Abstract
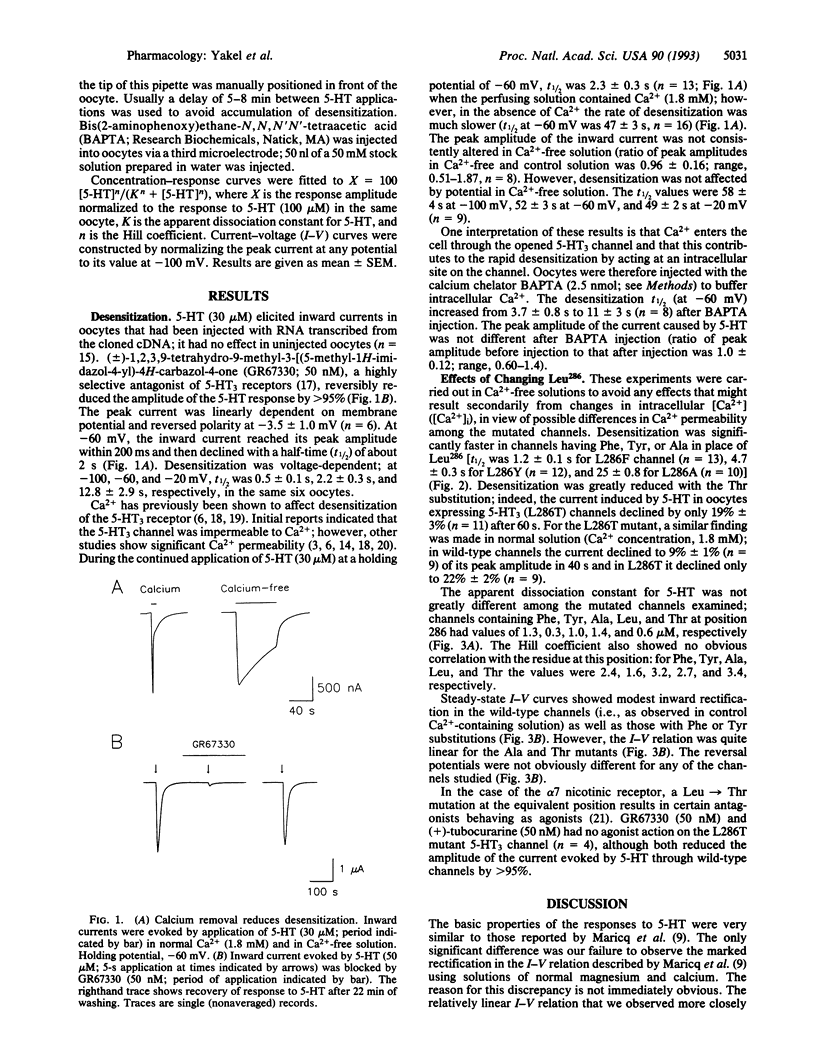
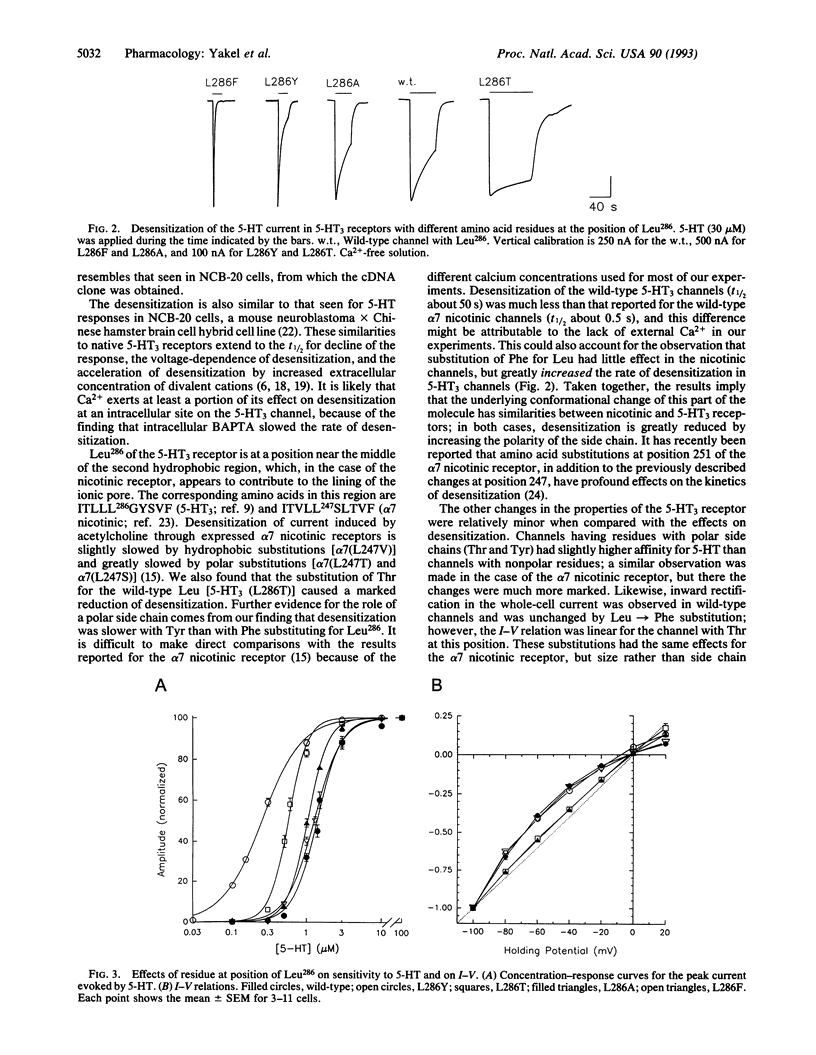
5-Hydroxytryptamine type 3 receptors were expressed in Xenopus oocytes from a cloned cDNA. The peak inward current evoked by 5-hydroxytryptamine (30 microM) was linearly related to the holding potential (-100 to +20 mV) and reversed near 0 mV. The inward current (at -60 mV) declined during the continued presence of 5-hydroxytryptamine with a half-time of about 2 s; this desensitization was 20 times slower in calcium-free solution. Desensitization was markedly different in channels in which Leu286 was changed by site-directed mutagenesis; this residue is thought to lie near the middle of the M2 segment. Desensitization was faster with Phe, Tyr, or Ala in this position and slower with Thr. Phe and Thr substitutions in the equivalent position of the nicotinic acetylcholine receptor have similar effects on desensitization, suggesting that the underlying conformational change might be common to ligand-gated channels.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertrand D., Devillers-Thiéry A., Revah F., Galzi J. L., Hussy N., Mulle C., Bertrand S., Ballivet M., Changeux J. P. Unconventional pharmacology of a neuronal nicotinic receptor mutated in the channel domain. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1261–1265. doi: 10.1073/pnas.89.4.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettler B., Boulter J., Hermans-Borgmeyer I., O'Shea-Greenfield A., Deneris E. S., Moll C., Borgmeyer U., Hollmann M., Heinemann S. Cloning of a novel glutamate receptor subunit, GluR5: expression in the nervous system during development. Neuron. 1990 Nov;5(5):583–595. doi: 10.1016/0896-6273(90)90213-y. [DOI] [PubMed] [Google Scholar]
- Christie M. J., Adelman J. P., Douglass J., North R. A. Expression of a cloned rat brain potassium channel in Xenopus oocytes. Science. 1989 Apr 14;244(4901):221–224. doi: 10.1126/science.2539643. [DOI] [PubMed] [Google Scholar]
- Egebjerg J., Bettler B., Hermans-Borgmeyer I., Heinemann S. Cloning of a cDNA for a glutamate receptor subunit activated by kainate but not AMPA. Nature. 1991 Jun 27;351(6329):745–748. doi: 10.1038/351745a0. [DOI] [PubMed] [Google Scholar]
