ABSTRACT
Accelerated hyperfractionated radiotherapy was performed as treatment for patients with T1 glottic cancer, and its utility was evaluated based on treatment outcomes and adverse effects. Fifty-eight men who had undergone radiotherapy were retrospectively reviewed. Tumor classification was Tis in 4 patients, T1a in 38, and T1b in 16. Histological examination revealed squamous cell carcinoma in 55 patients. Travel time from home to hospital was 0–1 hour for 24 patients, 1–2 hours for 9, and >2 hours for 25. Laser vaporization was performed prior to radiotherapy in 38 patients, and 19 patients received concurrent chemotherapy with an agent such as S-1. Patients were irradiated twice daily using an irradiation container. Most patients received a dose of 1.5 Gy/fraction up to a total of 60 Gy. The median overall treatment time was 30 days, with a median observation period of 59.6 months. A complete response was observed in all patients. The 5-year overall survival, disease-free survival, and local control rates were 97.2%, 93.2%, and 97.8%, respectively. Although grade 3 pharyngeal mucositis was observed in 2 patients, there were no other grade 3 or higher acute adverse events. As late toxicity, grade 2 laryngeal edema and grade 1 laryngeal hemorrhage were observed in 1 patient each, but no serious events such as laryngeal necrosis or laryngeal stenosis were observed. In conclusion, this treatment method brings excellent outcome and will substantially reduce the treatment duration among patients who need to stay at nearby hotels while undergoing treatment at hospitals in rural areas.
Key Words: glottic cancer, accelerated hyperftactionted radiotherapy, overall treatment time, local control rate, safety
INTRODUCTION
Curative radiotherapy is currently the standard treatment for T1 glottic cancer. Irradiation is generally performed once daily to a total radiation dose of 60–70 Gy, and the 5-year local control rate (LCR) is around 82–93%.1-12) Because radiotherapy for T1 glottic cancer does not affect the entire body due to the small radiation field, hospitals in large cities offer the treatment mainly on an outpatient basis. On the other hand, in rural areas, it is often difficult for patients to undergo outpatient radiotherapy because of the long distance between their home and the treatment hospital. In countries such as the United States, there are a lot of hotels where patients can stay, around the hospitals. However, this is not yet common in Japan where patients living far from the hospital are often admitted for radiotherapy.
Because patients often request a shorter hospital stay for inpatient radiotherapy, we try to meet this need as much as possible. To do this, we decided to incorporate hyperfractionated radiotherapy in treatment which involves irradiation twice daily. This approach should shorten overall treatment time (OTT) without affecting treatment outcome, thereby benefiting both patients and hospitals. There were some papers about outcomes of hyperfractionated radiotherapy or accelerated hyperfractionated radiotherapy (AHF) for patients with T2 glottic cancer13-15), but there were few reports on AHF with T1 glottic cancer.
In this study, we investigated the outcomes, feasibility, and adverse events of AHF for T1 glottic cancer to reveal the utility and validity of the treatment, retrospectively.
MATERIALS AND METHODS
Patients
Subjects were 58 patients with glottic cancer who underwent curative radiotherapy at Shimane University Hospital between January 2000 and November 2013. Clinical stage was determined according to the Union for International Cancer Control 2009 staging system. The patients were diagnosed with clinical TisN0M0 or T1N0M0 disease based on the findings of laryngoscopy and computed tomography (CT), and all were indicated for hyperfractionated radiotherapy. Our study was conducted in accordance with the Declaration of Helsinki. Patients were informed in detail of the procedure and provided written consent. Conventional radiotherapy with irradiation once daily was performed for patients who did not consent to hyperfractionated radiotherapy.
Table 1 shows the patient characteristics (median age, 70 years; range, 48–83 years). According to the definitions recommended by the Europe Clinical Oncology Group (ECOG), performance status (PS) was 0 and 1 in 35 and 23 patients, respectively. None of the patients had a PS of 2 or higher. Histological findings revealed squamous cell carcinoma in 55 patients and other cancer in 3 patients such as adenocarcinoma. With regard to T stage, 4 patients had Tis, 38 had T1a, and 16 had T1b. The travel time between home and hospital was ≤1 hour for 24 (41.4%) patients, 1–2 hours for 9 (15.5%) patients, and ≥2 hours for 25 (43.1%) patients, respectively.
