Clinically important antiarrhythmic drugs may act by modifying primarily physical properties of the cell membrane.
Abstract
Amiodarone is a widely prescribed antiarrhythmic drug used to treat the most prevalent type of arrhythmia, atrial fibrillation (AF). At therapeutic concentrations, amiodarone alters the function of many diverse membrane proteins, which results in complex therapeutic and toxicity profiles. Other antiarrhythmics, such as dronedarone, similarly alter the function of multiple membrane proteins, suggesting that a multipronged mechanism may be beneficial for treating AF, but raising questions about how these antiarrhythmics regulate a diverse range of membrane proteins at similar concentrations. One possible mechanism is that these molecules regulate membrane protein function by altering the common environment provided by the host lipid bilayer. We took advantage of the gramicidin (gA) channels’ sensitivity to changes in bilayer properties to determine whether commonly used antiarrhythmics—amiodarone, dronedarone, propranolol, and pindolol, whose pharmacological modes of action range from multi-target to specific—perturb lipid bilayer properties at therapeutic concentrations. Using a gA-based fluorescence assay, we found that amiodarone and dronedarone are potent bilayer modifiers at therapeutic concentrations; propranolol alters bilayer properties only at supratherapeutic concentration, and pindolol has little effect. Using single-channel electrophysiology, we found that amiodarone and dronedarone, but not propranolol or pindolol, increase bilayer elasticity. The overlap between therapeutic and bilayer-altering concentrations, which is observed also using plasma membrane–like lipid mixtures, underscores the need to explore the role of the bilayer in therapeutic as well as toxic effects of antiarrhythmic agents.
INTRODUCTION
Cardiac arrhythmias are major causes of morbidity and mortality (Benjamin et al., 1998), with atrial fibrillation, the most prevalent cardiac arrhythmia, being a major risk factor for stroke (Mozaffarian et al., 2015). Amiodarone is the most commonly prescribed antiarrhythmic drug owing to its efficacy and minimal proarrhythmic side effects (Zimetbaum, 2012). Amiodarone acts through multiple mechanisms—prolongation of repolarization, reduction of excitability, and slowing of conduction (Singh, 1983)—exerting these effects by altering the function of diverse membrane proteins: ion channels, ion exchangers, and adrenergic receptors (Heijman et al., 2013b). This multi-target therapeutic mechanism is a feature shared by other antiarrhythmics in current use (Dobrev et al., 2012; Grunnet et al., 2012), suggesting that a multipronged mechanism of action may be a desired feature of antiarrhythmic drugs, although the mechanism for such target promiscuity is unclear.
In the case of membrane proteins, the concurrent regulation of many different proteins could be caused by a common mechanism arising from drug-dependent changes in lipid bilayer properties that alter the energetic coupling between membrane proteins and their host bilayer (Rusinova et al., 2011). This bilayer-mediated mechanism results from hydrophobic coupling between membrane proteins and the surrounding lipid bilayer (Lundbæk et al., 2010b).
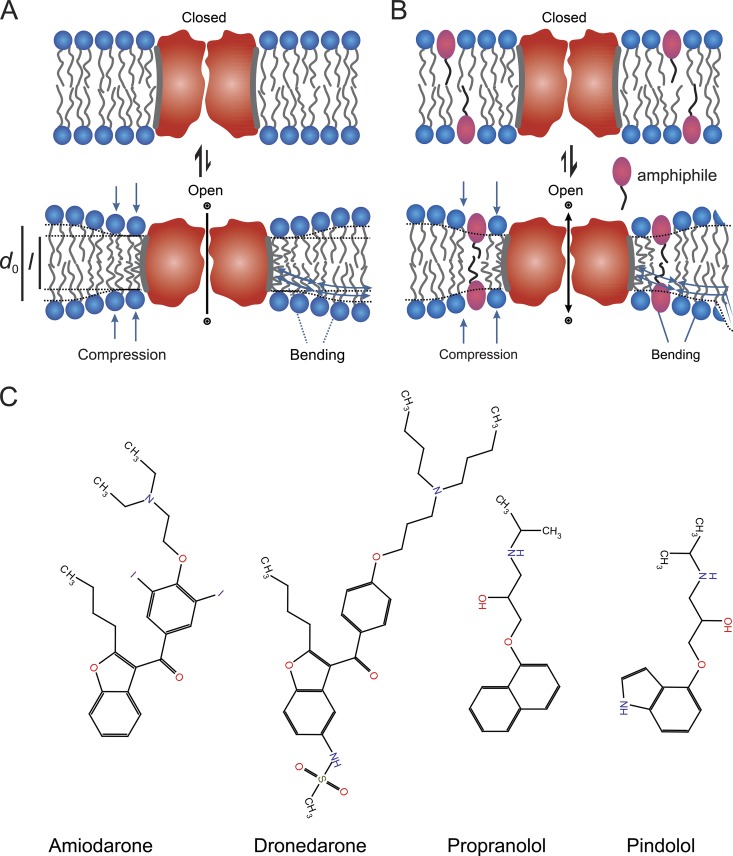
The lipid bilayer adaptation to a membrane protein’s hydrophobic domain has an associated energetic cost, the bilayer deformation energy (), which varies with changes in protein shape and lipid bilayer properties (Nielsen et al., 1998; Nielsen and Andersen, 2000; Partenskii and Jordan, 2002). Different protein conformations (e.g., Lundbæk et al., 2010a) are thus likely to be associated with different (Fig. 1 A). This concept is important because the free energy cost () for a conformational change (between states I and II) is determined by the contributions from the membrane protein () and the bilayer () (e.g., Lundbæk et al., 2010a):
| (1) |
where and vary with changes in bilayer elasticity, thickness, and intrinsic lipid curvature (Andersen et al., 2007), which in turn means that (and therefore ) will vary with changes in bilayer properties (except when the changes in equal the changes in ).
Figure 1.
A schematic illustration of how amphiphilic drugs can modulate membrane protein function by a bilayer-mediated mechanism and structures of the antiarrhythmics. (A) Schematic representation of the bilayer-mediated regulation of membrane protein function, which arises because the reversible partitioning of the amphiphiles between the aqueous solution and the bilayer–solution interface alters lipid bilayer properties, including the elasticity (Evans et al., 1995; Zhelev, 1998; Bruno et al., 2013) and thus ΔGdef (and therefore ). In the figure, conformations I and II are denoted as “closed” and “open,” respectively. (B) Molecular structures of the antiarrhythmics amiodarone, dronedarone, propranolol, and pindolol.
Drug-induced changes in lipid bilayer properties thus will shift a protein’s conformational distribution by changing thereby producing changes in protein function (Fig. 1 B). Such bilayer-mediated regulation is exemplified, for example, in the effects of amphiphiles on voltage-gated sodium (NaV) channels (Lundbæk et al., 2004; Rusinova et al., 2011; Ingólfsson et al., 2014), mechanosensitive MscL, voltage-gated potassium (KV2.1) channels (Ingólfsson et al., 2014), and the proton-gated prokaryotic potassium channel KcsA (Rusinova et al., 2014). Importantly, because the relative changes in function will vary among membrane proteins.
We used gramicidin (gA) channels to quantify how amiodarone, dronedarone, propranolol, and pindolol (Fig. 1 C) alter The bilayer deformation resulting from channel formation makes gA channels powerful probes because changes in the gA monomer↔dimer equilibrium, as reflected in changes in channel activity, can be directly related to changes in bilayer properties, making it possible to quantify (Lundbæk et al., 2010a).
Using a gA-based fluorescence assay (GBFA), we determined the bilayer-modifying potency of each drug (Ingólfsson and Andersen, 2010) and used gA single-channel electrophysiology (Lundbæk et al., 2010a; Rusinova et al., 2011) to obtain detailed information about which bilayer properties are affected and We examined whether the antiarrhythmics alter the properties of bilayers formed from either 1,2-dioleoyl-sn-glycero-3-phosphocholine (DC18:1PC) or the ternary DC18:1PC/bovine sphingomyelin/cholesterol (DC18:1PC/porcine brain sphingomyelin [bSM]/Chol) mixture that mimics the cell membrane outer leaflet. All the antiarrhythmics tested alter lipid bilayer properties, with amiodarone and dronedarone doing so at clinically relevant concentrations. Our results show that, in addition to direct effects on specific targets, amiodarone and dronedarone may alter the function of diverse membrane proteins by a general bilayer-mediated mechanism.
MATERIALS AND METHODS
Materials
DC18:1PC, 1,2-dierucoyl-sn-glycero-3-phosphocholine (DC22:1PC), bSM, and plant cholesterol were from Avanti Polar Lipids, Inc. Dronedarone, amiodarone, propranolol, pindolol (all ≥98% pure), and sodium thiosulfate (Na2S2O3; 99.999% pure) were from Sigma-Aldrich. Iodine (I2; 99.8% pure) was from VWR International. 8-Aminonaphthalene-1,3,6-trisulfonate (ANTS) was from Invitrogen. The gA analogues [Ala1]gA and gA(15), and the sequence-shortened, left-handed des-d-Val-Gly-gA− and gA−(13), used for the single-channel experiments, were synthesized and purified as described previously (Greathouse et al., 1999; see sequences in Table S1). The gA used in the fluorescence quench experiments was the naturally occurring mixture, which is 80–85% gA plus gAs B and C (Abo-Riziq et al., 2006). (The mixture is often called gA D [gD] after R. Dubos, who discovered the gAs [Dubos, 1939].)
