Abstract
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system and is associated with demyelination, neurodegeneration, and sensitivity to oxidative stress. In this work, we administered a nanodroplet formulation of pomegranate seed oil (PSO), denominated Nano-PSO, to mice induced for experimental autoimmune encephalomyelitis (EAE), an established model of MS. PSO comprises high levels of punicic acid, a unique polyunsaturated fatty acid considered as one of the strongest natural antioxidants. We show here that while EAE-induced mice treated with natural PSO presented some reduction in disease burden, this beneficial effect increased significantly when EAE mice were treated with Nano-PSO of specific size nanodroplets at much lower concentrations of the oil. Pathological examinations revealed that Nano-PSO administration dramatically reduced demyelination and oxidation of lipids in the brains of the affected animals, which are hallmarks of this severe neurological disease. We propose that novel formulations of natural antioxidants such as Nano-PSO may be considered for the treatment of patients suffering from demyelinating diseases. On the mechanistic side, our results demonstrate that lipid oxidation may be a seminal feature in both demyelination and neurodegeneration.
Keywords: nanodrops, PSO, EAE, oxidative stress, neurodegeneration
Introduction
Sensitivity to oxidative stress is a common pathological feature in all neurodegenerative diseases.1 In fact, most of the key disease proteins forming aggregates in such conditions are oxidized,2,3 and in prion diseases, oxidation of Met residues in PrP Helix3 precedes its acquisition of protease resistance.4 Also brain lipids are oxidized in these and other brain diseases,5,6 suggesting that lipid oxidation may play an important role in the pathogenesis of neurodegenerative diseases.7–9 Oxidized phospholipids generate compounds, such as 4-oxo-2-nonenal and acrolein, which are predominantly toxic to brain cells.10 Recent evidence suggests that, in addition to autoimmune pathways, the neurological damage in demyelinating diseases such as multiple sclerosis (MS)11 may also be of neurodegenerative nature.12–14 Indeed, oxidation of brain proteins was shown to occur both in MS and in experimental autoimmune encephalomyelitis (EAE),15 a well-studied animal model of MS. Most importantly, lipids in MS plaques are oxidized, as demonstrated by immunostaining with an antibody against oxidized phospholipids.6,16
We have recently shown that administration of a nanodroplet formulation of pomegranate seed oil (PSO), denominated Nano-PSO, can significantly reduce the oxidation of lipids in a transgenic mouse model of genetic prion disease.17 In the TgMHu2ME199K mice, reduction in lipid oxidation, as well as reduced neuronal death, occurred concomitantly with a significant delay in disease onset, constituting a proof of concept that an advanced formulation of a natural antioxidant may fight neurodegeneration. No apparent side effects were observed in the time frame (6–8 months) and doses of Nano-PSO administered to the mice in these experiments.
PSO comprises a unique component named punicic acid (PA), a polyunsaturated fatty acid also considered as one of the strongest natural antioxidants.18 The oil-in-water (O/W) nanoemulsion of PSO denominated as Nano-PSO19 may increase the bioability and activity of PSO. This approach, as is the case for delivery systems, such as phospholipid micelles or nanodroplets,20–22 may allow the distribution of the oil components to organs other than the liver, thereby enabling a longer circulation that may increase the levels of PA available to pass the blood–brain barrier (BBB). Indeed, similar unsaturated fatty acids, such as linoleic acid, were shown to readily cross the BBB.23–25 PA is present only in PSO (60%–80%) and Trichosanthes kirilowii (40%)26 and was effective in protecting tissue lipid profiles in inflammatory disease models.27 PSO’s lack of toxicity and partial bioavailability was already established in humans.26 An additional antioxidant, β-sitosterol, which was demonstrated to accumulate in the plasma membrane of brain cells,28 is present in PSO at significantly higher concentrations as compared to oils from other plants,29 indicating PSO may constitute a natural compound with stronger antioxidant activities than its individual components.
In this work, we describe the clinical and pathological effects of Nano-PSO in the treatment of EAE-induced mice. We show that while administration of large doses of PSO in food can reduce disease burden in the EAE mice, Nano-PSO exerts a wider effect at a much lower dose. We also established an optimal size for the activity of PSO lipid droplets in this clinical model. Concomitant with decreased disease burden, pathological examination of brain sections from Nano-PSO-treated mice also revealed reduced demyelination and almost eradication of brain lipid oxidation. Our results therefore reinforce the notion that lipid oxidation is an important factor in demyelinating diseases and show that advanced formulations of natural antioxidants may be both safe and efficient in the long-term treatment of diseases such as MS.
Materials and methods
Animal experiments
All animal experiments were conducted under the guidelines and supervision of the Hebrew University Ethical Committee, which approved the methods employed in this project (Permit Number: MD-13-13772-5).
Induction of EAE
Induction of myelin oligodendrocyte glycoprotein (MOG) EAE was done as previously described.30,31 Shortly, 6- to 8-week-old female C57BL/6 mice were immunized with an emulsion containing 200 μg of MOG35–55 (70% purified; synthesized at Hebrew University, Jerusalem, Israel) in saline and an equal volume of complete Freund’s adjuvant containing 5 mg/mL H37RA (Difco Laboratories, Detroit, MI, USA). The inoculum (0.2 mL) was injected subcutaneously into right and left flanks. One hundred nanograms of pertussis toxin (List Biological Labs, Campbell, CA, USA) in 0.1 mL saline was also injected intraperitoneally on day 0 and 48 hours later.
EAE scoring system
Mice were observed daily for the appearance of neurological symptoms, which were scored as follows: 0, asymptomatic; 1, partial loss of tail tonicity; 1.5, limp tail; 2, hind limb weakness (right reflex); 3, ataxia; 4, early paralysis; 5, full paralysis; and 6, moribund or dead.
Preparation of PSO-enriched food
One kilogram of mouse-pelleted food (Harlan, Teklad) was dissolved in water and subsequently supplemented with 25 or 75 mL of PSO (Flavex, Rehlingen, Germany). The mixture was next reassembled and dehydrated as pellets.
