Abstract
Since the 2006 European ban on the use of antibiotics as growth promoters in animal feed, numerous studies have been published describing alternative strategies to prevent diseases in animals. A particular focus has been on prevention of necrotic enteritis in poultry caused by Clostridium perfringens by the use of microbes or microbe-derived products. Microbes produce a plethora of molecules with antimicrobial properties and they can also have beneficial effects through interactions with their host. Here we review recent developments in novel preventive treatments against C. perfringens-induced necrotic enteritis in broiler chickens that employ yeasts, bacteria and bacteriophages or secondary metabolites and other microbial products in disease control.
Keywords: Clostridium perfringens, necrotic enteritis, broiler chicken, antimicrobials, probiotic, competitive exclusion, bacteriocin
Introduction
Clostridium Perfringens, the causative agent for necrotic enteritis
Clostridium perfringens is a spore-forming, anaerobic, Gram-positive bacterium, found in the environment and also in the gastro-intestinal (GI) tract of humans and animals (Songer, 1996; Van Immerseel et al., 2004; Popoff, 2013). It is one of the most common causes of foodborne illnesses in humans, but it also poses an important threat for animals (Uzal et al., 2010; Grass et al., 2013). Indeed, C. perfringens is responsible for severe infections in animals, such as enterotoxaemia, gangrenous dermatitis and necrotic enteritis (NE), especially in pigs and poultry (Songer, 1996; Van Immerseel et al., 2004; Timbermont et al., 2011).
C. perfringens strains can produce up to 17 different toxins (the majors toxins α, β, β2, ε, ι and the enterotoxin CPE), recently reviewed by Uzal et al. (2014). C. perfringens isolates are classified in 5 toxinogroups, based on their toxin production (Songer, 1996; Van Immerseel et al., 2009), each set of toxins being responsible for a specific disease (Uzal et al., 2010). For examples, type B strains, which produce α, β, and ε toxins cause lamb dysentery and type D strains, which only produce the α and ε toxins are responsible enterotoxaemia in those animals (Songer, 1996; Uzal et al., 2010, 2014; Popoff, 2013). In poultry, necrotic enteritis is caused mainly by type A strains, which produce the α toxin and the pore-forming toxin NetB (for NE B-like) (Engström et al., 2003; Keyburn et al., 2008; Cooper and Songer, 2009). The α toxin was long thought to be responsible for necrotic enteritis but several reports have since established that the NetB toxin alone can cause the disease (Keyburn et al., 2008, 2010; Van Immerseel et al., 2009).
Another notable mechanism contributing to the virulence of C. perfringens is the production of bacteriocins. Virulent strains of C. perfringens have been shown to inhibit the growth of other C. perfringens strains in order to take advantage during competition for nutrients (Barbara et al., 2008; Timbermont et al., 2009). Recently, Timbermont and colleagues identified perfrin, a novel 11.5 kDa bacteriocin that is produced by a NetB-positive strain isolated from a chicken with NE. Intriguingly, perfrin has no sequence homology to other bacteriocin proteins, suggesting that this is the paradigm for a new class of bacteriocin (Timbermont et al., 2014). It is likely that further bacteriocins remain to be discovered.
Necrotic enteritis and broiler chickens
C. perfringens-induced NE in chickens leads to sudden death, with mortality rates up to 50% (Kaldhusdal and Løvland, 2000; McDevitt et al., 2006; Lee et al., 2011b). More importantly, C. perfringens is also responsible for subclinical infections, associated with chronic damage of the intestinal mucosa. Such subclinical infections cause problems such as lower performance and reduced weight gain, which have dramatic economic consequences (Elwinger et al., 1992; Kaldhusdal et al., 2001; Skinner et al., 2010). The cost of NE worldwide was estimated to 2 billion dollars per year, which includes not only direct loss due to broilers deaths, but also veterinary and cleaning costs (Van der Sluis, 2000; Timbermont et al., 2011).
C. perfringens is almost always found in healthy chickens, although at levels less than 105 cfu/g intestinal content. The ability of the bacterium to cause disease is linked to several predisposing factors that affect intestinal conditions and create a favorable environment for proliferation. Perhaps the most important of these factors is the incidence of coccidiosis (Al-Sheikhly and Al-Saieg, 1980; Craven et al., 2001; Williams, 2005; Si et al., 2007). NE incidence and the mortality rates are higher when chickens are co-infected with Eimeria, a causal agent of coccidiosis (Shane et al., 1985; Baba et al., 1992). The feeding diet has been shown to be another factor favoring disease, through an influence on the properties of the intestinal content such as viscosity and the presence of non-digestible polysaccharides, the GI tract transit time and the intestinal pH (Annett et al., 2002; Drew et al., 2004; Moran, 2014). For example, diets rich in wheat or fish proteins are known to increase the risk of necrotic enteritis (Annett et al., 2002; Drew et al., 2004).
Animals are often infected through bacterial cells or spores present in their feed, from contaminated litter or by cross-contamination with infected animals at the early stages of life. Young animals, which have immature immune systems and no established commensal flora, are primarily at risk. Infected animals show severe lesions of the jejunum and ileum, the small intestine presenting a degenerated mucosa and being distended by gases produced by C. perfringens. Signs of infection include the animal looking depressed, moving less and having diarrhea, which is the most visibly obvious symptom. For a more detailed coverage of C. perfringens pathogenicity and clinical signs of NE, the reader is directed to a number of other articles (Helmboldt and Bryant, 1971; Van Immerseel et al., 2004; Timbermont et al., 2011; Paiva and McElroy, 2014).
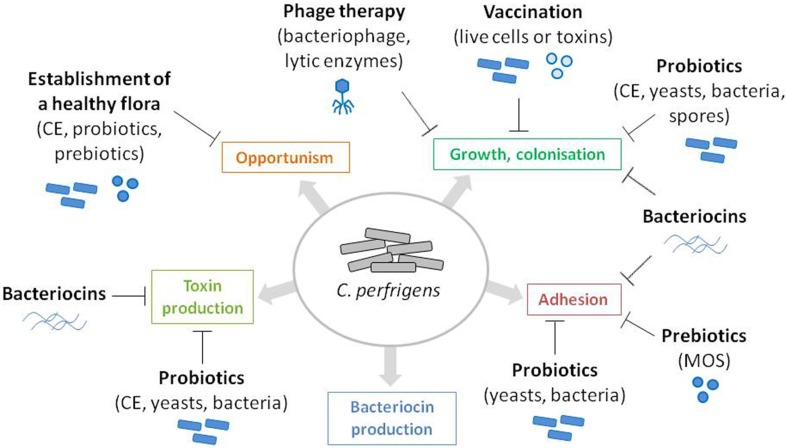
The rapid death (within 24 h) of chickens with NE often prevents the treatment of the disease. Antibiotics have been commonly used worldwide as growth promoters and for prophylactic treatment of C. perfringens-induced NE in poultry. However, with the European ban on antibiotics (feed additives regulation 1831/2003/EC), which took effect in January 2006, alternatives to antibiotics became essential in order to prevent NE occurrence and the consequent economic losses for the poultry industry. Preventive treatments can take the form of actions on predisposing factors, such as coccidiosis prevention, diet modifications, or improving overall cleanliness and hygiene. Alternatively they can directly target the causal agent of the disease by controlling the proliferation, colonization and persistence of virulent strains of C. perfringens or interfering with virulence and pathogenicity factors (Figure 1). C. perfringens infections can be reduced or abolished by using natural feed additives, such as probiotics (yeasts or bacteria), plants (Engberg et al., 2012), molecules of plant origin [for example, essential oils (Mitsch et al., 2004; Timbermont et al., 2010) or Annatto extracts (Galindo-Cuspinera et al., 2003)], organic acids (Geier et al., 2010; Timbermont et al., 2010), enzymes (Jackson et al., 2003; Engberg et al., 2004), lysozyme (Liu et al., 2010), or molecules of microbial origin, such as yeast extract and antimicrobial peptides (Figure 1). Here we give an overview of these preventive treatments, by focusing on micro-organisms and molecules or products of microbial origins that affects C. perfringens growth and pathogenicity.
Figure 1.
Identification of C. perfringens virulence and pathogenicity factors as potential targets for NE prevention. C. perfringens virulence and pathogenicity factors are represented as colored boxes. Antagonistic action of the microbes and microbe-derived products discussed in this review are represented by flat-end arrows.
Feeding “live” bacteria and yeasts
Supplementation of the broilers' diet with one or several beneficial bacteria has proven to be efficient to prevent the overgrowth of pathogens and the subsequent diseases. Several bacterial strains have been shown to increase broiler chickens performance (health, weight gain, feed conversion) and to prevent or reduce the incidence of diseases caused by pathogenic bacteria (reviewed by Patterson and Burkholder, 2003; Lutful Kabir, 2009; Chaucheyras-Durand and Durand, 2010). Probiotics, or direct-fed microbials, and competitive exclusion (CE) cultures are thus commonly used in broiler farms. There are several commercially available products that have been shown to be efficient against C. perfringens and NE in poultry (Table 1).
Table 1.