GBFA
Large unilamellar vesicles (LUVs) were made from DC22:1PC as described in Ingólfsson and Andersen (2010), with the following modifications. Phospholipids in chloroform and gD in methanol (1,000:1 lipid/gA weight ratio) were mixed, the chloroform and methanol were evaporated under nitrogen stream, and the lipid/gD mix was left overnight in a desiccator under vacuum to remove trace amounts of solvent. Because of the inevitable variation in LUV sizes, the time course of the fluorescence quenching cannot be described by a single-exponential decay, and the quench rates were obtained by fitting a stretched exponential (Berberan-Santos et al., 2005):
| (2) |
to the fluorescence quench time course from each mixing reaction (where F(t) and F(0) denote the fluorescence at times t and 0, respectively; τ0 is a parameter with the dimensions of time; and β, the parameter that defines the deviation from the single exponential, is 0 < β ≤ 1) and evaluating the quench rate at 2 ms (the instrumental dead time is ∼1.5 ms):
| (3) |
To test the drugs’ bilayer-modifying potency, we added aliquots of 50–100-mM stock solutions of the drugs in DMSO—dronedarone, amiodarone (±Na2S2O3), propranolol, pindolol, as well as iodine (±Na2S2O3)—and incubated the LUVs with the drug in question for 10 min at 25°C before determining the fluorescence quench time courses. The DMSO concentration did not exceed 0.5% (vol/vol), a concentration at which it does not alter lipid bilayer properties (Ingólfsson and Andersen, 2010). Each measurement consisted of (four to eight) individual mixing reactions, and the rates for each mixing reaction were averaged and normalized to the control rate in the absence of drug.
Single-channel gA current measurements
gA single-channel recordings were done at 25°C using the bilayer punch method (Andersen, 1983; Rusinova et al., 2011). In brief, planar bilayers were formed from DC18:1PC in n-decane or a DC18:1PC/bSM/Chol in n-decane across a hole in a Teflon partition that separated two 1.0-M NaCl (buffered to pH 7.0 with HEPES), as described previously (Greathouse et al., 1999; Rusinova et al., 2011). The membranes were doped with gA(15) and gA−(13), and control channel activity was recorded after a 30-min equilibration. Aliquots of 10–100-mM DMSO stock solutions of dronedarone, amiodarone (±Na2S2O3), propranolol, pindolol, and I2 (in H2O) were then added to both sides of the bilayer; the solutions were equilibrated with the membrane for 10 min before recording. DMSO did not exceed 1% (vol/vol), a concentration that has no effect on gA channel function (Ingólfsson and Andersen, 2010). Appearance frequencies (f) were determined only if the bilayer remained intact for the duration of the entire experiment (before and after drug addition). The number of gA channel events from one to two recordings before drug addition was determined from the number of channel appearances obtained from the analysis of single-channel lifetimes and divided by the total recording time (≥5 min) to give the control fcntrl. If the bilayer remained intact, in a similar procedure to the one above, fdrug was determined from one to two recordings immediately after a 10-min equilibration with the drug. Single-channel lifetimes (τ) were determined by fitting survivor histograms with a single-exponential distribution (N(t)/N(0) = exp[−t/τ], where N(t) is the number of channels with lifetime longer than time t) using Origin 6.1 (OriginLab). The results represent mean ± SD (n = 2–4) or mean ± range (for n = 2). Relative changes in bilayer deformation energy were calculated as (Artigas et al., 2006; Rusinova et al., 2011):
| (4) |
where fdrug and τdrug denote the gA channel appearance frequency and lifetime in the presence of an antiarrhythmic.
Online supplemental material
Fig. S1 plots the relative changes in gA−(13) and gA(15) τ and f with increasing concentrations of amiodarone, dronedarone, propranolol, and pindolol. Fig. S2 shows effects of antiarrhythmics on gA−(13) and gA(15) current transition amplitude. Fig. S3 (A and B) shows changes in and gA−(13) and gA(15) current transition amplitudes as a function of antiarrhythmic mole fraction in the bilayer. Fig. S3 C plots changes in as a function of changes in gA−(13) and gA(15) current transition amplitudes. Fig. S4 shows time dependence of changes in gA−(13) and gA(15) τ by amiodarone in the absence and presence of Na2S2O3, which reduces I2 to I−. Fig. S5 shows absence of amiodarone-effect time dependence in GBFA experiments. Table S1 lists the sequence and channel hydrophobic length of gA analogues used in this study. The online supplemental material is available at http://www.jgp.org/cgi/content/full/jgp.201511470/DC1.
RESULTS
Effects of antiarrhythmics on lipid bilayer properties
We tested the bilayer-modulating effects of amiodarone, propranolol, and pindolol at concentrations where they have acute effects on cardiac excitability (Kodama et al., 1997; Heijman et al., 2013b) and alter sarcolemmal enzyme activities (Chatelain et al., 1989), and dronedarone at its clinical concentrations (Table 1) using the GBFA.
Table 1.
Nominal and free antiarrhythmic concentrations
| Antiarrhythmic | Clinical plasma concentrations (µM) | LogPa | mmb | ||
| Experimental concentrations (µM) | |||||
| Nominal | Free | ||||
| Dronedarone | 0.15–0.3c | 10 | 7 × 10−5 | 8.75 | 4.0 × 10−2 |
| Amiodarone | 1d | 30 | 1 × 10−3 | 7.8 | 10−1 |
| Propranolol | 0.01–1e | 200 | 90 | 3.48 | 2.5 × 10−1 |
| Pindolol | 0.04f | 300 | 300 | 1.75 | 1.5 × 10−2 |
Estimated following Bruno et al. (2007), Ingólfsson et al. (2007), and Rusinova et al. (2011).
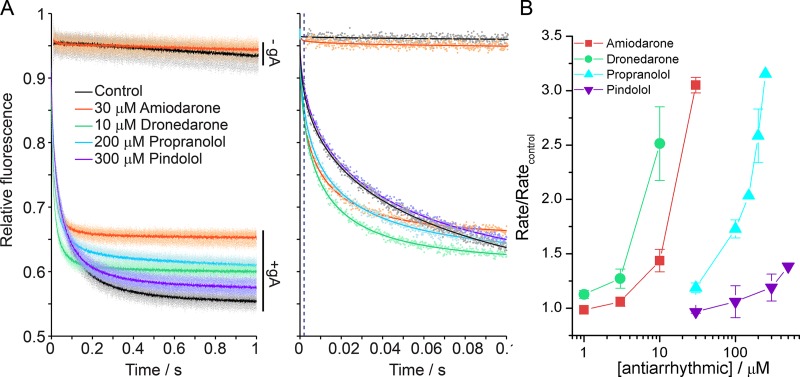
The GBFA takes advantage of gA channels’ permeability to Tl+, a quencher of the water-soluble fluorophore ANTS, where the rate of influx is a function of the time-averaged number of gA channels in the LUV membrane (Ingólfsson and Andersen, 2010). In the absence of gA (top horizontal traces in Fig. 2 A), the drugs have no effect on the rate of fluorescence quenching, meaning that the compounds did not compromise lipid bilayer stability at the concentrations tested. In the presence of the drugs, the gA-dependent fluorescence quench rate increases (bottom traces in Fig. 2 A), demonstrating that the antiarrhythmics increase the rate of Tl+ influx into the LUVs. This increase is caused by a shift in the gA monomer↔dimer equilibrium toward the conducting dimer state as antiarrhythmics decrease and thereby the free energy of dimerization (Andersen et al., 2007):
| (5) |
The relative potency of each antiarrhythmic was quantified by plotting the change in quench rate (normalized to the rate in the absence of drug) as a function of drug concentration (Fig. 2 B).
Figure 2.
Antiarrhythmics alter lipid bilayer properties. (A, left) Fluorescence quench traces showing Tl+ quenching of ANTS fluorescence in DC22:1PC LUVs without gA (−gA; the top two traces) and with gA (+gA; the bottom five traces) in the absence of drug (black, control) or with dronedarone (green), amiodarone (orange), propranolol (cyan), and pindolol (purple). Amiodarone, dronedarone, and propranolol increase the fluorescence signal up to 12% depending on the concentration, but the flux rate measurements were not affected. The results for each drug represent five to eight repeats (dots) and their averages (solid lines). (Right) Single repeats (dots) with stretched exponential fit (solid line). (B) Normalized quench rates determined from the stretched exponential fits at varying antiarrhythmic concentrations. Error bars represent mean ± SD (n = 3 – 5).
The antiarrhythmics decreased with rank order: dronedarone ≥ amiodarone > propranolol > pindolol.
The concentrations used in Fig. 2 are the nominal concentrations, which are not corrected for drug distribution between the aqueous and membrane phases. We estimated the aqueous concentrations following Bruno et al. (2007) using measured partition coefficients into lipid bilayers, LogP, and compared those to the free plasma concentrations at therapeutic doses (Table 1). The free concentrations of amiodarone and dronedarone, the most hydrophobic compounds, are comparable to the clinical concentrations, whereas the free concentrations of the less hydrophobic propranolol and pindolol are orders of magnitude higher than the clinical concentrations (Anavekar et al., 1975; Woosley et al., 1979), although there is overlap between the concentration range where propranolol alters lipid bilayer properties and the concentration range where it alters the function of voltage-dependent sodium channels (Wang et al., 2010).
