Abstract
Lysosomal storage diseases (LSDs) encompass a wide range of disorders characterized by inborn errors of lysosomal function. The majority of LSDs result from genetic defects in lysosomal enzymes, although some arise from mutations in lysosomal proteins that lack known enzymatic activity. Neuropathological abnormalities are a feature of several LSDs and when severe, represent an important determinant in disease outcome. Glial dysfunction, particularly in astrocytes, is also observed in numerous LSDs and has been suggested to impact neurodegeneration. This review will discuss the potential role of astrocytes in LSDs and highlight the possibility of targeting glia as a beneficial strategy to counteract the neuropathology associated with LSDs.
Keywords: Astrocytes, lysosomal storage diseases, mitochondrial dysfunction, neurodegeneration, reactive astrocytosis
INTRODUCTION
Lysosomes are essential organelles of eukaryotic cells whose function is degradation and recycling of macromolecules that are channeled through endocytosis, phagocytosis, and autophagy (Kroemer and Jaattela, 2005). Defects in lysosomal function may curtail degradation, which can result in the accumulation of substances within the lysosome. Lysosomal storage diseases (LSDs) represent a subgroup of inborn errors of metabolism primarily resulting from a deficiency of one or more lysosomal enzymes involved in macromolecule degradation, (for review see (Schultz et al., 2011, Cox and Cachon-Gonzalez, 2012, Platt et al., 2012, Boustany, 2013), although in some LSDs, the function of mutated protein(s) has yet to be determined (Bruun et al., 1991, Rakheja et al., 2007). Since the discovery of lysosomes by Christian de Duve (De Duve, 1963, 1966), over 60 distinct LSDs have been described, with a collective incidence estimated at 1:5,000 live births world-wide (Fuller et al., 2006). Roughly two thirds to three quarters of LSDs are neuropathic, which can affect multiple brain regions depending on the disease type. A few examples of LSDs that are associated with CNS pathology include, Gaucher disease, Krabbe disease, Sandhoff disease, Niemann-Pick Type C, and the group of neuronal ceroid lipofuscinoses (commonly referred to as Batten Disease; (Prada and Grabowski, 2013). This review will highlight select LSDs that affect the CNS, the neuropathological events associated with these disorders, and potential roles of reactive astrocytes in disease progression. The various enzymes/proteins that are mutated in the LSDs discussed in this review are all expressed in astrocytes, since they play a critical role in lysosomal homeostasis/function. However, an intriguing finding is that not all LSDs have dramatic CNS pathology, which brings into question the functional importance of mutated genes in the brain compared to other organs, even though all nucleated cells contain lysosomes. Even within the CNS, neuronal loss/dysfunction in many LSDs is often restricted to specific brain regions, which remains another enigma, since typically the mutated gene is ubiquitously expressed, although it is possible that differences in expression levels may dictate susceptibility. Another variable to consider is the cell type-specific impact of the mutation in neurons, astrocytes, microglia, or other populations, such as endothelial cells and how this influences pathology via autonomous or non-autonomous pathways. Alternatively, regional changes in the expression of other molecules that normally associate with the affected protein may be differentially regulated and could conceivably influence neuronal susceptibility in LSDs.
LSDs associated with enzyme deficiencies
Gaucher disease
Gaucher disease is caused by a deficiency in glucocerebrosidase (GBA), a lysosomal enzyme responsible for the degradation of glucocerebroside, an intermediate in glycolipid metabolism (Kampine et al., 1967, Jmoudiak and Futerman, 2005). Nearly 300 GBA mutations have been identified, including missense, nonsense, and frameshift mutations in addition to deletions, insertions, and complex alleles. Collectively, these mutations have been linked to 3 forms of Gaucher disease, classified as Type 1–3 (Grabowski et al., 1985). Type 1, also referred to as non-neuronopathic or adult Gaucher disease, is generally late onset and represents the most common form, with an ethnic predilection among Ashkenazi Jews (Gan-Or et al., 2008). Type 2 has the earliest onset, typically by 3 to 6 months of age, with death usually occurring by 2 years. Type 3 is a juvenile disease with an onset in early childhood. As a result of GBA deficiency, lysosomes accumulate several glycolipids, including glucocerebroside and glucosylsphingosine (Conradi et al., 1984, Farfel-Becker et al., 2014). The major cell type affected in Gaucher disease is the macrophage, where resident macrophage populations in the spleen and liver have perturbed homeostatic functions (Conradi et al., 1988). As a result, there is a marked splenomegaly, which destroys hematopoietic cells leading to anemia (Mandlebaum, 1912, Appel and Markowitz, 1971).
In terms of the CNS, the neuronopathic form of Gaucher disease has been associated with neurodegeneration in layer V of the cerebral cortex, lateral globus pallidus, various thalamic nuclei, and hippocampal CA2-CA4 regions (Conradi et al., 1984, Wong et al., 2004, Farfel-Becker et al., 2014). It is currently not well understood why these particular brain regions are selectively targeted given the ubiquitous expression of GBA; however, the collective evidence clearly indicates that it is not due to storage material accumulation (Vitner et al., 2012, Farfel-Becker et al., 2014, Vitner et al., 2014). The neuronopathic forms of Gaucher disease are also characterized by microglial proliferation, astrocytosis, and a robust neuroinflammatory response (Vitner et al., 2012, Vitner and Futerman, 2013). A mouse model of Gaucher disease where GBA was selectively deleted in neurons and glial cells resulted in increased expression of the lysosomal enzyme cathepsin D in reactive astrocytes (Vitner et al., 2010a), which may represent a compensatory mechanism to offset GBA deficiency. However, the consequences of exaggerated cathepsin D expression in this model and the overall functional role that astrocytes play in Gaucher disease still remains to be identified. Interestingly, although the disease is known to target macrophage functions in the periphery, little information is available regarding the impact of GBA deficiency in microglia, although it has been shown that wild type microglia cannot rescue neurodegeneration associated with Gaucher disease (Enquist et al., 2007).
Krabbe disease
Krabbe disease, also known as globoid cell leukodystrophy (GLD), results from β-galactocerebrosidase deficiency, which catalyzes the hydrolysis of galactose from several sphingolipids, including galactosylceramide, lactosylceramide, and galactosylsphingosine, to generate ceramide and sphingosine (Andrews and Cancilla, 1970, Andrews et al., 1971). β-galactocerebrosidase loss leads to the accumulation of the toxic glycosphingolipid psychosine (Suzuki and Suzuki, 1985). Krabbe disease is an early onset LSD, with symptoms typically presenting around 6 months of age and mortality occurring by 2 years (Wenger et al., 1997). Krabbe disease primarily affects the CNS, resulting in extensive demyelination of cerebral white matter tracts leading to spasticity, ataxia, blindness, seizures, and severe dementia (Husain et al., 2004, Kohlschutter, 2013). The neuropathology associated with Krabbe disease has been attributed, in large part, to the abnormal accumulation of psychosine in the brain (Igisu and Suzuki, 1984b, a, Cantuti Castelvetri et al., 2013). The disease is typified by abnormal axonal transport and severe axonal loss, which is accompanied by astrogliosis (Jesionek-Kupnicka et al., 1997, Castelvetri et al., 2011). Metabolic alterations in astrocytes have been reported in a mouse model of Krabbe disease, which included increased glutamine levels and upregulation of lactate-specific monocarboxylic acid transporters (Meisingset et al., 2013). Additionally, primary astrocytes isolated from Krabbe disease mice displayed increased prostaglandin receptor (DP1 and DP2) expression (Mohri et al., 2006) and IL-6 production was elevated in reactive astrocytes in the CNS (LeVine and Brown, 1997). However, the functional significance of these alterations in astrocyte properties in Krabbe disease and how they may impact neuron survival or function remain unknown.
Microglial activation has also been reported in patients with Krabbe disease, which is consistent with a prominent neuroinflammatory response (Smith et al., 2014). This robust inflammatory response likely results from pronounced cell loss and release of danger-associated molecular patterns (DAMPs) from damaged/dying neurons that can trigger inflammatory pathways, which in turn, further exacerbate neuron damage. Indeed, psychosine has been reported to exert inflammatory and apoptotic effects in glia (Giri et al., 2002).
Sandhoff disease
Sandhoff disease is caused by a deficiency in β-hexosaminidases A and B, resulting in the excessive lysosomal accumulation of GM2 gangliosides and oligosaccharides containing glucosamine residues (Itoh et al., 1984). There are three forms of Sandhoff disease, namely infantile, juvenile, and adult onset (O’Dowd et al., 1986). The infantile form is the most aggressive and typically presents between 2–9 months age, with death occurring before 3 years (O’Dowd et al., 1986). The juvenile form of Sandhoff disease is less common than the infantile variant, with clinical symptoms evident between the ages of 3–10 years, which include organomegaly, bone deformations, and CNS manifestations, such as speech disabilities, cerebral ataxia, and severe psychomotor disturbances (O’Dowd et al., 1986). Neuropathological abnormalities associated with Sandhoff disease include prominent cerebellar atrophy and ventricular dilatation. Histologically, neurons harbor membranous cytoplasmic bodies (MCBs) formed by the accumulation of GM gangliosides and other lipopigments in the lysosome (Itoh et al., 1984). Lectin staining has revealed the prominent accumulation of complex carbohydrates, such as N-acetylglucosamine, in astrocytes (Alroy et al., 1988) and MCBs are also observed in children with Sandhoff disease (Itoh et al., 1984). Astrogliosis is a prominent feature of Sandhoff disease, although the functional implications of reactive astrocytes remain unknown (Myerowitz et al., 2002). An earlier report examining primary astrocytes isolated from a mouse model of Sandhoff disease demonstrated increased proliferation that was associated with elevated ERK phosphorylation and sphingosine-1-phosphate (S1P) synthesis (Kawashima et al., 2009). These changes in astrocytes were dependent on GM2 ganglioside accumulation within the lysosome. Additionally, a direct relationship between S1P metabolism and reactive astrocytosis has been reported in a mouse model of Sandhoff disease, where the deletion of sphingosine kinase (which synthesizes S1P) or sphingosine-1 phosphate receptor reduced astrocyte proliferation and reactive astrocytosis (Wu et al., 2008). Interestingly, S1P has recently emerged as a key neuroinflammatory mediator in multiple sclerosis and is being explored as a potential therapeutic target to attenuate disease severity (Brinkmann, 2009, Graler, 2010, Chun and Brinkmann, 2011). Accordingly, astrocytes from Sandhoff disease mice produced the chemokine macrophage-inflammatory protein 1α (MIP-1α) (Wu and Proia, 2004), which when deleted improved the neurological status and lifespan of these animals (Wu and Proia, 2004). These observations support a pathological role for astrocytes in neuronal dysfunction and Sandhoff disease progression.