- Galzi J. L., Devillers-Thiéry A., Hussy N., Bertrand S., Changeux J. P., Bertrand D. Mutations in the channel domain of a neuronal nicotinic receptor convert ion selectivity from cationic to anionic. Nature. 1992 Oct 8;359(6395):500–505. doi: 10.1038/359500a0. [DOI] [PubMed] [Google Scholar]
- Higashi H., Nishi S. 5-Hydroxytryptamine receptors of visceral primary afferent neurones on rabbit nodose ganglia. J Physiol. 1982 Feb;323:543–567. doi: 10.1113/jphysiol.1982.sp014091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M., O'Shea-Greenfield A., Rogers S. W., Heinemann S. Cloning by functional expression of a member of the glutamate receptor family. Nature. 1989 Dec 7;342(6250):643–648. doi: 10.1038/342643a0. [DOI] [PubMed] [Google Scholar]
- Huganir R. L., Delcour A. H., Greengard P., Hess G. P. Phosphorylation of the nicotinic acetylcholine receptor regulates its rate of desensitization. Nature. 1986 Jun 19;321(6072):774–776. doi: 10.1038/321774a0. [DOI] [PubMed] [Google Scholar]
- Huganir R. L., Greengard P. Regulation of neurotransmitter receptor desensitization by protein phosphorylation. Neuron. 1990 Nov;5(5):555–567. doi: 10.1016/0896-6273(90)90211-w. [DOI] [PubMed] [Google Scholar]
- Isaacson J. S., Nicoll R. A. Aniracetam reduces glutamate receptor desensitization and slows the decay of fast excitatory synaptic currents in the hippocampus. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10936–10940. doi: 10.1073/pnas.88.23.10936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinänen K., Wisden W., Sommer B., Werner P., Herb A., Verdoorn T. A., Sakmann B., Seeburg P. H. A family of AMPA-selective glutamate receptors. Science. 1990 Aug 3;249(4968):556–560. doi: 10.1126/science.2166337. [DOI] [PubMed] [Google Scholar]
- Kilpatrick G. J., Butler A., Hagan R. M., Jones B. J., Tyers M. B. [3H] GR67330, a very high affinity ligand for 5-HT3 receptors. Naunyn Schmiedebergs Arch Pharmacol. 1990 Jul;342(1):22–30. doi: 10.1007/BF00178967. [DOI] [PubMed] [Google Scholar]
- Kutsuwada T., Kashiwabuchi N., Mori H., Sakimura K., Kushiya E., Araki K., Meguro H., Masaki H., Kumanishi T., Arakawa M. Molecular diversity of the NMDA receptor channel. Nature. 1992 Jul 2;358(6381):36–41. doi: 10.1038/358036a0. [DOI] [PubMed] [Google Scholar]