Examples of commercially available microbial feed additives for NE prevention in poultry.
| Product | Company | Composition | Origin | Activity | Selected references |
|---|---|---|---|---|---|
| Aviguard® | MSD Animal Health | Over 200 bacterial species | Healthy, adult chickens | Competitive exclusion | Hofacre et al., 1998 |
| BROILACT® | Nimrod Veterinary products | Complex mixture of bacteria | Intestine of a normal adult fowl | Competitive exclusion | Kaldhusdal et al., 2001 |
| PoultryStar® | Biomin | 6 bacterial species and 1 prebiotic (FOS) | Unknown | Competitive exclusion | McReynolds et al., 2009 |
| MSC™ | Continental Grain Co. | Bacteria | Caeca and caecal sections | Competitive exclusion | Craven et al., 1999 |
| Finelact™ | QTI Animal Health | L. reuteri | Live, healthy chicken | Probiotic | Tested in a field trial (manufacturer's product data) |
| FloraMax® B-11 | Pacific Vet Group, USA | 11 lactic acid bacteria and inactivated Saccharomyces cerevisiae | Poultry intestine | Probiotic | Layton et al., 2013 |
| NuPro® | Alltech Inc. | Yeast extract | Yeast | Immunostimulation, antimicrobial activity | Thanissery et al., 2010 |
| SafMannan® | Phileo Lesaffre Animal Care | Yeast Extract | S. cerevisiae | Immunostimulation, antimicrobial activity | Abudabos and Yehia, 2013 |
Probiotics
A probiotic is defined as “a live microbial food supplement that beneficially affects the host by improving the intestinal microbial balance” (Fuller, 1999). Indeed, probiotics can interact with the host to improve immunity and intestinal morphology or stimulate the metabolism, thus reducing the risk of infection by opportunistic pathogens. Probiotic bacteria have also been shown to produce molecules with antimicrobial activities, such as bacteriocins, that target specific pathogens, or even inhibit the adhesion of pathogens or the production of pathogenic toxins (Joerger, 2003; Pan and Yu, 2014). Moreover, beneficial bacteria can act as competition against pathogenic strains within the host. The concept of competitive exclusion will be discussed further below. For the purpose of this section, we have chosen to focus on strains that were shown to have an effect on NE incidence in poultry, through a targeted antagonistic activity against C. perfringens.
A large number of studies described the isolation of microorganisms with anti-C. perfringens activity in vitro (Table 2). Most of these strains belong to the genera Bacillus and Lactobacillus. Very few reports discussed the deployment of live yeasts with antagonistic activity against C. perfringens, their use in NE prevention being limited to inactivated yeast or yeast extract (Tables 1, 2).
Table 2.
Examples of probiotic strains with anti-C. perfringens activity in vitro and in vivo.
| Strain | Origin | Anti-Cp activity in vitro | Anti-Cp activity in vivo (poultry model) | Mode of action | References |
|---|---|---|---|---|---|
| Bacillus | |||||
| Bacillus cereus 8A | n.s. | + | n.t. | Bacteriocin | Bizani and Brandelli, 2002 |
| Bacillus licheniformis | Broiler GI tract | + | n.t. | n.s. | Barbosa et al., 2005 |
| n.s. | n.t. | + | Spores | Knap et al., 2010 | |
| B. pumilus | Broiler GI tract | + | n.t. | n.s. | Barbosa et al., 2005 |
| B. subtilis | Broiler GI tract | + | n.t. | n.s. | Barbosa et al., 2005 |
| Porcine intestine | + | n.t. | Bacteriocin | Klose et al., 2010 | |
| Healthy chicken GI tract | + | + | Protein | Teo and Tan, 2005; Jayaraman et al., 2013 | |
| n.s. | − | + | Spores | La Ragione and Woodward, 2003 | |
| Enterococci | |||||
| E. faecium | Porcine intestine | + | n.t. | Lactate and H2O2 | Klose et al., 2010 |
| Fermented food | + | n.t. | 3,000 Da BLIS | Chen et al., 2007 | |
| Broiler GI tract | + | n.t. | Enterocin A/B | Shin et al., 2008 | |
| n.s. | + | n.s. | Cao et al., 2013 | ||
| E. faecalis | Human | + | n.t. | Bacteriocin | Bottone et al., 1974 |
| Human | + | n.t. | n.s. | Stark, 1960 | |
| n.s. | −a | + | Toxin inhibition | Fukata et al., 1991 | |
| E. durans | Human | + | n.t. | n.s. | Stark, 1960 |
| Bifidobacteria | |||||
| B. animalis ssp lactis | Commercial strain | + | n.t. | NS molecule | Schoster et al., 2013 |
| B. infantis | n.s. | + | n.t. | n.s. | Gibson and Wang, 1994 |
| B. thermoacidophilum | Porcine intestine | + | n.t. | Lactate and H2O2 | Kim et al., 2007; Klose et al., 2010 |
| Lactobacilli | |||||
| Lactobacillus sp. | Chicken feces | + | + | n.s. | Gérard et al., 2008 |
| L. acidophilus | n.s. | −a | + | Toxin inhibition | Fukata et al., 1991 |
| L. amylovorus | Porcine intestine | + | n.t. | Lactate and H2O2 | Kim et al., 2007; Klose et al., 2010 |
| L. animalis | Dog feces | + | +b | n.s. | Biagi et al., 2007 |
| L. fermentum | Porcine epithelium | + | n.t. | Toxin inhibition | Allaart et al., 2011 |
| Reference strain | n.t. | +c | n.t. | Cao et al., 2012 | |
| L. johnsonii FI9785 | Poultry | − | + | n.t. | La Ragione et al., 2004 |
| L. mucosae | Porcine intestine | + | n.t. | Lactate and H2O2 | Klose et al., 2010 |
| L. plantarum | Commercial strain | + | n.t. | BS molecule | Schoster et al., 2013 |
| L. reuteri | Porcine intestine | + | n.t. | Lactate and H2O2 | Kim et al., 2007; Klose et al., 2010 |
| L. salivarius | Chicken intestine | + | n.t. | Lactate and H2O2 | Kim et al., 2007; Klose et al., 2010 |
Active against toxin production.
Active in a canine model.
Reduced inflammation.
n.s., not specified; n.t., not tested; BLIS, bacteriocin-like inhibitory substance; NS, narrow spectrum; BS, broad spectrum.
Yeasts
Despite being under-represented in the literature as anti-C. perfringens agents, yeasts are known to have antimicrobial properties, which were recently reviewed (Hatoum et al., 2012). In addition the cell wall is, for many types of yeast, rich in beta-glucans, which have immunomodulatory properties (Novak and Vetvicka, 2008). On top of the beneficial effects they have on the host, yeasts can constitute a protection against pathogens by (i) producing mycocins, (ii) secreting enzymes that degrade bacterial toxins, (iii) preventing adhesion to epithelial cells, or (iv) by acting as a competitive exclusion agent (reviewed by Hatoum et al., 2012). For example, Debaromyces hansenii secretes molecules with anti-C. butyricum activity (Fatichenti et al., 1983), and Saccharomyces boulardii secretes a serine protease that inhibits the action of C. difficile toxins in vivo and in vitro (Castagliuolo et al., 1996, 1999).
Field trials using live S. boulardii as feed additives obtained positive results on performance and intestinal health improvement in healthy chickens (Rajput et al., 2013) and in chickens infected with Salmonella Enteritidis (Gil de los santos et al., 2005). Moreover, another study by the same authors showed that using a recombinant strain of Pichia pastoris carrying the gene coding for the C. perfringens α toxin, not only improved broiler chickens performance, but also increased the secretion of anti-C. perfringens antibodies (Gil de los santos et al., 2012). It would be interesting to test the effects of these strains on the mortality and C. perfringens counts in C. perfringens-induced NE challenged birds. It is also worth noting that two fungi of the genus Fusarium were reported to produce mycocins with anti-C. perfringens activity (enniatin B of F. tricinctum and the beauvericin of F. proliferatum), which were active at low concentrations (20 μg/ml and 0.1 μg/ml, respectively; Meca et al., 2010, 2011).
Bacillus species
Several strains of Bacillus have been shown to have antagonistic activity against C. perfringens (Table 2). In most studies, the activity was linked to the production of bacteriocins. Indeed, within the Bacillus genus, several species are known to produce bacteriocins and antimicrobial peptides (Stein, 2005; Lee and Kim, 2011; Mongkolthanaruk, 2012; Cochrane and Vederas, 2014). For example, B. thuringiensis produces thuricin which is active against C. difficile (Rea et al., 2010).
The antagonistic species described in the literature include B. cereus, B. licheniformis, B. pumilus, and B. subtilis, which was the most represented. In a study involving over 200 Bacillus strains isolated from broiler feces, Barbosa et al. (2005) identified several species (licheniformis, pumilus, subtilis) with activity against C. perfringens in vitro (Barbosa et al., 2005). A Bacillus cereus strain, isolated from a soil sample in Brazil, also showed antagonism against C. perfringens. The activity of the strain was ascribed to the production of a bacteriocin during the exponential phase of growth (Bizani and Brandelli, 2002). Teo and Tan (2005) isolated B. subtilis strain SP6 and showed that it had anti-C. perfringens activity in vitro (Teo and Tan, 2005). The authors identified the molecule responsible for the antagonistic activity as a 960–983 Da molecule of proteinacious nature that was highly heat-stable (Teo and Tan, 2005). The same strain was used in a NE challenge field trial involving 216 chicks and was shown to reduce mortality by half, to improve intestinal health (as measured by villi length) and to significantly reduce C. perfringens counts (Jayaraman et al., 2013).
The supplementation of animal feed with Bacillus spores was also tested and proven to be an efficient alternative to the use of antibiotics. When 20 day old chicks, inoculated with low amounts of C. perfringens, were given a single dose of 109 B. subtilis spores, colonization and persistence of C. perfringens were abolished, although the B. subtilis strain alone was shown to be unable to affect C. perfringens in vitro (La Ragione and Woodward, 2003). In another field trial, Knap et al. (2010) tested the effect of adding B. licheniformis spores to the diet, but used larger amounts and for longer periods of time (Knap et al., 2010). They observed increased performance and reduced mortality in the group of chicks treated with the spores.
