ABSTRACT
Carbon ion radiotherapy has recently emerged as an alternative choice of treatment for malignant tumors of the head and neck. However, it is still in the infant stages and its influence on subsequent salvage surgery remains unclear. Here we report the case of a 43-year-old woman who underwent salvage surgery for left frontal bone osteosarcoma recurrence following carbon ion radiotherapy. Tumor resection was performed with a wide margin including the tissue considered to have been damaged by carbon ion radiotherapy. The dural defect was reconstructed using a fascia lata graft and pedicled galeal pericranial flap. The soft tissue defect was reconstructed using an anterolateral thigh flap anastomosed to the ipsilateral neck interposed by the radial forearm flap. As the patient developed no postoperative wound complications, she was able to initiate adjuvant chemotherapy early. Carbon ion radiotherapy is useful for its focused distribution and strong biological effects. Although the affected field may be limited, its high potency may severely damage adjacent normal tissue and lead to serious postoperative complications. Despite these concerns, satisfactory results were achieved in this case.
Key Words: carbon ion radiotherapy, salvage surgery, microsurgery, skull base, osteosarcoma
Surgery and radiation therapy are the two main treatment options for head and neck malignant tumor. Recently, carbon ion radiotherapy has emerged as an alternative choice of treatment. Carbon ion radiotherapy is useful given its focused distribution and strong biological effects. However, its high potency may severely damage adjacent normal tissue, although the damaged region is limited. Consequently, this modality may pose serious risks to subsequent salvage surgery.
Here we report an unusual case of salvage skull base surgery with free flap reconstruction for recurrence of osteosarcoma after carbon ion radiotherapy.
CASE REPORT
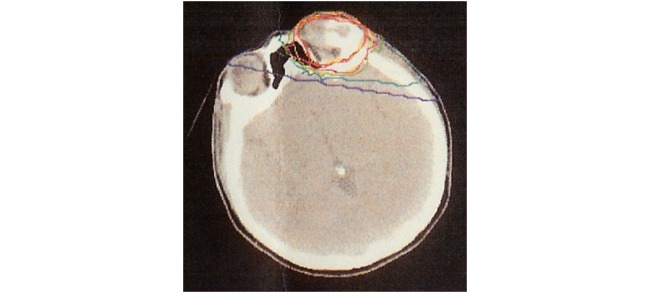
A 43-year-old woman developed recurrent osteosarcoma in the left supraorbital area. While originally recommended to undergo surgery, she declined and underwent carbon ion radiotherapy at another institution. A total dose of 70.4 gray equivalents (GyE) in 16 fractions was administered over 4 weeks. Irradiation was performed according to dose distributions based on planning computed tomography (CT) (Figure 1). Stable disease was maintained for 15 months after primary treatment.
Fig. 1.
Carbon ion dose distribution. The yellow line indicates the target volume; red line, 100% dose; pink line, 95% dose; orange line, 90% dose; green line, 50% dose; blue line, 30% dose; and purple line, 10% dose.
Physical examination revealed a hard, 2×4 cm non-tender mass located in the left supraorbital area. The protrusion measured 1 cm in length. The area of skin that had been exposed to carbon ion irradiation was slightly red. Vision in her left eye was poor due to cataract, and her left frontal lobe was edematous, possibly due to a reaction to carbon ion radiotherapy. She was administered a steroid for her frontal lobe edema.