- Lambert J. J., Peters J. A., Hales T. G., Dempster J. The properties of 5-HT3 receptors in clonal cell lines studied by patch-clamp techniques. Br J Pharmacol. 1989 May;97(1):27–40. doi: 10.1111/j.1476-5381.1989.tb11920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maricq A. V., Peterson A. S., Brake A. J., Myers R. M., Julius D. Primary structure and functional expression of the 5HT3 receptor, a serotonin-gated ion channel. Science. 1991 Oct 18;254(5030):432–437. doi: 10.1126/science.1718042. [DOI] [PubMed] [Google Scholar]
- Meguro H., Mori H., Araki K., Kushiya E., Kutsuwada T., Yamazaki M., Kumanishi T., Arakawa M., Sakimura K., Mishina M. Functional characterization of a heteromeric NMDA receptor channel expressed from cloned cDNAs. Nature. 1992 May 7;357(6373):70–74. doi: 10.1038/357070a0. [DOI] [PubMed] [Google Scholar]
- Moriyoshi K., Masu M., Ishii T., Shigemoto R., Mizuno N., Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991 Nov 7;354(6348):31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- Neijt H. C., Plomp J. J., Vijverberg H. P. Kinetics of the membrane current mediated by serotonin 5-HT3 receptors in cultured mouse neuroblastoma cells. J Physiol. 1989 Apr;411:257–269. doi: 10.1113/jphysiol.1989.sp017572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neijt H. C., te Duits I. J., Vijverberg H. P. Pharmacological characterization of serotonin 5-HT3 receptor-mediated electrical response in cultured mouse neuroblastoma cells. Neuropharmacology. 1988 Mar;27(3):301–307. doi: 10.1016/0028-3908(88)90048-2. [DOI] [PubMed] [Google Scholar]
- Peters J. A., Hales T. G., Lambert J. J. Divalent cations modulate 5-HT3 receptor-induced currents in N1E-115 neuroblastoma cells. Eur J Pharmacol. 1988 Jul 14;151(3):491–495. doi: 10.1016/0014-2999(88)90550-x. [DOI] [PubMed] [Google Scholar]
- Revah F., Bertrand D., Galzi J. L., Devillers-Thiéry A., Mulle C., Hussy N., Bertrand S., Ballivet M., Changeux J. P. Mutations in the channel domain alter desensitization of a neuronal nicotinic receptor. Nature. 1991 Oct 31;353(6347):846–849. doi: 10.1038/353846a0. [DOI] [PubMed] [Google Scholar]
