ABSTRACT
The purposes of this study on prostate cancer are to demonstrate the time course of International Prostate Symptom Score (IPSS) after intensity-modulated radiation therapy (IMRT) combined with androgen deprivation therapy (ADT) and to examine the factor associated with the IPSS change. This study included 216 patients treated with IMRT between 2006 and 2010. Patients were evaluated in three groups according to baseline IPSS as defined by the American Urological Association classification, where IPSSs of 0 to 7, 8 to 19, and 20 to 35 represent mild (n = 124), moderate (n = 70), and severe (n = 22) symptom groups, respectively. The average IPSSs ± standard deviation at baseline vs. those at 24 months after IMRT were 3.5 ± 2.1 vs. 5.1 ± 3.6 in the mild group (P < 0.001), 12.6 ± 3.4 vs. 10.0 ± 6.0 in the moderate group (P = 0.0015), and 23.8 ± 2.9 vs. 14.4 ± 9.1 in the severe group (P < 0.001). Among factors of patient and treatment characteristics, age, IPSS classification, pretreatment GU medications, and positive biopsy rates were associated with the IPSS difference between baseline and 24 months (P = 0.023, < 0.001, 0.044, and 0.028, respectively). In conclusion, patients with moderate to severe urinary symptoms can exhibit improvement in urinary function after IMRT, whereas patients with mild symptoms may have slightly worsened functions. Age, baseline IPSS, GU medications, and tumor burden in the prostate can have an effect on the IPSS changes.
Key Words: IPSS, prostate cancer, genitourinary toxicity, IMRT, ADT
INTRODUCTION
Major concerns associated with high-dose external beam radiotherapy (EBRT) for prostate cancer are rectal and urinary toxicities. Factors increasing the risk of rectal toxicity in this treatment approach are considered to be dose-volume parameters of the rectum in the radiotherapy planning and some clinical characteristics.1) Thus, intensity-modulated radiation therapy (IMRT) has reduced rectal toxicity in this curative treatment.2, 3) On the other hand, clinical factors related to genitourinary (GU) toxicities have not been fully elucidated. GU toxicities were enhanced by dose escalation with IMRT. We should pay more attention to detailed quality of life analysis, not only with respect to rectal bleeding but also other specific symptoms such as urinary incontinence.4) Reliable dose–volume models of GU toxicity also remain unavailable because of the variable bladder filling occurring between computed tomography (CT) simulation and irradiation.5)
In the case of brachytherapy, the pretreatment International Prostate Symptom Score (IPSS) has a high correlation with urinary function after treatment.6) It is established that patients with pretreatment high IPSS and poor urinary function are typically not candidates for brachytherapy.7) However, the significance of pretreatment IPSS and the time course of IPSS have not been clearly demonstrated in EBRT for prostate cancer. The purpose of this study was to demonstrate the IPSS course of prostate cancer patients after IMRT combined with androgen deprivation therapy (ADT) and to examine the relationship between the GU toxicity and the clinical characteristics with pretreatment IPSS.
MATERIALS AND METHODS
Patients
Of 241 patients treated with IMRT for clinically localized prostate cancer between June 2006 and December 2010 at our hospital, 216 who had continuous IPSSs were included in this retrospective study. Twenty-five patients excluded from this study lacked continuous IPSS data, mainly at the baseline. Informed consent was obtained for IMRT and data exploitation under adequate privacy control from all patients before treatment. This study was approved by the institutional review board.
Androgen deprivation therapy and radiotherapy
All patients were given neoadjuvant ADT consisting of a combination of a luteinizing hormone-releasing hormone (LHRH) analogue and anti-androgen treatment. The median time of neoadjuvant ADT was 10 months (range, 2–68 months). Most patients (96.8%) also received adjuvant ADT consisting of only the LHRH analogue. The median time of adjuvant ADT was 19 months (range, 1–37 months). The details of ADT are described in our previous report.2)
The entire bladder was delineated on radiation planning CT as an organ at risk. The bladder V70, V40, and V20 means the percentage of the bladder covered by at least 70 Gy, 40 Gy, and 20 Gy, respectively. The definitions of the planning target volume (PTV) and other normal structures and other details for IMRT methods are described in our previous report.2) Whole-pelvic radiotherapy was not used. Patients basically received 74 Gy in the low-risk group and 78 Gy in intermediate- and high-risk groups according to the National Comprehensive Cancer Network (NCCN) criteria.
