Abstract
Major depressive disorder (MDD) will affect one out of every five people in their lifetime and is the leading cause of disability worldwide. Nevertheless, mechanisms associated with the pathogenesis of MDD have yet to be completely understood and current treatments remain ineffective in a large subset of patients. In this review, we summarize the most recent discoveries and insights for which parallel findings have been obtained in human depressed subjects and rodent models of mood disorders in order to examine the potential etiology of depression. These mechanisms range from synaptic plasticity mechanisms to epigenetics and the immune system where there is strong evidence to support a functional role in the development of specific depression symptomology. Ultimately we conclude by discussing how novel therapeutic strategies targeting central and peripheral processes might ultimately aid in the development of effective new treatments for MDD and related stress disorders.
Keywords: major depressive disorders, synaptic plasticity, epigenetics, immune system, cytokines, astrocytes
1. Introduction
Mood disorders affect ~20% of the American population over their lifetime and yearly prevalence rates are close to 10%, including 6.7% for major depressive disorder (MDD) (Kessler et al., 1993, Kessler et al., 2005). This represents approximately 35 million adults in the US that will experience an episode of major depression in their lifetime (Kessler et al., 2003), with women having a higher risk of first onset and twice the occurrence of men (Kessler et al., 1993). Moreover, depression is the leading cause of disability worldwide (Lopez and Murray, 1998) — the economic burden of depression was estimated to be 83.1 billion USD in the year 2000 (Greenberg et al., 2003). MDD is characterized by symptoms of emotional, motivational, cognitive and physiological domains making it a complex disease to treat. Furthermore, depression is often associated with chronic illnesses (Evans et al., 2005) or other mood disorders such as co-morbid anxiety, all of which can be predictors of poor response to antidepressant treatments (Brent et al., 1998). In fact, it is estimated that only 50% of depressed patients are responsive to currently available antidepressant treatments (Rush et al., 2006). This may reflect the fact that depression diagnosis is based solely on behavioral symptoms and the drugs used to treat these symptoms are not specific to the underlying disease pathology (Berton and Nestler, 2006). Here we provide an overview of insights from human and rodent studies identifying new disease mechanisms that are relevant to developing novel, efficient treatments of depression.
2. Animal models of depression
Modeling human depression in animals is challenging considering the subjective nature of the multiple psychological and physiological symptoms and the lack of objective biomarkers (for review, see (Nestler and Hyman, 2010)). Validity of an animal model is generally evaluated with the following criteria: 1) manifestation of symptoms are reasonably analogous to the human disorder, 2) behavioral changes can be monitored objectively and 3) behavior changes can be reversed with therapeutically effective antidepressant treatments (McKinney and Bunney, 1969). None of the animal models developed so far perfectly reproduce the depression-like phenotype observed in humans (for review, see (Berton and Nestler, 2006)). Moreover, unlike other psychiatric disorders, genome wide association studies have not identified a single risk allele associated with MDD, thus, the field lacks true genetic models of the disease. Nevertheless, overlapping studies in human post mortem samples and translational rodent models have led to significant discoveries over the last few decades and drug discovery efforts are now geared towards testing the efficacy of drugs targeting known disease mechanisms. The next sections will briefly describe the relevance of established rodent depression models within the context of recent disease mechanisms identified in human MDD subjects.
2.1. Olfactory bulbectomy
Surgical bilateral olfactory bulbectomy (OBX) has been used to screen antidepressant drugs for the past 40 years in both rats (van Riezen et al., 1976, Kelly et al., 1997, Mar et al., 2002) and mice (Han et al., 2009). OBX induces major dysfunction of the cortical-hippocampal-amygdala circuits (for review, see (Russo and Nestler, 2013)) affecting behaviors such as locomotion, food seeking and avoidance (for review, see (Song and Leonard, 2005)). Cognitive deficits, loss of libido, reduced social interaction and exploration of a novel environment was associated with cortical neuronal degeneration in OBX rats (Wang et al., 2007). Impaired structural plasticity of the hippocampus was also recently linked to emotional and spatial memory deficits in this model (Morales-Medina et al., 2013). Chronic antidepressant treatments can reverse both behavioral and structural changes (Song and Leonard, 2005). While the direct link between OBX and human MDD is controversial, clinical studies have reported decreased olfactory sensitivity in acute major depression (Atanasova et al., 2008) and there is a significant negative correlation between olfactory bulb volume and depression severity (Negoias et al., 2010). Still, less invasive and more ethologically valid models have been characterized.
2.2. Maternal separation and models of early life stress
Early life traumatic events contribute to the development of individual differences in the ability to react and cope with subsequent stressful events. Victims of childhood abuse or parental neglect have significantly higher probabilities of developing mood disorders (for review, see (Anacker et al., 2014)) and studies of institutionalized children indicate that global deprivation induces persistent behavioral and cognitive deficits (Chugani et al., 2001). Rodent models of maternal separation suggest that offspring of separated pups are more submissive and generally adopt a more passive coping strategy in response to stress throughout life (Gardner et al., 2005). These early life events permanently alter endocrine responses to stress (Plotsky et al., 2005) and confer vulnerability to drug abuse in adulthood (for review, see (Moffett et al., 2007)). Additionally, individual differences in maternal behaviors, such as licking and grooming, can correspond with offspring vulnerability and promote resilience (Fleming et al., 1999, Meaney, 2001, Weaver et al., 2004, Weinstock, 2008). As adults, the offspring of high licking and grooming mothers are less fearful and characterized by lower hypothalamic-pituitary-adrenal (HPA) responses to stress (Liu et al., 1997, Caldji et al., 1998, Francis et al., 1999a, Tang et al., 2014). In an elegant study, Weaver et al. (2004) showed that mother-pup contacts alter the offspring epigenome thus affecting regulation of the glucocorticoid receptor (GR) promoter in the hippocampus. Moreover, mother-pup licking and grooming behaviors are stably transmitted across generations (Francis et al., 1999a), and cross-fostering studies suggest that these might be inherited through the nursing mother and not the biological one (Champagne and Meaney, 2001). Interestingly, social deprivation and maternal care models seem to have distinct behavioral and neurochemical consequences depending upon the developmental stage of the offspring (Hall, 1998). This highlights the importance of future studies to identify causative factors in the development of depressive behaviors during these critical windows in order to translate findings to human disease.
2.3. Learned helplessness
In humans and rodents, learned helplessness (LH) is defined as a deficit to escape an aversive stimulus induced by prior exposure to uncontrollable stress (Pryce et al., 2011). It is interpreted as a depression-like coping deficit in avoidable situations (Vollmayr and Gass, 2013). The original protocol consisting of inescapable foot shock was first developed in dogs (Overmier and Seligman, 1967) and later adapted to rodents (Maier, 1990, Chourbaji et al., 2005). It is associated with the cognitive theory of depression (Overmier and Seligman, 1967). Helpless rats are characterized by a broad range of behavioral, physiological and hormonal changes reversed only by chronic antidepressant treatments (Sherman et al., 1982, Takamori et al., 2001). Though rat strains bred for congenital vulnerability to LH exhibit normal memory acquisition and retrieval, they exhibit reduced sucrose preference, which is analogous to anhedonia in human depression (Vollmayr et al., 2004). Much like in humans, susceptibility to LH is observed in only a subset of the male rats exposed to inescapable stress, marked by an increase in depressive-like behaviors (Vollmayr et al., 2003). On the other hand, female rodents never develop traditional LH (Dalla et al., 2008b), hence the importance to keep gender in mind when looking for insights from animal models to develop novel, efficient drugs.
2.4. Repeated restraint stress
Repeated restraint stress is an inescapable paradigm similar to LH where the animal is enclosed daily in a narrow tube (1–6h/day) for up to 21 consecutive days (Watanabe et al., 1992, Magarinos and McEwen, 1995, Kim and Han, 2006, Ulloa et al., 2010, Yu et al., 2012, Lee et al., 2013, Voorhees et al., 2013, Cheng et al., 2014). Adult rodents exposed to repeated restraint stress exhibit a depression-like phenotype marked by reduced sucrose preference, anxiety-like exploratory deficits and increased immobility in the forced swim test (Kim and Han, 2006, Ulloa et al., 2010, Lee et al., 2013, Voorhees et al., 2013), Some of these phenotypes can only be reversed by chronic treatments with antidepressants (Stone et al., 1984, Ulloa et al., 2010, Yu et al., 2012). Stress-related hormones, notably corticosterone, initially increase following restraint stress (Magarinos and McEwen, 1995, Kim and Han, 2006, Lee et al., 2013, Voorhees et al., 2013). However, following repeated stress, the HPA response is desensitized and no significant increase of corticosterone level is observed after 21 days (Magarinos and McEwen, 1995). Validity of this animal model for MDD etiology in human is not as clear as for other paradigms however, over the years findings in line with other rodent models as well as human depression have been reported and are discussed in the following sections.
2.5. Chronic unpredictable/mild stress
Chronic mild stress (CMS) is an animal model centered on the idea that low level chronic and unpredictable stressors, similar to what a human might experience in everyday life, induce depression in vulnerable individuals (for review, see (Willner, 2005)). Like other depression models, CMS induces a wide range of behavioral deficits including decreased sucrose preference, motivation for reward stimuli and sexual behaviors along with increased aggression, anxiety-like behaviors and altered sleep patterns. Many of the behavioral alterations are reversed by chronic, but not acute, treatments with standard antidepressants (Willner, 2005, Mutlu et al., 2012). CMS disrupts the HPA axis and induces a depression-like phenotype in both male and female rats (Grippo et al., 2005b). Interestingly, a 4-week paradigm of CMS decreases the olfactory bulb volume and function in rats (Yang et al., 2011). Strain-related differences have been recently reported with C57BL/6J mice displaying greater anxiety- and depression-like behaviors than the ICR (CD-1) outbred strain (Jung et al., 2014). Despite the fact that the CMS model is widely used and effective, it can be cumbersome and time consuming. Furthermore, because the types of stressors and length of stress can vary widely from lab to lab, the behavioral outcomes may not always fully replicate.
2.6 Social isolation
Human beings are social by nature and a lower sense of belongingness is associated with higher risk of developing mood disorders and depression-like symptoms (Hagerty et al., 1996). In this regard, grief and partner loss in later life is especially relevant to depression in the elderly (Costello and Kendrick, 2000). Significant associations have been reported between social skills, levels of self-esteem and depression in adolescent substance abusers (Van Hasselt et al., 1993). Depression-like behaviors and related lack of social skills in adulthood may also favor alcohol (Windle and Windle, 2012) and drug abuse (Mueser and Gingerich, 2013). Indeed, co-morbid mood disorder is present in 30–40% of individuals suffering from addiction (Conway et al., 2006). Rodents are also by nature social creatures and several studies have been conducted with social isolation used to mimic loneliness. Deprivation of contact with peers enhances alcohol consumption (Ehlers et al., 2007) and vulnerability to drug abuse in male rats, particularly in stressful conditions (Ahmed et al., 1995). In female prairie voles, a socially monogamous rodent model, chronic social isolation induces depression-like behaviors, notably anhedonia, and higher neuroendocrine response to a resident intruder (Grippo et al., 2007). Social isolation might even exert deleterious effects on normally beneficial experiences such as the induction of neurogenesis in the hippocampus following voluntary physical exercise (Stranahan et al., 2006). Anxiety- and anhedonia-like symptoms of social isolation in adults can be reversed by chronic, but not acute, treatments with antidepressants (Wallace et al., 2009) reinforcing the relevance of this model to study depression and antidepressant responses.
2.7 Chronic social defeat stress
Social defeat stress has been extensively studied in humans, particularly in the context of bullying, which has gained attention recently as a major public health risk (Bjorkqvist, 2001). Mood disorders are highly prevalent in victims of bullying. Adults who reported bullying in childhood are more than twice as likely to attempt suicide later in life (Meltzer et al., 2011). Excessive competition behaviors in a social environment may also increase overall vulnerability to stress leading to depression-like symptoms (Gilbert et al., 2009). In rodents, repeated exposures to social defeat stress (chronic social defeat stress, CSDS) induces a depression-like phenotype characterized by anhedonia and social-avoidance behaviors (Kudryavtseva et al., 1991, Berton et al., 2006, Krishnan et al., 2007, Golden et al., 2011), which can be reversed by chronic treatment with antidepressants (Rygula et al., 2006a, Rygula et al., 2006b). Interestingly, in C57BL/6J mice subjected to CSDS there are individual differences in vulnerability (Berton et al., 2006, Krishnan et al., 2007, Golden et al., 2011, Hodes et al., 2014), which makes this model particularly relevant to study human stress vulnerability and depression. Indeed, a majority of humans exposed to stressful events are resilient and do not develop depression or post-traumatic stress disorders (PTSD) (for review, see (Southwick and Charney, 2012)). In addition, endocrine disruptions similar to those observed in humans with depression have been identified as markers of vulnerability to social stress in rodents (Korte et al., 1995, Berton et al., 1999, Jochems et al., 2015). While much work has determined the mechanisms of vulnerability in these mice, more recent studies have highlighted active neurobiological mechanisms associated with resilience (for reviews, see (Russo et al., 2012, Pfau and Russo, 2015)). It is thought that identification of neurobiological changes associated with active coping to stress normally seen in resilient individuals will allow the development of novel more effective classes of antidepressant medications.
2.8 Witness defeat
Witnessing a traumatic event is sufficient to induce PTSD symptoms in vulnerable humans (Kessler et al., 1995) and rodents ((Warren et al., 2013, Patki et al., 2014)). Warren et al. (2013) showed that witnessing stressful events induces long-lasting behaviors associated with a depression-/anxiety-like phenotype and enhanced corticosterone serum level in adult mice. More importantly, gene expression changes in the ventral tegmental area were similar to those of susceptible mice following the more traditional CSDS (Warren et al., 2013). Witness defeat was also associated with depression-/anxiety-like behaviors, memory deficits and elevated corticosterone levels in rats (Patki et al., 2014). This animal model is particularly relevant for combat-related trauma witnessed by soldiers that develop PTSD (Daskalakis and Yehuda, 2014). Some have even argued that witnessing human atrocities may in fact have a greater impact than self-exposure (Yehuda et al., 1992).
2.9 Genetic and pharmacological rodent models
Despite the fact that genome wide association studies in humans have not identified a single risk allele associated with MDD, there are clearly genetic factors that contribute to stress vulnerability in rodent (Levinson, 2006, Flint and Kendler, 2014). Several groups discovered that depression-like phenotypes differ dramatically between specific rat strains. For example, the Wistar Kyoto rat strain exhibits a pronounced pro-depressant phenotype including behavioral despair, social avoidance and anhedonia (Pare, 1994, Solberg et al., 2004, Nam et al., 2014). Chronic treatment with antidepressants, electroconvulsive therapy or deep brain stimulation can reverse depressive-like behaviors in these animals (Jeannotte et al., 2009, Falowski et al., 2011, Kyeremanteng et al., 2012, Tizabi et al., 2012, Belujon and Grace, 2014, Kyeremanteng et al., 2014). In line with this concept the Flinders sensitive rat line, which was originally bred according to their resistance to the anticholinesterase agent diisopropyl fluorophosphate, exhibit some similarities to depressed humans (for extensive review, please refer to (Overstreet and Wegener, 2013)), such as increased immobility in the forced swim test (Overstreet et al., 1992), altered coping response in the social interaction test (Overstreet et al., 2004) and reduced maternal behaviors (Lavi-Avnon et al., 2005, Friedman et al., 2006). This depression-like phenotype can be partially reversed by chronic antidepressant treatments (Schiller et al., 1992, Pucilowski and Overstreet, 1993, Overstreet et al., 2004). Surprisingly, Flinders sensitive rats do not exhibit a greater degree of anhedonia, (Pucilowski et al., 1993) nor do they show increased anxiety in a novel environment such as the elevated plus maze (Schiller et al., 1991). Thus, while this rat model recapitulates aspects of human depression, it may be more limited in understanding anhedonia or depression without comorbidity of anxiety.
Genetic models have also been developed based on polymorphisms identified in depressed patients although we should caution that none of these polymorphisms have survived corrections for multiple comparisons across more stringent population based genome-wide analysis in MDD patients (Flint and Kendler, 2014). Rather, most were identified in bipolar and schizophrenia patients where MDD comorbidity is high (Levinson, 2006, Flint and Kendler, 2014, Gatt et al., 2015). As an example, a DNA variant in the vicinity of the brain-derived neurotrophic factor (BDNF) gene has been linked to increased susceptibility to bipolar disorder in a family-based association study (Neves-Pereira et al., 2002). In parallel, single-nucleotide polymorphisms within the BDNF gene were reported in bipolar patients (Sklar et al., 2002). Genetic variants of the BDNF gene have also been shown to be associated with greater risk for MDD in a subset of patients with a history of schizophrenia (Schumacher et al., 2005). Additional studies in animal have shown that BDNF polymorphisms are implicated in stress response and the effects of antidepressants (Nibuya et al., 1995, Smith et al., 1995, Duman and Monteggia, 2006). Genetically modified mice expressing a methionine substitution for valine at codon 66 of the BDNF gene (Val66Met) reproduces phenotypic hallmarks of depression as observed in humans (Chen et al., 2006).
Pharmacological interventions such as chronic corticosterone administration can also induce long lasting depressive-like behaviors in rodents by mimicking the increased stress hormone production observed in a subset of depressed patients (Gourley and Taylor, 2009, Sterner and Kalynchuk, 2010). In addition, serotonin deficiency induced by tryptophan depletion or tryptophan hydroxylase inhibition can cause depression in both humans (Young, 2013) and rodent models of depression (Berton and Nestler, 2006), implicating reduced serotonergic function in depression. For a more detailed discussion of these models and their utility for studying depression we refer the reader to the following reviews (Berton and Nestler, 2006, Sterner and Kalynchuk, 2010, Jacobsen et al., 2012).
3. Validity for human disorders
Clinical features of MDD are heterogeneous and it is becoming increasingly clear that patients can be stratified to some extent based upon underlying etiologies (Kessler et al., 1996, Frazer and Morilak, 2005, Matthews et al., 2005). Despite the fact that none of the animal models currently used fully recapitulate the entire human depression syndrome, as we discuss throughout the review each has advantages and disadvantages (for review, see (Berton and Nestler, 2006)) by capturing different aspects of the human condition.
Most of the animal models of depression have been developed using male rodents and were later applied to their female counterparts. Consequently some models, for example CSDS, are less appropriate to study female depression, since females of most rodent strains do not readily fight. Furthermore, female rats do not express LH as males do (Dalla et al., 2008b) and display a differential response and adaptation to stress in the FST and CMS paradigms (Dalla et al., 2008a). In this regard, behavioral effects of antidepressant treatment might be gender-specific (Kokras et al., 2009, Dalla et al., 2010). Indeed, women may respond better to selective serotonin reuptake inhibitors (Kornstein et al., 2000), which might be related to sexual dimorphisms of the serotonergic system (Rubinow et al., 1998). In contrast, men and postmenopausal women show a more favorable response to tricyclic antidepressants, highlighting a possible role for sex hormones in response to antidepressant treatment (Kornstein et al., 2000, Kornstein et al., 2010). For extensive reviews on sex differences in animal models of mood disorders readers should refer to (Dalla et al., 2011, Kreiner et al., 2013, Kokras and Dalla, 2014, Seney and Sibille, 2014, Pfau and Russo, 2015).
Nevertheless, significant progress has been made in the last few decades using the animal models presented above and a growing number of studies are including human samples to validate results obtained in rodents. In the next sections, we will summarize converging evidence from both depressed humans and rodent depression models related to synaptic remodeling, transcription factors and epigenetics, immunity and inflammation and astrocyte function (Table 1).
Table 1.
Promising targets to treat depression validated in human and rodent studies.
3.1 Neuronal and synaptic remodeling
In the late 1990s, decreased brain activity was linked to a reduction in cortical volume in patients suffering from bipolar and unipolar depression (Drevets et al., 1997). Since then, multiple groups have investigated neuroanatomical and cellular alterations in the brain of depressed patients and suicide subjects (for reviews, see (Manji et al., 2001, Hercher et al., 2009, Russo and Nestler, 2013)). One of the consistent findings from histopathological studies is lower cortical thickness and cell densities in the prefrontal cortex (PFC) of depressed patients (Rajkowska et al., 1999). Microarray gene profiling has revealed decreased expression of synapse-related genes and loss of excitatory synapses in both the PFC (Kang et al., 2012) and hippocampus (Duric et al., 2013) of MDD subjects. Interestingly, experience-dependent structural and functional changes identified in human MDD are strongly affected in many of the rodent stress models described above and have been linked to depression-/anxiety-like behavioral phenotypes (Shansky et al., 2009, Shansky et al., 2010, Christoffel et al., 2011b, Davidson and McEwen, 2012, McEwen, 2012, McEwen and Morrison, 2013)(Christoffel et al., in press). As a side note, sex hormones can influence adaptive structural plasticity and have been shown to mediate the effects of stress on the brain (for reviews, see (Shansky, 2009, McEwen, 2010)). It has been argued that sex hormone mediated restructuring of synaptic contacts within the brain might be linked to gender differences in MDD prevalence (Shansky, 2009).
