Abstract
Quiescence is a ubiquitous cell cycle stage conserved from microbes through humans and is essential to normal cellular function and response to changing environmental conditions. We recently reported a massive repressive event associated with quiescence in Saccharomyces cerevisiae, where Rpd3 establishes repressive chromatin structure that drives transcriptional shutoff [6]. Here, we describe in detail the experimental procedures, data collection, and data analysis related to our characterization of transcriptional quiescence in budding yeast (GEO: GSE67151). Our results provide a bona fide molecular event driven by widespread changes in chromatin structure through action of Rpd3 that distinguishes quiescence as a unique cell cycle stage in S. cerevisiae.
Keywords: Chromatin, Histone deacetylase, Nucleosome positions, Yeast quiescence, Transcription
| Specifications | |
|---|---|
| Organism/cell line/tissue | Saccharomyces cerevisiae, strain W303 |
| Sex | (Mating Type) MATa |
| Sequencer or array type | Illumina HiSeq 2500 |
| Data format | Raw: merged fastq.gz files Analyzed: RNA-seq: normalized transcript abundance (FPKM values),.xls file MNase-seq: normalized per-base dyad counts,.txt files ChIP-seq: normalized per-base coverage files,.txt files |
| Experimental factors | Cell cycle stage: exponentially-growing, diauxic shift, or isolated quiescent cells Treatment (for ChIP-seq): rapamycin, thiolutin, or none Digestion extent (for MNase-seq): 50% mononucleosomes or 80% mononucleosomes Genotype: wild type, Δrpd3 |
| Experimental features | Analysis of transcript abundance, nucleosome positions, histone density, histone acetylation status, polymerase binding, TFIIB binding, or Rpd3 binding was performed for yeast cells grown to OD600 = 0.4–0.6 (“log cells”), 2 h after glucose was exhausted from rich media (“DS” or “diauxic shift cells”), or after Percoll separation of quiescent cells from 7-day stationary phase cultures (“Q” or “quiescent”) [1]. Control experiments were performed for cells treated with thiolutin or rapamycin to inhibit transcription or mimic starvation, respectively. |
| Consent | Not applicable |
| Sample source location | Not applicable |
1. Direct link to deposited data
Strand-specific RNA sequencing for wild type and Δrpd3 yeast entering quiescence [RNA-seq].
http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE67149.
Genome-wide maps of nucleosome positions in purified quiescent S. cerevisiae cells [MNase-seq].
http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE67148.
Characterization of H3 density, H3 or H4 acetylation, Rpd3 binding, TFIIB binding, and Rpb3(pol II) binding in wild type and rpd3 cells as they transition from logarithmic growth to diauxid shift to quiescence (ChIP-seq).
2. Experimental design, materials and methods
2.1. Methods
2.1.1. Growth conditions and quiescent cell isolation
Cells were grown in YPD from overnight cultures diluted to OD600 = 0.02. Log cells were grown for ~ 8 h to OD600 = 0.4–0.6; diauxic shift cells were grown for ~ 16 h and were harvested exactly 2 h after glucose was no longer detected in the media by glucose test strips (Precision Labs, Inc.); Q cells were purified from stationary phase cultures after 7 days. For thiolutin or rapamycin treatment, cells were grown to OD600 = 0.6 and rapamycin (Millipore, 100 nM final) or thiolutin (abcam, 3 μg/mL final) was added for 60 min at 30 °C. Q cells were separated by Percoll gradient as follows: 9 mL Percoll (GE Healthcare) was combined with 1 mL (1500 mM) NaCl to a final concentration of 150 mM NaCl and spun in 30 mL glass centrifuge tubes at 10,000 × g for 15 min at 4 °C to establish a density gradient. Stationary phase cultures (25 mL) were pelleted and resuspended in 1 mL 10 mM Tris pH 7.5. Resuspended cells were gently layered on top of the Percoll gradient and spun at 400 × g for 60 min at 4 °C [1]. The upper layer of NQ cells was removed and the bottom layer of Q cells was combined with 10 mM Tris pH 7.5, pelleted, and OD600 was measured to determine yield. For rpd3 Q cell isolation, up to 250 mL of cells were grown in 25 mL batches, separated individually, and combined after Q cell purification for further analyses.