The lipid bilayer–modifying potency of the tested antiarrhythmics need not scale with the compounds’ bilayer-partitioning coefficient (Bruno et al., 2007; Rusinova et al., 2011). At the given partitioning coefficient and concentration, antiarrhythmics reach varying mole fraction in the membrane (Table 1, mm) to achieve similar magnitudes of bilayer-modifying effects (Fig. 2 B), where dronedarone requires approximately an order of magnitude lower mm than propranolol for comparable effects; to achieve a comparable effect with pindolol would require a much higher mm than for the other compounds.
Antiarrhythmics increase bilayer elasticity, which contributes to the decrease in ΔGbilayer
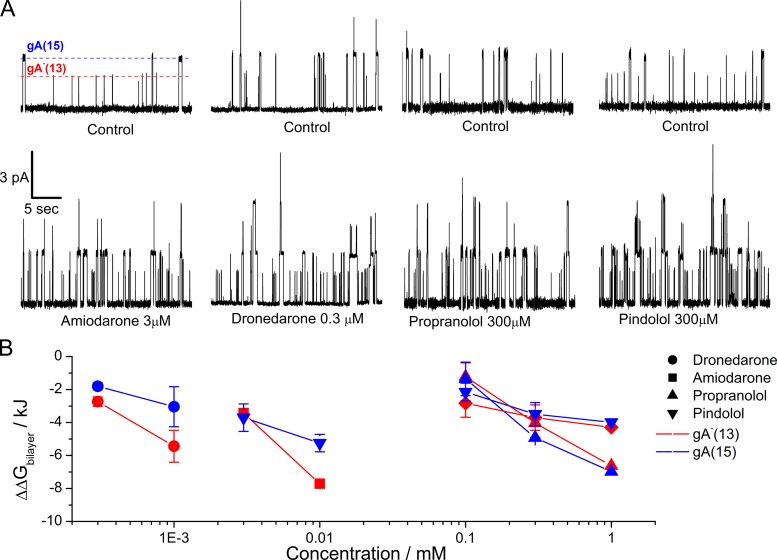
To determine what bilayer properties the antiarrhythmics alter, we determined the effects on the average single-channel lifetimes (τ), appearance frequencies (f), and current transition amplitudes (compare Andersen et al., 2007; Lundbæk et al., 2010a; Rusinova et al., 2011). The gA single-channel τ is the inverse of the gA dimer dissociation rate constant, and f is proportional to monomer association rate constant. Changes in τ and f in the presence of a bilayer-modifying amphiphile reflect the amphiphile’s effect on the bilayer deformation energy, and the relative changes in τ and f allow us to estimate the amphiphile-induced change in the bilayer deformation energy, ΔΔGbilayer (Eq. 4). Because the experiments were done with gA analogues of different lengths, the 15–amino acid gA(15) and the enantiomeric, sequence-shortened analogue gA−(13), respectively, which form channels of different lengths (Table S1) and thus different bilayer-channel hydrophobic mismatches. Consequently, if a drug alters bilayer elasticity, the magnitude of the resulting changes in τ and f will be larger for the channels with the larger hydrophobic mismatch (in this case those formed by the gA−(13) subunits). Conversely, if the changes in bilayer deformation are similar for channels of different length, the drugs have minimal effects on bilayer elasticity.
All the antiarrhythmics increased τ and f (Figs. 3 A and S1, and Table 2). The initial experiments with amiodarone showed a time-dependent increase in channel activity (Fig. S4), which we surmised might be caused by the iodine in amiodarone. We therefore did experiments in which we added Na2S2O3, which reduces I2 to I− (Finkelstein and Cass, 1968), together with the amiodarone. Na2S2O3 did indeed abolish the time-dependent increase in channel activity (lifetime), but the effect of amiodarone could not be mimicked by adding I2 (Fig. S4, legend). In any case, all the experiments with amiodarone were done in the presence of 50 µM Na2S2O3. Dronedarone and amiodarone produced larger changes in τ and f for the shorter gA−(13) channels, as compared with the longer gA(15) channels; within experimental error, propranolol and pindolol produced similar changes in τ and f for both channels (Table 2 and Fig. S1).
Figure 3.
Antiarrhythmics increase gA single-channel activity and decrease the bilayer deformation energy (). (A) gA single-channel traces without (top row) and with (bottom row) the antiarrhythmics at the indicated concentrations; red and blue dashed lines indicate the average gA−(13) and gA(15) single-channel current amplitudes. (B) Changes in which were estimated from the ratio of the time-averaged number of gA channels in the presence (τdrug · fdrug) and absence (τ · f) of the antiarrhythmic (compare Eq. 4). Blue symbols denote results for gA(15) channels, and red symbols denote results for gA−(13) channels. Error bars represent mean ± SD, if n ≥ 3; mean ± range/2, if n = 2.
Table 2.
Relative increases in the antiarrhythmic-induced gA channel appearance frequencies and lifetimes
| gA single-channel properties | Amiodarone | Dronedarone | Propranolol | Pindolol | |||||||
| 3 µM | 10 µM | 0.3 µM | 1 µM | 3 µM | 100 µM | 300 µM | 1,000 µM | 100 µM | 300 µM | 1,000 µM | |
| f13/f13cntrl | 2.5 ± 0.7 | 6.5 ± 0.3 | 2.1 ± 0.1 | 4.2 ± 1.2 | 1.6 ± 0.4 | 4.4 ± 0.3 | 9 | 3 ± 1 | 4.0 ± 1.2 | 5 | |
| f15/f15cntrl | 3.1 ± 0.5 | 3.6 ± 1.2 | 1.7 ± 0.03 | 2.2 ± 0.7 | 1.8 ± 0.6 | 6.5 ± 0.2 | 11 | 2.2 ± 0.4 | 3.4 ± 1.1 | 4 | |
| τ13/τ13cntrl | 1.6 ± 0.2 | 3.5 ± 0.2 | 1.5 ± 0.1 | 2.2 ± 0.2 | 4.6 ± 0.8 | 1.1 ± 0.1 | 1.2 ± 0.03 | 1.8 ± 0.2 | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.4 ± 0.3 |
| τ15/τ15cntrl | 1.5 ± 0.3 | 2.3 ± 0.04 | 1.2 ± 0.1 | 1.6 ± 0.3 | 3.0 ± 0.8 | 1.0 ± 0.05 | 1.1 ± 0.1 | 1.4 ± 0.02 | 1.1 ± 0.1 | 1.2 ± 0.2 | 1.3 ± 0.1 |
Values represent relative changes in gA channels’ single-channel appearance frequencies (f) and lifetimes (τ), where f or τ in the presence of antiarrhythmics is normalized to that in the absence of antiarrhythmic in the same experiment.
Knowing the relative changes in τ and f (Table 2), we can estimate the changes in ΔGbilayer following Artigas et al. (2006), Andersen et al. (2007), Lundbæk et al. (2010a), Rusinova et al. (2011), Eq. 4, and Fig. 3 B. All the antiarrhythmics decreased ΔGbilayer, with dronedarone being the most, and pindolol the least, potent, whether they increased or decreased membrane fluidity (Chatelain et al., 1985).
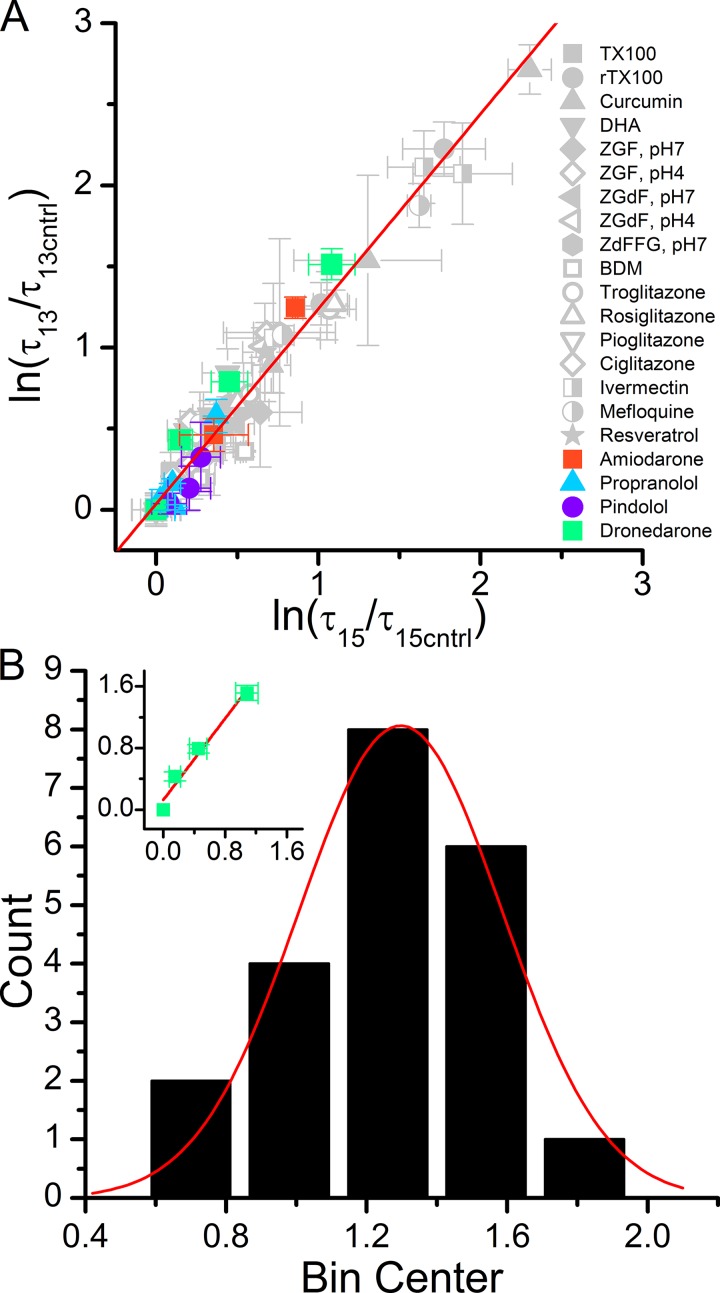
Confirming previous studies (Lundbæk et al., 2010b; Rusinova et al., 2011), structurally diverse compounds produce remarkably similar relative increases in the lifetimes of the short gA−(13) channels relative to the long gA(15) channels. Fig. 4 A shows the linear relation between the natural logarithms of the changes in τ for the short gA−(13) channels ln[τ13/τ13cntrl] versus the changes for the long gA(15) channels ln[τ15/τ15cntrl], where we included earlier results from Rusinova et al. (2011) and Ingólfsson et al. (2014).