In rats exposed to the LH paradigm, changes in depressive behaviors are closely related to persistent remodeling of hippocampal spines and synapses (Hajszan et al., 2009) (Figure 1). Dendritic atrophy has also been reported in the hippocampus of OBX rats and was associated with depression-like behaviors (Morales-Medina et al., 2013). In addition, chronic daily restraint stress induces similar effects in the rat medial PFC (Radley et al., 2004, Radley et al., 2006) and even a brief episode of uncontrollable stress causes dendritic retraction in the PFC of mice (Izquierdo et al., 2006). Moreover, experience-dependent structural plasticity and synaptic functioning in the rat hippocampus are influenced by the degree and quality of maternal care (Champagne et al., 2008, Bagot et al., 2009), which under severe circumstances can be extremely persistent throughout adulthood (Baudin et al., 2012, Sousa et al., 2014). Unlike the hippocampus and PFC, chronic stress increases spine number and dendrite complexity within limbic regions such as the nucleus accumbens (NAc) and basolateral amygdala (BLA) (Vyas et al., 2006, Christoffel et al., 2011a, Warren et al., 2014). For example, 21-day immobilization stress (2h/day) enhances dendritic arborization and elongation in the BLA inducing anxiety-like behaviors in the elevated plus maze (Vyas et al., 2006). In addition, restraint stress induces anhedonia and modifies synaptic strength of NAc medium spiny neurons (MSN) (Lim et al., 2012). Interestingly, chronic gestational stress induces persistent depression-like behaviors and alters spine density and dendrite morphology in the NAc (Haim et al., 2014) and the PFC (Leuner et al., 2014) of female rats.
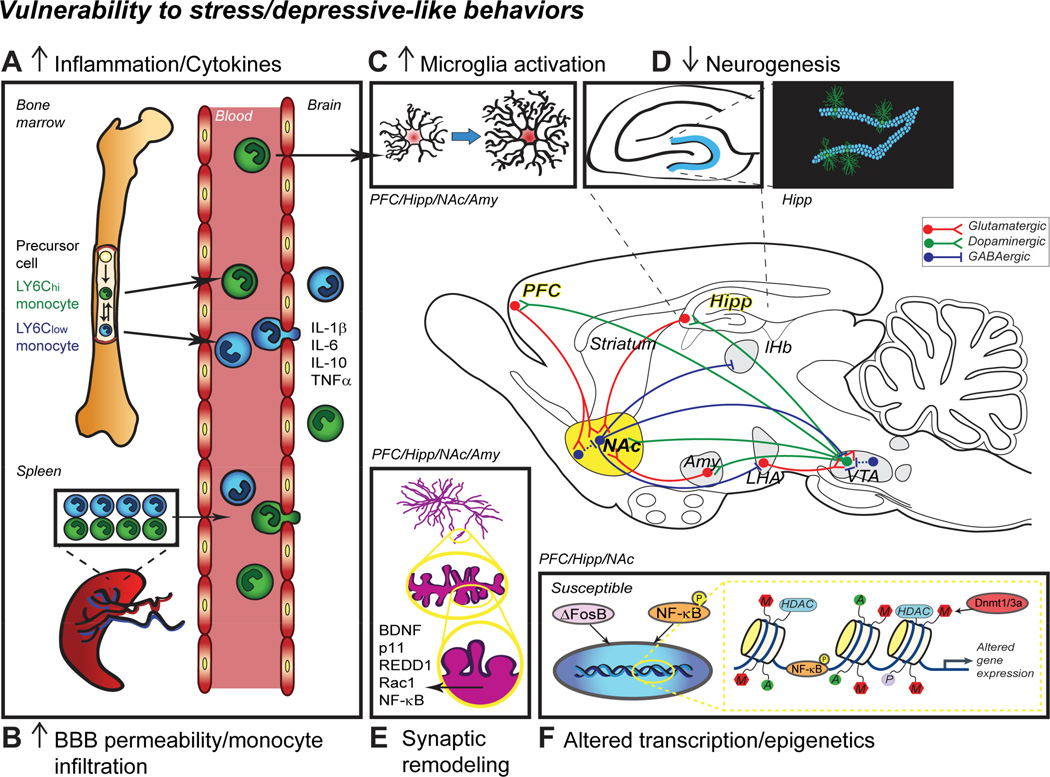
Figure 1. Mechanisms associated with MDD pathogenesis.
A) Pro-inflammatory cytokine (interleukin, IL; tumor necrosis factor alpha, TNFα) levels are increased in the blood of depressed patients and animal models of MDD suggesting enhanced monocyte production through the bone marrow or release from the spleen reservoir. Moreover, reduction of the bloodbrain barrier (BBB) permeability may favor monocyte infiltration in the brain (B) leading to inflammation and increased microglia activation (C). The MDD brain is characterized by lower neurogenesis in the hippocampus (HIPP) (D) and altered synaptic remodeling in multiple structures including the prefrontal cortex (PFC), HIPP and nucleus accumbens (NAc) (E). Changes have also been reported in other brain regions such as the amygdala (AMY) and ventral tegmental area (VTA) but are not discussed here. All these brain regions with various functions are highly interconnected highlighting the complexity of MDD treatment. Experience-dependent synaptic remodeling and dendritic spine formation is affected by chronic stress and regulated through multiple signaling pathways including brain-derived neurotrophic factor (BDNF), its downstream target p11, regulated in development and DNA damage response-1 (REDD1), small Rho GTPase RAS-related C3 botulinum toxin substrate 1 (Rac1) and nuclear factor kappa-B (NF-ΚB). F) Heritability and chronicity of MDD also imply persistent changes at the molecular level and recent studies reported altered transcription and epigenetics through pathologically modified expression of ΔFosB and DNA methyltransferases (Dnmt), respectively. LHA, lateral hypothalamus; lHb, lateral habenula.
Collectively, these studies show that stress-induced changes in synapse structure and function within the reward circuitry (Figure 1) drive symptoms of anhedonia and greater addiction liability seen in depressed patients (for review, see (Russo and Nestler, 2013)). Thus, it may be possible in the future to target neuronal and cellular plasticity mechanisms to promote a resilient phenotype and improve treatment in drug-resistant patients (Manji et al., 2003, Vidal et al., 2011).
In addition to synaptic plasticity, previous research has implicated other forms of neuronal plasticity such as the birth and integration of new neurons in the hippocampus as being particularly important for antidepressant drug responses (Banasr and Duman, 2007). Two neurogenic niches have been identified in rodents: the subgranular zone of the dentate gyrus in the hippocampus and the subventricular zone of the lateral ventricles. New neurons generated in the hippocampus become dentate granule cells while those produced in the subventricular zone migrate to the olfactory bulb (Imayoshi et al., 2009, Borsini et al., 2015). Within the adult rodent brain, repeated stress reduces cell proliferation and total neuron number in the dentate gyrus (Pham et al., 2003). Conversely physical activity (van Praag et al., 1999) and environmental enrichment (Kempermann et al., 1997), both of which are antidepressant in humans, promote the formation of new neurons from a pool of neural progenitor cells (Cameron and Gould, 1994, Zhao et al., 2008, Imayoshi et al., 2009, Borsini et al., 2015). Recent imaging studies suggest that experience-dependent neurogenesis may be a biomarker of a healthy and well-functioning brain as reduced neurogenesis has been observed in numerous human and rodent studies related to depression and PTSD (for reviews, see (Dranovsky and Hen, 2006, Becker and Wojtowicz, 2007, Eisch and Petrik, 2012, Kheirbek et al., 2012)) (Figure 1). A greater understanding of the mechanisms mediating such reduced neurogenesis in depression and related disorders may help to improve diagnosis and treatment outcome.
3.1.1 BDNF
The neurotrophic theory of depression and antidepressant responses argues that low levels of neurotrophic factors, such as BDNF, increase stress vulnerability through their effects on nerve cells within forebrain structures (Duman et al., 1997) (Figure 1). Over the years, the story has become more complex with multiple groups showing that stress and antidepressant treatments have opposite effects on BDNF expression in the brain depending upon structure (for review, see (Duman and Monteggia, 2006)). BDNF (Val66Met) gene polymorphism has been associated with altered hippocampal and cortical morphology (Pezawas et al., 2004), reduced hippocampal volume and increased depression prevalence in humans (Szeszko et al., 2005, Bueller et al., 2006). Mice expressing the Val66Met gene variant also exhibit increased anxiety-related behaviors and decreased hippocampal volume and dendritic complexity that are not normalized by fluoxetine treatment (Chen et al., 2006). Interestingly, desipramine has antidepressants effects in these same mice suggesting that this polymorphism could influence individual response to drug treatment (Yu et al., 2012). BDNF has recently been targeted as a promising marker for successful antidepressant response in a personalized-medicine approach to treat depression (Cattaneo et al., 2013).
BDNF is important for hippocampal dendritic remodeling following chronic restraint stress in mice (Magarinos et al., 2011). Reduced BDNF levels have been reported in the hippocampus of rats bred for vulnerability to LH (Aznar et al., 2010). The rapid action of ketamine, an antagonist of glutamate N-methyl-D-aspartate (NMDA) receptors, which acts as an antidepressant in treatment-resistant patients (Murrough, 2012) seems to be mediated by the release of BDNF in the PFC (Lepack et al., 2014). Electroconvulsive seizure therapy (ECS), a clinical intervention for depressed patients resistant to antidepressant treatments, induces production of neurotrophic and angiogenic factors in the rat hippocampus (Newton et al., 2003). Conversely, chronic stress increases BDNF levels in the mesolimbic dopamine system to promote a pro-depressant phenotype (Eisch et al., 2003, Berton et al., 2006), while both standard treatment with antidepressants, as well as knockdown of BDNF in the VTA-NAc pathway, prevents depressionlike behavior induced by CSDS (Berton et al., 2006, Walsh et al., 2014). Post mortem studies in humans have verified increased BDNF within the NAc suggesting that this may reflect an important disease mechanism to target for treatment (Krishnan et al., 2007). Despite this, the heterogeneous effects of BDNF throughout mesolimbic and forebrain circuitry make it a very challenging molecule to target globally.
3.1.2 p11
Following the promising results reported in BNDF studies, downstream targets of this neurotrophic factor, such as the protein p11 (also called S100A10), have been considered as potential targets to improve MDD treatments and predict antidepressant response (Svenningsson et al., 2014) (Figure 1). p11, a calcium effector protein modulating serotonin receptor signal transduction (for reviews, see (Svenningsson and Greengard, 2007, Svenningsson et al., 2013)), is down-regulated in the brain of depressed patients (Svenningsson et al., 2006) and subjects who commit suicide (Anisman et al., 2008). In patients suffering from bipolar disorders, p11 mRNA level in peripheral mononuclear blood cells is positively correlated with the number of depressive episodes (Zhang et al., 2011a). It has also been proposed as a biomarker to distinguish PTSD from other psychiatric disorders (Su et al., 2009) and could be useful to identify patients at high risk of suicide (Zhang et al., 2011b).
In rodents, therapies such as ECS (Svenningsson et al., 2006, Perez-Caballero et al., 2014) or chronic antidepressant treatment (Svenningsson et al., 2006) increase p11 expression. Furthermore, overexpression of p11 in the NAc induces antidepressant-like behavioral effects (Svenningsson et al., 2006, Alexander et al., 2010). Conversely, p11 knockout mice exhibit a pro-depressant phenotype and are insensitive to the antidepressant actions of BDNF (Warner-Schmidt et al., 2010). Corticostriatal projection neurons strongly express p11 and seem to be required for the antidepressant effects of serotonin reuptake inhibitors (Schmidt et al., 2012). Interestingly, excessive cytokine production, which may be a risk factor for depression (Hodes et al., 2014), mediates anti-depressant increases of p11 level in the frontal cortex and common antiinflammatory drugs have been shown to antagonize beneficial antidepressant responses in mice (Warner-Schmidt et al., 2011). This last observation seems to be related to a direct drug interaction, whereby over-the-counter anti-inflammatory compounds inhibit availability of certain monoaminergic antidepressants in blood, a finding subsequently confirmed in a human cohort raising concerns for clinicians (Warner-Schmidt et al., 2011).
3.1.3 REDD1
As mentioned previously chronic stress and MDD has been associated with atrophy in various brain regions. The molecular mechanisms involved in these morphological alterations are still poorly understood. However, Ota et al. (2014) recently proposed a crucial role for REDD1 (regulated in development and DNA damage response-1) (Figure 1). REDD1 expression is increased in the PFC following stress thereby inhibiting mTOR (mammalian target of rapamycin) (Corradetti et al., 2005, Ota et al., 2014), which is implicated in BDNF-induced protein synthesis (Takei et al., 2004) and experience-dependent plasticity (Hoeffer and Klann, 2010). In mice, deletion of the gene encoding REDD1 promotes resilience in the CMS paradigm while overexpression in the PFC induces neuronal atrophy as well as anxiety- and depression-like behaviors (Ota et al., 2014). Accordingly, REDD1 is increased in postmortem tissue from the PFC of depressed patients (Ota et al., 2014), whereas mTOR protein is significantly reduced (Jernigan et al., 2011). Glucocorticoids released in response to stress have also been shown to negatively regulate mTOR activity (Polman et al., 2012) and mTOR-dependent synapse formation, which is thought to underlie the rapid antidepressant effects of ketamine (Li et al., 2010, Li et al., 2011).
3.1.4 Rac1
Our group recently identified Rac1 (RAS-related C3 botulinum toxin substrate 1), a small Rho GTPase involved in actin-dependent synaptic remodeling (Nakayama et al., 2000, Ng et al., 2002, Tolias et al., 2011, Um et al., 2014), as a promising target for MDD treatment (Figure 1). Golden et al. (2013) reported a sustained reduction of Rac1 expression in the NAc of stress-susceptible mice following CSDS. Interestingly these findings were corroborated in NAc of postmortem tissue from depressed human subjects (Golden et al., 2013). Reduction of Rac1 expression or inhibition of its activity specifically in the NAc increase social avoidance and anhedonia and induce the formation of immature stubby excitatory spines on MSN (Golden et al., 2013). Overexpression of constitutively active Rac1 prevents formation of these immature stubby excitatory spines and reduces the expression of depressive-like behaviors following CSDS (Golden et al., 2013).
3.1.5 NF-κB
IKK-NF-κB signaling has been associated with regulation of neuronal morphology and is necessary and sufficient to induce anxiety- and depressive-like behaviors in mice including social avoidance and anhedonia (Christoffel et al., 2011a, Christoffel et al., 2012). CSDS induces accumulation of IKK protein leading to phosphorylation and activation of NF-κB. Inhibition of NF-κB by expressing a dominant-negative IKK decreases basal dendritic spine number and prevents CSDS-induced spine formation in the NAc whereas constitutive activation has the opposite effect (Russo et al., 2009) (Figure 1). Brain-specific NF-ΚB activators include glutamate and neurotrophic factors (for reviews, see (O'Neill and Kaltschmidt, 1997, Meffert and Baltimore, 2005)) and thus this transcription factor has been closely associated with neuronal function (O'Mahony et al., 2006) and synaptic plasticity underlying normal brain function (Meffert et al., 2003, Kaltschmidt et al., 2006). Hormonal regulation of NF-ΚB seems to occur as its expression is upregulated in ovariectomized female mice promoting decreased susceptibility to stress as assessed in the CMS paradigm (LaPlant et al., 2009).
In human, higher levels of NF-ΚB has been reported in the postmortem frontal cortex from bipolar disorder patients (Sun et al., 2001), although equivalent postmortem studies in MDD subjects are still lacking. Increased peripheral NF-ΚB activity has been shown in women with childhood abuse-related PTSD (Pace et al., 2012) and NF-ΚB DNA binding is enhanced in leukocytes from depressed male patients following a stressful social event when compared to controls (Pace et al., 2006). Together, these findings highlight an important role for IKK and NF-κB in the development of mood disorders and underlying neuronal and synaptic remodeling.
3.2 Transcription and epigenetics
Given the disappointment surrounding genome wide linkage studies in MDD, the field has largely focused on mechanism of epigenetics that refer to either inherited or environmentally induced post-translational modifications on the DNA that can mediate long-term changes in gene expression independent of DNA sequence changes (Bagot et al., 2014). Recent evidence suggest that stress sensitivity can be transmitted through generations in rodents (Dietz et al., 2011) and human (Yehuda et al., 2014). Moreover, molecular changes are different if the stressful events occurred early or late in life (Malki et al., 2014) suggesting that long-term alterations in transcription potential may be relevant to mood disorders. Accordingly, epigenetic mechanisms have been linked to the persistent synaptic plasticity changes associated with depressive-like behaviors (Bagot et al., 2014, Fass et al., 2014) and gender differences in stress responses (Hodes, 2013, Hunter and McEwen, 2013). In the next sections, we will summarize recent findings related to the transcription factor ΔFosB, microRNA regulation by β-catenin and histone modifications (Figure 1) that might be used to augment current MDD treatment.
3.2.1 ΔFosB
Immediate early gene expression is differentially regulated in the brain by stress (Melia et al., 1994). However, while acute stress is associated with elevated c-Fos and FosB expression in the NAc and PFC, only ΔFosB, a stable splice variant of the FosB gene, is induced by restraint stress, CMS (Perrotti et al., 2004) and CSDS (Hinwood et al., 2011). ΔFosB owes its stability, in part, to a splicing mediated absence of degron domains (Carle et al., 2007) and phosphorylation sites (Ulery et al., 2006) that prevent a rapid proteosomal degradation. This unique stability makes it a good candidate to explain the persistence of behaviors associated with exposure to chronic stimuli (for review, see (Nestler, 2014)). Furthermore, ΔFosB expression affects synaptic strength and dendritic spine morphology (Grueter et al., 2013). Interestingly, resilience to social defeat stress has been associated with ΔFosB induction in D1 MSNs in NAc of mice (Vialou et al., 2010a, Vialou et al., 2010b) and humans (Vialou et al., 2010a) and is required for antidepressant behavioral responses (Vialou et al., 2010b). Although ΔFosB seems to be induced in the NAc of susceptible mice to a lesser extent than resilient mice, its induction is specific to D2 MSNs (Lobo et al., 2013). Interestingly, natural reward behaviors such as sucrose drinking and sexual behaviors also increase ΔFosB expression in the NAc (Wallace et al., 2008). Unlike in NAc, increased expression of ΔFosB in the PFC leads to anhedonia, social avoidance behavior and immobility suggesting that it has region specific effects (Vialou et al., 2014). Environmental enrichment, which is known to dampen stress-related behaviors and promote resilience (Greenwood and Fleshner, 2008, Schloesser et al., 2010), affects ΔFosB expression in both NAc (Zhang et al., 2014) and PFC (Lehmann and Herkenham, 2011). Molecules regulating ΔFosB (Wang et al., 2012) or its downstream targets look promising to develop novel antidepressant treatments. As an example, expression of the opioid peptide dynorphin is suppressed by ΔFosB (Zachariou et al., 2006) and knockdown of prodynorphin gene protects against age-related increases in anxiety and altered synaptic plasticity (Menard et al., 2013), raising the possibility that dynorphin downregulation may promote stress resilience.
3.2.2 β-catenin
The multi-functional protein β-catenin has recently been identified as a key regulator in the development of behavioral resilience (Dias et al., 2014) Selective knockdown of β-catenin in the NAc during chronic stress promotes the establishment of depressive-like behaviors (Dias et al., 2014). One of the first studies highlighting a role for β-catenin in depression showed that chronic ECS up-regulates expression and promotes neurogenesis in the rat hippocampus (Madsen et al., 2003). β-catenin regulates downstream Wnt signaling, which has also been associated with stress susceptibility in the CSDS model (Wilkinson et al., 2011). In humans, β-catenin protein level is significantly reduced in the PFC of postmortem tissue from MDD patients (Karege et al., 2012). Interestingly, overexpression of β-catenin mimics the effect of lithium (Gould et al., 2007), which is commonly used to treat bipolar disorder and acts by inhibiting glycogen synthase kinase 3β (GSK-3Β) (Beaulieu et al., 2004). GSK-3Β phosphorylation is negatively correlated with β-catenin level in the PFC of depressed patients (Karege et al., 2012). In vivo inhibition of GSK-3Β upregulates β-catenin level in the mouse hippocampus and has antidepressant effects (Kaidanovich-Beilin et al., 2004). Furthermore, selective deletion of GSK-3Β in the forebrain has anxiolytic and pro-social effects (Latapy et al., 2012) highlighting the fact that GSK-3Β may play a central role in mood regulation (for review, see (Saus et al., 2010)).
