis that AMPK activators have disease-modifying effects in chronic pain states (Price and Dussor 2013).
An alternative approach would be to target mechanisms in the CNS responsible for the maintenance of plasticity resulting in priming. These mechanisms share similarities to molecules involved in learning and memory; hence, they carry inherent risks that deserve appropriate caution (Price and Ghosh 2013). Having said this, innovative approaches to therapeutic intervention may lead to disease-modifying effects in chronic pain patients resulting from a permanent reversal of plasticity in dorsal horn circuitry. From this perspective, we propose that BDNF/trkB signaling is an attractive candidate. As mentioned above, short-term disruption of BDNF action at trkB receptors leads to a permanent reversal of a primed state (Melemedjian et al. 2013a). This suggests that in the clinical arena, therapeutics targeting this pathway could be used for a brief period of time to achieve long-lasting effects on chronic pain. TrkB-based therapeutics are under investigation for a wide variety of neurological disorders (Cazorla et al. 2011); therefore, clinical opportunities along this front may arise in the near future. In the longer term, continued research into the role of aPKCs in pain chronification may lead to important insight into a specific role for certain aPKC isoforms in the maintenance of priming. Because it now appears to be the case that PKMζ is not required for late LTP or learning and memory rostral to the spinal cord, an intriguing possibility is that this aPKC isoform plays a specific role in the pain pathway (Lee et al. 2013; Price and Ghosh 2013; Volk et al. 2013). This scenario could lead to therapeutic opportunities that would have decreased liability for disruption of plasticity in brain regions involved in learning and memory.
A final opportunity worth noting concerns the complex interrelationship of opioids with chronic pain. As discussed at length above, there is evidence that opioid analgesics can augment priming when given during the time of surgery (Rivat et al. 2013). On the other hand, endogenous opioid mechanisms may be crucial for masking hyperalgesia that would otherwise persist following injury. How can these mechanisms be resolved for better therapeutics? A clear opportunity exists in the development of non-opioid analgesics for the treatment of pain. This is a long-standing goal of research in the pain arena and continues to be the impetus for most target-based drug discovery in the field. From the perspective of MOR constitutive activity, which likely serves a beneficial function, targeting AC1 to avoid cAMP superactivation when this mechanism is disengaged could avoid the adverse consequences resulting from this MOR plasticity (Corder et al. 2013).
In closing, we have summarized research into hyperalgesic priming highlighting our current understanding of plasticity mechanisms in the peripheral nociceptor and in the CNS that govern this preclinical model of the transition from acute to chronic pain. The advent and subsequent proliferation of research into this model has led to a great expansion of our understanding of plasticity in the nociceptive system. Our view is that these findings provide great insight into therapeutic opportunities not only for the treatment of chronic pain but also for its potential reversal. Continued work in this area holds great promise for the development of revolutionary therapeutics for the permanent alleviation of chronic pain states.
Acknowledgments
This work was supported by NIH grants NS065926 and GM102575 to T.J.P.
Contributor Information
Ram Kandasamy, Department of Pharmacology, The University of Arizona, Tucson, AZ 85721, USA.
Theodore J. Price, Email: theodore.price@utdallas.edu, Department of Pharmacology, The University of Arizona, Tucson, AZ 85721, USA; Bio5 Institute, The University of Arizona, Tucson, AZ 85721, USA; Graduate Interdisciplinary Program in Neuroscience, The University of Arizona, Tucson, AZ 85721, USA; School of Brain and Behavioral Sciences, The University of Texas at Dallas, Richardson, TX 75080, USA.