Enterococci
A strain of E. faecium when fed to chicks on day of hatch was shown to reduce numbers of C. perfringens along with other pathogens after 28 days, and concomitantly to increase the counts of lactic acid bacteria (Lactobacilli and Bifidobacteria) (Cao et al., 2013). Klose et al. (2010) tested a number of Enterococcus strains, isolated from various animals, for their antagonism against C. perfringens and found that almost all had anti-C. perfringens activity, which could be attributed to the production of acids and hydrogen peroxide (Klose et al., 2010). Enterococci are known to produce a wide-range of bacteriocins, called enterocins, which are active against Gram-positive and Gram-negative bacteria (Franz et al., 2007). Shin et al. (2008) isolated a strain of E. faecium from broiler intestines that was active against C. perfringens in vitro, and identified the antimicrobial molecules as enterocins, with high homology to enterocins A and B (Shin et al., 2008). Strains of E. faecalis were also active against C. perfringens in vivo (Stark, 1960; Bottone et al., 1974; Fukata et al., 1991) (Table 2). One strain even prevented C. perfringens proliferation in vivo and inhibited α toxin production in vitro (Fukata et al., 1991). However, the potentially pathogenic nature of E. faecalis could prevent its use as a probiotic feed additive.
Lactic acid bacteria
Lactic acid bacteria (LAB) are also very good probiotic candidates, as they display antimicrobial activities, but also have beneficial effects for the host. Cao et al. (2012) showed that adding L. fermentum I.2029 to the diet of young chicks reduced the occurrence of C. perfringens-induced ileal lesions and inflammation. However, the effect on C. perfringens numbers was not measured in this study. Cao and colleagues also showed that the addition of the probiotic stimulated the host immune system, as seen by increased levels of cytokine expression, measured by real-time PCR (Cao et al., 2012). Many LAB isolates have exhibited anti-C. perfringens activity in vitro (Table 2). For example, in a screening experiment involving 104 Lactobacillus strains isolated from geese feces, 84 strains were active against C. perfringens (Dec et al., 2014). Related to that, Kim et al. (2007) isolated several LAB (Lactobacillus and Bifidobacterium) from pig intestines that had antagonistic action against C. perfringens, with an L. amylovorus strain presenting properties amenable to be a potential probiotic candidate (Kim et al., 2007). The antimicrobial action of LAB is often attributed to the secretion of bacteriocins or the production of organic acids. Schoster et al. (2013) tested the inhibitory activities of several commercial strains against C. perfringens in vitro. These authors identified 2 LAB strains, L. plantarum, and B. animalis spp. lactis, with antagonism against reference strains, but also against clinical isolates (Schoster et al., 2013). The L. plantarum strain was active against Gram negative and Gram positive strains, whereas the Bifidobacterium isolate had a narrower spectrum of activity (Schoster et al., 2013).
The ability of a potential probiotic strain to survive in the host without affecting the bacterial balance or the beneficial flora is of major importance. In this regard, the in vitro studies that report the isolation of potential probiotic strains almost always test the strain for their ability to survive in the host and exert their action in vivo, through assessment of acid and bile resistance, auto-aggregation and adhesion to epithelial cells. A strain of Lactobacillus species that reduced C. perfringens numbers in chickens was shown not to affect the commensal flora (Gérard et al., 2008). In a trial conducted by La Ragione et al. (2004), 20 day old chicks were fed a strain of L. johnsonii (FI9785). This strain was able persist in the chicks for the duration of the experiment, caused a reduced colonization by C. perfringens after 15 days, although no direct anti-C. perfringens activity was evident in vitro (La Ragione et al., 2004). Layton and colleagues tested the efficacy of the probiotic FloraMax-B11 (FM-B11), which consists of several LAB strains (Table 1) on chicks challenged with E. maxima, S. typhimurium and C. perfringens (Layton et al., 2013). The chicks started receiving the probiotic on day 14 and were infected 7 days later. After 10 days, they observed a high reduction of mortality in the group treated with FM-B11, along with reduced intestinal lesions and C. perfringens counts. Furthermore, FM-B11 was shown to be active against C. perfringens in vitro (Layton et al., 2013). Allaart et al. (2011) described an example of an antagonistic action that targeted pathogenicity via the inhibition of toxin production. A probiotic strain of L. fermentum of porcine origin negatively regulated the production of the β2 toxin by C. perfringens, without affecting cell growth (Allaart et al., 2011). The action of L. fermentum on β2 toxin production appeared to occur at the transcription level and was exerted through an effect on the environmental pH. The exact mechanism is however still unclear and it is not known whether the same effect would be observed in vivo (Allaart et al., 2011).
Competitive exclusion
The concept of competitive exclusion (CE) was originally described by Nurmi and Rantala in 1973, when feeding young chicks with bacteria isolated from a healthy adult chicken prevented colonization by Salmonella infantis (Nurmi and Rantala, 1973; Rantala and Nurmi, 1973). The exact mechanisms of action of CE remain unclear. However, it is now well known that implanting a “healthy” flora in the early days of life accelerates the establishment of the intestinal flora and creates a competition for nutrients within the intestine, thus preventing colonization by pathogens (Joerger, 2003; Schneitz, 2005). Moreover, the beneficial effects can be due to the intrinsic properties of the bacteria composing the mixture, as described above in the “Probiotics” section. CE products were initially described against Salmonella in chickens. Since then several studies focusing on the effect of CE to prevent C. perfringens-associated CE have been published, and several commercial products with proven effects on C. perfringens-induced NE are available (Table 1).
The first reports discussing the use of caecal contents from healthy chickens to prevent NE in young chicks date from the early 80 s. Barnes et al. (1980) described experiments in which 1-day-old chicks were fed caecal samples from healthy hens, containing, among others, several Lactobacillus, Streptococcus faecalis, S. faecium (now called Enterococcus faecalis and E. faecium) and Bacteroides hypermegas (Barnes et al., 1980). After 3 days, they observed a reduction in the number of C. perfringens, ranging from 100 to 1000 times lower in treated animals. Since then, several reports have been published, reporting a globally better intestinal health, a reduction in the number of C. perfringens and lower mortality, after administration of a CE product. The composition and efficacy of commercialized CE cultures have been the focus of several studies (Table 1). Elwinger and colleagues showed that the use of Broilact® reduced the mortality and occurrence of NE, with less C. perfringens in the caecum of animals in the treated group (Elwinger et al., 1992). Another field trial involving Broilact® was performed by Kaldhusdal et al. (2001) where chicks were sprayed with this CE product on the day of hatch. They observed a reduction in C. perfringens counts, lower incidence of NE and NE-associated gut lesions, with a most significant effect observed in the early weeks of life (Kaldhusdal et al., 2001). Another research group tested the efficacy of MSC™ (for Mucosal Starter Culture) which is another commercial product consisting of bacteria isolated from the gut of a healthy chicken (Craven et al., 1999). They used several virulent strains of C. perfringens to challenge the animals and fed them MSC in their first 3 weeks of life; interestingly, although they did not observe an effect on the number of C. perfringens, they detected less enterotoxin in the treated group compared to the control one, suggesting a selection for less virulent C. perfringens strains. In another trial, they additionally fed the birds a diet known to predispose them to NE, which resulted in a reduction in toxins present and but also in the numbers of C. perfringens (Craven et al., 1999). Hofacre et al. (1998) performed a trial involving 900 chicks which were challenged with C. perfringens and E. acervulina when 14 days old. The chicks were treated with Aviguard®, two other CE products and one probiotic 3 days later. They observed reduced mortality in the chickens treated with CE cultures compared to the ones that only received the probiotic. Moreover, the CE-treated animals had a reduction in lesions to the intestinal mucosa and displayed overall increased feed conversion (Hofacre et al., 1998). The same researchers later tested a different CE product containing L. acidophilus, L. plantarum, E. faecium and Pediococcus acidilactia (All-Lac XCL, Alltech) in combination with a prebiotic (MOS, Alltech) and observed a reduction of mortality by half compared to the untreated animals, with effects comparable to those of bacitracin (Hofacre et al., 2003). McReynolds et al. (2009) also tested the effects of CE cultures in association with a prebiotic containing essential oils and fructo-oligosaccharides (FOS) on chicks given an immunosuppressant vaccine, inoculated with C. perfringens and in dietary conditions favorable to NE development (McReynolds et al., 2009). Both the prebiotic and the CE cultures led to reduced C. perfringens counts, a reduction in the intestinal lesions and lower mortality (McReynolds et al., 2009). Overall, the use of CE cultures in combination with other products, probiotics and prebiotics that have a more targeted action on C. perfringens appears to be more effective to prevent NE occurrence in poultry.
Molecules of microbial origin
Prebiotics
Prebiotics are additives that will stimulate the commensal flora and enhance the beneficial effects of probiotics within the host and are mostly indigestible oligosaccharides (Patel and Goyal, 2012). Numerous molecules have been described, with mannan-oligosaccharides (MOS) being the main prebiotic of microbial origin. MOS are components within the yeast cell wall and constitute the main active ingredient of yeast extract (YE) for disease control. They are often used as feed additives in broiler diets (Table 1) where they have been shown to improve intestinal health and immune response, and also inhibit pathogen colonization by reducing adhesion. The addition of MOS to broiler feed was shown to improve overall performance as measured by productivity and weight gain (Fowler et al., 2015). Thanissery and colleagues tested the effect of adding 2% yeast extract (NuPro, Alltech) to broiler feed, for the first 10 days of life, before a challenge with type A C. perfringens strains (Thanissery et al., 2010). Overall, animals treated with NuPro had fewer lesions in the duodenum compared to the untreated ones, to a degree comparable to the group treated with the antibiotic bacitracin (Thanissery et al., 2010). The C. perfringens counts were one to two logs lower in the treated groups; however, the difference was not statistically significant; the authors suggested the use of NuPro for longer periods in order to improve its efficiency. Recently, Abudabos and Yehia (2013) tested another commercial yeast extract additive, Saf-Mannan, in a field trial for its ability to protect broiler chickens against NE (Abudabos and Yehia, 2013). They performed a C. perfringens challenge on 16 days-old birds that were fed 0.05% Saf-Mannan since hatching, and compared their performance, gut health and C. perfringens counts on day 30. The chicks that were given the yeast extract showed overall better intestinal health (based on villi height) and had improved performance (measured by body weight gain and feed conversion ratio), which are consistent with the known beneficial effect of yeast extract on broiler performance. Moreover, the animals treated with Saf-Mannan had a 5 log reduction of C. perfringens numbers in the small intestine in comparison to the untreated animals (Abudabos and Yehia, 2013). However, caution is required when discussing the anti-C. perfringens of MOS or YE, as the antagonistic effect seems to be highly variable and dependent on a number of variables, such as dose, length of treatment or even diet (Biggs et al., 2007; Jacobs and Parsons, 2009; Kim et al., 2011).
