When muscles become paralyzed in crises of hyperkalemic periodic paralysis, patients do not stop breathing. Here is why.
Abstract
The diaphragm muscle of hyperkalemic periodic paralysis (HyperKPP) patients and of the M1592V HyperKPP mouse model rarely suffers from the myotonic and paralytic symptoms that occur in limb muscles. Enigmatically, HyperKPP diaphragm expresses the mutant NaV1.4 channel and, more importantly, has an abnormally high Na+ influx similar to that in extensor digitorum longus (EDL) and soleus, two hindlimb muscles suffering from the robust HyperKPP abnormalities. The objective was to uncover the physiological mechanisms that render HyperKPP diaphragm asymptomatic. A first mechanism involves efficient maintenance of resting membrane polarization in HyperKPP diaphragm at various extracellular K+ concentrations compared with larger membrane depolarizations in HyperKPP EDL and soleus. The improved resting membrane potential (EM) results from significantly increased Na+ K+ pump electrogenic activity, and not from an increased protein content. Action potential amplitude was greater in HyperKPP diaphragm than in HyperKPP soleus and EDL, providing a second mechanism for the asymptomatic behavior of the HyperKPP diaphragm. One suggested mechanism for the greater action potential amplitude is lower intracellular Na+ concentration because of greater Na+ K+ pump activity, allowing better Na+ current during the action potential depolarization phase. Finally, HyperKPP diaphragm had a greater capacity to generate force at depolarized EM compared with wild-type diaphragm. Action potential amplitude was not different between wild-type and HyperKPP diaphragm. There was also no evidence for an increased activity of the Na+–Ca2+ exchanger working in the reverse mode in the HyperKPP diaphragm compared with the wild-type diaphragm. So, a third mechanism remains to be elucidated to fully understand how HyperKPP diaphragm generates more force compared with wild type. Although the mechanism for the greater force at depolarized resting EM remains to be determined, this study provides support for the modulation of the Na+ K+ pump as a component of therapy to alleviate weakness in HyperKPP.
INTRODUCTION
Hyperkalemic periodic paralysis (HyperKPP) is an autosomal-dominant disease with nearly complete penetrance (Gamstorp et al., 1957; Bradley et al., 1990). The disease manifests with periods of myotonic discharge and episodic paralytic attacks (Lehmann-Horn et al., 1983; Bradley et al., 1990; Miller et al., 2004). Weakness is prominent in the limbs and may completely incapacitate patients for hours at a time, with the frequency of paralytic attacks ranging from 3 to 28 per month (Miller et al., 2004). A key hallmark of the disease is the precipitation of paralytic attacks after potassium ingestion (Gamstorp et al., 1957; Poskanzer and Kerr, 1961; Streeten et al., 1971; Wang and Clausen, 1976; Bradley et al., 1990). Paralysis is in some cases associated with plasma [K+] increasing from the normal 4 mM to 6–8 mM (Gamstorp et al., 1957; Lehmann-Horn et al., 1983; Miller et al., 2004), whereas in other cases there is no increase (Poskanzer and Kerr, 1961; Rüdel and Ricker, 1985; Chinnery et al., 2002). Most of the available treatments for HyperKPP are limited either by partial effectiveness or declining efficacy over time (Clausen et al., 1980; Lehmann-Horn et al., 1983).
HyperKPP is caused by missense mutations in the SCN4A gene that encodes for the α subunit of the Nav1.4 sodium channel expressed in adult skeletal muscles (Cannon, 2006). Most HyperKPP cases (∼66%) result from two mutations: threonine to methionine at residue 704 (T704M) or methionine to valine at residue 1592 (M1592V); the remaining cases are related to seven other mutations (Miller et al., 2004). The mutations cause three primary functional defects in the Nav1.4 channel. First, the steady-state activation curve shifts toward more negative membrane potentials (EMs; Rojas et al., 1999), which lowers action potential threshold. Second, the steady-state slow inactivation shifts toward less negative EMs (Hayward et al., 1999). Third, mutant channels enter a non-inactivation mode upon membrane depolarization at elevated extracellular K+ concentration ([K+]e), an effect not observed in normal channels (Cannon et al., 1991).
As a consequence of these defects, Nav1.4 channels open at greater frequency at rest, resulting in large Na+ influx (Clausen et al., 2011; Lucas et al., 2014), which then depolarizes the cell membrane (Lehmann-Horn et al., 1983; Ricker et al., 1989; Clausen et al., 2011; Lucas et al., 2014). Myotonic discharges occur when the EM approaches a threshold for sustained firing. [K+]e increases as several action potentials are generated. In normal muscles, increases in [K+]e cause a membrane depolarization that inactivates NaV1.4 channels, thereby reducing action potential amplitude (Yensen et al., 2002). As a consequence of lower action potential amplitude, less Ca2+ is released from the sarcoplasmic reticulum, resulting in decreased force or sarcomere shortening as [Ca2+]i becomes submaximal (Lucas et al., 2014; Zhu et al., 2014). In HyperKPP limb muscles, the effects of increased K+ on EM and force are greater than in normal muscles (Wang and Clausen, 1976; Lehmann-Horn et al., 1983, 1987; Hayward et al., 2008; Clausen et al., 2011; Lucas et al., 2014), often causing complete loss of membrane excitability. In fact, this sensitivity to elevated K+ constitutes a key feature of HyperKPP.
HyperKPP patients primarily suffer from limb weakness or paralysis but, surprisingly, only ∼25% of patients experience respiratory distress (Charles et al., 2013). In one study (Lucas et al., 2014), only two of eight tested diaphragms from the M1592V HyperKPP knock-in mouse model completely stopped contracting upon stimulation while developing a prolonged contracture (in the absence of stimulation) when [K+]e was increased to 10 mM, whereas the remaining six HyperKPP diaphragms had similar tetanic force at 4.7 and 10 mM K+ when compared with their wild-type counterpart. Furthermore, in this study, neither a sudden loss in the ability to respond to stimuli nor a prolonged contracture was observed from the 44 tested HyperKPP diaphragms during an experiment at any [K+]e, in the presence of ouabain to inhibit the Na+–K+ ATPase pump (NKA) or ORM-10103 to inhibit the Na+–Ca2+ exchanger (NCX). Thus, the susceptibility to paralysis of HyperKPP diaphragm appears to be very low, at least in the context of the M1592V mutation. An asymptomatic diaphragm muscle is surprising for two reasons. First, diaphragm expresses the NaV1.4 channel protein at 75% of the level in extensor digitorum longus (EDL) and twice the level in soleus, being two muscles suffering from HyperKPP symptoms (Zhou and Hoffman, 1994; Lucas et al., 2014). Second, the tetrodotoxin (TTX)-sensitive Na+ influx in the diaphragm is the largest of these three HyperKPP muscles (Lucas et al., 2014). A better understanding of the mechanisms by which HyperKPP diaphragm maintains its force may help us develop better and more effective treatments for HyperKPP patients.
The objective of this study was to clarify the physiological mechanisms that render diaphragm muscle less vulnerable to the ionic perturbations produced by HyperKPP mutant Na channel expression. We compared how various [K+]e affect the resting EM, action potential, and tetanic force, as well as the protein content and electrogenic contribution of the α1 and α2 isoforms of the NKA in EDL, soleus, and diaphragm. The results showed that the HyperKPP diaphragm is resistant to weakness because (a) the NKA electrogenic contribution to resting EM was greater in HyperKPP than in wild-type diaphragm, HyperKPP EDL, and soleus; (b) at depolarized resting EM, HyperKPP diaphragm generated an action potential with greater amplitude than HyperKPP soleus and EDL; and (c) it generated greater tetanic force than its wild-type counterpart.
MATERIALS AND METHODS
Animals and approval for animal studies
HyperKPP mice (strain FVB.129S4(B6)-Scn4atm1.1Ljh/J) were generated by knocking in the equivalent of human missense mutation M1592V into the mouse genome; i.e., at position 1585 as described previously by Hayward et al. (2008). The FVB strain was used as a wild-type mouse. All mice were 2–3-mo old and weighed 20–25 g. The homozygous mutants generally do not survive beyond postnatal day 5, so knock-in mice were maintained as heterozygotes by crossbreeding with FVB mice. Mice were fed ad libitum and housed according to the guidelines of the Canadian Council for Animal Care. The Animal Care Committee of the University of Ottawa approved all experimental procedures used in this study. Before muscle excision, 2–3-mo-old mice were anaesthetized with a single intraperitoneal injection of 2.2 mg ketamine/0.4 mg xylazine/0.22 mg acepromazine per 10 g of animal body weight, and sacrificed by cervical dislocation. EDL, soleus, flexor digitorum brevis (FDB), or diaphragm was then dissected out. For force measurements, 5–7-mm wide diaphragm strips were used.
Genotyping
A 2-mm tail piece was incubated overnight with 500 µl of tail digestion buffer (0.2 mM Na2EDTA and 25 mM NaOH, pH 12.3) and 50 µl proteinase K (1 mg/ml) at 56°C. DNA extraction involved the addition of 650 µl of 1:1 phenol/CIA and centrifuged at 12,000 g for 10 min. 650 µl of CIA was added twice to the pellet and centrifuged before suspending the resulting pellet in 750 µl isopropyl alcohol. After 10 min, the solution was centrifuged for 15 min at 15,000 g. The alcohol was removed and the pellet was suspended in 750 µl of 70% ethanol and centrifuged. After removing the alcohol, the pellet was left to dry for 30 min before the addition of 200 µl TE buffer (10 mM Tris and 1 mM EDTA, pH 8.0) and incubated at 65°C for 2 h. PCR was then completed using the previously extracted DNA and the following primers: NC1F (forward): 5′-TGTCTAACTTCGCCTACGTCAA-3′ and NC2R (reverse): 5′-GAGTCACCCAGTACCTCTTTGG-3′.
PCR products were digested for 6 h using the restriction digest enzyme NspI. The mutation that is knocked in to the HyperKPP mice causes the removal of one NspI cut site that is easily detected by agarose gel electrophoresis; two bands were visualized for wild-type mice, which carry the cut site on both alleles, and three bands were seen for heterozygous HyperKPP mice harboring one normal allele and one mutant allele.