References
- Abraham WC, Williams JM. LTP maintenance and its protein synthesis-dependence. Neurobiol Learn Mem. 2008;89:260–268. doi: 10.1016/j.nlm.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Aley KO, Levine JD. Role of protein kinase A in the maintenance of inflammatory pain. J Neurosci. 1999;19:2181–2186. doi: 10.1523/JNEUROSCI.19-06-02181.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aley KO, Messing RO, Mochly-Rosen D, Levine JD. Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. J Neurosci. 2000;20:4680–4685. doi: 10.1523/JNEUROSCI.20-12-04680.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althaus A, Hinrichs-Rocker A, Chapman R, Arranz Becker O, Lefering R, Simanski C, Weber F, Moser KH, Joppich R, Trojan S, Gutzeit N, Neugebauer E. Development of a risk index for the prediction of chronic post-surgical pain. Eur J Pain. 2012;16:901–910. doi: 10.1002/j.1532-2149.2011.00090.x. [DOI] [PubMed] [Google Scholar]
- Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354:581–585. doi: 10.1016/S0140-6736(99)01312-4. [DOI] [PubMed] [Google Scholar]
- Asiedu MN, Tillu DV, Melemedjian OK, Shy A, Sanoja R, Bodell B, Ghosh S, Porreca F, Price TJ. Spinal protein kinase M zeta underlies the maintenance mechanism of persistent nociceptive sensitization. J Neurosci. 2011;31:6646–6653. doi: 10.1523/JNEUROSCI.6286-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins CM, Davare MA, Oh MC, Derkach V, Soderling TR. Bidirectional regulation of cytoplasmic polyadenylation element-binding protein phosphorylation by Ca2+/calmodulin-dependent protein kinase II and protein phosphatase 1 during hippocampal long-term potentiation. J Neurosci. 2005;25:5604–5610. doi: 10.1523/JNEUROSCI.5051-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkowiec A, Katz DM. Activity-dependent release of endogenous brain-derived neurotrophic factor from primary sensory neurons detected by ELISA in situ. J Neurosci. 2000;20:7417–7423. doi: 10.1523/JNEUROSCI.20-19-07417.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartleson JD. Low back pain. Curr Treat Options Neurol. 2001;3:159–168. doi: 10.1007/s11940-001-0051-4. [DOI] [PubMed] [Google Scholar]
- Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigal ME, Lipton RB. Clinical course in migraine: conceptualizing migraine transformation. Neurology. 2008;71:848–855. doi: 10.1212/01.wnl.0000325565.63526.d2. [DOI] [PubMed] [Google Scholar]
- Bogen O, Alessandri-Haber N, Chu C, Gear RW, Levine JD. Generation of a pain memory in the primary afferent nociceptor triggered by PKCε activation of CPEB. J Neurosci. 2012;32:2018–2026. doi: 10.1523/JNEUROSCI.5138-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabanero D, Campillo A, Celerier E, Romero A, Puig MM. Pronociceptive effects of remifentanil in a mouse model of postsurgical pain: effect of a second surgery. Anesthesiology. 2009a;111:1334–1345. doi: 10.1097/ALN.0b013e3181bfab61. [DOI] [PubMed] [Google Scholar]
- Cabanero D, Celerier E, Garcia-Nogales P, Mata M, Roques BP, Maldonado R, Puig MM. The pro-nociceptive effects of remifentanil or surgical injury in mice are associated with a decrease in delta-opioid receptor mRNA levels: prevention of the nociceptive response by on-site delivery of enkephalins. Pain. 2009b;141:88–96. doi: 10.1016/j.pain.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Carey TS, Garrett JM, Jackman A, Hadler N. Recurrence and care seeking after acute back pain: results of a long-term follow-up study. North Carolina Back Pain Project. Med Care. 1999;37:157–164. doi: 10.1097/00005650-199902000-00006. [DOI] [PubMed] [Google Scholar]