Multiple sulfatase deficiency
Multiple sulfatase deficiency (MSD) is caused by a mutation in sulfatase modifying factor 1 (Sumf1) that results in post-translational defects in lysosomal sulfatases and the pathological accumulation of mucopolysaccharides (Austin, 1973, Eto et al., 1980). There are three types of MSD, namely neonatal, late-infantile, and juvenile (Busche et al., 2009). The infantile form of MSD is the most aggressive, with symptoms beginning soon after birth. Clinical manifestations include coarsened facial features, ichthyosis, deafness, splenomegaly, and hepatomegaly (Burk et al., 1984, Macaulay et al., 1998, Diaz-Font et al., 2005). Children with MSD develop leukodystrophy, leading to movement disorders and developmental delay with occasional seizures (Guerra et al., 1990, Incecik et al., 2013). The late-infantile form is the most common type of MSD. These children have normal cognitive development in early childhood but experience a rapid decline in motor and cognitive abilities that are attributed to progressive leukodystrophy (Kohlschutter, 2013). Neuroimaging studies have revealed periventricular lesions extending into the corpus callosum and brain stem (Guerra et al., 1990). MSD is also typified by extensive demyelination, with the accumulation of metachromatic material composed of cholesterol and galactolipid pigments in CNS glia and peripheral macrophages (Annunziata et al., 2007). A recent study demonstrated that the targeted deletion of Sumf1 in astrocytes using a Cre-Lox mouse model resulted in severe lysosomal storage material deposition and cortical neuron loss in vivo (Di Malta et al., 2012b). Sumf1-deficient astrocytes also failed to promote the survival and function of wild type neurons, suggesting a non-cell autonomous mechanism for neurodegeneration. This study was the first to demonstrate that astrocytes play a key role in MSD neuropathology (Di Malta et al., 2012b); however, the mechanisms responsible for astrocyte-mediated neuronal dysfunction and death remain to be identified.
LSDs associated with other lysosomal defects
Niemann-Pick Type C
Niemann-Pick type C (NPC) is caused by mutations in one of two genes, NPC1 or NPC2, which manifest as severe abnormalities in lipid trafficking (Lloyd-Evans and Platt, 2010). Both NPC1 and NPC2 are predicted to encode proteins involved in cholesterol homeostasis, which accounts for cholesterol and glycosphingolipid accumulation within the lysosome (Palmer et al., 1985, Sokol et al., 1988, Zervas et al., 2001a, Zervas et al., 2001b, Zhou et al., 2011, Mengel et al., 2013, Platt et al., 2014). NPC affects both peripheral organs and the CNS, with symptoms ranging from splenomegaly and hepatomegaly to neurological abnormalities, including psychomotor disturbances, ataxia, and seizures (Lloyd-Evans and Platt, 2010, Mengel et al., 2013). Neuroimaging studies have revealed diffuse cerebral atrophy and white matter changes (Palmer et al., 1985). In chronic progressive cases, neurofibrillary tangles similar to those in Alzheimer’s disease (AD) have been reported (Ohm et al., 2003). Astrocyte and microglial activation is also associated with NPC (Patel et al., 1999, German et al., 2002, Suzuki et al., 2003), where astrocytes exhibit reduced gap junction communication and mitochondrial dysfunction (Saez et al., 2013). Increased levels of IL-1β as well as increased ApoE, a genetic risk factor for Alzheimer’s disease, have been reported in animals models of NPC (Yan et al., 2014). Consistent with this study, increased expression of amyloid precursor protein (APP) as well as β- and γ-secretase were found in reactive astrocytes in mice with NPC disease (Kodam et al., 2010) suggesting an association between NPC and AD.
Neuronal ceroid lipofuscinosis (NCL)
Neuronal ceroid lipofuscinosis (NCL), commonly referred to as Batten disease, represents a family of disorders caused by mutations in ceroid lipofuscinosis (CLN) genes (Getty et al., 2007, Aberg et al., 2009). To date, mutations in 14 different CLN genes have been identified that are broadly classified into infantile, late-infantile, juvenile, and adult onset forms (Aberg et al., 2009, Lebrun et al., 2011). The childhood forms of Batten disease are characterized by blindness, behavioral deficits, seizures, and progressive cognitive and motor impairment that leads to premature death (Sinha et al., 2004, Cotman et al., 2013). A histopathological hallmark of all NCLs is the lysosomal accumulation of autofluorescent lipopigments and proteins; however, the structural presentation of inclusion material varies according to each disease type (Palmer et al., 1986). Biochemical characterization of storage material has also identified lipophilic proteins, including subunit C of mitochondrial ATP synthase (SCAMS, primarily in Juvenile NCL) or sphingolipid pigments (in other forms of NCL) (Pardo et al., 1994, Goebel et al., 1999, Fossale et al., 2004).
Infantile NCL (INCL) is the most aggressive NCL form, with a life expectancy of 2–6 years (Hawkins-Salsbury et al., 2013). INCL is caused by a mutation in CLN1, which encodes for palmitoyl protein thioesterase (PPT1), an enzyme responsible for the cleavage of long-chain fatty acid residues on several proteins containing cysteine moieties (Vesa et al., 1995, Hofmann et al., 1997). Clinical symptoms of INCL can manifest as early as 6 months of age, which rapidly progress to severe motor and cognitive deficits, seizures, and premature death (for review see (Cotman et al., 2013). Late-infantile NCL (LINCL) is caused by mutations in CLN2 that encodes the lysosomal enzyme tripeptidyl peptidase I (TPPI), which cleaves tripeptides from the terminal amine groups of partially unfolded proteins (Sleat et al., 1997, Sleat et al., 1999). Juvenile NCL (JNCL) results from mutations in the CLN3 gene (for review see (Cotman et al., 2013). The precise function of CLN3 remains unknown; however, based on functional analysis in yeast and mammalian cell culture models, CLN3 has been predicted to regulate lysosomal acidification, endocytic and vesicle trafficking, and proper maintenance of mitochondrial function (Pearce et al., 1999, Kim et al., 2003, Luiro et al., 2004). Similar to INCL and LINCL, JNCL also presents with visual impairment, seizures, and progressive cognitive and motor decline, but with an advanced onset, typically between 5–10 years of age (Cotman et al., 2013). Astrocyte and microglial involvement in NCLs will be discussed in more detail in a later section.
NEURODEGENERATION IN LSDs
Many LSDs are associated with neurodegeneration, which manifests as moderate to severe neuronal death in multiple brain regions, including the thalamus, cerebral cortex, hippocampus and cerebellum (Folkerth, 1999, Prada and Grabowski, 2013). Neuron loss is usually accompanied by reactive astrocytosis that has been suggested to further exacerbate neuronal abnormalities (Jesionek-Kupnicka et al., 1997, Vitner et al., 2010b). While the mechanisms responsible for neurodegeneration in many LSDs is not completely understood, the abnormal accumulation of lysosomal storage material due to defective degradation mechanisms was originally thought to contribute to neuronal loss in LSDs (Kiselyov et al., 2007, Settembre et al., 2008, Lieberman et al., 2012) as well as in other neurodegenerative disorders typified by protein aggregation, such as AD and Parkinson’s Disease (PD) (Matsuda and Tanaka, 2010, Tan et al., 2014). However, this possibility has recently been called into question in LSDs based on the finding that lysosomal storage material accumulation is typically widespread in neurons throughout the brain, yet only select neuron populations die. Nevertheless, neurons are post-mitotic and unable to eliminate unwanted organelles and macromolecules by cell division. Therefore, neurons rely heavily on functional lysosomes to efficiently clear these substances by a process referred to as autophagy. Autophagy delivers cytosolic components to lysosomes by enveloping them into autophagosomes, which fuse with lysosomes to facilitate the degradation of vesicle contents (Nixon, 2006, Lieberman et al., 2012). Autophagic defects have been reported in several LSDs, including Pompe disease, MSD, NPC, Gaucher disease, and NCLs (Lieberman et al., 2012), which have been suggested to contribute to neurodegeneration. Additionally, lysosomal membrane permeability (LMP) has been demonstrated in several LSDs, including late-infantile NCL (Micsenyi et al., 2013) and mucopolysaccharidosis type I (Pereira et al., 2010). Aberrant autophagy has been suggested as a link to LMP, which has been shown to enhance protein aggregates and may trigger neurodegeneration (Rodriguez-Muela et al., 2015).
Several lines of evidence also support mitochondrial dysfunction in CNS cells in various LSDs, including Gaucher disease, MSD, NPC, and mucopolysaccharidoses (Yu et al., 2005, Osellame and Duchen, 2014). In addition, perturbed mitochondrial Ca2+ homeostasis has also been observed in the aforementioned LSDs, including decreased Ca2+ buffering capacity and reduced ATP production as well as mitochondrial fragmentation (Kiselyov et al., 2007, de Pablo-Latorre et al., 2012, Chandrachud et al., 2015). A reduction in mitochondrial membrane potential and concomitant decrease in ATP was shown in a mouse model of NPC1 (Yu et al., 2005). Decreased oxygen consumption and mitochondrial electron transport chain enzymes have been reported in neurons from a mouse model of JNCL (CLN3−/−) (Luiro et al., 2006). Similarly, enlarged mitochondria were observed in a cerebellar neuronal granular cell line derived from JNCL mice (Fossale et al., 2004); however, it should be noted that this cell type is not lost in the disease. Mitochondrial dysfunction in astrocytes has also been observed in several LSDs, including Gaucher, GM1-gangliosidosis, and NPC (Takamura et al., 2008, Osellame and Duchen, 2014). Therefore, mitochondrial abnormalities may represent a common feature of LSDs, raising the possibility that an energy crisis could be one mechanism responsible for the neurodegenerative process. The nature and purported functions of reactive astrocytes in the context of LSDs will be discussed in greater detail in the following section.
DO REACTIVE ASTROCYTES CONTRIBUTE TO NEURONAL LOSS IN LSDs?
Reactive astrocytosis occurs in response to a variety of insults/stimuli, which can accompany either acute (i.e. traumatic brain injury, cerebral ischemia) or chronic neurological conditions (i.e. AD, PD, and LSDs; for review see (Burda and Sofroniew, 2014, Pekny and Pekna, 2014). While several studies have reported extensive neuronal loss in humans and animal models of LSDs (Folkerth, 1999, Prada and Grabowski, 2013), the involvement of other CNS cells, particularly astrocytes, in the pathogenesis of LSDs have not been extensively investigated. Reactive astrocytosis is a common sequelae in a large number of LSDs; however, its functional consequences remain unclear. It is possible that astrocytes respond to neuronal dysfunction in a protective manner in an attempt to restore homeostasis. Alternatively, activated astrocytes may exacerbate neuron loss by diverting their homeostatic functions (i.e. glutamate uptake and metabolism, pH/ion buffering) towards other pathways that are not conducive to neuron survival, as they also express the same genes that are mutated in neurons. Numerous signals can elicit reactive astrocytosis; however, in the context of LSDs, danger-associated molecular patterns (DAMPs) released from damaged or dying cells appear to represent the most physiological relevant stimuli to trigger astrocyte acitvation (Vincent et al., 2007).
Upon activation, astrocytes undergo a morphological transformation and alter their gene expression profiles. A major hallmark of reactive astrocytosis is increased expression of the intermediate filament proteins glial fibrillary acidic protein (GFAP) and vimentin (for review, see (Hol and Pekny, 2015). The precise function of augmenting these proteins is not clear; however, studies have shown that both GFAP and vimentin participate in limiting the extent of CNS damage by sequestering affected areas following stroke or spinal cord injury (Pekny et al., 1999, Li et al., 2008). Along these lines, a recent study has reported that reactive astrocytes play a protective role in infantile NCL (Macauley et al., 2011). Specifically, CLN1/GFAP/vimentin triple mutant mice displayed more aggressive disease progression than WT animals, which correlated with increased neuroinflammation (Macauley et al., 2011, Shyng and Sands, 2014). Because of the selectivity of GFAP and vimentin to astrocytes, protective responses were predicted to be astrocyte-derived; however, it remains possible that additional factors may have contributed to the accelerated disease phenotype in these mice. For example, it is known that astrocytes display a significant degree of crosstalk with neurons and even microglia (Bezzi et al., 2001, Liu et al., 2011). Therefore, if astrocyte function was impacted by the loss of GFAP and vimentin in the context of CLN1 mutation, this would have worsened neuron survival and contributed to more aggressive disease, although this possibility remains speculative.