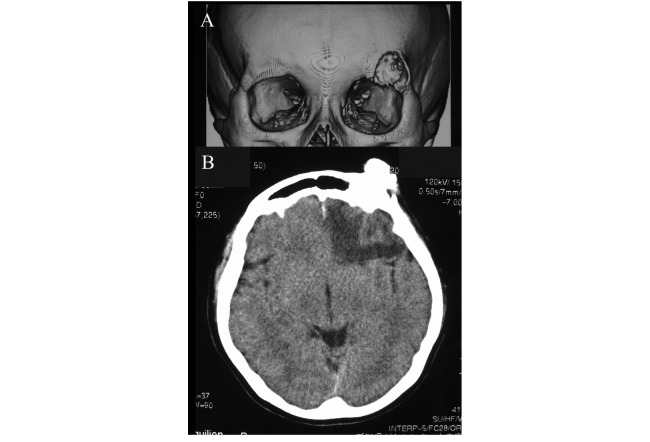
CT of the head revealed a 1.8×2.5 cm frontal bone mass (Figures 2A, B). A low-density area indicated edema in the left frontal lobe (Figure 2B). Compared to the images obtained after treatment, the mass was larger in size, with increased calcification. Needle biopsy was performed, and the pathologic diagnosis of osteosarcoma was confirmed. Stable disease was maintained for a prolonged period; the mass was clinically diagnosed as recurrence of osteosarcoma rather than residual disease.
Fig. 2.
Computed tomography scan of the head showing a frontal bone mass. (A) The mass was located in the left supraorbital area. (B) Low-density area indicated edema in the left frontal lobe.
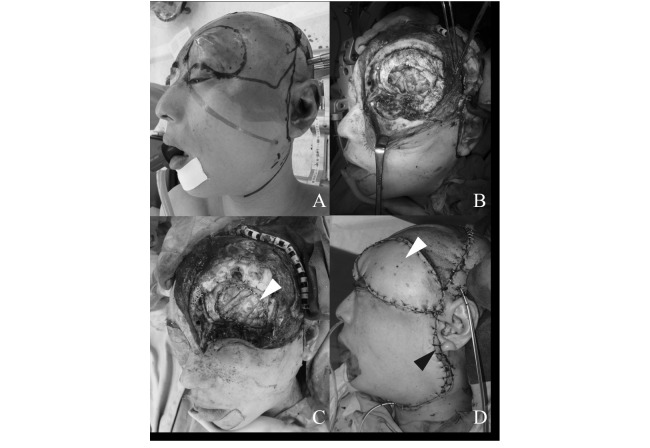
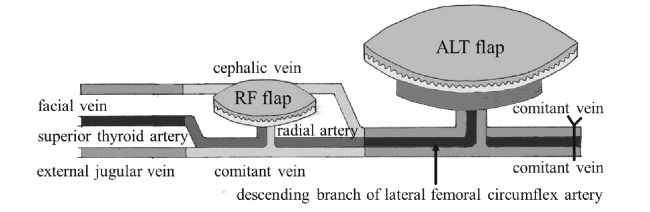
The patient underwent wide resection of the mass and normal tissue considered to have been damaged by carbon ion radiotherapy. Wide resection was performed with a 2-cm margin from the edge of swelling (Figure 3A), and included the left eyeball, part of the frontal bone, dura mater, the left frontal skull base, and the region of reddish tissue damaged by carbon ion radiotherapy (Figure 3B). The dural defect was reconstructed with a free non-vascularized fascia lata graft (Figure 3C). A galeal pericranial flap pedicled on the ipsilateral superficial temporal artery was applied over the fascia lata. Although reconstruction of the defect required free tissue transfer, the distance between the defect and recipient vessels was too long. To overcome this problem, we harvested a radial forearm (RF) flap and anterolateral thigh (ALT) flap. The RF flap was anastomosed end-to-end to the recipient vessels in the ipsilateral neck (superior thyroid artery, external jugular vein, and facial vein), and the ALT flap was anastomosed end-to-end to the distal end of the RF flap (Figure 3D, 4).
Fig. 3.
(A) Wide resection with a 2-cm margin from the edge of swelling was performed. (B) The left eye ball, dura mater, left frontal skull base, and the region of reddish tissue damaged by carbon ion radiotherapy were resected. (C) The dural defect was reconstructed using a free non-vascularized fascia lata graft (white arrow). (D) The soft tissue defect was reconstructed using a radial forearm flap (black arrow) and an anterolateral thigh flap (white arrow).
Fig. 4.