Regulation of microRNA expression by β-catenin in the context of CSDS (Dias et al., 2014) is intriguing since these non-coding transcripts are abundant in the nervous system and seem to play a role in synaptic function and plasticity (for reviews, see (Kosik, 2006, Im and Kenny, 2012)). β-catenin itself is modulated by microRNAs (Veronese et al., 2011) and polymorphisms in these microRNAs have been associated with MDD in human (Saus et al., 2010, Xu et al., 2010). Lastly, given that chronic treatment with antidepressants affects microRNA levels in serotoninergic neurons (Baudry et al., 2010), this raises the possibility that they make be targeted in new MDD treatment strategies.
3.2.3 DNA and histone modifications
Long-lasting changes of gene regulation associated with the establishment of depressive-like behaviors are highly similar between different animal models of depression, whereas the patterns observed in resilient rodents generally overlap with those of animals treated with antidepressants (Hunter et al., 2009, Wilkinson et al., 2009, Sun et al., 2013, Bagot et al., 2014). As for brain structural remodeling, sex differences in epigenetic mechanisms may influence susceptibility to stress and thus MDD prevalence (Sterrenburg et al., 2011, Hodes, 2013, Hunter and McEwen, 2013). Prenatal stress may even have a sexually dimorphic influence on stress-coping strategies later in life (Mueller and Bale, 2007, 2008). Here we will briefly discuss recent findings on DNA and histone modifications associated with depression (for a more extensive reviews on the topic, please refer to (Hodes, 2013, Hunter and McEwen, 2013, Sun et al., 2013, Bagot et al., 2014).
A genome-wide study conducted in 58 mother-child dyads reveals small DNA methylation differences between neonates exposed to non-medicated maternal depression when compared to controls (Non et al., 2014). Early life experience such as maternal care greatly affects stress susceptibility later in life. In rats, behavioral programming of the offspring epigenome includes changes in DNA methylation and histone acetylation, which promote either depressive-like behaviors or resilience (Weaver et al., 2004) and influences synaptic plasticity (Bagot et al., 2012a, Bagot et al., 2012b). Prenatal stress or prolonged maternal separation modifies DNA methylation of the corticotropin-releasing factor (CRF) gene, an effect that persists in adulthood (Mueller and Bale, 2008, van der Doelen et al., 2015). CRF, which is secreted by neurons of the hypothalamic paraventricular nucleus (PVN) in response to stress (Herman et al., 2008), mediates the effects of early life experience on stress response in adulthood and may be involved in vulnerability to mood disorders across generations (Francis et al., 1999b). Social stressors such as CSDS also affect DNA methylation in adult mice and decreased methylation of the CRF promoter has been reported in susceptible, but not resilient, mice (Elliott et al., 2010). These findings are interesting in light of the fact that a subset of MDD patients exhibit HPA axis hyperactivity which might be driven by increased production of CRF (Arborelius et al., 1999). Hence, an increased number of CRF neurons have been reported in the PVN of depressed subjects in postmortem studies (Raadsheer et al., 1994, Raadsheer et al., 1995, Wang et al., 2008) leading to the hypothesis that CRF receptor antagonists may represent a potential novel class of antidepressants.
DNA methyltransferase (Dnmt) enzymes catalyze the transfer of methyl groups to the DNA, are abundant in neurons (Feng et al., 2005) and are involved in experience-dependent synaptic plasticity and function (Levenson et al., 2006, Feng et al., 2010). In line with altered DNA methylation, Dnmt3a expression is upregulated in the NAc following CSDS and promotes greater stress vulnerability (LaPlant et al., 2010). In addition, infusion of R108, a potent non-nucleoside inhibitor of DNA methylation, promoted resilience similar to chronic intraperitoneal injection with the antidepressant fluoxetine (LaPlant et al., 2010). Decreased level of p11 in the PFC of Flinders Sensitive Line, a genetic rat model of depression, was linked to hypermethylation in its promoter region, a pattern that could be reversed by chronic treatment with escitalopram, a selective serotonin reuptake inhibitor (Melas et al., 2012). Interestingly, escitalopram-induced hypomethylation was associated with reduction of Dnmt1 and Dnmt3a levels (Melas et al., 2012) reinforcing the interest in targeting these enzymes as novel treatments for MDD.
In addition to DNA methylation, increased methylation at the BDNF promoter has been linked to reduced BDNF levels and increased risk for suicide in humans (Kang et al., 2013). In rodents, downregulation of BDNF following CSDS is associated with a similar repressive histone methylation in the hippocampus and antidepressant treatment upregulates BDNF expression through histone acetylation (Tsankova et al., 2006). Acute or repeated ECS treatment affects histone acetylation in the rat hippocampus modulating cFos and BDNF gene expression (Tsankova et al., 2004). Conversely in NAc, CSDS induces a persistent increase in acetylated histone H3 level associated with a decrease of histone deacetylase 2 (HDAC2), which can be reversed by HDAC inhibitors to promote resilience (Covington et al., 2009). In line with these findings, HDAC2 protein level is reduced in the NAc of postmortem tissue from depressed patients (Covington et al., 2009). On the other hand, HDAC2 expression is increased in peripheral white blood cells of depressed patients when compared to controls during a depressive episode, but not during the remissive state (Hobara et al., 2010), suggesting that aberrant transcriptional regulation in mood disorders may vary between the central nervous system and periphery.
3.3 Immune system and inflammation
Alterations within the peripheral immune system and subsequent over activation of pro-inflammatory cytokines has long been associated with mood disorders (for reviews, see (Capuron and Miller, 2011, Maes et al., 2011)) leading to the proposal of a macrophage theory of depression (Smith, 1991). In addition, continuous activation of the peripheral immune system induced by various forms of cancer, systemic infection or autoimmune disease may promote the development of major depression in vulnerable individuals (for review, see (Dantzer et al., 2008)). Disturbances in leukocyte function and/or leukocyte number and elevated cytokine expression have been proposed as potential biomarkers of depression (Maes et al., 1992, Raison et al., 2006, Dowlati et al., 2010, Baune et al., 2012, Hodes et al., 2014) and PTSD (Pace and Heim, 2011). There is also increasing evidence that anti-inflammatory drugs may have antidepressant effects in MDD patients (Muller et al., 2006, Tyring et al., 2006, Raison et al., 2013, Kohler et al., 2014). Interestingly, there are gender differences with regard to the immune system and depression that might make females more vulnerable to both inflammatory illnesses and depression (Maes et al., 1992, Vogelzangs et al., 2012). In the next sections, we will summarize findings related to cytokines, microglia activation and monocyte infiltration through reduced blood-brain barrier (BBB) permeability (Figure 1) in the context of MDD.
3.3.1 Cytokines
Multiples human studies have associated increased peripheral cytokine production with the development of mood disorders (Maes et al., 1992, Maes et al., 1997, Lanquillon et al., 2000, Dean et al., 2010, Dowlati et al., 2010, Maes et al., 2011, Fagundes et al., 2013) and PSTD (Baker et al., 2001, Gill et al., 2008, Newton et al., 2014). Systemic injection of pro-inflammatory cytokines induce depression-like behaviors (Dantzer et al., 2008), whereas antidepressant treatment may normalize elevated cytokine levels (Sluzewska et al., 1995, Kubera et al., 2011). Altered genetic status at the interleukin 6 (IL-6) gene locus has been recently identified by computational analyses as a risk for stress vulnerability (Cole et al., 2010). Nevertheless the mechanisms and sources of increased inflammation and behavioral disturbance in depression are not yet well understood.
Cytokines can be generally divided into either pro- or anti-inflammatory in nature (for review, see (Kubera et al., 2011)). Pro-inflammatory cytokines such as interleukin 1 (IL-1) and tumor necrosis factor alpha (TNFα) affect synaptic plasticity leading to the establishment of depression-like behaviors and mood disorders (Khairova et al., 2009). In rodents, CSDS-, CMS- and LH-induced depression-like behaviors have been correlated with high levels of pro-inflammatory IL-1β, IL-6 and TNFα (Grippo et al., 2005a, Kubera et al., 2011, Wohleb et al., 2011, Hodes et al., 2014). In contrast, anti-inflammatory IL-10 is reduced in the rat cortex and hippocampus following chronic restraint stress and depression-like behaviors can be reversed by administration of recombinant IL-10 (Voorhees et al., 2013). Activation of the immune system and elevated pro-inflammatory cytokine production affects multiple biological targets associated with depression, including cell proliferation, neurogenesis, gliogenesis and apoptosis (for reviews, see (Kubera et al., 2011, Borsini et al., 2015)). Stress-related activation of NF-ΚB signaling and IL-1β inhibit neurogenesis in the hippocampus and promote depression-like behaviors in the CMS paradigm (Koo et al., 2010). Interestingly, chronic light deprivation induces IL-6-dependent depression-like behaviors also through activation of NF-ΚB signaling and may be relevant to seasonal affective disorder marked by depressive symptoms (Monje et al., 2011). Our group recently reported that preexisting individual differences in stress-responsive IL-6 release from leukocytes contributes to CSDS susceptibility in adult mice (Hodes et al., 2014). Leukocyte-derived IL-6 release prior to stress exposure predicts susceptibility versus resilience in the CSDS paradigm (Hodes et al., 2014). Furthermore, while inhibiting peripheral IL-6 production promotes resilience, transplantation of bone marrow from stress-susceptible mice leads to greater social avoidance following subthreshold defeat or witness defeat (Hodes et al., 2014). Surprisingly, a recent study reported that transplantation of lymph node cell suspensions from chronically stressed mice into stress-naïve lymphocyte-deficient mice produces antidepressant-like effects. These interesting results may suggest that there are differences in the innate (monocytes and macrophages) versus adaptive (lymphocytes) immune system that differentially contribute to susceptibility and resilience (Brachman et al., 2015).
A major issue related to studies investigating the role of the immune system in CSDS-induced depressive behaviors is the possibility of individual variation in wounding. Especially given the fact that wounding during social stress can affect antiviral immunity (de Groot et al., 2002) and modulate splenic immune responses (Merlot et al., 2003). Nevertheless, we found that peripheral IL-6 also promotes greater vulnerability to a non-physical form of social defeat stress in which the experimental mouse simply witness the traumatic event without experiencing any physical attack themselves (Hodes et al., 2014), suggesting that psychological stress is sufficient to induce immune dysregulation and depression. Lastly, enhanced vulnerability to stressful events in older rodents has been associated with persistent activation of the peripheral immune system (Godbout et al., 2008), which highlights the relevance of these findings in the context of the worldwide population of aging adults.
3.3.2 Microglia
Elevated cytokine production in the periphery and/or central nervous system is accompanied by activation of microglia, the immunocompetent cells of the brain. This in turn has been associated with altered synaptic plasticity, neurogenesis and emotional behaviors (for reviews, see (Kettenmann et al., 2013, Delpech et al., 2015)). On the other hand the antibiotic minocycline, which reduced microglia responses, prevents stress-induced IL-1β release in LH rats (Blandino et al., 2006, Blandino et al., 2009), suggesting that cytokines and microglia may be involved in stress responses. Leukocytes from stress-susceptible mice in the CSDS paradigm release more IL-6 following LPS stimulation than resilient mice (Hodes et al., 2014) and similarly, isolated microglia from LH rats or stress-susceptible mice produced higher levels of IL-1β or IL-6, respectively, in response to the same stimulation (Frank et al., 2007, Wohleb et al., 2011). As is the case for cytokines, higher microglia immunoreactivity has been reported in the cortex of depressed patients (Steiner et al., 2011) and subjects who commit suicide (Steiner et al., 2008). In an elegant study, Setiawan et al. (2015) recently showed that microglia activation is enhanced during a major depressive episode using positron emission tomography. These changes were significant in the PFC, anterior cingular cortex and insula and greater microglia activation in the insula was correlated with depression severity (Setiawan et al., 2015).
In rats, chronic restraint stress affects density and morphology of microglia in stress-sensitive brain regions (Tynan et al., 2010) and can be reversed by treatment with minocycline (Hinwood et al., 2013). Repeated social defeat (RSD), a modified version of the CSDS paradigm, is also associated with microglia hypertrophy in the mouse hippocampus, PFC and amygdala and an increase of inflammatory markers on microglia and CNS macrophages (Wohleb et al., 2011). Furthermore, RSD promotes the infiltration of monocytes originating from the periphery in the brain (Wohleb et al., 2013), suggesting that blood brain-barrier (BBB) permeability may be affected by stress and could contribute to the establishment of depressive-like behaviors and mood disorders (Wohleb et al., 2014b, Reader et al., 2015).
3.3.3 BBB permeability and monocyte infiltration
Several weeks after RSD, acute stress exposure is sufficient to re-establish monocyte trafficking from the spleen to the brain and alter emotional behaviors in mice (Wohleb et al., 2014a). Monocytes, which originate from progenitor cells in the bone marrow or spleen, can traffic to the CNS via the bloodstream when the BBB is compromised (for review, see (Gordon and Taylor, 2005)). The BBB, which is formed by endothelial cells sealed by tight junctions, pericytes and astrocytes, seems to be compromised in patients suffering from mood disorders as assessed by the ratio of cerebrospinal fluid to serum of various markers including albumin (Gudmundsson et al., 2007, Bechter et al., 2010).
While the mechanisms of increased immune infiltration and BBB integrity are not well understand, recent evidence suggests that altered gut microbiota, which plays an active role in immunity and inflammatory diseases (for review, see (Kamada et al., 2013)), may influence BBB permeability early in life and throughout adulthood (Braniste et al., 2014). Multiple groups have now reported that manipulation of the microbiome status affects anxiety- and depressivelike behaviors in rodents (for review, see (Foster and McVey Neufeld, 2013)) and these behavioral changes can be reversed by treatment with probiotics (Foster and McVey Neufeld, 2013). This concomitant involvement of both CNS and the peripheral immune system in stress responses suggests that depression is a complex disease requiring interventions at multiple levels. In this regard, selective ablation of astrocytes seem to facilitate leukocyte infiltration in the brain and delay BBB repair (Bush et al., 1999) making these glial cells an interesting target to develop innovative new treatments for human depression.
3.4 Astrocytes
Histopathological studies showed that glial cell density is reduced in the PFC, hippocampus and amygdala of MDD patients (for review see (Russo and Nestler, 2013)). Accordingly, astrocytic gene expression such as structural glial-fibrillary-acidic-protein (GFAP) is markedly decreased in PFC samples of postmortem tissue from depressed patients (Nagy et al., 2014) (Figure 2). In rats, GFAP protein expression is reduced following maternal deprivation (Leventopoulos et al., 2007) or a 5-week social defeat paradigm (Araya-Callis et al., 2012), while S100β mRNA, a marker of astrocytes and oligodendrocytes, is decreased in a genetic model of depression (Strenn et al., 2015). Astrocytes can be classified into three types according to their morphology and spatial organization: 1) first radial astrocytes have long unbranched processes and surround blood vessels, 2) protoplasmic astrocytes are present mostly in gray matter and display highly branched short processes and finally 3) fibrous astrocytes have a classic stellar shape and are enriched in the white matter (Chen and Swanson, 2003). Chronic stress seems to disrupt the GFAP+ astrocytic network (process length, branching and volume) leading to structural atrophy but maybe not loss of the astrocytes themselves as assessed with S100β and Nissl staining (Tynan et al., 2013). GFAP is necessary for normal fibrous astrocyte function, which includes maintenance of BBB integrity (Kakinuma et al., 1998) (Figure 2). In adult rats, toxin-mediated glial loss in the PFC is sufficient to induce depressive-like behaviors (Banasr and Duman, 2008). In mice, epigenetic modifications of the glial cell-derived neurotrophic factor (Gdnf) gene in the ventral striatum have been associated with susceptibility to CMS (Uchida et al., 2011), while treatments with standard antidepressants have multiple effects on astrocytes including upregulation of structural proteins, activation of intracellular pathways and alterations of receptor expression (for review, see (Czeh and Di Benedetto, 2013)). S100β has been proposed as a biomarker for depression since it is elevated in the serum of patients suffering from depressive symptoms (Schroeter et al., 2008, Kim et al., 2012) (Figure 2). Moreover, antidepressant treatment decreases S100β level correlating with clinical improvement (Schroeter et al., 2008).
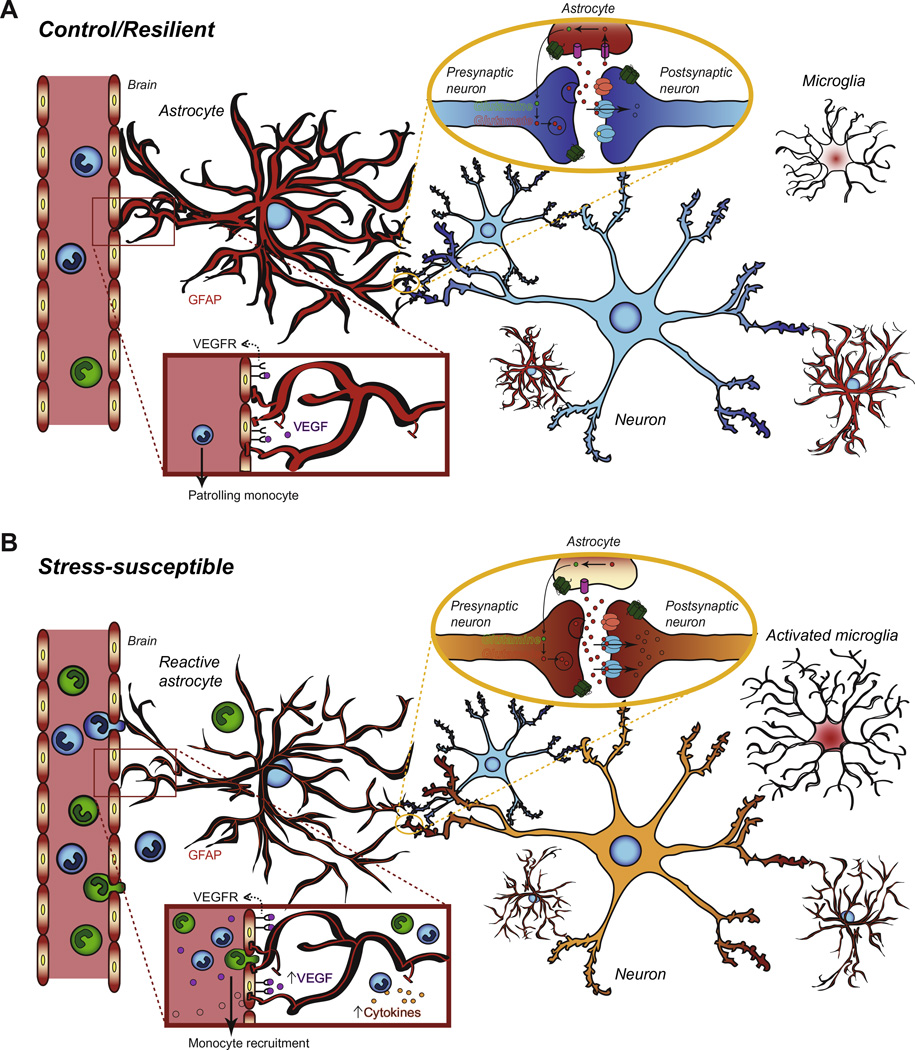
Figure 2. Potential role of astrocyte function in stress susceptibility and MDD pathogenesis.
A) Under normal conditions, astrocytes mediate neuronal function at excitatory synapses via glutamate uptake and maintain BBB permability through tight intercellular junctions with blood vessel endothelial cells. They also participate in brain metabolism by transporting nutrients through end-feet that physically connect blood vessels on one end and neuronal cells on the other. Within few hours of a brain injury, expression of structural proteins such as GFAP, is increased in reactive astrocytes initiating astrogliosis, a protective mechanism that might be deficient in stress-susceptible animals and patients suffering from MDD. B) The astrocytic network integrity seems to be impaired in MDD as mutiple groups reported lower glial fibrillary acidic protein (GFAP) expression in the PFC and hippocampus of depressed patients and rodents vulnerable to stress. Therefore, glutamate recapture is reduced affecting neurotransmission and possibly leading to glutamate receptor (AMPA, NMDA, mGluR) overactivation and excitotoxicity. Concomitantly, chronic stress may favor BBB breakdown allowing the infiltration of peripheral monocytes. Once in the brain, these monocytes release pro-inflammatory cytokines activating astrocytes and microglia. Reactive glial cells also produce and release proinflammatory cytokines exacerbating stress response. Overexpression of astrocyte-derived growth factor VEGF (vesicular endothelial growth factor) affects BBB permeability and may initiate or reinforce BBB breakdown but its exact role remains unclear. Finally, glial-specific calcium-binding protein S100β is secreted in the bloodstream by astrocytes following injury. Both VEGF and S100β have been proposed as biomarkers of MDD according to clinical studies.