Preparation of O/W pomegranate oil nanoemulsion by sonication
A nanoemulsion with 10.8% oil fraction was prepared as follows: 1.56 g of pomegranate oil, 0.65 g of Tween 80, and 0.39 g of glyceryl monooleate were mixed by a magnetic stirrer for 20 minutes. Approximately 2.5 g of the aforementioned mixture was mixed with 0.277 g of glycerol using a magnetic stirrer for 15 minutes. Then, 2 g of the glycerol mixture was added drop wise to 8 g of deionized water. A crude white emulsion was obtained. At the second stage, this crude emulsion was sonicated using a horn sonicator (model Vibra-Cell; Sonics & Materials, Inc., USA) for 10 minutes at 750 W. The samples were cooled in an ice water bath during the sonication process. A bluish emulsion was obtained.
Preparation of 30 nm self-emulsifying nanodroplets
A mixture comprising 26.3% PSO, 31.57% Tween 80, and 42.13% Cremophor RH 40 was mixed using a magnetic stirrer for 20 minutes. Then, 10 μL of the mixture was added to 3 mL of deionized water. After vortexing for 30–60 seconds, an emulsion with nanosized droplets was obtained. The size of the O/W nanoemulsion droplets was 101 nm; Z average, peak 1: 30 nm, 92.5%; peak 2: 334 nm, 7.1%; peak 3: 4,796 nm, 0.4%; and polydispersity index (Pdi): 0.403.
Thiobarbituric acid reactive substances measurement
Lipid peroxidation was evaluated by measuring levels of malonaldehyde (MDA). A total of 0.15 g of brain tissue was homogenized in 1.35 mL lysis buffer containing 1% deionized Triton X-100 in 25 mM Tris–HCl pH 7.5, 150 mM NaCl, and 5 mM ethylenediaminetetraacetic acid and centrifuged at 3,000 rpm for 15 minutes at 4°C. Then, 100 μL of the supernatant was added to 50 μL of 8.1% sodium dodecyl sulfate, 20 μL of 20% acetic acid pH 3.5, 10 μL of 1.33% thiobarbituric acid, and 120 μL deionized distilled water and incubated for an hour at 95°C. After cooling, 300 μL of n-butanol:pyridine (15:1) was added, and the mixture was centrifuged at 10,000 rpm for 5 minutes at room temperature. Absorbance of the organic phase was measured using spectrophotometer at λ=532 nm. The amount of thiobarbituric acid reactive substances (TBARS) was determined according to a standard calibration curve generated from MDA.
Cryo-TEM
The nanodroplets were imaged using a transmission electron microscope Tecnai G2Spirit Twin T-12. The microscope was equipped with an FEI 4k Eagle CCD camera. Sample preparation was done using Vitrobot Mark IV (FEI).
Dynamic light scattering
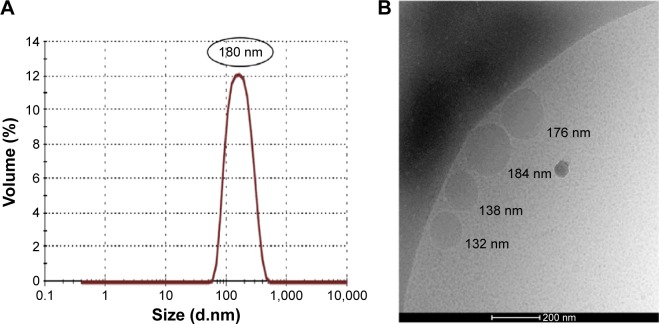
Size measurements of the droplets were performed with a Zetasizer Nano S (Malvern Instruments, Malvern, UK) in triplicates after dilution of the emulsion in water. The size of the coarse white emulsion droplets was in the range of few microns. The average size of the droplets of the O/W nanoemulsion used in these experiments was 30 and 180 nm.
Pathological examinations
Histological evaluations were performed on paraffin- embedded sections of brain samples. Sections were stained with Luxol fast blue/periodic acid Schiff (EMD Millipore, Billerica, MA, USA) to assess demyelination. Paraffin-embedded sections of brains were used for immunohistochemistry with EO6 an antibody against oxidized lipids. Consecutive section was stained with hematoxylin and eosin to recognize infiltrates.
Materials
Most chemicals were from Sigma-Aldrich Co. (St Louis, MO, USA). PSO was from Flavex, and EO6 was from Avanti Polar Lipids, Alabaster, AL, USA.
Statistical studies
Analyses of EAE score graphs were performed with the Microsoft Excel software (2010). Graphs represent the mean and respective standard error of clinical scores of groups of mice. The differences between experimental groups were assessed by one-way analysis of variance followed by the paired two-tailed Student’s t-test. Also the statistical analysis of TBARS experiments was done in the same way.
Quantification of pathology immunostaining was performed by measuring the stain-positive area in different fields at a magnification ×40. Stained pixels were measured using image pro analyzer 3D software, Media Cybernetics.
Results
Low levels of Nano-PSO significantly reduced disease burden in EAE-induced mice
Nano-PSO droplets were prepared as described in the “Materials and methods” section and characterized by dynamic light scattering measurement of droplet size as well as by Cryo-TEM imaging (Figure 1A and B). Unless stipulated otherwise, mice suffering from EAE were treated with Nano-PSO droplets with an average size of 180 nm.
Figure 1.
Droplet size analysis.
Notes: (A) DLS results for Nano-PSO and (B) cryo-TEM image of Nano-PSO.
Abbreviations: DLS, dynamic light scattering; PSO, pomegranate seed oil; cryo-TEM, cryogenic transmission electron microscope.