Follow-up and data analysis
Follow-up evaluations were performed at 3-month intervals. IPSS consisting of seven domains, for evaluating incomplete emptying, frequency, weak stream, intermittency, urgency, straining, and nocturia, was measured on a 0 to 5 scale at each follow-up. The length of follow-up was calculated from the start date of IMRT. IPSSs at baseline (i.e., before the initiation of any therapy), and about 3, 6, 12, and 24 months after IMRT were reviewed. Patients were divided and evaluated in three groups according to baseline IPSS as defined by the American Urological Association (AUA) classification, where IPSSs of 0 to 7, 8 to 19, and 20 to 35 represent mild, moderate, and severe symptom groups, respectively. Toxicity was scored according to the Radiation Therapy Oncology Group morbidity grading scale.8) The Kruskal-Wallis test was used in the comparison of patient and treatment characteristics among the three AUA groups. The Friedman test was used to detect the changes of IPSS from baseline to 24 months after IMRT. The Wilcoxon signed-rank test was used in the comparison of IPSS at baseline and at 24 months. The effects of patient characteristics and treatment parameters on the IPSS difference between baseline and 24 months were analyzed by the Mann-Whitney U test and the multiple linear regression model. The Fisher’s exact test was used in the analysis of potential factors associated with acute and late GU toxicity. A logistic regression model was used to identify significant predictors of acute and late GU toxicity and the IPSS difference between baseline and 24 months. All factors were adjusted in the analyses of the multiple linear regression model and a logistic regression model. Median values were basically adopted as cut-off values for these factors. The following factors were analyzed: age (≤ 69 vs. ≥ 70), T-stage (≤ T2 vs. ≥ T3), positive rate in needle biopsy (≤ 50% vs. > 50%), pretreatment GU medications, diabetes, neoadjuvant ADT time (≤ 10 months vs. > 10 months), total ADT time (≤ 27 months vs. > 27 months), prostate volume (≤ 20 cc vs. > 20 cc), PTV maximum dose (≤ 81.9 Gy vs. > 81.9 Gy), PTV volume (≤ 60 cc vs. > 60 cc), bladder V70 (≤ 10% vs. > 10%), bladder V40 (≤ 39% vs. > 39%), bladder V20 (≤ 75% vs. > 75%), bladder maximum dose (≤ 81 Gy vs. > 81 Gy). A P-value of < 0.05 was considered significant. All statistical analyses were performed with EZR,9) which is a graphical user interface for R (The R Foundation for Statistical Computing).
RESULTS
Patient and treatment characteristics
The median age of the subjects was 69 years. More than half of the patients (115, 53.2%) had T3a or higher T-stage. The median baseline IPSS was 6. Forty patients (18.5%) took GU medications before the initiation of any therapy. All pretreatment GU medications were alpha-blockers. The numbers of patients were 124 in the mild (baseline IPSS ≤ 7), 70 in the moderate (baseline IPSS ≥ 8 and ≤ 19), and 22 in the severe group (baseline IPSS ≥ 20). During IMRT, 43 patients (20.0%) started GU medications and five patients (2.4%) changed their medications or needed the addition of the new drug. Of these 48 patients in total, 21, 21, and 6 patients were the mild, the moderate, and the severe group, respectively. The used drugs were mainly alpha-blockers and two patients (0.9%) took anticholinergic drug. After IMRT, 12 patients (5.6%) started GU medications and six patients (2.8%) changed their medications or needed the addition of the new drug. Of these 18 patients in total, 7, 7, and 4 patients were the mild, the moderate, and the severe group, respectively. The used drugs were mainly alpha-blockers and seven patients (3.2%) took anticholinergic drug. The median follow-up time from the start date of IMRT was 34 months (range, 18–66 months). Therefore, IPSSs at 18 or 21 months after IMRT were used as alternatives to those at 24 months for 14 patients (6.5%). Table 1 describes patient and treatment characteristics. The rates of GU medications were significantly higher in the following order: mild, moderate, and then the severe group (P < 0.001). The prostate volumes measured on radiotherapy planning CT tended to be larger in the following order: mild, moderate, and then the severe group (P = 0.053). The PTV volumes were significantly larger in the following order: mild, moderate, and then the severe group (P = 0.010).
