Abstract
Objective
Neuropsychiatric (NP) events occur unpredictably in systemic lupus erythematosus (SLE) and most biomarker associations remain to be prospectively validated. We examined a disease inception cohort of 1047 SLE patients to determine which autoantibodies at enrollment predicted subsequent NP events.
Methods
Patients with recent SLE diagnosis were assessed prospectively for up to 10 years for NP events using ACR case definitions. Decision rules of graded stringency determined whether NP events were attributable to SLE. Associations between the first NP event and baseline autoantibodies (lupus anticoagulant, anticardiolipin, anti-β2 glycoprotein-I, anti-ribosomal P and anti-NR2 glutamate receptor) were tested by Cox proportional hazards regression.
Results
Disease duration at enrollment was 5.4±4.2 months, followup was 3.6±2.6 years. Patients were 89.1% female with mean (±SD) age 35.2±13.7 years. 495/1047 (47.3%) developed ≥1 NP event (total 917 events). NP events attributed to SLE were 15.4% (model A) and 28.2% (model B). At enrollment 21.9% of patients had lupus anticoagulant, 13.4% anticardiolipin, 15.1% anti-β2 glycoprotein-I, 9.2% anti-ribosomal P and 13.7% anti-NR2 antibodies. Lupus anticoagulant at baseline was associated with subsequent intracranial thrombosis (total n=22) attributed to SLE (model B) (Hazard ratio, HR 2.54 (95% CI: 1.08–5.94). Anti-ribosomal P antibody was associated with subsequent psychosis (total n=14) attributed to SLE (model B) (HR: 3.92 (95% CI:1.23–12.5); p=0.02). Other autoantibodies did not predict NP events.
Conclusion
In a prospective study of 1047 recently diagnosed SLE patients, lupus anticoagulant and anti-ribosomal P antibodies are associated with an increased future risk for intracranial thrombosis and lupus psychosis respectively
Keywords: Systemic lupus erythematosus, Neuropsychiatric, Inception cohort, Autoanibodies, Biomarkers
Nervous system involvement in systemic lupus erythematosus (SLE) encompasses a variety of neurological (N) and psychiatric (P) features. Using the American College of Rheumatology (ACR) case definitions (1), the prevalence of neuropsychiatric (NP) disease in SLE varies from 21–95%, but only 19–38% of eventsare a ttributable to lupus (2–6). NP events present or reoccur at any time in the disease course, although the majority occurs around the time of diagnosis of SLE, particularly those attributable to SLE (7–11). The identification of biomarkers at the time of diagnosis to quantify the subsequent risk of NP events attributable toSLE (NPSLE) would be helpful.
NPSLE is likely mediated by autoantibodies, microvasculopathy, and the intracranial production of inflammatory mediators (12–15), ofter in combination. Lupus related autoantibodies most frequently associated with NPSLE include antiphospholipid antibodies, anti-ribosomal P antibodies and autoantibodies which bind to neuronal antigens such as the N-methyl-D-aspartate (NMDA) glutamate receptor (anti-NR2) (16). Although there is biological plausibility and experimental data (16–20) to implicate these autoantibodies in the causality of nervous system disease, studies of human SLE have provided inconsistent findings (21–25). Limitations of previous studies include their cross-sectional design, inclusion of patients with variable disease duration, and lack of standardization in both the classification of NP events and the methodology used for autoantibody detection. We have assembled an international, inception cohort of SLE patients to examine the association between a panel of autoantibodies measured within a mean of 6 months of the time of diagnosis and subsequent nervous system events over a mean followup of 3.6 years. Attribution models of different stringency were used to distinguish NP events attributed to SLE and non-SLE causes.
Patients and Methods
Research study network
The study was conducted by members of the Systemic Lupus International Collaborating Clinics (SLICC) (26) a network of 37 investigators in 30 international academic medical centres in 11 countries. Twenty-one centres participated in the study. Data were collected prospectively on patients presenting with a new diagnosis of SLE. All information was submitted to the coordinating centre in Halifax, Nova Scotia, Canada and entered into a centralized Access database. Appropriate procedures ensured data quality, management and security. The studyl was approved by the Capital Health Research Ethics Board, Halifax, Nova Scotia, Canada and by each of the participating centre’s own institutional research ethics review boards.
Patients
Patients fulfilled the ACR classification criteria for SLE (27) provided written informed consent. The date of diagnosis was when these cumulative criteria were first recognized. Enrollment was permitted up to 15 months following the diagnosis. Variables collected included age, gender, ethnicity, education and medication history. Lupus-related variables included the ACR classification criteria for SLE (27), the SLE Disease Activity Index (SLEDAI) (28) and the SLICC/ACR damage index (SDI) (29). Laboratory variables were hematology, serum and urine chemistry and immunologic variables (including anti-DNA antibodies) required for the generation of SLEDAI and SDI scores.
Neuropsychiatric (NP) events
An enrollment window within which all NP events were captured extended from 6 months prior to the diagnosis of SLE up to the enrollment date. NP events were characterized using the ACR nomenclature and case definitions for 19 NP syndromes (1). These were diagnosed by clinical evaluation and investigations were performed if clinically warranted.
Patients were reviewed annually with a 6-month window around the anticipated assessment date. New NP events since the previous study visit and their attribution were determined.
Supplementary information was recorded as per the ACR glossary for NP syndromes (1) to identify other potential causes (“exclusions”) or contributing factors (“associations”) for each of the NP events. These “non-SLE factors” were used in part to determine the attribution of NP events. Patients could have more than one type of NP event and repeated episodes of the same event within the enrollment window or within a follow-up assessment period were recorded once. The date of the first episode was taken as the onset of the NP event within the particular time frame.