Table 1.
Patient characteristics
| No. of cases | 58 | |
|---|---|---|
| Age, years (median) |
48–83 (70) |
|
| Male | 58 | (100%) |
| Female | 0 | (0%) |
| PS (ECOG) | ||
| 0 | 35 | (60.3%) |
| 1 | 23 | (39.7%) |
| 2 – 4 | 0 | (0%) |
| Brinkman index | ||
| 0 | 3 | (5.2%) |
| 1 – 500 | 12 | (20.7%) |
| 501 – 1000 | 15 | (25.9%) |
| 1001 – 1500 | 12 | (20.7%) |
| 1501 – 2000 | 2 | (3.4%) |
| > 2001 | 6 | (10.3%) |
| not description | 8 | (13.8%) |
| Travel time from home to hospital (one way) | ||
| 0 – 0.5 hr | 11 | (19.0%) |
| 0.5 – 1 hr | 13 | (22.4%) |
| 1 – 2 hrs | 9 | (15.5%) |
| 2 – 3 hrs | 13 | (22.4%) |
| 3 < hrs | 12 | (20.7%) |
| T stage | ||
| Tis | 4 | (6.9%) |
| T1a | 38 | (65.5%) |
| T1b | 16 | (27.6%) |
| Histology | ||
| squamous cell carcinoma | 55 | (94.8%) |
| adenocarcinoma | 1 | (1.7%) |
| others | 2 | (3.4%) |
| Chemotherapy | ||
| Yes | 19 | (32.8%) |
| S-1 | 17 | (29.3%) |
| CBDCA | 2 | (3.4%) |
| No | 39 | (67.2%) |
| Laser vaporization | ||
| Yes | 38 | (65.5%) |
| No | 20 | (34.5%) |
PS; performance status, ECOG; Europe Clinical Oncology Group, hr; hour(s), CBDCA; carboplatin
Laser vaporization and chemotherapy
38 (65.5%) patients underwent laser vaporization before radiotherapy. At our hospital, we generally perform laser vaporization concurrently with biopsy. However, laser vaporization was not performed in patients who were treated a long time earlier, underwent biopsy at other hospitals, were at a high risk for general anesthesia, or whose neck was short. In laser vaporization, ablation is provided only down to the submucosal layer to prevent adverse effects such as hoarseness. In other words, laser vaporization is provided for tumor volume reduction, not for radical cure in our hospital.
In addition, while 39 (67.2%) patients received no chemotherapy, 19 (34.5%) whose tumor was relatively larger or extended to the anterior commissure underwent chemotherapy with S-1 (17 patients) or carboplatin (2 patients). Chemotherapy is performed in patients with tumors at specific locations such as anterior cerebral commissure or with large diameters. In addition, chemotherapy is conducted based on the judgment of individual otolaryngologists after a departmental conference. Patients are fully informed and provide consent before undergoing concurrent chemotherapy. Chemotherapy was performed in 8 and 11 patients with T1a and T1b, respectively, but in no patients with Tis. On the other hand, 4 patients with Tis, 30 with T1a, and 5 with T1b did not receive chemotherapy.
Radiotherapy
Radiotherapy was performed using 6MV X-ray beams with a 5–6 × 5–6 cm radiation field. Irradiation was performed twice daily (morning and evening) with an interval of 6 hours for 5 days a week for a total of 10 irradiations per week. Most cases (48patients, 82.8%) received a dose of 1.5 Gy/fraction up to a total of 60 Gy (40 fractions). And, 1 patient (1.7%) received total of 51 Gy (34 fractions), 2 patients (3.4%) total of 66 Gy (44 fractions), and 1 patient (1.7%) total of 69 Gy (46 fractions). Moreover, 1 patients (1.7%) received a dose of 1.2 Gy/fraction up to a total of 60 Gy (50 fractions), 1 patient (1.7%) total of 63.6 Gy (53 fractions), and 4 patients (6.9%) total of 69.6 Gy (58 fractions) ,respectively. Because radiotherapy performed at a single dose of 1.2 Gy in the early phase of this study resulted in only minor adverse events, the single radiation dose was increased to 1.5 Gy. In patients with lesions in the anterior commissure, a bolus was used as necessary.