Despite the fact that numerous LSDs are typified by reactive astrocytosis, the stimuli responsible for astrocyte activation are not completely known. Although intrinsic changes resulting from the accumulation of lysosomal material could be one trigger, it is possible that non-autonomous signals from neighboring cells, such as neurons or microglia, could drive astrocyte activation by sensing DAMPs released from dysfunctional or dying cells (Figure 1). This possibility is feasible, since inflammatory mediators and other DAMPs are known to induce reactive astrocytosis in various neurological conditions (Vincent et al., 2007). Relevant to LSDs, microglia in a mouse model of Juvenile Batten Disease (JNCL) have been shown to exist in a “primed” state, producing exaggerated levels of numerous proinflammatory mediators in response to DAMPs, whereas wild type microglia were relatively non-responsive (Xiong and Kielian, 2013). By extension, these microglial-derived inflammatory mediators can trigger astrocyte activation to establish a perpetual inflammatory cycle that can induce neuronal death under chronic conditions. Indeed, CLN3 mutant neurons were more susceptible to cytokine-induced death compared to WT neurons (Xiong and Kielian, 2013). It will be important to investigate the other consequences of CLN3 deficiency for astrocytes and microglia and the potential consequences this may have for neurons. This will be achieved with the use of CLN3 conditional knockout mice that are currently in development, where CLN3 is selectively excised in astrocytes or microglia using glial-specific Cre-deleter lines.
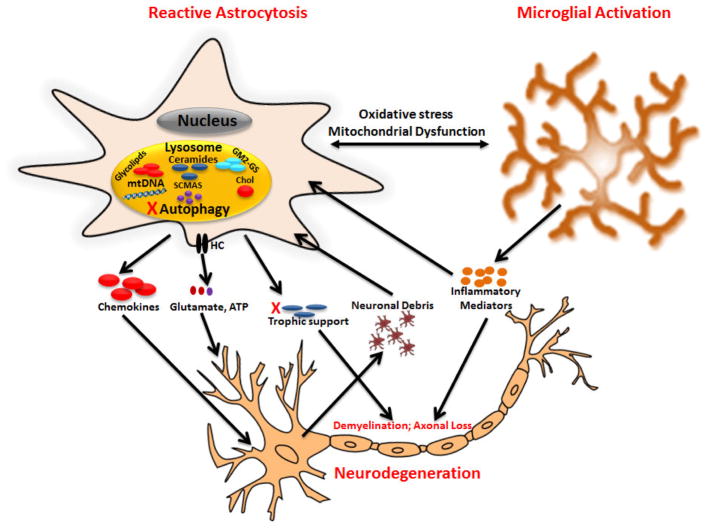
Figure 1. Potential contribution of reactive astrocytes to neurodegeneration in LSDs.
Lysosomal dysfunction in astrocytes and microglia likely contributes to neuron death in a non-cell autonomous manner through the release of damaging mediators and/or loss of trophic support. In addition, disruption of lysosomal homeostasis in neurons also regulates cell death in a cell autonomous manner. GM2-GS, GM2-gangliosides, HC, hemichannels, mtDNA, mitochondrial DNA, chol, cholesterol.
In terms of the potential molecular mechanisms whereby LSDs alter astrocyte properties and how these changes affect neuronal survival or function, we can look to other neurodegenerative diseases for examples, since as mentioned above, common themes have recently emerged. For example, various signaling pathways have been shown to contribute to reactive astrocytosis in acute and chronic neurological conditions (Pekny and Nilsson, 2005, Burda and Sofroniew, 2014). These include JAK/STAT3 signaling and ERK1/2 phosphorylation (Sriram et al., 2004). Notably, JAK/STAT3 activation was observed in a mouse model of Sandhoff disease (Hexb−/−) that was mediated by microglial-derived TNF-α production. Inhibition of TNF-α in Hexb/Tnf-α double knockout mice significantly inhibited astrocyte activation and reduced neuronal death. These changes coincided with significantly increased lifespan, enhanced sensorimotor coordination, and improved neurological function. Interestingly, these improvements in Hexb/Tnf-α double knockout animals were not accompanied by alterations in ganglioside accumulation in neurons (Abo-Ouf et al., 2013). Likewise, increased ERK phosphorylation was shown in a sheep model of infantile NCL, where reactive astrocytes are a prominent feature and associated with aggressive neurodegeneration (Kanninen et al., 2013).
Many LSDs have been associated with defects in autophagy (Kiselyov et al., 2007, Settembre et al., 2008, Lieberman et al., 2012), which could represent another pathway to induce astrocytosis, since cells are unable to clear cellular debris (Lee et al., 2009, Di Malta et al., 2012b). In addition, autophagic inhibition could lead to cytoplasmic DAMP release from damaged/senescent organelles to trigger astrocyte activation (Figure 1). Consistent with this tenet, increased lactosylceramide (LacCer), a glycolipid shown to be dysregulated in mice with experimental autoimmune encephalomyelitis (EAE), a model of multiple sclerosis, was shown to activate astrocytes and cause neurodegeneration (Chen et al., 1999). Inhibition of excessive LacCer formation not only reduced astrocytosis but also diminished neurodegeneration in these animals (Chen et al., 1999). Numerous LSDs, including NPC, GM1 gangliosidosis, acid lipase deficiency, and mucopolysaccharidoses, are typified by lysosomal LacCer accumulation (Di Malta et al., 2012b) and it is intriguing to consider whether this could represent a mechanism to trigger astrocyte activation. Finally, an autophagic block could also create nutrient and energy stresses on the cell that could elicit reactive astrocytosis.
Additional molecules that accumulate within astrocytes in various LSDs may also contribute to astrocyte activation and associated pathology. For example, ceramide species known to accrue in select LSDs can induce reactive astrocytosis (Bassi et al., 2006) by activating NF-κB (Calatayud et al., 2005) and MAPKs (Oh et al., 2006). In addition, ceramides have well-described pro-apoptotic activity (Jatana et al., 2002, Oh et al., 2006), which could contribute to astrocyte loss during more advanced stages of disease, although this remains speculative and has not yet been demonstrated in any LSD to date. Cathepsin D is another molecule that is elevated in astrocytes during Gaucher disease and was implicated in astrocyte dysfunction (Choi et al., 2002). Furthermore, astrocytes from Sandhoff disease mice have been shown to possess inflammatory activity via chemokine production (Wu and Proia, 2004) that may amplify CNS inflammation and collateral neuronal damage. To date, only a few studies have directly demonstrated a role for reactive astrocytes in neurodegeneration in select LSDs. As mentioned earlier, a recent report showed that targeted deletion of Sumf1 (mutation in MSD) in astrocytes using a Cre-Lox mouse model resulted in severe lysosomal storage and induced cortical neuron loss in vivo (Di Malta et al., 2012b, a). Sumf1-deficient astrocytes also failed to promote the survival and function of wild type neurons, suggesting a non-cell autonomous mechanism for neurodegeneration. Indirect evidence also suggests that astrocyte dysfunction in LSDs may exert detrimental effects on neurons. Such information has been largely derived from targeted gene therapy, where the re-insertion of deficient genes into astrocytes was shown to limit neurodegeneration. For example, NPC1 gene transduction into astrocytes was shown to enhance survival, decrease neuronal cholesterol accumulation, and reduce neurodegeneration in a NPC mouse model (Zhang et al., 2008). Likewise, retroviral-mediated transduction of the human GBA gene into astrocytes and oligodendrocytes in Twitcher mice was shown to correct the neuronal phenotype in these animals (Luddi et al., 2001).
Additionally, several lines of evidence indicate that reactive astrocytosis precedes neuronal loss in some LSDs, including various forms of Batten disease. For example, studies have shown early prominent astrocyte activation, as indicated by increased GFAP expression, in JNCL mouse models that predates neuronal loss in these mice that is not evident until around 12 months of age (Pontikis et al., 2004, Burkovetskaya et al., 2014). Likewise, a sheep model of variant late-infantile NCL (CLN6) displays overt astrocyte activation in several brain regions prior to any neurodegeneration (Oswald et al., 2005). Astrocyte activation also precedes neuron loss in other NCL forms, including CLN1 and CLN8 disease (Macauley et al., 2009, Kuronen et al., 2012).
Recent evidence suggests that astrocytes express functional neurotransmitter receptors that actively participate in synaptic transmission at the tripartite synapse (Perea et al., 2009) and also influence neuronal synapses by releasing gliotransmitters (Perea et al., 2009, Araque et al., 2014). One proposed mechanism for astrocyte gliotransmitter release is through hemichannels (HCs; for review see (Bosch and Kielian, 2014, Montero and Orellana, 2015). A HC is composed of a hexameric ring of connexin proteins and constitutes one-half of a gap junction channel. HCs form gap junctions when they align with adjacent HCs in neighboring cells; however, HCs can also be formed by pannexins that are incapable of forming gap junctions (Saez and Leybaert, 2014). HCs have been reported to open under both physiological and pathological conditions to facilitate the release of various small m.w. metabolites and gliotransmitters from astrocytes, including ATP, glucose, glutathione, glutamate, GABA, and D-serine (Giaume et al., 2013, Abudara et al., 2014, Abudara et al., 2015, Montero and Orellana, 2015). Transient HC opening may provide a mechanism for fine tuning changes in intracellular and extracellular molecular gradients across the astrocyte membrane; however, chronic HC activity can have catastrophic consequences by dissipating CNS chemical gradients that require a significant energetic cost to establish (Saez et al., 2013, Bosch and Kielian, 2014). For example, opening of connexin 43 hemichannels in astrocytes and subsequent release of ATP and glutamate has been shown to induce neuronal death during hypoxic conditions (Orellana et al., 2011a), in an in vitro slice culture model of Alzheimer’s disease (Orellana et al., 2011b), as well as during HIV and bacterial infection (Karpuk et al., 2011, Orellana et al., 2014). In the context of LSD, astrocyte HC activity was transiently increased in acute brain slices derived from a mouse model of JNCL at an early postnatal age (1 month) that significantly preceded neuronal loss, which occurs around 12 months in this model (Burkovetskaya et al., 2014). Interestingly, the expression of astrocyte-specific glutamate transporters and glutamine synthetase was significantly decreased with increasing age of CLN3 mutant mice, suggesting a decline in astrocyte function. This possibility was reinforced by electrophysiological findings in acute brain slices, demonstrating abnormalities in resting membrane potential and conductance in CLN3 mutant astrocytes (Burkovetskaya et al., 2014). It is noteworthy that in light of these changes, astrocytes in the CLN3 mutant mouse were shown to be activated as typified by increased GFAP expression. Blockade of HC activity using the novel carbenoxolone-based inhibitor INI-0602 enhanced gap junction communication and led to significant reductions in lysosomal storage material in the brains of CLN3 mutant mice (Burkovetskaya et al., 2014). Collectively, this supports the premise that astrocyte dysfunction likely influences neuropathology in LSDs, although it remains to be determined whether this is a universal phenomenon or restricted to specific LSDs.