Table 1.
Patient and treatment characteristics
| AUA classification | ||||||
|---|---|---|---|---|---|---|
| Characteristic | All patients n = 216 |
mild n = 124 |
moderate n = 70 |
severe n = 22 |
P value | |
| Age (years) | 69 (49–81) | 68.2 | 69.5 | 68.7 | 0.51 | |
| PSA level (ng/ml) | 14.13 (1.40–319.00) | 30.0 | 28.0 | 25.8 | 0.32 | |
| Gleason score | 7 (5–10) | 7.4 | 7.3 | 7.6 | 0.71 | |
| Tumor stage | T1-T2 | 101 (46.8%) | 57 | 33 | 11 | 0.93 |
| T3-T4 | 115 (53.2%) | 67 | 37 | 11 | ||
| Positive biopsy rate (%) | 50.0 (3.0–100.0) | 46.4 | 50.3 | 48.0 | 0.70 | |
| Positive biopsy > 50% (%) | 35.1 | 31.5 | 41.4 | 36.4 | 0.38 | |
| Baseline IPSS | 6 (0–32) | 3.5 | 12.6 | 23.8 | < 0.001 | |
| GU medications (%) | 40 (18.5%) | 9.7 | 27.1 | 40.9 | < 0.001 | |
| Diabetes (%) | 21 (9.7%) | 7.3 | 12.9 | 13.6 | 0.36 | |
| Neoadjuvant ADT (month) | 10 (2–68) | 10.9 | 10.4 | 10.9 | 0.65 | |
| Total ADT (month) | 27 (4–94) | 27.7 | 26.5 | 27.4 | 0.53 | |
| Prostate volume (cc) | 20.8 (8.0–100.8) | 21.7 | 23.9 | 24.4 | 0.053 | |
| PTV volume (cc) | 59.4 (22.1–190.9) | 59.2 | 67.1 | 68.1 | 0.010 | |
| Prescribed dose (Gy) | 78 (70.0–78.0) | 77.7 | 77.4 | 77.3 | 0.16 | |
| Bladder V70 (%) | 10.0 (2.0–34.0) | 10.7 | 10.0 | 12.8 | 0.056 | |
| Bladder V40 (%) | 38.9 (12.9–88.7) | 40.6 | 37.6 | 45.0 | 0.13 | |
| Bladder V20 (%) | 73.5 (28.0–100.0) | 74.1 | 69.2 | 73.9 | 0.063 | |
| Bladder max. dose (Gy) | 80.9 (73.6–84.1) | 80.7 | 80.5 | 80.5 | 0.85 | |
| Follow-up (month) | 34 (18–66) | 36.3 | 34.4 | 34.8 | 0.70 | |
Data of all patients and each group are presented as median and mean, respectively.
Data of positive rate in needle biopsy were missing in 3 patients (2 in the mild group and 1 in the moderate group).
