Abstract
The incidence of breast cancer among women is high and increasing. This study investigated the inhibitory effect of an extract from bamboo Phyllostachys edulis on the development of 7,12-dimethylbenz[a]anthracene (DMBA)-induced breast cancer in female Sprague-Dawley rats. The rats were fed with bamboo extract (BEX) supplemented diet or control diet, and treated with DMBA after 3 weeks of the dietary regime. The incidence of mammary tumors was monitored by palpation for the next 11 weeks. At the end of the experiment, blood samples were collected for total antioxidant capacities (TAC) assay and liver samples for phase II enzyme activity assays. The TAC values, total contents of phenolics and flavonoids of BEX were also measured. The results showed that BEX delayed the onset of mammary tumor by 1 week, decreased the tumor incidence by 44% and tumor multiplicity by 67%, and increased the total sulfotransferases (SULT) activity by 63%. BEX showed high levels of TAC, total phenolic and total flavonoids. However, the serum TAC values were not affected by BEX supplementation. In summary, the results indicate that BEX possesses a potent anti-breast cancer effect, and the upregulation of SULT activity, therefore estrogen metabolism may be the underlying mechanism.
Keywords: breast cancer; 7,12-dimethylbenz[a]anthracene (DMBA); bamboo extract (BEX); sulfotransferases (SULT); glutathione-S-transferase (GST); UDP-glucuronosyltransferases (UGT); total antioxidant capacity (TAC); flavonoids
INTRODUCTION
In the USA, a woman’s lifetime risk of breast cancer has nearly tripled during the past four decades, rendering it the most common cancer among women in this country. Worldwide rates of breast cancer have been rising steadily since the 1940s with over a million new cases diagnosed worldwide each year (Parkin et al., 2005). 7,12-Dimethylbenz[a]anthracene (DMBA) efficiently induces breast cancer in female rats. Oral delivery of DMBA to rats, a model mimicking the dietary intake of carcinogens in humans, has been used intensively for carcinogenic studies since the 1960s (Heimann et al., 1968). The natural estrogen 17β-estradiol (E2) is also known to have a dual role in the carcinogenesis of breast cancer. It is a carcinogen that becomes genotoxic after metabolic activation (Mitrunen and Hirvonen, 2003). E2 also interacts with estrogen receptors to stimulate the proliferation of mammary epithelial cells thereby enhancing the mutagenesis of DNA damage (Katzenellenbogen, 1996). Recent cohort studies revealed strong relationships between elevated levels of circulating estrogens and the risk of breast cancer in humans (Bernstein, 1998).
When carcinogens enter a cell, phase I enzymes (cytochrome P450-dependent monooxygenases) catalyse the addition of an oxygen atom to increase their water solubility. Some of the metabolites formed at this step can covalently bind to DNA forming DNA adducts. The metabolites formed by phase I enzymes can be detoxified by phase II enzymes, which conjugate the genotoxic intermediate to endogenous polar substrates to further increase its water solubility therefore excretability. Glutathione-S-transferase (GST) constitutes a major group of phase II enzymes that use glutathione as a substrate. The expression of GST is controlled by multiple transcription factors and is highly inducible by dietary components (Pool-Zobel et al., 2005). Polymorphisms of GSTs have been found correlated with elevated carcinogen-DNA adducts in human breast tissue and the incidence and mortality of breast cancer (Rundle et al., 2000; Chang et al., 2006). UDP-glucuronosyltransferase (UGT) is another group of phase II enzymes transferring a nucleotide sugar to small, hydrophobic molecules. Several of the UGTs are integral in the conjugation and excretion of steroid hormones, e.g. UGT1A1 conjugates E2, and UGT2B15 conjugates androgens (Lampe, 2007). Polymorphisms in UGT1A1 and UGT2B15 have been associated with higher serum estradiol concentrations in post-menopausal women (Sparks et al., 2004). Sulfotransferase (SULT) is a family of phase II enzymes transferring a sulfate group from 3"-phosphoadenylylsulfate to the hydroxyl group of an acceptor. SULT metabolizes estrone, E2 and catecholestrogens (Weinshilboum et al., 1997), with SULT1E1 having the highest affinity for these substrates (Adjei and Weinshilboum, 2002). SULT1E1 is expressed in the liver and breast, but not in malignant breast cells, potentially signaling that SULT is vital for normal cellular proliferation and lack or absence of SULT may be integral to estrogen-mediated carcinogenesis (Falany and Falany, 1996).
Patients with breast cancer are under systemic oxidative stress which is indicated by the elevated levels of reactive oxygen species, DNA damage, and lipid peroxidation, and decreased total antioxidant capacities (TAC) in the blood (Tas et al., 2005). Persistent oxidative stress may be involved in early mutagenic events, as well as an increasing mutation rate, accelerating tumor progression and increasing risk of metastasis (Halliwell, 2007).