The RF flap was anastomosed end-to-end to recipient vessels in the ipsilateral neck, and the ALT flap was anastomosed end-to-end to the distal end of the RF flap.
The wound healed and the postoperative course was uneventful. Histopathological examination of the resected specimen revealed increased dysplastic spindle cells, leading to a histological diagnosis of osteoblastic osteosarcoma. The degree of reaction to carbon ion radiotherapy, such as fibrosis, was comparatively low. Systemic adjuvant chemotherapy with ifosfamide, carboplatin, and etoposide was administered for 4 months. The patient survived and has remained well for 2 years postoperatively (Figure 5).
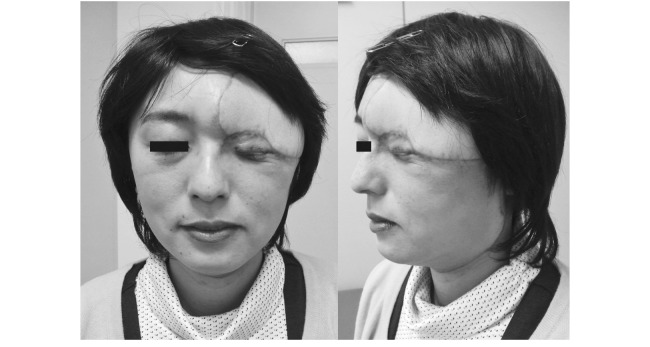
Fig. 5.
Two years postoperatively, the wound healed and the postoperative course was uneventful.
DISCUSSION
Carbon ion radiotherapy offers several physical and biological advantages over conventional photon radiotherapy. Physically, carbon ion beams allow for increased energy deposition with increasing depth, and the penetration depth achieves a sharp maximum at the end of its range to form a Bragg peak. Biologically, cellular damage caused by carbon ions is different from those caused by protons and photons, in that the rate of cancer cell recovery from sublethal damage is lower and variations in sensitivity by cell-cycle stage and changes in oxygen concentration are smaller. Carbon ion radiotherapy can also deliver a large mean energy per unit length of path in the body.1-4) Thus, strong biological effects can be achieved with improved dose distribution, thereby permitting dose escalation within the tumor region without causing extensive damage to neighboring normal tissue.
Our patient received carbon ion radiotherapy with a total dose of 70.4 GyE in 16 fractions over 4 weeks. This dose was determined based on a report by Kamada et al., who conducted a phase I/II dose escalation study on carbon ion radiotherapy in patients with bone and soft tissue sarcomas.5) They found that this irradiation schedule was appropriate for patients with subcutaneous tumor involvement. Furthermore, acute and late skin reactions were observed in all patients treated with the same total dose of 70.4 GyE.5)
With the exception of a skin reaction, Schulz-Ertner et al. also reported that 4% of patients who had undergone carbon ion radiotherapy with a prescribed target dose of 57–70 GyE (median, 60) for skull base chondrosarcoma developed white matter changes in the temporal lobe within the high-dose area near the tumor site without developing symptoms.6) Our patient presented with mild late skin toxicity, eye toxicity, and asymptomatic white matter changes on CT.
A few English reports have been published on salvage surgery cases following carbon ion radiotherapy.6, 7) However, the impact of preceding carbon ion radiotherapy on salvage surgery is not discussed and is currently unclear. Moreover, it is also unclear how postoperative complications can be reduced. Some studies involving conventional radiotherapy have been reported. For instance, Patel et al. conducted an international collaborative study on craniofacial surgery for malignant skull base tumors and found that prior history of radiotherapy was significantly associated with the development of postoperative complications.8) Thus, attention should be paid to both tumor resection and defect reconstruction.