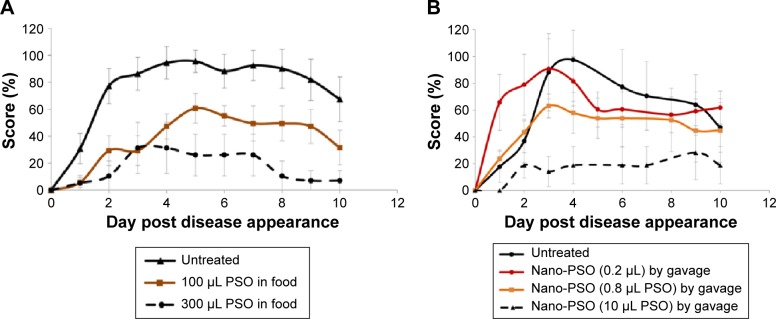
To induce EAE, groups of C57Bl naïve female mice were immunized with an emulsion comprising MOG35–55 peptide and complete Freund’s adjuvant, resulting in an acute neurological paralytic disease followed by partial remission.32,33 Following the induction, mice were scored daily for disease signs as described in the “Materials and methods” section. Figure 2A shows results from an experiment in which EAE-induced mice were fed from the day of induction either with normal chow (untreated) or with chow to which increasing concentrations of PSO (see levels of oil in graph legend) were added per 3 g of chow, which is the average daily consumption of food by each mouse. The figure shows that daily doses of 100–300 μL of PSO were effective in reducing disease burden in the EAE mice (60% of the highest score in the EAE untreated mice for the 100 μL PSO dose and 36% for the 300 μL dose; P<0.05 for PSO-treated groups versus untreated groups). Lower levels of PSO were not effective against the disease. While this constitutes a proof of principle that high levels of PSO can have an anti-EAE effect, such levels of oil, in particular when converted to human doses (4–12 mL of oil/d), are difficult to consume on a daily basis.
Figure 2.
Nano-PSO as an α-EAE agent.
Notes: Mice were induced for EAE and treated from day 1 of the induction either with PSO or with Nano-PSO. (A) Designated EAE-induced groups were fed either with normal mouse chow (untreated group; n=10) or with chow enriched with PSO at the concentration in which 3 g (daily intake) comprises the levels designated in the figure insert: 100 (n=10) or 300 μL (n=8) PSO. P<0.05 for all PSO-treated groups versus the untreated group. (B) Designated EAE-induced groups were either left untreated or treated (by gavage) with 150 μL solution comprising 0.2, 0.8 (n=6), or 10 μL (n=7) PSO in the form of Nano-PSO. P<0.05 for 0.8 and 10 μL PSO-treated group versus the untreated group.
Abbreviations: PSO, pomegranate seed oil; EAE, experimental autoimmune encephalomyelitis.
To overcome this limitation, we next tested the α-EAE activity of Nano-PSO at different concentrations. As opposed to the continuous consumption of PSO in food, Nano-PSO was administered once a day by gavage (diluted into 150 μL of water). Figure 2B shows that a 10% dose of the lowest active concentration of PSO in food mostly abolished disease presentation when administered as Nano-PSO (dashed line) (P<0.05). Moreover, even lower levels of Nano-PSO (0.8 μL/d) were effective against EAE. This indicates that Nano-PSO is a much more effective formulation than the natural oil in the treatment of EAE, as was the case for the delay of disease onset of genetic CJD in a transgenic model.17
Anti-EAE activity of Nano-PSO components
As stated in the “Introduction” section, the main component of PSO is PA, a 18:3 polyunsaturated fatty acid. This is also the main or only compound that differentiates PSO from other vegetable oils,29 in particular soybean oil. To test whether PA is indeed the Nano-PSO component that exerts most of the α-EAE effect, EAE-induced mice were treated from the day of the induction with a comparable Nano-Soya formulation, as well as with a mixture of the emulsifying agents. Figure 3 shows that only Nano-PSO (2 μL PSO/d) exerted a beneficial clinical effect on the EAE-induced mice (P<0.05), while groups treated with Nano-Soya or the surfactants alone behave similarly in disease pattern and score to the untreated EAE mice.
Figure 3.
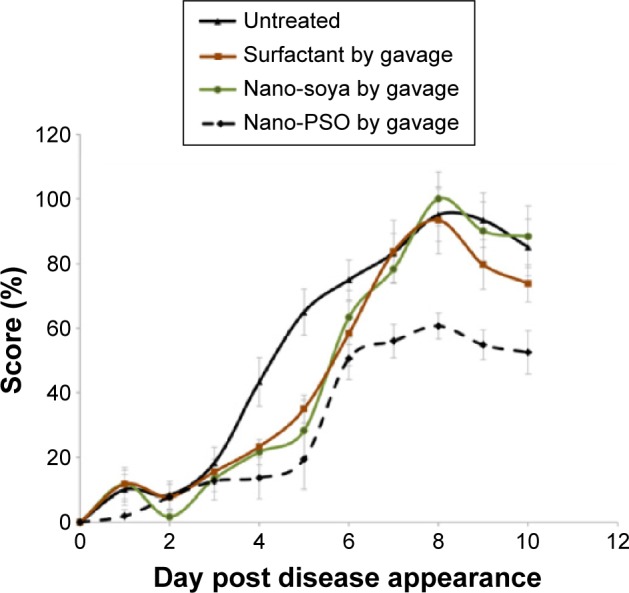
Individual α-EAE activity of Nano-PSO ingredients.
Notes: Mice were induced for EAE and treated from day 1 of induction (by gavage) with the reagents described in the insert of the figure (n=7 for each of the groups). Nano-PSO was administrated at a dose of 2 μL PSO per 150 μL solution. Mice were scored daily for EAE signs for 2 additional weeks. P<0.05 for the results in the Nano-PSO group versus all others.
Abbreviations: PSO, pomegranate seed oil; EAE, experimental autoimmune encephalomyelitis.