Various natural products have been tested for their inhibition of DMBA-induced breast cancer with mixed results (Singletary et al., 1996; Rogers et al., 1998; Funahashi et al., 2001; Kavanagh et al., 2001; Kim et al., 2004; Jung et al., 2006). In Asian countries different parts of bamboo have been used for medicinal purposes to treat hypertension, arteriosclerosis, cardiovascular disease and cancers. This study tested the inhibitory effects of an ethanol/water extract from bamboo Phyllostachys edulis as a dietary supplement on the development of DMBA-induced breast cancer in rats. The influences of the bamboo extract (BEX) on food consumption, body weight, incidence and multiplicity of mammary tumors, serum TAC levels and total enzymatic activities of GST, UGT and SULT in liver were investigated.
MATERIALS AND METHODS
Bamboo extract
BEX used in this study was provided by Golden Basin LLC (Hau’ula, HI). It was made from fresh leaves and small branches of bamboo Phyllostachys edulis by ethanol/water (70/30, v/v) extraction.
BEX supplemented diet
The raw bamboo extract was freeze-dried and ground to fine powder. The powder was sent to Research Diets (New Brunswick, NJ) to be incorporated into a standard rodent diet D10001 at a concentration of 5 g/kg (0.5%). D10001 was used as control diet.
Animals
Female Sprague-Dawley (SD) rats of 4-week old were purchased from Charles River Laboratories and hosted at the University of Hawaii Laboratory Animal Services facilities. The experiment procedure was approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Hawaii. The rats were hosted one per cage, with free access to food and water except when treated with DMBA (as described below). The room temperature was controlled at 20 °C and humidity at 65%. Lighting was turned on and off with 12 h intervals for day and night using a timer-driven switch.
Dietary regime
After 1 week of acclimation with regular laboratory rodent chow, nine rats were assigned to the BEX diet and ten rats were assigned to the control diet. The dietary regime was maintained throughout the rest of the experiment.
Food intake
During the first week of the special diet regime, the food intake of each rat was monitored daily.
Body weight
The body weight of each rat was monitored weekly throughout the experiment.
DMBA treatment
After 3 weeks of the special diet regime, the rats were fasted overnight, then DMBA dissolved in sesame oil was intragastrically delivered to each rat at a dosage of 75 mg/kg body weight. Rats were further restrained from food for 6 h before being returned to normal feeding.
Tumor examination
The incidence of mammary tumor was examined by palpation weekly. The diameter of tumors was measured to monitor the growth. At week 14, the rats were terminated by CO2 euthanasia.
Tissue harvesting and storage
Blood was withdrawn from the tail vein at week 12, centrifuged at 2000 × g for 10 min, serum was then aliquoted and stored in −80 °C until assay. Liver was excised after euthanasia and immediately frozen in liquid nitrogen, then transferred to −80 °C for storage until assay.
BEX solution
Freeze-dried BEX powder was mixed with ethanol at a ratio of 100 mg/mL and agitated at ambient temperature for 3 h, and then centrifuged at 10 000 × g for 10 min. The supernatant was used for the ABTS, FRAP and total phenolics/flavonoids assays.
FRAP assay
The Fe3+-reducing ability was measured by the ferric-reducing ability of plasma (FRAP) procedure with modification (Benzie and Strain, 1996). The reagent contained 0.83 mM TPTZ (2, 4, 6-tri-pyridyl-s-triazine) and 1.67 mM ferric chloride in 0.1 M acetate buffer (pH 3.6). A suitable amount of BEX solution or serum was mixed with 400 μL of reagent and incubated at room temperature for 10 min. Reduction of Fe3+-TPTZ to Fe2+-TPTZ was monitored by the absorbance increase at 595 nm by spectrophotometry. Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) was used as a standard. The results are expressed as the Trolox equivalent antioxidant capacity (TEAC).
ABTS assay
The 2,2"-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) radical scavenging ability was measured by the ABTS method with modification (Pellegrini et al., 1999). To prepare the ABTS radical, an ABTS solution was oxidized in water by potassium persulfate (molar ratio = 1:0.35) for 12–16 h in the dark, and then diluted in PBS (pH 7.4) prior to assay, giving an absorbance of 0.70 ± 0.02 at 730 nm. A suitable amount of BEX solution or serum was added to 400 μL of reagent, followed by incubation at room temperature for 10 min. Scavenging of the ABTS radical was then monitored by the absorbance decrease at 730 nm. Trolox was used as the standard and the results were expressed as TEAC.