Attribution of NP events
Decision rules were used to determine the attribution of all NP events. Factors considered in the decision rules included: (i) onset of NP event(s) prior to the diagnosis of SLE; (ii) concurrent non-SLE factor(s) identified from the ACR glossary for each NP syndrome and (iii); “common” NP events which are frequent in normal population controls as described by Ainiala et al (30). These include all headaches, anxiety, mild depression (mood disorders failing to meet criteria for “major depressive-like episodes”), mild cognitive impairment (deficits in less than 3 of the 8 specified cognitive domains) and polyneuropathy without electrophysiological confirmation.
Attribution of NP events was determined by the central application of decision rules of different stringency (models A and B) as described in detail elsewhere (31, 32). NP events which fulfilled the criteria for model A (the most stringent) or for model B (the least stringent) were attributed to SLE. By definition, all NP events attributed to SLE using model A were included in the group of NP events attributed to SLE using model B. Those events which did not fulfill these criteria were attributed to non-SLE causes
Determination of autoantibodies
Autoantibodies were measured in Dr. Joan Merrill’s laboratory at the Oklahoma Medical Research Foundation, USA. Autoantibody determinations were made without knowledge of the occurrence of NP events or their attribution in individual patients.
ELISA for anti-NR2 antibodies
NR2 human peptide sequence, (Asp Trp Glu Tyr Ser Val Trp Leu Ser Asn)8 Lys 4 Lys2 Lys-β Ala, was synthesized using f-moc chemistry, purified by HPLC and confirmed by Edman degradation at the Molecular Biology Proteomics Facility of the University of Oklahoma Health Sciences Centre, Oklahoma City, OK. High binding, Nunc 96-well polystyrene plates were coated with 5 ug/mL of NR2 peptide in borate buffered saline and blocked with borate buffered saline, bovine serum albumin (Fraction V, Sigma) and 1.2% Tween 80. Patient sera, positive and negative controls were added, diluted 1/100 in the same blocking buffer. Plates were washed with borate buffered saline between each step with vigorous pounding to eliminate non-specific binding. Secondary antibody was an alkaline phosphatase conjugated goat anti-human IgG (Sigma) with the addition of goat serum to block non-specific binding (donor herd, Sigma). Plates were developed using p-NPP substrate buffer (Sigma). Optical density of the enzyme-linked immune assay were read at 405 (primary wavelength) and 450 (secondary wavelength). Serial dilutions of a high binding positive control were used as a calibrator.
Antiphosphilipid, anti-β2 glycoprotein-I, and anti-ribosomal P antibodies
Lupus anticoagulant (LA) and ELISAs for anticardiolipin, anti-β2 glycoprotein-I and anti-ribosomal P protein were performed as previously described (33–35). The LA assay was performed using screen and confirm reagents from Rainbow Scientific, 83 Maple Ave., Windsor, CT. Each reagent was standardized against20 plasmasamples (collected in citrate) from healthy donors. A normal reference range was derived from calculating two standard deviations above the mean of healthy controls on the screen and confirm (phospholipid quenched) tests and calculating the ratio of screen value/confirm value. Patient’s clotting time for LA Screen was divided by the LA Confirm’s clotting time. If this number was above the normal reference range, the patient was considered positive for LA. β2 glycoprotein-I, purified from human plasma, was the gift of Drs. Naomi and Charles Esmon, and ribosomal P protein was provided by Dr. Morris Reichlin, Oklahoma Medical Research Foundation. Each ELISA was validated against a curve, constructed using serial dilutions of a high binding serum. In the case of anticardiolipin and anti-ribosomal P protein, these calibrators were previously established in Dr. Reichlin’s laboratory. In the case of anti-β2 glycoprotein-I the calibrator was established by the Registry for the Antiphospholipid Syndrome at Oklahoma. The cutoff for positive was defined as 2 SD above the mean of 60 healthy controls and/or position on the flat part of the calibrator curve, whichever was associated with the higher O.D. On each ELISA plate, positive and negative control sera (established previously from the laboratory collection and frozen at −80°C in assay specific aliquots) were run to ensure a valid assay.
Statistical analysis
Chi-square tests were used to examine the association of autoantibody prevalence at enrollment with geographical regions or ethnic/racial groups. The associations of autoantibodies at enrollment with the time to the first occurrence of NP events overall, or events attributed to SLE (model A or model B) as well as the time to the first occurrence of individual events (cerebrovascular disease and psychosis) were examined using Coxproportional hazards regression.
Results
Patients
A total of 1047 patients were recruited between October 1999 and April 2010. The median (range) number of patients enrolled in each of the 21 centres was 31 (6–161). The patients were predominantly women, with a mean (±SD) age of 35.2±13.7 years and a wide ethnic distribution although predominantly Caucasian (Table 1).
Table 1.