2.1.2. RNA-seq
RNA was purified as follows: 100 OD600 units of cells were ground with chilled mortar and pestle in the presence of glass beads in liquid nitrogen until the bead/cell mixture was a fine white powder. Lysed cells were resuspended in 300 μL of TES buffer (10 mM Tris pH 7.5, 10 mM EDTA, 0.5% SDS) and combined with 300 μL acid phenol. Lysate was incubated at 65 °C for 30 min with vortexing every 10 min, then centrifuged 16,000 × g for 10 min at 4 °C. The aqueous layer was extracted once more with 300 μL acid phenol then once with 300 μL chloroform. RNA was precipitated by ethanol precipitation then quantified by NanoDrop. RNA was cleaned using the RNeasy kit with on-column DNase treatment (Qiagen) per the manufacturer protocol. Purified RNA (3 μg) was combined with 1.5 μL of a 1:10 dilution of ERCC Spike-in control mix 1 (Life Technologies) and depleted of rRNA per the manufacturer protocol (Ribo-Zero, Epicenter).
Strand-specific sequencing libraries were constructed from rRNA-depleted samples using the TruSeq RNA Sample Prep Kit v2 (Illumnia) with the following modifications: Superscript III (Invitrogen) was used for the first strand synthesis. Phenol/chloroform extraction, ethanol precipitation, and resuspension in 104 μL of RNase-free water were performed prior to second strand synthesis. Second strand synthesis was performed by first adding second strand buffer (30 μL), 4 μL of 10 mM dNTP mix with dUTP replacing dTTP, 4 μL first-strand buffer, and 2 μL (100 mM) DTT and incubating on ice for 5 min. Then 1 μL RNase H (NEB) and 5 μL DNA Polymerase I (NEB) were added, mixed, and incubated for 2.5 h at 16 °C. cDNA was purified by Qiaquick PCR purification (Qiagen) and eluted into 50 μL buffer EB prior to library construction. After adapter ligation, cDNA was gel-purified (excised between 200 and 300 bp size range) and resuspended in 50 μL buffer EB, then 19 μL of cDNA was incubated with 1 μL USER enzyme (NEB) for 15 min at 37 °C and heat-inactivated for 5 min at 95 °C. All 20 μL of USER-treated cDNA was subject to 15 cycles of amplification according to the TruSeq protocol, then a second gel extraction and selection between 200 and 300 bp was performed prior to sequencing.
Paired end sequencing (50 cycles) was performed with an Illumina HiSeq 2500 on high output mode. Base calling was performed using Illumina CASAVA software. Reads were mapped to the Saccharomyces cerevisiae reference genome [2] (Saccharomyces_cerevisiae.EF4.65.dna.toplevel.fa) appended with sequences from the ERCC control provided by the manufacturer (https://tools.lifetechnologies.com/content/sfs/manuals/ERCC92.zip) using TopHat2 [3]. Aligned reads were filtered for properly mapped primary alignments using SAMtools (− f 3 − F 256) [4]. Biological replicates were highly reproducible and were merged into a single file for downstream analyses. For visualization of data, strands were computationally separated using SAMtools with flags − f 83 and − f 163 for Watson strands or − f 99 and − f 147 for Crick strands, then visualized using Integrated Genome Browser. Initial differential transcript analysis to calculate FPKM prior to global normalization was performed using CuffDiff [9] with a maskfile for tRNA, rRNA, and snRNA. Reads mapping to ERCC spike-in controls were aligned between samples such that all ERCC control transcript FPKM values were equivalent across data sets. RNA content per cell was measured in triplicate to determine the relative number of cells required for equal RNA yield. FPKM values were then scaled to reflect the relative RNA content per cell. Scaling factors were determined to be 5.0 for wild type log cells, 2.5 for wild type DS cells, 1.0 for WT Q cells, 5.0 for Δrpd3 log cells, 3.5 for Δrpd3 DS cells, and 3.0 for Δrpd3 Q cells. For example the ratio of 5.0 to 1.0 for wild type log to wild type Q signifies that log cells contain 5 times as much RNA per cell as Q cells, so after ERCC FPKM values were set equal, FPKM values in log cells were further scaled by a factor of 5 to reflect the global shift in RNA content per cell. We found this to be equivalent and more straightforward than initially adding the RNA spike in relative ratios before library preparation.