Follow up
After radiotherapy, patients were examined for recurrence during follow-up observation, which included tumor marker testing, laryngoscopy, CT, and positron emission tomography, at the Department of Radiotherapy or Otorhinolaryngology at our hospital. Adverse events were evaluated using the Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0.
Statistical analysis
Kaplan-Meier curves were generated to analyze overall survival rate (OS), disease-free survival rate (DFS), LCR, and cause-specific survival rate (CSS).
RESULTS
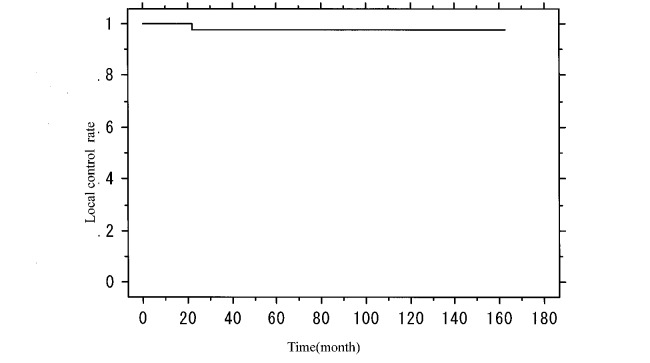
The median OTT was 30 days (range, 26–45 days), and the median follow-up duration was 59.6 months. At the response evaluation after radiotherapy, no tumors were observed in any of the patients, indicating a complete response in all cases. As shown in Figure 1, 5-year and 7-year OS were 97.2% and 86.1%, respectively, while 5-year and 7-year DFS were 93.2% and 82.2%, respectively (Fig. 2). In addition, 5-year and 7-year LCR were both 97.8% (Fig. 3), and 5-year and 7-year CSS were both 100%. During follow up, 8 (13.8%) patients developed second malignancy: gastric, esophageal, and lung cancer in 2 patients each and bronchial and gallbladder cancer in 1 patient each. Seven (12.1%) of the 58 patients died during the observation period, but none due to glottic cancer (the primary cancer), even though 3 died of other cancer. The remaining 4 patients died of other disease: pneumonia in 2 cases and acute exacerbation of chronic hepatitis B and chronic obstructive pulmonary disease in 1 case each.
Fig. 1.
Kaplan-Meier analysis of overall survival in patients with T1 glottic cancer
Fig. 2.
Kaplan-Meier analysis of disease-free survival in patients with T1 glottic cancer
Fig. 3.
Kaplan-Meier analysis of local control in patients with T1 glottic cancer
The 5-year OS rates in patients with and without laser vaporization were 100% and 92.9%, respectively, with no significant difference between the groups. The 5-year OS rates in patients with and without chemotherapy were 100% and 95.5%, respectively, again with no significant difference between the groups.
Analysis by primary tumor (T) subgroup revealed that for T1a and Tis 5-year OS was 95.8%, 7-year OS was 85.2%, 5-year and 7-year DFS were 93.3% and 78.0%, respectively, and 5-year and 7-year LCR were both 100%. In patients with T1b, 5-year and 7-year OS were 100% and 87.5%, respectively, while 5-year and 7-year DFS and LCR were all 92.9%. No significant differences were observed between the groups.
Acute adverse events were grade 3 pharyngeal mucositis in 2 patients (3.4%), with no cases of adverse effects of grade 4 or higher. None of the patients had grade 3 or higher dermatitis or hoarseness. And, reduced food intake observed in a small number of patients was quickly improved. Details are shown in Table 2.
Table 2.
Acute adverse events
| Pharyngeal mucositis | ||
| Grade 0 | 11 | (19.0%) |
| Grade 1 | 18 | (31.0%) |
| Grade 2 | 27 | (46.6%) |
| Grade 3 | 2 | (3.4%) |
| Grade 4 and more | 0 | (0%) |
| Radiation induced dermatitis | ||
| Grade 0 | 11 | (19.0%) |
| Grade 1 | 30 | (51.7%) |
| Grade 2 | 17 | (29.3%) |
| Grade 3 | 0 | (0%) |
| Grade 4 and more | 0 | (0%) |
| Hoarseness | ||
| Grade 0 | 14 | (24.1%) |
| Grade 1 | 29 | (50.0%) |
| Grade 2 | 15 | (25.9%) |
| Grade 3 | 0 | (0%) |
| Grade 4 and more | 0 | (0%) |
Although no grade 3 or higher late adverse event was observed, grade 2 laryngeal edema and grade 1 laryngeal hemorrhage were observed in 1 patient each. No other adverse events or late toxicity, including skin disorder, subcutaneous tissue disorder, laryngeal necrosis, or laryngeal stenosis, and so on, were observed.