Bacteriocins
Bacteriocins are small ribosomally synthesized antimicrobial peptides that are produced by a large number of bacteria. They are classified based on their size, structure and post-translational modifications (Cotter et al., 2013). One of the main benefits of the use of bacteriocins is that some of them present a highly specific antimicrobial activity, so that they can be used to treat specific infections without altering the commensal gut flora. As discussed previously, the action of many probiotic strains is exerted through the secretion of bacteriocins (Table 2). Several examples of well-described bacteriocins with beneficial effects for broilers can be found. These include pediocin A, produced by Pediococcus pentosaceus, and divercin of Carnobacterium divergens, which were shown to improve broiler performance in a field trial (Grilli et al., 2009; Józefiak et al., 2012) as well as the well-characterized nisin produced by Lactococcus lactis that was shown to affect C. perfringens cells and spores in vitro (Udompijitkul et al., 2012). A strain of Ruminococcus gnavus, isolated from an healthy human feces, was shown to produce a 2.6 kDa bacteriocin (Ruminococcin A, class IIA lantibiotics) that was highly active against C. perfringens in vitro (MIC = 75 μg/ml) (Dabard et al., 2001). However, ruminococcin A is poorly expressed in vivo as tested in R. gnavus-inoculated rats, a potential limit on its usefulness (Crost et al., 2011). In contrast, another bacteriocin (Ruminococcin C) identified by the same research group, which was active against C. perfringens with a MIC of 40 μg/ml, was expressed in vivo (Crost et al., 2011). Lee et al. (2011a) identified an anti-C. perfringens lantibiotic produced by Bifidobacterium longum that had a broad range of inhibition. Sharma and colleagues identified a strain of Brevibacillus borstelensis with anti-C. perfringens activity that was associated with a thermostable bacteriocin-like inhibitory substance (BLIS) of 12 kDa, which was active under the physiological conditions expected in the GI tract (Sharma et al., 2014).
The use of purified bacteriocins or the producing strains as feed additives represents a realistic alternative to conventional antibiotics. Thorough characterizations are required, however, to confirm the synthesis and the integrity of the molecule in GI tract conditions. Moreover, the potential development of resistance in the C. perfringens target organism needs to be taken into account.
Bacteriophages
Bacteriophages are highly species-specific viruses that infect and kill bacteria. Upon replication within the bacterial cell, phages produce endolysins, which target peptidoglycans and lyse the bacterial cell wall, freeing the phages and allowing them to spread to other cells (Nakonieczna et al., 2015). Phages were first discovered and described a century ago (Twort, 1915; d'Hérelles, 1917). Phage therapy was widely used to treat bacterial infections until the 40 s, and has seen a recent upsurge in interest with the growing need for alternatives to antibiotic treatments to treat diseases caused by antibiotic-resistant bacteria.
Many bacteriophages of C. perfringens have been described and sequenced (Morales et al., 2012; Seal et al., 2012; Volozhantsev et al., 2012), including several that were isolated from strains of poultry origin and that had specific anti-Clostridium activity (Zimmer et al., 2002a; Seal et al., 2011; Volozhantsev et al., 2011; Seal, 2013). The use of bacteriophages to limit C. perfringens infection has proven efficient in field trials. For example, Miller et al. (2010) showed that feeding broilers with a mixture of six bacteriophages reduced mortality in an NE challenge by over 90% and improved overall performance assessed as weight gain and feed conversion (Miller et al., 2010).
A number of studies focused on the use of bacteriophage endolysins as antimicrobials, rather than the phage itself (Zimmer et al., 2002b; Tillman et al., 2013; Gervasi et al., 2014a; Swift et al., 2015). The use of phage proteins instead of bacteriophages eliminates complications that can arise with phage therapy. Indeed, several studies have described bacteria becoming resistant to phage infection, by developing mechanisms to prevent the entry of the phage in the cell or by degrading the injected DNA (Nobrega et al., 2015). A purified recombinant endolysin of bacteriophage ϕ3626, isolated from a type strain of C. perfringens, was shown to have lytic activity against over 40 strains of C. perfringens, without affecting other Clostridium species or species of different genera, such as Lactobacillus, Enterococcus or Bacillus (30 and 34 strains tested respectively) (Zimmer et al., 2002a,b). Recently, a modified endolysin was shown to be active against C. perfringens even at high temperatures, making it a suitable candidate as an antimicrobial additive for NE prevention (Swift et al., 2015). Another research group characterized the endolysin CP25L, isolated from a C. perfringens bacteriophage, which was active against C. perfringens in vitro (Gervasi et al., 2013, 2014a). The authors were able to over-express the enzyme in a modified L. johnsonii strain, strain which was discussed earlier in this review as active against C. perfringens in vivo (La Ragione et al., 2004; Gervasi et al., 2014a,b). This strain was able to survive in GI tract-like conditions, but the expression of the endolysin and the control of C. perfingens growth in co-culture were inconsistent (Gervasi et al., 2014b). The use of probiotic strains to deliver antagonist molecules within the GI tract is a promising alternative; however, the application can be problematic. Indeed, it is hard to predict the behavior of the molecule in vivo and many factors can interfere with its synthesis by affecting the producer strain.
Vaccination against C. perfringens
A large number of trials tested the efficacy of broiler vaccination as a prophylactic treatment against C. perfringens-induced NE. For the purpose of this review, we will limit this section to an overview of the recent advances regarding vaccines against C. perfringens. The reader is also directed to a recent review by the Van Immerseel lab (Mot et al., 2014). Several strategies have been used to vaccinate broilers against C. perfringens to include use of live bacteria or inactivated toxins. Vaccines can be delivered by spraying chicks upon hatching, by addition to the feed or the drinking water, or even injected in ovo (Sharma, 1999; Muir et al., 2000; Mot et al., 2014). Vaccination using non-virulent C. perfringens strains have proven to be inefficient, and it has been shown that strains used in vaccines need to remain mildly virulent. Thompson et al. (2006) showed that strains with a mutation in the gene coding for the α toxin that were still virulent (but less than the wild-type) were able to protect chickens against NE, whereas an avirulent strain of C. perfringens did not have any immunizing effects (Thompson et al., 2006).
Several trials have shown that chickens could be protected against C. perfringens-induced NE by injection with inactive and active toxins (Kulkarni et al., 2007; Jang et al., 2012) and antigenic proteins (Jiang et al., 2009). Since the discovery of its role in NE, the NetB toxin has been intensively studied with regards to vaccination, with some promising results (Fernandes da Costa et al., 2013; Keyburn et al., 2013a,b).
Conclusions and perspectives
A number of studies have now shown that the use of live micro-organisms and molecules produced by microbes represent potential alternatives to the use of conventional antibiotics for the prevention of C. perfringens-induced NE in broiler chickens. Although a large number of probiotic bacterial strains with C. perfringens antagonism have been described, studies and trials using live yeasts are surprisingly sparse and in our opinion warrant further investigations. Antimicrobial molecules, such as bacteriocins or phage endolysins, are also good candidates for new antimicrobials. Research for new antimicrobials is, however, limited by regulations and applicability and mainly focuses on the use of GRAS micro-organisms, with restricted use of genetically modified organisms (GMOs). Nanoparticles could also be used as vectors for delivery of these new antimicrobial molecules, thus avoiding the expression and regulatory issues that arise with the use of live cells and GMOs.
It is difficult to identify a single “ideal” solution within this wealth of options for NE disease control. Several microbes and molecules of microbial origins, some already available commercially, represent promising agents that conceivably could be used in conjunction with one another to formulate highly effective synergic antimicrobials. For example, a product consisting of a CE culture, a probiotic strain producing a targeted anti-C. perfringens molecule and a prebiotic product would constitute a robust formulation that could prevent the overgrowth of C. perfringens in vivo and maintain a healthy GI tract flora at the same time.
Several criteria must be taken into account when developing feed additives or preventive treatments for the animal industry. The financial cost of the product is a major criterion, especially for small animals with low market value, like broiler chickens. A thorough genetic characterization of candidate strains is essential in order to confirm the safety of the bacterial strain and ensure the lack of virulence and antibiotic resistance genes. The chosen molecule or strain must be able to stay active in the host and withstand industrial treatments. One must also keep in mind that the GI tract is a highly complex environment with numerous bacterial species that can affect the efficacy of these antimicrobials, perhaps in a different manner from one animal to another. Moreover, C. perfringens and other bacteria are highly adaptable micro-organisms. It is thus of high importance to develop and use products in a rational manner in order to avoid the appearance of strains resistant to these novel antimicrobials, as has occurred with conventional antibiotics.
Author contributions
DC, RD, EA, and DD contributed to the conception and the design of the review and researched and wrote the review.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by a research grant from Société Industrielle Lesaffre. We would like to thank Dr J. Maxwell Dow for his critical review of this manuscript.