2.1.3. MNase-seq
Cells were grown and isolated at the appropriate cell cycle stage, then crosslinked with 1% formaldehyde for 20 min at 30 °C with shaking. Crosslinking was quenched with glycine (125 mM final) then cells were pelleted and washed twice with H2O. Cells were resuspended in 20 mL of [1 M sorbitol, 10 mM beta-mercaptoethanol, 50 mM Tris pH 7.5] and treated with zymolyase (100 T, amsbio). Cells harvested from exponentially-growing cultures in YPD (~ 100OD600 units) or after diauxic shift (~ 125OD600 units) were treated with 2 mg of zymolyase for approximately 20 min at 30 °C. Q cells were treated with ~ 10 mg of zymolyase for 60–90 min at 30 °C. Complete spheroplast production was determined microscopically. Spheroplasts were pelleted at 4000 × g for 20 min and resuspended in 2 mL [1 M sorbitol, 50 mM NaCl, 10 mM Tris pH 7.5, 5 mM MgCl2, 1 mM CaCl2, 0.075% (v/v) Igepal, 0.5 mM spermidine, 1 mM beta-mercaptoethanol]. Three separate digestion reactions (600 μL) were prepared with 30 units of Exonuclease III and 10, 20, or 40 units of Micrococcal Nuclease (MNase, Worthington) for 10 min at 37 °C. Reactions were quenched with 150 μL [5% (w/v) SDS, 50 mM EDTA] and incubated with 0.2 mg Proteinase K overnight at 65 °C. DNA was purified by phenol/chloroform extraction, ethanol precipitated, and resuspended in 10 μg RNase A in 60 μL (1 ×) NEB Buffer 2. Reactions with desired digestion extents (eg 50% mononucleosomes, 80% mononucleosomes) were determined by 2% agarose gel electrophoresis and mononucleosomes were gel-purified for library preparation. Mononucleosomal DNA was resuspended in 50 μL of 1 × NEB Buffer 3 and treated with 10 units alkaline phosphatase (CIP, New England Biolabs) for 1 h at 37 °C. DNA was purified by MinElute PCR Purification kit (Qiagen) and sequencing libraries were constructed using the Illumina TruSeq RNA Sample Prep Kit v2 beginning at the End Repair step according to the manufacturer protocol [8].
Paired end sequencing (50 cycles) was performed with an Illumina HiSeq 2500 on high output mode. Base calling was performed with Illumina CASAVA software and reads were mapped to the S. cerevisiae reference genome (Saccharomyces_cerevisiae.EF4.65.dna.toplevel.fa) using Bowtie2. Aligned reads were filtered for properly paired primary alignments with SAMtools (− f 3 − F 256). For paired reads with an insert size 100 < insert < 200, the midpoint of each read was calculated as a single dyad position (assuming MNase digestion is roughly equal on each side of the nucleosome). Raw dyad counts were piled up into a single coverage file, then data sets were normalized such that the average dyad count at any genomic position (excluding the rDNA locus) was 1.0. The final processed data files are normalized dyad coverage file with an average per-base count of 1. For analysis at transcription start sites (TSS), data within 1 kb of annotated TSS [7] were binned while preserving gene orientation to give average nucleosome dyad signal at a given TSS. For calling of + 1 or − 1 nucleosomes, a 140 bp sliding window was passed through the genome to find local maxima within 100 base pairs of annotated TSS with a threshold average value of 1.0. These conservative criteria identified + 1 and − 1 nucleosomes for 80% of transcription start sites. Nucleosome depleted region (NDR) ranking analysis was performed by calculating the difference in signal between Q cells and log cells from TSS-200 to TSS for each annotated transcription start site.