IPSS courses after IMRT
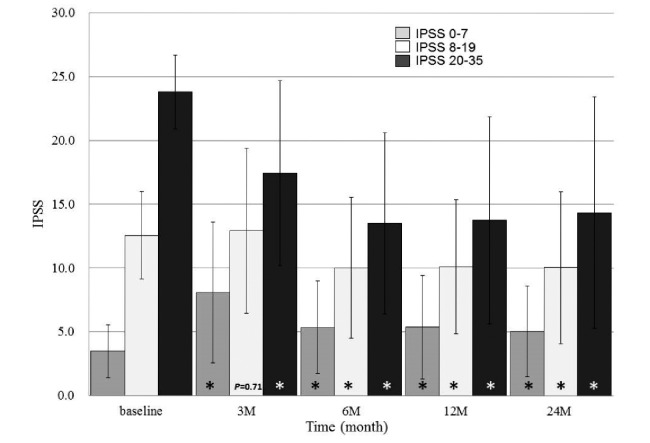
IPSS significantly changed over time in all patients (P < 0.001). The average IPSS was highest at three months after IMRT and lowest at 24 months after IMRT. Figure 1 the shows IPSS courses in the three groups according to the AUA classification. The average IPSS significantly changed over time in all three groups (all P < 0.001). The average IPSS was lowest at baseline (3.5 ± 2.1), highest at three months after IMRT (8.1 ± 5.5), and second lowest at 24 months after IMRT (5.1 ± 3.6) in the mild group. The average IPSS at 24 months after IMRT was significantly higher than that at baseline in the mild group (5.1 ± 3.6 vs. 3.5 ± 2.1, P < 0.001). In the moderate group, the highest average IPSS was at three months after IMRT (12.9 ± 6.5) and the lowest was at 24 months after IMRT (10.0 ± 6.0). The average IPSS at 24 months after IMRT was significantly lower than that at baseline in the moderate group (10.0 ± 6.0 vs. 12.6 ± 3.4, P = 0.0015). In the severe group, the average IPSS was highest at baseline (23.8 ± 2.9) and lowest at 6 months after IMRT (13.5 ± 7.1). The average IPSS at 24 months after IMRT was significantly lower than that at baseline in the severe group (14.4 ± 9.1 vs. 23.8 ± 2.9, P < 0.001).
Fig. 1.
IPSS trends of three groups after IMRT combined with ADT according to the AUA classification.
Bars and lines represent mean IPSSs and standard deviations at each time.
* means statistically significant (P < 0.01) compared with those at baseline in each group.
The average IPSS difference between baseline and 24 months was – 0.9 ± 6.2 in all the patients. Table 2 shows the effects of patient and treatment characteristics on the IPSS difference between baseline and 24 months. IPSS classification, pretreatment GU medications, and positive biopsy rates were associated with the IPSS difference between baseline and 24 months by the Mann-Whitney U test (P < 0.001, P < 0.001, and P = 0.0078, respectively). In the multiple linear regression model, age, IPSS classification, pretreatment GU medications, and positive biopsy rates were associated with the IPSS difference between baseline and 24 months (P = 0.023, < 0.001, 0.044, and 0.028, respectively).
Table 2.
Effects of patient characteristics and treatment parameters on the IPSS difference between baseline and 24 months in the Mann-Whitney U test and the multiple linear regression model
| Characteristic | Mann-Whitney U test | Multiple linear regression model | |||
|---|---|---|---|---|---|
| P value | RC | 95% CI | P value | ||
| Age | 0.51 | – 1.7 | – 3.1– – 0.24 | 0.023 | |
| Tumor stage | 0.21 | – 0.039 | – 1.7 –1.6 | 0.96 | |
| Baseline IPSS | < 0.001 | 4.8 | 3.7–5.9 | < 0.001 | |
| GU medications | < 0.001 | 1.9 | 0.060–3.8 | 0.044 | |
| Diabetes | 0.071 | 0.56 | – 1.8–2.9 | ||
| ADT | 0.55 | 0.81 | – 0.73–2.3 | 0.30 | |
| Prostate volume | 0.93 | – 0.34 | – 2.1–1.4 | 0.70 | |
| PTV volume | 0.66 | – 0.39 | – 2.1–1.3 | 0.66 | |
| PTV max. dose | 0.65 | 0.35 | – 1.3–2.0 | 0.68 | |
| Bladder V70 | 0.27 | – 0.58 | – 1.9–1.8 | 0.53 | |
| Bladder V40 | 0.55 | 1.4 | – 0.69–3.5 | 0.19 | |
| Bladder V20 | 0.085 | – 1.7 | – 3.7–0.20 | 0.080 | |
| Bladder max. dose | 0.45 | 0.13 | – 1.5–1.8 | 0.87 | |
| Positive biopsy rate | 0.0078 | 1.9 | 0.22–3.5 | 0.028 | |
RC = regression coefficient CI = confidence interval
Urinary toxicity
Table 3 shows the incidence of acute and late GU toxicities. Of 51 patients (23.6%) who developed acute Grade 2 urinary toxicity, most of the symptoms (46, 21.3%) were dysuria, such as urinary frequency. Table 4 shows the effects of patient characteristics and treatment parameters on acute and late GU toxicities. In Fisher’s exact test, only baseline IPSS classification was associated with acute GU toxicity (P = 0.0089). Multiple logistic regression analysis also showed that baseline IPSS classification was an independent factor associated with acute GU toxicity (P = 0.0089). A one-unit increase of the AUA classification doubled the acute GU toxicity risk.