SIMILARITIES BETWEEN LSDs AND PARKINSON’S DISEASE
While LSDs are a distinct class of disorders resulting from inborn errors in lysosomal metabolism, neuropathological similarities between LSDs and PD exist (for review see (Deng et al., 2014). For example, clinical symptoms such as bradykinesia, rigidity, and tremors characteristic of PD are also found in some of the LSDs, including JNCL (Aberg et al., 2000, Valadares et al., 2011). Further, extensive reports describing astrocyte activation (Schneider and Denaro, 1988, Blunt et al., 1992, Renkawek et al., 1999, Episcopo et al., 2013) and dysfunction in PD, including aberrant autophagy (Osellame et al., 2013), proteasomal defects (Jansen et al., 2014) and neuroinflammation (Tilleux and Hermans, 2007) are also common features of various LSDs (Jesionek-Kupnicka et al., 1997, Pontikis et al., 2004, Vitner et al., 2010b, Di Malta et al., 2012a, b, Burkovetskaya et al., 2014). Additionally, both JNCL and PD share certain similarities in the brain regions affected, including nigrostriatal pathology, as well as abnormal substrate accumulation, either lysosomal storage or α-synuclein, respectively (Jarvela et al., 1997, Weimer et al., 2007). There is also growing evidence that heterozygous carriers of Gaucher and NPC mutations have increased risk for developing PD, which clearly highlights a link between these diseases (Neudorfer et al., 1996, Volders et al., 2002, Halperin et al., 2006, Sidransky et al., 2009b, Goker-Alpan et al., 2012). Indeed, a multicenter analysis examining GBA mutations in PD patients revealed an odds ratio of 5.43 compared to controls. PD patients harboring a GBA mutation had an earlier disease onset (on average 4 years earlier) and were more likely to experience atypical clinical disease, making GBA mutations among the most common genetic risk factors for PD identified to date (Sidransky et al., 2009a). Similar to GD, patients with Niemann-Pick display α-synucleopathy (Coleman et al., 1988, Josephs et al., 2004, Saito et al., 2004).
LSDs and PD also share attributes at the subcellular level, suggesting that some aspects of pathology may overlap. Specifically, both disorders display evidence of disturbed autophagy-lysosomal pathways, proteasome defects, mitochondrial abnormalities, and intracellular accumulation of macromolecules (Pan et al., 2008, Osellame and Duchen, 2014). Other features, such as glial activation and selective neuron loss in specific brain regions are also common between LSDs and PD (Aberg et al., 2000, Wong et al., 2004). Therefore, it is possible that treatment strategies designed for PD may also exert beneficial effects to counteract neurological abnormalities in patients with specific LSDs, although this remains to be determined.
CONCLUDING REMARKS
Lysosomal storage diseases are debilitating disorders caused by inborn errors in lysosomal proteins. Numerous LSDs are associated with CNS pathology, with prominent neurodegeneration typically occurring in early childhood, which reduces life expectancy. Unfortunately, there is no cure for these diseases, which has resulted in aggressive attempts for gene/enzyme replacement therapy to counteract the pathological consequences of these diseases. Available evidence suggests that astrocyte activation is an early indicator of CNS pathology in these disorders, which conceivably contributes to neurodegeneration in later stages of disease progression. However, whether astrocyte dysfunction is a primary cause of neuronal loss or rather a consequence of reactivity to diseased neurons in the context of LSDs remains the proverbial “chicken and the egg” question. Recent studies employing selective enzyme/gene replacement strategies in astrocytes have revealed significant reductions in neuronal loss as well as improved survival, which strongly suggest that neurodegeneration in LSDs may in part be a consequence of astrocyte dysfunction. However, it remains to be determined if astrocyte involvement in neuronal demise is a universal phenomenon across several LSDs or rather, is only applicable to select degenerative processes. Nevertheless, targeting glial dysfunction in these LSDs may have a therapeutic benefit, an avenue that is currently being pursued.
HIGHLIGHTS.
Several lysosomal storage diseases (LSDs) are associated with CNS neuron loss.
Astrocyte dysfunction is observed in numerous LSDs and is suggested to impact neurodegeneration.
Enzyme/gene replacement in astrocytes reduces neuron loss, implicating astrocyte dysfunction in LSD pathology.
Acknowledgments
This work was supported by the National Institute of Neurological Disorders and Stroke (NINDS) 1R21NS084392-01A1 to T.K. The authors thank Dr. Jonathan Cooper at King’s College London for critical review of the manuscript.
ABBREVIATIONS
- AD
Alzheimer’s Disease
- CNS
central nervous system
- DAMP
danger associated molecular pattern
- GBA
glucocerebrosidase
- GLD
globoid cell leukodystrophy
- GFAP
glial fibrillary acidic protein
- INCL
Infantile Neuronal Ceroid Lipofuscinosis
- JNCL
Juvenile Neuronal Ceroid Lipofuscinosis
- LINCL
Late Infantile Neuronal Ceroid Lipofuscinosis
- LMP
lysosomal membrane permeability
- LSD
lysosomal storage disease
- MSD
multiple sulfatase deficiency
- NPC
Niemann-Pick type C
- PAMP
pathogen associated molecular pattern
- PPT1
palmitoyl protein thioesterase
- TPP1
tripeptidyl peptidase I
- PD
Parkinson’s Disease
- SCMAS
subunit C mitochondrial ATP synthase
References
- Aberg L, Lauronen L, Hamalainen J, Mole SE, Autti T. A 30-year follow-up of a neuronal ceroid lipofuscinosis patient with mutations in CLN3 and protracted disease course. Pediatr Neurol. 2009;40:134–137. doi: 10.1016/j.pediatrneurol.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Aberg L, Liewendahl K, Nikkinen P, Autti T, Rinne JO, Santavuori P. Decreased striatal dopamine transporter density in JNCL patients with parkinsonian symptoms. Neurology. 2000;54:1069–1074. doi: 10.1212/wnl.54.5.1069. [DOI] [PubMed] [Google Scholar]
- Abo-Ouf H, Hooper AW, White EJ, van Rensburg HJ, Trigatti BL, Igdoura SA. Deletion of tumor necrosis factor-alpha ameliorates neurodegeneration in Sandhoff disease mice. Hum Mol Genet. 2013;22:3960–3975. doi: 10.1093/hmg/ddt250. [DOI] [PubMed] [Google Scholar]
- Abudara V, Bechberger J, Freitas-Andrade M, De Bock M, Wang N, Bultynck G, Naus CC, Leybaert L, Giaume C. The connexin43 mimetic peptide Gap19 inhibits hemichannels without altering gap junctional communication in astrocytes. Front Cell Neurosci. 2014;8:306. doi: 10.3389/fncel.2014.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abudara V, Roux L, Dallerac G, Matias I, Dulong J, Mothet JP, Rouach N, Giaume C. Activated microglia impairs neuroglial interaction by opening Cx43 hemichannels in hippocampal astrocytes. Glia. 2015;63:795–811. doi: 10.1002/glia.22785. [DOI] [PubMed] [Google Scholar]
- Alroy J, Adelman LS, Warren CD. Lectin histochemistry of gangliosidosis. II. Neurovisceral tissues from patients with Sandhoff’s disease. Acta Neuropathol. 1988;76:359–365. doi: 10.1007/BF00686972. [DOI] [PubMed] [Google Scholar]
- Andrews JM, Cancilla PA. Cytoplasmic inclusions in human globoid cell leukodystrophy. Krabbe’s disease. Arch Pathol. 1970;89:53–55. [PubMed] [Google Scholar]
- Andrews JM, Cancilla PA, Grippo J, Menkes JH. Globoid cell leukodystrophy (Krabbe’s disease): morphological and biochemical studies. Neurology. 1971;21:337–352. doi: 10.1212/wnl.21.4.329-a. [DOI] [PubMed] [Google Scholar]
- Annunziata I, Bouche V, Lombardi A, Settembre C, Ballabio A. Multiple sulfatase deficiency is due to hypomorphic mutations of the SUMF1 gene. Hum Mutat. 2007;28:928. doi: 10.1002/humu.9504. [DOI] [PubMed] [Google Scholar]
- Appel MF, Markowitz AM. Massive splenomegaly in Gaucher’s disease. JAMA. 1971;217:343–344. [PubMed] [Google Scholar]
- Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A. Gliotransmitters travel in time and space. Neuron. 2014;81:728–739. doi: 10.1016/j.neuron.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin JH. Studies in metachromatic leukodystrophy. XII. Multiple sulfatase deficiency. Arch Neurol. 1973;28:258–264. doi: 10.1001/archneur.1973.00490220066010. [DOI] [PubMed] [Google Scholar]
- Bassi R, Anelli V, Giussani P, Tettamanti G, Viani P, Riboni L. Sphingosine-1-phosphate is released by cerebellar astrocytes in response to bFGF and induces astrocyte proliferation through Gi-protein-coupled receptors. Glia. 2006;53:621–630. doi: 10.1002/glia.20324. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Domercq M, Vesce S, Volterra A. Neuron-astrocyte cross-talk during synaptic transmission: physiological and neuropathological implications. Prog Brain Res. 2001;132:255–265. doi: 10.1016/S0079-6123(01)32081-2. [DOI] [PubMed] [Google Scholar]
- Blunt SB, Jenner P, Marsden CD. Motor function, graft survival and gliosis in rats with 6-OHDA lesions and foetal ventral mesencephalic grafts chronically treated with L-dopa and carbidopa. Exp Brain Res. 1992;88:326–340. doi: 10.1007/BF02259108. [DOI] [PubMed] [Google Scholar]
- Bosch M, Kielian T. Hemichannels in neurodegenerative diseases: is there a link to pathology? Front Cell Neurosci. 2014;8:242. doi: 10.3389/fncel.2014.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boustany RM. Lysosomal storage diseases--the horizon expands. Nat Rev Neurol. 2013;9:583–598. doi: 10.1038/nrneurol.2013.163. [DOI] [PubMed] [Google Scholar]
- Brinkmann V. FTY720 (fingolimod) in Multiple Sclerosis: therapeutic effects in the immune and the central nervous system. Br J Pharmacol. 2009;158:1173–1182. doi: 10.1111/j.1476-5381.2009.00451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruun I, Reske-Nielsen E, Oster S. Juvenile ceroid-lipofuscinosis and calcifications of the CNS. Acta Neurol Scand. 1991;83:1–8. doi: 10.1111/j.1600-0404.1991.tb03951.x. [DOI] [PubMed] [Google Scholar]
- Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron. 2014;81:229–248. doi: 10.1016/j.neuron.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk RD, Valle D, Thomas GH, Miller C, Moser A, Moser H, Rosenbaum KN. Early manifestations of multiple sulfatase deficiency. J Pediatr. 1984;104:574–578. doi: 10.1016/s0022-3476(84)80550-8. [DOI] [PubMed] [Google Scholar]