Cytokines and mediators of inflammation modulate astrocyte signaling through cell surface receptors, which, in turn, regulate behavior and synaptic function (for review, see (Sofroniew, 2014)). Astrocytic transcriptome profiles change dramatically in response to stimulation with cytokines and/or inflammatory mediators and most of the up-regulated genes are chemokines, cytokines and related growth factors (Pang et al., 2001, Meeuwsen et al., 2003, John et al., 2005, Hamby et al., 2012) (Figure 2). Chronic production of IL-6 by astrocytes reduces hippocampal neurogenesis (Vallieres et al., 2002), which may then increase vulnerability to stress as discussed previously. Regulation of astrocytes by glycolipids may also drive chronic inflammation in the brain by over-activating microglia and facilitating monocyte infiltration (Mayo et al., 2014). Still, astrocyte reactivity is not homogenous (Anderson et al., 2014) and more studies are required to define the precise mechanisms between astrocytes and stress-related immune responses in the context of human depression.
3.4.1 VEGF
Persistent clinical observations suggest a relationship between cardiovascular diseases and depression: vascular diseases elevate the rates of depression-like symptoms and depression is a risk factor for the development of cardiovascular pathologies (Musselman et al., 1998, Carney et al., 2003, Carney and Freedland, 2003, Plante, 2005, Salaycik et al., 2007, Teper and O'Brien, 2008, Newton et al., 2013). Astrocytes closely interact with blood vessels and transient calcium increases in astrocyte end-feet causes cerebrovascular constrictions affecting cerebral blood flow and neuronal activity (Mulligan and MacVicar, 2004) (Figure 2). A recent study reported that activation of astrocytes in the olfactory bulb in response to odor stimulation mediates hyperemia onset indicating an active role in neurovascular coupling (Otsu et al., 2015). Overexpression of the astrocyte-derived neurotrophic factor VEGF (vesicular endothelial growth factor), a growth factor involved in angiogenesis, has been associated with breakdown of the BBB permeability and down-regulation of the tight junction proteins claudin-5 and occludin in CNS inflammatory diseases (Argaw et al., 2009, Argaw et al., 2012) (Figure 2). Interestingly, VEGF has been proposed as a potential target to treat depression (Warner-Schmidt and Duman, 2008, Fournier and Duman, 2012). Indeed, VEGF signaling is necessary for hippocampal cell proliferation and neurogenesis induced by ECS (Segi-Nishida et al., 2008) or treatment with antidepressants (Warner-Schmidt and Duman, 2007). Limited enhancement of BBB permeability through VEGF up-regulation may facilitate drug delivery in the brain (Jiang et al., 2014). Nevertheless, tight manipulation of VEGF overexpression to favor angiogenesis and drug delivery without affecting BBB permeability and neuronal function appears to be challenging. However, despite conflicting results in clinical studies (for review, see (Clark-Raymond and Halaris, 2013)) VEGF has been proposed as a potential biomarker to diagnose human depression (Arnold et al., 2012) and may be useful to evaluate the timeline of antidepressant treatment efficacy (Fornaro et al., 2013) (Figure 2).
3.4.2 Neuronal function regulation
Astrocyte perivascular end-feet establish a concomitant physical link with blood vessels (Kacem et al., 1998) and neurons to allow the transfer of metabolic substrates (for reviews, see (Tsacopoulos and Magistretti, 1996, Haydon and Carmignoto, 2006)) (Figure 2). This metabolic network is associated with activity-dependent synaptic transmission (Rouach et al., 2008) and plasticity (Gordon et al., 2009). Mice susceptible to CSDS are characterized by lower astrocytic ATP level and normalization of astrocyte-derived ATP release reversed depression-like behaviors such as social avoidance, coat deterioration, anhedonia and immobility in the FST test (Cao et al., 2013). Antidepressant treatments may not only prevent stress-induced decreases in GFAP expression (Czeh et al., 2006), but also rescue gap junction dysfunction (Sun et al., 2012) and promote gliogenesis (Kusakawa et al., 2010). A role for the astrocytic network has also been proposed as a therapeutic mechanisms in deep brain stimulation (for review, see (Fenoy et al., 2014)). Pathological activation of the lateral habenula has been reported in depressed patients (Morris et al., 1999, Roiser et al., 2009) and this phenomenon might be associated with astrocyte-mediated disruption of glutamate clearance (Figure 2), promoting susceptibility to chronic stress and depressive-like behaviors in mice (Cui et al., 2014). In line with this idea, reduced expression of glial glutamate transporters was observed in the hippocampus and cortex of LH rats (Zink et al., 2010) and hippocampal NMDA receptor function is robustly increased in stress-susceptible offspring of mothers displaying low maternal care (Bagot et al., 2012a) (Figure 2). Targeting glial cells may be a promising avenue to develop novel antidepressant treatments (Sanacora and Banasr, 2013) considering the central role of astrocytes in immune responses, homeostasis, metabolism and neuronal transmission.
4. Conclusions
Despite decades of active research using various animal models of depression combined with analysis of postmortem tissue from depressed subjects, only a handful of new treatments have been developed for MDD. Fortunately, new genetic, epigenetic and optogenetic tools have extensively broadened the toolbox of neuroscientists to better understand the cells and circuits involved in depression-like behaviors. In addition, several groups are now investigating the biology of resilience, which could lead to unexpected discoveries in the near future and novel therapeutic strategies aimed at promoting active coping. Profound individual differences are observed in both human and rodents when confronted with stressful events and these could be related to early life experiences, prenatal environment and transgenerational inheritance of epigenetic marks that confer resilience. The immune system may also play an active role in the development of mood disorders as demonstrated by recent findings from our group and others raising the intriguing possibility of novel therapeutics that target peripheral immune cells. Finally, the involvement of astrocytes in neurovascular coupling and neuronal function are still poorly understood and requires more focus considering their potential as mediators of central and peripheral processes. While these insights raise a lot of questions they also offer an opportunity for a more efficient, personalized approach to medicine for patients suffering from MDD that are unresponsive to currently available treatments.
Highlights.
-
✓
Discoveries related to major depressive disorders (MDD) obtained in rodent models and validated in human depressed subjects
-
✓
Recent findings on neuronal and synaptic remodeling, transcription and epigenetics
-
✓
Functional role of the immune system in the development of depression symptomology
-
✓
Involvement of glial cells in MDD pathogenesis
-
✓
Necessity of novel therapeutic strategies targeting central and peripheral processes
Acknowledgements
This research was supported by grants from the National Institutes of Health R01 MH090264 and R01 MH104559 to SJR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH, Stinus L, Le Moal M, Cador M. Social deprivation enhances the vulnerability of male Wistar rats to stressor- and amphetamine-induced behavioral sensitization. Psychopharmacology (Berl) 1995;117:116–124. doi: 10.1007/BF02245106. [DOI] [PubMed] [Google Scholar]
- Alexander B, Warner-Schmidt J, Eriksson T, Tamminga C, Arango-Lievano M, Ghose S, Vernov M, Stavarache M, Musatov S, Flajolet M, Svenningsson P, Greengard P, Kaplitt MG. Reversal of depressed behaviors in mice by p11 gene therapy in the nucleus accumbens. Sci Transl Med. 2010;2:54ra76. doi: 10.1126/scitranslmed.3001079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker C, O'Donnell KJ, Meaney MJ. Early life adversity and the epigenetic programming of hypothalamic-pituitary-adrenal function. Dialogues Clin Neurosci. 2014;16:321–333. doi: 10.31887/DCNS.2014.16.3/canacker. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MA, Ao Y, Sofroniew MV. Heterogeneity of reactive astrocytes. Neurosci Lett. 2014;565:23–29. doi: 10.1016/j.neulet.2013.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisman H, Du L, Palkovits M, Faludi G, Kovacs GG, Szontagh-Kishazi P, Merali Z, Poulter MO. Serotonin receptor subtype and p11 mRNA expression in stress-relevant brain regions of suicide and control subjects. J Psychiatry Neurosci. 2008;33:131–141. [PMC free article] [PubMed] [Google Scholar]
- Araya-Callis C, Hiemke C, Abumaria N, Flugge G. Chronic psychosocial stress and citalopram modulate the expression of the glial proteins GFAP and NDRG2 in the hippocampus. Psychopharmacology (Berl) 2012;224:209–222. doi: 10.1007/s00213-012-2741-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- Argaw AT, Asp L, Zhang J, Navrazhina K, Pham T, Mariani JN, Mahase S, Dutta DJ, Seto J, Kramer EG, Ferrara N, Sofroniew MV, John GR. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J Clin Invest. 2012;122:2454–2468. doi: 10.1172/JCI60842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc Natl Acad Sci U S A. 2009;106:1977–1982. doi: 10.1073/pnas.0808698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SE, Xie SX, Leung YY, Wang LS, Kling MA, Han X, Kim EJ, Wolk DA, Bennett DA, Chen-Plotkin A, Grossman M, Hu W, Lee VM, Mackin RS, Trojanowski JQ, Wilson RS, Shaw LM. Plasma biomarkers of depressive symptoms in older adults. Transl Psychiatry. 2012;2:e65. doi: 10.1038/tp.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanasova B, Graux J, El Hage W, Hommet C, Camus V, Belzung C. Olfaction: a potential cognitive marker of psychiatric disorders. Neurosci Biobehav Rev. 2008;32:1315–1325. doi: 10.1016/j.neubiorev.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Aznar S, Klein AB, Santini MA, Knudsen GM, Henn F, Gass P, Vollmayr B. Aging and depression vulnerability interaction results in decreased serotonin innervation associated with reduced BDNF levels in hippocampus of rats bred for learned helplessness. Synapse. 2010;64:561–565. doi: 10.1002/syn.20773. [DOI] [PubMed] [Google Scholar]
- Bagot RC, Labonte B, Pena CJ, Nestler EJ. Epigenetic signaling in psychiatric disorders: stress and depression. Dialogues Clin Neurosci. 2014;16:281–295. doi: 10.31887/DCNS.2014.16.3/rbagot. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot RC, Tse YC, Nguyen HB, Wong AS, Meaney MJ, Wong TP. Maternal care influences hippocampal N-methyl-D-aspartate receptor function and dynamic regulation by corticosterone in adulthood. Biol Psychiatry. 2012a;72:491–498. doi: 10.1016/j.biopsych.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Bagot RC, van Hasselt FN, Champagne DL, Meaney MJ, Krugers HJ, Joels M. Maternal care determines rapid effects of stress mediators on synaptic plasticity in adult rat hippocampal dentate gyrus. Neurobiol Learn Mem. 2009;92:292–300. doi: 10.1016/j.nlm.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Bagot RC, Zhang TY, Wen X, Nguyen TT, Nguyen HB, Diorio J, Wong TP, Meaney MJ. Variations in postnatal maternal care and the epigenetic regulation of metabotropic glutamate receptor 1 expression and hippocampal function in the rat. Proc Natl Acad Sci U S A. 2012b;109(Suppl 2):17200–17207. doi: 10.1073/pnas.1204599109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DG, Ekhator NN, Kasckow JW, Hill KK, Zoumakis E, Dashevsky BA, Chrousos GP, Geracioti TD., Jr Plasma and cerebrospinal fluid interleukin-6 concentrations in posttraumatic stress disorder. Neuroimmunomodulation. 2001;9:209–217. doi: 10.1159/000049028. [DOI] [PubMed] [Google Scholar]
- Banasr M, Duman RS. Regulation of neurogenesis and gliogenesis by stress and antidepressant treatment. CNS Neurol Disord Drug Targets. 2007;6:311–320. doi: 10.2174/187152707783220929. [DOI] [PubMed] [Google Scholar]
- Banasr M, Duman RS. Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biol Psychiatry. 2008;64:863–870. doi: 10.1016/j.biopsych.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudin A, Blot K, Verney C, Estevez L, Santamaria J, Gressens P, Giros B, Otani S, Dauge V, Naudon L. Maternal deprivation induces deficits in temporal memory and cognitive flexibility and exaggerates synaptic plasticity in the rat medial prefrontal cortex. Neurobiol Learn Mem. 2012;98:207–214. doi: 10.1016/j.nlm.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Baudry A, Mouillet-Richard S, Schneider B, Launay JM, Kellermann O. miR-16 targets the serotonin transporter: a new facet for adaptive responses to antidepressants. Science. 2010;329:1537–1541. doi: 10.1126/science.1193692. [DOI] [PubMed] [Google Scholar]
- Baune BT, Smith E, Reppermund S, Air T, Samaras K, Lux O, Brodaty H, Sachdev P, Trollor JN. Inflammatory biomarkers predict depressive, but not anxiety symptoms during aging: the prospective Sydney Memory and Aging Study. Psychoneuroendocrinology. 2012;37:1521–1530. doi: 10.1016/j.psyneuen.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Yao WD, Kockeritz L, Woodgett JR, Gainetdinov RR, Caron MG. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc Natl Acad Sci U S A. 2004;101:5099–5104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechter K, Reiber H, Herzog S, Fuchs D, Tumani H, Maxeiner HG. Cerebrospinal fluid analysis in affective and schizophrenic spectrum disorders: identification of subgroups with immune responses and blood-CSF barrier dysfunction. J Psychiatr Res. 2010;44:321–330. doi: 10.1016/j.jpsychires.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Becker S, Wojtowicz JM. A model of hippocampal neurogenesis in memory and mood disorders. Trends Cogn Sci. 2007;11:70–76. doi: 10.1016/j.tics.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Belujon P, Grace AA. Restoring mood balance in depression: ketamine reverses deficit in dopamine-dependent synaptic plasticity. Biol Psychiatry. 2014;76:927–936. doi: 10.1016/j.biopsych.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O, Durand M, Aguerre S, Mormede P, Chaouloff F. Behavioral, neuroendocrine and serotonergic consequences of single social defeat and repeated fluoxetine pretreatment in the Lewis rat strain. Neuroscience. 1999;92:327–341. doi: 10.1016/s0306-4522(98)00742-8. [DOI] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci. 2006;7:137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- Bjorkqvist K. Social defeat as a stressor in humans. Physiol Behav. 2001;73:435–442. doi: 10.1016/s0031-9384(01)00490-5. [DOI] [PubMed] [Google Scholar]
- Blandino P, Jr, Barnum CJ, Deak T. The involvement of norepinephrine and microglia in hypothalamic and splenic IL-1beta responses to stress. J Neuroimmunol. 2006;173:87–95. doi: 10.1016/j.jneuroim.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Blandino P, Jr, Barnum CJ, Solomon LG, Larish Y, Lankow BS, Deak T. Gene expression changes in the hypothalamus provide evidence for regionally-selective changes in IL-1 and microglial markers after acute stress. Brain Behav Immun. 2009;23:958–968. doi: 10.1016/j.bbi.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Borsini A, Zunszain PA, Thuret S, Pariante CM. The role of inflammatory cytokines as key modulators of neurogenesis. Trends Neurosci. 2015 doi: 10.1016/j.tins.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Brachman RA, Lehmann ML, Maric D, Herkenham M. Lymphocytes from chronically stressed mice confer antidepressant-like effects to naive mice. J Neurosci. 2015;35:1530–1538. doi: 10.1523/JNEUROSCI.2278-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Toth M, Korecka A, Bakocevic N, Guan NL, Kundu P, Gulyas B, Halldin C, Hultenby K, Nilsson H, Hebert H, Volpe BT, Diamond B, Pettersson S. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6:263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent DA, Kolko DJ, Birmaher B, Baugher M, Bridge J, Roth C, Holder D. Predictors of treatment efficacy in a clinical trial of three psychosocial treatments for adolescent depression. J Am Acad Child Adolesc Psychiatry. 1998;37:906–914. doi: 10.1097/00004583-199809000-00010. [DOI] [PubMed] [Google Scholar]
- Bueller JA, Aftab M, Sen S, Gomez-Hassan D, Burmeister M, Zubieta JK. BDNF Val66Met allele is associated with reduced hippocampal volume in healthy subjects. Biol Psychiatry. 2006;59:812–815. doi: 10.1016/j.biopsych.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, Mucke L, Johnson MH, Sofroniew MV. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308. doi: 10.1016/s0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci U S A. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Cao X, Li LP, Wang Q, Wu Q, Hu HH, Zhang M, Fang YY, Zhang J, Li SJ, Xiong WC, Yan HC, Gao YB, Liu JH, Li XW, Sun LR, Zeng YN, Zhu XH, Gao TM. Astrocyte-derived ATP modulates depressive-like behaviors. Nat Med. 2013;19:773–777. doi: 10.1038/nm.3162. [DOI] [PubMed] [Google Scholar]
- Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. 2011;130:226–238. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carle TL, Ohnishi YN, Ohnishi YH, Alibhai IN, Wilkinson MB, Kumar A, Nestler EJ. Proteasome-dependent and -independent mechanisms for FosB destabilization: identification of FosB degron domains and implications for DeltaFosB stability. Eur J Neurosci. 2007;25:3009–3019. doi: 10.1111/j.1460-9568.2007.05575.x. [DOI] [PubMed] [Google Scholar]
- Carney RM, Blumenthal JA, Catellier D, Freedland KE, Berkman LF, Watkins LL, Czajkowski SM, Hayano J, Jaffe AS. Depression as a risk factor for mortality after acute myocardial infarction. Am J Cardiol. 2003;92:1277–1281. doi: 10.1016/j.amjcard.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Carney RM, Freedland KE. Depression, mortality, and medical morbidity in patients with coronary heart disease. Biol Psychiatry. 2003;54:241–247. doi: 10.1016/s0006-3223(03)00111-2. [DOI] [PubMed] [Google Scholar]
- Cattaneo A, Gennarelli M, Uher R, Breen G, Farmer A, Aitchison KJ, Craig IW, Anacker C, Zunsztain PA, McGuffin P, Pariante CM. Candidate genes expression profile associated with antidepressants response in the GENDEP study: differentiating between baseline 'predictors' and longitudinal 'targets'. Neuropsychopharmacology. 2013;38:377–385. doi: 10.1038/npp.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne DL, Bagot RC, van Hasselt F, Ramakers G, Meaney MJ, de Kloet ER, Joels M, Krugers H. Maternal care and hippocampal plasticity: evidence for experiencedependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J Neurosci. 2008;28:6037–6045. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F, Meaney MJ. Like mother, like daughter: evidence for non-genomic transmission of parental behavior and stress responsivity. Prog Brain Res. 2001;133:287–302. doi: 10.1016/s0079-6123(01)33022-4. [DOI] [PubMed] [Google Scholar]
- Chen Y, Swanson RA. Astrocytes and brain injury. J Cereb Blood Flow Metab. 2003;23:137–149. doi: 10.1097/01.WCB.0000044631.80210.3C. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, Hempstead BL, Lee FS. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Rodriguiz RM, Murthy SR, Senatorov V, Thouennon E, Cawley NX, Aryal DK, Ahn S, Lecka-Czernik B, Wetsel WC, Loh YP. Neurotrophic factor-alpha1 prevents stress-induced depression through enhancement of neurogenesis and is activated by rosiglitazone. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chourbaji S, Zacher C, Sanchis-Segura C, Dormann C, Vollmayr B, Gass P. Learned helplessness: validity and reliability of depressive-like states in mice. Brain Res Brain Res Protoc. 2005;16:70–78. doi: 10.1016/j.brainresprot.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Dumitriu D, Robison AJ, Janssen WG, Ahn HF, Krishnan V, Reyes CM, Han MH, Ables JL, Eisch AJ, Dietz DM, Ferguson D, Neve RL, Greengard P, Kim Y, Morrison JH, Russo SJ. IkappaB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J Neurosci. 2011a;31:314–321. doi: 10.1523/JNEUROSCI.4763-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Guise KG, Walsh JJ, Heshmati M, Friedman AK, Dey A, Smith M, Rebusi N, Pfau M, Ables JL, Hodes GE, Ben-Dor GA, Deisseroth K, Shapiro ML, Malenka RC, Ibanez-Tallon I, Han MH, Russo SJ. Excitatory transmission at thalamo-striatal synapses mediates susceptibility to social stress. Nature Neuroscience. 2015 doi: 10.1038/nn.4034. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Heshmati M, Graham A, Birnbaum S, Neve RL, Hodes GE, Russo SJ. Effects of inhibitor of kappaB kinase activity in the nucleus accumbens on emotional behavior. Neuropsychopharmacology. 2012;37:2615–2623. doi: 10.1038/npp.2012.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Russo SJ. Structural and synaptic plasticity in stress-related disorders. Rev Neurosci. 2011b;22:535–549. doi: 10.1515/RNS.2011.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani HT, Behen ME, Muzik O, Juhasz C, Nagy F, Chugani DC. Local brain functional activity following early deprivation: a study of postinstitutionalized Romanian orphans. Neuroimage. 2001;14:1290–1301. doi: 10.1006/nimg.2001.0917. [DOI] [PubMed] [Google Scholar]