References
- Abudabos A. M., Yehia H. M. (2013). Effect of dietary mannan oligosaccharide from Saccharomyces cerevisiae on live performance of broilers under Clostridium perfringens challenge. Ital. J. Anim. Sci. 12, 231–235. 10.4081/ijas.2013.e38 [DOI] [Google Scholar]
- Allaart J. G., van Asten A. J., Vernooij J. C., Gröne A. (2011). Effect of Lactobacillus fermentum on beta2 toxin production by Clostridium perfringens. Appl. Environ. Microbiol. 77, 4406–4411. 10.1128/AEM.03002-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sheikhly F., Al-Saieg A. (1980). Role of Coccidia in the occurrence of necrotic enteritis of chickens. Avian Dis. 24, 324–333. 10.2307/1589700 [DOI] [PubMed] [Google Scholar]
- Annett C. B., Viste J. R., Chirino-Trejo M., Classen H. L., Middleton D. M., Simko E. (2002). Necrotic enteritis: effect of barley, wheat and corn diets on proliferation of Clostridium perfringens type A. Avian Pathol. 31, 598–601. 10.1080/0307945021000024544 [DOI] [PubMed] [Google Scholar]
- Baba E., Wakeshima H., Fukui K., Fukata T., Arakawa A. (1992). Adhesion of bacteria to the cecal mucosal surface of conventional and germ-free chickens infected with Eimeria tenella. Am. J. Vet. Res. 53, 194–197. [PubMed] [Google Scholar]
- Barbara A. J., Trinh H. T., Glock R. D., Glenn Songer J. (2008). Necrotic enteritis-producing strains of Clostridium perfringens displace non-necrotic enteritis strains from the gut of chicks. Vet. Microbiol. 126, 377–382. 10.1016/j.vetmic.2007.07.019 [DOI] [PubMed] [Google Scholar]
- Barbosa T. M., Serra C. R., La Ragione R. M., Woodward M. J., Henriques A. O. (2005). Screening for bacillus isolates in the broiler gastrointestinal tract. Appl. Environ. Microbiol. 71, 968–978. 10.1128/AEM.71.2.968-978.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes E. M., Impey C. S., Cooper D. M. (1980). Manipulation of the crop and intestinal flora of the newly hatched chick. Am. J. Clin. Nutr. 33, 2426–2433. [DOI] [PubMed] [Google Scholar]
- Biagi G., Cipollini I., Pompei A., Zaghini G., Matteuzzi D. (2007). Effect of a Lactobacillus animalis strain on composition and metabolism of the intestinal microflora in adult dogs. Vet. Microbiol. 124, 160–165. 10.1016/j.vetmic.2007.03.013 [DOI] [PubMed] [Google Scholar]
- Biggs P., Parsons C. M., Fahey G. C. (2007). The effects of several oligosaccharides on growth performance, nutrient digestibilities, and cecal microbial populations in young chicks. Poult. Sci. 86, 2327–2336. 10.3382/ps.2007-00427 [DOI] [PubMed] [Google Scholar]
- Bizani D., Brandelli A. (2002). Characterization of a bacteriocin produced by a newly isolated Bacillus sp. strain 8 A. J. Appl. Microbiol. 93, 512–519. 10.1046/j.1365-2672.2002.01720.x [DOI] [PubMed] [Google Scholar]
- Bottone E., Allerhand J., Pisano M. A. (1974). Effects of a bacteriocin produced by Streptococcus faecalis var. zymogenes (E1) on susceptible microorganisms. Antonie Van Leeuwenhoek 40, 385–392. 10.1007/BF00399350 [DOI] [PubMed] [Google Scholar]
- Cao G. T., Zeng X. F., Chen A. G., Zhou L., Zhang L., Xiao Y. P., et al. (2013). Effects of a probiotic, Enterococcus faecium, on growth performance, intestinal morphology, immune response, and cecal microflora in broiler chickens challenged with Escherichia coli K88. Poult. Sci. 92, 2949–2955. 10.3382/ps.2013-03366 [DOI] [PubMed] [Google Scholar]
- Cao L., Yang X. J., Li Z. J., Sun F. F., Wu X. H., Yao J. H. (2012). Reduced lesions in chickens with Clostridium perfringens-induced necrotic enteritis by Lactobacillus fermentum 1.20291. Poult. Sci. 91, 3065–3071. 10.3382/ps.2012-02548 [DOI] [PubMed] [Google Scholar]
- Castagliuolo I., LaMont J. T., Nikulasson S. T., Pothoulakis C. (1996). Saccharomyces boulardii protease inhibits Clostridium difficile toxin A effects in the rat ileum. Infect. Immun. 64, 5225–5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagliuolo I., Riegler M. F., Valenick L., LaMont J. T., Pothoulakis C. (1999). Saccharomyces boulardii protease inhibits the effects of Clostridium difficile toxins A and B in human colonic mucosa. Infect. Immun. 67, 302–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaucheyras-Durand F., Durand H. (2010). Probiotics in animal nutrition and health. Benef. Microbes. 1, 3–9. 10.3920/BM2008.1002 [DOI] [PubMed] [Google Scholar]
- Chen Y. S., Yanagida F., Srionnual S. (2007). Characteristics of bacteriocin-like inhibitory substances from dochi-isolated Enterococcus faecium D081821 and D081833. Lett. Appl. Microbiol. 44, 320–325. 10.1111/j.1472-765X.2006.02058.x [DOI] [PubMed] [Google Scholar]
- Cochrane S. A., Vederas J. C. (2014). Lipopeptides from Bacillus and Paenibacillus spp.: a gold mine of antibiotic candidates. Med. Res. Rev. [Epub ahead of print]. 10.1002/med.21321 [DOI] [PubMed] [Google Scholar]
- Cooper K. K., Songer J. G. (2009). Necrotic enteritis in chickens: a paradigm of enteric infection by Clostridium perfringens type A. Anaerobe 15, 55–60. 10.1016/j.anaerobe.2009.01.006 [DOI] [PubMed] [Google Scholar]
- Cotter P. D., Ross R. P., Hill C. (2013). Bacteriocins—a viable alternative to antibiotics? Nat. Rev. Microbiol. 11, 95–105. 10.1038/nrmicro2937 [DOI] [PubMed] [Google Scholar]
- Craven S. E., Stern N. J., Bailey J. S., Cox N. A. (2001). Incidence of Clostridium perfringens in broiler chickens and their environment during production and processing. Avian Dis. 45, 887–896. 10.2307/1592868 [DOI] [PubMed] [Google Scholar]
- Craven S. E., Stern N. J., Cox N. A., Bailey J. S., Berrang M. (1999). Cecal carriage of Clostridium perfringens in broiler chickens given mucosal starter culture. Avian Dis. 43, 484–490. 10.2307/1592646 [DOI] [PubMed] [Google Scholar]
- Crost E. H., Ajandouz E. H., Villard C., Geraert P. A., Puigserver A., Fons M. (2011). Ruminococcin, C, a new anti-Clostridium perfringens bacteriocin produced in the gut by the commensal bacterium Ruminococcus gnavus E1. Biochimie 93, 1487–1494. 10.1016/j.biochi.2011.05.001 [DOI] [PubMed] [Google Scholar]
- Dabard J., Bridonneau C., Phillipe C., Anglade P., Molle D., Nardi M., et al. (2001). Ruminococcin, A., a new lantibiotic produced by a Ruminococcus gnavus strain isolated from human feces. Appl. Environ. Microbiol. 67, 4111–4118. 10.1128/AEM.67.9.4111-4118.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dec M., Puchalski A., Urban-Chmiel R., Wernicki A. (2014). Screening of Lactobacillus strains of domestic goose origin against bacterial poultry pathogens for use as probiotics. Poult. Sci. 93, 2464–2472. 10.3382/ps.2014-04025 [DOI] [PubMed] [Google Scholar]
- d'Hérelles F. (1917). Sur un microbe invisible antagoniste des bacilles dysentériques. Comptes rendus Acad. Sci. Paris 165, 373–375. [Google Scholar]
- Drew M. D., Syed N. A., Goldade B. G., Laarveld B., Van Kessel A. G. (2004). Effects of dietary protein source and level on intestinal populations of Clostridium perfringens in broiler chickens. Poult. Sci. 83, 414–420. 10.1093/ps/83.3.414 [DOI] [PubMed] [Google Scholar]
- Elwinger K., Schneitz C., Berndtson E., Fossum O., Teglöf B., Engstöm B. (1992). Factors affecting the incidence of necrotic enteritis, caecal carriage of Clostridium perfringens and bird performance in broiler chicks. Acta Vet. Scand. 33, 369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg R. M., Grevsen K., Ivarsen E., Fretté X., Christensen L. P., Højberg O., et al. (2012). The effect of Artemisia annua on broiler performance, on intestinal microbiota and on the course of a Clostridium perfringens infection applying a necrotic enteritis disease model. Avian Pathol. 41, 369–376. 10.1080/03079457.2012.696185 [DOI] [PubMed] [Google Scholar]
- Engberg R. M., Hedemann M. S., Steenfeldt S., Jensen B. B. (2004). Influence of whole wheat and xylanase on broiler performance and microbial composition and activity in the digestive tract. Poult. Sci. 83, 925–938. 10.1093/ps/83.6.925 [DOI] [PubMed] [Google Scholar]
- Engström B. E., Fermér C., Lindberg A., Saarinen E., Båverud V., Gunnarsson A. (2003). Molecular typing of isolates of Clostridium perfringens from healthy and diseased poultry. Vet. Microbiol. 94, 225–235. 10.1016/S0378-1135(03)00106-8 [DOI] [PubMed] [Google Scholar]
- Fatichenti F., Bergere J. L., Deiana P., Farris G. A. (1983). Antagonistic activity of Debaryomyces hansenii towards Clostridium tyrobutyricum and Cl. butyricum. J. Dairy Res. 50, 449–457. 10.1017/S0022029900032684 [DOI] [PubMed] [Google Scholar]
- Fernandes da Costa S. P., Mot D., Bokori-Brown M., Savva C. G., Basak A. K., Van Immerseel F., et al. (2013). Protection against avian necrotic enteritis after immunisation with NetB genetic or formaldehyde toxoids. Vaccine 31, 4003–4008. 10.1016/j.vaccine.2013.05.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler J., Kakani R., Haq A., Byrd J. A., Bailey C. A. (2015). Growth promoting effects of prebiotic yeast cell wall products in starter broilers under an immune stress and Clostridium perfringens challenge. J. Appl. Poult. Res 24, 66–72. 10.3382/japr/pfv010 [DOI] [Google Scholar]