Treatment as compared to prevention in EAE-induced mice
Next, we tested whether Nano-PSO can reduce neurological damage already inflicted by EAE induction, as opposed to prevent/delay EAE onset. To this effect, we compared the clinical effect of Nano-PSO administration from the day of induction (10 μL PSO/d) to that of the same dose administered from day 7 postinduction (graph results from day 10 postinduction). It is well established that at this time point of EAE induction, activated immune cells are already formed and infiltrated into the central nervous system (CNS).34 Figure 4 shows that Nano-PSO administration exerts a beneficial effect at both time points (P<0.05); however, while the early treatment shows both delay in disease onset and reduced disease burden, the latter treatment only shows reduced scores but a similar kinetics of disease presentation as the untreated mice. This is a very encouraging result, indicating Nano-PSO could be beneficial to humans already suffering from early signs of demyelinating diseases such as MS.
Figure 4.
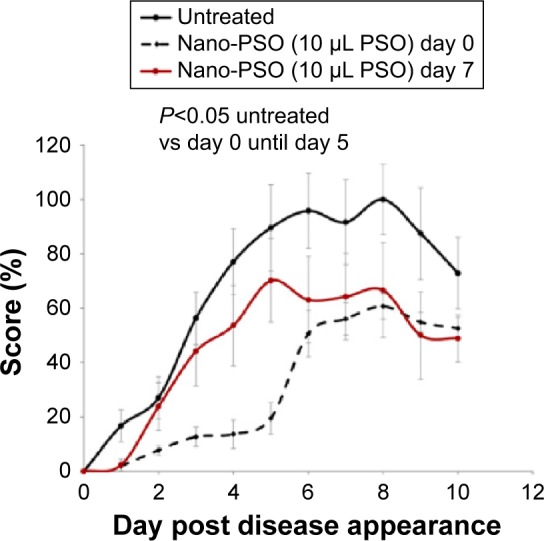
Nano-PSO in the prevention and treatment of EAE.
Notes: Mice induced for EAE were administered Nano-PSO in two different start points. As shown in the insert, while one group of induced mice was left untreated (n=8), a second group was treated with Nano-PSO from day 1 of the induction (n=6) and a third group from day 7 of the induction (n=7). Mice were scored daily for EAE signs for 2 additional weeks. P<0.05 for both Nano-PSO treatments.
Abbreviations: PSO, pomegranate seed oil; EAE, experimental autoimmune encephalomyelitis.
Effect of size of Nano-PSO droplet on its α-EAE activity
Since the rational for using PSO nanodroplets in these experiments lies in the possibility that such entities, as opposed to the large drops of natural PSO, may escape the liver trap on their first passage, we next asked whether the size of such droplets is important. All batches of Nano-PSO in the previous experiments comprised droplets of 180 nm (Figure 1) since this size of nanoparticles was shown to be successful for other brain conditions.35,36 We now compared the α-EAE activity of the 180 nm nanodrops with that of a Nano-PSO formulation in which the average diameter of the droplets is close to 30 nm, a size used for the targeting of RNA and drugs containing lipid droplets to peripheral organs, such as liver37 or lungs.38 To prepare these 30 nm droplets formulation, a self-emulsifying system was developed according to the procedure described in the “Materials and methods” section and in patent No. 14/523,408.39 PSO nanodroplets of both sizes were administered to EAE-induced mice from day 1 of the induction at a dose of 10 μL of PSO per day. Figure 5 shows that the formulation comprising the lower size droplets had no beneficial effect in EAE-induced mice, as opposed to the strong response of the sick mice to the 180 nm formulation (P<0.05). The 30 nm formulation was even less active than the 10 μL dose of PSO in its natural form (Figure 1). It is possible that lower size nanodroplets may be absorbed by peripheral organs soon after ingestion and have no opportunity to pass the BBB from the blood and generate a beneficial effect in the CNS. Also, smaller droplets may be more prone to oxidation than the larger ones. Whether small Nano-PSO droplets can or cannot generate a beneficial effect in other clinical settings outside the brain remains to be established.
Figure 5.
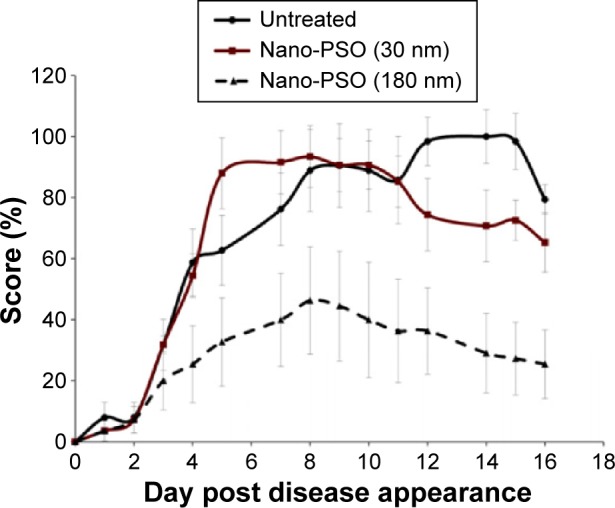
Small Nano-PSO particles are inactive against EAE.
Notes: Mice induced for EAE were treated with Nano-PSO in different droplet sizes. As shown in the insert, while one group of induced mice was left untreated (n=8), a second group was treated with 180 nm droplets of Nano-PSO (n=7), and a third group with 30 nm Nano-PSO droplets (n=7). P<0.05 for the untreated group versus the group treated with 180 nm droplets of Nano-PSO.
Abbreviations: PSO, pomegranate seed oil; EAE, experimental autoimmune encephalomyelitis.