Figure 4.
The antiarrhythmic-induced changes in the single-channel lifetimes of gA−(13) channels versus the changes in the lifetimes of gA(15) channels. (A) Natural logarithm of relative changes in τ13 (ln(τ13/τ13cntrl)) versus the natural logarithm of relative changes in τ15 (ln(τ15/τ15cntrl)) observed for dronedarone (green), amiodarone (orange circle), propranolol (blue triangle), and pindolol (black square) plotted together with results from Lundbæk et al. (2010b) and Rusinova et al. (2011). The points cluster around a straight line with slope 1.2 ± 0.03 (error bars represent mean ± SE). (B) Distribution of the slopes for the lnτ13 versus lnτ15 relations for the individual compounds in A. The distribution is fit by Gaussian function with a mean ± SD (σ calculated from the fit) of 1.3 ± 0.2, σ = 0.3. Changes in the histogram bin size result in the median slope ranging between 1.2 and 1.3. Inset illustrates an individual linear fit to the (ln(τ13/τ13cntrl)) versus ln(τ15/τ15cntrl) in the presence of dronedarone (green symbols). Slopes of the linear fits, such as that in the inset, obtained for each compound were used to construct the distribution in B.
The overall similarity among the ln[τ13/τ13,cntrl] versus ln[τ15/τ15,cntrl] relations for different compounds, together with the similar effects on left- and right-handed channels (the channels formed by gA−(13) and gA(15) have opposite chirality), shows that the changes in gA activity primarily are caused by changes in general bilayer properties that are insensitive to the structural characteristics of the compounds, as opposed to direct interactions with gA itself. The slope of the ln[τ13/τ13,cntrl] versus ln[τ15/τ15,cntrl] relation is 1.20 ± 0.03 (lower and upper confidence limits: 1.14 and 1.25), regardless of the particular compound’s bilayer-modifying potency (Fig. 4 A). Focusing on the individual antiarrhythmics, the slopes of the ln[τ13/τ13,cntrl] versus ln[τ15/τ15,cntrl] relationships are: 1.3 ± 0.2 for dronedarone, 1.5 ± 0.1 for amiodarone, 1.5 ± 0.3 for propranolol (Fig. 4 B, inset), and 1.1 ± 0.3 for pindolol. In the case of propranolol, however, given the miniscule changes in τ (Table 2 and Fig. S1), slope of the ln[τ13/τ13,cntrl] versus ln[τ15/τ15,cntrl] relation does not differ from 1. A histogram of individual slopes can be fit with a Gaussian with a mean of 1.30 ± 0.02, σ = 0.3 (Fig. 4 B), suggesting that there may be additional compound structure–dependent effects on the lipid bilayer, because of their varying effects on the acyl chain packing and dynamics in the bilayer core, in addition to the increase in bilayer elasticity that arises for thermodynamic reasons (Evans et al., 1995; Needham et al., 1998; Bruno et al., 2013).
In addition to the changes in τ and f, the antiarrhythmics reduced the current transition amplitudes of both gA(15) and gA−(13) channels (Fig. S2). The changes in current transition amplitudes most likely arise because charged amphiphiles that adsorb to the bilayer–water interface in the vicinity of a gA channel will impart a surface potential that will alter the local ion concentrations and thus the single-channel current amplitude transitions (Apell et al., 1979; Lundbæk et al., 1997; Bruno et al., 2007, 2013; Rusinova et al., 2011). The antiarrhythmics are secondary and tertiary amines with pKs of ∼9, and will thus impart a positive surface charge when they partition into the membrane–solution interface at pH 7.0 (Froud et al., 1986). The positive charge will reduce the cation concentration near the pore entrance, which would account for the reduction in current transition amplitudes, which is opposite to the increases that were observed with the negatively charged polyunsaturated fatty acid, docosahexaenoic acid, which increases the current transition amplitudes (Bruno et al., 2013). We cannot completely rule out the possibility that the antiarrhythmics interact directly with gA channels, but the similar effects on left- and right-handed channels, the correlation between the magnitude of the current transition amplitude reduction and the antiarrhythmic’s LogP (Table 1), as well as the correlation between antiarrhythmic mole fractions at which the current transition amplitude shift occurs (Fig. S3 A) and at which they decrease ΔGbilayer (Fig. S3 B) all suggest that accumulation of surface charge is a primary determinant of the decreased current transition amplitudes. Plotting the changes in ΔGbilayer as a function of the changes in current transition amplitude, (icntrl − i)/icntrl (Fig. S3 C), show that all the antiarrhythmics produce similar slopes in the ΔGbilayer versus (icntrl − i)/icntrl relations for the gA(15) channels, indicating that the two parameters correlate and depend in a similar manner on the mole fraction of the compound in the bilayer. For the gA−(13) channels, the slopes of the ΔGbilayer versus (icntrl − i)/icntrl relations for pindolol and propranolol are similar to those for gA(15), whereas the slopes for dronedarone and amiodarone are larger (as would be expected because dronedarone and amiodarone increase bilayer elasticity).
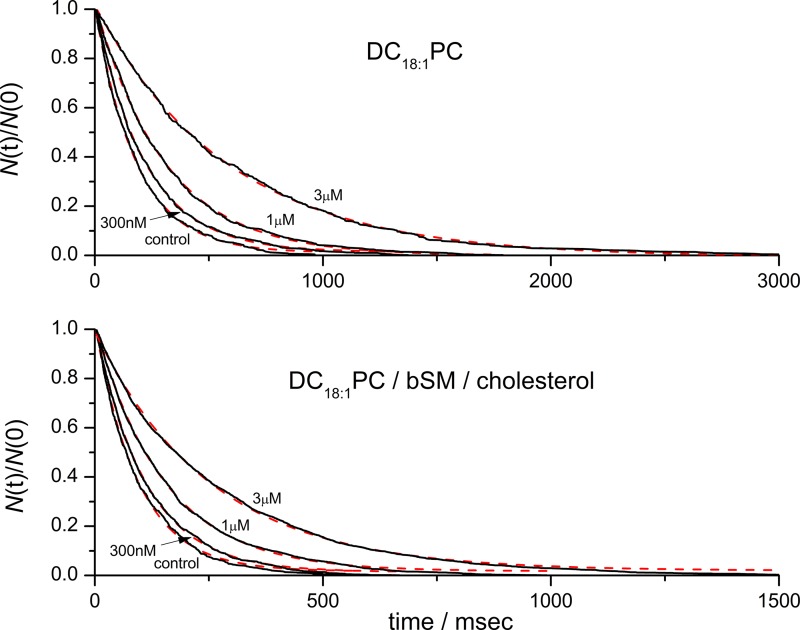
Experiments with ternary lipid bilayer mixtures
Experiments in DC18:1PC bilayers yield unambiguous information that can be used to calculate changes in the bilayer deformation energy caused by reversible partitioning (Artigas et al., 2006; Andersen et al., 2007; Lundbæk et al., 2010a; Rusinova et al., 2011) of drugs without the complications of domain reorganization and heterogeneous phospholipid mixing that may confound the quantification of effects on more complex bilayers. The bilayers of cell membranes, however, have complex lipid compositions (Wenk, 2005; van Meer et al., 2008). To explore whether the antiarrhythmics alter bilayer properties in bilayers with heterogenous lipid composition, where their effects could be dampened or obscured (or, maybe, enhanced) by lipid redistribution and changes in domain organization (e.g., Heerklotz, 2002; Heerklotz et al., 2003), we did experiments using DC18:1PC/bSM/Chol (1:1:1) mixtures, where there is coexistence of liquid-ordered and liquid-disordered domains (Veatch and Keller, 2005; Baumgart et al., 2007; Petruzielo et al., 2013). If gA forms conducting dimers in both the liquid-ordered and liquid-disordered domains that have different properties (i.e., stiffness), we would expect this to differentially alter gA single-channel properties, which would be observed as two populations of channels with different average lifetimes (τ). We observe only a single, kinetically homogeneous channel population, however (Fig. 5). The parsimonious interpretation of these results is that in the stiffer liquid-ordered domains is so much larger than in the liquid-disordered domains (e.g., Lundbæk et al., 2003) that we observe gA channel activity only in the liquid-disordered domains—and that the antiarrhythmics do not soften the liquid-ordered domains sufficiently to allow for channel formation.
Figure 5.
Survivor single-channel lifetime histograms for gA(15) in DC18:1PC and DC18:1PC/bSM/Chol bilayers with single-exponential fits. There is no evidence for the existence of more than one population of channels in either membrane.