Table 3.
Incidence of acute and late Grade 2 or higher genitourinary (GU) toxicity among patients treated with intensity-modulated radiation therapy (IMRT)
| AUA classification | AUA classification | |||||||
|---|---|---|---|---|---|---|---|---|
| Acute GU toxicity |
mild | moderate | severe | Late GU toxicity |
mild | moderate | severe | |
| n = 216 | n = 124 | n = 70 | n = 22 | n = 216 | n = 124 | n = 70 | n = 22 | |
| Total | 51 (23.6%) |
21 (16.9%) |
22 (31.4%) |
8 (36.4%) |
19 (8.8%) |
10 (8.1%) |
7 (10.0%) |
2 (9.1%) |
| Grade 2 | ||||||||
| dysuria | 46 (21.3%) |
21 (16.9%) |
18 (25.7%) |
7 (31.8%) |
13 (6.0%) |
4 (3.2%) |
6 (8.6%) |
2 (9.1%) |
| hematuria | 3 (1.4%) | 0 | 2 (2.9%) | 1 (4.5%) | 1 (0.5%) | 1 (0.8%) | 0 | 0 |
| pain on urination | 2 (0.9%) | 0 | 2 (2.9%) | 0 | 0 | 0 | 0 | 0 |
| cystis | 0 | 0 | 0 | 0 | 2 (0.9%) | 2 (1.6%) | 0 | 0 |
| Grade 3 | ||||||||
| urinary retention | 0 | 0 | 0 | 0 | 2 (0.9%) | 1 (0.8%) | 1 (1.4%) | 0 |
| bladder ulcer | 0 | 0 | 0 | 0 | 1 (0.5%) | 1 (0.8%) | 0 | 0 |
| Grade 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Rates are represented within each group.
Table 4.
Effects of patient characteristics and treatment parameters on acute and late genitourinary (GU) toxicity in Fisher’s exact test and logistic regression analysis
| Characteristic | Effects on acute GU toxicity | Effects on late GU toxicity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fisher’s exact test |
Logistic regression model | Fisher’s exact test |
Logistic regression model | ||||||||
| P value | OR | 95% CI | P value | P value | OR | 95% CI | P value | ||||
| Age | 0.11 | 1.6 | 0.77–3.1 | 0.22 | 1 | 1.1 | 0.38–3.2 | 0.88 | |||
| Tumor stage | 0.055 | 0.53 | 0.26 –1.1 | 0.071 | 0.81 | 1.2 | 0.38–4.0 | 0.73 | |||
| Baseline IPSS | 0.0089 | 2.0 | 1.2–3.3 | 0.0089 | 0.88 | 1.6 | 0.72–3.4 | 0.27 | |||
| GU medications | 0.68 | 0.51 | 0.20–1.3 | 0.17 | 0.21 | 0.18 | 0.021–1.6 | 0.12 | |||
| Diabetes | 1 | 0.81 | 0.26–2.5 | 0.72 | 0.23 | NE | NE | NE | |||
| ADT | 0.33 | 0.57 | 0.28–1.2 | 0.12 | 0.35 | 1.5 | 0.46–4.8 | 0.50 | |||
| Prostate volume | 0.43 | 1.2 | 0.51–2.8 | 0.68 | 0.34 | 1.8 | 0.50–6.2 | 0.38 | |||
| PTV volume | 0.52 | 0.89 | 0.40–2.0 | 0.79 | 0.64 | 0.55 | 0.16–1.9 | 0.33 | |||
| PTV max. dose | 0.63 | 2.1 | 0.91–5.0 | 0.083 | 0.81 | 0.66 | 0.20–2.2 | 0.50 | |||
| Bladder V70 | 0.63 | 1.5 | 0.63–3.5 | 0.37 | 0.090 | 4.1 | 1.12–15 | 0.033 | |||
| Bladder V40 | 1 | 0.96 | 0.37–2.5 | 0.94 | 0.81 | 1.0 | 0.23–4.6 | 0.97 | |||
| Bladder V20 | 0.63 | 0.80 | 0.33–2.0 | 0.64 | 1 | 0.44 | 0.10–1.9 | 0.28 | |||
| Bladder max. dose | 0.27 | 0.43 | 0.18–1.0 | 0.053 | 0.81 | 1.4 | 0.43–4.6 | 0.57 | |||
In the analysis of the effects on acute GU toxicity, ADT means neoadjuvant ADT CI = confidence interval