DISCUSSION
In this study, 5-year OS and LCR were 97.2% and 97.8%, respectively, and tumors disappeared in all patients at the response evaluation after radiotherapy. These findings indicate excellent treatment outcomes in all patients. The LCRs obtained in the present study are better than those reported in the literature2,8-12,16) (Table 3). Although such a simple comparison cannot be used to draw a definitive conclusion because there were few reports about irradiation twice daily, the results suggest that the present method is extremely useful for managing T1 glottic cancer. No significant differences in treatment outcomes are apparent between these studies because the outcomes of treatment for T1 glottic cancer are originally good, suggesting that any treatment method is acceptable. This also means that patients may select an appropriate treatment modality based on social and other related factors, for example travel time from home to hospital. Furthermore, only 1 patient had local recurrence. None of the deaths were caused by glottic cancer, but by other cancer or disease. This alone suggests the utility of the present treatment.
Table 3.
Other studies of radiation therapy for T1N0 glottic cancer
| Author | Dose per fr | Number of fr | Total dose | Schedule | OTT | Local control rate |
|---|---|---|---|---|---|---|
| (Gy) | (Gy) | (day) | (%) | |||
| Reddy et al.2) | 2.0 | 33 | 66 | once daily | 45 | 89 |
| Fein et al.8) | 2.0 | 33 | 66 | once daily | 49 | 89 |
| Yamazaki et al.9) | 2.25 | 25 | 56.25 | once daily | 35 | 92 |
| van der Voet et al.10) | 2.4 | 25 | 60 | once daily | 35 | 91 |
| Motegi et al.16) | 2.4 | 25 | 60 | once daily | 37 | 93 |
| Onimaru et al.11) | 2.5 | 26 | 65 | once daily | 45† | 91.9 |
| Growda et al.12) | 3.125 | 16 | 50 | once daily | 21 | 93 |
| Present study | 1.5 | 40 | 60 | twice daily | 30 | 97.8 |
fr; fraction(s), OTT; Overall treatment time, †; slightly increased overall treatment time due to the 4-weekly irradiation schedule
The occurrence of second malignancy in this study was 13.8% (8 patients), while the occurrence of secondary cancer was similarly 13.4% (21 of 157 patients) in the study by Kim et al.17) We expect the number of secondary cancers to increase as we continue follow-up observations. Therefore, it is important to perform periodic screenings for not only the larynx and lymph nodes, but also the gastrointestinal tract and mediastinum at follow up evaluations.
In this study, 32.8% of the patients underwent chemotherapy due, for example, to relatively large tumor size. In most of these patients, S-1 was administered as the drug of choice with no major adverse events such as hematological toxicity. Although laser vaporization was performed only to reduce tumor size in 65.5% of the patients prior to radiotherapy, this supplementary procedure for tumor reduction appears to have contributed to the excellent treatment outcomes in this study.
No significant differences were observed between Tis-T1a and T1b in the present study. Nomiya et al.18) reported LCRs of 92.3% and 85.0% in T1a and T1b patients, respectively. In the present study, the 5-year LCR in T1b patients was 92.9%, with potential for better outcomes if chemotherapy with S-1, for example, had been used in many T1b cases. Hirasawa et al.19) reported there was a tendency for the LCRs of chemoradiotherapy group to be more favorable than those of the radiotherapy alone group with the patients of the early glottic cancer. In this study, chemotherapy may be proactively recommended provided it does not affect the patient’s general condition or interfere with the completion of radiotherapy in T1b patients, especially.