- Burkovetskaya M, Karpuk N, Xiong J, Bosch M, Boska MD, Takeuchi H, Suzumura A, Kielian T. Evidence for aberrant astrocyte hemichannel activity in Juvenile Neuronal Ceroid Lipofuscinosis (JNCL) PLoS One. 2014;9:e95023. doi: 10.1371/journal.pone.0095023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busche A, Hennermann JB, Burger F, Proquitte H, Dierks T, von Arnim-Baas A, Horn D. Neonatal manifestation of multiple sulfatase deficiency. Eur J Pediatr. 2009;168:969–973. doi: 10.1007/s00431-008-0871-2. [DOI] [PubMed] [Google Scholar]
- Calatayud CA, Pasquini LA, Pasquini JM, Soto EF. Involvement of the ubiquitin-mediated proteolytic system in the signaling pathway induced by ceramide in primary astrocyte cultures. Dev Neurosci. 2005;27:397–407. doi: 10.1159/000088454. [DOI] [PubMed] [Google Scholar]
- Cantuti Castelvetri L, Givogri MI, Hebert A, Smith B, Song Y, Kaminska A, Lopez-Rosas A, Morfini G, Pigino G, Sands M, Brady ST, Bongarzone ER. The sphingolipid psychosine inhibits fast axonal transport in Krabbe disease by activation of GSK3beta and deregulation of molecular motors. J Neurosci. 2013;33:10048–10056. doi: 10.1523/JNEUROSCI.0217-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelvetri LC, Givogri MI, Zhu H, Smith B, Lopez-Rosas A, Qiu X, van Breemen R, Bongarzone ER. Axonopathy is a compounding factor in the pathogenesis of Krabbe disease. Acta Neuropathol. 2011;122:35–48. doi: 10.1007/s00401-011-0814-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrachud U, Walker MW, Simas AM, Heetveld S, Petcherski A, Klein M, Oh H, Wolf P, Zhao WN, Norton S, Haggarty SJ, Lloyd-Evans E, Cotman SL. Unbiased Cell-Based Screening in a Neuronal Cell Model of Batten Disease Highlights an Interaction Between Ca2+ Homeostasis, Autophagy, and CLN3 Function. J Biol Chem. 2015 doi: 10.1074/jbc.M114.621706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CS, Patterson MC, Wheatley CL, O’Brien JF, Pagano RE. Broad screening test for sphingolipid-storage diseases. Lancet. 1999;354:901–905. doi: 10.1016/S0140-6736(98)10034-X. [DOI] [PubMed] [Google Scholar]
- Choi SH, Choi DH, Lee JJ, Park MS, Chun BG. Imidazoline drugs stabilize lysosomes and inhibit oxidative cytotoxicity in astrocytes. Free Radic Biol Med. 2002;32:394–405. doi: 10.1016/s0891-5849(01)00819-x. [DOI] [PubMed] [Google Scholar]
- Chun J, Brinkmann V. A mechanistically novel, first oral therapy for multiple sclerosis: the development of fingolimod (FTY720, Gilenya) Discov Med. 2011;12:213–228. [PMC free article] [PubMed] [Google Scholar]
- Coleman RJ, Robb SA, Lake BD, Brett EM, Harding AE. The diverse neurological features of Niemann-Pick disease type C: a report of two cases. Mov Disord. 1988;3:295–299. doi: 10.1002/mds.870030403. [DOI] [PubMed] [Google Scholar]
- Conradi NG, Kalimo H, Sourander P. Reactions of vessel walls and brain parenchyma to the accumulation of Gaucher cells in the Norrbottnian type (type III) of Gaucher disease. Acta Neuropathol. 1988;75:385–390. doi: 10.1007/BF00687792. [DOI] [PubMed] [Google Scholar]
- Conradi NG, Sourander P, Nilsson O, Svennerholm L, Erikson A. Neuropathology of the Norrbottnian type of Gaucher disease. Morphological and biochemical studies. Acta Neuropathol. 1984;65:99–109. doi: 10.1007/BF00690463. [DOI] [PubMed] [Google Scholar]
- Cotman SL, Karaa A, Staropoli JF, Sims KB. Neuronal ceroid lipofuscinosis: impact of recent genetic advances and expansion of the clinicopathologic spectrum. Curr Neurol Neurosci Rep. 2013;13:366. doi: 10.1007/s11910-013-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox TM, Cachon-Gonzalez MB. The cellular pathology of lysosomal diseases. J Pathol. 2012;226:241–254. doi: 10.1002/path.3021. [DOI] [PubMed] [Google Scholar]
- De Duve C. The lysosome. Sci Am. 1963;208:64–72. doi: 10.1038/scientificamerican0563-64. [DOI] [PubMed] [Google Scholar]
- De Duve C. The significance of lysosomes in pathology and medicine. Proc Inst Med Chic. 1966;26:73–76. [PubMed] [Google Scholar]
- de Pablo-Latorre R, Saide A, Polishhuck EV, Nusco E, Fraldi A, Ballabio A. Impaired parkin-mediated mitochondrial targeting to autophagosomes differentially contributes to tissue pathology in lysosomal storage diseases. Hum Mol Genet. 2012;21:1770–1781. doi: 10.1093/hmg/ddr610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H, Xiu X, Jankovic J. Genetic Convergence of Parkinson’s Disease and Lysosomal Storage Disorders. Mol Neurobiol. 2014 doi: 10.1007/s12035-014-8832-4. [DOI] [PubMed] [Google Scholar]
- Di Malta C, Fryer JD, Settembre C, Ballabio A. Astrocyte dysfunction triggers neurodegeneration in a lysosomal storage disorder. Proc Natl Acad Sci U S A. 2012a;109:E2334–2342. doi: 10.1073/pnas.1209577109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Malta C, Fryer JD, Settembre C, Ballabio A. Autophagy in astrocytes: a novel culprit in lysosomal storage disorders. Autophagy. 2012b;8:1871–1872. doi: 10.4161/auto.22184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Font A, Santamaria R, Cozar M, Blanco M, Chamoles N, Coll MJ, Chabas A, Vilageliu L, Grinberg D. Clinical and mutational characterization of three patients with multiple sulfatase deficiency: report of a new splicing mutation. Mol Genet Metab. 2005;86:206–211. doi: 10.1016/j.ymgme.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Enquist IB, Lo Bianco C, Ooka A, Nilsson E, Mansson JE, Ehinger M, Richter J, Brady RO, Kirik D, Karlsson S. Murine models of acute neuronopathic Gaucher disease. Proc Natl Acad Sci U S A. 2007;104:17483–17488. doi: 10.1073/pnas.0708086104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Episcopo FL, Tirolo C, Testa N, Caniglia S, Morale MC, Marchetti B. Reactive astrocytes are key players in nigrostriatal dopaminergic neurorepair in the MPTP mouse model of Parkinson’s disease: focus on endogenous neurorestoration. Curr Aging Sci. 2013;6:45–55. doi: 10.2174/1874609811306010007. [DOI] [PubMed] [Google Scholar]
- Eto Y, Numaguchi S, Tahara T, Rennert OM. Multiple sulfatase deficiency (mucosulfatidosis): impaired degradation of labeled sulfated compounds in cultured skin fibroblasts in vivo. Eur J Pediatr. 1980;135:85–89. doi: 10.1007/BF00445900. [DOI] [PubMed] [Google Scholar]
- Farfel-Becker T, Vitner EB, Kelly SL, Bame JR, Duan J, Shinder V, Merrill AH, Jr, Dobrenis K, Futerman AH. Neuronal accumulation of glucosylceramide in a mouse model of neuronopathic Gaucher disease leads to neurodegeneration. Hum Mol Genet. 2014;23:843–854. doi: 10.1093/hmg/ddt468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkerth RD. Abnormalities of developing white matter in lysosomal storage diseases. J Neuropathol Exp Neurol. 1999;58:887–902. doi: 10.1097/00005072-199909000-00001. [DOI] [PubMed] [Google Scholar]
- Fossale E, Wolf P, Espinola JA, Lubicz-Nawrocka T, Teed AM, Gao H, Rigamonti D, Cattaneo E, MacDonald ME, Cotman SL. Membrane trafficking and mitochondrial abnormalities precede subunit c deposition in a cerebellar cell model of juvenile neuronal ceroid lipofuscinosis. BMC Neurosci. 2004;5:57. doi: 10.1186/1471-2202-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller M, Meikle PJ, Hopwood JJ. Epidemiology of lysosomal storage diseases: an overview. In: Mehta A, et al., editors. Fabry Disease: Perspectives from 5 Years of FOS. Oxford: 2006. [PubMed] [Google Scholar]
- Gan-Or Z, Giladi N, Rozovski U, Shifrin C, Rosner S, Gurevich T, Bar-Shira A, Orr-Urtreger A. Genotype-phenotype correlations between GBA mutations and Parkinson disease risk and onset. Neurology. 2008;70:2277–2283. doi: 10.1212/01.wnl.0000304039.11891.29. [DOI] [PubMed] [Google Scholar]
- German DC, Liang CL, Song T, Yazdani U, Xie C, Dietschy JM. Neurodegeneration in the Niemann-Pick C mouse: glial involvement. Neuroscience. 2002;109:437–450. doi: 10.1016/s0306-4522(01)00517-6. [DOI] [PubMed] [Google Scholar]
- Getty AL, Rothberg PG, Pearce DA. Diagnosis of neuronal ceroid lipofuscinosis: mutation detection strategies. Expert Opin Med Diagn. 2007;1:351–362. doi: 10.1517/17530059.1.3.351. [DOI] [PubMed] [Google Scholar]
- Giaume C, Leybaert L, Naus CC, Saez JC. Connexin and pannexin hemichannels in brain glial cells: properties, pharmacology, and roles. Front Pharmacol. 2013;4:88. doi: 10.3389/fphar.2013.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri S, Jatana M, Rattan R, Won JS, Singh I, Singh AK. Galactosylsphingosine (psychosine)-induced expression of cytokine-mediated inducible nitric oxide synthases via AP-1 and C/EBP: implications for Krabbe disease. FASEB J. 2002;16:661–672. doi: 10.1096/fj.01-0798com. [DOI] [PubMed] [Google Scholar]
- Goebel HH, Schochet SS, Jaynes M, Bruck W, Kohlschutter A, Hentati F. Progress in neuropathology of the neuronal ceroid lipofuscinoses. Mol Genet Metab. 1999;66:367–372. doi: 10.1006/mgme.1999.2808. [DOI] [PubMed] [Google Scholar]
- Goker-Alpan O, Masdeu JC, Kohn PD, Ianni A, Lopez G, Groden C, Chapman MC, Cropp B, Eisenberg DP, Maniwang ED, Davis J, Wiggs E, Sidransky E, Berman KF. The neurobiology of glucocerebrosidase-associated parkinsonism: a positron emission tomography study of dopamine synthesis and regional cerebral blood flow. Brain. 2012;135:2440–2448. doi: 10.1093/brain/aws174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski GA, Goldblatt J, Dinur T, Kruse J, Svennerholm L, Gatt S, Desnick RJ. Genetic heterogeneity in Gaucher disease: physicokinetic and immunologic studies of the residual enzyme in cultured fibroblasts from non-neuronopathic and neuronopathic patients. Am J Med Genet. 1985;21:529–549. doi: 10.1002/ajmg.1320210316. [DOI] [PubMed] [Google Scholar]
- Graler MH. Targeting sphingosine 1-phosphate (S1P) levels and S1P receptor functions for therapeutic immune interventions. Cell Physiol Biochem. 2010;26:79–86. doi: 10.1159/000315108. [DOI] [PubMed] [Google Scholar]
- Guerra WF, Verity MA, Fluharty AL, Nguyen HT, Philippart M. Multiple sulfatase deficiency: clinical, neuropathological, ultrastructural and biochemical studies. J Neuropathol Exp Neurol. 1990;49:406–423. doi: 10.1097/00005072-199007000-00005. [DOI] [PubMed] [Google Scholar]