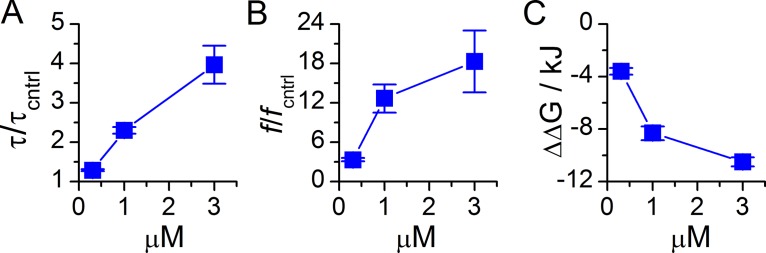
Dronedarone produced similar changes in the reference DC18:1PC bilayers and in bilayers formed from DC18:1PC/bSM/Chol 1:1:1 (Fig. 6), as a mimic of the extracellular leaflet of the plasma membrane (Feigenson, 2007). Dronedarone increased both τ and f for gA(15) channels over a similar concentration range as in DC18:1PC membranes, but to a greater extent (Table 2 and Figs. S1 and 6, A and B): at 3 µM dronedarone, ΔΔGbilayer was doubled from 5.2 kJ/mole in DC18:1PC to 10.5 kJ/mole in DC18:1PC/bSM/Chol (compare Figs. 3 B and 6 C). The increased effect on ΔΔGbilayer most likely reflects that the liquid-ordered domains in the ternary mixture are stiffer than the DC18:1PC bilayers, which would lead to larger absolute changes in the amphiphile-induced changes in bilayer elasticity (Bruno et al., 2013). In any case, these results show that there are no qualitative differences in the antiarrhythmics’ effects on single-component and multicomponent bilayers.
Figure 6.
Dronedarone alters gA(15) channel function in bilayers formed from ternary DC18:1PC/bSM/Chol 1:1:1 mixtures. (A) Relative changes in τ. (B) Relative changes in f. (C) The decrease in Error bars represent mean ± range/2 (n = 2).
DISCUSSION
A hallmark of many current and in-development antiarrhythmics is their effects on numerous, diverse membrane proteins (Singh, 1983; Dobrev et al., 2012; Zimetbaum, 2012), as is the case for amiodarone and dronedarone (Kodama et al., 1997; Zimetbaum, 2012; Heijman et al., 2013a). Although well established, the mechanism(s) underlying these multi-target effects remains unresolved. The prevailing paradigm attributes drug regulation of protein function to direct interactions (binding) of a drug to its target, but this paradigm does not readily explain the multi-targeting behavior over a narrow effective concentration range. So, alternatively, amiodarone and dronedarone could act through a common, more general mechanism such as drug-induced changes in the energetic coupling between the bilayer-embedded proteins and their host bilayer (Sackmann et al., 1984; Andersen et al., 1992; Keller et al., 1993; Lundbæk and Andersen, 1994). To explore this possibility, we took advantage of the gA channels’ sensitivity to changes in lipid bilayer properties to determine whether amiodarone and dronedarone, as well as propranolol and pindolol (Chatelain et al., 1989), might alter the energetic coupling between a well-defined reporter channel and the host lipid bilayer.
Amiodarone and dronedarone alter lipid bilayer properties at their clinical, pharmacologically relevant (Kodama et al., 1997; Zimetbaum, 2012; Heijman et al., 2013a) concentrations (Table 1). In contrast, propranolol and pindolol block β-adrenergic receptors (Zimetbaum, 2012) and have their clinical effects in the micromolar concentration range (Anavekar et al., 1975; Woosley et al., 1979), which does not overlap with the concentrations at which they alter lipid bilayer properties (Table 1).
We first discuss the generality of drug-induced changes in lipid bilayer properties. We then consider how, despite the generality, different amphiphiles alter different bilayer properties and the time-dependent effects of amiodarone. We finally discuss the implications of our results for target promiscuity and a bilayer-dependent mechanism for polypharmacology.
Generality of amphiphile-induced changes in lipid bilayer properties
Dronedarone is a more potent bilayer modifier in DC18:1PC/bSM/Chol 1:1:1 bilayers, producing a twofold larger reduction in than in DC18:1PC bilayers (Figs. 3 B and 6 C). Similarly, we have shown previously that amphiphiles produce greater increases in gA channel activity in cholesterol-containing bilayers, as compared with cholesterol-free membranes (Bruno et al., 2007; Rusinova et al., 2011). The increased effect in cholesterol-containing bilayers reflects that cholesterol increases bilayer thickness (Simon et al., 1982; Gandhavadi et al., 2002) and elastic moduli (Needham and Nunn, 1990), making it energetically more costly to deform cholesterol-containing bilayers (Lundbæk et al., 2003). The increased stiffness further causes the amphiphile-induced changes in bilayer stiffness to be increased (Bruno et al., 2013), which also contributes to the greater changes in channel function.
As is the case for other amphiphiles (Lundbæk et al., 2005, 2010b; Greisen et al., 2011), all four antiarrhythmics produce greater relative changes in f than in τ, consistent with the notion that gA dimer formation involves a large decrease in local membrane thickness, whereas dimer dissociation involves only a modest axial separation of the monomers (Huang, 1986; Lundbæk et al., 2010b). This is evident also when comparing the effects of dronedarone on gA(15) in DC18:1PC and DC18:1PC/bSM/Chol bilayers. Indeed, 1 µM dronedarone increased f by a factor of 2.2 in DC18:1PC versus 12.7 in DC18:1PC/bSM/Chol bilayers; yet the relative changes in τ were only 1.6 versus 2.3, respectively (Table 2 and Figs. S1 and 6, A and B), reflecting the greater decrease in ΔGbilayer (and greater stiffness) in the ternary mixture as compared with DC18:1PC bilayers (Figs. 3 B and 5 C). Not surprisingly, therefore the ΔGbilayer versus (icntrl − i)/icntrl relation for gA(15) channels in DC18:1PC/bSM/Chol bilayers is steeper than the relation in DC18:1PC bilayers (Fig. S3 C).
We conclude that amphiphiles alter lipid bilayer properties generally, but that the magnitude of the changes in ΔGbilayer varies with changes in the membrane lipid composition. We further note that amphiphile-induced changes in integral membrane protein function are related to the changes in gA channel function in single-component bilayers (Hwang et al., 2003; Lundbæk et al., 2005; Bruno et al., 2007; Ingólfsson et al., 2007, 2014; Rusinova et al., 2011), and changes in (NaV) function in cell membranes scale with the changes in gA function in single-component bilayers (Lundbæk et al., 2005; Rusinova et al., 2011; Herold et al., 2014). That is, the results with gA channels in well-defined bilayer systems relate well to the results in the much more complex situation in cellular membranes, even though the conformational changes in membrane proteins are far more complex than the gA monomer↔dimer transitions. In either case, however, the bilayer deformation energies for the different states are functions of the same changes in bilayer properties, which allows for the use of gA channels as probes for changes in lipid bilayer properties and in membrane protein function.
What bilayer properties are altered by amphiphiles?
Amphiphile-induced changes in lipid bilayer properties (Schreier et al., 2000) have often been attributed to changes in bilayer fluidity (e.g., Gordon et al., 1980; Chatelain et al., 1989), but changes in membrane fluidity per se cannot account for changes in the energetics of membrane protein conformational change or membrane protein function (Lee, 1991). Moreover, apparent changes in fluidity may reflect changes in the free volume of the membrane, which would be proportional to changes in membrane tension (Markin and Sachs, 2015) and, using the polymer brush model (Rawicz et al., 2000), also the elastic moduli. Increases in membrane tension increase gA activity by effectively reducing the hydrophobic mismatch (Huang, 1986; Goulian et al., 1998). Indeed, Chatelain et al. (1989) found that amiodarone and propranolol alter bilayer fluidity in opposite directions but have similar effects on membrane protein function, which is not consistent with either changes in fluidity per se or changes in tension/bilayer thickness being the primary determinant of the changes in membrane protein function.
Amiodarone and dronedarone alter channel τ and f in a hydrophobic mismatch-dependent manner, indicating that amiodarone and dronedarone increase bilayer elasticity and thereby reduce the bilayer deformation energy associated with channel formation (Lundbæk et al., 2005; Andersen et al., 2007). Propranolol and pindolol, in contrast, produced similar increases in f and minimal increases in τ for the short gA−(13) and the long gA(15) channels (Table 2 and Fig. S1), with the relative increases in f being larger than the increases in τ (pindolol produced no increase in τ). That is, propranolol and pindolol do not increase bilayer elasticity. Within the framework provided by the theory of elastic bilayer deformations, they alter the intrinsic curvature: positive changes in curvature will decrease (Nielsen and Andersen, 2000).
Time-dependent effects of amiodarone
Chronic use of amiodarone is associated with toxicity (Kodama et al., 1997) and prolongation of action potential duration, presumably caused by decreased IKs and Ito current densities (Kodama et al., 1999; Kamiya et al., 2001). The prevention of structural remodeling of the atria by chronic amiodarone is thought to result from gene expression regulation by amiodarone’s antagonism of thyroid hormone (triiodothyronine) action on nuclear receptors (Kodama et al., 1997). Changes in mRNA levels for the channels responsible for IKs and Ito, however, do not account for all the effects observed with chronic amiodarone (Kamiya et al., 2001). Some of these effects may be caused by the iodine in amiodarone and the known toxicity of iodine (Wolff and Chaikoff, 1948). Specifically, iodine can oxidize carbon–carbon double bonds in fatty acids (Knothe, 2002), for example, the oleic acid in DC18:1PC, and we sought to determine whether the time-dependent amiodarone effects (Fig. S4) could be caused by such changes in the acyl chains in DC18:1PC. Although amiodarone produced time-dependent increases in channel activity planar lipid bilayers (Fig. S4), it did not do so in LUVs (Fig. S5), where the lipid/amiodarone ratio is 10-fold higher, which would tend to reduce the effect of any covalent changes in the acyl chains. More importantly, I2 alone did not mimic the effect of amiodarone in planar bilayers (Fig. S4, legend) or in LUVs (Fig. S5). It is not clear whether the time-dependent effects of amiodarone on planar lipid bilayers could underlie some of its pharmacologic/toxic effects during chronic use.