Western blots of Na+ K+ ATPase α1 and α2
Muscles were homogenized in buffer containing (mM): 50 Tris, 150 NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, and protease inhibitor cocktail, pH 8.0. Samples were rotated end-over-end for 1 h. Homogenates were centrifuged for 30 min at 17,500 g and 4°C. Protein concentration was determined in supernatants using the BCA assay method (Thermo Fisher Scientific). 40 μg of protein aliquots of the muscle lysates was diluted with Laemmli sample buffer and heated for 20 min at 56°C. Proteins were separated on 8% acrylamide gel at 100 V and transferred onto nitrocellulose membranes (Mini-PROTEAN 3 apparatus; Bio-Rad Laboratories). Equal protein loading was verified with Ponceau S (MP Biomedicals). Membranes were blocked overnight at 4°C with 5% skim milk powder in PBS containing 0.1% Tween, washed three times (10 min each) with PBS, and incubated overnight with either rabbit anti-NKAα1 antibody (Cell Signaling Technology) diluted at 1:1,000 in PBS containing 5% BSA or rabbit anti-NKAα2 antibody (EMD Millipore) diluted at 1:5,000 in PBS containing 5% skim milk. Membranes were washed three times (10 min each) with PBS and incubated for 1 h with horseradish peroxidase–conjugated goat anti–rabbit antibody (Jackson ImmunoResearch Laboratories, Inc.) diluted at 1:10,000 in PBS containing 5% skim milk. Bands were visualized by chemiluminescence using the ECL kit (PerkinElmer) on Cl-Xposure film (Thermo Fisher Scientific). Cl-Xposure films were scanned (MP 600 PIXMA; Cannon) and quantified using ImageJ software (National Institutes of Health).
Physiological measurements
Solutions.
Control solution contained (mM): 118.5 NaCl, 4.7 KCl, 1.3 CaCl2, 3.1 MgCl2, 25 NaHCO3, 2 NaH2PO4, and 5.5 d-glucose. Solutions containing different K+ concentrations were prepared by adding the appropriate amount of KCl. Solutions containing ouabain, an NKA inhibitor (Sigma-Aldrich), were prepared by dissolving ouabain directly in the control solution. Solutions containing 2-[(3,4-dihydro-2-phenyl-2H-1-benzopyran-6-yl)oxy]-5-nitro-pyridine (ORM-10103; an inhibitor of the NCX; Sigma-Aldrich) were prepared by first dissolving ORM-10103 in DMSO before it was added to the control solution. For the experiments involving ORM-10103, the final DMSO concentration was 0.1% (vol/vol) including the control solution. Solutions were continuously bubbled with 95% O2–5% CO2 to maintain a pH of 7.4. Experimental temperature was 37°C. Total flow of solutions in the muscle chamber was 15 ml/min being split just above and below the muscle to prevent any buildup of reactive oxygen species, which is quite large at 37°C (Edwards et al., 2007).
Force measurement.
Muscle length was adjusted to give maximal tetanic force. Muscles were positioned horizontally in a Plexiglas chamber. One end of the muscle was fixed to a stationary hook, whereas the other end was attached to a force transducer (model 400A; Aurora Scientific Canada). The transducer was connected to a data acquisition system (KCP13104; Keithley), and data were recorded at 5 kHz. Tetanic force was defined as the force developed while muscles were electrically stimulated and was calculated as the difference between the maximum force during a contraction and the baseline force measured 5 ms before stimulation. Electrical stimulations were applied across two platinum wires (4 mm apart) located on opposite sides of the fibers. They were connected to a Grass S88 stimulator and a Grass SIU5 isolation unit (Grass Technologies). Tetanic contractions were elicited with 200-ms trains of 0.3-ms, 10-V (supramaximal voltage) pulses. Stimulation frequencies were set to give maximum tetanic force: 140 Hz for soleus and 200 Hz for EDL and diaphragm. Tetanic contractions were elicited every 5 min.
Resting EM and action potential measurements.
Resting EM and action potential were measured using glass microelectrodes. Microelectrode tip resistances were 7∼15 MΩ, and that of the reference electrode was ∼1 MΩ. All electrodes were filled with 2 M K-citrate. A recording was rejected when the change in potential upon penetration was not a sharp drop or when the microelectrode potential did not return to zero upon withdrawal from the fiber. Single action potentials were elicited using fine platinum wires placed along the surface fibers using a single 10-V, 0.3-ms square pulse.
Statistics
Data are expressed as mean ± SEM (SEM). For statistical differences, two-way ANOVAs were used for Western blot measurements. Statistical comparisons between wild-type and HyperKPP muscles involved different mice, and for such comparison, force and EMs were independent from one another. Force and EMs were also measured at different [K+]e or ouabain concentration using the same muscles; in this case, the measurements are not independent from one another. As a consequence of the experimental design, we had two error terms: (1) the population error from the variability between mice when comparing wild-type and HyperKPP, and (2) within muscle error when comparing the effects of K+ and ouabain. So, statistical analyses were performed using spit-plot ANOVA designs as described by Steel and Torrie (1980). For this, the comparison between wild-type and HyperKPP was part of the whole plot, which used the population error, whereas the comparison for the K+ and ouabain effect was part of the split plot, which used the within muscle error. Calculations were made using the general linear model procedures of Statistical Analysis Software (version 9.3; SAS Institute Inc.). When a main effect or an interaction was significant, the least square difference (LSD) was used to locate the significant differences. The word “significant” refers only to a statistical difference (P < 0.05).
RESULTS
[K+]e effects on tetanic force and resting EM
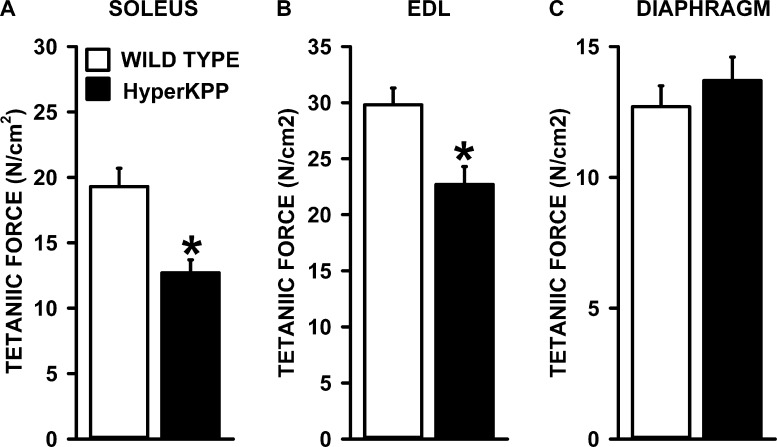
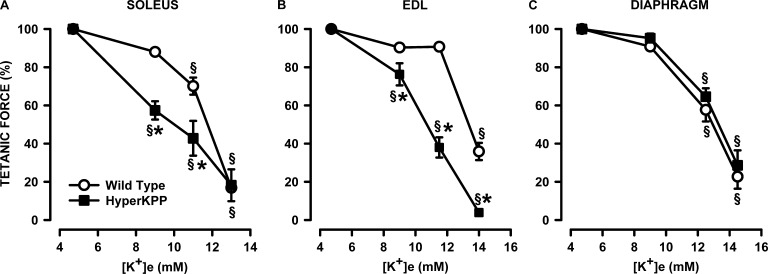
As mentioned in the Introduction, the K+-induced force depression starts with a membrane depolarization. So, we first analyzed the K+ effects on tetanic force and resting EM to construct tetanic force–resting EM relationships to document how much of the force losses in HyperKPP muscles compared with wild type are related to membrane depolarization. At 4.7 mM K+ (control), mean tetanic forces using the data from all tested HyperKPP soleus and EDL in this study were significantly lower than their wild-type counterparts. Wild-type and HyperKPP soleus, respectively, generated on average a tetanic force of 19.3 and 12.7 N/cm2; i.e., mean tetanic force of HyperKPP soleus was 66% of the wild-type force (Fig. 1 A). Wild-type and HyperKPP EDL, respectively, generated 29.8 and 22.7 N/cm2 for a HyperKPP force being 76% of wild type (Fig. 1 B). For the diaphragm, however, there was no significant difference between wild type and HyperKPP (Fig. 1 C). The relative decreases in mean tetanic force of HyperKPP soleus and EDL at 9–11 mM K+ were significantly greater in HyperKPP than in wild-type EDL and soleus (Fig. 2, A and B) but not in the diaphragm, as there was no difference between wild-type and HyperKPP (Fig. 2 C). Thus, HyperKPP diaphragms do not have a greater sensitivity to the K+-induced force depression as observed with EDL and soleus.
Figure 1.
HyperKPP soleus and EDL but not diaphragm generated less tetanic force than wild-type muscles. (A–C) Tetanic force was elicited with a 200-msec train of 0.3 msec, 10-V square pulses at 140 Hz for the soleus and 200 Hz for the EDL and diaphragm. Data were acquired immediately after adjusting the length for maximum force at the beginning of an experiment. The tetanic forces from every experiment including those for EM measurements were used to calculate mean tetanic force. Error bars represent the SEM of 24 solei, 34 EDL, and 44 diaphragms. *, mean tetanic force of HyperKPP muscles was significantly different from that of wild type; ANOVA and LSD; P < 0.05.
Figure 2.
HyperKPP soleus and EDL but not diaphragm were more sensitive to the K+-induced force depression. (A–C) Each muscle was tested at only one elevated [K+]e. Tetanic force is expressed as a percentage of the force at 4.7 mM K+. Error bars represent the SEM of five muscles. §, mean tetanic force was significantly different from the mean force at 4.7 mM K+; *, mean tetanic force of HyperKPP muscle was significantly different from the mean force of wild-type muscle; ANOVA and LSD; P < 0.05.
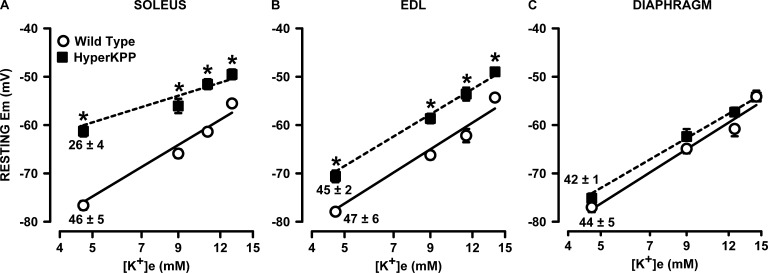
HyperKPP muscle fibers are known to have less negative resting EM than normal fibers (Lehmann-Horn et al., 1983, 1987; Ricker et al., 1989; Clausen et al., 2011). Furthermore, the K+-induced force depression is caused by a depolarization of the cell membrane, which then causes an inactivation of NaV1.4 channels (Renaud and Light, 1992; Cairns et al., 1997; Yensen et al., 2002). To better understand the importance of the membrane depolarization in the lower force generated by HyperKPP EDL and soleus or the conservation of force for the diaphragm, we measured resting EM in 10–15 fibers chosen at random from the muscle surface at different [K+]e (note here that action potentials were not measured to determine if a fiber was excitable or not). At 4.7 mM K+, mean resting EM was 15 mV lower in HyperKPP than in wild-type soleus, a difference that became smaller as [K+]e was increased because the K+-induced membrane depolarization per [K+]e decade was smaller in HyperKPP soleus (Fig. 3 A). For EDL, the difference in resting EM between wild-type and HyperKPP was smaller, being 5–9 mV among the different [K+]e as the slopes of the membrane depolarization were not different between wild type and HyperKPP (Fig. 3 B). The smallest difference in resting EM between wild type and HyperKPP was with the diaphragm, being only 2–4 mV and nonsignificant at all [K+]e (Fig. 3 C).