- Halperin A, Elstein D, Zimran A. Increased incidence of Parkinson disease among relatives of patients with Gaucher disease. Blood Cells Mol Dis. 2006;36:426–428. doi: 10.1016/j.bcmd.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Hawkins-Salsbury JA, Cooper JD, Sands MS. Pathogenesis and therapies for infantile neuronal ceroid lipofuscinosis (infantile CLN1 disease) Biochim Biophys Acta. 2013;1832:1906–1909. doi: 10.1016/j.bbadis.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SL, Lee LA, Lu JY, Verkruyse LA. Palmitoyl-protein thioesterase and the molecular pathogenesis of infantile neuronal ceroid lipofuscinosis. Neuropediatrics. 1997;28:27–30. doi: 10.1055/s-2007-973661. [DOI] [PubMed] [Google Scholar]
- Hol EM, Pekny M. Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr Opin Cell Biol. 2015;32C:121–130. doi: 10.1016/j.ceb.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Husain AM, Altuwaijri M, Aldosari M. Krabbe disease: neurophysiologic studies and MRI correlations. Neurology. 2004;63:617–620. doi: 10.1212/01.wnl.0000134651.38196.f8. [DOI] [PubMed] [Google Scholar]
- Igisu H, Suzuki K. Analysis of galactosylsphingosine (psychosine) in the brain. J Lipid Res. 1984a;25:1000–1006. [PubMed] [Google Scholar]
- Igisu H, Suzuki K. Progressive accumulation of toxic metabolite in a genetic leukodystrophy. Science. 1984b;224:753–755. doi: 10.1126/science.6719111. [DOI] [PubMed] [Google Scholar]
- Incecik F, Ozbek MN, Gungor S, Pepe S, Herguner OM, Mungan NO, Gungor S, Altunbasak S. Multiple sulfatase deficiency: A case series of four children. Ann Indian Acad Neurol. 2013;16:720–722. doi: 10.4103/0972-2327.120449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H, Tanaka J, Morihana Y, Tamaki T. The fine structure of cytoplasmic inclusions in brain and other visceral organs in Sandhoff disease. Brain Dev. 1984;6:467–474. doi: 10.1016/s0387-7604(84)80029-7. [DOI] [PubMed] [Google Scholar]
- Jansen AH, Reits EA, Hol EM. The ubiquitin proteasome system in glia and its role in neurodegenerative diseases. Front Mol Neurosci. 2014;7:73. doi: 10.3389/fnmol.2014.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvela I, Autti T, Lamminranta S, Aberg L, Raininko R, Santavuori P. Clinical and magnetic resonance imaging findings in Batten disease: analysis of the major mutation (1.02-kb deletion) Ann Neurol. 1997;42:799–802. doi: 10.1002/ana.410420517. [DOI] [PubMed] [Google Scholar]
- Jatana M, Giri S, Singh AK. Apoptotic positive cells in Krabbe brain and induction of apoptosis in rat C6 glial cells by psychosine. Neurosci Lett. 2002;330:183–187. doi: 10.1016/s0304-3940(02)00655-9. [DOI] [PubMed] [Google Scholar]
- Jesionek-Kupnicka D, Majchrowska A, Krawczyk J, Wendorff J, Barcikowska M, Lukaszek S, Liberski PP. Krabbe disease: an ultrastructural study of globoid cells and reactive astrocytes at the brain and optic nerves. Folia Neuropathol. 1997;35:155–162. [PubMed] [Google Scholar]
- Jmoudiak M, Futerman AH. Gaucher disease: pathological mechanisms and modern management. Br J Haematol. 2005;129:178–188. doi: 10.1111/j.1365-2141.2004.05351.x. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Matsumoto JY, Lindor NM. Heterozygous Niemann-Pick disease type C presenting with tremor. Neurology. 2004;63:2189–2190. doi: 10.1212/01.wnl.0000145710.25588.2f. [DOI] [PubMed] [Google Scholar]
- Kampine JP, Brady RO, Kanfer JN, Feld M, Shapiro D. Diagnosis of gaucher’s disease and niemann-pick disease with small samples of venous blood. Science. 1967;155:86–88. doi: 10.1126/science.155.3758.86. [DOI] [PubMed] [Google Scholar]
- Kanninen KM, Grubman A, Caragounis A, Duncan C, Parker SJ, Lidgerwood GE, Volitakis I, Ganio G, Crouch PJ, White AR. Altered biometal homeostasis is associated with CLN6 mRNA loss in mouse neuronal ceroid lipofuscinosis. Biol Open. 2013;2:635–646. doi: 10.1242/bio.20134804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpuk N, Burkovetskaya M, Fritz T, Angle A, Kielian T. Neuroinflammation leads to region-dependent alterations in astrocyte gap junction communication and hemichannel activity. J Neurosci. 2011;31:414–425. doi: 10.1523/JNEUROSCI.5247-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima N, Tsuji D, Okuda T, Itoh K, Nakayama K. Mechanism of abnormal growth in astrocytes derived from a mouse model of GM2 gangliosidosis. J Neurochem. 2009;111:1031–1041. doi: 10.1111/j.1471-4159.2009.06391.x. [DOI] [PubMed] [Google Scholar]
- Kim Y, Ramirez-Montealegre D, Pearce DA. A role in vacuolar arginine transport for yeast Btn1p and for human CLN3, the protein defective in Batten disease. Proc Natl Acad Sci U S A. 2003;100:15458–15462. doi: 10.1073/pnas.2136651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselyov K, Jennigs JJ, Jr, Rbaibi Y, Chu CT. Autophagy, mitochondria and cell death in lysosomal storage diseases. Autophagy. 2007;3:259–262. doi: 10.4161/auto.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodam A, Maulik M, Peake K, Amritraj A, Vetrivel KS, Thinakaran G, Vance JE, Kar S. Altered levels and distribution of amyloid precursor protein and its processing enzymes in Niemann-Pick type C1-deficient mouse brains. Glia. 2010;58:1267–1281. doi: 10.1002/glia.21001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlschutter A. Lysosomal leukodystrophies: Krabbe disease and metachromatic leukodystrophy. Handb Clin Neurol. 2013;113:1611–1618. doi: 10.1016/B978-0-444-59565-2.00029-0. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Jaattela M. Lysosomes and autophagy in cell death control. Nat Rev Cancer. 2005;5:886–897. doi: 10.1038/nrc1738. [DOI] [PubMed] [Google Scholar]
- Kuronen M, Lehesjoki AE, Jalanko A, Cooper JD, Kopra O. Selective spatiotemporal patterns of glial activation and neuron loss in the sensory thalamocortical pathways of neuronal ceroid lipofuscinosis 8 mice. Neurobiol Dis. 2012;47:444–457. doi: 10.1016/j.nbd.2012.04.018. [DOI] [PubMed] [Google Scholar]
- Lebrun AH, Moll-Khosrawi P, Pohl S, Makrypidi G, Storch S, Kilian D, Streichert T, Otto B, Mole SE, Ullrich K, Cotman S, Kohlschutter A, Braulke T, Schulz A. Analysis of potential biomarkers and modifier genes affecting the clinical course of CLN3 disease. Mol Med. 2011;17:1253–1261. doi: 10.2119/molmed.2010.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Cho KS, Koh JY. Oxidative injury triggers autophagy in astrocytes: the role of endogenous zinc. Glia. 2009;57:1351–1361. doi: 10.1002/glia.20854. [DOI] [PubMed] [Google Scholar]
- LeVine SM, Brown DC. IL-6 and TNFalpha expression in brains of twitcher, quaking and normal mice. J Neuroimmunol. 1997;73:47–56. doi: 10.1016/s0165-5728(96)00166-x. [DOI] [PubMed] [Google Scholar]
- Li L, Lundkvist A, Andersson D, Wilhelmsson U, Nagai N, Pardo AC, Nodin C, Stahlberg A, Aprico K, Larsson K, Yabe T, Moons L, Fotheringham A, Davies I, Carmeliet P, Schwartz JP, Pekna M, Kubista M, Blomstrand F, Maragakis N, Nilsson M, Pekny M. Protective role of reactive astrocytes in brain ischemia. J Cereb Blood Flow Metab. 2008;28:468–481. doi: 10.1038/sj.jcbfm.9600546. [DOI] [PubMed] [Google Scholar]
- Lieberman AP, Puertollano R, Raben N, Slaugenhaupt S, Walkley SU, Ballabio A. Autophagy in lysosomal storage disorders. Autophagy. 2012;8:719–730. doi: 10.4161/auto.19469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Tang Y, Feng J. Cross talk between activation of microglia and astrocytes in pathological conditions in the central nervous system. Life Sci. 2011;89:141–146. doi: 10.1016/j.lfs.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Lloyd-Evans E, Platt FM. Lipids on trial: the search for the offending metabolite in Niemann-Pick type C disease. Traffic. 2010;11:419–428. doi: 10.1111/j.1600-0854.2010.01032.x. [DOI] [PubMed] [Google Scholar]
- Luddi A, Volterrani M, Strazza M, Smorlesi A, Rafi MA, Datto J, Wenger DA, Costantino-Ceccarini E. Retrovirus-mediated gene transfer and galactocerebrosidase uptake into twitcher glial cells results in appropriate localization and phenotype correction. Neurobiol Dis. 2001;8:600–610. doi: 10.1006/nbdi.2001.0407. [DOI] [PubMed] [Google Scholar]
- Luiro K, Kopra O, Blom T, Gentile M, Mitchison HM, Hovatta I, Tornquist K, Jalanko A. Batten disease (JNCL) is linked to disturbances in mitochondrial, cytoskeletal, and synaptic compartments. J Neurosci Res. 2006;84:1124–1138. doi: 10.1002/jnr.21015. [DOI] [PubMed] [Google Scholar]
- Luiro K, Yliannala K, Ahtiainen L, Maunu H, Jarvela I, Kyttala A, Jalanko A. Interconnections of CLN3, Hook1 and Rab proteins link Batten disease to defects in the endocytic pathway. Hum Mol Genet. 2004;13:3017–3027. doi: 10.1093/hmg/ddh321. [DOI] [PubMed] [Google Scholar]
- Macaulay RJ, Lowry NJ, Casey RE. Pathologic findings of multiple sulfatase deficiency reflect the pattern of enzyme deficiencies. Pediatr Neurol. 1998;19:372–376. doi: 10.1016/s0887-8994(98)00073-3. [DOI] [PubMed] [Google Scholar]
- Macauley SL, Pekny M, Sands MS. The role of attenuated astrocyte activation in infantile neuronal ceroid lipofuscinosis. J Neurosci. 2011;31:15575–15585. doi: 10.1523/JNEUROSCI.3579-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macauley SL, Wozniak DF, Kielar C, Tan Y, Cooper JD, Sands MS. Cerebellar pathology and motor deficits in the palmitoyl protein thioesterase 1-deficient mouse. Exp Neurol. 2009;217:124–135. doi: 10.1016/j.expneurol.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandlebaum FS. A Contribution to the Pathology of Primary Splenomegaly (Gaucher Type), with the Report of an Autopsy on a Male Child Four and One Half Years of Age. J Exp Med. 1912;16:797–821. doi: 10.1084/jem.16.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda N, Tanaka K. Does impairment of the ubiquitin-proteasome system or the autophagy-lysosome pathway predispose individuals to neurodegenerative disorders such as Parkinson’s disease? J Alzheimers Dis. 2010;19:1–9. doi: 10.3233/JAD-2010-1231. [DOI] [PubMed] [Google Scholar]