Of 16 patients (7.4%) who developed late Grade 2 urinary toxicity, 13 patients (6.0%) experienced dysuria requiring medication or medication change at a median of 19 months (range, 8–47 months). Two patients (0.9%) experienced Grade 3 urinary retention requiring self-catheterization or dilation at 14 and 17 months after IMRT. One patient (0.5%) developed bladder ulcer requiring laser coagulation (Grade 3) at 14 months after IMRT. In the Fisher’s exact test, no factor was associated with ≥ Grade 2 late GU toxicity, as shown in Table 4. In the multiple logistic regression model, only bladder V70 was significantly associated with ≥ Grade 2 late GU toxicity (P = 0.033). Patients with bladder V70 > 10% had about four-fold higher risk of late GU toxicity than those with bladder V70 ≤ 10%.
DISCUSSION
The average IPSSs at baseline vs. those at 24 months after IMRT were 23.8 ± 2.9 vs. 14.4 ± 9.1 and 12.6 ± 3.4 vs. 10.0 ± 6.0 in the severe and moderate groups, respectively. These data show that patients with moderate to severe urinary symptoms can exhibit improvement in urinary function after IMRT combined with ADT. The IPSS in the group with positive biopsy rates > 50% improved significantly through 24 months compared with that in the group with positive biopsy rates ≤ 50%, as shown in Table 2. These data suggest that the urinary outcome might be improved more in patients with a larger tumor burden as a result of the treatment. Reduction in tumor burden by EBRT combined with ADT may be the first factor in the improvement of urinary function in the severe and moderate groups.
Prostate cytoreduction due to ADT may be the second factor in the improvement of urinary function in the severe and moderate groups, although ADT time had no effect on the IPSS difference between baseline and 24 months. At any rate, as the prostate volume tended to be larger in the order of mild, moderate, and then the severe group, baseline IPSS was considered to be associated with the enlargement of the prostate. In our study, more than half of the patients had T3a or higher T-stage and more than 70% of them belonged to the high-risk group. As ADT was used for as long as 27 months as the current standard treatment approach for patients with locally advanced prostate cancer,10) ADT time might have had no effect on the IPSS changes in our study. On the other hand, Feigenberg et al. reported increased rates of urinary morbidity with long-term ADT use.11) The use of 3 months of neoadjuvant ADT can decrease the volume of the prostate by 30–50% before EBRT.12) Feigenberg et al. argued that the problem with this effect is that the prostate probably continues to shrink during radiation. This could lead to an increased volume of the rectum and/or bladder exposed to a significant radiation dose. As neoadjuvant ADT was also used for as long as 10 months in our study, such long-term neoadjuvant ADT could rather improve urinary function along with the shrinkage of the prostate. Thus, ADT time might have had no effect on the risk of acute and late GU toxicity in our study.