Figure 3.
HyperKPP soleus and EDL but not diaphragm fibers were more depolarized than wild-type fibers. Each muscle was tested at one or two elevated [K+]e. Resting EM was measured in several fibers in each muscles. (A–C) An average resting EM was calculated for each muscle, and from the muscle averages a final mean was calculated. Error bars represent the SEM of 209–278 fibers/19 muscles at 4.7 mM K+ and 65–107 fibers/5–6 muscles at other [K+]e. Numbers below the resting EM at 4.7 mM K+ indicate the slope and SEM of the membrane depolarization (in mV/[K+]e decade) using the mean resting EM versus log([K+]e); calculations were performed using the LINEST function of Excel 2013 (correlation coefficients of the analyses ranged from 0.973 to 0.999). *, mean resting EM of HyperKPP muscle was significantly different from the mean value of wild-type muscle; ANOVA and LSD; P < 0.05.
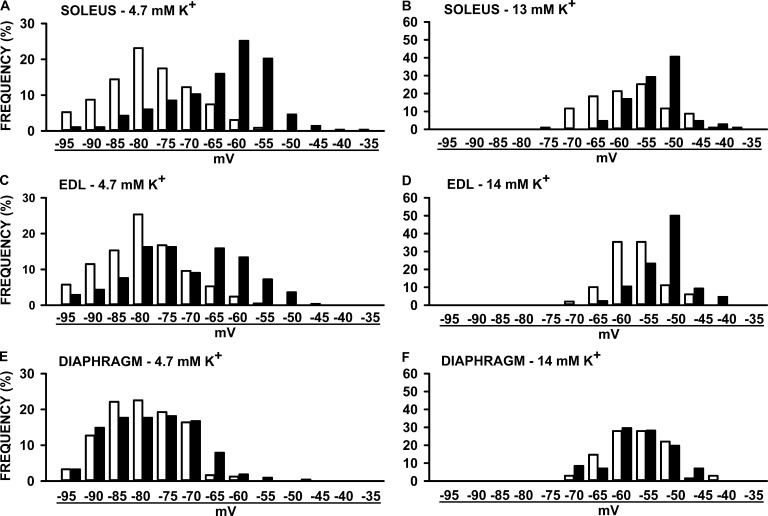
Resting EM varied tremendously among mammalian muscle fibers. Consequently, one cannot just use the mean resting EM to represent all fibers. The frequency distribution of resting EM was then documented by separating all measured values in bins of 5 mV. For soleus muscles, the frequency distribution for HyperKPP fibers was shifted toward less negative resting EM compared with wild-type fibers at both 4.7 and 13 mM K+ (Fig. 4, A and B). At 4.7 mM K+, 69% of all wild-type soleus fibers had a resting EM above −70 mV compared with only 21% for HyperKPP fibers. Muscles with mean resting EM of less than −55 mV lose their capacity to generate force (Renaud and Light, 1992; Cairns et al., 1997). At 4.7 mM K+, 27% of all HyperKPP soleus fibers had a resting EM below −55 mV compared with <1% for wild-type fibers. The proportion of HyperKPP soleus fibers with resting EM below −55 mV increases to 78% at 13 mM K+, whereas it increased only to 46% for wild-type fibers. A similar shift toward less negative resting EM in HyperKPP EDL fibers was also observed at 4.7 and 14 mM K+ (Fig. 4, C and D). There were, however, two major differences between HyperKPP soleus and EDL: at 4.7 mM K+, 47% of HyperKPP EDL fibers had a resting EM above −70 mV compared with only 21% for soleus fibers, whereas the proportion of fibers with a resting EM below −55 mV was just 11% for EDL compared with 27% for soleus. Contrary to hindlimb muscles, there was no major shift in the frequency distribution of resting EM between the wild-type and HyperKPP diaphragm (Fig. 4, E and F). Only small differences were observed where the number of fibers with a resting EM ranging between −65 and −75 mV was less in HyperKPP than in wild type, whereas the number of fibers with a resting EM between −55 and −60 mV was slightly higher in HyperKPP. These small differences explained the slightly less negative mean resting EM of HyperKPP fibers compared with wild-type fibers in Fig. 3 C.
Figure 4.
The frequency distribution of resting EM was shifted toward less negative resting EM in the HyperKPP soleus and EDL but not in the diaphragm when compared with wild-type muscles. Resting EM values were separated in a bin of 5 mV, and the number of fibers in each bin is expressed as a percentage of the total number of tested fibers. The resting EM values under each pair of bars represent the upper bound of each bin. The total number of fibers were 209–278 (A, C, and E) for 4.7 mM K+ and 68–106 at the elevated [K+]e (B, D, and F).
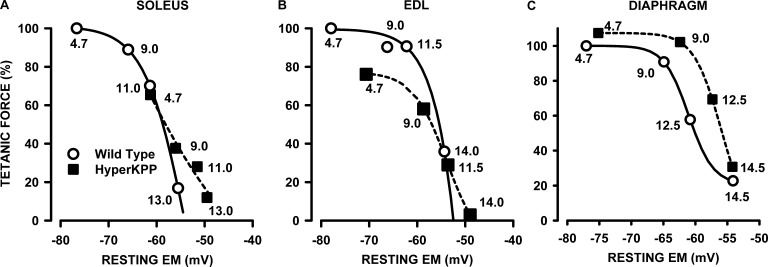
Next, we ascertained how much of the lower forces in HyperKPP EDL and soleus and the conservation of force in the HyperKPP diaphragm are related to resting EM. To do this, we took into consideration that the tetanic force from a whole muscle is a function of the mean resting EM of all fibers, as reported previously (Renaud and Light, 1992; Cairns et al., 1997). To construct a tetanic force–resting EM relationship, we calculated all mean absolute forces at different [K+]e as a percentage of the force generated by wild-type muscles at 4.7 mM K+. The force generated by HyperKPP soleus at 4.7 and 9 mM K+ fell very close to the tetanic force–resting EM of wild-type soleus, suggesting that the lower tetanic forces of HyperKPP soleus at those [K+]e are largely caused by less negative mean resting EM (Fig. 5 A). Interestingly, although tetanic force in wild-type soleus is expected to reach zero at −54 mV, HyperKPP soleus still generated some force between −55 and −49 mV.
Figure 5.
Tetanic force versus resting EM relationships. (A–C) Relationships were made by first expressing all mean tetanic forces of wild-type and HyperKPP muscles at various [K+]e as a percentage of the mean tetanic force of wild-type muscles at 4.7 mM K+ (taken as the normal maximum force these muscles can generate), and then plotting the relative values against the resting EM shown in Fig. 2. The numbers beside each symbol indicate the [K+]e at which tetanic force and resting EM were measured. The curves were plotted after fitting the data points to the following sigmoidal relationship: where a, b, c, and d are constants.
The tetanic force of HyperKPP EDL at 4.7 and 9 mM fell below the expected force from the force–EM relationship of wild-type EDL (Fig. 5 B). For example, HyperKPP EDL tetanic force at −71 mV (measured at 4.7 mM K+) was 76%, whereas at that EM, the expected tetanic force of wild-type EDL was 98%. It thus appears that the lower force in HyperKPP EDL is not just related to less negative resting EM. At resting EM smaller than −53 mV, HyperKPP EDL developed more force than wild type. For example, HyperKPP EDL force at −48 mV (measured at 14 mM K+) was 3%, whereas for wild-type, zero force was expected to occur at −52 mV. Remarkably, the tetanic force–resting EM was significantly shifted toward less negative resting EM in HyperKPP diaphragm (Fig. 5 C). The largest difference was observed at −59 mV, as the expected wild-type force was 41% compared with 86% for HyperKPP, representing a 45% difference.
The results so far revealed two major features in this model of HyperKPP that spare the diaphragm from altered function compared with the robust abnormalities observed in EDL and soleus. The first one is smaller membrane depolarization in HyperKPP diaphragm (this study) despite the fact that it has the largest TTX-sensitive Na+ influx of all three muscles (Lucas et al., 2014). The second factor is a greater capacity of the HyperKPP diaphragm to generate more force than wild type at depolarized resting EM. To further understand the lower membrane depolarization in the HyperKPP diaphragm, we determined how the content and electrogenic contribution of NKA differ between the HyperKPP diaphragm, EDL, and soleus. To further understand the capacity of the HyperKPP diaphragm to generate more force at depolarized EM, we tested (a) the possibility that the NCX works in the reverse mode when the membrane is depolarized, as has been suggested previously for the wild-type diaphragm (Zavecz and Anderson, 1992); and (b) whether the HyperKPP diaphragm fibers generate better action potentials than wild type when the membrane is depolarized by K+.