- Clark-Raymond A, Halaris A. VEGF and depression: a comprehensive assessment of clinical data. J Psychiatr Res. 2013;47:1080–1087. doi: 10.1016/j.jpsychires.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Cole SW, Arevalo JM, Takahashi R, Sloan EK, Lutgendorf SK, Sood AK, Sheridan JF, Seeman TE. Computational identification of gene-social environment interaction at the human IL6 locus. Proc Natl Acad Sci U S A. 2010;107:5681–5686. doi: 10.1073/pnas.0911515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway KP, Compton W, Stinson FS, Grant BF. Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2006;67:247–257. doi: 10.4088/jcp.v67n0211. [DOI] [PubMed] [Google Scholar]
- Corradetti MN, Inoki K, Guan KL. The stress-inducted proteins RTP801 and RTP801L are negative regulators of the mammalian target of rapamycin pathway. J Biol Chem. 2005;280:9769–9772. doi: 10.1074/jbc.C400557200. [DOI] [PubMed] [Google Scholar]
- Costello J, Kendrick K. Grief and older people: the making or breaking of emotional bonds following partner loss in later life. J Adv Nurs. 2000;32:1374–1382. doi: 10.1046/j.1365-2648.2000.01625.x. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, Berton O, Fass DM, Renthal W, Rush AJ, 3rd, Wu EY, Ghose S, Krishnan V, Russo SJ, Tamminga C, Haggarty SJ, Nestler EJ. Antidepressant actions of histone deacetylase inhibitors. J Neurosci. 2009;29:11451–11460. doi: 10.1523/JNEUROSCI.1758-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W, Mizukami H, Yanagisawa M, Aida T, Nomura M, Isomura Y, Takayanagi R, Ozawa K, Tanaka K, Aizawa H. Glial dysfunction in the mouse habenula causes depressive-like behaviors and sleep disturbance. J Neurosci. 2014;34:16273–16285. doi: 10.1523/JNEUROSCI.1465-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeh B, Di Benedetto B. Antidepressants act directly on astrocytes: evidences and functional consequences. Eur Neuropsychopharmacol. 2013;23:171–185. doi: 10.1016/j.euroneuro.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Czeh B, Simon M, Schmelting B, Hiemke C, Fuchs E. Astroglial plasticity in the hippocampus is affected by chronic psychosocial stress and concomitant fluoxetine treatment. Neuropsychopharmacology. 2006;31:1616–1626. doi: 10.1038/sj.npp.1300982. [DOI] [PubMed] [Google Scholar]
- Dalla C, Antoniou K, Kokras N, Drossopoulou G, Papathanasiou G, Bekris S, Daskas S, Papadopoulou-Daifoti Z. Sex differences in the effects of two stress paradigms on dopaminergic neurotransmission. Physiol Behav. 2008a;93:595–605. doi: 10.1016/j.physbeh.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Dalla C, Edgecomb C, Whetstone AS, Shors TJ. Females do not express learned helplessness like males do. Neuropsychopharmacology. 2008b;33:1559–1569. doi: 10.1038/sj.npp.1301533. [DOI] [PubMed] [Google Scholar]
- Dalla C, Pitychoutis PM, Kokras N, Papadopoulou-Daifoti Z. Sex differences in animal models of depression and antidepressant response. Basic Clin Pharmacol Toxicol. 2010;106:226–233. doi: 10.1111/j.1742-7843.2009.00516.x. [DOI] [PubMed] [Google Scholar]
- Dalla C, Pitychoutis PM, Kokras N, Papadopoulou-Daifoti Z. Sex differences in response to stress and expression of depressive-like behaviours in the rat. Curr Top Behav Neurosci. 2011;8:97–118. doi: 10.1007/7854_2010_94. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis NP, Yehuda R. Principles for developing animal models of military PTSD. Eur J Psychotraumatol. 2014;5 doi: 10.3402/ejpt.v5.23825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, McEwen BS. Social influences on neuroplasticity: stress and interventions to promote well-being. Nat Neurosci. 2012;15:689–695. doi: 10.1038/nn.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot J, Boersma WJ, Scholten JW, Koolhaas JM. Social stress in male mice impairs long-term antiviral immunity selectively in wounded subjects. Physiol Behav. 2002;75:277–285. doi: 10.1016/s0031-9384(01)00677-1. [DOI] [PubMed] [Google Scholar]
- Dean B, Tawadros N, Scarr E, Gibbons AS. Regionally-specific changes in levels of tumour necrosis factor in the dorsolateral prefrontal cortex obtained postmortem from subjects with major depressive disorder. J Affect Disord. 2010;120:245–248. doi: 10.1016/j.jad.2009.04.027. [DOI] [PubMed] [Google Scholar]
- Delpech JC, Madore C, Nadjar A, Joffre C, Wohleb E, Laye S. Microglia in neuronal plasticity: Influence of stress. Neuropharmacology. 2015 doi: 10.1016/j.neuropharm.2014.12.034. [DOI] [PubMed] [Google Scholar]
- Dias C, Feng J, Sun H, Shao NY, Mazei-Robison MS, Damez-Werno D, Scobie K, Bagot R, LaBonte B, Ribeiro E, Liu X, Kennedy P, Vialou V, Ferguson D, Pena C, Calipari ES, Koo JW, Mouzon E, Ghose S, Tamminga C, Neve R, Shen L, Nestler EJ. beta-catenin mediates stress resilience through Dicer1/microRNA regulation. Nature. 2014;516:51–55. doi: 10.1038/nature13976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz DM, Laplant Q, Watts EL, Hodes GE, Russo SJ, Feng J, Oosting RS, Vialou V, Nestler EJ. Paternal transmission of stress-induced pathologies. Biol Psychiatry. 2011;70:408–414. doi: 10.1016/j.biopsych.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Dranovsky A, Hen R. Hippocampal neurogenesis: regulation by stress and antidepressants. Biol Psychiatry. 2006;59:1136–1143. doi: 10.1016/j.biopsych.2006.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Duric V, Banasr M, Stockmeier CA, Simen AA, Newton SS, Overholser JC, Jurjus GJ, Dieter L, Duman RS. Altered expression of synapse and glutamate related genes in postmortem hippocampus of depressed subjects. Int J Neuropsychopharmacol. 2013;16:69–82. . doi: 10.1017/S1461145712000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Walker BM, Pian JP, Roth JL, Slawecki CJ. Increased alcohol drinking in isolate-housed alcohol-preferring rats. Behav Neurosci. 2007;121:111–119. doi: 10.1037/0735-7044.121.1.111. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Bolanos CA, de Wit J, Simonak RD, Pudiak CM, Barrot M, Verhaagen J, Nestler EJ. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: a role in depression. Biol Psychiatry. 2003;54:994–1005. doi: 10.1016/j.biopsych.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Petrik D. Depression and hippocampal neurogenesis: a road to remission? Science. 2012;338:72–75. doi: 10.1126/science.1222941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott E, Ezra-Nevo G, Regev L, Neufeld-Cohen A, Chen A. Resilience to social stress coincides with functional DNA methylation of the Crf gene in adult mice. Nat Neurosci. 2010;13:1351–1353. doi: 10.1038/nn.2642. [DOI] [PubMed] [Google Scholar]
- Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KR, Nemeroff CB, Bremner JD, Carney RM, Coyne JC, Delong MR, Frasure-Smith N, Glassman AH, Gold PW, Grant I, Gwyther L, Ironson G, Johnson RL, Kanner AM, Katon WJ, Kaufmann PG, Keefe FJ, Ketter T, Laughren TP, Leserman J, Lyketsos CG, McDonald WM, McEwen BS, Miller AH, Musselman D, O'Connor C, Petitto JM, Pollock BG, Robinson RG, Roose SP, Rowland J, Sheline Y, Sheps DS, Simon G, Spiegel D, Stunkard A, Sunderland T, Tibbits P, Jr, Valvo WJ. Mood disorders in the medically ill: scientific review and recommendations. Biol Psychiatry. 2005;58:175–189. doi: 10.1016/j.biopsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Fagundes CP, Glaser R, Hwang BS, Malarkey WB, Kiecolt-Glaser JK. Depressive symptoms enhance stress-induced inflammatory responses. Brain Behav Immun. 2013;31:172–176. doi: 10.1016/j.bbi.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falowski SM, Sharan A, Reyes BA, Sikkema C, Szot P, Van Bockstaele EJ. An evaluation of neuroplasticity and behavior after deep brain stimulation of the nucleus accumbens in an animal model of depression. Neurosurgery. 2011;69:1281–1290. doi: 10.1227/NEU.0b013e3182237346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fass DM, Schroeder FA, Perlis RH, Haggarty SJ. Epigenetic mechanisms in mood disorders: targeting neuroplasticity. Neuroscience. 2014;264:112–130. doi: 10.1016/j.neuroscience.2013.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Chang H, Li E, Fan G. Dynamic expression of de novo DNA methyltransferases Dnmt3a and Dnmt3b in the central nervous system. J Neurosci Res. 2005;79:734–746. doi: 10.1002/jnr.20404. [DOI] [PubMed] [Google Scholar]
- Feng J, Zhou Y, Campbell SL, Le T, Li E, Sweatt JD, Silva AJ, Fan G. Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nat Neurosci. 2010;13:423–430. doi: 10.1038/nn.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoy AJ, Goetz L, Chabardes S, Xia Y. Deep brain stimulation: are astrocytes a key driver behind the scene? CNS Neurosci Ther. 2014;20:191–201. doi: 10.1111/cns.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming AS, O'Day DH, Kraemer GW. Neurobiology of mother-infant interactions: experience and central nervous system plasticity across development and generations. Neurosci Biobehav Rev. 1999;23:673–685. doi: 10.1016/s0149-7634(99)00011-1. [DOI] [PubMed] [Google Scholar]
- Flint J, Kendler KS. The genetics of major depression. Neuron. 2014;81:484–503. doi: 10.1016/j.neuron.2014.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornaro M, Rocchi G, Escelsior A, Contini P, Ghio M, Colicchio S, De Berardis D, Amore M, Fornaro P, Martino M. VEGF plasma level variations in duloxetine-treated patients with major depression. J Affect Disord. 2013;151:590–595. doi: 10.1016/j.jad.2013.06.055. [DOI] [PubMed] [Google Scholar]
- Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Fournier NM, Duman RS. Role of vascular endothelial growth factor in adult hippocampal neurogenesis: implications for the pathophysiology and treatment of depression. Behav Brain Res. 2012;227:440–449. doi: 10.1016/j.bbr.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999a;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Francis DD, Caldji C, Champagne F, Plotsky PM, Meaney MJ. The role of corticotropin-releasing factor--norepinephrine systems in mediating the effects of early experience on the development of behavioral and endocrine responses to stress. Biol Psychiatry. 1999b;46:1153–1166. doi: 10.1016/s0006-3223(99)00237-1. [DOI] [PubMed] [Google Scholar]
- Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun. 2007;21:47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Frazer A, Morilak DA. What should animal models of depression model? Neurosci Biobehav Rev. 2005;29:515–523. doi: 10.1016/j.neubiorev.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Friedman E, Berman M, Overstreet D. Swim test immobility in a genetic rat model of depression is modified by maternal environment: a cross-foster study. Dev Psychobiol. 2006;48:169–177. doi: 10.1002/dev.20119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner KL, Thrivikraman KV, Lightman SL, Plotsky PM, Lowry CA. Early life experience alters behavior during social defeat: focus on serotonergic systems. Neuroscience. 2005;136:181–191. doi: 10.1016/j.neuroscience.2005.07.042. [DOI] [PubMed] [Google Scholar]
- Gatt JM, Burton KL, Williams LM, Schofield PR. Specific and common genes implicated across major mental disorders: a review of meta-analysis studies. J Psychiatr Res. 2015;60:1–13. doi: 10.1016/j.jpsychires.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Gilbert P, McEwan K, Bellew R, Mills A, Gale C. The dark side of competition: How competitive behaviour and striving to avoid inferiority are linked to depression, anxiety, stress and self-harm. Psychol Psychother. 2009;82:123–136. doi: 10.1348/147608308X379806. [DOI] [PubMed] [Google Scholar]
- Gill J, Vythilingam M, Page GG. Low cortisol, high DHEA, and high levels of stimulated TNF-alpha, and IL-6 in women with PTSD. J Trauma Stress. 2008;21:530–539. doi: 10.1002/jts.20372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout JP, Moreau M, Lestage J, Chen J, Sparkman NL, O'Connor J, Castanon N, Kelley KW, Dantzer R, Johnson RW. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology. 2008;33:2341–2351. doi: 10.1038/sj.npp.1301649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Christoffel DJ, Heshmati M, Hodes GE, Magida J, Davis K, Cahill ME, Dias C, Ribeiro E, Ables JL, Kennedy PJ, Robison AJ, Gonzalez-Maeso J, Neve RL, Turecki G, Ghose S, Tamminga CA, Russo SJ. Epigenetic regulation of RAC1 induces synaptic remodeling in stress disorders and depression. Nat Med. 2013;19:337–344. doi: 10.1038/nm.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Covington HE, 3rd, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6:1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon GR, Iremonger KJ, Kantevari S, Ellis-Davies GC, MacVicar BA, Bains JS. Astrocyte-mediated distributed plasticity at hypothalamic glutamate synapses. Neuron. 2009;64:391–403. doi: 10.1016/j.neuron.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- Gould TD, Einat H, O'Donnell KC, Picchini AM, Schloesser RJ, Manji HK. Beta-catenin overexpression in the mouse brain phenocopies lithium-sensitive behaviors. Neuropsychopharmacology. 2007;32:2173–2183. doi: 10.1038/sj.npp.1301338. [DOI] [PubMed] [Google Scholar]
- Gourley SL, Taylor JR. Recapitulation and reversal of a persistent depression-like syndrome in rodents. Curr Protoc Neurosci. 2009;Chapter 9(Unit 9):32. doi: 10.1002/0471142301.ns0932s49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, Lowe SW, Berglund PA, Corey-Lisle PK. The economic burden of depression in the United States: how did it change between 1990 and 2000? J Clin Psychiatry. 2003;64:1465–1475. doi: 10.4088/jcp.v64n1211. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Fleshner M. Exercise, learned helplessness, and the stress-resistant brain. Neuromolecular Med. 2008;10:81–98. doi: 10.1007/s12017-008-8029-y. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Francis J, Beltz TG, Felder RB, Johnson AK. Neuroendocrine and cytokine profile of chronic mild stress-induced anhedonia. Physiol Behav. 2005a;84:697–706. doi: 10.1016/j.physbeh.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, Carter CS. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology. 2007;32:966–980. doi: 10.1016/j.psyneuen.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Sullivan NR, Damjanoska KJ, Crane JW, Carrasco GA, Shi J, Chen Z, Garcia F, Muma NA, Van de Kar LD. Chronic mild stress induces behavioral and physiological changes, and may alter serotonin 1A receptor function, in male and cycling female rats. Psychopharmacology (Berl) 2005b;179:769–780. doi: 10.1007/s00213-004-2103-4. [DOI] [PubMed] [Google Scholar]
- Grueter BA, Robison AJ, Neve RL, Nestler EJ, Malenka RC. FosB differentially modulates nucleus accumbens direct and indirect pathway function. Proc Natl Acad Sci U S A. 2013;110:1923–1928. doi: 10.1073/pnas.1221742110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudmundsson P, Skoog I, Waern M, Blennow K, Palsson S, Rosengren L, Gustafson D. The relationship between cerebrospinal fluid biomarkers and depression in elderly women. Am J Geriatr Psychiatry. 2007;15:832–838. doi: 10.1097/JGP.0b013e3180547091. [DOI] [PubMed] [Google Scholar]
- Hagerty BM, Williams RA, Coyne JC, Early MR. Sense of belonging and indicators of social and psychological functioning. Arch Psychiatr Nurs. 1996;10:235–244. doi: 10.1016/s0883-9417(96)80029-x. [DOI] [PubMed] [Google Scholar]
- Haim A, Sherer M, Leuner B. Gestational stress induces persistent depressive-like behavior and structural modifications within the postpartum nucleus accumbens. Eur J Neurosci. 2014;40:3766–3773. doi: 10.1111/ejn.12752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajszan T, Dow A, Warner-Schmidt JL, Szigeti-Buck K, Sallam NL, Parducz A, Leranth C, Duman RS. Remodeling of hippocampal spine synapses in the rat learned helplessness model of depression. Biol Psychiatry. 2009;65:392–400. doi: 10.1016/j.biopsych.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall FS. Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical and behavioral consequences. Crit Rev Neurobiol. 1998;12:129–162. doi: 10.1615/critrevneurobiol.v12.i1-2.50. [DOI] [PubMed] [Google Scholar]
- Hamby ME, Coppola G, Ao Y, Geschwind DH, Khakh BS, Sofroniew MV. Inflammatory mediators alter the astrocyte transcriptome and calcium signaling elicited by multiple G-protein-coupled receptors. J Neurosci. 2012;32:14489–14510. doi: 10.1523/JNEUROSCI.1256-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han F, Nakano T, Yamamoto Y, Shioda N, Lu YM, Fukunaga K. Improvement of depressive behaviors by nefiracetam is associated with activation of CaM kinases in olfactory bulbectomized mice. Brain Res. 2009;1265:205–214. doi: 10.1016/j.brainres.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- Hercher C, Turecki G, Mechawar N. Through the looking glass: examining neuroanatomical evidence for cellular alterations in major depression. J Psychiatr Res. 2009;43:947–961. doi: 10.1016/j.jpsychires.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Herman JP, Flak J, Jankord R. Chronic stress plasticity in the hypothalamic paraventricular nucleus. Prog Brain Res. 2008;170:353–364. doi: 10.1016/S0079-6123(08)00429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinwood M, Tynan RJ, Charnley JL, Beynon SB, Day TA, Walker FR. Chronic stress induced remodeling of the prefrontal cortex: structural re-organization of microglia and the inhibitory effect of minocycline. Cereb Cortex. 2013;23:1784–1797. doi: 10.1093/cercor/bhs151. [DOI] [PubMed] [Google Scholar]
- Hinwood M, Tynan RJ, Day TA, Walker FR. Repeated social defeat selectively increases deltaFosB expression and histone H3 acetylation in the infralimbic medial prefrontal cortex. Cereb Cortex. 2011;21:262–271. doi: 10.1093/cercor/bhq080. [DOI] [PubMed] [Google Scholar]
- Hobara T, Uchida S, Otsuki K, Matsubara T, Funato H, Matsuo K, Suetsugi M, Watanabe Y. Altered gene expression of histone deacetylases in mood disorder patients. J Psychiatr Res. 2010;44:263–270. doi: 10.1016/j.jpsychires.2009.08.015. [DOI] [PubMed] [Google Scholar]
- Hodes GE. Sex, stress, and epigenetics: regulation of behavior in animal models of mood disorders. Biol Sex Differ. 2013;4:1. doi: 10.1186/2042-6410-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D, Rebusi N, Heshmati M, Aleyasin H, Warren BL, Lebonte B, Horn S, Lapidus KA, Stelzhammer V, Wong EH, Bahn S, Krishnan V, Bolanos-Guzman CA, Murrough JW, Merad M, Russo SJ. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci U S A. 2014;111:16136–16141. doi: 10.1073/pnas.1415191111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RG, McCarthy KJ, Milne TA, Pfaff DW, McEwen BS. Regulation of hippocampal H3 histone methylation by acute and chronic stress. Proc Natl Acad Sci U S A. 2009;106:20912–20917. doi: 10.1073/pnas.0911143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RG, McEwen BS. Stress and anxiety across the lifespan: structural plasticity and epigenetic regulation. Epigenomics. 2013;5:177–194. doi: 10.2217/epi.13.8. [DOI] [PubMed] [Google Scholar]