- Franz C. M., van Belkum M. J., Holzapfel W. H., Abriouel H., Gálvez A. (2007). Diversity of enterococcal bacteriocins and their grouping in a new classification scheme. FEMS Microbiol. Rev. 31, 293–310. 10.1111/j.1574-6976.2007.00064.x [DOI] [PubMed] [Google Scholar]
- Fukata T., Hadate Y., Baba E., Arakawa A. (1991). Influence of bacteria on Clostridium perfringens infections in young chickens. Avian Dis. 35, 224–227. 10.2307/1591319 [DOI] [PubMed] [Google Scholar]
- Fuller R. (1999). Probiotics for farm animals, in Probiotics: A Critical Review, ed Tannock G. W. (New York, NY: Horizon Scientific Press; ), 15–22. [Google Scholar]
- Galindo-Cuspinera V., Westhoff D. C., Rankin S. A. (2003). Antimicrobial properties of commercial annatto extracts against selected pathogenic, lactic acid, and spoilage microorganisms. J. Food. Prot. 66, 1074–1078. [DOI] [PubMed] [Google Scholar]
- Geier M. S., Mikkelsen L. L., Torok V. A., Allison G. E., Olnood C. G., Boulianne M., et al. (2010). Comparison of alternatives to in-feed antimicrobials for the prevention of clinical necrotic enteritis. J. Appl. Microbiol. 109, 1329–1338. 10.1111/j.1365-2672.2010.04758.x [DOI] [PubMed] [Google Scholar]
- Gérard P., Brézillon C., Quéré F., Salmon A., Rabot S. (2008). Characterization of cecal microbiota and response to an orally administered Lactobacillus probiotic strain in the broiler chicken. J. Mol. Microbiol. Biotechnol. 14, 115–122. 10.1159/000106090 [DOI] [PubMed] [Google Scholar]
- Gervasi T., Lo Curto R., Minniti E., Narbad A., Mayer M. J. (2014b). Application of Lactobacillus johnsonii expressing phage endolysin for control of Clostridium perfringens. Lett. Appl. Microbiol. 59, 355–361. 10.1111/lam.12298 [DOI] [PubMed] [Google Scholar]
- Gervasi T., Curto R. L., Narbad A., Mayer M. J. (2013). Complete genome sequence of PhiCP51, a temperate bacteriophage of Clostridium perfringens. Arch. Virol. 158, 2015–2017. 10.1007/s00705-013-1647-1 [DOI] [PubMed] [Google Scholar]
- Gervasi T., Horn N., Wegmann U., Dugo G., Narbad A., Mayer M. J. (2014a). Expression and delivery of an endolysin to combat Clostridium perfringens. Appl. Microbiol. Biotechnol. 98, 2495–2505. 10.1007/s00253-013-5128-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G. R., Wang X. (1994). Regulatory effects of bifidobacteria on the growth of other colonic bacteria. J. Appl. Bacteriol. 77, 412–420. 10.1111/j.1365-2672.1994.tb03443.x [DOI] [PubMed] [Google Scholar]
- Gil de los santos J. R., Storch O. B., Gil-turnes C. (2005). Bacillus cereus var. toyoii and Saccharomyces boulardii increased feed efficiency in broilers infected with Salmonella enteritidis. Br. Poult. Sci. 46, 494–497. 10.1080/00071660500181461 [DOI] [PubMed] [Google Scholar]
- Gil de los santos J. R., Storch O. B., Fernandes C. G., Gil-Turnes C. (2012). Evaluation in broilers of the probiotic properties of Pichia pastoris and a recombinant P. pastoris containing the Clostridium perfringens alpha toxin gene. Vet. Microbiol. 156, 448–451. 10.1016/j.vetmic.2011.11.019 [DOI] [PubMed] [Google Scholar]
- Grass J. E., Gould L. H., Mahon B. E. (2013). Epidemiology of foodborne disease outbreaks caused by Clostridium perfringens, United States, 1998-2010. Foodborne Pathog. Dis. 10, 131–136. 10.1089/fpd.2012.1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilli E., Messina M. R., Catelli E., Morlacchini M., Piva A. (2009). Pediocin A improves growth performance of broilers challenged with Clostridium perfringens. Poult. Sci. 88, 2152–2158. 10.3382/ps.2009-00160 [DOI] [PubMed] [Google Scholar]
- Hatoum R., Labrie S., Fliss I. (2012). Antimicrobial and probiotic properties of yeasts: from fundamental to novel applications. Front. Microbiol. 3:421. 10.3389/fmicb.2012.00421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmboldt C. F., Bryant E. S. (1971). The pathology of necrotic enteritis in domestic fowl. Avian Dis. 15, 775–780. 10.2307/1588866 [DOI] [PubMed] [Google Scholar]
- Hofacre C. L., Beacorn T., Collett S., Mathis G. (2003). Using competitive exclusion, mannanoligosaccharide and other intestinal products to control necrotic enteritis. J. Appl. Poult. Res. 12, 60–64. 10.1093/japr/12.1.60 [DOI] [Google Scholar]
- Hofacre C. L., Froyman R., Gautrias B., George B., Goodwin M. A., Brown J. (1998). Use of Aviguard and other intestinal bioproducts in experimental Clostridium perfringens-associated necrotizing enteritis in broiler chickens. Avian Dis. 42, 579–584. 10.2307/1592685 [DOI] [PubMed] [Google Scholar]
- Jackson M. E., Anderson D. M., Hsiao H. Y., Mathis G. F., Fodge D. W. (2003). Beneficial effect of beta-mannanase feed enzyme on performance of chicks challenged with Eimerla sp. and Clostridium perfringens. Avian Dis. 47, 759–763. 10.1637/7024 [DOI] [PubMed] [Google Scholar]
- Jacobs C. M., Parsons C. M. (2009). The effect of Grobiotic-P combined with yeast cell wall and gluconic acid on growth performance, nutrient digestibilities, and cecal microbial populations in young chicks. Poult. Sci. 88, 2360–2367. 10.3382/ps.2009-00037 [DOI] [PubMed] [Google Scholar]
- Jang S. I., Lillehoj H. S., Lee S. H., Lee K. W., Lillehoj E. P., Hong Y. H., et al. (2012). Vaccination with Clostridium perfringens recombinant proteins in combination with Montanide ISA 71 VG adjuvant increases protection against experimental necrotic enteritis in commercial broiler chickens. Vaccine 30, 5401–5406. 10.1016/j.vaccine.2012.06.007 [DOI] [PubMed] [Google Scholar]
- Jayaraman S., Thangavel G., Kurian H., Mani R., Mukkalil R., Chirakkal H. (2013). Bacillus subtilis PB6 improves intestinal health of broiler chickens challenged with Clostridium perfringens-induced necrotic enteritis. Poult. Sci. 92, 370–374. 10.3382/ps.2012-02528 [DOI] [PubMed] [Google Scholar]
- Jiang Y., Kulkarni R. R., Parreira V. R., Prescott J. F. (2009). Immunization of broiler chickens against Clostridium perfringens-induced necrotic enteritis using purified recombinant immunogenic proteins. Avian Dis. 53, 409–415. 10.1637/8656-021109-Reg.1 [DOI] [PubMed] [Google Scholar]
- Joerger R. D. (2003). Alternatives to antibiotics: bacteriocins, antimicrobial peptides and bacteriophages. Poult. Sci. 82, 640–647. 10.1093/ps/82.4.640 [DOI] [PubMed] [Google Scholar]
- Józefiak D., Sip A., Rutkowski A., Rawski M., Kaczmarek S., Wolun-Cholewa M., et al. (2012). Lyophilized Carnobacterium divergens AS7 bacteriocin preparation improves performance of broiler chickens challenged with Clostridium perfringens. Poult. Sci. 91, 1899–1907. 10.3382/ps.2012-02151 [DOI] [PubMed] [Google Scholar]
- Kaldhusdal M., Løvland A. (2000). The economical impact of Clostridium perfringens is greater than anticipated. World Poultry. 16, 50–51. [Google Scholar]
- Kaldhusdal M., Schneitz C., Hofshagen M., Skjerve E. (2001). Reduced incidence of Clostridium perfringens-associated lesions and improved performance in broiler chickens treated with normal intestinal bacteria from adult fowl. Avian Dis. 45, 149–156. 10.2307/1593022 [DOI] [PubMed] [Google Scholar]
- Keyburn A. L., Boyce J. D., Vaz P., Bannam T. L., Ford M. E., Parker D., et al. (2008). NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathog. 4:e26. 10.1371/journal.ppat.0040026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyburn A. L., Portela R. W., Ford M. E., Bannam T. L., Yan X. X., Rood J. I., et al. (2013a). Maternal immunization with vaccines containing recombinant NetB toxin partially protects progeny chickens from necrotic enteritis. Vet. Res. 44:108. 10.1186/1297-9716-44-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyburn A. L., Portela R. W., Sproat K., Ford M. E., Bannam T. L., Yan X., et al. (2013b). Vaccination with recombinant NetB toxin partially protects broiler chickens from necrotic enteritis. Vet. Res. 44:54. 10.1186/1297-9716-44-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyburn A. L., Yan X. X., Bannam T. L., Van Immerseel F., Rood J. I., Moore R. J. (2010). Association between avian necrotic enteritis and Clostridium perfringens strains expressing NetB toxin. Vet. Res. 41, 21. 10.1051/vetres/2009069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G. B., Seo Y. M., Kim C. H., Paik I. K. (2011). Effect of dietary prebiotic supplementation on the performance, intestinal microflora, and immune response of broilers. Poult. Sci. 90, 75–82. 10.3382/ps.2010-00732 [DOI] [PubMed] [Google Scholar]