Total content of phenolics
This was determined using the Folin-Ciocalteu assay with modification (Marinova et al., 2005). Briefly, a sample volume (in proper dilution) of 48 μL was mixed with 432 μL water, and 48 μL Folin-Ciocalteu reagent and incubated at room temperature for 5 min. Then 480 μL Na2CO3 (7%) and 192 μL water was added and the final mixture was incubated at room temperature for 90 min. The absorbance at 750 nm was measured using a spectrophotometer. Gallic acid was used as the standard.
Total content of flavonoids
This was analysed using the aluminum chloride colorimetric assay with modification (Marinova et al., 2005). Briefly, a sample volume (in proper dilution) of 120 μL was mixed with 480 μL water and 36 μL NaNO2 (5%) and incubated at room temperature for 5 min. Then 36 μL AlCl3 (10%) was added and the mixture was incubated for another 5 min. Finally, 240 μL NaOH (1 M) and 288 μL water was added and the absorbance at 510 nm was measured using a spectrophotometer. Catechin was used as the standard.
Preparation of subcellular fractions
Microsomes and cytosol were prepared from rat liver by differential centrifugation. Briefly, pieces of rat liver were retrieved from the −80 °C freezer, thawed and weighed. Livers were placed in a glass tissue grinder and ground manually with 1:4 (w/v) Tris-HCl buffer containing 5 mM MgCl2, pH 7.4. The whole liver homogenate was centrifuged at 20 000 × g for 20 min then the supernatant was transferred to a clean tube. Supernatants were centrifuged at 100 000 × g for 1 h. Subsequently the second supernatant (cytosolic fraction) was aliquoted and frozen at −80 °C until use in the GST and SULT assays. The pellet (microsomes) was re-suspended in 200 μL Tris-HCl with 5 mM MgCl2 (pH 7.4) by pipette mixing then aliquoted and frozen at −80 °C until use in UGT assays.
Determination of protein content
Protein content was measured with the bicinchoninic acid method using BSA as the protein standard (Smith et al., 1985).
Total GST activity assay
The assay for total glutathione-S-transferase (GST) activity was carried out using rat liver cytosol (0.5 mg/mL) and 100 μM 1-chloro-2,4-dinitrobenzene (CDNB) substrate as described previously (Gonzalez et al., 1989). Absorbance units (AU) were converted to concentrations using the extinction coefficient % = 9.4 mM/cm (Habig et al., 1974).
Total UGT activity assay
The assay for UGT activity was performed with rat liver microsomes (2 mg/mL) and 100 μM 4-methylumbelliferone (4MU) substrate. The method was carried out as described previously (Collier et al., 2000), except that alamethicin (20 μg/mL) was used as an activator. Fluorescence units (FU) were converted to concentrations by comparison with a standard curve of 4MU (0–200 μM). The average r2 and slopes of the standard curves were 0.993 ± 0.001 and 467 ± 73.6 FU/μM respectively (n = 3). The intra- and inter-assay CVs for the slopes of the standard curves were 3.8% and 15.7%, respectively. The limits of sensitivity and quantitation were the same at 0.1 μM (p < 0.001, t-test vs background, CV 4.8%).
Total SULT activity assay
The assay for total sulfotransferase (SULT) activity was carried out using rat liver cytosol (2 mg/mL) and 400 μM 4-nitrophenol (4NP) substrate as described previously (Mulder and Van Doom, 1975; Tabrett and Coughtrie, 2003). Absorbance units (AU) were converted to concentrations by comparison with a standard curve of 4NP (0– 600 μM). The average r2 and slopes of the standard curves were 0.9994 ± 3 × 10−4 and 4.04 ± 1 × 10−4 mAU/μM respectively (n = 3) with intra- and inter-assay CVs of 0.86% and 2.6%, respectively, for the slopes. The limits of sensitivity and quantitation of the assay were the same at 4 μM (p = 0.0435, t-test vs background, CV 3%).