2.1.4. ChIP-seq
Cells were grown to the appropriate cell cycle stage (~ 70 OD600 units log cells, ~ 125 OD600 units DS cells, or ~ 250 OD600 units purified Q cells) and crosslinked with 1% formaldehyde for 20 min at 30 °C before quenching with a final concentration of 125 mM glycine. Crosslinked cells were pelleted and washed twice with ice cold TBS (150 mM NaCl, 20 mM Tris pH 7.5). Pellets were resuspended in 300 μL [100 mM Tris pH 8.0, 20% (v/v) glycerol] with 300 μL glass beads and subject to 5 min of bead beating (log, DS) or 2 rounds of 5 min bead beating (Q cells). Lysate was combined with 1 mL FA buffer [50 mM HEPES pH 7.6, 150 mM NaCl, 1 mM EDTA, 1% (v/v) Triton X100, 0.1% (w/v) sodium deoxycholate] and centrifuged 16,000 × g for 5 min. Pellet was resuspended in 1 mL FA buffer and sonicated with a Bioruptor sonication bath (Diagenode UCD-200) in a recirculating ice-water bath for 30 min (max output, 30 s on/30 s off). Sheared chromatin was centrifuged 16,000 × g at 4 °C for 30 min and the supernatant was used for chromatin immunoprecipitation. Chromatin immunoprecipitation was performed with 150 μL of sonicated chromatin with 30 μL Protein G-coupled magnetic beads conjugated to the appropriate antibody as detailed in [8]. For each chromatin sample, 50 μL of chromatin was separately sequenced without immunoprecipitation as input DNA. Yield of samples was determined using the Quant-It picogreen dsDNA Assay (Life Technologies). Sequencing libraries were constructed using the NuGEN Ovation Ultralow System per the manufacturer protocol.
Single-end sequencing (50 cycles) was performed on an Illumina HiSeq 2500 on high output mode. Base calling was performed with Illumina CASAVA software and reads were mapped to the S. cerevisiae reference genome (Saccharomyces_cerevisiae.EF4.65.dna.toplevel.fa) using Bowtie2 on “very sensitive” mode. Aligned reads were filtered for primary alignments with SAMtools (− F 256) and converted to per base coverage files. Data were normalized such that the average signal at a genomic location was 1.0, then the ratio of normalized ChIP sample to sample-matched Input was calculated across the genome.
2.1.5. Data analysis
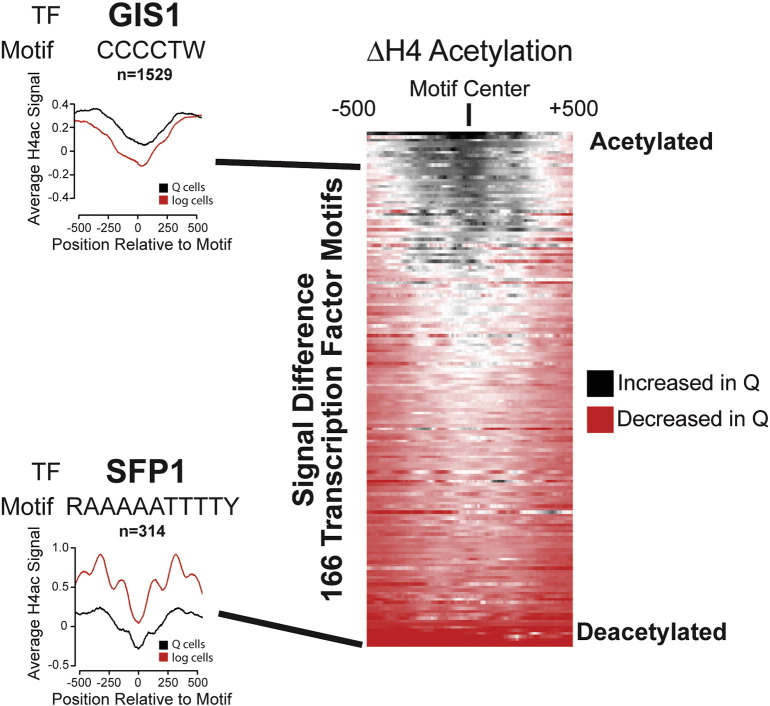
For analyzing features at predicted transcription factor (TF) binding sites, we obtained TF consensus sequences from the JASPAR CORE fungi database [5] (http://jaspar.genereg.net/). Instances of intergenic motifs (excluding the rDNA locus and mitochondrial genome) were obtained using the pattern matching tool from SGD (http://www.yeastgenome.org/cgi-bin/PATMATCH/nph-patmatch). Normalized coverage file data were binned within 500 bp of individual motifs and normalized to the number of instances for each motif to give the average signal (nucleosome dyads or ChIP) at a given motif. Motifs were then ranked based on difference between log and Q cells in within the 500 bp window (Fig. 1). Correlations between transcriptional shutoff, histone density, histone acetylation, and Rpd3 binding at transcription start sites were determined as follows: average acetylation ChIP signal or Rpd3 ChIP signal from (TSS − 300) to (TSS + 400) was calculated or average H3 ChIP signal from (TSS − 300) to (TSS + 200) was calculated for each TSS. Difference in transcription, H3, acetylation, or Rpd3 binding was determined for different growth conditions and Pearson correlations between relevant variables were determined using R software.