Treatment of EAE-induced mice with Nano-PSO inhibits demyelination and oxidation of brain lipids
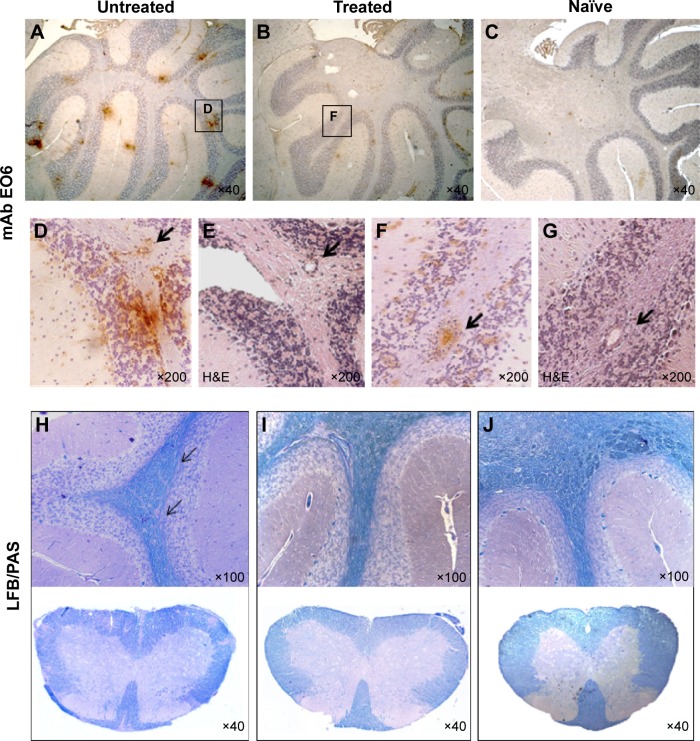
To test if the lower clinical scores resulting from Nano-PSO treatment of EAE-induced mice are consistent with reduced appearance of EAE pathological markers, we looked in the brains of treated and untreated mice for several parameters, such as infiltration of immune cells, demyelination, and lipid oxidation. Figure 6 shows that while immune infiltrates can be detected in both treated and untreated EAE brains (sections E and G), Nano-PSO administration significantly reduced lipid oxidation levels (sections A and B and enlarged in D and F), as detected by the EO6 antibody staining in both treated and untreated brains. As opposed to EAE mice, no EO6 immunostaining (section C) or immune infiltrates (not shown) could be observed in naïve mice. Nano-PSO administration also reduced demyelination levels, as can be seen by comparing Luxol fast blue staining for myelin in both brains and spinal cords of untreated, EAE and naïve mice (section H–J). Interestingly, while the EO6 antibody recognized in the untreated mice both a diffuse and a light staining as well as focal points stained intensely (section D), the latter one reminiscent of the plaques in the patients with MS,6 in the Nano-PSO-treated EAE brains, only the light and diffuse pattern of immunostaining was apparent (section F). Quantification of the levels of EO6 stain was performed by measuring the positive area in six different fields at a magnification ×40. Stained pixels were measured using image pro analyzer 3D software, Media Cybernetics (“Materials and methods” section). The quantification results show that while for EAE-treated sections the percentage of positive area was 1.11±0.08, for the nontreated samples, it was 5.17±1.13 and for the wild-type brains 0.07±0.03.
Figure 6.
Pathological markers of EAE in Nano-PSO treated and untreated mice.
Notes: Nano-PSO treated and untreated mice were sacrificed 3 weeks after induction of EAE, and their formalin-fixed, paraffin-embedded brain sections as well as those of age-matched naïve mice (C and J) were stained by mAb EO6 (A–D and F), H&E (E and G), and LFB/PAS (brains and spinal cords) (H–J). (D) and (F) represent an enlargement of the squares in (A) and (B); (E) and (G) are serial sections of (D) and (F), respectively. Arrows in (D–G) indicate immune infiltrates. Arrows in (H) represent demyelinated areas.
Abbreviations: EAE, experimental autoimmune encephalomyelitis; PSO, pomegranate seed oil; mAb, monoclonal antibody; H&E, hematoxylin and eosin; LFB, Luxol fast blue; PAS, periodic acid Schiff.
Interestingly, there was no colocalization between infiltrates and the oxidated plaques in the untreated EAE brains (compare sections D and E), indicating that while infiltration of activated immune cells may induce demyelination via oxidation of focal points, inhibition of such oxidation even in the presence of infiltrates may ameliorate disease signs (compare F and G). As we have shown previously,17 EO6 does not recognize any forms in naïve mice suggesting that oxidized phospholipids are a feature of brain disease (section C).
Administration of Nano-PSO reduced MDA levels in EAE-induced mice brains
MDA is a product of lipid peroxidation, and its levels can be empirically measured by the TBARS.40 It was recently shown to be increased in blood and saliva of MS patients.41 To establish whether MDA levels are elevated in the brains of EAE-induced mice and whether such elevation can be alleviated by Nano-PSO treatment, we subjected brain samples from six wild type, five EAE, and three treated EAE mice to the TBARS test (“Materials and methods” section). Figure 7 shows that while MDA levels are elevated significantly in the brains of EAE-induced mice (P<0.001 between naïve and untreated EAE brains), these are reduced almost to the levels of naïve brains following Nano-PSO treatment (P<0.663 between naïve and EAE-treated brains). These results, together with the results from Figure 6 also demonstrating reduced levels of oxidized phospholipids in Nano-PSO-treated EAE mice, strongly suggest that Nano-PSO may reduce lipid oxidation.
Figure 7.
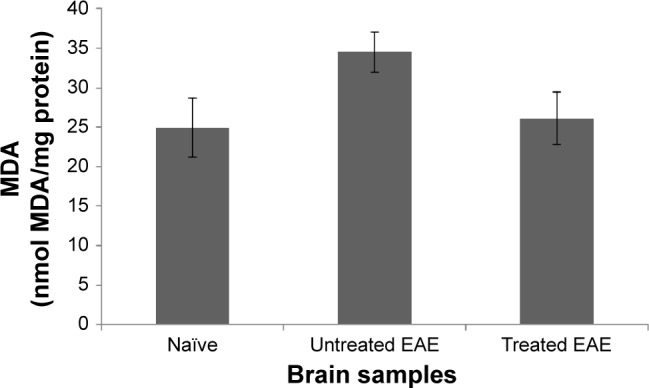
TBARS levels in EAE brains.