RESULTS
Food intake and body weight
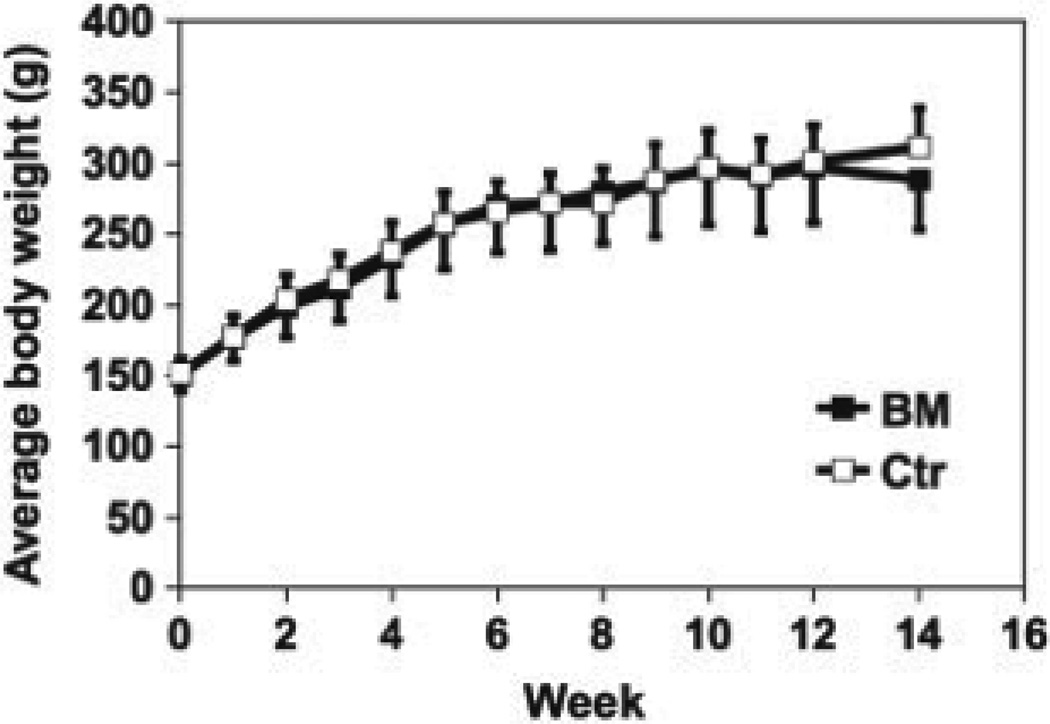
During the first week of the special diet regime, the average food intake in the BEX group was 16.6 ± 1.5 g/rat/day, and that of the control group was 16.9 ± 1.0 g. No significant difference was observed between the two groups, indicating that the content of BEX in the diet did not affect the appetite of the rats. The record of body weight of each group during the 14-week experiment is summarized in Fig. 1, showing that BEX supplementation did not affect the growth rate of the rats.
Figure 1.
Body weight of rats on BEX and control diets during the 14-week experiment. Average and SD are shown. n = 9 for BEX; n = 10 for control.
Tumor incidence
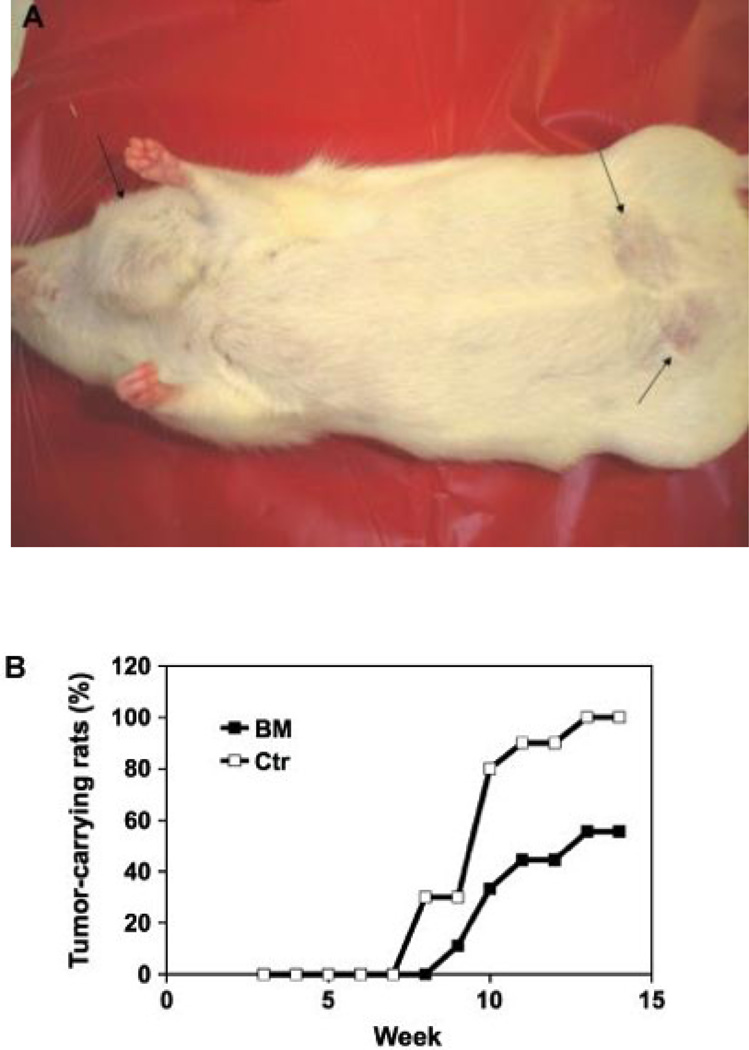
Five weeks after DMBA treatment, three rats in the control group had tumors (3, 8 and 11 mm in diameter). One week later, the first tumor (1.5 mm in diameter) appeared in the BEX group. Figure 2A shows the appearance of mammary tumors in a rat, and Fig. 2B shows that the BEX diet delayed the tumor onset by 1 week and decreased the tumor incidence by 44% at week 14.