NKA
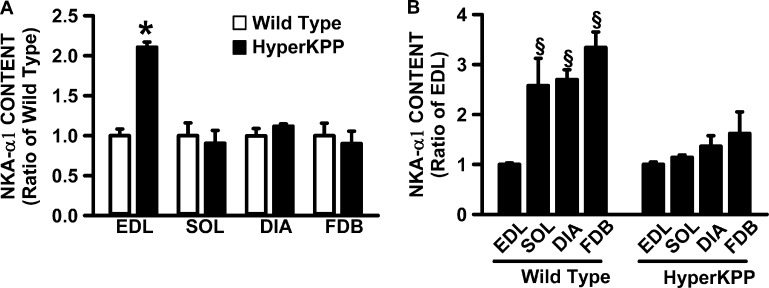
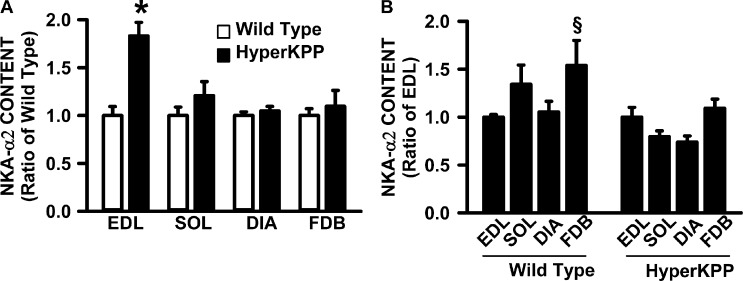
For the measurements of NKAα1 and NKAα2 protein content, we included the FDB because it is also an asymptomatic muscle, but contrary to the diaphragm, it has a very low TTX-sensitive Na+ influx even though its NaV1.4 channel protein content is comparable to that of soleus (Lucas et al., 2014). Here, we first determined whether the NKA protein content is greater in HyperKPP than in the wild-type diaphragm, as has been reported for HyperKPP EDL (Clausen et al., 2011). HyperKPP EDL was the only muscle with significantly greater NKAα1 protein content than in the wild-type counterpart (Fig. 6 A). Next, we determined how the large increase in NKAα1 protein content in HyperKPP EDL affected the relative differences between muscles in wild type and HyperKPP. Although wild-type EDL had two to three times less NKAα1 content compared with soleus, diaphragm, and FDB, the NKAα1 content in HyperKPP EDL was no longer different from the other three muscles (Fig. 6 B). The situation was the same for the NKAα2 protein content. That is, HyperKPP EDL had a greater NKAα2 content than in wild type (Fig. 7 A). As a consequence of the increase, the wild-type diaphragm had similar NKAα2 protein content to wild-type EDL, whereas for HyperKPP NKAα2, the content was less in the diaphragm than in EDL (Fig. 7 B). It was also noted that the 83% higher content in HyperKPP than in wild-type EDL as well as the 21% higher content in HyperKPP soleus were similar to the values reported from ouabain-binding studies (Clausen et al., 2011).
Figure 6.
NKAα1 protein content was significantly higher in HyperKPP than in wild-type EDL, whereas there was no difference for the soleus, diaphragm, and FDB muscles. (A) For each muscle, NKAα1 contents were calculated as a ratio of the content in wild-type muscle. (B) For each of wild-type and HyperKPP, NKAα1 contents were calculated as a ratio of the EDL content. Error bars represent the SEM of five muscles. §, mean NKAα1 content significantly different from mean content in EDL; *, mean NKAα1 content was significantly different from wild-type content; ANOVA and LSD; P < 0.05.
Figure 7.
NKAα2 protein content was significantly higher in HyperKPP than in wild-type EDL, whereas there was no difference for the soleus, diaphragm, and FDB muscles. (A) For each muscle, NKAα2 contents were calculated as a ratio of the content in wild-type muscle. (B) For each of wild type and HyperKPP, NKAα2 contents were calculated as a ratio of the EDL content. Error bars represent the SEM of 8–10 muscles. §, mean NKAα2 content significantly different from mean content in EDL; *, mean NKAα2 content was significantly different from wild-type content; ANOVA and LSD; P < 0.05.
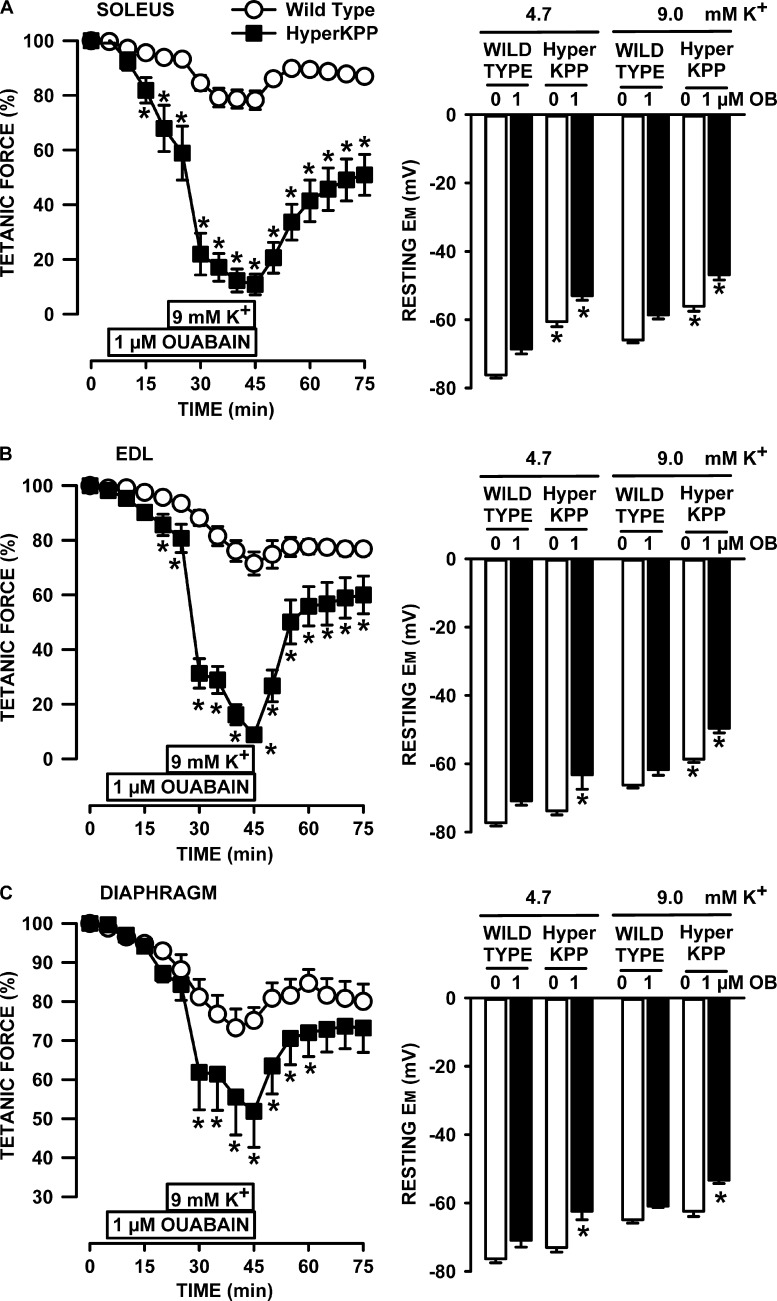
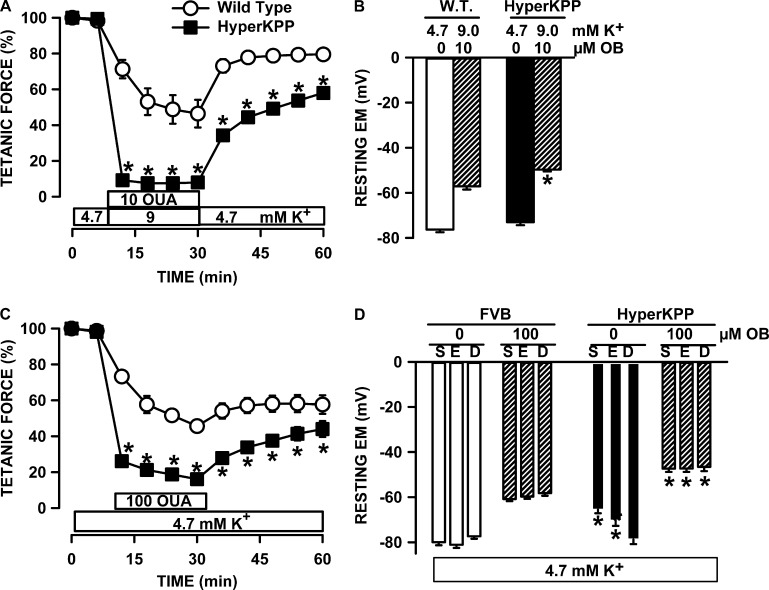
Next, we assessed how inhibiting NKA activity with ouabain affected tetanic force and resting EM. We first used ouabain at a concentration of 1 µM to reduce NKAα2 activity by 92% and that of NKAα1 by 6% according to the ouabain Ki values reported by Chibalin et al. (2012) that were measured from changes in rat diaphragm resting EM after exposure to various ouabain concentrations. At 4.7 mM K+, 1 µM ouabain reduced mean tetanic force of wild-type soleus by 7%, whereas resting EM depolarized by 8 mV (Fig. 8 A). The decrease in tetanic force for HyperKPP soleus was much larger at 42% despite a similar depolarization of 7 mV. Ouabain also caused a greater decrease in force at 9 mM K+ in HyperKPP soleus, even though the ouabain-induced membrane depolarization was 7 mV in wild type and 9 mV in HyperKPP. The apparent greater ouabain effect on HyperKPP soleus force despite a similar extent of depolarizations was because in the absence of ouabain, resting EM at 4.7 mM K+ was −75 and −60 mV in wild-type and HyperKPP soleus, respectively. As per the tetanic force–resting EM curve (Fig. 5 A), small 7–9-mV depolarization is expected to have small effects on wild-type force, whereas in HyperKPP soleus, the effects were greater because the starting resting EM was in the steepest portion of the curve. The ouabain and K+ effects in EDL (Fig. 8 B) resembled those of soleus.
Figure 8.
Ouabain caused greater loss of tetanic force and membrane depolarization in HyperKPP, EDL, and soleus than in diaphragm. (A–C) All muscles were allowed a 30-min equilibrium at 4.7 mM K+ before any measurements or changes in [K+]e or ouabain. For force measurements, muscles were exposed 20 min to ouabain while being exposed to 4.7 mM K+ before [K+]e was increased to 9 mM, still in the presence of ouabain as indicated in the figures. A similar approach was used for resting EM with half the muscles; for the other half, muscles were exposed to ouabain only after [K+]e had been raised to 9 mM K+. The resting EM at 9 mM and 1 µM ouabain was not different between the two approaches, so the data were pooled. Error bars represent SEM; force of five muscles; resting EM: 70–93 fibers/9 muscles at 4.7 mM K+ and 39–80 fibers/6 muscles for all other conditions. *, Mean tetanic force or resting EM of HyperKPP muscle was significantly different from the mean values of wild-type muscle, ANOVA, and LSD, P < 0.05.