Notes: Samples from naïve, as well as treated and untreated EAE brains were subjected to the TBARS test.
Abbreviations: TBARS, thiobarbituric acid reactive substances; EAE, experimental autoimmune encephalomyelitis; MDA, malonaldehyde.
Discussion
We have shown here that, as is the case for some dietary unsaturated lipids,42 administration of PSO comprising high levels of PA, a polyunsaturated fatty acid, can be clinically beneficial to mice induced for EAE, a model of MS. However, while the oil in its natural form could only reduce disease burden significantly when given at very high doses, a superior clinical effect was achieved when 1% of these PSO levels were administrated to the EAE-induced mice in the form of emulsified nanodroplets, denominated Nano-PSO. Nano-PSO was also beneficial to the EAE mice when treatment commenced close to disease manifestation (day 7) and not only when administered concomitant with disease induction. This suggests that our reagent may be effective not only for disease prevention but also for abrogation of disease progression. We have also demonstrated that the size of the nanodroplets is important for their therapeutic effect since 30 nm droplets of PSO were inactive in this clinical setting as opposed to those approximately 200 nm size. Indeed, the small droplets may succumb to disruption by biosurfactants such as bile salts at a different rate than larger droplets,43,44 which may remain intact for longer periods of time, and subsequently cross the BBB.
On the mechanistic side, we have shown here that Nano-PSO can inhibit both demyelination and lipid oxidation in EAE brains even in the presence of immune infiltrates in the CNS. These results indicate that while oxidized lipids may not feature in the initial activation of immune cells, they may well play a central role in the subsequent demyelination, as previously suggested.45 Since oxidized lipoproteins are neurotoxic and have proinflammatory properties, lipid peroxidation products could be involved in demyelination and axonal injury in MS.46,47 In addition, the activity of Nano-PSO, at least at the particle size shown to be beneficial for EAE, may be mostly confined to the CNS, thereby interfering with brain oxidation features and demyelination and less with inhibition of infiltration. Owing to the lack of toxicity of these reagents, Nano-PSO may be a good choice for individuals at initial stages of demyelinating diseases such as MS. At later stages, Nano-PSO may be used in combination with advanced MS treatments such as Natalizumab (Tysabri), which reduces the migration of lymphocytes to the CNS by binding to the α4 integrin very late antigen,48 or in combination of other antioxidant formulations.49 In addition to demyelinating diseases, oxidative stress is also an important feature of most neurodegenerative conditions.6,14 Indeed, the EO6 antibody shown here to stain plaques in the untreated EAE brains as it was shown to mark human MS plaques6 also reacts extensively with brain slices from TgMHu2ME199K mice, a mouse model of genetic prion disease,17 suggesting that lipid oxidation may be a general feature of neurodegeneration. Most importantly, Nano-PSO was also beneficial in the prevention and treatment of genetic prion disease in the TgMHu2ME199K model, indicating that reagents that can prevent lipid oxidation may be beneficial for an array of neurodegenerative diseases. A significant number of natural antioxidants are ubiquitously present in a healthy human diet. Many of them, such as sulforaphane from broccoli, curcumin, and epigallocatechin gallate from green tea, were recognized for their neuroprotective properties in cells and tested in appropriate animal models.50–52 However, their in vivo activity was limited by the subpharmacological doses presented in food, their poor bioavailability to humans, rapid chemical degradation, and reduced distribution to different organs in the body, in particular, the CNS. In this work, and after PSO by itself was found to be clinically active in its natural form, we made an effort to overcome such limitations by tailoring a more active formulation in the form of Nano-PSO. The results presented in this work indeed indicate that natural compounds can be formulated into active drugs.
Acknowledgments
This work was funded by a grant from the Agnes Ginges Center for Human Neurogenetics and from a gift from the Darmoni family. We thank Dr Y Levi-Kalisman from the Nanocenter of the Hebrew University for her help in the cryo-TEM studies.
Footnotes
Disclosure
Based on the results described in this manuscript, we are in the process of developing a commercial venture to test Nano-PSO in patients. The authors report no conflicts of interest in this work.
References
- 1.Butterfield DA, Kanski J. Brain protein oxidation in age-related neurodegenerative disorders that are associated with aggregated proteins. Mech Ageing Dev. 2001;122(9):945–962. doi: 10.1016/s0047-6374(01)00249-4. [DOI] [PubMed] [Google Scholar]
- 2.Aguzzi A, O’Connor T. Protein aggregation diseases: pathogenicity and therapeutic perspectives. Nat Rev Drug Discov. 2010;9(3):237–248. doi: 10.1038/nrd3050. [DOI] [PubMed] [Google Scholar]