Target-promiscuity and bilayer-mediated mechanisms for altering membrane protein function
The concepts of drug promiscuity (Insel, 1988; Mencher and Wang, 2005) and polypharmacology (Roth et al., 2004; Peters, 2013) denote the effects of a drug on multiple targets, usually with an assumption of direct binding to the target proteins. Our results, together with those in earlier studies (Hwang et al., 2003; Lundbæk et al., 2005; Bruno et al., 2007; Ingólfsson et al., 2007, 2014; Rusinova et al., 2011), suggest an alternative mechanism for drug promiscuity/polypharmacology for membrane proteins, namely that the drug in question alters the physical properties of the common feature among all membrane proteins: their host lipid bilayer. Amiodarone and dronedarone alter bilayer properties at clinically relevant concentrations and alter the function of multiple membrane proteins, suggesting that their promiscuity, and maybe even their clinical effects, may be due, at least in part, to their effects on the lipid bilayer, thus representing a novel form of polypharmacology. Although the list of protein targets for amiodarone and dronedarone is effectively the same, dronedarone’s efficacy is disputable and, considering its low bioavailability (Heijman et al., 2013a) compared with amiodarone (Latini et al., 1984), pharmacokinetics may play an important role in the differences in efficacy between these two drugs.
Many other current drugs similarly exhibit multichannel effects (Heijman et al., 2013b), and some drug targets, such as NaV1.5, are mechanosensitive (Morris and Juranka, 2007; Beyder et al., 2010). For example, ranolazine, a novel multichannel antiarrhythmic (Heijman et al., 2013b), reduces the mechanosensitivity of NaV1.5 channels in mouse cardiomyocytes, which may be caused by changes in lipid bilayer properties (Beyder et al., 2012), as well as the shear-stretch sensitivity of endogenous voltage-dependent currents in cell lines derived from human atrial myocytes (Strege et al., 2012). (Table 2 in Morris and Juranka [2007] summarizes drug effects on a variety of NaV channels.) We have previously found a correlation between drug off-target effects and bilayer-modifying potency in a family of insulin-sensitizing drugs—the thiazolidinediones—where they altered bilayer properties at the same concentration as their effects on the NaV (Rusinova et al., 2011). Moreover, the rank order of the bilayer-modifying potency coincided with severity of their side effects, with troglitazone being the most effective but also the most toxic and the most bilayer modifying.
Conclusion
The bilayer-mediated regulation of membrane protein function is fundamentally nonspecific, meaning that drugs that alter bilayer properties will have unintended off-target and pleiotropic effects that may negatively impact their toxicity profiles but also may be beneficial in the case of drugs that alter system properties, such as antiarrhythmics.
Despite the promiscuity that is implicit with a common bilayer-mediated mechanism, the relative changes in the function of any specific protein will depend on the amphiphile-induced changes in for that protein. Our results thus suggest that a bilayer-mediated mechanism may explain the ability of antiarrhythmics (and other amphiphilic drugs) to regulate multiple targets within a narrow concentration range. These results furthermore underscore the importance of considering bilayer-mediated effects in drug development as contributing to both beneficial and detrimental off-target and pleiotropic effects.
Supplementary Material
Acknowledgments
We thank E. Ashley Hobart for assistance with some of the initial experiments. We thank Douglas B. Sawyer for suggesting that we explore the effects of amiodarone on lipid bilayers, and an anonymous reviewer for comments that helped us improve the presentation.
This work was supported by National Institutes of Health grant GM021342 to O.S. Andersen.
The authors declare no competing financial interests.
Donald W. Hilgemann served as guest editor.
Footnotes
Abbreviations used in this paper:
- ANTS
- 8-aminonaphthalene-1,3,6-trisulfonate
- bSM
- porcine brain sphingomyelin
- DC18:1PC
- 1,2-dioleoyl-sn-glycero-3-phosphocholine
- DC22:1PC
- 1,2-dierucoyl-sn-glycero-3-phosphocholine
- gA
- gramicidin A
- GBFA
- gA-based fluorescence assay
- LUV
- large unilamellar vesicle
- NaV
- voltage-gated sodium
References
- Abo-Riziq A., Crews B.O., Callahan M.P., Grace L., and de Vries M.S.. 2006. Spectroscopy of isolated gramicidin peptides. Angew. Chem. Int. Ed. Engl. 45:5166–5169. 10.1002/anie.200601516 [DOI] [PubMed] [Google Scholar]
- Anavekar S.-N., Louis W.J., Morgan T.O., Doyle A.E., and Johnston C.I.. 1975. The relationship of plasma levels of pindolol in hypertensive patients to effects on blood pressure, plasma renin and plasma noradrenaline levels. Clin. Exp. Pharmacol. Physiol. 2:203–212. 10.1111/j.1440-1681.1975.tb03026.x [DOI] [PubMed] [Google Scholar]
- Andersen O.S. 1983. Ion movement through gramicidin A channels. Single-channel measurements at very high potentials. Biophys. J. 41:119–133. 10.1016/S0006-3495(83)84414-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen O.S., Sawyer D.B., and Koeppe R.E. II. 1992. Modulation of channel function by the host bilayer. Biomembrane Structure and Function. Easwaran K.R.K. and Gaber B., Adenine Press, Schenectady, NY: 227–244. [Google Scholar]
- Andersen O.S., Bruno M.J., Sun H., and Koeppe R.E.I. II. 2007. Single-molecule methods for monitoring changes in bilayer elastic properties. Methods Mol. Biol. 400:543–570. 10.1007/978-1-59745-519-0_37 [DOI] [PubMed] [Google Scholar]
- Apell H.J., Bamberg E., and Läuger P.. 1979. Effects of surface charge on the conductance of the gramicidin channel. Biochim. Biophys. Acta. 552:369–378. [DOI] [PubMed] [Google Scholar]
- Artigas P., Al’aref S.J., Hobart E.A., Díaz L.F., Sakaguchi M., Straw S., and Andersen O.S.. 2006. 2,3-butanedione monoxime affects cystic fibrosis transmembrane conductance regulator channel function through phosphorylation-dependent and phosphorylation-independent mechanisms: The role of bilayer material properties. Mol. Pharmacol. 70:2015–2026. 10.1124/mol.106.026070 [DOI] [PubMed] [Google Scholar]
- Baumgart T., Hammond A.T., Sengupta P., Hess S.T., Holowka D.A., Baird B.A., and Webb W.W.. 2007. Large-scale fluid/fluid phase separation of proteins and lipids in giant plasma membrane vesicles. Proc. Natl. Acad. Sci. USA. 104:3165–3170. 10.1073/pnas.0611357104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benet L.Z., Broccatelli F., and Oprea T.I.. 2011. BDDCS applied to over 900 drugs. AAPS J. 13:519–547. 10.1208/s12248-011-9290-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin E.J., Wolf P.A., D’Agostino R.B., Silbershatz H., Kannel W.B., and Levy D.. 1998. Impact of atrial fibrillation on the risk of death: The Framingham Heart Study. Circulation. 98:946–952. 10.1161/01.CIR.98.10.946 [DOI] [PubMed] [Google Scholar]
- Berberan-Santos M., Bodunov E., and Valeur B.. 2005. Mathematical functions for the analysis of luminescence decays with underlying distributions 1. Kohlrausch decay function (stretched exponential). Chem. Phys. 315:171–182. 10.1016/j.chemphys.2005.04.006 [DOI] [Google Scholar]
- Beyder A., Rae J.L., Bernard C., Strege P.R., Sachs F., and Farrugia G.. 2010. Mechanosensitivity of Nav1.5, a voltage-sensitive sodium channel. J. Physiol. 588:4969–4985. 10.1113/jphysiol.2010.199034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyder A., Strege P.R., Reyes S., Bernard C.E., Terzic A., Makielski J., Ackerman M.J., and Farrugia G.. 2012. Ranolazine decreases mechanosensitivity of the voltage-gated sodium ion channel Nav1.5: A novel mechanism of drug action. Circulation. 125:2698–2706. 10.1161/CIRCULATIONAHA.112.094714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno M.J., Koeppe R.E.I. II, and Andersen O.S.. 2007. Docosahexaenoic acid alters bilayer elastic properties. Proc. Natl. Acad. Sci. USA. 104:9638–9643. 10.1073/pnas.0701015104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno M.J., Rusinova R., Gleason N.J., Koeppe R.E. II, and Andersen O.S.. 2013. Interactions of drugs and amphiphiles with membranes: modulation of lipid bilayer elastic properties by changes in acyl chain unsaturation and protonation. Faraday Discuss. 161:461–480. 10.1039/C2FD20092A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channer K.S., James M.A., MacConnell T., and Rees J.R.. 1994. Beta-adrenoceptor blockers in atrial fibrillation: the importance of partial agonist activity. Br. J. Clin. Pharmacol. 37:53–57. 10.1111/j.1365-2125.1994.tb04238.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatelain P., Laruel R., and Gillard M.. 1985. Effect of amiodarone on membrane fluidity and Na+/K+ ATPase activity in rat-brain synaptic membranes. Biochem. Biophys. Res. Commun. 129:148–154. 10.1016/0006-291X(85)91415-9 [DOI] [PubMed] [Google Scholar]