The situation was very different with the diaphragm (Fig. 8 C). First, the decrease in force upon exposure to 1 µM ouabain at 4.7 mM K+ was not different between the wild-type and HyperKPP diaphragm because resting EM in the presence of ouabain did not decrease below −72 mV for wild type and −63 mV for HyperKPP; i.e., resting EM remained in a range for which there is little effect on tetanic force (Fig. 5 C). Second, although the decrease in tetanic force in the wild-type diaphragm upon raising [K+]e to 9 mM at 1 µM ouabain was similar to that of wild-type soleus and EDL, the decrease in the HyperKPP diaphragm was only 42% compared with 89–91% in soleus and EDL. For a complete loss of force in the HyperKPP diaphragm at 9 mM K+, the HyperKPP diaphragm had to be exposed to 10 µM ouabain (Fig. 9 A), which fully inhibits NKAα2 and NKAα1 by 37% (Chibalin et al., 2012). The large decrease in force in that condition was related to a membrane depolarization to −50 mV (Fig. 9 B), a EM at which tetanic force is expected to be zero in the HyperKPP diaphragm (Fig. 5 C).
Figure 9.
Effect of 10 µM ouabain at 9 mM K+ and 100 µM ouabain at 4.7 mM K+. Effects of 10 µM ouabain added as [K+]e was increased to 9 mM on (A) tetanic force and (B) resting EM of diaphragm. Effects of 100 µM ouabain at 4.7 mM K+ on (C) tetanic force in diaphragm and (D) resting EM of soleus (S), EDL (E), and diaphragm (D). Error bars represent the SEM of 5 muscles (A and B) and 86–177 fibers/5 muscles (C and D). *, mean tetanic force or resting EM of HyperKPP muscle was significantly different from the mean values of wild-type muscle; ANOVA and LSD, P < 0.05.
At 100 µM ouabain, both NKAα1 and NKAα2 are fully inhibited (Chibalin et al., 2012). At 4.7 mM K+, 100 µM ouabain reduced the tetanic force of the HyperKPP diaphragm by 84% (Fig. 9 C; similar experiments were not performed with EDL and soleus because 10 µM ouabain is sufficient to completely abolish tetanic force in HyperKPP EDL and soleus; Lucas et al., 2014). Although the HyperKPP diaphragm had the most negative and the HyperKPP soleus had the least negative resting EM at 4.7 mM K+, an exposure to 100 µM ouabain depolarized the membrane to the same level in all three muscles, i.e., −47 mV (Fig. 9 D).
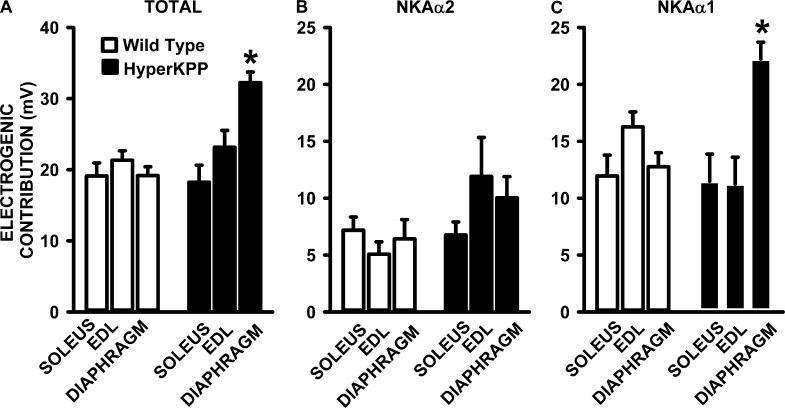
The total electrogenic contribution of NKAα1 and NKAα2, calculated from the differences in resting EM in the absence and presence of 100 µM ouabain, was 19–21 mV in wild-type muscles and was not significantly different from the 18–23-mV contribution in the HyperKPP soleus and EDL (Fig. 10 A). In the HyperKPP diaphragm, however, the total NKA contribution was significantly greater at 32 mV compared with only 19 mV in the wild-type diaphragm. The NKAα2 electrogenic contribution, calculated from the depolarization caused by 1 µM ouabain, was similar in wild-type and HyperKPP soleus and higher in the HyperKPP EDL and diaphragm than in their wild-type counterparts, even though the differences were not significant (Fig. 10 B). The NKAα1 electrogenic contribution, or the difference between total and NKAα2 contribution, was not different between wild-type and HyperKPP EDL and soleus, whereas it was significantly greater in HyperKPP than in the wild-type diaphragm (Fig. 10 C).
Figure 10.
The NKA electrogenic contribution at 4.7 mM K+ was significantly greater in the HyperKPP diaphragm than in the EDL and soleus. (A) Total NKA electrogenic contribution calculated from the difference in resting EM in the absence and presence of 100 µM ouabain, which fully inhibits NKAα1 and NKAα2 activity. (B) NKAα2 electrogenic contribution calculated from the difference in resting EM in the absence and presence of 1 µM ouabain, which reduced the activity of NKAα2 by 92% and that of NKAα1 by 6%. (C) NKAα1 electrogenic contribution calculated from the difference in total and NKAα2 electrogenic contribution. Error bars represent the SEM for the number of fibers and muscles given in Fig. 8. *, mean electrogenic contribution in HyperKPP was significantly different from the mean value for wild type; ANOVA and LSD; P < 0.05.
NCX
Increasing [Ca2+]e improves force generation of HyperKPP muscles (Creutzfeldt et al., 1963; Lucas et al., 2014). Furthermore, contrary to wild-type EDL and soleus, the diaphragm depends on Ca2+ influx to maintain twitch force (Viirès et al., 1988), and there is evidence that NCX plays a role in diaphragm contractility (Zavecz et al., 1991; Zavecz and Anderson, 1992). In cardiac muscle, membrane depolarization and increases in [Na+]i are two mechanisms by which [Ca2+]i is elevated during contraction, as NCX works in the reverse mode (Janvier and Boyett, 1996) and a similar mechanism has been proposed for the diaphragm (Zavecz and Anderson, 1992). We therefore investigated whether the greater force in the HyperKPP diaphragm at less negative resting EM involves higher NCX activity in the reverse mode. To test this, we measured tetanic force while exposing muscles to 3 µM ORM-10103, an NCX inhibitor (Jost et al., 2013).
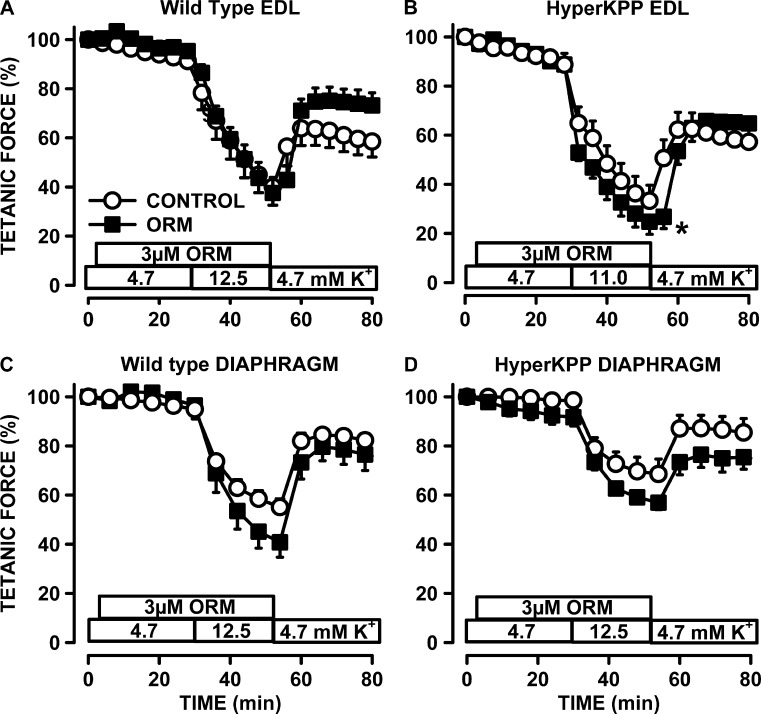
The effects of ORM-10103 were first tested in wild-type EDL, as this muscle is the least dependent on extracellular Ca2+ (Viirès et al., 1988) and for which there is no evidence for a role of NCX during contractions (Blaustein and Lederer, 1999). As expected, ORM-10103 had no effect on tetanic force of that muscle at 4.7 and 12.5 mM K+ (Fig. 11 A). ORM-10103 had no effect on HyperKPP EDL while exposed to 4.7 mM K+, but the force loss at 11 mM K+ was greater in the presence of ORM-10103 (Fig. 11 B). Similarly, the decrease in tetanic force in the wild-type and HyperKPP diaphragm at 12.5 mM K+ was greater in the presence than in the absence of ORM-10103 (Fig. 11, C and D). However, the difference in force in the absence and presence of ORM-10103 at 12.5 mM K+ was the same for the wild-type and HyperKPP diaphragm, suggesting that an increased NCX activity in the reverse mode is not a mechanism that can explain the greater force in the HyperKPP diaphragm at depolarized resting EM (Fig. 5 C).
Figure 11.
ORM-10103 caused small decreases in tetanic force in the wild-type diaphragm and in the HyperKPP EDL and diaphragm, but not in wild type EDL. (A–D) Muscles were first exposed to 3 µM ORM-10103, an NCX inhibitor, while being exposed to 4.7 mM K+. The increase in [K+]e was to 12.5 mM for all muscles, except for the HyperKPP EDL, for which the increase was to 11 mM K+, as this muscle was more sensitive to the K+-induced force depression. Error bars represent the SEM of 5 muscles. *, mean tetanic force of ORM-10103–exposed muscles was significantly less than the mean value of control; ANOVA and LSD; P < 0.05.