- Carling D, Thornton C, Woods A, Sanders MJ. AMP-activated protein kinase: new regulation, new roles? Biochem J. 2012;445:11–27. doi: 10.1042/BJ20120546. [DOI] [PubMed] [Google Scholar]
- Cassidy JD, Cote P, Carroll LJ, Kristman V. Incidence and course of low back pain episodes in the general population. Spine (Phila Pa 1976) 2005;30:2817–2823. doi: 10.1097/01.brs.0000190448.69091.53. [DOI] [PubMed] [Google Scholar]
- Cazorla M, Premont J, Mann A, Girard N, Kellendonk C, Rognan D. Identification of a low-molecular weight TrkB antagonist with anxiolytic and antidepressant activity in mice. J Clin Invest. 2011;121:1846–1857. doi: 10.1172/JCI43992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celerier E, Rivat C, Jun Y, Laulin JP, Larcher A, Reynier P, Simonnet G. Long-lasting hyperalgesia induced by fentanyl in rats: preventive effect of ketamine. Anesthesiology. 2000;92:465–472. doi: 10.1097/00000542-200002000-00029. [DOI] [PubMed] [Google Scholar]
- Celerier E, Laulin JP, Corcuff JB, Le Moal M, Simonnet G. Progressive enhancement of delayed hyperalgesia induced by repeated heroin administration: a sensitization process. J Neurosci. 2001;21:4074–4080. doi: 10.1523/JNEUROSCI.21-11-04074.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder G, Doolen S, Donahue RR, Winter MK, Jutras BL, He Y, Hu X, Wieskopf JS, Mogil JS, Storm DR, Wang ZJ, McCarson KE, Taylor BK. Constitutive mu-opioid receptor activity leads to long-term endogenous analgesia and dependence. Science. 2013;341:1394–1399. doi: 10.1126/science.1239403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft PR, Lewis M, Papageorgiou AC, Thomas E, Jayson MI, Macfarlane GJ, Silman AJ. Risk factors for neck pain: a longitudinal study in the general population. Pain. 2001;93:317–325. doi: 10.1016/S0304-3959(01)00334-7. [DOI] [PubMed] [Google Scholar]
- Dina OA, Khasar SG, Gear RW, Levine JD. Activation of Gi induces mechanical hyperalgesia poststress or inflammation. Neuroscience. 2009;160:501–507. doi: 10.1016/j.neuroscience.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drdla R, Gassner M, Gingl E, Sandkuhler J. Induction of synaptic long-term potentiation after opioid withdrawal. Science. 2009;325:207–210. doi: 10.1126/science.1171759. [DOI] [PubMed] [Google Scholar]
- Drdla-Schutting R, Benrath J, Wunderbaldinger G, Sandkuhler J. Erasure of a spinal memory trace of pain by a brief, high-dose opioid administration. Science. 2012;335:235–238. doi: 10.1126/science.1211726. [DOI] [PubMed] [Google Scholar]
- Eijkelkamp N, Wang H, Garza-Carbajal A, Willemen HL, Zwartkruis FJ, Wood JN, Dantzer R, Kelley KW, Heijnen CJ, Kavelaars A. Low nociceptor GRK2 prolongs prostaglandin E2 hyperalgesia via biased cAMP signaling to Epac/Rap1, protein kinase Cepsilon, and MEK/ERK. J Neurosci. 2010;30:12806–12815. doi: 10.1523/JNEUROSCI.3142-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari LF, Bogen O, Alessandri-Haber N, Levine E, Gear RW, Levine JD. Transient decrease in nociceptor GRK2 expression produces long-term enhancement in inflammatory pain. Neuroscience. 2012;222:392–403. doi: 10.1016/j.neuroscience.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari LF, Bogen O, Levine JD. Role of nociceptor alphaCaMKII in transition from acute to chronic pain (hyperalgesic priming) in male and female rats. J Neurosci. 2013a;33:11002–11011. doi: 10.1523/JNEUROSCI.1785-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari LF, Bogen O, Chu C, Levine JD. Peripheral administration of translation inhibitors reverses increased hyperalgesia in a model of chronic pain in the rat. J Pain. 2013b;14:731–738. doi: 10.1016/j.jpain.2013.01.