- Kim P. I., Jung M. Y., Chang Y. H., Kim S., Kim S. J., Park Y. H. (2007). Probiotic properties of Lactobacillus and Bifidobacterium strains isolated from porcine gastrointestinal tract. Appl. Microbiol. Biotechnol. 74, 1103–1111. 10.1007/s00253-006-0741-7 [DOI] [PubMed] [Google Scholar]
- Klose V., Bayer K., Bruckbeck R., Schatzmayr G., Loibner A. P. (2010). In vitro antagonistic activities of animal intestinal strains against swine-associated pathogens. Vet. Microbiol. 144, 515–521. 10.1016/j.vetmic.2010.02.025 [DOI] [PubMed] [Google Scholar]
- Knap I., Lund B., Kehlet A. B., Hofacre C., Mathis G. (2010). Bacillus licheniformis prevents necrotic enteritis in broiler chickens. Avian Dis. 54, 931–935. 10.1637/9106-101509-ResNote.1 [DOI] [PubMed] [Google Scholar]
- Kulkarni R. R., Parreira V. R., Sharif S., Prescott J. F. (2007). Immunization of broiler chickens against Clostridium perfringens-induced necrotic enteritis. Clin Vaccine Immunol. 14, 1070–1077. 10.1128/CVI.00162-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Ragione R. M., Narbad A., Gasson M. J., Woodward M. J. (2004). In vivo characterization of Lactobacillus johnsonii FI9785 for use as a defined competitive exclusion agent against bacterial pathogens in poultry. Lett. Appl. Microbiol. 38, 197–205. 10.1111/j.1472-765X.2004.01474.x [DOI] [PubMed] [Google Scholar]
- La Ragione R. M., Woodward M. J. (2003). Competitive exclusion by Bacillus subtilis spores of Salmonella enterica serotype Enteritidis and Clostridium perfringens in young chickens. Vet. Microbiol. 94, 245–256. 10.1016/S0378-1135(03)00077-4 [DOI] [PubMed] [Google Scholar]
- Layton S. L., Hernandez-Velasco X., Chaitanya S., Xavier J., Menconi A., Latorre J. D., et al. (2013). The effect of a Lactobacillus-based probiotic for the control of necrotic enteritis in broilers. Food Nutr. Sci. 4, 1–7. 10.4236/fns.2013.411A001 [DOI] [Google Scholar]
- Lee H., Kim H. Y. (2011). Lantibiotics, class I bacteriocins from the genus Bacillus. J. Microbiol. Biotechnol. 21, 229–235. [PubMed] [Google Scholar]
- Lee J. H., Li X., O'sullivan D. J. (2011a). Transcription analysis of a lantibiotic gene cluster from Bifidobacterium longum DJO10A. Appl. Environ. Microbiol. 77, 5879–5887. 10.1128/AEM.00571-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. W., Lillehoj H. S., Jeong W., Jeoung H. Y., An D. J. (2011b). Avian necrotic enteritis: experimental models, host immunity, pathogenesis, risk factors, and vaccine development. Poult. Sci. 90, 1381–1390. 10.3382/ps.2010-01319 [DOI] [PubMed] [Google Scholar]
- Liu D., Guo Y., Wang Z., Yuan J. (2010). Exogenous lysozyme influences Clostridium perfringens colonization and intestinal barrier function in broiler chickens. Avian Pathol. 39, 17–24. 10.1080/03079450903447404 [DOI] [PubMed] [Google Scholar]
- Lutful Kabir S. M. (2009). The role of probiotics in the poultry industry. Int. J. Mol. Sci. 10, 3531–3546. 10.3390/ijms10083531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt R. M., Brooker J. D., Acamovic T., Sparks N. H. C. (2006). Necrotic enteritis: a continuing challenge for the poultry industry. World's Poult. Sci. J. 62, 221–247. 10.1079/WPS200593 [DOI] [Google Scholar]
- McReynolds J., Waneck C., Byrd J., Genovese K., Duke S., Nisbet D. (2009). Efficacy of multistrain direct-fed microbial and phytogenetic products in reducing necrotic enteritis in commercial broilers. Poult. Sci. 88, 2075–2080. 10.3382/ps.2009-00106 [DOI] [PubMed] [Google Scholar]
- Meca G., Sospedra I., Soriano J. M., Ritieni A., Moretti A., Mañes J. (2010). Antibacterial effect of the bioactive compound beauvericin produced by Fusarium proliferatum on solid medium of wheat. Toxicon 56, 349–354. 10.1016/j.toxicon.2010.03.022 [DOI] [PubMed] [Google Scholar]
- Meca G., Sospedra I., Valero M. A., Mañes J., Font G., Ruiz M. J. (2011). Antibacterial activity of the enniatin B, produced by Fusarium tricinctum in liquid culture, and cytotoxic effects on Caco-2 cells. Toxicol. Mech. Methods 21, 503–512. 10.3109/15376516.2011.556202 [DOI] [PubMed] [Google Scholar]
- Miller R. W., Skinner E. J., Sulakvelidze A., Mathis G. F., Hofacre C. L. (2010). Bacteriophage therapy for control of necrotic enteritis of broiler chickens experimentally infected with Clostridium perfringens. Avian Dis. 54, 33–40. 10.1637/8953-060509-Reg.1 [DOI] [PubMed] [Google Scholar]
- Mitsch P., Zitterl-Eglseer K., Köhler B., Gabler C., Losa R., Zimpernik I. (2004). The effect of two different blends of essential oil components on the proliferation of Clostridium perfringens in the intestines of broiler chickens. Poult. Sci. 83, 669–675. 10.1093/ps/83.4.669 [DOI] [PubMed] [Google Scholar]
- Mongkolthanaruk W. (2012). Classification of Bacillus beneficial substances related to plants, humans and animals. J. Microbiol. Biotechnol. 22, 1597–1604. 10.4014/jmb.1204.04013 [DOI] [PubMed] [Google Scholar]
- Morales C. A., Oakley B. B., Garrish J. K., Siragusa G. R., Ard M. B., Seal B. S. (2012). Complete genome sequence of the podoviral bacteriophage PhiCP24R, which is virulent for Clostridium perfringens. Arch. Virol. 157, 769–772. 10.1007/s00705-011-1218-2 [DOI] [PubMed] [Google Scholar]
- Moran E. T., Jr. (2014). Intestinal events and nutritional dynamics predispose Clostridium perfringens virulence in broilers. Poult. Sci. 93, 3028–3036. 10.3382/ps.2014-04313 [DOI] [PubMed] [Google Scholar]
- Mot D., Timbermont L., Haesebrouck F., Ducatelle R., Van Immerseel F. (2014). Progress and problems in vaccination against necrotic enteritis in broiler chickens. Avian Pathol. 43, 290–300. 10.1080/03079457.2014.939942 [DOI] [PubMed] [Google Scholar]
- Muir W. I., Bryden W. L., Husband A. J. (2000). Immunity, vaccination and the avian intestinal tract. Dev. Comp. Immunol. 24, 325–342. 10.1016/S0145-305X(99)00081-6 [DOI] [PubMed] [Google Scholar]
- Nakonieczna A., Cooper C. J., Gryko R. (2015). Bacteriophages and bacteriophage-derived endolysins as potential therapeutics to combat Gram-positive spore forming bacteria. J. Appl. Microbiol. 119, 620–631. 10.1111/jam.12881 [DOI] [PubMed] [Google Scholar]
- Nobrega F. L., Costa A. R., Kluskens L. D., Azeredo J. (2015). Revisiting phage therapy: new applications for old resources. Trends Microbiol. 23, 185–191. 10.1016/j.tim.2015.01.006 [DOI] [PubMed] [Google Scholar]
- Novak M., Vetvicka V. (2008). Beta-glucans, history, and the present: immunomodulatory aspects and mechanisms of action. J. Immunotoxicol. 5, 47–57. 10.1080/15476910802019045 [DOI] [PubMed] [Google Scholar]
- Nurmi E., Rantala M. (1973). New aspects of Salmonella infection in broiler production. Nature 241, 210–211. 10.1038/241210a0 [DOI] [PubMed] [Google Scholar]
- Paiva D., McElroy A. (2014). Necrotic enteritis: applications for the poultry industry. J. Appl. Poult. Res. 23, 557–566. 10.3382/japr.2013-00925 [DOI] [Google Scholar]
- Pan D., Yu Z. (2014). Intestinal microbiome of poultry and its interaction with host and diet. Gut. Microbes. 5, 108–119. 10.4161/gmic.26945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S., Goyal A. (2012). The current trends and future perspectives of prebiotics research: a review. 3 Biotech. 2, 115–125. 10.1007/s13205-012-0044-x [DOI] [Google Scholar]
- Patterson J. A., Burkholder K. M. (2003). Application of prebiotics and probiotics in poultry production. Poult. Sci. 82, 627–631. 10.1093/ps/82.4.627 [DOI] [PubMed] [Google Scholar]
- Popoff M. R. (2013). Clostridium, in Sécurité Sanitaire des Aliments: Epidémiologie et lutte Contre les Contaminants Zoonotiques, eds Drider D., Salvat G. (Paris: Economica; ), 165–189. [Google Scholar]
- Rajput I. R., Li L. Y., Xin X., Wu B. B., Juan Z. L., Cui Z. W., et al. (2013). Effect of Saccharomyces boulardii and Bacillus subtilis B10 on intestinal ultrastructure modulation and mucosal immunity development mechanism in broiler chickens. Poult. Sci. 92, 956–965. 10.3382/ps.2012-02845 [DOI] [PubMed] [Google Scholar]
- Rantala M., Nurmi E. (1973). Prevention of the growth of Salmonella infantis in chicks by the flora of the alimentary tract of chickens. Br. Poult. Sci. 14, 627–630. 10.1080/00071667308416073 [DOI] [PubMed] [Google Scholar]
- Rea M. C., Sit C. S., Clayton E., O'Connor P. M., Whittal R. M., Zheng J., et al. (2010). Thuricin, C. D., a posttranslationally modified bacteriocin with a narrow spectrum of activity against Clostridium difficile. Proc. Natl. Acad. Sci. U.S.A. 107, 9352–9357. 10.1073/pnas.0913554107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneitz C. (2005). Competitive exclusion in poultry—30 years of research. Food Contr. 16, 657–667. 10.1016/j.foodcont.2004.06.002 [DOI] [Google Scholar]
- Schoster A., Kokotovic B., Permin A., Pedersen P. D., Dal Bello F., Guardabassi L. (2013). In vitro inhibition of Clostridium difficile and Clostridium perfringens by commercial probiotic strains. Anaerobe 20, 36–41. 10.1016/j.anaerobe.2013.02.006 [DOI] [PubMed] [Google Scholar]