- Im HI, Kenny PJ. MicroRNAs in neuronal function and dysfunction. Trends Neurosci. 2012;35:325–334. doi: 10.1016/j.tins.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Kageyama R. Continuous neurogenesis in the adult brain. Dev Growth Differ. 2009;51:379–386. doi: 10.1111/j.1440-169X.2009.01094.x. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci. 2006;26:5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen JP, Medvedev IO, Caron MG. The 5-HT deficiency theory of depression: perspectives from a naturalistic 5-HT deficiency model, the tryptophan hydroxylase 2Arg439His knockin mouse. Philos Trans R Soc Lond B Biol Sci. 2012;367:2444–2459. doi: 10.1098/rstb.2012.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannotte AM, McCarthy JG, Redei EE, Sidhu A. Desipramine modulation of alpha-, gamma-synuclein, and the norepinephrine transporter in an animal model of depression. Neuropsychopharmacology. 2009;34:987–998. doi: 10.1038/npp.2008.146. [DOI] [PubMed] [Google Scholar]
- Jernigan CS, Goswami DB, Austin MC, Iyo AH, Chandran A, Stockmeier CA, Karolewicz B. The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1774–1779. doi: 10.1016/j.pnpbp.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Xia R, Jiang Y, Wang L, Gao F. Vascular endothelial growth factors enhance the permeability of the mouse blood-brain barrier. PLoS One. 2014;9:e86407. doi: 10.1371/journal.pone.0086407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochems J, Teegarden SL, Chen Y, Boulden J, Challis C, Ben-Dor GA, Kim SF, Berton O. Enhancement of stress resilience through histone deacetylase 6-mediated regulation of glucocorticoid receptor chaperone dynamics. Biol Psychiatry. 2015;77:345–355. doi: 10.1016/j.biopsych.2014.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John GR, Lee SC, Song X, Rivieccio M, Brosnan CF. IL-1-regulated responses in astrocytes: relevance to injury and recovery. Glia. 2005;49:161–176. doi: 10.1002/glia.20109. [DOI] [PubMed] [Google Scholar]
- Jung YH, Hong SI, Ma SX, Hwang JY, Kim JS, Lee JH, Seo JY, Lee SY, Jang CG. Strain differences in the chronic mild stress animal model of depression and anxiety in mice. Biomol Ther (Seoul) 2014;22:453–459. doi: 10.4062/biomolther.2014.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacem K, Lacombe P, Seylaz J, Bonvento G. Structural organization of the perivascular astrocyte endfeet and their relationship with the endothelial glucose transporter: a confocal microscopy study. Glia. 1998;23:1–10. [PubMed] [Google Scholar]
- Kaidanovich-Beilin O, Milman A, Weizman A, Pick CG, Eldar-Finkelman H. Rapid antidepressive-like activity of specific glycogen synthase kinase-3 inhibitor and its effect on beta-catenin in mouse hippocampus. Biol Psychiatry. 2004;55:781–784. doi: 10.1016/j.biopsych.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Kakinuma Y, Hama H, Sugiyama F, Yagami K, Goto K, Murakami K, Fukamizu A. Impaired blood-brain barrier function in angiotensinogen-deficient mice. Nat Med. 1998;4:1078–1080. doi: 10.1038/2070. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt B, Ndiaye D, Korte M, Pothion S, Arbibe L, Prullage M, Pfeiffer J, Lindecke A, Staiger V, Israel A, Kaltschmidt C, Memet S. NF-kappaB regulates spatial memory formation and synaptic plasticity through protein kinase A/CREB signaling. Mol Cell Biol. 2006;26:2936–2946. doi: 10.1128/MCB.26.8.2936-2946.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada N, Seo SU, Chen GY, Nunez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13:321–335. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Kim JM, Lee JY, Kim SY, Bae KY, Kim SW, Shin IS, Kim HR, Shin MG, Yoon JS. BDNF promoter methylation and suicidal behavior in depressive patients. J Affect Disord. 2013;151:679–685. doi: 10.1016/j.jad.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier CA, Licznerski P, Lepack A, Majik MS, Jeong LS, Banasr M, Son H, Duman RS. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med. 2012;18:1413–1417. doi: 10.1038/nm.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karege F, Perroud N, Burkhardt S, Fernandez R, Ballmann E, La Harpe R, Malafosse A. Protein levels of beta-catenin and activation state of glycogen synthase kinase-3beta in major depression. A study with postmortem prefrontal cortex. J Affect Disord. 2012;136:185–188. doi: 10.1016/j.jad.2011.09.024. [DOI] [PubMed] [Google Scholar]
- Kelly JP, Wrynn AS, Leonard BE. The olfactory bulbectomized rat as a model of depression: an update. Pharmacol Ther. 1997;74:299–316. doi: 10.1016/s0163-7258(97)00004-1. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Nelson CB, McGonagle KA, Liu J, Swartz M, Blazer DG. Comorbidity of DSM-III-R major depressive disorder in the general population: results from the US National Comorbidity Survey. Br J Psychiatry. 1996;(Suppl):17–30. [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Kirchhoff F, Verkhratsky A. Microglia: new roles for the synaptic stripper. Neuron. 2013;77:10–18. doi: 10.1016/j.neuron.2012.12.023. [DOI] [PubMed] [Google Scholar]
- Khairova RA, Machado-Vieira R, Du J, Manji HK. A potential role for pro-inflammatory cytokines in regulating synaptic plasticity in major depressive disorder. Int J Neuropsychopharmacol. 2009;12:561–578. doi: 10.1017/S1461145709009924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirbek MA, Klemenhagen KC, Sahay A, Hen R. Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders. Nat Neurosci. 2012;15:1613–1620. doi: 10.1038/nn.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JK, Kim SG, Kim HJ, Song YR. Serum S100B protein is associated with depressive symptoms in patients with end-stage renal disease. Clin Biochem. 2012;45:1573–1577. doi: 10.1016/j.clinbiochem.2012.08.014. [DOI] [PubMed] [Google Scholar]
- Kim KS, Han PL. Optimization of chronic stress paradigms using anxiety- and depression-like behavioral parameters. J Neurosci Res. 2006;83:497–507. doi: 10.1002/jnr.20754. [DOI] [PubMed] [Google Scholar]
- Kohler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, Mors O, Krogh J. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71:1381–1391. doi: 10.1001/jamapsychiatry.2014.1611. [DOI] [PubMed] [Google Scholar]
- Kokras N, Antoniou K, Dalla C, Bekris S, Xagoraris M, Ovestreet DH, Papadopoulou-Daifoti Z. Sex-related differential response to clomipramine treatment in a rat model of depression. J Psychopharmacol. 2009;23:945–956. doi: 10.1177/0269881108095914. [DOI] [PubMed] [Google Scholar]
- Kokras N, Dalla C. Sex differences in animal models of psychiatric disorders. Br J Pharmacol. 2014;171:4595–4619. doi: 10.1111/bph.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci U S A. 2010;107:2669–2674. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornstein SG, Schatzberg AF, Thase ME, Yonkers KA, McCullough JP, Keitner GI, Gelenberg AJ, Davis SM, Harrison WM, Keller MB. Gender differences in treatment response to sertraline versus imipramine in chronic depression. Am J Psychiatry. 2000;157:1445–1452. doi: 10.1176/appi.ajp.157.9.1445. [DOI] [PubMed] [Google Scholar]
- Kornstein SG, Young EA, Harvey AT, Wisniewski SR, Barkin JL, Thase ME, Trivedi MH, Nierenberg AA, Rush AJ. The influence of menopause status and postmenopausal use of hormone therapy on presentation of major depression in women. Menopause. 2010;17:828–839. doi: 10.1097/gme.0b013e3181d770a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte SM, Buwalda B, Meijer O, De Kloet ER, Bohus B. Socially defeated male rats display a blunted adrenocortical response to a low dose of 8-OH-DPAT. Eur J Pharmacol. 1995;272:45–50. doi: 10.1016/0014-2999(94)00621-d. [DOI] [PubMed] [Google Scholar]
- Kosik KS. The neuronal microRNA system. Nat Rev Neurosci. 2006;7:911–920. doi: 10.1038/nrn2037. [DOI] [PubMed] [Google Scholar]
- Kreiner G, Chmielarz P, Roman A, Nalepa I. Gender differences in genetic mouse models evaluated for depressive-like and antidepressant behavior. Pharmacol Rep. 2013;65:1580–1590. doi: 10.1016/s1734-1140(13)71519-6. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Kubera M, Obuchowicz E, Goehler L, Brzeszcz J, Maes M. In animal models, psychosocial stress-induced (neuro)inflammation, apoptosis and reduced neurogenesis are associated to the onset of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:744–759. doi: 10.1016/j.pnpbp.2010.08.026. [DOI] [PubMed] [Google Scholar]
- Kudryavtseva NN, Bakshtanovskaya IV, Koryakina LA. Social model of depression in mice of C57BL/6J strain. Pharmacol Biochem Behav. 1991;38:315–320. doi: 10.1016/0091-3057(91)90284-9. [DOI] [PubMed] [Google Scholar]
- Kusakawa S, Nakamura K, Miyamoto Y, Sanbe A, Torii T, Yamauchi J, Tanoue A. Fluoxetine promotes gliogenesis during neural differentiation in mouse embryonic stem cells. J Neurosci Res. 2010;88:3479–3487. doi: 10.1002/jnr.22509. [DOI] [PubMed] [Google Scholar]
- Kyeremanteng C, James J, Mackay J, Merali Z. A study of brain and serum brain-derived neurotrophic factor protein in Wistar and Wistar-Kyoto rat strains after electroconvulsive stimulus. Pharmacopsychiatry. 2012;45:244–249. doi: 10.1055/s-0032-1306278. [DOI] [PubMed] [Google Scholar]
- Kyeremanteng C, MacKay JC, James JS, Kent P, Cayer C, Anisman H, Merali Z. Effects of electroconvulsive seizures on depression-related behavior, memory and neurochemical changes in Wistar and Wistar-Kyoto rats. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54:170–178. doi: 10.1016/j.pnpbp.2014.05.012. [DOI] [PubMed] [Google Scholar]
- Lanquillon S, Krieg JC, Bening-Abu-Shach U, Vedder H. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology. 2000;22:370–379. doi: 10.1016/S0893-133X(99)00134-7. [DOI] [PubMed] [Google Scholar]
- LaPlant Q, Chakravarty S, Vialou V, Mukherjee S, Koo JW, Kalahasti G, Bradbury KR, Taylor SV, Maze I, Kumar A, Graham A, Birnbaum SG, Krishnan V, Truong HT, Neve RL, Nestler EJ, Russo SJ. Role of nuclear factor kappaB in ovarian hormone-mediated stress hypersensitivity in female mice. Biol Psychiatry. 2009;65:874–880. doi: 10.1016/j.biopsych.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPlant Q, Vialou V, Covington HE, 3rd, Dumitriu D, Feng J, Warren BL, Maze I, Dietz DM, Watts EL, Iniguez SD, Koo JW, Mouzon E, Renthal W, Hollis F, Wang H, Noonan MA, Ren Y, Eisch AJ, Bolanos CA, Kabbaj M, Xiao G, Neve RL, Hurd YL, Oosting RS, Fan G, Morrison JH, Nestler EJ. Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nat Neurosci. 2010;13:1137–1143. doi: 10.1038/nn.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latapy C, Rioux V, Guitton MJ, Beaulieu JM. Selective deletion of forebrain glycogen synthase kinase 3beta reveals a central role in serotonin-sensitive anxiety and social behaviour. Philos Trans R Soc Lond B Biol Sci. 2012;367:2460–2474. doi: 10.1098/rstb.2012.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi-Avnon Y, Yadid G, Overstreet DH, Weller A. Abnormal patterns of maternal behavior in a genetic animal model of depression. Physiol Behav. 2005;84:607–615. doi: 10.1016/j.physbeh.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Lee B, Sur B, Park J, Kim SH, Kwon S, Yeom M, Shim I, Lee H, Hahm DH. Chronic administration of baicalein decreases depression-like behavior induced by repeated restraint stress in rats. Korean J Physiol Pharmacol. 2013;17:393–403. doi: 10.4196/kjpp.2013.17.5.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann ML, Herkenham M. Environmental enrichment confers stress resiliency to social defeat through an infralimbic cortex-dependent neuroanatomical pathway. J Neurosci. 2011;31:6159–6173. doi: 10.1523/JNEUROSCI.0577-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS. BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol. 2014;18 doi: 10.1093/ijnp/pyu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Fredericks PJ, Nealer C, Albin-Brooks C. Chronic gestational stress leads to depressive-like behavior and compromises medial prefrontal cortex structure and function during the postpartum period. PLoS One. 2014;9:e89912. doi: 10.1371/journal.pone.0089912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson JM, Roth TL, Lubin FD, Miller CA, Huang IC, Desai P, Malone LM, Sweatt JD. Evidence that DNA (cytosine-5) methyltransferase regulates synaptic plasticity in the hippocampus. J Biol Chem. 2006;281:15763–15773. doi: 10.1074/jbc.M511767200. [DOI] [PubMed] [Google Scholar]
- Leventopoulos M, Ruedi-Bettschen D, Knuesel I, Feldon J, Pryce CR, Opacka-Juffry J. Long-term effects of early life deprivation on brain glia in Fischer rats. Brain Res. 2007;1142:119–126. doi: 10.1016/j.brainres.2007.01.039. [DOI] [PubMed] [Google Scholar]
- Levinson DF. The genetics of depression: a review. Biol Psychiatry. 2006;60:84–92. doi: 10.1016/j.biopsych.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim BK, Huang KW, Grueter BA, Rothwell PE, Malenka RC. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature. 2012;487:183–189. doi: 10.1038/nature11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Lobo MK, Zaman S, Damez-Werno DM, Koo JW, Bagot RC, DiNieri JA, Nugent A, Finkel E, Chaudhury D, Chandra R, Riberio E, Rabkin J, Mouzon E, Cachope R, Cheer JF, Han MH, Dietz DM, Self DW, Hurd YL, Vialou V, Nestler EJ. DeltaFosB induction in striatal medium spiny neuron subtypes in response to chronic pharmacological, emotional, and optogenetic stimuli. J Neurosci. 2013;33:18381–18395. doi: 10.1523/JNEUROSCI.1875-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez AD, Murray CC. The global burden of disease, 1990–2020. Nat Med. 1998;4:1241–1243. doi: 10.1038/3218. [DOI] [PubMed] [Google Scholar]
- Madsen TM, Newton SS, Eaton ME, Russell DS, Duman RS. Chronic electroconvulsive seizure up-regulates beta-catenin expression in rat hippocampus: role in adult neurogenesis. Biol Psychiatry. 2003;54:1006–1014. doi: 10.1016/s0006-3223(03)00700-5. [DOI] [PubMed] [Google Scholar]
- Maes M, Bosmans E, De Jongh R, Kenis G, Vandoolaeghe E, Neels H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9:853–858. doi: 10.1006/cyto.1997.0238. [DOI] [PubMed] [Google Scholar]
- Maes M, Kubera M, Obuchowiczwa E, Goehler L, Brzeszcz J. Depression's multiple comorbidities explained by (neuro)inflammatory and oxidative & nitrosative stress pathways. Neuro Endocrinol Lett. 2011;32:7–24. [PubMed] [Google Scholar]
- Maes M, Van der Planken M, Stevens WJ, Peeters D, DeClerck LS, Bridts CH, Schotte C, Cosyns P. Leukocytosis, monocytosis and neutrophilia: hallmarks of severe depression. J Psychiatr Res. 1992;26:125–134. doi: 10.1016/0022-3956(92)90004-8. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, Li CJ, Gal Toth J, Bath KG, Jing D, Lee FS, McEwen BS. Effect of brain-derived neurotrophic factor haploinsufficiency on stress-induced remodeling of hippocampal neurons. Hippocampus. 2011;21:253–264. doi: 10.1002/hipo.20744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: comparison of stressors. Neuroscience. 1995;69:83–88. doi: 10.1016/0306-4522(95)00256-i. [DOI] [PubMed] [Google Scholar]
- Maier SF. Role of fear in mediating shuttle escape learning deficit produced by inescapable shock. J Exp Psychol Anim Behav Process. 1990;16:137–149. [PubMed] [Google Scholar]
- Malki K, Keers R, Tosto MG, Lourdusamy A, Carboni L, Domenici E, Uher R, McGuffin P, Schalkwyk LC. The endogenous and reactive depression subtypes revisited: integrative animal and human studies implicate multiple distinct molecular mechanisms underlying major depressive disorder. BMC Med. 2014;12:73. doi: 10.1186/1741-7015-12-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7:541–547. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- Manji HK, Quiroz JA, Sporn J, Payne JL, Denicoff K, N AG, Zarate CA, Jr, Charney DS. Enhancing neuronal plasticity and cellular resilience to develop novel, improved therapeutics for difficult-to-treat depression. Biol Psychiatry. 2003;53:707–742. doi: 10.1016/s0006-3223(03)00117-3. [DOI] [PubMed] [Google Scholar]
- Mar A, Spreekmeester E, Rochford J. Fluoxetine-induced increases in open-field habituation in the olfactory bulbectomized rat depend on test aversiveness but not on anxiety. Pharmacol Biochem Behav. 2002;73:703–712. doi: 10.1016/s0091-3057(02)00881-x. [DOI] [PubMed] [Google Scholar]
- Matthews K, Christmas D, Swan J, Sorrell E. Animal models of depression: navigating through the clinical fog. Neurosci Biobehav Rev. 2005;29:503–513. doi: 10.1016/j.neubiorev.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Mayo L, Trauger SA, Blain M, Nadeau M, Patel B, Alvarez JI, Mascanfroni ID, Yeste A, Kivisakk P, Kallas K, Ellezam B, Bakshi R, Prat A, Antel JP, Weiner HL, Quintana FJ. Regulation of astrocyte activation by glycolipids drives chronic CNS inflammation. Nat Med. 2014;20:1147–1156. doi: 10.1038/nm.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress, sex, and neural adaptation to a changing environment: mechanisms of neuronal remodeling. Ann N Y Acad Sci. 2010;1204(Suppl):E38–E59. doi: 10.1111/j.1749-6632.2010.05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. The ever-changing brain: cellular and molecular mechanisms for the effects of stressful experiences. Dev Neurobiol. 2012;72:878–890. doi: 10.1002/dneu.20968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Morrison JH. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79:16–29. doi: 10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney WT, Jr, Bunney WE., Jr Animal model of depression. I. Review of evidence: implications for research. Arch Gen Psychiatry. 1969;21:240–248. doi: 10.1001/archpsyc.1969.01740200112015. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Meeuwsen S, Persoon-Deen C, Bsibsi M, Ravid R, van Noort JM. Cytokine, chemokine and growth factor gene profiling of cultured human astrocytes after exposure to proinflammatory stimuli. Glia. 2003;43:243–253. doi: 10.1002/glia.10259. [DOI] [PubMed] [Google Scholar]
- Meffert MK, Baltimore D. Physiological functions for brain NF-kappaB. Trends Neurosci. 2005;28:37–43. doi: 10.1016/j.tins.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Meffert MK, Chang JM, Wiltgen BJ, Fanselow MS, Baltimore D. NF-kappa B functions in synaptic signaling and behavior. Nat Neurosci. 2003;6:1072–1078. doi: 10.1038/nn1110. [DOI] [PubMed] [Google Scholar]
- Melas PA, Rogdaki M, Lennartsson A, Bjork K, Qi H, Witasp A, Werme M, Wegener G, Mathe AA, Svenningsson P, Lavebratt C. Antidepressant treatment is associated with epigenetic alterations in the promoter of P11 in a genetic model of depression. Int J Neuropsychopharmacol. 2012;15:669–679. doi: 10.1017/S1461145711000940. [DOI] [PubMed] [Google Scholar]
- Melia KR, Ryabinin AE, Schroeder R, Bloom FE, Wilson MC. Induction and habituation of immediate early gene expression in rat brain by acute and repeated restraint stress. J Neurosci. 1994;14:5929–5938. doi: 10.1523/JNEUROSCI.14-10-05929.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer H, Vostanis P, Ford T, Bebbington P, Dennis MS. Victims of bullying in childhood and suicide attempts in adulthood. Eur Psychiatry. 2011;26:498–503. doi: 10.1016/j.eurpsy.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Menard C, Tse YC, Cavanagh C, Chabot JG, Herzog H, Schwarzer C, Wong TP, Quirion R. Knockdown of prodynorphin gene prevents cognitive decline, reduces anxiety, and rescues loss of group 1 metabotropic glutamate receptor function in aging. J Neurosci. 2013;33:12792–12804. doi: 10.1523/JNEUROSCI.0290-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlot E, Moze E, Dantzer R, Neveu PJ. Importance of fighting in the immune effects of social defeat. Physiol Behav. 2003;80:351–357. doi: 10.1016/j.physbeh.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Moffett MC, Vicentic A, Kozel M, Plotsky P, Francis DD, Kuhar MJ. Maternal separation alters drug intake patterns in adulthood in rats. Biochem Pharmacol. 2007;73:321–330. doi: 10.1016/j.bcp.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje FJ, Cabatic M, Divisch I, Kim EJ, Herkner KR, Binder BR, Pollak DD. Constant darkness induces IL-6-dependent depression-like behavior through the NF-kappaB signaling pathway. J Neurosci. 2011;31:9075–9083. doi: 10.1523/JNEUROSCI.1537-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Medina JC, Juarez I, Venancio-Garcia E, Cabrera SN, Menard C, Yu W, Flores G, Mechawar N, Quirion R. Impaired structural hippocampal plasticity is associated with emotional and memory deficits in the olfactory bulbectomized rat. Neuroscience. 2013;236:233–243. doi: 10.1016/j.neuroscience.2013.01.037. [DOI] [PubMed] [Google Scholar]