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardell LR, Wang R, Burgess SE, Ossipov MH, Vanderah TW, Malan TP, Jr, Lai J, Porreca F. Sustained morphine exposure induces a spinal dynorphin-dependent enhancement of excitatory transmitter release from primary afferent fibers. J Neurosci. 2002;22:6747–6755. doi: 10.1523/JNEUROSCI.22-15-06747.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardell LR, King T, Ossipov MH, Rice KC, Lai J, Vanderah TW, Porreca F. Opioid receptor-mediated hyperalgesia and antinociceptive tolerance induced by sustained opiate delivery. Neurosci Lett. 2006;396:44–49. doi: 10.1016/j.neulet.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Garraway SM, Petruska JC, Mendell LM. BDNF sensitizes the response of lamina II neurons to high threshold primary afferent inputs. Eur J Neurosci. 2003;18:2467–2476. doi: 10.1046/j.1460-9568.2003.02982.x. [DOI] [PubMed] [Google Scholar]
- Harris W. Persistent pain in lesions of the peripheral and central nervous system. Br Med J. 1921;2:896–900. doi: 10.1136/bmj.2.3178.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdy B, et al. Translational control of the activation of transcription factor NF-kappaB and production of type I interferon by phosphorylation of the translation factor eIF4E. Nat Immunol. 2012;13:543–550. doi: 10.1038/ni.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hucho TB, Dina OA, Levine JD. Epac mediates a cAMP-to-PKC signaling in inflammatory pain: an isolectin B4(+) neuron-specific mechanism. J Neurosci. 2005;25:6119–6126. doi: 10.1523/JNEUROSCI.0285-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huganir RL, Nicoll RA. AMPARs and synaptic plasticity: the last 25 years. Neuron. 2013;80:704–717. doi: 10.1016/j.neuron.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EK, Levine JD. Hyperalgesic priming is restricted to isolectin B4-positive nociceptors. Neuroscience. 2010;169:431–435. doi: 10.1016/j.neuroscience.2010.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EK, Parada CA, Levine JD. Hyperalgesic priming in the rat demonstrates marked sexual dimorphism. Pain. 2003;105:143–150. doi: 10.1016/s0304-3959(03)00175-1. [DOI] [PubMed] [Google Scholar]
- Joseph EK, Bogen O, Alessandri-Haber N, Levine JD. PLC-beta 3 signals upstream of PKC epsilon in acute and chronic inflammatory hyperalgesia. Pain. 2007;132:67–73. doi: 10.1016/j.pain.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Joseph EK, Reichling DB, Levine JD. Shared mechanisms for opioid tolerance and a transition to chronic pain. J Neurosci. 2010;30:4660–4666. doi: 10.1523/JNEUROSCI.5530-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr BJ, Bradbury EJ, Bennett DL, Trivedi PM, Dassan P, French J, Shelton DB, McMahon SB, Thompson SW. Brain-derived neurotrophic factor modulates nociceptive sensory inputs and NMDA-evoked responses in the rat spinal cord. J Neurosci. 1999;19:5138–5148. doi: 10.1523/JNEUROSCI.19-12-05138.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasar SG, Burkham J, Dina OA, Brown AS, Bogen O, Alessandri-Haber N, Green PG, Reichling DB, Levine JD. Stress induces a switch of intracellular signaling in sensory neurons in a model of generalized pain. J Neurosci. 2008;28:5721–5730. doi: 10.1523/JNEUROSCI.0256-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein T, Stahn S, Magerl W, Treede RD. The role of heterosynaptic facilitation in long-term potentiation (LTP) of human pain sensation. Pain. 2008;139:507–519. doi: 10.1016/j.pain.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Kolb E, Canjuga M, Bauer GF, Laubli T. Course of back pain across 5 years: a retrospective cohort study in the general population of Switzerland. Spine (Phila Pa 1976) 2011;36:E268–E273. doi: 10.1097/BRS.0b013e3181f324b5. [DOI] [PubMed] [Google Scholar]