- Chatelain P., Laruel R., Vic P., and Brotelle R.. 1989. Differential effects of amiodarone and propranolol on lipid dynamics and enzymatic activities in cardiac sarcolemmal membranes. Biochem. Pharmacol. 38:1231–1239. 10.1016/0006-2952(89)90328-6 [DOI] [PubMed] [Google Scholar]
- Dobrev D., Carlsson L., and Nattel S.. 2012. Novel molecular targets for atrial fibrillation therapy. Nat. Rev. Drug Discov. 11:275–291. 10.1038/nrd3682 [DOI] [PubMed] [Google Scholar]
- Dubos R.J. 1939. Studies on a bactericidal agent extracted from a soil bacillus I. Preparation of the agent. Its activity in vitro. J. Exp. Med. 70:1–10. 10.1084/jem.70.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E., Rawicz W., and Hofmann A.F.. 1995. Lipid bilayer expansion and mechanical disruption in solutions of water-soluble bile acid. Bile Acids in Gastroenterology: Basic and Clinical Advances. Hofmann A.F., Paumgartner G., and Stiehl A., Kluwer Academic Publishers, Dordrecht, Netherlands: 59–68. [Google Scholar]
- Feigenson G.W. 2007. Phase boundaries and biological membranes. Annu. Rev. Biophys. Biomol. Struct. 36:63–77. 10.1146/annurev.biophys.36.040306.132721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein A., and Cass A.. 1968. Permeability and electrical properties of thin lipid membranes. J. Gen. Physiol. 52:145–172. 10.1085/jgp.52.1.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froud R.J., East J.M., Rooney E.K., and Lee A.G.. 1986. Binding of long-chain alkyl derivatives to lipid bilayers and to (Ca2+-Mg2+)-ATPase. Biochemistry. 25:7535–7544. [DOI] [PubMed] [Google Scholar]
- Gandhavadi M., Allende D., Vidal A., Simon S.A., and McIntosh T.J.. 2002. Structure, composition, and peptide binding properties of detergent soluble bilayers and detergent resistant rafts. Biophys. J. 82:1469–1482. 10.1016/S0006-3495(02)75501-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon L.M., Sauerheber R.D., Esgate J.A., Dipple I., Marchmont R.J., and Houslay M.D.. 1980. The increase in bilayer fluidity of rat liver plasma membranes achieved by the local anesthetic benzyl alcohol affects the activity of intrinsic membrane enzymes. J. Biol. Chem. 255:4519–4527. [PubMed] [Google Scholar]
- Goulian M., Mesquita O.N., Fygenson D.K., Nielsen C., Andersen O.S., and Libchaber A.. 1998. Gramicidin channel kinetics under tension. Biophys. J. 74:328–337. 10.1016/S0006-3495(98)77790-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greathouse D.V., Koeppe R.E. II, Providence L.L., Shobana S., and Andersen O.S.. 1999. Design and characterization of gramicidin channels. Methods Enzymol. 294:525–550. 10.1016/S0076-6879(99)94031-4 [DOI] [PubMed] [Google Scholar]
- Greisen P. Jr., Lum K., Ashrafuzzaman M., Greathouse D.V., Andersen O.S., and Lundbæk J.A.. 2011. Linear rate-equilibrium relations arising from ion channel-bilayer energetic coupling. Proc. Natl. Acad. Sci. USA. 108:12717–12722. 10.1073/pnas.1103192108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunnet M., Bentzen B.H., Sørensen U.S., and Diness J.G.. 2012. Cardiac ion channels and mechanisms for protection against atrial fibrillation. Rev. Physiol. Biochem. Pharmacol. 162:1–58. [DOI] [PubMed] [Google Scholar]
- Haffajee C.I., Love J.C., Canada A.T., Lesko L.J., Asdourian G., and Alpert J.S.. 1983. Clinical pharmacokinetics and efficacy of amiodarone for refractory tachyarrhythmias. Circulation. 67:1347–1355. 10.1161/01.CIR.67.6.1347 [DOI] [PubMed] [Google Scholar]
- Heerklotz H. 2002. Triton promotes domain formation in lipid raft mixtures. Biophys. J. 83:2693–2701. 10.1016/S0006-3495(02)75278-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerklotz H., Szadkowska H., Anderson T., and Seelig J.. 2003. The sensitivity of lipid domains to small perturbations demonstrated by the effect of Triton. J. Mol. Biol. 329:793–799. 10.1016/S0022-2836(03)00504-7 [DOI] [PubMed] [Google Scholar]
- Heijman J., Heusch G., and Dobrev D.. 2013a. Pleiotropic effects of antiarrhythmic agents: dronedarone in the treatment of atrial fibrillation. Clin. Med. Insights Cardiol. 7:127–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijman J., Voigt N., and Dobrev D.. 2013b. New directions in antiarrhythmic drug therapy for atrial fibrillation. Future Cardiol. 9:71–88. 10.2217/fca.12.78 [DOI] [PubMed] [Google Scholar]
- Herold K.F., Sanford R.L., Lee W., Schultz M.F., Ingólfsson H.I., Andersen O.S., and Hemmings H.C. Jr. 2014. Volatile anesthetics inhibit sodium channels without altering bulk lipid bilayer properties. J. Gen. Physiol. 144:545–560. 10.1085/jgp.201411172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H.W. 1986. Deformation free energy of bilayer membrane and its effect on gramicidin channel lifetime. Biophys. J. 50:1061–1070. 10.1016/S0006-3495(86)83550-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang T.C., Koeppe R.E.I. II, and Andersen O.S.. 2003. Genistein can modulate channel function by a phosphorylation-independent mechanism: importance of hydrophobic mismatch and bilayer mechanics. Biochemistry. 42:13646–13658. 10.1021/bi034887y [DOI] [PubMed] [Google Scholar]
- Ingólfsson H.I., and Andersen O.S.. 2010. Screening for small molecules’ bilayer-modifying potential using a gramicidin-based fluorescence assay. Assay Drug Dev. Technol. 8:427–436. 10.1089/adt.2009.0250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingólfsson H.I., Koeppe R.E.I. II, and Andersen O.S.. 2007. Curcumin is a modulator of bilayer material properties. Biochemistry. 46:10384–10391. 10.1021/bi701013n [DOI] [PubMed] [Google Scholar]
- Ingólfsson H.I., Thakur P., Herold K.F., Hobart E.A., Ramsey N.B., Periole X., de Jong D.H., Zwama M., Yilmaz D., Hall K., et al. 2014. Phytochemicals perturb membranes and promiscuously alter protein function. ACS Chem. Biol. 9:1788–1798. 10.1021/cb500086e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel P.A. 1988. Phenylalkylamines are promiscuous receptor blockers. Trends Pharmacol. Sci. 9:10 10.1016/0165-6147(88)90231-3 [DOI] [PubMed] [Google Scholar]
- Kamiya K., Nishiyama A., Yasui K., Hojo M., Sanguinetti M.C., and Kodama I.. 2001. Short- and long-term effects of amiodarone on the two components of cardiac delayed rectifier K+ current. Circulation. 103:1317–1324. 10.1161/01.CIR.103.9.1317 [DOI] [PubMed] [Google Scholar]
- Keller S.L., Bezrukov S.M., Gruner S.M., Tate M.W., Vodyanoy I., and Parsegian V.A.. 1993. Probability of alamethicin conductance states varies with nonlamellar tendency of bilayer phospholipids. Biophys. J. 65:23–27. 10.1016/S0006-3495(93)81040-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knothe G. 2002. Structure indices in FA chemistry. How relevant is the iodine value? J. Am. Oil Chem. Soc. 79:847–854. 10.1007/s11746-002-0569-4 [DOI] [Google Scholar]
- Kodama I., Kamiya K., and Toyama J.. 1997. Cellular electropharmacology of amiodarone. Cardiovasc. Res. 35:13–29. 10.1016/S0008-6363(97)00114-4 [DOI] [PubMed] [Google Scholar]
- Kodama I., Kamiya K., and Toyama J.. 1999. Amiodarone: ionic and cellular mechanisms of action of the most promising class III agent. Am. J. Cardiol. 84:20–28. 10.1016/S0002-9149(99)00698-0 [DOI] [PubMed] [Google Scholar]
- Latini R., Tognoni G., and Kates R.E.. 1984. Clinical pharmacokinetics of amiodarone. Clin. Pharmacokinet. 9:136–156. 10.2165/00003088-198409020-00002 [DOI] [PubMed] [Google Scholar]
- Lee A.G. 1991. Lipids and their effects on membrane proteins: Evidence against a role for fluidity. Prog. Lipid Res. 30:323–348. 10.1016/0163-7827(91)90002-M [DOI] [PubMed] [Google Scholar]
- Lundbæk J.A., and Andersen O.S.. 1994. Lysophospholipids modulate channel function by altering the mechanical properties of lipid bilayers. J. Gen. Physiol. 104:645–673. 10.1085/jgp.104.4.645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundbæk J.A., Maer A.M., and Andersen O.S.. 1997. Lipid bilayer electrostatic energy, curvature stress, and assembly of gramicidin channels. Biochemistry. 36:5695–5701. 10.1021/bi9619841 [DOI] [PubMed] [Google Scholar]