The adverse events observed in the present study were acceptable overall. Although a small number of patients developed grade 3 pharyngeal mucositis, this acute adverse event was readily controlled. No grade 3 or higher dermatitis or hoarseness was observed, and reduced food intake observed in a small number of patients was quickly improved. By nature, AHF is associated with moderate to severe acute adverse events. However, the adverse effects of AHF in this study were considered acceptable as the patients were able to undergo the treatment while maintaining daily life. One reason is that the irradiation field for treating T1 glottic cancer is relatively small, reducing the occurrence of acute adverse events. Second, as a reason of mild radiation induced dermatitis, we administered azelastine actively. Azelastine inhibits the release of chemical mediators such as histamine, serotonin and leukotriene, pharmacologically. And, it was reported that administration of azelastine reduces the degree of acute radiation induced dermatitis.20) Moreover, no serious late adverse events such as grade 3 or higher laryngeal edema were observed, presumably because the single dose used in this study was 1.5 Gy, not 2 Gy or higher. As a well-known radiobiological phenomenon, late toxicity can be controlled by reducing the single dose.
In addition, OTT is an extremely important factor in radiotherapy, especially for cancer in the head and neck area, and accelerated repopulation has been known as a factor associated with this phenomenon.21) For example, in squamous cell carcinoma of the head and neck, accelerated repopulation happens approximately 21–28 days after the initiation of irradiation.22) Furthermore, increasing the treatment duration leads to poorer treatment outcomes due to a reduction in radiosensitivity caused by the regeneration of tumor tissue and fibrosis of stromal cells.23) These events serve as the basis for the biological superiority of AHF. It has also been revealed that the risk of recurrence increases linearly as the duration of treatment extends beyond 28 days.24)
Even in glottic cancers, Onimaru et al. reported a 5-year LCR of 91.8% in patients with OTT ≤46 days compared with an LCR of 82.6% in patients with OTT ≥47, demonstrating a significant reduction in LCR.11)
The median OTT in this study was 30 days by using AHF technique, which is drastically shorter than the median OTT observed with conventional treatment methods, and we regard this to be a major factor contributing to the good treatment results. To shorten OTT and improve treatment outcomes, many studies have increased the dose per fraction rather than actively utilizing AHF method. Yamazaki et al. successfully shortened OTT and achieved a 5-year LCR of 92% by increasing the dose per fraction to 2.25 Gy.9) On the other hand, Gultekin et al.25) reported a 5-year LCR of 81% by increasing the dose to 2.3 Gy/fraction, while van der Voet et al.10) achieved a 5-year LCR of 89% by increasing the dose per fraction to 2.4 Gy (60 Gy in total). Although the dose per fraction in this study was 1.5 Gy, the daily dose was 3 Gy because irradiation was performed twice daily, resulting in the overwhelming reduction in OTT. Therefore, the major merits of the present method are the improvement in treatment outcomes through reducing OTT and controlling adverse events by reducing dose per fraction.
Furthermore, medical facilities located in rural areas, like our hospital, are responsible for a wide area and often provide radiotherapy on an inpatient basis. However, hospital management demands shorter hospital stays, for which AHF is also beneficial because it can reduce such stay by reducing OTT. Many hospitals in the United States and Europe are surrounded by hotels that can accommodate patients and their families, but even so, a twice-daily irradiation regimen which shortens the treatment time is a clear benefit for patients who undergo radiotherapy while staying at a hotel.
This study has a number of limitations. First, because it was a retrospective study, the reliability of the data obtained may be inferior to that obtained in prospective studies. Furthermore, because some patients could not be reached for follow-up observations, our results may have slight inaccuracies. Second, single and total doses were not consistent among the patients, and the present treatment outcome was not due to single radiotherapy alone because patients underwent radiotherapy with and without chemotherapy or laser vaporization. We plan to fix the dose to 1.5 Gy/fraction in future studies and redesign the treatment approach for T1b patients by including chemotherapy with S-1.
Despite these limitations, because of high LCR and reasonable adverse events, AHF performed for T1 glottic cancer is a useful treatment modality. Furthermore, the benefit of shortening OTT extends beyond improving treatment outcomes. By proactively introducing the present method, hospitals in rural areas can reduce the hospital stay of patients who need to travel far for hospital visits, thereby offering clear advantages to patients. This would also benefit hospitals that use the bundled payment system. We plan to increase the number of cases and conduct prospective studies to continue the investigation.
In conclusion, the outcome of AHF for T1 glottic cancer was excellent, and the adverse events were acceptable. The present treatment method will substantially reduce the treatment duration among patients who need to stay at nearby hotels while undergoing treatment at hospitals in rural areas.