One of the main problems with wide resection is the management of irradiated normal tissue adjacent to the tumor. Theoretically, carbon ion radiotherapy can deliver more focused beams to the tumor compared to other types of radiotherapy. However, its potency is so high that even the limited field adjacent to the tumor can be seriously damaged. The healing ability of irradiated normal tissue exhibiting mild skin reaction is unclear and should not be clinically disregarded. To avoid postoperative wound complications, it is better to resect tissue that has been "heavily injured" along with the tumor. Given the lack of pertinent literature, much weight was placed on safety and thus the tumor was resected with a wide safety margin in the present case. However, considering the nature of carbon ion radiotherapy, it may be sufficient to resect a more limited region if only taking into account tissue affected by carbon ion radiotherapy. In other words, although the resection was sufficiently conservative, there is the possibility of resecting slightly more than actually necessary. Actually, in our case, histopathological change as a reaction to carbon ion radiotherapy was low. Use of preoperative carbon ion radiotherapy has been reported in the fields of esophageal and pancreatic cancers by Akutsu et al. and Shinoto et al.9, 10) While these two studies used a low dose of carbon ion radiotherapy relative to ours and had a short duration between irradiation and surgery, they found little fibrosis and no adverse findings in the normal tissues of resected specimens in the radiation field. These studies also found that carbon ion radiotherapy did not affect peri- and postoperative morbidity.
Reconstruction in the present case involved dural and soft tissue reconstruction. Dural reconstruction is important in separating the oropharynx from the meninges and preventing cerebrospinal fluid leak, which is potentially life threatening. Accordingly, we utilized a fascia lata graft to achieve watertight dural closure, and applied a pedicled galeal pericranial flap with abundant blood supply over the fascia. As for soft tissue reconstruction, numerous studies have demonstrated that complication rates are acceptably low when free flaps are used to reconstruct large-volume defects, and furthermore, this technique has been proven effective for low-volume skull base defects.11-14) Aggressive use of microvascular repair is recommended in patients who have received a full course of radiation to the skull base.13, 15, 16) Free flaps, which provide highly vascularized and voluminous tissue, can fill dead space with separation of the oropharynx from meninges and fulfill the reconstructive goal of primary healing. Accordingly, we performed soft tissue reconstruction using an ALT flap, and to address the issue of long-distance, we used a RF flap as a bridge flap. The patient achieved primary wound healing with no postoperative complications.
CONCLUSION
We performed salvage and reconstructive surgery for the recurrence of osteosarcoma following carbon ion radiotherapy. The irradiated field was determined, and tumor resection was performed with a wide margin, including tissue considered to have been damaged by carbon ion radiotherapy. The defects were reconstructed using well-vascularized flaps. The patient developed no postoperative complications, including wound-healing delay, which enabled early initiation of adjuvant chemotherapy.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest concerning this article.
REFERENCES
- 1).Kanai T, Endo M, Minohara S, Miyahara N, Koyama-ito H, Tomura H, Matsufuji N, Futami Y, Fukumura A, Hiraoka T, Furusawa Y, Ando K, Suzuki M, Soga F, Kawachi K. Biophysical characteristics of HIMAC clinical irradiation system for heavy-ion radiation therapy. Int J Radiat Oncol Biol Phys, 1999; 44: 201–210. [DOI] [PubMed]