Action potential–resting EM relationship
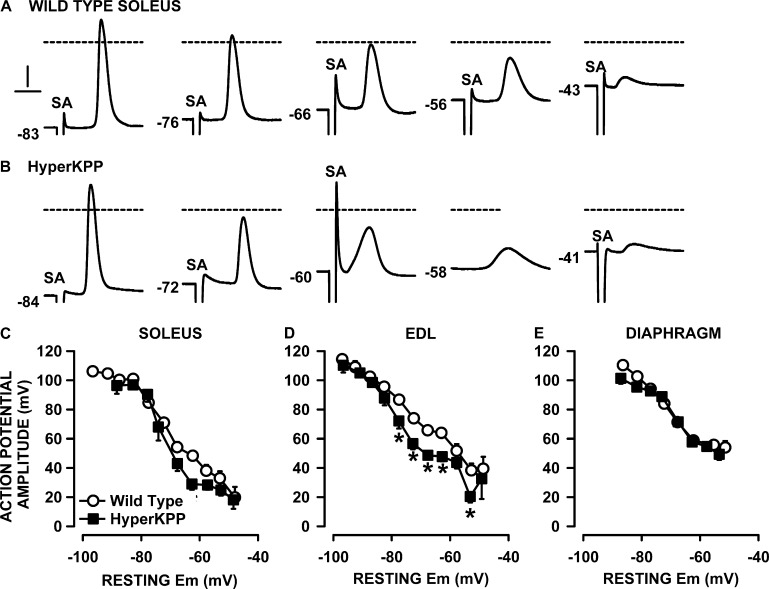
Next, we measured action potentials in soleus, EDL, and diaphragm fibers. In general, at 4.7 mM K+, action potentials were easily triggered in wild-type muscles, for which >95% of fibers generated an action potential upon stimulation. The situation was very different in HyperKPP soleus and EDL. At 4.7 mM K+, 30% of HyperKPP soleus fibers failed to generate an action potential upon stimulation, while 27% of HyperKPP EDL did the same. Among the excitable fibers, action potential shapes were quite similar between wild-type and HyperKPP EDL fibers when resting EM was greater than −80 mV (measured at 4.7 mM K+; Fig. 12, A and B). At less negative resting EM, such as between −55 and −75 mV (8–10 mM K+), HyperKPP EDL fibers had action potentials with lower amplitude than their wild-type counterparts. Very few fibers generated action potential below a resting EM of −55 mV (12–15 mM K+), and for those that did, the amplitude was very small.
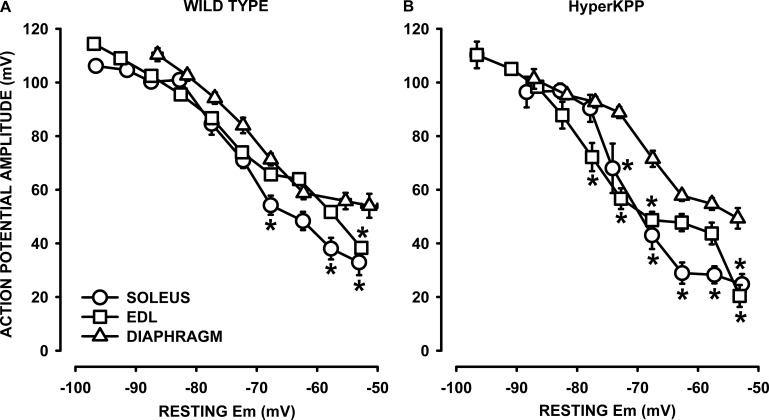
Figure 12.
Action potential amplitudes were lower in HyperKPP than in wild type, soleus, and EDL but not diaphragm. Examples of action potential traces from (A) wild-type and (B) HyperKPP EDL. Numbers at the start of each trace represent the starting resting EM. SA is for the 0.3-msec-long stimulus artifact. Dashed horizontal lines represent 0 mV. Vertical and horizontal bars represent 20 mV and 1 msec, respectively. (C–E) Resting EM and action potentials were measured from fibers located at the muscle surface. Each muscle was tested at 2 or 3 [K+]e. Data from all fibers were pooled together and separated according to their resting EM in a bin of 5 mV. For each bin, resting EM and action potential amplitudes were averaged. Vertical and horizontal bars represent the SEM of action potential amplitude and resting EM, respectively (not shown if smaller than symbol). The total number of samples varied between 158 and 385 fibers from 5 to 11 muscles. *, mean action potential amplitude from HyperKPP fibers was significantly different from that of wild type; ANOVA and LSD; P < 0.05.
A major aim here was to test whether the shift in the tetanic force versus resting EM relationship in the HyperKPP diaphragm versus wild type (Fig. 5) can be explained by action potentials with greater amplitude in the HyperKPP than wild-type diaphragm at depolarized resting EM. To do this, we first took into consideration the large variability in resting EM (Fig. 4). That is, action potentials were measured in several fibers while being exposed at [K+]e varying between 4.7 and 14.5 mM. Fibers were then separated according to their resting EM in bins of 5 mV. For each bin, mean resting EM, action potential amplitude, and peak were averaged to construct action potential amplitude versus resting EM relationship.
For soleus and EDL, mean action potential amplitudes were similar between wild type and HyperKPP at resting EM above −70 mV (Fig. 12, C and D). At resting EM less negative than −70 mV, action potential amplitude became smaller in HyperKPP soleus and EDL compared with their wild-type counterpart. Contrary to the situation with soleus and EDL, mean action potential amplitudes at various resting EMs were not different between the wild-type and HyperKPP diaphragm (Fig. 12 E).
We then compared the action potential amplitude versus resting EM between muscles for each of wild type and HyperKPP. For wild type, mean action potential amplitudes were lower in soleus and EDL compared with the diaphragm, with significant differences at depolarized resting EM (Fig. 13 A). The differences between diaphragm and hindlimb muscles were more pronounced in HyperKPP (Fig. 13 B). Similar analyses for action potential peak versus resting EM relationships gave rise to similar differences between wild type and HyperKPP and between EDL, soleus, and diaphragm to those observed for the action potential amplitude versus resting EM relationships (not depicted).
Figure 13.
At depolarized resting EM, action potential amplitude becomes significantly less in soleus and EDL than in the diaphragm, especially for HyperKPP. (A and B) The data are the same as in Fig. 12 but replotted to show differences between muscles. *, mean action potential amplitude from EDL or soleus fibers was significantly different from that of diaphragm fibers; ANOVA and LSD; P < 0.05.
DISCUSSION
Individuals with HyperKPP rarely suffer from respiratory distress, although this can occur more frequently after procedures or exposure to anesthetic agents (Charles et al., 2013). In two of our studies (this study and Lucas et al., 2014), only 2 of the 52 tested diaphragm muscles from the M1592V HyperKPP mouse model suddenly stopped contracting upon stimulation as if they had become paralyzed in the course of an in vitro experiment. The objective of this study was to identify physiological mechanisms that render HyperKPP diaphragm resistant to weakness triggered by elevated [K+]e. The major findings of this study were: (a) contrary to HyperKPP soleus and EDL fibers, which were highly depolarized compared with their wild-type counterparts, there was no significant difference in mean or in the frequency distribution of resting EM between diaphragm fibers from HyperKPP compared with wild type; (b) the entire tetanic force–resting EM relationship of the HyperKPP diaphragm was shifted to less negative resting EM when compared with the wild-type relationship, whereas only a partial shift was observed for HyperKPP soleus and EDL; (c) NKAα1 and NKAα2 protein content was the same in the wild-type and HyperKPP diaphragm; (d) the NKA electrogenic contribution, especially the α1 subunit, was greater in the HyperKPP diaphragm, whereas for soleus and EDL, the total NKAα1 and NKAα2 electrogenic contribution was not different between wild type and HyperKPP; (e) the action potential amplitude versus resting EM relationship of the HyperKPP diaphragm was similar to that of the wild-type diaphragm, whereas it was shifted toward more depolarized resting EM when compared with HyperKPP soleus and EDL.
Protection of resting EM by NKA as one mechanism
It is well known that resting EM is more depolarized in HyperKPP muscles from both mouse and patients, which likely is a major contributor to muscle weakness (Lehmann-Horn et al., 1983, 1987; Ricker et al., 1989; Clausen et al., 2011). The importance of less polarization of the resting EM contributing to the lower force is further confirmed in this study from three points of view. First, there was a major shift in the frequency distribution of resting EM toward lower EM values in HyperKPP soleus and EDL (Fig. 4, A and B). Second, many fibers had resting EM lower than −55 mV, an EM below which muscles failed to generate force (Renaud and Light, 1992; Cairns et al., 1997). In that regard, 30% of HyperKPP soleus fibers failed to generate an action potential upon stimulation, a value that was close to the 27% of fibers with a resting EM below −55 mV. Third, the tetanic force generated at 4.7 and 9 mM K+ by HyperKPP soleus was very close to the expected force from the tetanic force versus resting EM relationship of wild-type soleus.
At 4.7 mM K+, the relative difference in tetanic force between wild-type and HyperKPP was less for EDL (24%) than soleus (34%). We can again demonstrate that the difference between the two muscles is in part related to differences in resting EM. That is, HyperKPP EDL had a greater mean resting EM because of a larger proportion of fibers having resting EM above −70 mV and less below −55 mV than in HyperKPP soleus. However, contrary to HyperKPP soleus, the tetanic forces generated by HyperKPP EDL at 4.7 and 9 mM K+ was less than the expected forces from the mean resting EM and the tetanic force versus resting EM relationship of wild-type EDL, suggesting that perhaps other factors are involved in lowering force in HyperKPP EDL, as discussed in the section below, Importance of stronger action potential.
The situation in the HyperKPP diaphragm was completely different to that of hindlimb muscles. The differences in mean resting EM between wild type and HyperKPP at various [K+]e ranged between 2 and 4 mV in the diaphragm compared with 6–15 mV in the soleus and 5–9 mV in the EDL. Furthermore, contrary to HyperKPP soleus and EDL, there were only small differences in the frequency distribution of resting EM between the wild-type and HyperKPP diaphragm. The importance of better resting EM in the HyperKPP diaphragm than in the soleus and EDL especially at high [K+]e can be illustrated as follows. For HyperKPP soleus at 9 mM K+, resting EM was −56 mV and tetanic force was 40% of wild-type force at 4.7 mM K+ (used as the maximum force normal muscle can generate; Fig. 5). At the same [K+]e, resting EM of the HyperKPP diaphragm was −62 mV and tetanic force was 96% of maximum force, representing a 56% difference in force between the HyperKPP soleus and diaphragm. If, on the other hand, the HyperKPP diaphragm had depolarized to −56 mV, then according to the tetanic force–resting EM relationship shown in Fig. 5 C, tetanic force would have only been 50%, representing just a 10% difference with soleus. When similar calculations are performed for EDL, the differences in tetanic force at 9 mM K+ with the HyperKPP diaphragm is 39%, being reduced to 21% if the diaphragm-resting EM had depolarized to −59 mV as observed in EDL instead of the measured −62 mV. Thus, a better maintenance of resting EM (this study) despite large Na+ influx (Lucas et al., 2014) is one important mechanism that protects the HyperKPP diaphragm from the robust symptoms observed in the HyperKPP soleus and EDL.