- 3.Morales R, Green KM, Soto C. Cross currents in protein misfolding disorders: interactions and therapy. CNS Neurol Disord Drug Targets. 2009;8(5):363–371. doi: 10.2174/187152709789541998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canello T, Frid K, Gabizon R, et al. Oxidation of Helix-3 methionines precedes the formation of PK resistant PrP. PLoS Pathog. 2010;6(7):e1000977. doi: 10.1371/journal.ppat.1000977. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7(1):65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haider L, Fischer MT, Frischer JM, et al. Oxidative damage in multiple sclerosis lesions. Brain. 2011;134(pt 7):1914–1924. doi: 10.1093/brain/awr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perluigi M, Coccia R, Butterfield DA. 4-Hydroxy-2-nonenal, a reactive product of lipid peroxidation, and neurodegenerative diseases: a toxic combination illuminated by redox proteomics studies. Antioxid Redox Signal. 2012;17(11):1590–1609. doi: 10.1089/ars.2011.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adibhatla RM, Hatcher JF. Lipid oxidation and peroxidation in CNS health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2010;12(1):125–169. doi: 10.1089/ars.2009.2668. [DOI] [PubMed] [Google Scholar]
- 9.Reed TT. Lipid peroxidation and neurodegenerative disease. Free Radic Biol Med. 2011;51(7):1302–1319. doi: 10.1016/j.freeradbiomed.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 10.Singh M, Dang TN, Arseneault M, Ramassamy C. Role of by-products of lipid oxidation in Alzheimer’s disease brain: a focus on acrolein. J Alzheimers Dis. 2010;21(3):741–756. doi: 10.3233/JAD-2010-100405. [DOI] [PubMed] [Google Scholar]
- 11.Lassmann H. Mechanisms of inflammation induced tissue injury in multiple sclerosis. J Neurol Sci. 2008;274(1–2):45–47. doi: 10.1016/j.jns.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Ellwardt E, Zipp F. Molecular mechanisms linking neuroinflammation and neurodegeneration in MS. Exp Neurol. 2014;262(pt A):8–17. doi: 10.1016/j.expneurol.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Herz J, Zipp F, Siffrin V. Neurodegeneration in autoimmune CNS inflammation. Exp Neurol. 2010;225(1):9–17. doi: 10.1016/j.expneurol.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 14.Witte ME, Bol JG, Gerritsen WH, et al. Parkinson’s disease-associated parkin colocalizes with Alzheimer’s disease and multiple sclerosis brain lesions. Neurobiol Dis. 2009;36(3):445–452. doi: 10.1016/j.nbd.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Castegna A, Palmieri L, Spera I, et al. Oxidative stress and reduced glutamine synthetase activity in the absence of inflammation in the cortex of mice with experimental allergic encephalomyelitis. Neuroscience. 2011;185:97–105. doi: 10.1016/j.neuroscience.2011.04.041. [DOI] [PubMed] [Google Scholar]
- 16.Palinski W, Hörkkö S, Miller E, et al. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest. 1996;98(3):800–814. doi: 10.1172/JCI118853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizrahi M, Friedman-Levi Y, Larush L, et al. Pomegranate seed oil nanoemulsions for the prevention and treatment of neurodegenerative diseases: the case of genetic CJD. Nanomedicine. 2014;10(6):1353–1363. doi: 10.1016/j.nano.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 18.Schubert SY, Lansky EP, Neeman I. Antioxidant and eicosanoid enzyme inhibition properties of pomegranate seed oil and fermented juice flavonoids. J Ethnopharmacol. 1999;66(1):11–17. doi: 10.1016/s0378-8741(98)00222-0. [DOI] [PubMed] [Google Scholar]
- 19.Sawant RR, Torchilin VP. Multifunctionality of lipid-core micelles for drug delivery and tumour targeting. Mol Membr Biol. 2010;27(7):232–246. doi: 10.3109/09687688.2010.516276. [DOI] [PubMed] [Google Scholar]
- 20.Merian J, Boisgard R, Decleves X, Theze B, Texier I, Tavitian B. Synthetic lipid nanoparticles targeting steroid organs. J Nucl Med. 2013;54(11):1996–2003. doi: 10.2967/jnumed.113.121657. [DOI] [PubMed] [Google Scholar]
- 21.Margulis-Goshen K, Magdassi S. Formation of simvastatin nanoparticles from microemulsion. Nanomedicine. 2009;5(3):274–281. doi: 10.1016/j.nano.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Kim D, Park JH, Kweon DJ, Han GD. Bioavailability of nanoemulsified conjugated linoleic acid for an antiobesity effect. Int J Nanomedicine. 2013;8:451–459. doi: 10.2147/IJN.S38430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhopeshwarkar GA, Mead JF. Uptake and transport of fatty acids into the brain and the role of the blood–brain barrier system. Adv Lipid Res. 1973;11(0):109–142. doi: 10.1016/b978-0-12-024911-4.50010-6. [DOI] [PubMed] [Google Scholar]
- 24.Spector R. Fatty acid transport through the blood–brain barrier. J Neurochem. 1988;50(2):639–643. doi: 10.1111/j.1471-4159.1988.tb02958.x. [DOI] [PubMed] [Google Scholar]
- 25.Avellini L, Terracina L, Gaiti A. Linoleic acid passage through the blood–brain barrier and a possible effect of age. Neurochem Res. 1994;19(2):129–133. doi: 10.1007/BF00966806. [DOI] [PubMed] [Google Scholar]
- 26.Yuan G, Sinclair AJ, Xu C, Li D. Incorporation and metabolism of punicic acid in healthy young humans. Mol Nutr Food Res. 2009;53(10):1336–1342. doi: 10.1002/mnfr.200800520. [DOI] [PubMed] [Google Scholar]
- 27.Saha SS, Ghosh M. Antioxidant effect of vegetable oils containing conjugated linolenic acid isomers against induced tissue lipid peroxidation and inflammation in rat model. Chem Biol Interact. 2011;190(2–3):109–120. doi: 10.1016/j.cbi.2011.02.030. [DOI] [PubMed] [Google Scholar]
- 28.Shi C, Wu F, Zhu XC, Xu J. Incorporation of beta-sitosterol into the membrane increases resistance to oxidative stress and lipid peroxidation via estrogen receptor-mediated PI3K/GSK3beta signaling. Biochim Biophys Acta. 2013;1830(3):2538–2544. doi: 10.1016/j.bbagen.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 29.Kaufman M, Wiesman Z. Pomegranate oil analysis with emphasis on MALDI-TOF/MS triacylglycerol fingerprinting. J Agric Food Chem. 2007;55(25):10405–10413. doi: 10.1021/jf072741q. [DOI] [PubMed] [Google Scholar]