- 2).Suzuki M, Kase Y, Yamaguchi H, Kanai T, Ando K; Relative biological effectiveness for cell-killing effect on various human cell lines irradiated with heavy-ion medical accelerator in Chiba (HIMAC) carbon-ion beams. Int J Radiat Oncol Biol Phys, 2000; 48: 241–250. [DOI] [PubMed]
- 3).Koike S, Ando K, Oohira C, Fukawa T, Lee R, Takai N, Monobe M, Furusawa Y, Aoki M, Yamada S, Shimizu W, Nojima K, Majima H. Relative biological effectiveness of 290 MeV/u carbon ions for the growth delay of a radioresistant murine Fibrosarcoma. J Radiat Res, 2002; 43: 247–255. [DOI] [PubMed]
- 4).Smith IE, Courtenay VD, Mills J, Peckham MJ; In vitro radiation response of cells from four human tumors propagated in immune-suppressed mice. Cancer Res, 1978; 38: 390–392. [PubMed]
- 5).Kamada T, Tsujii H, Tsuji H, Yanagi T, Mizoe JE, Miyamoto T, Kato H, Yamada S, Morita S, Yoshikawa K, Kandatsu S, Tateishi A. Efficacy and safety of carbon ion radiotherapy in bone and soft tissue sarcomas. J Clin Oncol, 2002; 20: 4466–4471. [DOI] [PubMed]
- 6).Schulz-Ertner D, Nikoghosyan A, Hof H, Didinger B, Combs SE, Jäkel O, Karger CP, Edler L, Debus J. Carbon ion radiotherapy of skull base chondrosarcomas. Int J Radiat Oncol Biol Phys, 2007; 67: 171–177. [DOI] [PubMed]
- 7).Matsumoto T, Imagama S, Ito Z, Imai R, Kamada T, Shimoyama Y, Matsuyama Y, Ishiguro N. Total spondylectomy following carbon ion radiotherapy to treat chordoma of the mobile spine. Bone Joint J, 2013; 95: 1392–1395. [DOI] [PubMed]
- 8).Patel SG, Singh B, Polluri A, Bridger PG, Cantu G, Cheesman AD, deSa GM, Donald P, Fliss D, Gullane P, Janecka I, Kamata SE, Kowalski LP, Kraus DH, Levine PA, dos Santos LR, Pradhan S, Schramm V, Snyderman C, Wei WI, Shah JP. Craniofacial surgery for malignant skull base tumors: report of an international collaborative study. Cancer, 2003; 98: 1179–1187. [DOI] [PubMed]
- 9).Akutsu Y, Yasuda S, Nagata M, Izumi Y, Okazumi S, Shimada H, Nakatani Y, Tsujii H, Kamada T, Yamada S, Matsubara H. A phase I/II clinical trial of preoperative short-course carbon-ion radiotherapy for patients with squamous cell carcinoma of the esophagus. J Surg Oncol, 2012; 105: 750–755. [DOI] [PubMed]
- 10).Shinoto M, Yamada S, Yasuda S, Imada H, Shioyama Y, Honda H, Kamada T, Tsujii H, Saisho H; Working Group for Pancreas Cancer. Phase 1 trial of preoperative, short-course carbon-ion radiotherapy for patients with resectable pancreatic cancer. Cancer, 2013; 119: 45–51. [DOI] [PubMed]
- 11).Chepeha DB, Wang SJ, Marentette LJ, Thompson BG, Prince ME, Teknos TN. Radial forearm free tissue transfer reduces complications in salvage skull base surgery. Otolaryngol Head Neck Surg, 2004; 131: 958–963. [DOI] [PubMed]
- 12).Neligan PC, Mulholland S, Irish J, Gullane PJ, Boyd JB, Gentili F, Brown D, Freeman J. Flap selection in cranial base reconstruction. Plast Reconstr Surg, 1996; 98: 1159–1166. [DOI] [PubMed]
- 13).Newman J, O'Malley BW Jr, Chalian A, Brown MT. Microvascular reconstruction of cranial base defects: An evaluation of complication and survival rates to justify the use of this repair. Arch Otolaryngol Head Neck Surg, 2006; 132: 381–384. [DOI] [PubMed]
- 14).Kraus DH, Shah JP, Arbit E, Galicich JH, Strong EW. Complications of craniofacial resection for tumors involving the anterior skull base. Head Neck, 1994; 16: 307–312. [DOI] [PubMed]
- 15).Chang DW, Langstein HN, Gupta A, De Monte F, Do KA, Wang X, Robb G. Reconstructive management of cranial base defects after tumor ablation. Plast Reconstr Surg, 2001; 107: 1346–1355. [DOI] [PubMed]
- 16).Georgantopoulou A, Hodgkinson PD, Gerber CJ. Cranial-base surgery: a reconstructive algorithm. Br J Plast Surg, 2003; 56: 10–13. [DOI] [PubMed]