Demographic and clinical manifestations of SLE patients at enrollment
| Number of Patients | 1047 |
| Gender | |
| Female | 933 (89.1%) |
| Male | 114 (10.9%) |
| Age (years) (mean ± SD) | 35.2 ± 13.7 |
| Race/Ethnicity: | |
| Caucasian | 545 (52.1%) |
| Asian | 225 (21.5%) |
| African | 160 (15.3%) |
| Hispanic | 73 (7.0%) |
| Other | 44 (4.2%) |
| Single/Married/Other | 481 (45.9%)/435 (41.6%)/131(12.5%) |
| Post secondary education | 672 (66.9%) (range 36.4–100%) |
| Disease duration (months) (mean ± SD) | 5.4 ± 4.2 |
| Number of ACR criteria (mean ± SD) | 4.9 ± 1.0 |
| Cumulative ACR manifestations | |
| Malar rash | 364 (34.8%) |
| Discoid rash | 118 (11.3%) |
| Photosensitivity | 368 (35.1 %) |
| Oral/nasopharyngeal ulcers | 391 (37.3%) |
| Serositis | 280 (26.7%) |
| Arthritis | 759 (72.5%) |
| Renal disorder | 278 (26.6%) |
| Neurological disorder | 57 (5.4%) |
| Hematologic disorder | 639 (61.0%) |
| Immunologic disorder | 807 (77.1%) |
| Antinuclear antibody | 1017 (97.1%) |
| SLEDAI score (mean ± SD) | 5.5 ± 5.4 |
| SLICC/ACR damage index score (mean ± SD) | 0.24 ± 0.67 |
| Medications | |
| Corticosteroids | 718 (68.6%) |
| Antimalarials | 690 (65.9%) |
| Immunosuppressants | 402 (38.4%) |
| ASA | 144 (13.8%) |
| Antidepressants | 104 (9.9%) |
| Warfarin | 58 (5.5%) |
| Anticonvulsants | 37 (3.5%) |
| Antipsychotics | 6 (0.6%) |
At enrollment the mean disease duration was only 5.4±4.2 months despite the opportunity to recruit patients up to 15 months following the diagnosis of SLE. The prevalence of individual ACR classification criteria at enrollment reflected an unselected patient population. The mean SLEDAI and SDI scores revealed moderate global disease activity and minimal cumulative organ damage respectively. Therapy at enrollment reflected the typical range of lupus medications. The number of assessments in individual patients varied from 1 to 10 over a mean followup 3.6 ± 2.6 years.
Neuropsychiatric (NP) manifestations
495/1047(47.2%) patients had ≥1 NP event and 226/1047(21.5%) had ≥2 events. The events and their attribution are summarized in Table 2.
Table 2.
Characteristics of cumulative neuropsychiatric syndromes over the study period in SLE patients with ≥1 autoantibody measurement at enrollment (n=1047). The number of NP events and their attribution are indicated using attribution models A and B
| NP events (%)regardless of attribution | NP events due to SLE (model A) | NP events due to SLE (Model B) | NP events due to non-SLE causes | |
|---|---|---|---|---|
| Headache | 477 (52.0) | 0 | 0 | 477 |
| Mood disorders | 132 (14.4) | 23 | 54 | 78 |
| Seizure disorder | 53 (5.8) | 32 | 44 | 9 |
| Anxiety disorder | 52 (5.7) | 0 | 0 | 52 |
| Cerebrovascular disease | 47 (5.1) | 22 | 46 | 1 |
| Cognitive dysfunction | 41 (4.5) | 10 | 28 | 13 |
| Polyneuropathy | 23 (2.5) | 7 | 11 | 12 |
| Acute confusional state | 21 (2.3) | 10 | 15 | 6 |
| Mononeuropathy | 16 (1.7) | 6 | 16 | 0 |
| Psychosis | 16 (1.7) | 9 | 16 | 0 |
| Cranial neuropathy | 11 (1.2) | 8 | 8 | 3 |
| Movement disorder | 8 (0.9) | 1 | 5 | 3 |
| Myelopathy | 8 (0.9) | 4 | 7 | 1 |
| Aseptic meningitis | 6 (0.7) | 4 | 4 | 2 |
| Demyelinating syndrome | 4 (0.4) | 1 | 4 | 0 |
| Autonomic disorder | 1 (0.1) | 1 | 1 | 0 |
| Plexopathy | 1 (0.1) | 0 | 0 | 1 |
| Guillain-Barre syndrome | 0 | 0 | 0 | 0 |
| Myasthenia gravis | 0 | 0 | 0 | 0 |
| Total | 917 | 141 | 259 | 658 |
| % among 917 NP events | 15.4 | 28.2 | 71.8 |
- Attribution Model A: NP events which had their onset within the enrollment window and had no “exclusions” or “associations” and were not one of the NP events identified by Ainiala (30) were attributed to SLE.
- Attribution Model B: NP events which had their onset within 10 years of the diagnosis of SLE and were still present within the enrollment window and had no “exclusions” and were not one of the NP events identified by Ainiala (30)were attributed to SLE.
There were 917 NP events, encompassing 17 of the 19 NP syndromes: headache (52.0%), mood disorders (14.4%), seizure disorder (5.8%), anxiety disorder (5.7%), cerebrovascular disease (5.1%), cognitive dysfunction (4.5%), polyneuropathy (2.5%), acute confusional state (2.3%), mononeuropathy (1.7%), psychosis (1.7%), cranial neuropathy (1.2%), movement disorder (0.9%), myelopathy (0.9%), aseptic meningitis (0.7%), demyelinating syndrome (0.4%), autonomic neuropathy (0.1%) and plexopathy (0.1%). The proportion of NP events attributed to SLE varied from 15.4% – 28.2% using alternate attribution models and occurred in 9.7 % [model A] – 16.5 % [model B] of patients. There were no patients with Guillain-Barré syndrome or myasthenia gravis. Of the 917 NP events 865 (94.3%) affected the central nervous system and 52 (5.7%) involved the peripheral nervous system. The classification of events into diffuse and focal was 749 (81.7%) and 168 (18.3 %), respectively.