This study now provides evidence for an increased electrogenic contribution of NKA, primarily its α1 isoform, to the resting EM of the HyperKPP diaphragm. We suggest that the increase in NKA electrogenic contribution is related to greater NKA activity in HyperKPP than in the wild-type diaphragm, as we did not observe a difference in the NKAα1 or NKAα2 protein content between the wild-type and HyperKPP diaphragm. In fact, it appears that changes in NKA content does very little, as the NKA electrogenic contribution in the HyperKPP EDL was similar to that in the wild-type EDL despite a 1.5- to 2.0-fold greater NKAα1 and NKAα2 content in HyperKPP (Figs. 6 A and 7 A; Clausen et al., 2011). Also, an increased electrogenic contribution was observed only in the HyperKPP diaphragm despite the fact that the NKA content was no longer different between the HyperKPP soleus, EDL, and diaphragm as it was for wild-type muscles (Figs. 6 B and 7 B). A possible mechanism for the difference in NKA activity between the diaphragm and limb muscles is the fact that the former is constantly active. Reducing the time interval between contractions to 1 min not only acutely increases NKA activity in wild-type and HyperKPP muscles, but it also allows for an increase in tetanic force in the HyperKPP soleus toward the wild-type level (Overgaard et al., 1999; Clausen et al., 2011). This is in agreement with the fact that HyperKPP patients sometimes can avoid a weakness or paralytic attack with mild exercise (Poskanzer and Kerr, 1961).
An activation of NKA by the β-adrenergic agonist salbutamol has been shown to eliminate muscle weakness in hindlimb muscles of patients and mice suffering from HyperKPP (Wang and Clausen, 1976; Clausen et al., 2011). Altogether it would appear that a salbutamol treatment would help in keeping a higher NKA activity in hindlimb muscles and thus prevent weakness. Unfortunately, the efficacy of salbutamol decreases over time in human patients (Clausen et al., 1980). The loss of effectiveness over time suggests that there is a fundamental difference in the regulation of NKA activity between HyperKPP hindlimb and diaphragm muscles, and a better understanding of this regulation in future studies will help us develop more effective and sustained pharmacological strategies to treat HyperKPP patients.
Importance of stronger action potential
As discussed above, the mean tetanic forces generated by the HyperKPP soleus at resting EM between −55 and −60 mV (or at 4.7 or 9 mM K+) were close to our expectation from the tetanic force versus resting EM relationship of wild-type soleus. In EDL, on the other hand, the mean tetanic forces between −60 and −70 mV were less than the expectation. One possible reason for this difference may be in the generation of action potentials. HyperKPP soleus fibers generated action potentials of similar amplitude as their wild-type counterpart when resting EM was greater than −70 mV, with just a small tendency for lower amplitude below −70 mV. For EDL, the differences between wild-type and HyperKPP were more pronounced and started when resting EM was less than −80 mV. Considering the shift in the resting EM frequency distribution toward a lower potential, we suggest that even at 4.7 mM K+, HyperKPP EDL has many fibers with resting EM at which it generates lower action potential amplitude than wild type, which then most likely results in lower Ca2+ release and force generation. This difference between the HyperKPP soleus and EDL cannot be explained from our results. Lower action potential amplitude can be related to higher [Na+]i, which are known to be higher in HyperKPP muscles (Lehmann-Horn et al., 1987; Ricker et al., 1989; Amarteifio et al., 2012). Higher [Na+]i then lowers action potential amplitude as the decreased Na+ concentration gradient is reduced (Cairns et al., 2003). However, this explanation is questionable if we consider the lack of any significant difference in NKA electrogenic contribution (Fig. 10) and Na+ influx between the HyperKPP soleus and EDL (Lucas et al., 2014). Another possibility is the difference in ClC-1 Cl− channel activity between these two muscles, and varying this channel activity at elevated [K+]e significantly impacts the capacity of muscle to generate action potentials and force (Pedersen et al., 2005, 2009a, b).
Interestingly, HyperKPP EDL and soleus still generated a small amount of force when resting EM became below −55 mV, a potential at which no more force was generated by wild-type muscles as reported previously (Renaud and Light, 1992; Cairns et al., 1997). At those resting EMs, action potential measurements became very difficult because of a very large number of unexcitable fibers. Furthermore, although many fibers failed to generate single action potentials, the situation may be different during a tetanic stimulation train during which a few action potentials may eventually be generated considering that the steady-state slow inactivation never reaches zero (Hayward et al., 1999) and that K+ at a concentration as low as 10 mM provokes a non-inactivation mode in the mutant NaV1.4 channel (Cannon et al., 1991). So, at a resting EM below −55 mV, few action potentials are generated by mutant NaV1.4 channels during a tetanus giving rise to small force development.
The situation was again different with the diaphragm. Contrary to soleus and EDL, the HyperKPP diaphragm was slightly less sensitive to the K+-induced force depression than the wild-type diaphragm; although the effect was not significant, it was constantly observed in all experiments. The lower sensitivity to K+ was not accompanied by smaller membrane depolarizations when [K+]e was increased. In fact, the reverse was observed as the mean resting EMs were slightly lower in the HyperKPP than in the wild-type diaphragm. Moreover, there was little difference in the resting EM frequency distribution. As a consequence of this situation, the tetanic force versus mean resting EM was significantly shifted toward less negative resting EM so that at depolarized EM, the HyperKPP diaphragm generated greater tetanic force than the wild-type diaphragm.
The shift cannot be explained by the generation of an action potential with greater amplitude in HyperKPP than in wild type because there was no difference in the action potential amplitude versus resting EM relationship, at least in regards to the generation of single action potentials. Another possibility is greater increases in [Ca2+]i during a contraction in HyperKPP diaphragm fibers in the absence of any difference in action potential amplitude. An NCX inhibition resulted in lower force in both the wild-type and HyperKPP diaphragm at 12.5 mM K+ (Fig. 11), supporting a role for NCX working in the reverse mode to increase [Ca2+]i during contraction, which is in agreement with previous studies (Zavecz et al., 1991; Zavecz and Anderson, 1992; Blaustein and Lederer, 1999). However, the force reduction upon NCX inhibition was the same in wild type and HyperKPP. So, if there is a greater [Ca2+]i during contraction in the HyperKPP diaphragm, it is unlikely that the mechanism involves NCX. Another possibility is a greater Ca2+ entry via the store-operated Ca2+ entry to maintain high Ca2+ content in the sarcoplasmic reticulum as reported previously (Lucas et al., 2014) or greater Ca2+ release by the sarcoplasmic reticulum when CaV1.1 and RyR1 channels are activated by action potentials.
Although there was no difference in the action potential amplitude versus resting EM between the wild-type and HyperKPP diaphragm, we observed that this relationship in the diaphragm was shifted toward less negative resting EM when compared with EDL and soleus. The shift was small with few significant differences for wild-type muscles when resting EM was less than −70 mV. For HyperKPP muscles, on the other hand, the differences were much greater and became significant when resting EM was less than −80 mV. It thus appears that the HyperKPP diaphragm has a better capacity of generating normal action potentials than HyperKPP hindlimb muscles. One possible mechanism for the improved action potential amplitude is lower [Na+]i than in the hindlimb muscles because of greater NKA activity. The better action potentials also constitute a second mechanism that renders the HyperKPP diaphragm asymptomatic.
In conclusion, we provide evidence here that compared with the HyperKPP soleus and EDL, (a) the HyperKPP diaphragm muscle better maintains its resting EM at various [K+]e despite very large Na+ influx through defective NaV1.4 channels and (b) generates larger action potentials at depolarized EM, providing two essential mechanisms that minimize weakness in the HyperKPP diaphragm. The improved resting EM is related to significant increases in the electrogenic contribution (or activity) of the NKAα1 isoform rather than an increase in NKA protein content. The improved capacity of the HyperKPP diaphragm to generate action potentials may also be caused by the higher NKA activity that maintains low [Na+]i. Finally, compared with wild type, the HyperKPP diaphragm generated more force at depolarized resting EM. The mechanism for this improved force generation could not be discerned here as it was not related to the generation of stronger action potentials compared with wild type nor to a greater contribution of NCX working in the reverse mode to the increase in [Ca2+]i during contraction. Future studies will be necessary to understand the mechanisms responsible for the higher NKA activity in the HyperKPP diaphragm and to examine whether facilitating these pathways in hindlimb muscles could improve function for HyperKPP patients.
Acknowledgments
This study was supported by a grant from the Canadian Institute of Health Research to J.-M. Renaud.
The authors declare no competing financial interests.
Eduardo Rios served as editor.