- Meisingset TW, Ricca A, Neri M, Sonnewald U, Gritti A. Region- and age-dependent alterations of glial-neuronal metabolic interactions correlate with CNS pathology in a mouse model of globoid cell leukodystrophy. J Cereb Blood Flow Metab. 2013;33:1127–1137. doi: 10.1038/jcbfm.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengel E, Klunemann HH, Lourenco CM, Hendriksz CJ, Sedel F, Walterfang M, Kolb SA. Niemann-Pick disease type C symptomatology: an expert-based clinical description. Orphanet J Rare Dis. 2013;8:166. doi: 10.1186/1750-1172-8-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micsenyi MC, Sikora J, Stephney G, Dobrenis K, Walkley SU. Lysosomal membrane permeability stimulates protein aggregate formation in neurons of a lysosomal disease. J Neurosci. 2013;33:10815–10827. doi: 10.1523/JNEUROSCI.0987-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohri I, Taniike M, Taniguchi H, Kanekiyo T, Aritake K, Inui T, Fukumoto N, Eguchi N, Kushi A, Sasai H, Kanaoka Y, Ozono K, Narumiya S, Suzuki K, Urade Y. Prostaglandin D2-mediated microglia/astrocyte interaction enhances astrogliosis and demyelination in twitcher. J Neurosci. 2006;26:4383–4393. doi: 10.1523/JNEUROSCI.4531-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero TD, Orellana JA. Hemichannels: new pathways for gliotransmitter release. Neuroscience. 2015;286:45–59. doi: 10.1016/j.neuroscience.2014.11.048. [DOI] [PubMed] [Google Scholar]
- Myerowitz R, Lawson D, Mizukami H, Mi Y, Tifft CJ, Proia RL. Molecular pathophysiology in Tay-Sachs and Sandhoff diseases as revealed by gene expression profiling. Hum Mol Genet. 2002;11:1343–1350. doi: 10.1093/hmg/11.11.1343. [DOI] [PubMed] [Google Scholar]
- Neudorfer O, Giladi N, Elstein D, Abrahamov A, Turezkite T, Aghai E, Reches A, Bembi B, Zimran A. Occurrence of Parkinson’s syndrome in type I Gaucher disease. QJM. 1996;89:691–694. doi: 10.1093/qjmed/89.9.691. [DOI] [PubMed] [Google Scholar]
- Nixon RA. Autophagy in neurodegenerative disease: friend, foe or turncoat? Trends Neurosci. 2006;29:528–535. doi: 10.1016/j.tins.2006.07.003. [DOI] [PubMed] [Google Scholar]
- O’Dowd BF, Klavins MH, Willard HF, Gravel R, Lowden JA, Mahuran DJ. Molecular heterogeneity in the infantile and juvenile forms of Sandhoff disease (O-variant GM2 gangliosidosis) J Biol Chem. 1986;261:12680–12685. [PubMed] [Google Scholar]
- Oh HL, Seok JY, Kwon CH, Kang SK, Kim YK. Role of MAPK in ceramide-induced cell death in primary cultured astrocytes from mouse embryonic brain. Neurotoxicology. 2006;27:31–38. doi: 10.1016/j.neuro.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Ohm TG, Treiber-Held S, Distl R, Glockner F, Schonheit B, Tamanai M, Meske V. Cholesterol and tau protein--findings in Alzheimer’s and Niemann Pick C’s disease. Pharmacopsychiatry. 2003;36(Suppl 2):S120–126. doi: 10.1055/s-2003-43060. [DOI] [PubMed] [Google Scholar]
- Orellana JA, Froger N, Ezan P, Jiang JX, Bennett MV, Naus CC, Giaume C, Saez JC. ATP and glutamate released via astroglial connexin 43 hemichannels mediate neuronal death through activation of pannexin 1 hemichannels. J Neurochem. 2011a;118:826–840. doi: 10.1111/j.1471-4159.2011.07210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana JA, Saez JC, Bennett MV, Berman JW, Morgello S, Eugenin EA. HIV increases the release of dickkopf-1 protein from human astrocytes by a Cx43 hemichannel-dependent mechanism. J Neurochem. 2014;128:752–763. doi: 10.1111/jnc.12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana JA, Shoji KF, Abudara V, Ezan P, Amigou E, Saez PJ, Jiang JX, Naus CC, Saez JC, Giaume C. Amyloid beta-induced death in neurons involves glial and neuronal hemichannels. J Neurosci. 2011b;31:4962–4977. doi: 10.1523/JNEUROSCI.6417-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osellame LD, Duchen MR. Quality control gone wrong: mitochondria, lysosomal storage disorders and neurodegeneration. Br J Pharmacol. 2014;171:1958–1972. doi: 10.1111/bph.12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osellame LD, Rahim AA, Hargreaves IP, Gegg ME, Richard-Londt A, Brandner S, Waddington SN, Schapira AH, Duchen MR. Mitochondria and quality control defects in a mouse model of Gaucher disease--links to Parkinson’s disease. Cell Metab. 2013;17:941–953. doi: 10.1016/j.cmet.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald MJ, Palmer DN, Kay GW, Shemilt SJ, Rezaie P, Cooper JD. Glial activation spreads from specific cerebral foci and precedes neurodegeneration in presymptomatic ovine neuronal ceroid lipofuscinosis (CLN6) Neurobiol Dis. 2005;20:49–63. doi: 10.1016/j.nbd.2005.01.025. [DOI] [PubMed] [Google Scholar]
- Palmer DN, Barns G, Husbands DR, Jolly RD. Ceroid lipofuscinosis in sheep. II. The major component of the lipopigment in liver, kidney, pancreas, and brain is low molecular weight protein. J Biol Chem. 1986;261:1773–1777. [PubMed] [Google Scholar]
- Palmer DN, Husbands DR, Jolly RD. Phospholipid fatty acids in brains of normal sheep and sheep with ceroid-lipofuscinosis. Biochim Biophys Acta. 1985;834:159–163. doi: 10.1016/0005-2760(85)90151-1. [DOI] [PubMed] [Google Scholar]
- Pan T, Kondo S, Le W, Jankovic J. The role of autophagy-lysosome pathway in neurodegeneration associated with Parkinson’s disease. Brain. 2008;131:1969–1978. doi: 10.1093/brain/awm318. [DOI] [PubMed] [Google Scholar]
- Pardo CA, Rabin BA, Palmer DN, Price DL. Accumulation of the adenosine triphosphate synthase subunit C in the mnd mutant mouse. A model for neuronal ceroid lipofuscinosis. Am J Pathol. 1994;144:829–835. [PMC free article] [PubMed] [Google Scholar]
- Patel SC, Suresh S, Kumar U, Hu CY, Cooney A, Blanchette-Mackie EJ, Neufeld EB, Patel RC, Brady RO, Patel YC, Pentchev PG, Ong WY. Localization of Niemann-Pick C1 protein in astrocytes: implications for neuronal degeneration in Niemann-Pick type C disease. Proc Natl Acad Sci U S A. 1999;96:1657–1662. doi: 10.1073/pnas.96.4.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce DA, Carr CJ, Das B, Sherman F. Phenotypic reversal of the btn1 defects in yeast by chloroquine: a yeast model for Batten disease. Proc Natl Acad Sci U S A. 1999;96:11341–11345. doi: 10.1073/pnas.96.20.11341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekny M, Eliasson C, Siushansian R, Ding M, Dixon SJ, Pekna M, Wilson JX, Hamberger A. The impact of genetic removal of GFAP and/or vimentin on glutamine levels and transport of glucose and ascorbate in astrocytes. Neurochem Res. 1999;24:1357–1362. doi: 10.1023/a:1022572304626. [DOI] [PubMed] [Google Scholar]
- Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- Pekny M, Pekna M. Astrocyte reactivity and reactive astrogliosis: costs and benefits. Physiol Rev. 2014;94:1077–1098. doi: 10.1152/physrev.00041.2013. [DOI] [PubMed] [Google Scholar]
- Perea G, Navarrete M, Araque A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci. 2009;32:421–431. doi: 10.1016/j.tins.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Pereira VG, Gazarini ML, Rodrigues LC, da Silva FH, Han SW, Martins AM, Tersariol IL, D’Almeida V. Evidence of lysosomal membrane permeabilization in mucopolysaccharidosis type I: rupture of calcium and proton homeostasis. J Cell Physiol. 2010;223:335–342. doi: 10.1002/jcp.22039. [DOI] [PubMed] [Google Scholar]
- Platt FM, Boland B, van der Spoel AC. The cell biology of disease: lysosomal storage disorders: the cellular impact of lysosomal dysfunction. J Cell Biol. 2012;199:723–734. doi: 10.1083/jcb.201208152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt FM, Wassif C, Colaco A, Dardis A, Lloyd-Evans E, Bembi B, Porter FD. Disorders of cholesterol metabolism and their unanticipated convergent mechanisms of disease. Annu Rev Genomics Hum Genet. 2014;15:173–194. doi: 10.1146/annurev-genom-091212-153412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontikis CC, Cella CV, Parihar N, Lim MJ, Chakrabarti S, Mitchison HM, Mobley WC, Rezaie P, Pearce DA, Cooper JD. Late onset neurodegeneration in the Cln3−/− mouse model of juvenile neuronal ceroid lipofuscinosis is preceded by low level glial activation. Brain Res. 2004;1023:231–242. doi: 10.1016/j.brainres.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Prada CE, Grabowski GA. Neuronopathic lysosomal storage diseases: clinical and pathologic findings. Dev Disabil Res Rev. 2013;17:226–246. doi: 10.1002/ddrr.1116. [DOI] [PubMed] [Google Scholar]
- Rakheja D, Narayan SB, Bennett MJ. Juvenile neuronal ceroid-lipofuscinosis (Batten disease): a brief review and update. Curr Mol Med. 2007;7:603–608. doi: 10.2174/156652407781695729. [DOI] [PubMed] [Google Scholar]
- Renkawek K, Stege GJ, Bosman GJ. Dementia, gliosis and expression of the small heat shock proteins hsp27 and alpha B-crystallin in Parkinson’s disease. Neuroreport. 1999;10:2273–2276. doi: 10.1097/00001756-199908020-00009. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Muela N, Hernandez-Pinto AM, Serrano-Puebla A, Garcia-Ledo L, Latorre SH, de la Rosa EJ, Boya P. Lysosomal membrane permeabilization and autophagy blockade contribute to photoreceptor cell death in a mouse model of retinitis pigmentosa. Cell Death Differ. 2015;22:476–487. doi: 10.1038/cdd.2014.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez JC, Leybaert L. Hunting for connexin hemichannels. FEBS Lett. 2014;588:1205–1211. doi: 10.1016/j.febslet.2014.03.004. [DOI] [PubMed] [Google Scholar]
- Saez PJ, Orellana JA, Vega-Riveros N, Figueroa VA, Hernandez DE, Castro JF, Klein AD, Jiang JX, Zanlungo S, Saez JC. Disruption in connexin-based communication is associated with intracellular Ca(2)(+) signal alterations in astrocytes from Niemann-Pick type C mice. PLoS One. 2013;8:e71361. doi: 10.1371/journal.pone.0071361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Suzuki K, Hulette CM, Murayama S. Aberrant phosphorylation of alpha-synuclein in human Niemann-Pick type C1 disease. J Neuropathol Exp Neurol. 2004;63:323–328. doi: 10.1093/jnen/63.4.323. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Denaro FJ. Astrocytic responses to the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in cat and mouse brain. J Neuropathol Exp Neurol. 1988;47:452–458. doi: 10.1097/00005072-198807000-00006. [DOI] [PubMed] [Google Scholar]
- Schultz ML, Tecedor L, Chang M, Davidson BL. Clarifying lysosomal storage diseases. Trends Neurosci. 2011;34:401–410. doi: 10.1016/j.tins.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C, Fraldi A, Rubinsztein DC, Ballabio A. Lysosomal storage diseases as disorders of autophagy. Autophagy. 2008;4:113–114. doi: 10.4161/auto.5227. [DOI] [PubMed] [Google Scholar]