- Laferriere A, Pitcher MH, Haldane A, Huang Y, Cornea V, Kumar N, Sacktor TC, Cervero F, Coderre TJ. PKMzeta is essential for spinal plasticity underlying the maintenance of persistent pain. Mol Pain. 2011;7:99. doi: 10.1186/1744-8069-7-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AM, Kanter BR, Wang D, Lim JP, Zou ME, Qiu C, McMahon T, Dadgar J, Fischbach-Weiss SC, Messing RO. Prkcz null mice show normal learning and memory. Nature. 2013;493:416–419. doi: 10.1038/nature11803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton RB. Tracing transformation: chronic migraine classification, progression, and epidemiology. Neurology. 2009;72:S3–S7. doi: 10.1212/WNL.0b013e3181974b19. [DOI] [PubMed] [Google Scholar]
- Lu Y, Christian K, Lu B. BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol Learn Mem. 2008;89:312–323. doi: 10.1016/j.nlm.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Ribeiro-da-Silva A, De Koninck Y, Radhakrishnan V, Cuello AC, Henry JL. Substance P and enkephalin immunoreactivities in axonal boutons presynaptic to physiologically identified dorsal horn neurons. An ultrastructural multiple-labelling study in the cat. Neuroscience. 1997;77:793–811. doi: 10.1016/s0306-4522(96)00510-6. [DOI] [PubMed] [Google Scholar]
- Macrae WA. Chronic pain after surgery. Br J Anaesth. 2001;87:88–98. doi: 10.1093/bja/87.1.88. [DOI] [PubMed] [Google Scholar]
- Mannion RJ, Costigan M, Decosterd I, Amaya F, Ma QP, Holstege JC, Ji RR, Acheson A, Lindsay RM, Wilkinson GA, Woolf CJ. Neurotrophins: peripherally and centrally acting modulators of tactile stimulus-induced inflammatory pain hypersensitivity. Proc Natl Acad Sci U S A. 1999;96:9385–9390. doi: 10.1073/pnas.96.16.9385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, Price DD, Mayer DJ. Mechanisms of hyperalgesia and morphine tolerance: a current view of their possible interactions. Pain. 1995;62:259–274. doi: 10.1016/0304-3959(95)00073-2. [DOI] [PubMed] [Google Scholar]
- Matayoshi S, Jiang N, Katafuchi T, Koga K, Furue H, Yasaka T, Nakatsuka T, Zhou XF, Kawasaki Y, Tanaka N, Yoshimura M. Actions of brain-derived neurotrophic factor on spinal nociceptive transmission during inflammation in the rat. J Physiol. 2005;569:685–695. doi: 10.1113/jphysiol.2005.095331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melemedjian OK, Asiedu MN, Tillu DV, Peebles KA, Yan J, Ertz N, Dussor GO, Price TJ. IL-6- and NGF-induced rapid control of protein synthesis and nociceptive plasticity via convergent signaling to the eIF4F complex. J Neurosci. 2010;30:15113–15123. doi: 10.1523/JNEUROSCI.3947-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melemedjian OK, Asiedu MN, Tillu DV, Sanoja R, Yan J, Lark A, Khoutorsky A, Johnson J, Peebles KA, Lepow T, Sonenberg N, Dussor G, Price TJ. Targeting adenosine monophosphate-activated protein kinase (AMPK) in preclinical models reveals a potential mechanism for the treatment of neuropathic pain. Mol Pain. 2011;7:70. doi: 10.1186/1744-8069-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melemedjian OK, Tillu DV, Asiedu MN, Mandell EK, Moy JK, Blute VM, Taylor CJ, Ghosh S, Price TJ. BDNF regulates atypical PKC at spinal synapses to initiate and maintain a centralized chronic pain state. Mol Pain. 2013a;9:12. doi: 10.1186/1744-8069-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melemedjian OK, Khoutorsky A, Sorge RE, Yan J, Asiedu MN, Valdez A, Ghosh S, Dussor G, Mogil JS, Sonenberg N, Price TJ. mTORC1 inhibition induces pain via IRS-1-dependent feedback activation of ERK. Pain. 2013b;154(7):1080–1091. doi: 10.1016/j.pain.