The average IPSS at baseline vs. that at 24 months after IMRT was 3.5 ± 2.1 vs. 5.1 ± 3.6 in the mild group. This indicates that patients with mild symptoms may have slightly worse symptoms after IMRT combined with ADT. As the prostate volume of patients with mild symptoms tended to be smaller than that of moderate and severe groups, these patients might get smaller benefits of prostate cytoreduction from ADT. The factor causing the worse IPSS in the mild group may be the late toxicity of EBRT itself. In fact, analysis of the effects of patient characteristics and IMRT parameters on late GU toxicities indicated that only bladder V70 was associated with late ≥ Grade 2 GU toxicity. Therefore, the late RT effect might offset the advantage of prostate cytoreduction and reduction in tumor burden by treatment for patients in the mild group. An appropriate dose–volume evaluation of GU toxicity depends on the precise bladder filling between CT simulation and irradiation. In this study, all patients defecated when possible every morning and discharged urine about one hour both before CT simulation and before IMRT to minimize daily variations in the shape and anatomical location of the prostate, and we checked these situations carefully in the daily treatment. The use of image-guided radiation therapy (IGRT) may make it possible to perform an accurate survey for dose–volume models of GU toxicity.
To our knowledge, only one study has investigated the urinary outcome of men treated for prostate cancer with EBRT using IPSS.13) Malik et al. reported that patients with pretreatment high IPSS are not at a significantly increased risk of severe GU toxicity or obstructive uropathy compared with patients with lower pretreatment IPSS. Patients with IPSS ≥ 15 can have modest improvement in their baseline urinary function over time. In addition, they has reported that potential mechanisms for urinary symptom improvement after EBRT combined with ADT may be related to (1) prostate cytoreduction from neoadjuvant ADT and/or RT, (2) reduction in disease burden, (3) GU medication use, and (4) patient bias. These results are in good agreement with our current study. The results of our current study showed that a one-unit increase of the AUA classification doubled the acute GU toxicity risk. This result is also congruent with that reported by Malik et al.13) In the current study, the IPSS of the group ≤ 69 years improved significantly between baseline and 24 months compared with that of the group ≥ 70 years, as shown in Table 2. This indicates that younger patients may exhibit better improvement in their urinary function.
Localized prostate cancer patients usually have some radical treatment choices such as radical prostatectomy, brachytherapy, and IMRT. The results of our study provide useful data for the understanding of urinary function after IMRT combined with long-term ADT and the basis of comparison with other treatments. Three essential points need to be considered when interpreting the results of this study. At first, the lack of IPSS data just before EBRT. Therefore, the result of the current study has to be interpreted as reflecting the results of IMRT combined with long-term ADT. Secondly, when considering the natural course of urinary symptoms in 69 years old men, IPSS scores will increase during the period of two years as natural course. Thirdly, IPSSs of this study were not divided into voiding and storage symptom groups. Voiding symptom may reflect the obstruction caused by pathological changes of the prostate, and storage symptoms may reflect the bladder function such as overactivity caused by IMRT. In order to investigate the factors affecting the symptom change, it is necessary to analyze the IPSS by dividing them into voiding and storage symptom groups.
In conclusion, we present the IPSS courses and the relationships of GU toxicity and the clinical characteristics with pretreatment IPSS in IMRT combined with ADT for localized prostate cancer. Patients with moderate to severe urinary symptoms can exhibit improvement in urinary function after IMRT combined with ADT, whereas patients with mild symptoms may have slightly worse urinary function. Age, baseline IPSS, GU medications, and tumor burden in the prostate are considered to have an effect on the IPSS changes. The risk of acute GU toxicity will increase in patients with higher baseline IPSS. Late toxicity may be associated with the bladder volume exposed to high doses of IMRT.