Figure 2.
Mammary tumor induced by DMBA in rats. (A) The appearance of mammary tumors in a live rat on control diet (the tumors are shown by black arrows); and (B) the tumor incidence of rats was inhibited by BEX (n = 9 for BEX, n = 10 for control).
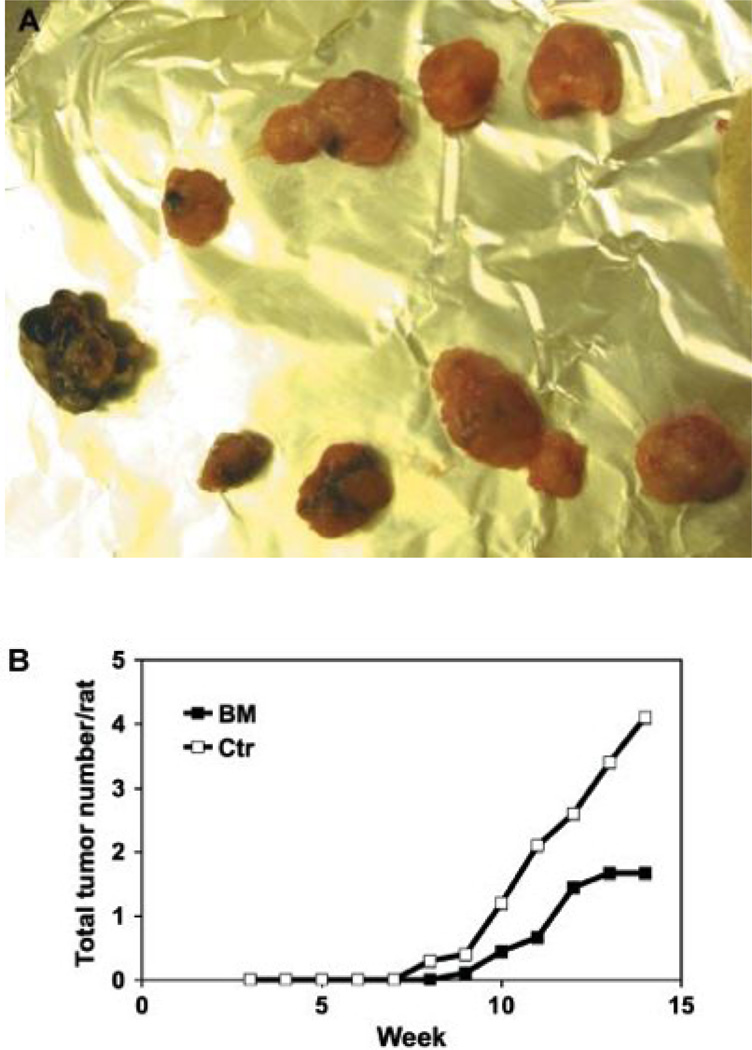
Tumor multiplicity
The number of tumors in each rat was recoded weekly. Figure 3A shows that as many as 11 tumors could occur in a rat in the control group, and Fig. 3B shows the dramatic inhibitory effect of BEX on tumor multiplication.
Figure 3.
The multiplicity of mammary tumors in rats. (A) Multiple tumors often occurred in rats on control diet; and (B) a comparison of tumor multiplication in rats on BEX (n = 9) or control (n = 10) diets.
TAC levels in serum
Blood samples were collected from the rats at week 12 and TAC values were measured in sera. The ABTS assay showed 0.97 ± 0.10 mM TEAC for the BEX group and 1.04 ± 0.23 for the control group, while the FRAP assay showed 0.33 ± 0.085 mM TEAC for the BEX and 0.37 ± 0.093 for the control. No significant difference was detected between the two groups.