Footnotes
Abbreviations used in this paper:
- EDL
- extensor digitorum longus
- EM
- membrane potential
- FDB
- flexor digitorum brevis
- HyperKPP
- hyperkalemic periodic paralysis
- LSD
- least square difference
- NCX
- Na+–Ca2+ exchanger
- NKA
- Na+–K+ ATPase pump
- TTX
- tetrodotoxin
References
- Amarteifio E., Nagel A.M., Weber M.A., Jurkat-Rott K., and Lehmann-Horn F.. 2012. Hyperkalemic periodic paralysis and permanent weakness: 3-T MR imaging depicts intracellular 23Na overload—initial results. Radiology. 264:154–163. 10.1148/radiol.12110980 [DOI] [PubMed] [Google Scholar]
- Blaustein M.P., and Lederer W.J.. 1999. Sodium/calcium exchange: its physiological implications. Physiol. Rev. 79:763–854. [DOI] [PubMed] [Google Scholar]
- Bradley W.G., Taylor R., Rice D.R., Hausmanowa-Petruzewicz I., Adelman L.S., Jenkison M., Jedrzejowska H., Drac H., and Pendlebury W.W.. 1990. Progressive myopathy in hyperkalemic periodic paralysis. Arch. Neurol. 47:1013–1017. 10.1001/archneur.1990.00530090091018 [DOI] [PubMed] [Google Scholar]
- Cairns S.P., Hing W.A., Slack J.R., Mills R.G., and Loiselle D.S.. 1997. Different effects of raised [K+]o on membrane potential and contraction in mouse fast- and slow-twitch muscle. Am. J. Physiol. 273:C598–C611. [DOI] [PubMed] [Google Scholar]
- Cairns S.P., Buller S.J., Loiselle D.S., and Renaud J.M.. 2003. Changes of action potentials and force at lowered [Na+]o in mouse skeletal muscle: implications for fatigue. Am. J. Physiol. Cell Physiol. 285:C1131–C1141. 10.1152/ajpcell.00401.2002 [DOI] [PubMed] [Google Scholar]
- Cannon S.C. 2006. Pathomechanisms in channelopathies of skeletal muscle and brain. Annu. Rev. Neurosci. 29:387–415. 10.1146/annurev.neuro.29.051605.112815 [DOI] [PubMed] [Google Scholar]
- Cannon S.C., Brown R.H. Jr, and Corey D.P.. 1991. A sodium channel defect in hyperkalemic periodic paralysis: Potassium-induced failure of inactivation. Neuron. 6:619–626. 10.1016/0896-6273(91)90064-7 [DOI] [PubMed] [Google Scholar]
- Charles G., Zheng C., Lehmann-Horn F., Jurkat-Rott K., and Levitt J.. 2013. Characterization of hyperkalemic periodic paralysis: a survey of genetically diagnosed individuals. J. Neurol. 260:2606–2613. 10.1007/s00415-013-7025-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibalin A.V., Heiny J.A., Benziane B., Prokofiev A.V., Vasiliev A.V., Kravtsova V.V., and Krivoi I.I.. 2012. Chronic nicotine modifies skeletal muscle Na,K-ATPase activity through its interaction with the nicotinic acetylcholine receptor and phospholemman. PLoS One. 7:e33719 10.1371/journal.pone.0033719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnery P.F., Walls T.J., Hanna M.G., Bates D., and Fawcett P.R.. 2002. Normokalemic periodic paralysis revisited: Does it exist? Ann. Neurol. 52:251–252. 10.1002/ana.10257 [DOI] [PubMed] [Google Scholar]
- Clausen T., Wang P., Orskov H., and Kristensen O.. 1980. Hyperkalemic periodic paralysis. Relationships between changes in plasma water, electrolytes, insulin and catecholamines during attacks. Scand. J. Clin. Lab. Invest. 40:211–220. 10.3109/00365518009095569 [DOI] [PubMed] [Google Scholar]
- Clausen T., Nielsen O.B., Clausen J.D., Pedersen T.H., and Hayward L.J.. 2011. Na+,K+-pump stimulation improves contractility in isolated muscles of mice with hyperkalemic periodic paralysis. J. Gen. Physiol. 138:117–130. 10.1085/jgp.201010586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutzfeldt O.D., Abbott B.C., Fowler W.M., and Pearson C.M.. 1963. Muscle membrane potentials in episodic adynamia. Electroencephalogr. Clin. Neurophysiol. 15:508–519. 10.1016/0013-4694(63)90071-3 [DOI] [PubMed] [Google Scholar]
- Edwards J.N., Macdonald W.A., van der Poel C., and Stephenson D.G.. 2007. O2(*-) production at 37°C plays a critical role in depressing tetanic force of isolated rat and mouse skeletal muscle. Am. J. Physiol. Cell Physiol. 293:C650–C660. 10.1152/ajpcell.00037.2007 [DOI] [PubMed] [Google Scholar]
- Gamstorp I., Hauge M., Helweglarsen H.F., Mjönes H., and Sagild U.. 1957. Adynamia episodica hereditaria: A disease clinically resembling familial periodic paralysis but characterized by increasing serum potassium during the paralytic attacks. Am. J. Med. 23:385–390. 10.1016/0002-9343(57)90318-2 [DOI] [PubMed] [Google Scholar]
- Hayward L.J., Sandoval G.M., and Cannon S.C.. 1999. Defective slow inactivation of sodium channels contributes to familial periodic paralysis. Neurology. 52:1447–1453. 10.1212/WNL.52.7.1447 [DOI] [PubMed] [Google Scholar]
- Hayward L.J., Kim J.S., Lee M.-Y., Zhou H., Kim J.W., Misra K., Salajegheh M., Wu F.-F., Matsuda C., Reid V., et al. . 2008. Targeted mutation of mouse skeletal muscle sodium channel produces myotonia and potassium-sensitive weakness. J. Clin. Invest. 118:1437–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janvier N.C., and Boyett M.R.. 1996. The role of Na-Ca exchange current in the cardiac action potential. Cardiovasc. Res. 32:69–84. 10.1016/0008-6363(96)00017-X [DOI] [PubMed] [Google Scholar]
- Jost N., Nagy N., Corici C., Kohajda Z., Horváth A., Acsai K., Biliczki P., Levijoki J., Pollesello P., Koskelainen T., et al. . 2013. ORM-10103, a novel specific inhibitor of the Na+/Ca2+ exchanger, decreases early and delayed afterdepolarizations in the canine heart. Br. J. Pharmacol. 170:768–778. 10.1111/bph.12228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann-Horn F., Rüdel R., Ricker K., Lorković H., Dengler R., and Hopf H.C.. 1983. Two cases of adynamia episodica hereditaria: In vitro investigation of muscle cell membrane and contraction parameters. Muscle Nerve. 6:113–121. 10.1002/mus.880060206 [DOI] [PubMed] [Google Scholar]
- Lehmann-Horn F., Küther G., Ricker K., Grafe P., Ballanyi K., and Rüdel R.. 1987. Adynamia episodica hereditaria with myotonia: A non-inactivating sodium current and the effect of extracellular pH. Muscle Nerve. 10:363–374. 10.1002/mus.880100414 [DOI] [PubMed] [Google Scholar]
- Lucas B., Ammar T., Khogali S., DeJong D., Barbalinardo M., Nishi C., Hayward L.J., and Renaud J.M.. 2014. Contractile abnormalities of mouse muscles expressing hyperkalemic periodic paralysis mutant NaV1.4 channels do not correlate with Na+ influx or channel content. Physiol. Genomics. 46:385–397. 10.1152/physiolgenomics.00166.2013 [DOI] [PubMed] [Google Scholar]
- Miller T.M., Dias da Silva M.R., Miller H.A., Kwiecinski H., Mendell J.R., Tawil R., McManis P., Griggs R.C., Angelini C., Servidei S., et al. . 2004. Correlating phenotype and genotype in the periodic paralyses. Neurology. 63:1647–1655. 10.1212/01.WNL.0000143383.91137.00 [DOI] [PubMed] [Google Scholar]
- Overgaard K., Nielsen O.B., Flatman J.A., and Clausen T.. 1999. Relations between excitability and contractility in rat soleus muscle: role of the Na+-K+ pump and Na+/K+ gradients. J. Physiol. 518:215–225. 10.1111/j.1469-7793.1999.0215r.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen T.H., de Paoli F., and Nielsen O.B.. 2005. Increased excitability of acidified skeletal muscle: Role of chloride conductance. J. Gen. Physiol. 125:237–246. 10.1085/jgp.200409173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen T.H., de Paoli F.V., Flatman J.A., and Nielsen O.B.. 2009a. Regulation of ClC-1 and KATP channels in action potential–firing fast-twitch muscle fibers. J. Gen. Physiol. 134:309–322. 10.1085/jgp.200910290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen T.H., Macdonald W.A., de Paoli F.V., Gurung I.S., and Nielsen O.B.. 2009b. Comparison of regulated passive membrane conductance in action potential–firing fast- and slow-twitch muscle. J. Gen. Physiol. 134:323–337. 10.1085/jgp.200910291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poskanzer D.C., and Kerr D.N.. 1961. A third type of periodic paralysis, with normokalemia and favourable response to sodium chloride. Am. J. Med. 31:328–342. 10.1016/0002-9343(61)90122-X [DOI] [PubMed] [Google Scholar]
- Renaud J.M., and Light P.. 1992. Effects of K+ on the twitch and tetanic contraction in the sartorius muscle of the frog, Rana pipiens. Implication for fatigue in vivo. Can. J. Physiol. Pharmacol. 70:1236–1246. 10.1139/y92-172 [DOI] [PubMed] [Google Scholar]
- Ricker K., Camacho L.M., Grafe P., Lehmann-Horn F., and Rüdel R.. 1989. Adynamia episodica hereditaria: What causes the weakness? Muscle Nerve. 12:883–891. 10.1002/mus.880121103 [DOI] [PubMed] [Google Scholar]
- Rojas C.V., Neely A., Velasco-Loyden G., Palma V., and Kukuljan M.. 1999. Hyperkalemic periodic paralysis M1592V mutation modifies activation in human skeletal muscle Na+ channel. Am. J. Physiol. 276:C259–C266. [DOI] [PubMed] [Google Scholar]
- Rüdel R., and Ricker K.. 1985. The primary periodic paralyses. Trends Neurosci. 8:467–470. 10.1016/0166-2236(85)90171-7 [DOI] [Google Scholar]
- Steel R.G.D., and Torrie J.H.. 1980. Principles and procedures of statistics. A biometrical approach. McGraw-Hill Book Company, New York: 481 pp. [Google Scholar]
- Streeten D.H.P., Dalakos T.G., and Fellerman H.. 1971. Studies on hyperkalemic periodic paralysis. Evidence of changes in plasma Na and Cl and induction of paralysis by adrenal glucocorticoids. J. Clin. Invest. 50:142–155. 10.1172/JCI106468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viirès N., Murciano D., Seta J.-P., Dureuil B., Pariente R., and Aubier M.. 1988. Effects of Ca2+ withdrawal on diaphragmatic fiber tension generation. J. Appl. Physiol. 64:26–30. [DOI] [PubMed] [Google Scholar]
- Wang P., and Clausen T.. 1976. Treatment of attacks in hyperkalaemic familial periodic paralysis by inhalation of salbutamol. Lancet. 1:221–223. 10.1016/S0140-6736(76)91340-4 [DOI] [PubMed] [Google Scholar]
- Yensen C., Matar W., and Renaud J.M.. 2002. K+-induced twitch potentiation is not due to longer action potential. Am. J. Physiol. Cell Physiol. 283:C169–C177. 10.1152/ajpcell.00549.2001 [DOI] [PubMed] [Google Scholar]
- Zavecz J.H., and Anderson W.M.. 1992. Role of extracellular Ca2+ in diaphragmatic contraction: effects of ouabain, monensin, and ryanodine. J. Appl. Physiol. 73:30–35. [DOI] [PubMed] [Google Scholar]
- Zavecz J.H., Anderson W.M., and Adams B.. 1991. Effect of amiloride on diaphragmatic contractility: evidence of a role for Na(+)-Ca2+ exchange. J. Appl. Physiol. 70:1309–1314. [DOI] [PubMed] [Google Scholar]
- Zhou J., and Hoffman E.P.. 1994. Pathophysiology of sodium channelopathies. Studies of sodium channel expression by quantitative multiplex fluorescence polymerase chain reaction. J. Biol. Chem. 269:18563–18571. [PubMed] [Google Scholar]
- Zhu Z., Sierra A., Burnett C.M., Chen B., Subbotina E., Koganti S.R., Gao Z., Wu Y., Anderson M.E., Song L.S., et al. . 2014. Sarcolemmal ATP-sensitive potassium channels modulate skeletal muscle function under low-intensity workloads. J. Gen. Physiol. 143:119–134. 10.1085/jgp.201311063 [DOI] [PMC free article] [PubMed] [Google Scholar]