Fig. 1.
Transcription factor binding site analysis
Individual profiles for ChIP-seq (log2 H4ac/Input is shown) or MNase-seq data were determined for logarithmically growing (log) cells or purified quiescent (Q) cells within 500 base pairs of intergenic instances of transcription factor binding motifs normalized to the number of motif instances (left). Differences in profiles were determined and ranked for 166 transcription factor motifs from the JASPAR database (right). These analyses were used to implicate transcription factors whose binding is likely regulated during the transition to quiescence in S. cerevisiae.
Acknowledgments
This work was supported by Leukemia & Lymphoma Society CDP-5078-14 for J.N.M., R01GM111428 and NCI P30-CA015704-38 for T.T.
References
- 1.Allen C., Buttner S., Aragon A.D., Thomas J.A., Meirelles O., Jaetao J.E., Benn D., Ruby S.W., Veenhuis M., Madeo F. Isolation of quiescent and nonquiescent cells from yeast stationary-phase cultures. J. Cell Biol. 2006;174:89–100. doi: 10.1083/jcb.200604072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flicek P., Amode M.R., Barrell D., Beal K., Billis K., Brent S., Carvalho-Silva D., Clapham P., Coates G., Fitzgerald S. Ensembl 2014. Nucleic Acids Res. 2014;42:D749–D755. doi: 10.1093/nar/gkt1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim D., Pertea G., Trapnell C., Pimentel H., Kelley R., Salzberg S.L. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14:R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., Marth G., Abecasis G., Durbin R., Genome Project Data Processing, S. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathelier A., Zhao X., Zhang A.W., Parcy F., Worsley-Hunt R., Arenillas D.J., Buchman S., Chen C.Y., Chou A., Ienasescu H. JASPAR 2014: an extensively expanded and updated open-access database of transcription factor binding profiles. Nucleic Acids Res. 2014;42:D142–D147. doi: 10.1093/nar/gkt997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKnight J.N., Boerma J.W., Breeden L.L., Tsukiyama T. Global promoter targeting of a conserved lysine deacetylase for transcriptional shutoff during quiescence entry. Mol. Cell. 2015;59:732–743. doi: 10.1016/j.molcel.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagalakshmi U., Wang Z., Waern K., Shou C., Raha D., Gerstein M., Snyder M. The transcriptional landscape of the yeast genome defined by RNA sequencing. Science. 2008;320:1344–1349. doi: 10.1126/science.1158441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez J., McKnight J.N., Tsukiyama T., Ausubel . Genome-wide Analysis of nucleosome positions, occupancy, and accessibility in yeast: nucleosome mapping, high-resolution histone ChIP, and NCAM. In: Frederick M., editor. Current protocols in molecular biology. Vol. 108. 2014. 21 28 21–21 28 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trapnell C., Roberts A., Goff L., Pertea G., Kim D., Kelley D.R., Pimentel H., Salzberg S.L., Rinn J.L., Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]