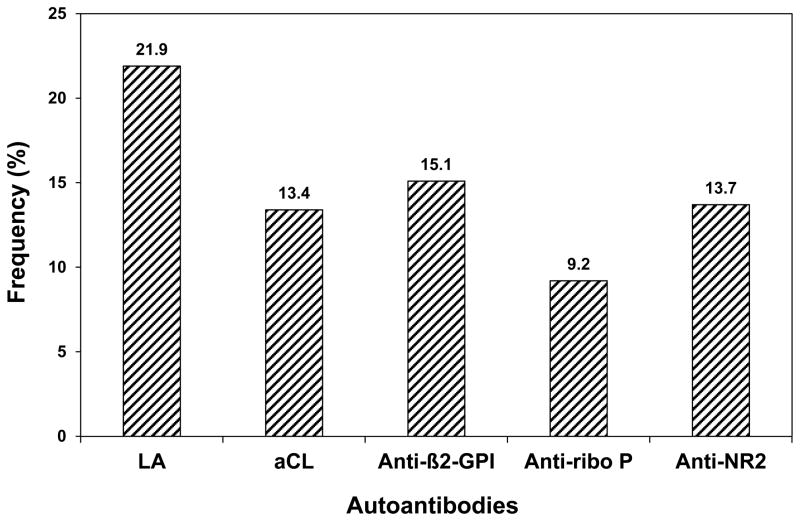
Autoantibodies and Racial/Ethnic Group
The prevalence of autoantibodies is illustrated in Figure 1. This varied from 9.2% (91/991) for anti-ribosomal P antibodies, 13.4% (133/995) for anticardiolipin, 13.7% (126/923) for anti-NR2, 15.1% (150/994) for anti-β2 glycoprotein-I and 21.9% (228/1042) for LA. The number of patients with 1, 2 or ≥3 positive antibody tests were 312, 107 and 61, respectively. The frequency of autoantibodies varied by geographical region. Specifically the frequency of LA was lower in Canadian centres (15.2%) compared to centres in the USA (26.0%), Europe (23.1%), Asia (22.6%) and Mexico (30.8%) (p=0.015), and the frequency of anti-ribosomal P antibodies was higher in Mexico (29.0%) compared to Canada (7.3%), USA (7.5%), Europe (8.0%) and Asia (13.7%) (p<0.001). In large part these findingswere due to the associationof racial/ethnic group with autoantibody frequencies (Table 3).
Figure 1.
Frequency of autoantibodies at enrollment. (LA=lupus anticoagulant; aCL=IgG anticardiolipin antibody; Anti-β2-GPI=IgG anti-β2 glycoprotein I antibody; Anti -ribo P=IgG anti-ribosomal P antibody; Anti-NR2=IgG anti-NR2 glutamate receptor antibody)
Table 3.
The association between autoantibody frequency and racial/ethnicgroup
| Caucasian | Asian | African | Hispanic | Other | P value | |
|---|---|---|---|---|---|---|
| Lupus anticoagulant (%) | 22.5 | 22.4 | 16.3 | 30.1 | 18.2 | 0.17 |
| Anticardiolipin (%) | 13.6 | 12.8 | 14.0 | 11.3 | 14.0 | 0.98 |
| Anti-β2-GPI (%) | 17.1 | 12.8 | 11.5 | 7.3 | 25.6 | 0.02 |
| Anti-ribosomal P (%) | 4.9 | 13.8 | 15.3 | 18.3 | 4.9 | <0.001 |
| Anti-NR2 (%) | 14.3 | 16.6 | 12.2 | 5.8 | 7.9 | 0.25 |
Autoantibodies and overall NP events
There was no significant positive association between autoantibodies and first occurrence of NP events overall, or events attributed to SLE (model A or model B). Clustering of NP events into diffuse/focal and central/peripheral manifestations did not change the outcome of this analysis. In keeping with our previous findings (36) the presence of anti-DNA antibodies measured at individual SLICC sites did not positively predict the occurrence of NP events (data not shown).
Autoantibodies and individual NP events
Analyses were also performed to examine specific a priori clinical-serologic associations. The association between antiphospholipid antibodies and cerebrovascular disease and between anti-ribosomal P antibodies and psychosiswere of particular interest (Table 4 and Figure 2).
Table 4.
The association between autoantibodies and the time to specific NP manifestations as indicated by hazard ratio (95% CI)
| LA | aCl | Anti-β2-GPI | Anti-β2-GPI or aCL or LA | |
|---|---|---|---|---|
| Any cerebrovascular Event (model B) | 1.84 (0.92–3.68) | 1.25 (0.52–3.02) | 1.14 (0.48–2.76) | 1.26 (0.69–2.30) |
| Stroke or sinus thrombosis (model B) | 2.54 (1.08–5.94) | 0.65 (0.15–2.78) | 0.27 (0.04–2.04) | 1.25 (0.58–2.72) |
| Anti-ribosomal P | ||||
| Psychosis (model B) | 3.92 (1.23–12.50) |
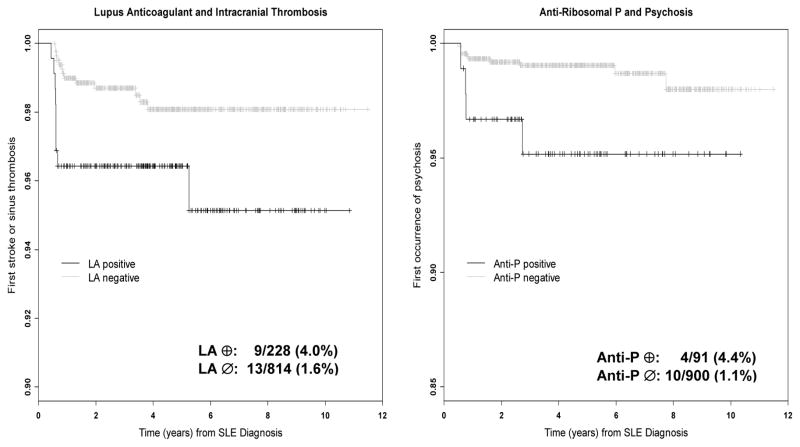
Figure 2.