ACKNOWLEDGEMENTS
The authors thank all staff members in the Division of Radiation Oncology, Shimane University Hospital for their valuable support.
CONFLICT OF INTEREST
All authors declare no actual or potential conflict of interest.
REFERENCES
- 1).Hayakawa K, Mitsuhashi N, Akimoto T, Maebayashi K, Ishikawa H, Hayakawa K, Sakurai H, Takahashi T, Kamei T, Niibe H. The effect of overall treatment time of radiation therapy on local control of T1-stage squamous cell carcinoma of the glottis. Laryngoscope, 1996; 106: 1545–1547. [DOI] [PubMed]
- 2).Reddy SP, Mohideen N, Marra S, Marks JE. Effect of tumor bulk on local control and survival of patients with T1 glottic cancer. Radiother Oncol, 1998; 47: 161–166. [DOI] [PubMed]
- 3).Skladowski K, Tarnawski R, Maciejewski B, Wygoda A, Slosarek K. Clinical radiobiology of glottic T1 squamous cell carcinoma. Int J Radiat Oncol Biol Phys, 1999; 43: 101–106. [DOI] [PubMed]
- 4).Khan MK, Koyfman SA, Hunter GK, Reddy CA, Saxton JP. Definitive radiotherapy for early (T1-T2) glottic squamous cell carcinoma: a 20 year Cleveland Clinic experience. Radiat Oncol, 2012; 7: 193. [DOI] [PMC free article] [PubMed]
- 5).Marshak G, Brenner B, Shvero J, Shapira J, Ophir D, Hochman I, Marshak G, Sulkes A, Rakowsky E. Prognostic factors for local control of early glottic cancer: the Rabin Medical Center retrospective study on 207 patients. Int J Radiat Oncol Biol Phys, 1999; 43: 1009–1013. [DOI] [PubMed]
- 6).Canaday DJ, Regine WF, Mohiuddin M, Zollinger W, Machtay M, Lee J, Schultz D, Rudoltz MS. Significance of pretreatment hemoglobin level in patients with T1 glottic cancer. Radiat Oncol Investig, 1999; 7: 42–48. [DOI] [PubMed]
- 7).Le QT, Fu KK, Kroll S, Ryu JK, Quivey JM, Meyler TS, Krieg RM, Phillips TL. Influence of fraction size, total dose, and overall time on local control of T1-T2 glottic carcinoma. Int J Radiat Oncol Biol Phys, 1997; 39: 115–126. [DOI] [PubMed]
- 8).Fein DA, Mendenhall WM, Parsons JT, Million RR. T1-T2 squamous cell carcinoma of the glottic larynx treated with radiotherapy: a multivariate analysis of variables potentially influencing local control. Int J Radiat Oncol Biol Phys, 1993; 25: 605–611. [DOI] [PubMed]
- 9).Yamazaki H, Nishiyama K, Tanaka E, Koizumi M, Chatani M. Radiotherapy for early glottic carcinoma (T1N0M0): results of prospective randomized study of radiation fraction size and overall treatment time. Int J Radiat Oncol Biol Phys, 2006; 64: 77–82. [DOI] [PubMed]
- 10).van der Voet JC, Keus RB, Hart AA, Hilgers FJ, Bartelink H. The impact of treatment time and smoking on local control and complications in T1 glottic cancer. Int J Radiat Oncol Biol Phys, 1998; 42: 247–255. [DOI] [PubMed]
- 11).Onimaru R, Hasegawa M, Yasuda K, Homma A, Oridate N, Fukuda S, Shirato H. Radiotherapy for glottic T1N0 carcinoma with slight hypofractionation and standard overall treatment time: importance of overall treatment time. Jpn J Clin Oncol, 2011; 41: 103–109. [DOI] [PubMed]
- 12).Gowda RV, Henk JM, Mais KL, Sykes AJ, Swindell R, Slevin NJ. Three weeks radiotherapy for T1 glottic cancer: the Christie and Royal Marsden Hospital Experience. Radiother Oncol, 2003; 68: 105–111. [DOI] [PubMed]