- Shyng C, Sands MS. Astrocytosis in infantile neuronal ceroid lipofuscinosis: friend or foe? Biochem Soc Trans. 2014;42:1282–1285. doi: 10.1042/BST20140188. [DOI] [PubMed] [Google Scholar]
- Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, Bar-Shira A, Berg D, Bras J, Brice A, Chen CM, Clark LN, Condroyer C, De Marco EV, Durr A, Eblan MJ, Fahn S, Farrer MJ, Fung HC, Gan-Or Z, Gasser T, Gershoni-Baruch R, Giladi N, Griffith A, Gurevich T, Januario C, Kropp P, Lang AE, Lee-Chen GJ, Lesage S, Marder K, Mata IF, Mirelman A, Mitsui J, Mizuta I, Nicoletti G, Oliveira C, Ottman R, Orr-Urtreger A, Pereira LV, Quattrone A, Rogaeva E, Rolfs A, Rosenbaum H, Rozenberg R, Samii A, Samaddar T, Schulte C, Sharma M, Singleton A, Spitz M, Tan EK, Tayebi N, Toda T, Troiano AR, Tsuji S, Wittstock M, Wolfsberg TG, Wu YR, Zabetian CP, Zhao Y, Ziegler SG. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N Engl J Med. 2009a;361:1651–1661. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidransky E, Samaddar T, Tayebi N. Mutations in GBA are associated with familial Parkinson disease susceptibility and age at onset. Neurology. 2009b;73:1424–1425. doi: 10.1212/WNL.0b013e3181b28601. author reply 1425–1426. [DOI] [PubMed] [Google Scholar]
- Sinha S, Satishchandra P, Santosh V, Gayatri N, Shankar SK. Neuronal ceroid lipofuscinosis: a clinicopathological study. Seizure. 2004;13:235–240. doi: 10.1016/S1059-1311(03)00163-8. [DOI] [PubMed] [Google Scholar]
- Sleat DE, Donnelly RJ, Lackland H, Liu CG, Sohar I, Pullarkat RK, Lobel P. Association of mutations in a lysosomal protein with classical late-infantile neuronal ceroid lipofuscinosis. Science. 1997;277:1802–1805. doi: 10.1126/science.277.5333.1802. [DOI] [PubMed] [Google Scholar]
- Sleat DE, Gin RM, Sohar I, Wisniewski K, Sklower-Brooks S, Pullarkat RK, Palmer DN, Lerner TJ, Boustany RM, Uldall P, Siakotos AN, Donnelly RJ, Lobel P. Mutational analysis of the defective protease in classic late-infantile neuronal ceroid lipofuscinosis, a neurodegenerative lysosomal storage disorder. Am J Hum Genet. 1999;64:1511–1523. doi: 10.1086/302427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BR, Santos MB, Marshall MS, Cantuti-Castelvetri L, Lopez-Rosas A, Li G, van Breemen R, Claycomb KI, Gallea JI, Celej MS, Crocker SJ, Givogri MI, Bongarzone ER. Neuronal inclusions of alpha-synuclein contribute to the pathogenesis of Krabbe disease. J Pathol. 2014;232:509–521. doi: 10.1002/path.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol J, Blanchette-Mackie J, Kruth HS, Dwyer NK, Amende LM, Butler JD, Robinson E, Patel S, Brady RO, Comly ME, et al. Type C Niemann-Pick disease. Lysosomal accumulation and defective intracellular mobilization of low density lipoprotein cholesterol. J Biol Chem. 1988;263:3411–3417. [PubMed] [Google Scholar]
- Sriram K, Benkovic SA, Hebert MA, Miller DB, O’Callaghan JP. Induction of gp130-related cytokines and activation of JAK2/STAT3 pathway in astrocytes precedes up-regulation of glial fibrillary acidic protein in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of neurodegeneration: key signaling pathway for astrogliosis in vivo? J Biol Chem. 2004;279:19936–19947. doi: 10.1074/jbc.M309304200. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Sakiyama T, Harada N, Abe M, Tadokoro M. Pathologic changes of glial cells in murine model of Niemann-Pick disease type C: immunohistochemical, lectin-histochemical and ultrastructural observations. Pediatr Int. 2003;45:1–4. doi: 10.1046/j.1442-200x.2003.01651.x. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Suzuki K. Genetic galactosylceramidase deficiency (globoid cell leukodystrophy, Krabbe disease) in different mammalian species. Neurochem Pathol. 1985;3:53–68. doi: 10.1007/BF02834075. [DOI] [PubMed] [Google Scholar]
- Takamura A, Higaki K, Kajimaki K, Otsuka S, Ninomiya H, Matsuda J, Ohno K, Suzuki Y, Nanba E. Enhanced autophagy and mitochondrial aberrations in murine G(M1)-gangliosidosis. Biochem Biophys Res Commun. 2008;367:616–622. doi: 10.1016/j.bbrc.2007.12.187. [DOI] [PubMed] [Google Scholar]
- Tan CC, Yu JT, Tan MS, Jiang T, Zhu XC, Tan L. Autophagy in aging and neurodegenerative diseases: implications for pathogenesis and therapy. Neurobiol Aging. 2014;35:941–957. doi: 10.1016/j.neurobiolaging.2013.11.019. [DOI] [PubMed] [Google Scholar]
- Tilleux S, Hermans E. Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. J Neurosci Res. 2007;85:2059–2070. doi: 10.1002/jnr.21325. [DOI] [PubMed] [Google Scholar]
- Valadares ER, Pizarro MX, Oliveira LR, Amorim RH, Pinheiro TM, Grieben U, Santos HH, Queiroz RR, de Lopes GC, Godard AL. Juvenile neuronal ceroid-lipofuscinosis: clinical and molecular investigation in a large family in Brazil. Arq Neuropsiquiatr. 2011;69:13–18. doi: 10.1590/s0004-282x2011000100004. [DOI] [PubMed] [Google Scholar]
- Vesa J, Hellsten E, Verkruyse LA, Camp LA, Rapola J, Santavuori P, Hofmann SL, Peltonen L. Mutations in the palmitoyl protein thioesterase gene causing infantile neuronal ceroid lipofuscinosis. Nature. 1995;376:584–587. doi: 10.1038/376584a0. [DOI] [PubMed] [Google Scholar]
- Vincent AJ, Choi-Lundberg DL, Harris JA, West AK, Chuah MI. Bacteria and PAMPs activate nuclear factor kappaB and Gro production in a subset of olfactory ensheathing cells and astrocytes but not in Schwann cells. Glia. 2007;55:905–916. doi: 10.1002/glia.20512. [DOI] [PubMed] [Google Scholar]
- Vitner EB, Dekel H, Zigdon H, Shachar T, Farfel-Becker T, Eilam R, Karlsson S, Futerman AH. Altered expression and distribution of cathepsins in neuronopathic forms of Gaucher disease and in other sphingolipidoses. Hum Mol Genet. 2010a;19:3583–3590. doi: 10.1093/hmg/ddq273. [DOI] [PubMed] [Google Scholar]
- Vitner EB, Farfel-Becker T, Eilam R, Biton I, Futerman AH. Contribution of brain inflammation to neuronal cell death in neuronopathic forms of Gaucher’s disease. Brain. 2012;135:1724–1735. doi: 10.1093/brain/aws095. [DOI] [PubMed] [Google Scholar]
- Vitner EB, Futerman AH. Neuronal forms of Gaucher disease. Handb Exp Pharmacol. 2013:405–419. doi: 10.1007/978-3-7091-1511-4_20. [DOI] [PubMed] [Google Scholar]
- Vitner EB, Platt FM, Futerman AH. Common and uncommon pathogenic cascades in lysosomal storage diseases. J Biol Chem. 2010b;285:20423–20427. doi: 10.1074/jbc.R110.134452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitner EB, Salomon R, Farfel-Becker T, Meshcheriakova A, Ali M, Klein AD, Platt FM, Cox TM, Futerman AH. RIPK3 as a potential therapeutic target for Gaucher’s disease. Nat Med. 2014;20:204–208. doi: 10.1038/nm.3449. [DOI] [PubMed] [Google Scholar]
- Volders P, Van Hove J, Lories RJ, Vandekerckhove P, Matthijs G, De Vos R, Vanier MT, Vincent MF, Westhovens R, Luyten FP. Niemann-Pick disease type B: an unusual clinical presentation with multiple vertebral fractures. Am J Med Genet. 2002;109:42–51. doi: 10.1002/ajmg.10278. [DOI] [PubMed] [Google Scholar]
- Weimer JM, Benedict JW, Elshatory YM, Short DW, Ramirez-Montealegre D, Ryan DA, Alexander NA, Federoff HJ, Cooper JD, Pearce DA. Alterations in striatal dopamine catabolism precede loss of substantia nigra neurons in a mouse model of juvenile neuronal ceroid lipofuscinosis. Brain Res. 2007;1162:98–112. doi: 10.1016/j.brainres.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger DA, Rafi MA, Luzi P. Molecular genetics of Krabbe disease (globoid cell leukodystrophy): diagnostic and clinical implications. Hum Mutat. 1997;10:268–279. doi: 10.1002/(SICI)1098-1004(1997)10:4<268::AID-HUMU2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Wong K, Sidransky E, Verma A, Mixon T, Sandberg GD, Wakefield LK, Morrison A, Lwin A, Colegial C, Allman JM, Schiffmann R. Neuropathology provides clues to the pathophysiology of Gaucher disease. Mol Genet Metab. 2004;82:192–207. doi: 10.1016/j.ymgme.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Wu YP, Mizugishi K, Bektas M, Sandhoff R, Proia RL. Sphingosine kinase 1/S1P receptor signaling axis controls glial proliferation in mice with Sandhoff disease. Hum Mol Genet. 2008;17:2257–2264. doi: 10.1093/hmg/ddn126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YP, Proia RL. Deletion of macrophage-inflammatory protein 1 alpha retards neurodegeneration in Sandhoff disease mice. Proc Natl Acad Sci U S A. 2004;101:8425–8430. doi: 10.1073/pnas.0400625101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, Kielian T. Microglia in juvenile neuronal ceroid lipofuscinosis are primed toward a pro-inflammatory phenotype. J Neurochem. 2013;127:245–258. doi: 10.1111/jnc.12385. [DOI] [PubMed] [Google Scholar]
- Yan X, Yang F, Lukas J, Witt M, Wree A, Rolfs A, Luo J. Hyperactive glial cells contribute to axonal pathologies in the spinal cord of Npc1 mutant mice. Glia. 2014;62:1024–1040. doi: 10.1002/glia.22659. [DOI] [PubMed] [Google Scholar]
- Yu W, Gong JS, Ko M, Garver WS, Yanagisawa K, Michikawa M. Altered cholesterol metabolism in Niemann-Pick type C1 mouse brains affects mitochondrial function. J Biol Chem. 2005;280:11731–11739. doi: 10.1074/jbc.M412898200. [DOI] [PubMed] [Google Scholar]
- Zervas M, Dobrenis K, Walkley SU. Neurons in Niemann-Pick disease type C accumulate gangliosides as well as unesterified cholesterol and undergo dendritic and axonal alterations. J Neuropathol Exp Neurol. 2001a;60:49–64. doi: 10.1093/jnen/60.1.49. [DOI] [PubMed] [Google Scholar]
- Zervas M, Somers KL, Thrall MA, Walkley SU. Critical role for glycosphingolipids in Niemann-Pick disease type C. Curr Biol. 2001b;11:1283–1287. doi: 10.1016/s0960-9822(01)00396-7. [DOI] [PubMed] [Google Scholar]
- Zhang M, Strnatka D, Donohue C, Hallows JL, Vincent I, Erickson RP. Astrocyte-only Npc1 reduces neuronal cholesterol and triples life span of Npc1−/− mice. J Neurosci Res. 2008;86:2848–2856. doi: 10.1002/jnr.21730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou SY, Xu SJ, Yan YG, Yu HM, Ling SC, Luo JH. Decreased purinergic inhibition of synaptic activity in a mouse model of Niemann-Pick disease type C. Hippocampus. 2011;21:212–219. doi: 10.1002/hipo.20741. [DOI] [PubMed] [Google Scholar]