Kaplan-Meier time-to-event curves for intracranial thrombosis in patients with and without lupus anticoagulant (LA) (left panel) and for psychosis in patients with and without anti-ribosomal P antibodies (Anti-P) (right panel).
Cerebrovascular disease includes stroke, transient ischemic attack, chronic multifocal disease, subarachnoid or intracranial hemorrhage and sinus thrombosis. There was no strong relationship demonstrated between cerebrovascular disease, so defined, and the presence of any one of either anti-β2 glycoprotein-I, anticardiolipin antibody or LA (Hazard ratio, HR 1.26 (95% CI 0.69–2.30). However, LA at baseline and the occurrence of cerebrovascular disease approached statistical significance (HR 1.84 (95% CI) 0.92–3.68) and the association with stroke/sinus thrombosis (total n=22) attributed to SLE (model B) was statistically significant (HR 2.54 (95% CI 1.08–5.94). The median (range) between the detection of LA and first stroke/sinus thrombosis was 5.02 (0–7.36) years. In addition, anti-ribosomal P antibody at baseline was associated with psychosis (total n=14) attributed to SLE (model B) (HR 3.92 (95% 1.23–12.5). Seven of the patients with psychosis were African, 5 Caucasian and 1 each Hispanic and Asian. Given the higher rate of psychosis in Africans (p<0.01), adjustment for racial/ethnic group (African/others) was undertaken. This led to a reduction in the hazard ratio for anti-ribosomal P antibody and subsequent psychosis to 3.1 with a corresponding shift in the confidence interval (0.95–9.99). The median (range) between the detection of anti-ribosomal P antibody and first episode of psychosis was 5.69 (0–9.16) years.
Discussion
We have evaluated the usefulness of measuring selected autoantibodies for predicting the occurrence of NPSLE in a large, international, inception cohort of SLE patients over the first 10 years of disease. Our findings provide some evidence that LA and anti-ribosomal P antibodies are significantly associated with specific manifestations of NP disease attributed to SLE, namely intracranial thrombosis and psychosis respectively. Variability in the frequency of some autoantibodies with racial/ethnic group supports previous observations of this kind (36, 37) and likely alters the risk profile for the occurrence of NP events in some groups of patients.
There several strengths to our study. In contrast to previous retrospective and cross-sectional clinical studies of NPSLE, ours was prospective to identify the characteristics and attribution of all NP events using a predefined annual data collection protocol. The multi-centre, international, longitudinal study design provides a basis for extrapolating our findings to the broader community of SLE patients. Although nervous system involvement by SLE has long been recognized, the lack of specificity of multiple individual manifestations and difficulty in identifying the correct attribution of the clinical NP events has been challenging. The ACR case definitions of 19 NP syndromes (1) which were developed over a decade ago have provided a much needed and now widely used platform for the classification of NP events in SLE cohorts. We have also used the accompanying ACR glossary with other information to derive decision rules for determining attribution of NP events to SLE or non-SLE causes (32). In previous studies the application of these decision rules has demonstrated significant correlations with clinical outcomes and selected autoantibodies (31, 32, 38, 39). The use of these rules in the current study provides an excellent platform for the prospective evaluation of potential biomarkers of NPSLE.
The search for biomarkers of NPSLE is based upon what is already known of the pathogenesis of the disease. There is robust evidence from several sources to implicate a pathogenic role for autoantibodies, microvasculopathy, and the intracranial production of inflammatory mediators. These studies have provided a menu of biomarker candidates, including autoantibodies, cytokines and other inflammatory molecules (40) as well as soluble markers of neuronal and glial degradation (41). Detailed discussion is not possible here but a few general observations are worthy of comment. First, given the multitude of clinical manifestations it is very unlikely that a single biomarker will reliably predict all NP events. Second, the anatomical location of biomarkers is important in some cases. For example the association of diffuse NP events with autoantibodies is significantly stronger if these are measured in cerebrospinal fluid (CSF), whereas autoantibodies associated with focal NP events are best studied in peripheral blood. An alternative to accessing CSF, is to find a biomarker of increased permeability of the blood-brain-barrier, a critical factor if some autoantibodies are to reach their target antigen and cause clinical disease. Finally, it is possible that combinations of biomarkers reflecting different components of the pathogenic model of NPSLE will best predict clinical events. To our knowledge, there are no previous studies which have set out to evaluate biomarkers of NPSLE in a large multi-ethnic cohort of patients specifically recruited as close to the diagnosis of SLE as possible and followed over an extended period. Some but not all previous cross-sectional studies with smaller sample sizes have found an association between lupus anticoagulant and intracranial thrombosis (42) and between anti-ribosomal P antibodies and lupus psychosis (24, 43–47). However, in these studies the autoantibodies were measured in close temporal proximity to the clinical event. In contrast, our study is the first to demonstrate the risk of a single autoantibody determination around the time of diagnosis of SLE for a subsequent and often remote NP event.