Phase II enzyme activities in liver
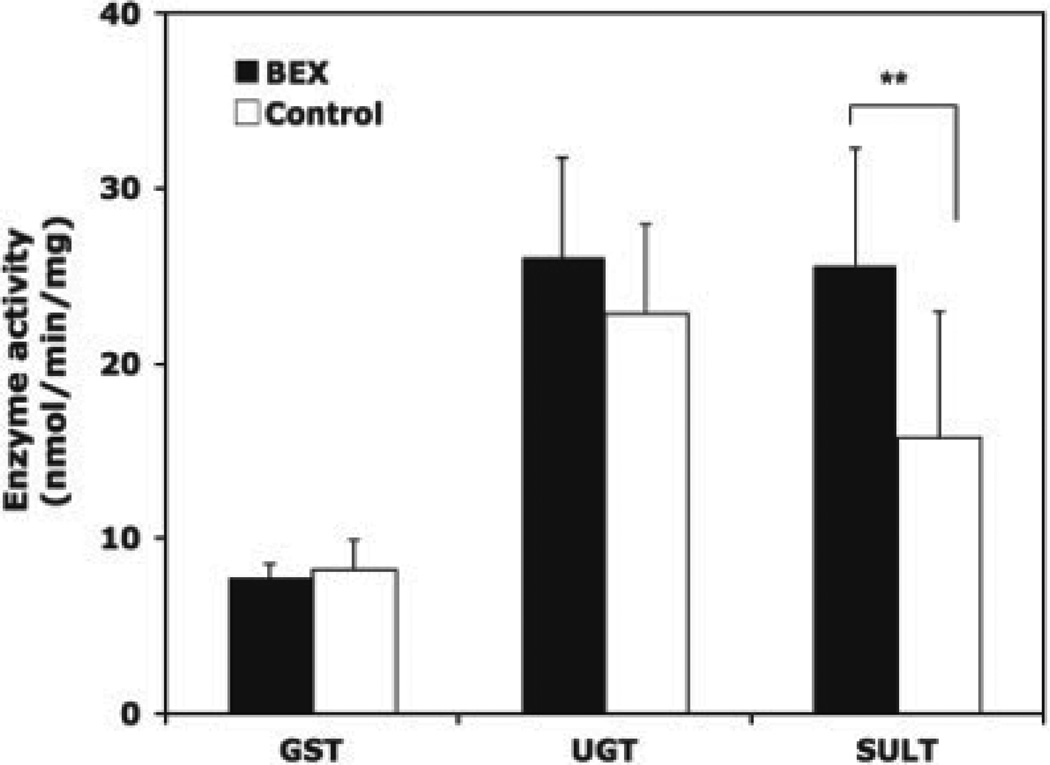
The liver samples were collected at week 14 after animal euthanasia, and the activities of three groups of phase II enzymes were analysed. In units of nmol/min/mg protein, the total GST activities were 7.78 ± 0.77 for the BEX group and 8.20 ± 1.69 for the control group; the total UGT activities were 26.07 ± 5.69 for the BEX and 22.81 ± 5.13 for the control; and the total SULT activities were 25.62 ± 6.62 for the BEX and 15.75 ± 7.23 for the control. BEX significantly upregulated the total SULT activity by 63% (p = 0.011), but had no significant influence on GST and UGT. This result is summarized in Fig. 4.
Figure 4.
GST, UGT and SULT activities in rat livers. The rats were fed BEX or control diet for 14 weeks and treated with DMBA for 11 weeks. Average and SD are shown, n = 9 for BEX, n = 10 for control.
TAC, total phenolics and total flavonoids in BEX
BEX showed high levels of TAC. At a concentration of 20 g dry mass per litter, the ABTS and FRAP assays revealed TAC values of 8 and 1.7 mM TEAC, respectively. The total content of phenolics in the BEX solution was 5.64 g of gallic acid equivalent per liter, and that of flavonoids was 1.96 g of catechin equivalent per liter.
DISCUSSION
BEX is characterized by its high TAC values and high contents of phenolics and flavonoids. Phenolics are plant metabolites widely spread throughout the plant kingdom. Flavonoids are an important class of phenolics. Epidemiological studies carried out in recent years have shown an inverse relationship between the risk of breast cancer and the dietary intake of certain forms of flavonoids (Peterson et al., 2003). Flavonoids are strong antioxidants in vitro but unlikely in vivo because the absorption of flavonoids is low, and flavonoids are extensively metabolized in the intestine and liver (Manach et al., 2005). Chronic or long-term consumption of flavonoid-rich foods does not result in the accumulation of significant amounts of flavonoids in plasma (Moon et al., 2000). This agrees with our observation that consumption of the BEX diet did not affect the TAC value of serum in the rats.