- Morris JS, Smith KA, Cowen PJ, Friston KJ, Dolan RJ. Covariation of activity in habenula and dorsal raphe nuclei following tryptophan depletion. Neuroimage. 1999;10:163–172. doi: 10.1006/nimg.1999.0455. [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Early prenatal stress impact on coping strategies and learning performance is sex dependent. Physiol Behav. 2007;91:55–65. doi: 10.1016/j.physbeh.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28:9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueser KT, Gingerich S. Treatment of co-occurring psychotic and substance use disorders. Soc Work Public Health. 2013;28:424–439. doi: 10.1080/19371918.2013.774676. [DOI] [PubMed] [Google Scholar]
- Muller N, Schwarz MJ, Dehning S, Douhe A, Cerovecki A, Goldstein-Muller B, Spellmann I, Hetzel G, Maino K, Kleindienst N, Moller HJ, Arolt V, Riedel M. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry. 2006;11:680–684. doi: 10.1038/sj.mp.4001805. [DOI] [PubMed] [Google Scholar]
- Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- Murrough JW. Ketamine as a novel antidepressant: from synapse to behavior. Clin Pharmacol Ther. 2012;91:303–309. doi: 10.1038/clpt.2011.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry. 1998;55:580–592. doi: 10.1001/archpsyc.55.7.580. [DOI] [PubMed] [Google Scholar]
- Mutlu O, Gumuslu E, Ulak G, Celikyurt IK, Kokturk S, Kir HM, Akar F, Erden F. Effects of fluoxetine, tianeptine and olanzapine on unpredictable chronic mild stress-induced depression-like behavior in mice. Life Sci. 2012;91:1252–1262. doi: 10.1016/j.lfs.2012.09.023. [DOI] [PubMed] [Google Scholar]
- Nagy C, Suderman M, Yang J, Szyf M, Mechawar N, Ernst C, Turecki G. Astrocytic abnormalities and global DNA methylation patterns in depression and suicide. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama AY, Harms MB, Luo L. Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J Neurosci. 2000;20:5329–5338. doi: 10.1523/JNEUROSCI.20-14-05329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam H, Clinton SM, Jackson NL, Kerman IA. Learned helplessness and social avoidance in the Wistar-Kyoto rat. Front Behav Neurosci. 2014;8:109. doi: 10.3389/fnbeh.2014.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negoias S, Croy I, Gerber J, Puschmann S, Petrowski K, Joraschky P, Hummel T. Reduced olfactory bulb volume and olfactory sensitivity in patients with acute major depression. Neuroscience. 2010;169:415–421. doi: 10.1016/j.neuroscience.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. FosB: A transcriptional regulator of stress and antidepressant responses. Eur J Pharmacol. 2014 doi: 10.1016/j.ejphar.2014.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves-Pereira M, Mundo E, Muglia P, King N, Macciardi F, Kennedy JL. The brain-derived neurotrophic factor gene confers susceptibility to bipolar disorder: evidence from a family-based association study. Am J Hum Genet. 2002;71:651–655. doi: 10.1086/342288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton SS, Collier EF, Hunsberger J, Adams D, Terwilliger R, Selvanayagam E, Duman RS. Gene profile of electroconvulsive seizures: induction of neurotrophic and angiogenic factors. J Neurosci. 2003;23:10841–10851. doi: 10.1523/JNEUROSCI.23-34-10841.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton SS, Fournier NM, Duman RS. Vascular growth factors in neuropsychiatry. Cell Mol Life Sci. 2013;70:1739–1752. doi: 10.1007/s00018-013-1281-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton TL, Fernandez-Botran R, Miller JJ, Burns VE. Interleukin-6 and soluble interleukin-6 receptor levels in posttraumatic stress disorder: associations with lifetime diagnostic status and psychological context. Biol Psychol. 2014;99:150–159. doi: 10.1016/j.biopsycho.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng J, Nardine T, Harms M, Tzu J, Goldstein A, Sun Y, Dietzl G, Dickson BJ, Luo L. Rac GTPases control axon growth, guidance and branching. Nature. 2002;416:442–447. doi: 10.1038/416442a. [DOI] [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Non AL, Binder AM, Kubzansky LD, Michels KB. Genome-wide DNA methylation in neonates exposed to maternal depression, anxiety, or SSRI medication during pregnancy. Epigenetics. 2014;9:964–972. doi: 10.4161/epi.28853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mahony A, Raber J, Montano M, Foehr E, Han V, Lu SM, Kwon H, LeFevour A, Chakraborty-Sett S, Greene WC. NF-kappaB/Rel regulates inhibitory and excitatory neuronal function and synaptic plasticity. Mol Cell Biol. 2006;26:7283–7298. doi: 10.1128/MCB.00510-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill LA, Kaltschmidt C. NF-kappaB: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 1997;20:252–258. doi: 10.1016/s0166-2236(96)01035-1. [DOI] [PubMed] [Google Scholar]
- Ota KT, Liu RJ, Voleti B, Maldonado-Aviles JG, Duric V, Iwata M, Dutheil S, Duman C, Boikess S, Lewis DA, Stockmeier CA, DiLeone RJ, Rex C, Aghajanian GK, Duman RS. REDD1 is essential for stress-induced synaptic loss and depressive behavior. Nat Med. 2014;20:531–535. doi: 10.1038/nm.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsu Y, Couchman K, Lyons DG, Collot M, Agarwal A, Mallet JM, Pfrieger FW, Bergles DE, Charpak S. Calcium dynamics in astrocyte processes during neurovascular coupling. Nat Neurosci. 2015;18:210–218. doi: 10.1038/nn.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overmier JB, Seligman ME. Effects of inescapable shock upon subsequent escape and avoidance responding. J Comp Physiol Psychol. 1967;63:28–33. doi: 10.1037/h0024166. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Keeney A, Hogg S. Antidepressant effects of citalopram and CRF receptor antagonist CP-154,526 in a rat model of depression. Eur J Pharmacol. 2004;492:195–201. doi: 10.1016/j.ejphar.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Rezvani AH, Janowsky DS. Genetic animal models of depression and ethanol preference provide support for cholinergic and serotonergic involvement in depression and alcoholism. Biol Psychiatry. 1992;31:919–936. doi: 10.1016/0006-3223(92)90118-j. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Wegener G. The flinders sensitive line rat model of depression--25 years and still producing. Pharmacol Rev. 2013;65:143–155. doi: 10.1124/pr.111.005397. [DOI] [PubMed] [Google Scholar]
- Pace TW, Heim CM. A short review on the psychoneuroimmunology of posttraumatic stress disorder: from risk factors to medical comorbidities. Brain Behav Immun. 2011;25:6–13. doi: 10.1016/j.bbi.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, Heim CM. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- Pace TW, Wingenfeld K, Schmidt I, Meinlschmidt G, Hellhammer DH, Heim CM. Increased peripheral NF-kappaB pathway activity in women with childhood abuse-related posttraumatic stress disorder. Brain Behav Immun. 2012;26:13–17. doi: 10.1016/j.bbi.2011.07.232. [DOI] [PubMed] [Google Scholar]
- Pang Y, Cai Z, Rhodes PG. Analysis of genes differentially expressed in astrocytes stimulated with lipopolysaccharide using cDNA arrays. Brain Res. 2001;914:15–22. doi: 10.1016/s0006-8993(01)02766-4. [DOI] [PubMed] [Google Scholar]
- Pare WP. Open field, learned helplessness, conditioned defensive burying, and forced-swim tests in WKY rats. Physiol Behav. 1994;55:433–439. doi: 10.1016/0031-9384(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Patki G, Solanki N, Salim S. Witnessing traumatic events causes severe behavioral impairments in rats. Int J Neuropsychopharmacol. 2014;17:2017–2029. doi: 10.1017/S1461145714000923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Caballero L, Perez-Egea R, Romero-Grimaldi C, Puigdemont D, Molet J, Caso JR, Mico JA, Perez V, Leza JC, Berrocoso E. Early responses to deep brain stimulation in depression are modulated by anti-inflammatory drugs. Mol Psychiatry. 2014;19:607–614. doi: 10.1038/mp.2013.63. [DOI] [PubMed] [Google Scholar]
- Perrotti LI, Hadeishi Y, Ulery PG, Barrot M, Monteggia L, Duman RS, Nestler EJ. Induction of deltaFosB in reward-related brain structures after chronic stress. J Neurosci. 2004;24:10594–10602. doi: 10.1523/JNEUROSCI.2542-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Verchinski BA, Mattay VS, Callicott JH, Kolachana BS, Straub RE, Egan MF, Meyer-Lindenberg A, Weinberger DR. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J Neurosci. 2004;24:10099–10102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfau ML, Russo SJ. Peripheral and Central Mechanisms of Stress Resilience. Neurobiol Stress. 2015;1:66–79. doi: 10.1016/j.ynstr.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham K, Nacher J, Hof PR, McEwen BS. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci. 2003;17:879–886. doi: 10.1046/j.1460-9568.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- Plante GE. Depression and cardiovascular disease: a reciprocal relationship. Metabolism. 2005;54:45–48. doi: 10.1016/j.metabol.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30:2192–2204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- Polman JA, Hunter RG, Speksnijder N, van den Oever JM, Korobko OB, McEwen BS, de Kloet ER, Datson NA. Glucocorticoids modulate the mTOR pathway in the hippocampus: differential effects depending on stress history. Endocrinology. 2012;153:4317–4327. doi: 10.1210/en.2012-1255. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Azzinnari D, Spinelli S, Seifritz E, Tegethoff M, Meinlschmidt G. Helplessness: a systematic translational review of theory and evidence for its relevance to understanding and treating depression. Pharmacol Ther. 2011;132:242–267. doi: 10.1016/j.pharmthera.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Pucilowski O, Overstreet DH. Effect of chronic antidepressant treatment on responses to apomorphine in selectively bred rat strains. Brain Res Bull. 1993;32:471–475. doi: 10.1016/0361-9230(93)90293-k. [DOI] [PubMed] [Google Scholar]
- Pucilowski O, Overstreet DH, Rezvani AH, Janowsky DS. Chronic mild stress-induced anhedonia: greater effect in a genetic rat model of depression. Physiol Behav. 1993;54:1215–1220. doi: 10.1016/0031-9384(93)90351-f. [DOI] [PubMed] [Google Scholar]
- Raadsheer FC, Hoogendijk WJ, Stam FC, Tilders FJ, Swaab DF. Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology. 1994;60:436–444. doi: 10.1159/000126778. [DOI] [PubMed] [Google Scholar]
- Raadsheer FC, van Heerikhuize JJ, Lucassen PJ, Hoogendijk WJ, Tilders FJ, Swaab DF. Corticotropin-releasing hormone mRNA levels in the paraventricular nucleus of patients with Alzheimer's disease and depression. Am J Psychiatry. 1995;152:1372–1376. doi: 10.1176/ajp.152.9.1372. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, McEwen BS, Morrison JH. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, Stockmeier CA. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Reader BF, Jarrett BL, McKim DB, Wohleb ES, Godbout JP, Sheridan JF. Peripheral and central effects of repeated social defeat stress: Monocyte trafficking, microglial activation, and anxiety. Neuroscience. 2015 doi: 10.1016/j.neuroscience.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiser JP, Levy J, Fromm SJ, Nugent AC, Talagala SL, Hasler G, Henn FA, Sahakian BJ, Drevets WC. The effects of tryptophan depletion on neural responses to emotional words in remitted depression. Biol Psychiatry. 2009;66:441–450. doi: 10.1016/j.biopsych.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322:1551–1555. doi: 10.1126/science.1164022. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Schmidt PJ, Roca CA. Estrogen-serotonin interactions: implications for affective regulation. Biol Psychiatry. 1998;44:839–850. doi: 10.1016/s0006-3223(98)00162-0. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Murrough JW, Han MH, Charney DS, Nestler EJ. Neurobiology of resilience. Nat Neurosci. 2012;15:1475–1484. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Wilkinson MB, Mazei-Robison MS, Dietz DM, Maze I, Krishnan V, Renthal W, Graham A, Birnbaum SG, Green TA, Robison B, Lesselyong A, Perrotti LI, Bolanos CA, Kumar A, Clark MS, Neumaier JF, Neve RL, Bhakar AL, Barker PA, Nestler EJ. Nuclear factor kappa B signaling regulates neuronal morphology and cocaine reward. J Neurosci. 2009;29:3529–3537. doi: 10.1523/JNEUROSCI.6173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Domenici E, Hiemke C, Fuchs E. Effects of fluoxetine on behavioral deficits evoked by chronic social stress in rats. Behav Brain Res. 2006a;174:188–192. doi: 10.1016/j.bbr.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Flugge G, Hiemke C, Fuchs E, Ruther E, Havemann-Reinecke U. Citalopram counteracts depressive-like symptoms evoked by chronic social stress in rats. Behav Pharmacol. 2006b;17:19–29. doi: 10.1097/01.fbp.0000186631.53851.71. [DOI] [PubMed] [Google Scholar]
- Salaycik KJ, Kelly-Hayes M, Beiser A, Nguyen AH, Brady SM, Kase CS, Wolf PA. Depressive symptoms and risk of stroke: the Framingham Study. Stroke. 2007;38:16–21. doi: 10.1161/01.STR.0000251695.39877.ca. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Banasr M. From pathophysiology to novel antidepressant drugs: glial contributions to the pathology and treatment of mood disorders. Biol Psychiatry. 2013;73:1172–1179. doi: 10.1016/j.biopsych.2013.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saus E, Soria V, Escaramis G, Vivarelli F, Crespo JM, Kagerbauer B, Menchon JM, Urretavizcaya M, Gratacos M, Estivill X. Genetic variants and abnormal processing of pre-miR-182, a circadian clock modulator, in major depression patients with late insomnia. Hum Mol Genet. 2010;19:4017–4025. doi: 10.1093/hmg/ddq316. [DOI] [PubMed] [Google Scholar]
- Schiller GD, Daws LC, Overstreet DH, Orbach J. Lack of anxiety in an animal model of depression with cholinergic supersensitivity. Brain Res Bull. 1991;26:433–435. doi: 10.1016/0361-9230(91)90019-g. [DOI] [PubMed] [Google Scholar]
- Schiller GD, Pucilowski O, Wienicke C, Overstreet DH. Immobility-reducing effects of antidepressants in a genetic animal model of depression. Brain Res Bull. 1992;28:821–823. doi: 10.1016/0361-9230(92)90267-2. [DOI] [PubMed] [Google Scholar]
- Schloesser RJ, Lehmann M, Martinowich K, Manji HK, Herkenham M. Environmental enrichment requires adult neurogenesis to facilitate the recovery from psychosocial stress. Mol Psychiatry. 2010;15:1152–1163. doi: 10.1038/mp.2010.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt EF, Warner-Schmidt JL, Otopalik BG, Pickett SB, Greengard P, Heintz N. Identification of the cortical neurons that mediate antidepressant responses. Cell. 2012;149:1152–1163. doi: 10.1016/j.cell.2012.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter ML, Abdul-Khaliq H, Krebs M, Diefenbacher A, Blasig IE. Serum markers support disease-specific glial pathology in major depression. J Affect Disord. 2008;111:271–280. doi: 10.1016/j.jad.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Schumacher J, Jamra RA, Becker T, Ohlraun S, Klopp N, Binder EB, Schulze TG, Deschner M, Schmal C, Hofels S, Zobel A, Illig T, Propping P, Holsboer F, Rietschel M, Nothen MM, Cichon S. Evidence for a relationship between genetic variants at the brain-derived neurotrophic factor (BDNF) locus and major depression. Biol Psychiatry. 2005;58:307–314. doi: 10.1016/j.biopsych.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Segi-Nishida E, Warner-Schmidt JL, Duman RS. Electroconvulsive seizure and VEGF increase the proliferation of neural stem-like cells in rat hippocampus. Proc Natl Acad Sci U S A. 2008;105:11352–11357. doi: 10.1073/pnas.0710858105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seney ML, Sibille E. Sex differences in mood disorders: perspectives from humans and rodent models. Biol Sex Differ. 2014;5:17. doi: 10.1186/s13293-014-0017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G, Suridjan I, Kennedy JL, Rekkas PV, Houle S, Meyer JH. Role of Translocator Protein Density, a Marker of Neuroinflammation, in the Brain During Major Depressive Episodes. JAMA Psychiatry. 2015 doi: 10.1001/jamapsychiatry.2014.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM. Estrogen, stress and the brain: progress toward unraveling gender discrepancies in major depressive disorder. Expert Rev Neurother. 2009;9:967–973. doi: 10.1586/ern.09.46. [DOI] [PubMed] [Google Scholar]
- Shansky RM, Hamo C, Hof PR, Lou W, McEwen BS, Morrison JH. Estrogen promotes stress sensitivity in a prefrontal cortex-amygdala pathway. Cereb Cortex. 2010;20:2560–2567. doi: 10.1093/cercor/bhq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Hamo C, Hof PR, McEwen BS, Morrison JH. Stress-induced dendritic remodeling in the prefrontal cortex is circuit specific. Cereb Cortex. 2009;19:2479–2484. doi: 10.1093/cercor/bhp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman AD, Sacquitne JL, Petty F. Specificity of the learned helplessness model of depression. Pharmacol Biochem Behav. 1982;16:449–454. doi: 10.1016/0091-3057(82)90451-8. [DOI] [PubMed] [Google Scholar]
- Sklar P, Gabriel SB, McInnis MG, Bennett P, Lim Y, Tsan G, Schaffner S, Kirov G, Jones I, Owen M, Craddock N, DePaulo JR, Lander ES. Family-based association study of 76 candidate genes in bipolar disorder: BDNF is a potential risk locus. Brain-derived neutrophic factor. Mol Psychiatry. 2002;7:579–593. doi: 10.1038/sj.mp.4001058. [DOI] [PubMed] [Google Scholar]
- Sluzewska A, Rybakowski JK, Laciak M, Mackiewicz A, Sobieska M, Wiktorowicz K. Interleukin-6 serum levels in depressed patients before and after treatment with fluoxetine. Ann N Y Acad Sci. 1995;762:474–476. doi: 10.1111/j.1749-6632.1995.tb32372.x. [DOI] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RS. The macrophage theory of depression. Med Hypotheses. 1991;35:298–306. doi: 10.1016/0306-9877(91)90272-z. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Multiple roles for astrocytes as effectors of cytokines and inflammatory mediators. Neuroscientist. 2014;20:160–172. doi: 10.1177/1073858413504466. [DOI] [PubMed] [Google Scholar]
- Solberg LC, Baum AE, Ahmadiyeh N, Shimomura K, Li R, Turek FW, Churchill GA, Takahashi JS, Redei EE. Sex- and lineage-specific inheritance of depression-like behavior in the rat. Mamm Genome. 2004;15:648–662. doi: 10.1007/s00335-004-2326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Leonard BE. The olfactory bulbectomised rat as a model of depression. Neurosci Biobehav Rev. 2005;29:627–647. doi: 10.1016/j.neubiorev.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Sousa VC, Vital J, Costenla AR, Batalha VL, Sebastiao AM, Ribeiro JA, Lopes LV. Maternal separation impairs long term-potentiation in CA1–CA3 synapses and hippocampal-dependent memory in old rats. Neurobiol Aging. 2014;35:1680–1685. doi: 10.1016/j.neurobiolaging.2014.01.024. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Charney DS. The science of resilience: implications for the prevention and treatment of depression. Science. 2012;338:79–82. doi: 10.1126/science.1222942. [DOI] [PubMed] [Google Scholar]
- Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, Bernstein HG, Bogerts B. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res. 2008;42:151–157. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Steiner J, Walter M, Gos T, Guillemin GJ, Bernstein HG, Sarnyai Z, Mawrin C, Brisch R, Bielau H, Meyer zu Schwabedissen L, Bogerts B, Myint AM. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? J Neuroinflammation. 2011;8:94. doi: 10.1186/1742-2094-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterner EY, Kalynchuk LE. Behavioral and neurobiological consequences of prolonged glucocorticoid exposure in rats: relevance to depression. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:777–790. doi: 10.1016/j.pnpbp.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Sterrenburg L, Gaszner B, Boerrigter J, Santbergen L, Bramini M, Elliott E, Chen A, Peeters BW, Roubos EW, Kozicz T. Chronic stress induces sex-specific alterations in methylation and expression of corticotropin-releasing factor gene in the rat. PLoS One. 2011;6:e28128. doi: 10.1371/journal.pone.0028128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EA, Trullas R, Platt JE. The effect of acute and chronic administration of desmethylimipramine on responses to stress in rats. Prog Neuropsychopharmacol Biol Psychiatry. 1984;8:587–592. doi: 10.1016/0278-5846(84)90019-8. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nat Neurosci. 2006;9:526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strenn N, Suchankova P, Nilsson S, Fischer C, Wegener G, Mathe AA, Ekman A. Expression of inflammatory markers in a genetic rodent model of depression. Behav Brain Res. 2015;281:348–357. doi: 10.1016/j.bbr.2014.09.025. [DOI] [PubMed] [Google Scholar]