There are several limitations to the current study. First, the number of autoantibodies studied was limited and CSF samples were not available. The selection of autoantibodies was based upon the evidence of their pathogenic role. Access to CSF samples in our study was infrequent and was restricted to situations when a lumbar puncture was clinically indicated. Also, the small number of specific NP events allowed little power for adjusted analyses. Second, as the study involved antibody determination at a single point in time, no information is yet available on the predictive value of sustained circulating levels of autoantibodies. Third, the classification of NP status was determined primarily by clinical assessment and using appropriate investigations only when clinically indicated. Specialized and sensitive investigations such as MRI neuroimaging studies and formal neuropsychological assessment of cognitive function were not routinely done on all patients. Their use would very likely have resulted in the recognition of additional structural and function abnormalities of the nervous system. However, many of the abnormalities would be of dubious clinical significance and the routine use of such investigations, although justified in the context of a clinical study, would not mirror what is most commonly done in clinical practice. Finally, the duration of followup in the current study does not reflect the lifetime experience with NP events experienced by the majority of SLE patients. We and others have previously reported that NP events, especially those attributed to SLE, occur most frequently within the first 2 years of the diagnosis of SLE (7–11), thus emphasizing the importance of this period of observation. Nevertheless further followup is required to identify the longer term association between autoantibodies detected either at baseline or over the duration of a patients’ illness and clinical expression of NPSLE. The long-term objective of our prospective study is to follow all patients for 10 years which will provide an excellent platform to find new and stronger associations with circulating serological biomarkers.
Acknowledgments
Financial support:
Dr. J.G. Hanly (Canadian Institutes of Health Research grant MOP-57752, Capital Health Research Fund)
Dr. M.B. Urowitz’s work was supported by the Canadian Institutes of Health Research (grant MOP-49529), The Lupus Foundation of Ontario, The Ontario Lupus Association, Lupus UK, The Lupus Foundation of America, The Lupus Alliance of Western New York, The Conn Smythe Foundation, The Lupus Flare Foundation, and The Tolfo Family of Toronto, Ontario, Canada.
Dr. Li Su (MRC(UK) grant U.1052.00.009) and Dr. V. Farewell (MRC(UK) grant U.1052.00.009).
Dr. Sang-Cheol Bae’s work was supported by the Korea Healthcare technology R & D project, Ministry for Health and Welfare, Republic of Korea (A080588).
The Montreal General Hospital Lupus Clinic is partially supported by the Singer Family Fund for Lupus Research. Dr. Clarke is a National Scholar of the Fonds de la recherché en santé de Quebec.
Dr. Paul R. Fortin is a Distinguished Senior Investigator of The Arthritis Society with additional support from the Arthritis Centre of Excellence, University of Toronto.
Dr. Ramsey-Goldman’s work was supported by the NIH (grants UL-1RR-025741, K24-AR-02318, and P60-AR-48098).
Dr. Ruiz-Irastorza is supported by the Department of Education, Universities and Research of the Basque Government.
References
- 1.The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 1999;42(4):599–608. doi: 10.1002/1529-0131(199904)42:4<599::AID-ANR2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 2.Ainiala H, Loukkola J, Peltola J, Korpela M, Hietaharju A. The prevalence of neuropsychiatric syndromes in systemic lupus erythematosus. Neurology. 2001;57(3):496–500. doi: 10.1212/wnl.57.3.496. [DOI] [PubMed] [Google Scholar]
- 3.Brey RL, Holliday SL, Saklad AR, Navarrete MG, Hermosillo-Romo D, Stallworth CL, et al. Neuropsychiatric syndromes in lupus: prevalence using standardized definitions. Neurology. 2002;58(8):1214–20. doi: 10.1212/wnl.58.8.1214. [DOI] [PubMed] [Google Scholar]
- 4.Hanly JG, McCurdy G, Fougere L, Douglas JA, Thompson K. Neuropsychiatric events in systemic lupus erythematosus: attribution and clinical significance. J Rheumatol. 2004;31(11):2156–62. [PubMed] [Google Scholar]
- 5.Sanna G, Bertolaccini ML, Cuadrado MJ, Laing H, Mathieu A, Hughes GR. Neuropsychiatric manifestations in systemic lupus erythematosus: prevalence and association with antiphospholipid antibodies. J Rheumatol. 2003;30(5):985–92. [PubMed] [Google Scholar]
- 6.Sibbitt WL, Jr, Brandt JR, Johnson CR, Maldonado ME, Patel SR, Ford CC, et al. The incidence and prevalence of neuropsychiatric syndromes in pediatric onset systemic lupus erythematosus. J Rheumatol. 2002;29(7):1536–42. [PubMed] [Google Scholar]