- 13).Trotti A 3rd, Zhang Q, Bentzen SM, Emami B, Hammond ME, Jones CU, Morrison WH, Sagar SM, Ridge JA, Fu KK, Ang KK. Randomized trial of hyperfractionation versus conventional fractionation in T2 squamous cell carcinoma of the vocal cord (RTOG 9512). Int J Radiat Oncol Biol Phys, 2014; 89: 958–963. [DOI] [PMC free article] [PubMed]
- 14).Tateya I, Hirano S, Kojima H, Omori K, Shoji K, Mitsumori M, Nagata Y, Ito J. Hyperfractionated radiotherapy for T2 glottic cancer for preservation of the larynx. Eur Arch Otorhinolaryngol, 2006; 263: 144–148. [DOI] [PubMed]
- 15).Okubo M, Nishimura Y, Shibata T, Nakamatsu K, Kanamori S, Tachibana I, Koike R, Nishikawa T, Mori K. Definitive radiation therapy for moderately advanced laryngeal cancer: effects of accelerated hyperfractionation. Jpn J Clin Oncol, 2010; 40: 944–948. [DOI] [PubMed]
- 16).Motegi A, Kawashima M, Arahira S, Zenda S, Toshima M, Onozawa M, Hayashi R, Akimoto T. Accelerated radiotherapy for T1 to T2 glottic cancer. Head Neck, 2015; 37: 5879–584. [DOI] [PubMed]
- 17).Kim TG, Ahn YC, Nam HR, Chung MK, Jeong HS, Son YI, Baek CH. Definitive radiation therapy for early glottic cancer: experience of two fractionation schedules. Clin Exp Otorhinolaryngol, 2012; 5: 94–100. [DOI] [PMC free article] [PubMed]
- 18).Nomiya T, Nemoto K, Wada H, Takai Y, Yamada S. Long-Term Results of Radiotherapy for T1a and T1bN0M0 Glottic Carcinoma. Laryngoscope, 2008; 118: 1417–1421. [DOI] [PubMed]
- 19).Hirasawa N, Itoh Y, Naganawa S, Ishihara S, Suzuki K, Koyama K, Murao T, Asano A, Nomoto Y, Horikawa Y, Sasaoka M, Obata Y. Multi-institutional analysis of early glottic cancer from 2000 to 2005. Radiat Oncol, 2012: 7: 122. [DOI] [PMC free article] [PubMed]
- 20).Furusawa M, Baba Y, Murakami R, Yokoyama T, Uozumi H, Nishimura R, Takada C, Takahashi M, Eura M, Ishikawa T. Azelastine: its clinical application for radiation dermatitis. Radiat Med, 1996; 14: 151–154. [PubMed]
- 21).Trott KR. The mechanisms of acceleration of repopulation in squamous epithelia during daily irradiation. Acta Oncol, 1999; 38: 153–157. [DOI] [PubMed]
- 22).Roberts SA, Hendry JH. The delay before onset of accelerated tumor cell repopulation during radiotherapy: a direct maximum-likelihood analysis of a collection of worldwide tumor-control data. Radiother Oncol, 1993; 29: 69–74. [DOI] [PubMed]
- 23).Hayakawa K, Mitsuhashi N, Tamaki Y, Takahashi M, Honjo J, Kamei T, Koyama I, Niibe H. Radiation therapy for stage I and II laryngeal cancer using 10 MV X-rays or cobalt-60 gamma-rays. Radiat Med, 1992; 10: 199–205. [PubMed]
- 24).Withers HR, Peters LJ, Taylor JM, Owen JB, Morrison WH, Schultheiss TE, Keane T, O’Sullivan B, van Dyk J, Gupta N, Wang CC, Jones CU, Doppke KP, Myint S, Thompson M, Parsons JT, Mendenhall WM, Dische S, Aird EGA, Henk JM, Bidmead MAM, Svoboda V, Chon Y, Hanlon AL, Peters TL, Hanks GE. Local control of carcinoma of the tonsil by radiation therapy: an analysis of patterns of fractionation in nine institutions. Int J Radiat Oncol Biol Phys, 1995; 33: 549–562. [DOI] [PubMed]
- 25).Gultekin M, Ozyar E, Cengiz M, Ozyigit G, Hayran M, Hosal S, Akyol F. High daily fraction dose external radiotherapy for T1 glottic carcinoma: treatment results and prognostic factors. Head Neck, 2012; 34: 1009–1014. [DOI] [PubMed]