Flavonoids are recognized by the body as xenobiotic compounds, and have been reported to induce phase II enzymes GST and UGT (Kong et al., 2001; Walle and Walle, 2002). However, in this study the total activities of these two enzymes were not affected by BEX supplementation. Since GST is a key part of the antioxidant cascade this may indicate that the primary mechanism of protection by BEX is not through antioxidant mechanisms. In contrast, the observation that SULT enzymes were induced by up to 63% in the livers of BEX treated animals is an interesting result and may imply that the mechanism of BEX protection involves altered balance of estrogen metabolism. SULT is one of the main enzyme families responsible for metabolizing steroids and terminates their actions within the cell allowing them to be cleared and excreted. Our observation of a greater SULT enzyme activity in BEX treated rat livers implies that BEX shifts the balance of estrogen metabolism towards greater clearance and removal of estrogen. Since excess estrogen is a hallmark of hormonal cancers, including breast cancer; this provides evidence that the mechanism by which BEX may be protective against cancer is through diminishing excess tissue estrogen.
In summary, this study demonstrated a potent protective effect of BEX on the development of DMBAinduced breast cancer in rats, and the regulatory effect of BEX on SULT activity, indicating that increased estrogen metabolism may be the underlying mechanism of the antibreast cancer function of BEX.
REFERENCES
- Adjei AA, Weinshilboum RM. Catecholestrogen sulfation: possible role in carcinogenesis. Biochem Biophys Res Commun. 2002;292:402–408. doi: 10.1006/bbrc.2002.6658. [DOI] [PubMed] [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bernstein L. The epidemiology of breast cancer. LOWAC J. 1998;1:7–13. [Google Scholar]
- Chang TW, Wang SM, Guo YL, Tsai PC, Huang CJ, Huang W. Glutathione S-transferase polymorphisms associated with risk of breast cancer in southern Taiwan. Breast. 2006;15:754–761. doi: 10.1016/j.breast.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Collier AC, Tingle MD, Keelan JA, Paxton JW, Mitchell MD. A highly sensitive fluorescent microplate method for the determination of UDP-glucuronosyl transferase activity in tissues and placental cell lines. Drug Metab Dispos. 2000;28:1184–1186. [PubMed] [Google Scholar]
- Falany JL, Falany CN. Expression of cytosolic sulfotransferases in normal mammary epithelial cells and breast cancer cell lines. Cancer Res. 1996;56:1551–1555. [PubMed] [Google Scholar]
- Funahashi H, Imai T, Mase T, et al. Seaweed prevents breast cancer? Jpn J Cancer Res. 2001;92:483–487. doi: 10.1111/j.1349-7006.2001.tb01119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez P, Tunon MJ, Manrique V, Garcia-Pardo LA, Gonzalez J. Changes in hepatic cytosolic glutathione-S-transferase enzymes induced by clotrimazole treatment in rats. Clin Exp Pharmacol Physiol. 1989;16:867–871. doi: 10.1111/j.1440-1681.1989.tb01526.x. [DOI] [PubMed] [Google Scholar]
- Habig WH, Pabst MJ, Jakoby WB. Glutathione-Stransferases. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- Halliwell B. Oxidative stress and cancer: have we moved forward? Biochem J. 2007;401:1–11. doi: 10.1042/BJ20061131. [DOI] [PubMed] [Google Scholar]
- Heimann R, Heuson JC, Coune A. Tumors developing in oophorectomized Sprague-Dawley rats after a single gastric instillation of 7,12-dimethylbenz(a)anthracene. Cancer Res. 1968;28:309–313. [PubMed] [Google Scholar]
- Jung KJ, Wallig MA, Singletary KW. Purple grape juice inhibits 7,12-dimethylbenz[a]anthracene (DMBA)-induced rat mammary tumorigenesis and in vivo DMBA-DNA adduct formation. Cancer Lett. 2006;233:279–288. doi: 10.1016/j.canlet.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen BS. Estrogen receptors: bioactivity and interactions with cell signaling pathways. Biol Reprod. 1996;54:287–293. doi: 10.1095/biolreprod54.2.287. [DOI] [PubMed] [Google Scholar]
- Kavanagh KT, Hafer LJ, Kim DW, et al. Green tea extracts decrease carcinogen-induced mammary tumor burden in rats and rate of breast cancer cell proliferation in culture. J Cell Biochem. 2001;82:387–398. doi: 10.1002/jcb.1164. [DOI] [PubMed] [Google Scholar]
- Kim H, Hall P, Smith M, et al. Chemoprevention by grape seed extract and genistein in carcinogen-induced mammary cancer in rats is diet dependent. J Nutr. 2004;134:3445S–3452S. doi: 10.1093/jn/134.12.3445S. [DOI] [PubMed] [Google Scholar]