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melemedjian OK, Mejia GL, Lepow TS, Zoph OK, Price TJ. Bidirectional regulation of P body formation mediated by eIF4F complex formation in sensory neurons. Neurosci Lett. 2014;563:169–174. doi: 10.1016/j.neulet.2013.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez R, Hake LE, Andresson T, Littlepage LE, Ruderman JV, Richter JD. Phosphorylation of CPE binding factor by Eg2 regulates translation of c-mos mRNA. Nature. 2000;404:302–307. doi: 10.1038/35005126. [DOI] [PubMed] [Google Scholar]
- Migues PV, Hardt O, Wu DC, Gamache K, Sacktor TC, Wang YT, Nader K. PKMzeta maintains memories by regulating GluR2-dependent AMPA receptor trafficking. Nat Neurosci. 2010;13:630–634. doi: 10.1038/nn.2531. [DOI] [PubMed] [Google Scholar]
- Misra UK, Pizzo SV. Epac1-induced cellular proliferation in prostate cancer cells is mediated by B-Raf/ERK and mTOR signaling cascades. J Cell Biochem. 2009;108:998–1011. doi: 10.1002/jcb.22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, Roche KW. Long-term potentiation: peeling the onion. Neuropharmacology. 2013;74:18–22. doi: 10.1016/j.neuropharm.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolet PS, Cote P, Cassidy JD, Carroll LJ. The association between a lifetime history of a neck injury in a motor vehicle collision and future neck pain: a population-based cohort study. Eur Spine J. 2010;19:972–981. doi: 10.1007/s00586-010-1344-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada CA, Yeh JJ, Reichling DB, Levine JD. Transient attenuation of protein kinase Cepsilon can terminate a chronic hyperalgesic state in the rat. Neuroscience. 2003;120:219–226. doi: 10.1016/s0306-4522(03)00267-7. [DOI] [PubMed] [Google Scholar]
- Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- Pezet S, Malcangio M, Lever IJ, Perkinton MS, Thompson SW, Williams RJ, McMahon SB. Noxious stimulation induces Trk receptor and downstream ERK phosphorylation in spinal dorsal horn. Mol Cell Neurosci. 2002;21:684–695. doi: 10.1006/mcne.2002.1205. [DOI] [PubMed] [Google Scholar]
- Pinto PR, McIntyre T, Ferrero R, Almeida A, Araujo-Soares V. Risk factors for moderate and severe persistent pain in patients undergoing total knee and hip arthroplasty: a prospective predictive study. PLoS One. 2013;8:e73917. doi: 10.1371/journal.pone.0073917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TJ, Dussor G. AMPK: an emerging target for modification of injury-induced pain plasticity. Neurosci Lett. 2013;557(Pt A):9–18. doi: 10.1016/j.neulet.2013.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TJ, Ghosh S. ZIPping to pain relief: the role (or not) of PKMzeta in chronic pain. Mol Pain. 2013;9:6. doi: 10.1186/1744-8069-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TJ, Melemedjian OK. Fragile X mental retardation protein (FMRP) and the spinal sensory system. Results Probl Cell Differ. 2012;54:41–59. doi: 10.1007/978-3-642-21649-7_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TJ, Rashid MH, Millecamps M, Sanoja R, Entrena JM, Cervero F. Decreased nociceptive sensitization in mice lacking the fragile X mental retardation protein: role of mGluR1/5 and mTOR. J Neurosci. 