- 7.Andrade RM, Alarcon GS, Gonzalez LA, Fernandez M, Apte M, Vila LM, et al. Seizures in patients with systemic lupus erythematosus: data from LUMINA, a multiethnic cohort (LUMINA LIV) Ann Rheum Dis. 2008;67(6):829–34. doi: 10.1136/ard.2007.077594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mackworth-Young CG, Hughes GR. Epilepsy: an early symptom of systemic lupus erythematosus. J Neurol Neurosurg Psychiatry. 1985;48(2):185. doi: 10.1136/jnnp.48.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toubi E, Khamashta MA, Panarra A, Hughes GR. Association of antiphospholipid antibodies with central nervous system disease in systemic lupus erythematosus. Am J Med. 1995;99(4):397–401. doi: 10.1016/s0002-9343(99)80188-0. [DOI] [PubMed] [Google Scholar]
- 10.Sibley JT, Olszynski WP, Decoteau WE, Sundaram MB. The incidence and prognosis of central nervous system disease in systemic lupus erythematosus. J Rheumatol. 1992;19(1):47–52. [PubMed] [Google Scholar]
- 11.Hanly JG, Su L, Farewell V, McCurdy G, Fougere L, Thompson K. Prospective study of neuropsychiatric events in systemic lupus erythematosus. J Rheumatol. 2009;36(7):1449–59. doi: 10.3899/jrheum.081133. [DOI] [PubMed] [Google Scholar]
- 12.Hanly JG. Neuropsychiatric lupus. Rheum Dis Clin North Am. 2005;31(2):273–98. doi: 10.1016/j.rdc.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Hermosillo-Romo D, Brey RL. Neuropsychiatric involvement in systemic lupus erythematosus. Curr Rheumatol Rep. 2002;4(4):337–44. doi: 10.1007/s11926-002-0043-8. [DOI] [PubMed] [Google Scholar]
- 14.Diamond B, Volpe B. On the track of neuropsychiatric lupus. Arthritis Rheum. 2003;48(10):2710–2. doi: 10.1002/art.11278. [DOI] [PubMed] [Google Scholar]
- 15.Senecal JL, Raymond Y. The pathogenesis of neuropsychiatric manifestations in systemic lupus erythematosus: a disease in search of autoantibodies, or autoantibodies in search of a disease? J Rheumatol. 2004;31(11):2093–8. [PubMed] [Google Scholar]
- 16.DeGiorgio LA, Konstantinov KN, Lee SC, Hardin JA, Volpe BT, Diamond B. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat Med. 2001;7(11):1189–93. doi: 10.1038/nm1101-1189. [DOI] [PubMed] [Google Scholar]
- 17.Chapman J, Cohen-Armon M, Shoenfeld Y, Korczyn AD. Antiphospholipid antibodies permeabilize and depolarize brain synaptoneurosomes. Lupus. 1999;8(2):127–33. doi: 10.1191/096120399678847524. [DOI] [PubMed] [Google Scholar]
- 18.Kowal C, DeGiorgio LA, Nakaoka T, Hetherington H, Huerta PT, Diamond B, et al. Cognition and immunity; antibody impairs memory. Immunity. 2004;21(2):179–88. doi: 10.1016/j.immuni.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Katzav A, Solodeev I, Brodsky O, Chapman J, Pick CG, Blank M, et al. Induction of autoimmune depression in mice by anti-ribosomal P antibodies via the limbic system. Arthritis Rheum. 2007;56(3):938–48. doi: 10.1002/art.22419. [DOI] [PubMed] [Google Scholar]
- 20.Katzav A, Chapman J, Shoenfeld Y. CNS dysfunction in the antiphospholipid syndrome. Lupus. 2003;12(12):903–7. doi: 10.1191/0961203303lu500oa. [DOI] [PubMed] [Google Scholar]
- 21.Denburg SD, Denburg JA. Cognitive dysfunction and antiphospholipid antibodies in systemic lupus erythematosus. Lupus. 2003;12(12):883–90. doi: 10.1191/0961203303lu497oa. [DOI] [PubMed] [Google Scholar]
- 22.Omdal R, Brokstad K, Waterloo K, Koldingsnes W, Jonsson R, Mellgren SI. Neuropsychiatric disturbances in SLE are associated with antibodies against NMDA receptors. Eur J Neurol. 2005;12(5):392–8. doi: 10.1111/j.1468-1331.2004.00976.x. [DOI] [PubMed] [Google Scholar]
- 23.Hanly JG, Robichaud J, Fisk JD. Anti -NR2 glutamate receptor antibodies and cognitive function in systemic lupus erythematosus. J Rheumatol. 2006;33(8):1553–8. [PubMed] [Google Scholar]
- 24.Arnett FC, Reveille JD, Moutsopoulos HM, Georgescu L, Elkon KB. Ribosomal P autoantibodies in systemic lupus erythematosus. Frequencies in different ethnic groups and clinical and immunogenetic associations. Arthritis Rheum. 1996;39(11):1833–9. doi: 10.1002/art.1780391109. [DOI] [PubMed] [Google Scholar]
- 25.Karassa FB, Afeltra A, Ambrozic A, Chang DM, De Keyser F, Doria A, et al. Accuracy of anti-ribosomal P protein antibody testing for the diagnosis of neuropsychiatric systemic lupus erythematosus: an international meta-analysis. Arthritis Rheum. 2006;54(1):312–24. doi: 10.1002/art.21539. [DOI] [PubMed] [Google Scholar]
- 26.Isenberg D, Ramsey-Goldman R. Systemic Lupus International Collaborating Group--onwards and upwards? Lupus. 2006;15(9):606–7. doi: 10.1177/0961203306071868. [DOI] [PubMed] [Google Scholar]
- 27.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 28.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35(6):630–40. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 29.Gladman D, Ginzler E, Goldsmith C, Fortin P, Liang M, Urowitz M, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996;39(3):363–9. doi: 10.1002/art.1780390303. [DOI] [PubMed] [Google Scholar]