- Kong AN, Owuor E, Yu R, et al. Induction of xenobiotic enzymes by the MAP kinase pathway and the antioxidant or electrophile response element (ARE/EpRE) Drug Metab Rev. 2001;33:255–271. doi: 10.1081/dmr-120000652. [DOI] [PubMed] [Google Scholar]
- Lampe JW. Diet, genetic polymorphisms, detoxification, and health risks. Altern Ther Health Med. 2007;13:S108–S111. [PubMed] [Google Scholar]
- Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans: I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- Marinova D, Ribarova F, Atanassova M. Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J Univ Chem Technol Metall. 2005;40:255–260. [Google Scholar]
- Mitrunen K, Hirvonen A. Molecular epidemiology of sporadic breast cancer. The role of polymorphic genes involved in oestrogen biosynthesis and metabolism. Mutat Res. 2003;544:9–41. doi: 10.1016/s1383-5742(03)00016-4. [DOI] [PubMed] [Google Scholar]
- Moon JH, Nakata R, Oshima S, Inakuma T, Terao J. Accumulation of quercetin conjugates in blood plasma after the short-term ingestion of onion by women. Am J Physiol Regul Integr Comp Physiol. 2000;279:R461–R467. doi: 10.1152/ajpregu.2000.279.2.R461. [DOI] [PubMed] [Google Scholar]
- Mulder GJ, Van Doorn ABD. A rapid NAD+-linked assay for microsomal uridine diphosphate glucuronosyl transferase of rat liver and some observations on substrate specificity of the enzyme. Biochem J. 1975;151:131–140. doi: 10.1042/bj1510131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Pellegrini N, Re R, Yang M, Rice-Evans C. Screening of dietary carotenoids and carotenoid-rich fruit extracts for antioxidant activities applying 2,2"-azinobis (3-ethylenebenzothiazoline-6-sulfonic acid) radical cation decoloration assay. Meth Enzymol. 1999;299:379–389. [Google Scholar]
- Peterson J, Lagiou P, Samoli E, et al. Flavonoid intake and breast cancer risk: a case–control study in Greece. Br J Cancer. 2003;89:1255–1259. doi: 10.1038/sj.bjc.6601271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pool-Zobel B, Veeriah S, Bohmer FD. Modulation of xenobiotic metabolising enzymes by anticarcinogens –focus on glutathione S-transferases and their role as targets of dietary chemoprevention in colorectal carcinogenesis. Mutat Res. 2005;591:74–92. doi: 10.1016/j.mrfmmm.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Rogers AE, Hafer LJ, Iskander YS, Yang S. Black tea and mammary gland carcinogenesis by 7,12-dimethylbenz[a]anthracene in rats fed control or high fat diets. Carcinogenesis. 1998;19:1269–1273. doi: 10.1093/carcin/19.7.1269. [DOI] [PubMed] [Google Scholar]
- Rundle A, Tang D, Zhou J, Cho S, Perera F. The association between glutathione S-transferase M1 genotype and polycyclic aromatic hydrocarbon-DNA adducts in breast tissue. Cancer Epidemiol Biomarkers Prev. 2000;9:1079–1085. [PubMed] [Google Scholar]
- Singletary K, MacDonald C, Wallig M. Inhibition by rosemary and carnosol of 7,12-dimethylbenz[a]anthracene (DMBA)-induced rat mammary tumorigenesis and in vivo DMBADNA adduct formation. Cancer Lett. 1996;104:43–48. doi: 10.1016/0304-3835(96)04227-9. [DOI] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Sparks R, Ulrich CM, Bigler J, et al. UDP-glucuronosyltransferase and sulfotransferase polymorphisms, sex hormone concentrations, and tumor receptor status in breast cancer patients. Breast Cancer Res. 2004;6:R488–R498. doi: 10.1186/bcr818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabrett CA, Coughtrie MW. Phenol sulfotransferase 1A1 activity in human liver: kinetic properties, interindividual variation and re-evaluation of the suitability of 4-nitrophenol as a probe substrate. Biochem Pharmacol. 2003;66:2089–2097. doi: 10.1016/s0006-2952(03)00582-3. [DOI] [PubMed] [Google Scholar]
- Tas F, Hansel H, Belce A, et al. Oxidative stress in breast cancer. Med Oncol. 2005;22:11–15. doi: 10.1385/MO:22:1:011. [DOI] [PubMed] [Google Scholar]
- Walle UK, Walle T. Induction of human UDP-glucuronosyltransferase UGT1A1 by flavonoids – structural requirements. Drug Metab Dispos. 2002;30:564–569. doi: 10.1124/dmd.30.5.564. [DOI] [PubMed] [Google Scholar]
- Weinshilboum RM, Otterness DM, Aksoy IA, Wood TC, Her C, Raftogianis RB. Sulfation and sulfotransferases 1: sulfotransferase molecular biology: cDNAs and genes. FASEB J. 1997;11:3–14. [PubMed] [Google Scholar]