2007;27:13958–13967. doi: 10.1523/JNEUROSCI.4383-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 2009;32:611–618. doi: 10.1016/j.tins.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichling DB, Green PG, Levine JD. The fundamental unit of pain is the cell. Pain. 2013;154(Suppl 1):S2–S9. doi: 10.1016/j.pain.2013.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro-da-Silva A, De Koninck Y, Cuello AC, Henry JL. Enkephalin-immunoreactive nociceptive neurons in the cat spinal cord. Neuroreport. 1992;3:25–28. doi: 10.1097/00001756-199201000-00006. [DOI] [PubMed] [Google Scholar]
- Richter JD. CPEB: a life in translation. Trends Biochem Sci. 2007;32:279–285. doi: 10.1016/j.tibs.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Rivat C, Laulin JP, Corcuff JB, Celerier E, Pain L, Simonnet G. Fentanyl enhancement of carrageenan-induced long-lasting hyperalgesia in rats: prevention by the N-methyl-D-aspartate receptor antagonist ketamine. Anesthesiology. 2002;96:381–391. doi: 10.1097/00000542-200202000-00025. [DOI] [PubMed] [Google Scholar]
- Rivat C, Laboureyras E, Laulin J-P, Le Roy C, Richebé P, Simonnet G. Non-nociceptive environmental stress induces hyperalgesia, not analgesia, in pain and opioid-experienced rats. Neuropsychopharmacology. 2007;32:2217–2228. doi: 10.1038/sj.npp.1301340. [DOI] [PubMed] [Google Scholar]
- Rivat C, Bollag L, Richebe P. Mechanisms of regional anaesthesia protection against hyperalgesia and pain chronicization. Curr Opin Anaesthesiol. 2013;26(5):621–625. doi: 10.1097/01.aco.0000432511.08070.de. [DOI] [PubMed] [Google Scholar]
- Russe OQ, Moser CV, Kynast KL, King TS, Stephan H, Geisslinger G, Niederberger E. Activation of the AMP-activated protein kinase reduces inflammatory nociception. J Pain. 2013;14:1330–1340. doi: 10.1016/j.jpain.2013.05.012. [DOI] [PubMed] [Google Scholar]
- Sacktor TC. How does PKMzeta maintain long-term memory? Nat Rev Neurosci. 2011;12:9–15. doi: 10.1038/nrn2949. [DOI] [PubMed] [Google Scholar]
- Sandkuhler J. Understanding LTP in pain pathways. Mol Pain. 2007;3:9. doi: 10.1186/1744-8069-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JE, McCleskey EW. Inhibition of Ca2+ currents by a mu-opioid in a defined subset of rat sensory neurons. J Neurosci. 1993;13:867–873. doi: 10.1523/JNEUROSCI.13-02-00867.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JE, Fischbach PS, Zheng D, McCleskey EW. Activation of mu opioid receptors inhibits transient high- and low-threshold Ca2+ currents, but spares a sustained current. Neuron. 1991;6:13–20. doi: 10.1016/0896-6273(91)90117-i. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen CH, Yuan P, Perez-Lorenzo R, Zhang Y, Lee SX, Ou Y, Asara JM, Cantley LC, Zheng B. Phosphorylation of BRAF by AMPK impairs BRAF-KSR1 association and cell proliferation. Mol Cell. 2013;52:161–172. doi: 10.1016/j.molcel.2013.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si K, Lindquist S, Kandel ER. A neuronal isoform of the aplysia CPEB has prion-like properties. Cell. 2003a;115:879–891. doi: 10.1016/s0092-8674(03)01020-1. [DOI] [PubMed] [Google Scholar]
- Si K, Giustetto M, Etkin A, Hsu R, Janisiewicz AM, Miniaci MC, Kim JH, Zhu H, Kandel ER. A neuronal isoform of CPEB regulates local protein synthesis and stabilizes synapse-specific long-term facilitation in aplysia. Cell. 2003b;115:893–904. doi: 10.1016/s0092-8674(03)01021-3. [DOI] [PubMed] [Google Scholar]
- Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136:731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamcan O, Mannion AF, Eisenring C, Horisberger B, Elfering A, Muller U. The course of chronic and recurrent low back pain in the general population. Pain. 2010;150:451–457. doi: 10.1016/j.pain.2010.05.019. [DOI] [PubMed] [Google Scholar]