- Su TP, Zhang L, Chung MY, Chen YS, Bi YM, Chou YH, Barker JL, Barrett JE, Maric D, Li XX, Li H, Webster MJ, Benedek D, Carlton JR, Ursano R. Levels of the potential biomarker p11 in peripheral blood cells distinguish patients with PTSD from those with other major psychiatric disorders. J Psychiatr Res. 2009;43:1078–1085. doi: 10.1016/j.jpsychires.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Sun H, Kennedy PJ, Nestler EJ. Epigenetics of the depressed brain: role of histone acetylation and methylation. Neuropsychopharmacology. 2013;38:124–137. doi: 10.1038/npp.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JD, Liu Y, Yuan YH, Li J, Chen NH. Gap junction dysfunction in the prefrontal cortex induces depressive-like behaviors in rats. Neuropsychopharmacology. 2012;37:1305–1320. doi: 10.1038/npp.2011.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Zhang L, Johnston NL, Torrey EF, Yolken RH. Serial analysis of gene expression in the frontal cortex of patients with bipolar disorder. Br J Psychiatry Suppl. 2001;41:s137–s141. doi: 10.1192/bjp.178.41.s137. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Berg L, Matthews D, Ionescu DF, Richards EM, Niciu MJ, Malinger A, Toups M, Manji H, Trivedi MH, Zarate CA, Jr, Greengard P. Preliminary evidence that early reduction in p11 levels in natural killer cells and monocytes predicts the likelihood of antidepressant response to chronic citalopram. Mol Psychiatry. 2014;19:962–964. doi: 10.1038/mp.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Chergui K, Rachleff I, Flajolet M, Zhang X, El Yacoubi M, Vaugeois JM, Nomikos GG, Greengard P. Alterations in 5-HT1B receptor function by p11 in depression-like states. Science. 2006;311:77–80. doi: 10.1126/science.1117571. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Greengard P. p11 (S100A10)--an inducible adaptor protein that modulates neuronal functions. Curr Opin Pharmacol. 2007;7:27–32. doi: 10.1016/j.coph.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Kim Y, Warner-Schmidt J, Oh YS, Greengard P. p11 and its role in depression and therapeutic responses to antidepressants. Nat Rev Neurosci. 2013;14:673–680. doi: 10.1038/nrn3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeszko PR, Lipsky R, Mentschel C, Robinson D, Gunduz-Bruce H, Sevy S, Ashtari M, Napolitano B, Bilder RM, Kane JM, Goldman D, Malhotra AK. Brain-derived neurotrophic factor val66met polymorphism and volume of the hippocampal formation. Mol Psychiatry. 2005;10:631–636. doi: 10.1038/sj.mp.4001656. [DOI] [PubMed] [Google Scholar]
- Takamori K, Yoshida S, Okuyama S. Repeated treatment with imipramine, fluvoxamine and tranylcypromine decreases the number of escape failures by activating dopaminergic systems in a rat learned helplessness test. Life Sci. 2001;69:1919–1926. doi: 10.1016/s0024-3205(01)01279-6. [DOI] [PubMed] [Google Scholar]
- Takei N, Inamura N, Kawamura M, Namba H, Hara K, Yonezawa K, Nawa H. Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. J Neurosci. 2004;24:9760–9769. doi: 10.1523/JNEUROSCI.1427-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang AC, Reeb-Sutherland BC, Romeo RD, McEwen BS. On the causes of early life experience effects: evaluating the role of mom. Front Neuroendocrinol. 2014;35:245–251. doi: 10.1016/j.yfrne.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Teper E, O'Brien JT. Vascular factors and depression. Int J Geriatr Psychiatry. 2008;23:993–1000. doi: 10.1002/gps.2020. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Bhatti BH, Manaye KF, Das JR, Akinfiresoye L. Antidepressant-like effects of low ketamine dose is associated with increased hippocampal AMPA/NMDA receptor density ratio in female Wistar-Kyoto rats. Neuroscience. 2012;213:72–80. doi: 10.1016/j.neuroscience.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolias KF, Duman JG, Um K. Control of synapse development and plasticity by Rho GTPase regulatory proteins. Prog Neurobiol. 2011;94:133–148. doi: 10.1016/j.pneurobio.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsacopoulos M, Magistretti PJ. Metabolic coupling between glia and neurons. J Neurosci. 1996;16:877–885. doi: 10.1523/JNEUROSCI.16-03-00877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Kumar A, Nestler EJ. Histone modifications at gene promoter regions in rat hippocampus after acute and chronic electroconvulsive seizures. J Neurosci. 2004;24:5603–5610. doi: 10.1523/JNEUROSCI.0589-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tynan RJ, Beynon SB, Hinwood M, Johnson SJ, Nilsson M, Woods JJ, Walker FR. Chronic stress-induced disruption of the astrocyte network is driven by structural atrophy and not loss of astrocytes. Acta Neuropathol. 2013;126:75–91. doi: 10.1007/s00401-013-1102-0. [DOI] [PubMed] [Google Scholar]
- Tynan RJ, Naicker S, Hinwood M, Nalivaiko E, Buller KM, Pow DV, Day TA, Walker FR. Chronic stress alters the density and morphology of microglia in a subset of stressresponsive brain regions. Brain Behav Immun. 2010;24:1058–1068. doi: 10.1016/j.bbi.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, Lalla D, Woolley M, Jahreis A, Zitnik R, Cella D, Krishnan R. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- Uchida S, Hara K, Kobayashi A, Otsuki K, Yamagata H, Hobara T, Suzuki T, Miyata N, Watanabe Y. Epigenetic status of Gdnf in the ventral striatum determines susceptibility and adaptation to daily stressful events. Neuron. 2011;69:359–372. doi: 10.1016/j.neuron.2010.12.023. [DOI] [PubMed] [Google Scholar]
- Ulery PG, Rudenko G, Nestler EJ. Regulation of DeltaFosB stability by phosphorylation. J Neurosci. 2006;26:5131–5142. doi: 10.1523/JNEUROSCI.4970-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloa JL, Castaneda P, Berrios C, Diaz-Veliz G, Mora S, Bravo JA, Araneda K, Menares C, Morales P, Fiedler JL. Comparison of the antidepressant sertraline on differential depression-like behaviors elicited by restraint stress and repeated corticosterone administration. Pharmacol Biochem Behav. 2010;97:213–221. doi: 10.1016/j.pbb.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Um K, Niu S, Duman JG, Cheng JX, Tu YK, Schwechter B, Liu F, Hiles L, Narayanan AS, Ash RT, Mulherkar S, Alpadi K, Smirnakis SM, Tolias KF. Dynamic control of excitatory synapse development by a Rac1 GEF/GAP regulatory complex. Dev Cell. 2014;29:701–715. doi: 10.1016/j.devcel.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallieres L, Campbell IL, Gage FH, Sawchenko PE. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J Neurosci. 2002;22:486–492. doi: 10.1523/JNEUROSCI.22-02-00486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Doelen RH, Arnoldussen IA, Ghareh H, van Och L, Homberg JR, Kozicz T. Early life adversity and serotonin transporter gene variation interact to affect DNA methylation of the corticotropin-releasing factor gene promoter region in the adult rat brain. Dev Psychopathol. 2015;27:123–135. doi: 10.1017/S0954579414001345. [DOI] [PubMed] [Google Scholar]
- Van Hasselt VB, Null JA, Kempton T, Bukstein OG. Social skills and depression in adolescent substance abusers. Addict Behav. 1993;18:9–18. doi: 10.1016/0306-4603(93)90004-s. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Riezen H, Schnieden H, Wren A. Behavioural changes following olfactory bulbectomy in rats: a possible model for the detection of antidepressant drugs [proceedings] Br J Pharmacol. 1976;57:426P–427P. [PMC free article] [PubMed] [Google Scholar]
- Veronese A, Visone R, Consiglio J, Acunzo M, Lupini L, Kim T, Ferracin M, Lovat F, Miotto E, Balatti V, D'Abundo L, Gramantieri L, Bolondi L, Pekarsky Y, Perrotti D, Negrini M, Croce CM. Mutated beta-catenin evades a microRNA-dependent regulatory loop. Proc Natl Acad Sci U S A. 2011;108:4840–4845. doi: 10.1073/pnas.1101734108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialou V, Bagot RC, Cahill ME, Ferguson D, Robison AJ, Dietz DM, Fallon B, Mazei-Robison M, Ku SM, Harrigan E, Winstanley CA, Joshi T, Feng J, Berton O, Nestler EJ. Prefrontal cortical circuit for depression- and anxiety-related behaviors mediated by cholecystokinin: role of DeltaFosB. J Neurosci. 2014;34:3878–3887. doi: 10.1523/JNEUROSCI.1787-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialou V, Maze I, Renthal W, LaPlant QC, Watts EL, Mouzon E, Ghose S, Tamminga CA, Nestler EJ. Serum response factor promotes resilience to chronic social stress through the induction of DeltaFosB. J Neurosci. 2010a;30:14585–14592. doi: 10.1523/JNEUROSCI.2496-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialou V, Robison AJ, Laplant QC, Covington HE, 3rd, Dietz DM, Ohnishi YN, Mouzon E, Rush AJ, 3rd, Watts EL, Wallace DL, Iniguez SD, Ohnishi YH, Steiner MA, Warren BL, Krishnan V, Bolanos CA, Neve RL, Ghose S, Berton O, Tamminga CA, Nestler EJ. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci. 2010b;13:745–752. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal R, Pilar-Cuellar F, dos Anjos S, Linge R, Treceno B, Vargas VI, Rodriguez-Gaztelumendi A, Mostany R, Castro E, Diaz A, Valdizan EM, Pazos A. New strategies in the development of antidepressants: towards the modulation of neuroplasticity pathways. Curr Pharm Des. 2011;17:521–533. doi: 10.2174/138161211795164086. [DOI] [PubMed] [Google Scholar]
- Vogelzangs N, Duivis HE, Beekman AT, Kluft C, Neuteboom J, Hoogendijk W, Smit JH, de Jonge P, Penninx BW. Association of depressive disorders, depression characteristics and antidepressant medication with inflammation. Transl Psychiatry. 2012;2:e79. doi: 10.1038/tp.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmayr B, Bachteler D, Vengeliene V, Gass P, Spanagel R, Henn F. Rats with congenital learned helplessness respond less to sucrose but show no deficits in activity or learning. Behav Brain Res. 2004;150:217–221. doi: 10.1016/S0166-4328(03)00259-6. [DOI] [PubMed] [Google Scholar]
- Vollmayr B, Gass P. Learned helplessness: unique features and translational value of a cognitive depression model. Cell Tissue Res. 2013;354:171–178. doi: 10.1007/s00441-013-1654-2. [DOI] [PubMed] [Google Scholar]
- Vollmayr B, Simonis C, Weber S, Gass P, Henn F. Reduced cell proliferation in the dentate gyrus is not correlated with the development of learned helplessness. Biol Psychiatry. 2003;54:1035–1040. doi: 10.1016/s0006-3223(03)00527-4. [DOI] [PubMed] [Google Scholar]
- Voorhees JL, Tarr AJ, Wohleb ES, Godbout JP, Mo X, Sheridan JF, Eubank TD, Marsh CB. Prolonged restraint stress increases IL-6, reduces IL-10, and causes persistent depressive-like behavior that is reversed by recombinant IL-10. PLoS One. 2013;8:e58488. doi: 10.1371/journal.pone.0058488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Jadhav S, Chattarji S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience. 2006;143:387–393. doi: 10.1016/j.neuroscience.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Wallace DL, Han MH, Graham DL, Green TA, Vialou V, Iniguez SD, Cao JL, Kirk A, Chakravarty S, Kumar A, Krishnan V, Neve RL, Cooper DC, Bolanos CA, Barrot M, McClung CA, Nestler EJ. CREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. Nat Neurosci. 2009;12:200–209. doi: 10.1038/nn.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DL, Vialou V, Rios L, Carle-Florence TL, Chakravarty S, Kumar A, Graham DL, Green TA, Kirk A, Iniguez SD, Perrotti LI, Barrot M, DiLeone RJ, Nestler EJ, Bolanos-Guzman CA. The influence of DeltaFosB in the nucleus accumbens on natural reward-related behavior. J Neurosci. 2008;28:10272–10277. doi: 10.1523/JNEUROSCI.1531-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JJ, Friedman AK, Sun H, Heller EA, Ku SM, Juarez B, Burnham VL, Mazei-Robison MS, Ferguson D, Golden SA, Koo JW, Chaudhury D, Christoffel DJ, Pomeranz L, Friedman JM, Russo SJ, Nestler EJ, Han MH. Stress and CRF gate neural activation of BDNF in the mesolimbic reward pathway. Nat Neurosci. 2014;17:27–29. doi: 10.1038/nn.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Noda Y, Tsunekawa H, Zhou Y, Miyazaki M, Senzaki K, Nabeshima T. Behavioural and neurochemical features of olfactory bulbectomized rats resembling depression with comorbid anxiety. Behav Brain Res. 2007;178:262–273. doi: 10.1016/j.bbr.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Wang SS, Kamphuis W, Huitinga I, Zhou JN, Swaab DF. Gene expression analysis in the human hypothalamus in depression by laser microdissection and real-time PCR: the presence of multiple receptor imbalances. Mol Psychiatry. 2008;13:786–799. 741. doi: 10.1038/mp.2008.38. [DOI] [PubMed] [Google Scholar]
- Wang Y, Cesena TI, Ohnishi Y, Burger-Caplan R, Lam V, Kirchhoff PD, Larsen SD, Larsen MJ, Nestler EJ, Rudenko G. Small molecule screening identifies regulators of the transcription factor DeltaFosB. ACS Chem Neurosci. 2012;3:546–556. doi: 10.1021/cn3000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Chen EY, Zhang X, Marshall JJ, Morozov A, Svenningsson P, Greengard P. A role for p11 in the antidepressant action of brain-derived neurotrophic factor. Biol Psychiatry. 2010;68:528–535. doi: 10.1016/j.biopsych.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Duman RS. VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc Natl Acad Sci U S A. 2007;104:4647–4652. doi: 10.1073/pnas.0610282104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Duman RS. VEGF as a potential target for therapeutic intervention in depression. Curr Opin Pharmacol. 2008;8:14–19. doi: 10.1016/j.coph.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Vanover KE, Chen EY, Marshall JJ, Greengard P. Antidepressant effects of selective serotonin reuptake inhibitors (SSRIs) are attenuated by antiinflammatory drugs in mice and humans. Proc Natl Acad Sci U S A. 2011;108:9262–9267. doi: 10.1073/pnas.1104836108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren BL, Sial OK, Alcantara LF, Greenwood MA, Brewer JS, Rozofsky JP, Parise EM, Bolanos-Guzman CA. Altered gene expression and spine density in nucleus accumbens of adolescent and adult male mice exposed to emotional and physical stress. Dev Neurosci. 2014;36:250–260. doi: 10.1159/000362875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren BL, Vialou VF, Iniguez SD, Alcantara LF, Wright KN, Feng J, Kennedy PJ, Laplant Q, Shen L, Nestler EJ, Bolanos-Guzman CA. Neurobiological sequelae of witnessing stressful events in adult mice. Biol Psychiatry. 2013;73:7–14. doi: 10.1016/j.biopsych.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weinstock M. The long-term behavioural consequences of prenatal stress. Neurosci Biobehav Rev. 2008;32:1073–1086. doi: 10.1016/j.neubiorev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Wilkinson MB, Dias C, Magida J, Mazei-Robison M, Lobo M, Kennedy P, Dietz D, Covington H, 3rd, Russo S, Neve R, Ghose S, Tamminga C, Nestler EJ. A novel role of the WNT-dishevelled-GSK3beta signaling cascade in the mouse nucleus accumbens in a social defeat model of depression. J Neurosci. 2011;31:9084–9092. doi: 10.1523/JNEUROSCI.0039-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson MB, Xiao G, Kumar A, LaPlant Q, Renthal W, Sikder D, Kodadek TJ, Nestler EJ. Imipramine treatment and resiliency exhibit similar chromatin regulation in the mouse nucleus accumbens in depression models. J Neurosci. 2009;29:7820–7832. doi: 10.1523/JNEUROSCI.0932-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- Windle M, Windle RC. Testing the specificity between social anxiety disorder and drinking motives. Addict Behav. 2012;37:1003–1008. doi: 10.1016/j.addbeh.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, Nelson RJ, Godbout JP, Sheridan JF. beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 2011;31:6277–6288. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, McKim DB, Shea DT, Powell ND, Tarr AJ, Sheridan JF, Godbout JP. Re-establishment of anxiety in stress-sensitized mice is caused by monocyte trafficking from the spleen to the brain. Biol Psychiatry. 2014a;75:970–981. doi: 10.1016/j.biopsych.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, McKim DB, Sheridan JF, Godbout JP. Monocyte trafficking to the brain with stress and inflammation: a novel axis of immune-to-brain communication that influences mood and behavior. Front Neurosci. 2014b;8:447. doi: 10.3389/fnins.2014.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Powell ND, Godbout JP, Sheridan JF. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci. 2013;33:13820–13833. doi: 10.1523/JNEUROSCI.1671-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Liu H, Li F, Sun N, Ren Y, Liu Z, Cao X, Wang Y, Liu P, Zhang K. A polymorphism in the microRNA-30e precursor associated with major depressive disorder risk and P300 waveform. J Affect Disord. 2010;127:332–336. doi: 10.1016/j.jad.2010.05.019. [DOI] [PubMed] [Google Scholar]
- Yang D, Li Q, Fang L, Cheng K, Zhang R, Zheng P, Zhan Q, Qi Z, Zhong S, Xie P. Reduced neurogenesis and pre-synaptic dysfunction in the olfactory bulb of a rat model of depression. Neuroscience. 2011;192:609–618. doi: 10.1016/j.neuroscience.2011.06.043. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Daskalakis NP, Lehrner A, Desarnaud F, Bader HN, Makotkine I, Flory JD, Bierer LM, Meaney MJ. Influences of maternal and paternal PTSD on epigenetic regulation of the glucocorticoid receptor gene in Holocaust survivor offspring. Am J Psychiatry. 2014;171:872–880. doi: 10.1176/appi.ajp.2014.13121571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Southwick SM, Giller EL., Jr Exposure to atrocities and severity of chronic posttraumatic stress disorder in Vietnam combat veterans. Am J Psychiatry. 1992;149:333–336. doi: 10.1176/ajp.149.3.333. [DOI] [PubMed] [Google Scholar]
- Young SN. The effect of raising and lowering tryptophan levels on human mood and social behaviour. Philos Trans R Soc Lond B Biol Sci. 2013;368:20110375. doi: 10.1098/rstb.2011.0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Wang DD, Wang Y, Liu T, Lee FS, Chen ZY. Variant brain-derived neurotrophic factor Val66Met polymorphism alters vulnerability to stress and response to antidepressants. J Neurosci. 2012;32:4092–4101. doi: 10.1523/JNEUROSCI.5048-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariou V, Bolanos CA, Selley DE, Theobald D, Cassidy MP, Kelz MB, Shaw-Lutchman T, Berton O, Sim-Selley LJ, Dileone RJ, Kumar A, Nestler EJ. An essential role for DeltaFosB in the nucleus accumbens in morphine action. Nat Neurosci. 2006;9:205–211. doi: 10.1038/nn1636. [DOI] [PubMed] [Google Scholar]
- Zhang L, Li CT, Su TP, Hu XZ, Lanius RA, Webster MJ, Chung MY, Chen YS, Bai YM, Barker JL, Barrett JE, Li XX, Li H, Benedek DM, Ursano R. P11 expression and PET in bipolar disorders. J Psychiatr Res. 2011a;45:1426–1431. doi: 10.1016/j.jpsychires.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Zhang L, Su TP, Choi K, Maree W, Li CT, Chung MY, Chen YS, Bai YM, Chou YH, Barker JL, Barrett JE, Li XX, Li H, Benedek DM, Ursano R. P11 (S100A10) as a potential biomarker of psychiatric patients at risk of suicide. J Psychiatr Res. 2011b;45:435–441. doi: 10.1016/j.jpsychires.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Crofton EJ, Li D, Lobo MK, Fan X, Nestler EJ, Green TA. Overexpression of DeltaFosB in nucleus accumbens mimics the protective addiction phenotype, but not the protective depression phenotype of environmental enrichment. Front Behav Neurosci. 2014;8:297. doi: 10.3389/fnbeh.2014.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zink M, Vollmayr B, Gebicke-Haerter PJ, Henn FA. Reduced expression of glutamate transporters vGluT1, EAAT2 and EAAT4 in learned helpless rats, an animal model of depression. Neuropharmacology. 2010;58:465–473. doi: 10.1016/j.neuropharm.2009.09.005. [DOI] [PubMed] [Google Scholar]