- Robertson B., Bevan S. Properties of 5-hydroxytryptamine3 receptor-gated currents in adult rat dorsal root ganglion neurones. Br J Pharmacol. 1991 Jan;102(1):272–276. doi: 10.1111/j.1476-5381.1991.tb12165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepfer R., Conroy W. G., Whiting P., Gore M., Lindstrom J. Brain alpha-bungarotoxin binding protein cDNAs and MAbs reveal subtypes of this branch of the ligand-gated ion channel gene superfamily. Neuron. 1990 Jul;5(1):35–48. doi: 10.1016/0896-6273(90)90031-a. [DOI] [PubMed] [Google Scholar]
- Shao X. M., Yakel J. L., Jackson M. B. Differentiation of NG108-15 cells alters channel conductance and desensitization kinetics of the 5-HT3 receptor. J Neurophysiol. 1991 Mar;65(3):630–638. doi: 10.1152/jn.1991.65.3.630. [DOI] [PubMed] [Google Scholar]
- Trussell L. O., Fischbach G. D. Glutamate receptor desensitization and its role in synaptic transmission. Neuron. 1989 Aug;3(2):209–218. doi: 10.1016/0896-6273(89)90034-2. [DOI] [PubMed] [Google Scholar]
- Unwin N. The structure of ion channels in membranes of excitable cells. Neuron. 1989 Dec;3(6):665–676. doi: 10.1016/0896-6273(89)90235-3. [DOI] [PubMed] [Google Scholar]
- Vyklicky L., Jr, Patneau D. K., Mayer M. L. Modulation of excitatory synaptic transmission by drugs that reduce desensitization at AMPA/kainate receptors. Neuron. 1991 Dec;7(6):971–984. doi: 10.1016/0896-6273(91)90342-w. [DOI] [PubMed] [Google Scholar]
- Yakel J. L., Jackson M. B. 5-HT3 receptors mediate rapid responses in cultured hippocampus and a clonal cell line. Neuron. 1988 Sep;1(7):615–621. doi: 10.1016/0896-6273(88)90111-0. [DOI] [PubMed] [Google Scholar]
- Yakel J. L., Shao X. M., Jackson M. B. Activation and desensitization of the 5-HT3 receptor in a rat glioma x mouse neuroblastoma hybrid cell. J Physiol. 1991 May;436:293–308. doi: 10.1113/jphysiol.1991.sp018551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakel J. L., Shao X. M., Jackson M. B. The selectivity of the channel coupled to the 5-HT3 receptor. Brain Res. 1990 Nov 12;533(1):46–52. doi: 10.1016/0006-8993(90)91793-g. [DOI] [PubMed] [Google Scholar]
- Yang J. Ion permeation through 5-hydroxytryptamine-gated channels in neuroblastoma N18 cells. J Gen Physiol. 1990 Dec;96(6):1177–1198. doi: 10.1085/jgp.96.6.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Mathie A., Hille B. 5-HT3 receptor channels in dissociated rat superior cervical ganglion neurons. J Physiol. 1992 Mar;448:237–256. doi: 10.1113/jphysiol.1992.sp019039. [DOI] [PMC free article] [PubMed] [Google Scholar]