ACKNOWLEDGEMENTS
This work was supported by a Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research, Grant Number 25871226.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1).Michalski JM, Gay H, Jackson A, Tucker SL, Deasy JO. Radiation dose-volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys, 2010; 76: 123–129. [DOI] [PMC free article] [PubMed]
- 2).Tomita N, Soga N, Ogura Y, Hayashi N, Shimizu H, Kubota T, Ito J, Hirata K, Ohshima Y, Tachibana H, Kodaira T. Preliminary results of intensity-modulated radiation therapy with helical tomotherapy for prostate cancer. J Cancer Res Clin Oncol, 2012; 138: 1931–1936. [DOI] [PMC free article] [PubMed]
- 3).Takeda K, Takai Y, Narazaki K, Mitsuya M, Umezawa R, Kadoya N, Fujita Y, Sugawara T, Kubozono M, Shimizu E, Abe K, Shirata Y, Ishikawa Y, Yamamoto T, Kozumi M, Dobashi S, Matsushita H, Chida K, Ishidoya S, Arai Y, Jingu K, Yamada S. Treatment outcome of high-dose image-guided intensity-modulated radiotherapy using intra-prostate fiducial markers for localized prostate cancer at a single institute in Japan. Radiat Oncol, 2012; 7: 105. [DOI] [PMC free article] [PubMed]
- 4).Yamazaki H, Nakamura S, Nishimura T, Yoshida K, Yoshioka Y, Koizumi M, Ogawa K. Transitioning from conventional radiotherapy to intensity-modulated radiotherapy for localized prostate cancer: changing focus from rectal bleeding to detailed quality of life analysis. J Radiat Res, 2014; 55: 1033–1047. [DOI] [PMC free article] [PubMed]
- 5).Valdagni R, Kattan MW, Rancati T, Yu C, Vavassori V, Fellin G, Cagna E, Gabriele P, Mauro FA, Baccolini M, Bianchi C, Menegotti L, Monti AF, Stasi M, Giganti MO, Fiorino C. Is it time to tailor the prediction of radio-induced toxicity in prostate cancer patients? Building the first set of nomograms for late rectal syndrome. Int J Radiat Oncol Biol Phys, 2012; 82: 1957–1966. [DOI] [PubMed]
- 6).Terk MD, Stock RG, Stone NN. Identification of patients at increased risk for prolonged urinary retention following radioactive seed implantation of the prostate. J Urol, 1998; 160: 1379–1382. [PubMed]
- 7).Nag S, Beyer D, Friedland J, Grimm P, Nath R. American Brachytherapy Society (ABS) recommendations for transperineal permanent brachytherapy of prostate cancer. Int J Radiat Oncol Biol Phys, 1999; 44: 789–799. [DOI] [PubMed]
- 8).Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys, 1995; 31: 1341–1346. [DOI] [PubMed]
- 9).Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant, 2013; 48: 452–458. [DOI] [PMC free article] [PubMed]
- 10).Sridharan S, Dal Pra A, Catton C, Bristow RG, Warde P. Locally advanced prostate cancer: current controversies and optimisation opportunities. Clin Oncol (R Coll Radiol), 2013; 25: 499–505. [DOI] [PubMed]
- 11).Feigenberg SJ, Hanlon AL, Horwitz EM, Uzzo RG, Eisenberg D, Pollack A. Long-term androgen deprivation increases Grade 2 and higher late morbidity in prostate cancer patients treated with three-dimensional conformal radiation therapy. Int J Radiat Oncol Biol Phys, 2005; 62: 397–405. [DOI] [PubMed]
- 12).Mason M, Maldonado Pijoan X, Steidle C, Guerif S, Wiegel T, van der Meulen E, Bergqvist PB, Khoo V. Neoadjuvant androgen deprivation therapy for prostate volume reduction, lower urinary tract symptom relief and quality of life improvement in men with intermediate- to high-risk prostate cancer: a randomised non-inferiority trial of degarelix versus goserelin plus bicalutamide. Clin Oncol (R Coll Radiol), 2013; 25: 190–196. [DOI] [PubMed]
- 13).Malik R, Jani AB, Liauw SL. External beam radiotherapy for prostate cancer: urinary outcomes for men with high International Prostate Symptom Scores (IPSS). Int J Radiat Oncol Biol Phys, 2011; 80: 1080–1086. [DOI] [PubMed]