- 30.Ainiala H, Hietaharju A, Loukkola J, Peltola J, Korpela M, Metsanoja R, et al. Validity of the new American College of Rheumatology criteria for neuropsychiatric lupus syndromes: a population-based evaluation. Arthritis Rheum. 2001;45(5):419–23. doi: 10.1002/1529-0131(200110)45:5<419::aid-art360>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 31.Hanly JG, Urowitz MB, Su L, Sanchez-Guerrero J, Bae SC, Gordon C, et al. Short-term outcome of neuropsychiatric events in systemic lupus erythematosus upon enrollment into an international inception cohort study. Arthritis Rheum. 2008;59(5):721–9. doi: 10.1002/art.23566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanly JG, Urowitz MB, Sanchez-Guerrero J, Bae SC, Gordon C, Wallace DJ, et al. Neuropsychiatric events at the time of diagnosis of systemic lupus erythematosus: an international inception cohort study. Arthritis Rheum. 2007;56(1):265–73. doi: 10.1002/art.22305. [DOI] [PubMed] [Google Scholar]
- 33.Merrill JT, Zhang HW, Shen C, Butman BT, Jeffries EP, Lahita RG, et al. Enhancement of protein S anticoagulant function by beta2-glycoprotein I, a major target antigen of antiphospholipid antibodies: beta2-glycoprotein I interferes with binding of protein S to its plasma inhibitor, C4b-binding protein. Thromb Haemost. 1999;81(5):748–57. [PubMed] [Google Scholar]
- 34.Merrill JT, Shen C, Gugnani M, Lahita RG, Mongey AB. High prevalence of antiphospholipid antibodies in patients taking procainamide. J Rheumatol. 1997;24(6):1083–8. [PubMed] [Google Scholar]
- 35.Erkan D, Zhang HW, Shriky RC, Merrill JT. Dual antibody reactivity to beta2-glycoprotein I and protein S: increased association with thrombotic events in the antiphospholipid syndrome. Lupus. 2002;11(4):215–20. doi: 10.1191/0961203302lu178oa. [DOI] [PubMed] [Google Scholar]
- 36.Teh LS, Lee MK, Wang F, Manivasagar M, Charles PJ, Nicholson GD, et al. Antiribosomal P protein antibodies in different populations of patients with systemic lupus erythematosus. Br J Rheumatol. 1993;32(8):663–5. doi: 10.1093/rheumatology/32.8.663. [DOI] [PubMed] [Google Scholar]
- 37.Arnett FC, Hamilton RG, Roebber MG, Harley JB, Reichlin M. Increased frequencies of Sm and nRNP autoantibodies in American blacks compared to whites with systemic lupus erythematosus. J Rheumatol. 1988;15(12):1773–6. [PubMed] [Google Scholar]
- 38.Hanly JG, Urowitz MB, Siannis F, Farewell V, Gordon C, Bae SC, et al. Autoantibodies and neuropsychiatric events at the time of systemic lupus erythematosus diagnosis: results from an international inception cohort study. Arthritis Rheum. 2008;58(3):843–53. doi: 10.1002/art.23218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanly JG, Urowitz MB, Su L, Bae SC, Gordon C, Wallace DJ, et al. Prospective analysis of neuropsychiatric events in an international disease inception cohort of SLE patients. Ann Rheum Dis. 2009 doi: 10.1136/ard.2008.106351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Efthimiou P, Blanco M. Pathogenesis of neuropsychiatric systemic lupus erythematosus and potential biomarkers. Mod Rheumatol. 2009;19(5):457–68. doi: 10.1007/s10165-009-0198-5. [DOI] [PubMed] [Google Scholar]
- 41.Trysberg E, Nylen K, Rosengren LE, Tarkowski A. Neuronal and astrocytic damage in systemic lupuserythematosus patients with central nervous system involvement. Arthritis Rheum. 2003;48(10):2881–7. doi: 10.1002/art.11279. [DOI] [PubMed] [Google Scholar]
- 42.Love PE, Santoro SA. Antiphospholipid antibodies: anticardiolipin and the lupus anticoagulant in systemic lupus erythematosus (SLE) and in non-SLE disorders. Prevalence and clinical significance. Ann Intern Med. 1990;112(9):682–98. doi: 10.7326/0003-4819-112-9-682. [DOI] [PubMed] [Google Scholar]
- 43.Bonfa E, Golombek SJ, Kaufman LD, Skelly S, Weissbach H, Brot N, et al. Association between lupus psychosis and anti-ribosomal P protein antibodies. N Engl J Med. 1987;317(5):265–71. doi: 10.1056/NEJM198707303170503. [DOI] [PubMed] [Google Scholar]
- 44.Nojima Y, Minota S, Yamada A, Takaku F, Aotsuka S, Yokohari R. Correlation of antibodies to ribosomal P protein with psychosis in patients with systemic lupus erythematosus. Ann Rheum Dis. 1992;51(9):1053–5. doi: 10.1136/ard.51.9.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Press J, Palayew K, Laxer RM, Elkon K, Eddy A, Rakoff D, et al. Antiribosomal P antibodies in pediatric patients with systemic lupus erythematosus and psychosis. Arthritis Rheum. 1996;39(4):671–6. doi: 10.1002/art.1780390420. [DOI] [PubMed] [Google Scholar]
- 46.Jonsen A, Bengtsson AA, Nived O, Ryberg B, Truedsson L, Ronnblom L, et al. The heterogeneity of neuropsychiatric systemic lupus erythematosus is reflected in lack of association with cerebrospinal fluid cytokine profiles. Lupus. 2003;12(11):846–50. doi: 10.1191/0961203303lu472sr. [DOI] [PubMed] [Google Scholar]
- 47.Briani C, Lucchetta M, Ghirardello A, Toffanin E, Zampieri S, Ruggero S, et al. Neurolupus is associated with anti-ribosomal P protein antibodies: an inception cohort study. J Autoimmun. 2009;32(2):79–84. doi: 10.1016/j.jaut.2008.12.002. [DOI] [PubMed] [Google Scholar]