- Seal B. S. (2013). Characterization of bacteriophages virulent for Clostridium perfringens and identification of phage lytic enzymes as alternatives to antibiotics for potential control of the bacterium. Poult. Sci. 92, 526–533. 10.3382/ps.2012-02708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal B. S., Fouts D. E., Simmons M., Garrish J. K., Kuntz R. L., Woolsey R., et al. (2011). Clostridium perfringens bacteriophages PhiCP39O and PhiCP26F: genomic organization and proteomic analysis of the virions. Arch. Virol. 156, 25–35. 10.1007/s00705-010-0812-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal B. S., Volozhantsev N. V., Oakley B. B., Morales C. A., Garrish J. K., Simmons M., et al. (2012). Bacteriophages of Clostridium perfringens, in Bacteriophages, ed Kurtboke I. (Rijeka: InTech; ), 215–236. [Google Scholar]
- Shane S. M., Gyimah J. E., Harrington K. S., Snider T. G., III. (1985). Etiology and pathogenesis of necrotic enteritis. Vet. Res. Commun. 9, 269–287. 10.1007/BF02215151 [DOI] [PubMed] [Google Scholar]
- Sharma J. M. (1999). Introduction to poultry vaccines and immunity. Adv. Vet. Med. 41, 481–494. 10.1016/S0065-3519(99)80036-6 [DOI] [PubMed] [Google Scholar]
- Sharma N., Gupta A., Gautam N. (2014). Characterization of bacteriocin like inhibitory substance produced by a new strain Brevibacillus borstelensis AG1 Isolated from ‘Marcha’. Braz. J. Microbiol. 45, 1007–1015. 10.1590/S1517-83822014000300033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin M. S., Han S. K., Ji A. R., Kim K. S., Lee W. K. (2008). Isolation and characterization of bacteriocin-producing bacteria from the gastrointestinal tract of broiler chickens for probiotic use. J. Appl. Microbiol. 105, 2203–2212. 10.1111/j.1365-2672.2008.03935.x [DOI] [PubMed] [Google Scholar]
- Si W., Gong J., Han Y., Yu H., Brennan J., Zhou H., et al. (2007). Quantification of cell proliferation and alpha-toxin gene expression of Clostridium perfringens in the development of necrotic enteritis in broiler chickens. Appl. Environ. Microbiol. 73, 7110–7113. 10.1128/AEM.01108-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner J. T., Bauer S., Young V., Pauling G., Wilson J. (2010). An economic analysis of the impact of subclinical (mild) necrotic enteritis in broiler chickens. Avian Dis. 54, 1237–1240. 10.1637/9399-052110-Reg.1 [DOI] [PubMed] [Google Scholar]
- Songer J. G. (1996). Clostridial enteric diseases of domestic animals. Clin. Microbiol. Rev. 9, 216–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark J. M. (1960). Antibiotic activity of haemolytic enterococci. Lancet. 1, 733–734. 10.1016/S0140-6736(60)90620-6 [DOI] [PubMed] [Google Scholar]
- Stein T. (2005). Bacillus subtilis antibiotics: structures, syntheses and specific functions. Mol. Microbiol. 56, 845–857. 10.1111/j.1365-2958.2005.04587.x [DOI] [PubMed] [Google Scholar]
- Swift S. M., Seal B. S., Garrish J. K., Oakley B. B., Hiett K., Yeh H. Y., et al. (2015). A thermophilic phage endolysin fusion to a Clostridium perfringens-specific cell wall binding domain creates an anti-Clostridium antimicrobial with improved thermostability. Viruses 7, 3019–3034. 10.3390/v7062758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo A. Y., Tan H. M. (2005). Inhibition of Clostridium perfringens by a novel strain of Bacillus subtilis isolated from the gastrointestinal tracts of healthy chickens. Appl. Environ. Microbiol. 71, 4185–4190. 10.1128/AEM.71.8.4185-4190.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanissery R., McReynolds J. L., Conner D. E., Macklin K. S., Curtis P. A., Fasina Y. O. (2010). Evaluation of the efficacy of yeast extract in reducing intestinal Clostridium perfringens levels in broiler chickens. Poult. Sci. 89, 2380–2388. 10.3382/ps.2010-00879 [DOI] [PubMed] [Google Scholar]
- Thompson D. R., Parreira V. R., Kulkarni R. R., Prescott J. F. (2006). Live attenuated vaccine-based control of necrotic enteritis of broiler chickens. Vet. Microbiol. 113, 25–34. 10.1016/j.vetmic.2005.10.015 [DOI] [PubMed] [Google Scholar]
- Tillman G. E., Simmons M., Garrish J. K., Seal B. S. (2013). Expression of a Clostridium perfringens genome-encoded putative N-acetylmuramoyl-L-alanine amidase as a potential antimicrobial to control the bacterium. Arch. Microbiol. 195, 675–681. 10.1007/s00203-013-0916-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timbermont L., De Smet L., Van Nieuwerburgh F., Parreira V. R., Van Driessche G., Haesebrouck F., et al. (2014). Perfrin, a novel bacteriocin associated with netB positive Clostridium perfringens strains from broilers with necrotic enteritis. Vet. Res. 45:40. 10.1186/1297-9716-45-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timbermont L., Haesebrouck F., Ducatelle R., Van Immerseel F. (2011). Necrotic enteritis in broilers: an updated review on the pathogenesis. Avian Pathol. 40, 341–347. 10.1080/03079457.2011.590967 [DOI] [PubMed] [Google Scholar]
- Timbermont L., Lanckriet A., Dewulf J., Nollet N., Schwarzer K., Haesebrouck F., et al. (2010). Control of Clostridium perfringens-induced necrotic enteritis in broilers by target-released butyric acid, fatty acids and essential oils. Avian Pathol. 39, 117–121. 10.1080/03079451003610586 [DOI] [PubMed] [Google Scholar]
- Timbermont L., Lanckriet A., Pasmans F., Haesebrouck F., Ducatelle R., Van Immerseel F. (2009). Intra-species growth-inhibition by Clostridium perfringens is a possible virulence trait in necrotic enteritis in broilers. Vet. Microbiol. 137, 388–391. 10.1016/j.vetmic.2009.01.017 [DOI] [PubMed] [Google Scholar]
- Twort F. W. (1915). An investigation of the nature of ultra-microscopic viruses. Lancet 2, 1241–1243. 10.1016/S0140-6736(01)20383-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udompijitkul P., Paredes-Sabja D., Sarker M. R. (2012). Inhibitory effects of nisin against Clostridium perfringens food poisoning and nonfood-borne isolates. J. Food Sci. 77, M51–M56. 10.1111/j.1750-3841.2011.02475.x [DOI] [PubMed] [Google Scholar]
- Uzal F. A., Freedman J. C., Shrestha A., Theoret J. R., Garcia J., Awad M. M., et al. (2014). Towards an understanding of the role of Clostridium perfringens toxins in human and animal disease. Future Microbiol. 9, 361–377. 10.2217/fmb.13.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzal F. A., Vidal J. E., McClane B. A., Gurjar A. A. (2010). Clostridium perfringens toxins involved in mammalian veterinary diseases. Open Toxinol. J. 2, 24–42. 10.2174/1875414701003020024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Sluis W. (2000). Clostridial enteritis is often an underestimated problem. World Poult. 16, 42–43. [Google Scholar]
- Van Immerseel F., De Buck J., Pasmans F., Huyghebaert G., Haesebrouck F., Ducatelle R. (2004). Clostridium perfringens in poultry: an emerging threat for animal and public health. Avian Pathol. 33, 537–549. 10.1080/03079450400013162 [DOI] [PubMed] [Google Scholar]
- Van Immerseel F., Rood J. I., Moore R. J., Titball R. W. (2009). Rethinking our understanding of the pathogenesis of necrotic enteritis in chickens. Trends Microbiol. 17, 32–36. 10.1016/j.tim.2008.09.005 [DOI] [PubMed] [Google Scholar]
- Volozhantsev N. V., Oakley B. B., Morales C. A., Verevkin V. V., Bannov V. A., Krasilnikova V. M., et al. (2012). Molecular characterization of podoviral bacteriophages virulent for Clostridium perfringens and their comparison with members of the Picovirinae. PLoS ONE 75:e38283. 10.1371/journal.pone.0038283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volozhantsev N. V., Verevkin V. V., Bannov V. A., Krasilnikova V. M., Myakinina V. P., Zhilenkov E. L., et al. (2011). The genome sequence and proteome of bacteriophage PhiCPV1 virulent for Clostridium perfringens. Virus Res. 155, 433–439. 10.1016/j.virusres.2010.11.012 [DOI] [PubMed] [Google Scholar]
- Williams R. B. (2005). Intercurrent coccidiosis and necrotic enteritis of chickens: rational, integrated disease management by maintenance of gut integrity. Avian Pathol. 34, 159–180. 10.1080/03079450500112195 [DOI] [PubMed] [Google Scholar]
- Zimmer M., Scherer S., Loessner M. J. (2002a). Genomic analysis of Clostridium perfringens bacteriophage phi3626, which integrates into guaA and possibly affects sporulation. J. Bacteriol. 184, 4359–4368. 10.1128/JB.184.16.4359-4368.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer M., Vukov N., Scherer S., Loessner M. J. (2002b). The murein hydrolase of the bacteriophage phi3626 dual lysis system is active against all tested Clostridium perfringens strains. Appl. Environ. Microbiol. 68, 5311–5317. 10.1128/AEM.68.11.5311-5317.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]