- Lundbæk J.A., Andersen O.S., Werge T., and Nielsen C.. 2003. Cholesterol-induced protein sorting: An analysis of energetic feasibility. Biophys. J. 84:2080–2089. 10.1016/S0006-3495(03)75015-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundbæk J.A., Birn P., Hansen A.J., Søgaard R., Nielsen C., Girshman J., Bruno M.J., Tape S.E., Egebjerg J., Greathouse D.V., et al. 2004. Regulation of sodium channel function by bilayer elasticity: The importance of hydrophobic coupling. Effects of Micelle-forming amphiphiles and cholesterol. J. Gen. Physiol. 123:599–621. 10.1085/jgp.200308996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundbæk J.A., Birn P., Tape S.E., Toombes G.E., Søgaard R., Koeppe R.E.I. II, Gruner S.M., Hansen A.J., and Andersen O.S.. 2005. Capsaicin regulates voltage-dependent sodium channels by altering lipid bilayer elasticity. Mol. Pharmacol. 68:680–689. [DOI] [PubMed] [Google Scholar]
- Lundbæk J.A., Collingwood S.A., Ingólfsson H.I., Kapoor R., and Andersen O.S.. 2010a. Lipid bilayer regulation of membrane protein function: gramicidin channels as molecular force probes. J. R. Soc. Interface. 7:373–395. 10.1098/rsif.2009.0443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundbæk J.A., Koeppe R.E. II, and Andersen O.S.. 2010b. Amphiphile regulation of ion channel function by changes in the bilayer spring constant. Proc. Natl. Acad. Sci. USA. 107:15427–15430. 10.1073/pnas.1007455107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markin V., and Sachs F.. 2015. Free volume in membranes: Viscosity or tension? Open J. Biophys. 5:80–83. 10.4236/ojbiphy.2015.53007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehvar R., and Brocks D.R.. 2001. Stereospecific pharmacokinetics and pharmacodynamics of beta-adrenergic blockers in humans. J. Pharm. Pharm. Sci. 4:185–200. [PubMed] [Google Scholar]
- Mencher S.K., and Wang L.G.. 2005. Promiscuous drugs compared to selective drugs (promiscuity can be a virtue). BMC Clin. Pharmacol. 5:3 10.1186/1472-6904-5-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C.E., and Juranka P.F.. 2007. Nav channel mechanosensitivity: Activation and inactivation accelerate reversibly with stretch. Biophys. J. 93:822–833. 10.1529/biophysj.106.101246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D., Benjamin E.J., Go A.S., Arnett D.K., Blaha M.J., Cushman M., de Ferranti S., Després J.P., Fullerton H.J., Howard V.J., et al. 2015. Heart disease and stroke statistics—2015 update: A report from the American Heart Association. Circulation. 131:e29–e322. 10.1161/CIR.0000000000000152 [DOI] [PubMed] [Google Scholar]
- Needham D., and Nunn R.S.. 1990. Elastic deformation and failure of lipid bilayer membranes containing cholesterol. Biophys. J. 58:997–1009. 10.1016/S0006-3495(90)82444-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham D., McIntosh T.J., Simon S.A., and Zhelev D.. 1998. Adsorption, molecular exchange and defect formation in membranes. Curr. Opin. Colloid Interface Sci. 3:511–517. 10.1016/S1359-0294(98)80026-5 [DOI] [Google Scholar]
- Nielsen C., and Andersen O.S.. 2000. Inclusion-induced bilayer deformations: Effects of monolayer equilibrium curvature. Biophys. J. 79:2583–2604. 10.1016/S0006-3495(00)76498-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen C., Goulian M., and Andersen O.S.. 1998. Energetics of inclusion-induced bilayer deformations. Biophys. J. 74:1966–1983. 10.1016/S0006-3495(98)77904-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partenskii M.B., and Jordan P.C.. 2002. Membrane deformation and the elastic energy of insertion: Perturbation of membrane elastic constants due to peptide insertion. J. Chem. Phys. 117:10768–10776. 10.1063/1.1519840 [DOI] [Google Scholar]
- Peters J.U. 2013. Polypharmacology—foe or friend? J. Med. Chem. 56:8955–8971. 10.1021/jm400856t [DOI] [PubMed] [Google Scholar]
- Petruzielo R.S., Heberle F.A., Drazba P., Katsaras J., and Feigenson G.W.. 2013. Phase behavior and domain size in sphingomyelin-containing lipid bilayers. Biochim. Biophys. Acta. 1828:1302–1313. 10.1016/j.bbamem.2013.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawicz W., Olbrich K.C., McIntosh T., Needham D., and Evans E.. 2000. Effect of chain length and unsaturation on elasticity of lipid bilayers. Biophys. J. 79:328–339. 10.1016/S0006-3495(00)76295-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth B.L., Sheffler D.J., and Kroeze W.K.. 2004. Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nat. Rev. Drug Discov. 3:353–359. 10.1038/nrd1346 [DOI] [PubMed] [Google Scholar]
- Rusinova R., Herold K.F., Sanford R.L., Greathouse D.V., Hemmings H.C.J. Jr., and Andersen O.S.. 2011. Thiazolidinedione insulin sensitizers alter lipid bilayer properties and voltage-dependent sodium channel function: Implications for drug discovery. J. Gen. Physiol. 138:249–270. 10.1085/jgp.201010529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusinova R., Kim D.M., Nimigean C.M., and Andersen O.S.. 2014. Regulation of ion channel function by the host lipid bilayer examined by a stopped-flow spectrofluorometric assay. Biophys. J. 106:1070–1078. 10.1016/j.bpj.2014.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackmann E., Kotulla R., and Heiszler F.-J.. 1984. On the role of lipid-bilayer elasticity for the lipid-protein interaction and the indirect protein-protein coupling. Can. J. Biochem. Cell Biol. 62:778–788. 10.1139/o84-099 [DOI] [PubMed] [Google Scholar]
- Schreier S., Malheiros S.V., and de Paula E.. 2000. Surface active drugs: self-association and interaction with membranes and surfactants. Physicochemical and biological aspects. Biochim. Biophys. Acta. 1508:210–234. 10.1016/S0304-4157(00)00012-5 [DOI] [PubMed] [Google Scholar]
- Seydel J.K. 2003. Drug-membrane interactions and pharmacodynamics. Drug-Membrane Interactions: Analysis, Drug Distribution, Modeling. Volume 15 Mannhold R., Kubinyi H., and Folkers G., Wiley, Hoboken, NJ: 217–289. [Google Scholar]
- Simon S.A., McIntosh T.J., and Latorre R.. 1982. Influence of cholesterol on water penetration into bilayers. Science. 216:65–67. 10.1126/science.7063872 [DOI] [PubMed] [Google Scholar]
- Singh B.N. 1983. Amiodarone: Historical development and pharmacologic profile. Am. Heart J. 106:788–797. 10.1016/0002-8703(83)90002-9 [DOI] [PubMed] [Google Scholar]
- Strege P., Beyder A., Bernard C., Crespo-Diaz R., Behfar A., Terzic A., Ackerman M., and Farrugia G.. 2012. Ranolazine inhibits shear sensitivity of endogenous Na+ current and spontaneous action potentials in HL-1 cells. Channels (Austin). 6:457–462. 10.4161/chan.22017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G., Voelker D.R., and Feigenson G.W.. 2008. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 9:112–124. 10.1038/nrm2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch S.L., and Keller S.L.. 2005. Seeing spots: Complex phase behavior in simple membranes. Biochim. Biophys. Acta. 1746:172–185. 10.1016/j.bbamcr.2005.06.010 [DOI] [PubMed] [Google Scholar]
- Wang D.W., Mistry A.M., Kahlig K.M., Kearney J.A., Xiang J., and George A.L.J. Jr. 2010. Propranolol blocks cardiac and neuronal voltage-gated sodium channels. Front. Pharmacol. 1:144 10.3389/fphar.2010.00144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk M.R. 2005. The emerging field of lipidomics. Nat. Rev. Drug Discov. 4:594–610. 10.1038/nrd1776 [DOI] [PubMed] [Google Scholar]
- Wilson T.W., Firor W.B., Johnson G.E., Holmes G.I., Tsianco M.C., Huber P.B., and Davies R.O.. 1982. Timolol and propranolol: Bioavailability, plasma concentrations, and beta blockade. Clin. Pharmacol. Ther. 32:676–685. 10.1038/clpt.1982.223 [DOI] [PubMed] [Google Scholar]
- Wolff J., and Chaikoff I.L.. 1948. Plasma inorganic iodide as a homeostatic regulator of thyroid function. J. Biol. Chem. 174:555–564. [PubMed] [Google Scholar]
- Woosley R.L., Kornhauser D., Smith R., Reele S., Higgins S.B., Nies A.S., Shand D.G., and Oates J.A.. 1979. Suppression of chronic ventricular arrhythmias with propranolol. Circulation. 60:819–827. 10.1161/01.CIR.60.4.819 [DOI] [PubMed] [Google Scholar]
- Zhelev D.V. 1998. Material property characteristics for lipid bilayers containing lysolipid. Biophys. J. 75:321–330. 10.1016/S0006-3495(98)77516-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimetbaum P. 2012. Antiarrhythmic drug therapy for atrial fibrillation. Circulation. 125:381–389. 10.1161/CIRCULATIONAHA.111.019927 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.