- 30.Ben-Nun A, Mendel I, Bakimer R, et al. The autoimmune reactivity to myelin oligodendrocyte glycoprotein (MOG) in multiple sclerosis is potentially pathogenic: effect of copolymer 1 on MOG-induced disease. J Neurol. 1996;243(4 suppl 1):S14–S22. doi: 10.1007/BF00873697. [DOI] [PubMed] [Google Scholar]
- 31.Friedman-Levi Y, Ovadia H, Hoftberger R, et al. Fatal neurological disease in scrapie-infected mice induced for experimental autoimmune encephalomyelitis. J Virol. 2007;81(18):9942–9949. doi: 10.1128/JVI.00780-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steinman L, Zamvil SS. Virtues and pitfalls of EAE for the development of therapies for multiple sclerosis. Trends Immunol. 2005;26(11):565–571. doi: 10.1016/j.it.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 33.Gold R, Linington C, Lassmann H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain. 2006;129(pt 8):1953–1971. doi: 10.1093/brain/awl075. [DOI] [PubMed] [Google Scholar]
- 34.Brown DA, Sawchenko PE. Time course and distribution of inflammatory and neurodegenerative events suggest structural bases for the pathogenesis of experimental autoimmune encephalomyelitis. J Comp Neurol. 2007;502(2):236–260. doi: 10.1002/cne.21307. [DOI] [PubMed] [Google Scholar]
- 35.Jose S, Anju SS, Cinu TA, Aleykutty NA, Thomas S, Souto EB. In vivo pharmacokinetics and biodistribution of resveratrol-loaded solid lipid nanoparticles for brain delivery. Int J Pharm. 2014;474(1–2):6–13. doi: 10.1016/j.ijpharm.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 36.Blasi P, Schoubben A, Traina G, et al. Lipid nanoparticles for brain targeting III. Long-term stability and in vivo toxicity. Int J Pharm. 2013;454(1):316–323. doi: 10.1016/j.ijpharm.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 37.Chen S, Tam YY, Lin PJ, Leung AK, Tam YK, Cullis PR. Development of lipid nanoparticle formulations of siRNA for hepatocyte gene silencing following subcutaneous administration. J Control Release. 2014;196:106–112. doi: 10.1016/j.jconrel.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 38.Wang P, Zhang L, Peng H, Li Y, Xiong J, Xu Z. The formulation and delivery of curcumin with solid lipid nanoparticles for the treatment of on non-small cell lung cancer both in vitro and in vivo. Mater Sci Eng C Mater Biol Appl. 2013;33(8):4802–4808. doi: 10.1016/j.msec.2013.07.047. [DOI] [PubMed] [Google Scholar]
- 39.Gabizon R, Ovadia H, Abramsky O, Magdassi S, Larush L, inventors. Hadasit Medical Ressearch Services And Development Ltd., Yissum Research Development Company Of Tthe Hebrew University Of Jerusalem Ltd., assignees Pomegranate Oil For Preventing And Treating Neurodegenerative Diseases. 20150044314 A1. United States patent US. 2015 Feb 12;
- 40.Guillen-Sans R, Guzman-Chozas M. The thiobarbituric acid (TBA) reaction in foods: a review. Crit Rev Food Sci Nutr. 1998;38(4):315–330. doi: 10.1080/10408699891274228. [DOI] [PubMed] [Google Scholar]
- 41.Karlik M, Valkovic P, Hancinova V, Krizova L, Tothova L, Celec P. Markers of oxidative stress in plasma and saliva in patients with multiple sclerosis. Clin Biochem. 2015;48(1–2):24–28. doi: 10.1016/j.clinbiochem.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 42.Kong W, Yen JH, Ganea D. Docosahexaenoic acid prevents dendritic cell maturation, inhibits antigen-specific Th1/Th17 differentiation and suppresses experimental autoimmune encephalomyelitis. Brain Behav Immun. 2013;25(5):872–882. doi: 10.1016/j.bbi.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Z, Gao F, Jiang S, et al. Bile salts enhance the intestinal absorption of lipophilic drug loaded lipid nanocarriers: mechanism and effect in rats. Int J Pharm. 2012;452(1–2):374–381. doi: 10.1016/j.ijpharm.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 44.Schubert R, Schmidt KH. Structural changes in vesicle membranes and mixed micelles of various lipid compositions after binding of different bile salts. Biochemistry. 1988;27(24):8787–8794. doi: 10.1021/bi00424a015. [DOI] [PubMed] [Google Scholar]
- 45.Ljubisavljevic S. Oxidative stress and neurobiology of demyelination. Mol Neurobiol. 2014 Dec 11; doi: 10.1007/s12035-014-9041-x. Epub. [DOI] [PubMed] [Google Scholar]
- 46.Ferretti G, Bacchetti T. Peroxidation of lipoproteins in multiple sclerosis. J Neurol Sci. 2011;311(1–2):92–97. doi: 10.1016/j.jns.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 47.Leitinger N. The role of phospholipid oxidation products in inflammatory and autoimmune diseases: evidence from animal models and in humans. Subcell Biochem. 2008;49:325–350. doi: 10.1007/978-1-4020-8830-8_12. [DOI] [PubMed] [Google Scholar]
- 48.Sellebjerg F, Sorensen PS. Therapeutic interference with leukocyte recirculation in multiple sclerosis. Eur J Neurol. 2015;22(3):434–442. doi: 10.1111/ene.12668. [DOI] [PubMed] [Google Scholar]
- 49.Kizelsztein P, Ovadia H, Garbuzenko O, Sigal A, Barenholz Y. Pegylated nanoliposomes remote-loaded with the antioxidant tempamine ameliorate experimental autoimmune encephalomyelitis. J Neuroimmunol. 2009;213(1–2):20–25. doi: 10.1016/j.jneuroim.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 50.Han JM, Lee YJ, Lee SY, et al. Protective effect of sulforaphane against dopaminergic cell death. J Pharmacol Exp Ther. 2007;321(1):249–256. doi: 10.1124/jpet.106.110866. [DOI] [PubMed] [Google Scholar]
- 51.Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65(11):1631–1652. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choi YT, Jung CH, Lee SR, et al. The green tea polyphenol (−)- epigallocatechin gallate attenuates beta-amyloid-induced neurotoxicity in cultured hippocampal neurons. Life Sci. 2001;70(5):603–614. doi: 10.1016/s0024-3205(01)01438-2. [DOI] [PubMed] [Google Scholar]