- Tao YX. AMPA receptor trafficking in inflammation-induced dorsal horn central sensitization. Neurosci Bull. 2012;28:111–120. doi: 10.1007/s12264-012-1204-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillu DV, Melemedjian OK, Asiedu MN, Qu N, De Felice M, Dussor G, Price TJ. Resveratrol engages AMPK to attenuate ERK and mTOR signaling in sensory neurons and inhibits incision-induced acute and chronic pain. Mol Pain. 2012;8:5. doi: 10.1186/1744-8069-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udagawa T, Swanger SA, Takeuchi K, Kim JH, Nalavadi V, Shin J, Lorenz LJ, Zukin RS, Bassell GJ, Richter JD. Bidirectional control of mRNA translation and synaptic plasticity by the cytoplasmic polyadenylation complex. Mol Cell. 2012;47:253–266. doi: 10.1016/j.molcel.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda T, Sasaki M, Elia AJ, Chio II, Hamada K, Fukunaga R, Mak TW. Combined deficiency for MAP kinase-interacting kinase 1 and 2 (Mnk1 and Mnk2) delays tumor development. Proc Natl Acad Sci U S A. 2010;107:13984–13990. doi: 10.1073/pnas.1008136107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderah TW, Suenaga NM, Ossipov MH, Malan TP, Jr, Lai J, Porreca F. Tonic descending facilitation from the rostral ventromedial medulla mediates opioid-induced abnormal pain and antinociceptive tolerance. J Neurosci. 2001;21:279–286. doi: 10.1523/JNEUROSCI.21-01-00279.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk LJ, Bachman JL, Johnson R, Yu Y, Huganir RL. PKM-zeta is not required for hippocampal synaptic plasticity, learning and memory. Nature. 2013;493:420–423. doi: 10.1038/nature11802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Korff M, Saunders K. The course of back pain in primary care. Spine (Phila Pa 1976) 1996;21:2833–2837. doi: 10.1097/00007632-199612150-00004. discussion 2838–2839. [DOI] [PubMed] [Google Scholar]
- Wang X, Flynn A, Waskiewicz AJ, Webb BL, Vries RG, Baines IA, Cooper JA, Proud CG. The phosphorylation of eukaryotic initiation factor eIF4E in response to phorbol esters, cell stresses, and cytokines is mediated by distinct MAP kinase pathways. J Biol Chem. 1998;273:9373–9377. doi: 10.1074/jbc.273.16.9373. [DOI] [PubMed] [Google Scholar]
- Wang H, Heijnen CJ, van Velthoven CT, Willemen HL, Ishikawa Y, Zhang X, Sood AK, Vroon A, Eijkelkamp N, Kavelaars A. Balancing GRK2 and EPAC1 levels prevents and relieves chronic pain. J Clin Invest. 2013;123(12):5023–5034. doi: 10.1172/JCI66241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Guo Y, Su Y, Zhang X, Luan H, Zhang X, Zhu H, He H, Wang X, Sun G, Sun X, Guo P, Zhu P. Cordycepin activates AMP-activated protein kinase (AMPK) via interaction with the gamma1 subunit. J Cell Mol Med. 2014;18(2):293–304. doi: 10.1111/jcmm.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Seereeram A, Nassar MA, Levato A, Pezet S, Hathaway G, Morenilla-Palao C, Stirling C, Fitzgerald M, McMahon SB, Rios M, Wood JN. Nociceptor-derived brain-derived neurotrophic factor regulates acute and inflammatory but not neuropathic pain. Mol Cell Neurosci. 2006;31:539–548. doi: 10.1016/j.mcn.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Zhou LJ, Zhong Y, Ren WJ, Li YY, Zhang T, Liu XG. BDNF induces late-phase LTP of C-fiber evoked field potentials in rat spinal dorsal horn. Exp Neurol. 2008;212:507–514. doi: 10.1016/j.expneurol.2008.04.034. [DOI] [PubMed] [Google Scholar]