Abstract
Aims
Primary percutaneous coronary intervention (PPCI) is the optimal treatment for patients presenting with ST-elevation myocardial infarction (STEMI). An elevated index of microcirculatory resistance (IMR) reflects microvascular function and when measured after PPCI, it can predict an adverse clinical outcome. We measured coronary microvascular function in STEMI patients and compared sequential changes before and after stent implantation.
Methods and results
In 85 STEMI patients, fractional flow reserve, coronary flow reserve, and IMR were measured using a pressure wire (Certus, St Jude Medical, St Paul, MN, USA) immediately before and after stent implantation. Stenting significantly improved all of the measured parameters of coronary physiology including IMR from 67.7 [interquartile range (IQR): 56.2–95.8] to 36.7 (IQR: 22.7–59.5), P < 0.001. However, after stenting, IMR remained elevated (>40) in 28 (32.9%) patients. In 15 of these patients (17.6% of the cohort), only a partial reduction in IMR occurred and these patients were more likely to be late presenters (pain to wire time >6 h). The extent of jeopardized myocardium [standardized beta: −0.26 (IMR unit/Bypass Angioplasty Revascularization Investigation score unit), P: 0.009] and pre-stenting IMR [standardized beta: −0.34 (IMR unit), P: 0.001] predicted a reduction in IMR after stenting (ΔIMR = post-stenting IMR − pre-stenting IMR), whereas thrombotic burden [standardized beta: 0.24 (IMR unit/thrombus score unit), P: 0.01] and deployed stent volume [standardized beta: 0.26 (IMR unit/mm3 of stent), P: 0.01] were associated with a potentially deleterious increase in IMR.
Conclusion
Improved perfusion of the myocardium by stent deployment during PPCI is not universal. The causes of impaired microvascular function at the completion of PPCI treatment are heterogeneous, but can reflect a later clinical presentation and/or the location and extent of the thrombotic burden.
Keywords: ST-elevation myocardial infarction, Stent, Index of microcirculatory resistance, Distal embolization
See page 3178 for the editorial comment on this article (doi:10.1093/eurheartj/ehv495)
Introduction
Rapid revascularization by primary percutaneous coronary intervention (PPCI) with stenting is considered to be the treatment of choice for patients with ST-elevation myocardial infarction (STEMI).1 However, immediate restoration of epicardial coronary artery patency does not always translate into restoration of ‘normal’ myocardial reperfusion, a phenomenon referred to as ‘no reflow’ and associated with profound coronary microvascular injury.2
Various pharmacological and procedural strategies have been proposed to minimize or prevent no reflow after stenting, with often conflicting results in part because of methodological limitations.3,4
Early identification of the subset of patients who are likely to experience suboptimal reperfusion would allow timely application of adjunctive or alternative therapeutic strategies. Consequently, clear understanding of the pathophysiology of the coronary microvasculature during MI is a critical step to improve PPCI outcomes.
The index of microcirculatory resistance (IMR) is a readily available thermodilution-derived parameter,5 which reflects the status of the coronary microvasculature. Index of microcirculatory resistance measured immediately after PPCI has been shown to predict infarct size and the occurrence of microvascular obstruction at cardiac magnetic resonance.6,7 Moreover, it has been recently demonstrated that a final IMR value of >40 after PPCI has prognostic relevance being significantly associated with increased rates of death and readmission for heart failure at 1 year.8
The assessment of coronary microcirculation before stenting and its consequences represent a critical gap in our understanding. Therefore, we aimed to systematically evaluate in STEMI patients the impact of stent deployment on coronary microvascular function and to ascertain which factors might predict a suboptimal outcome defined as a post-procedural IMR of >40.
Methods
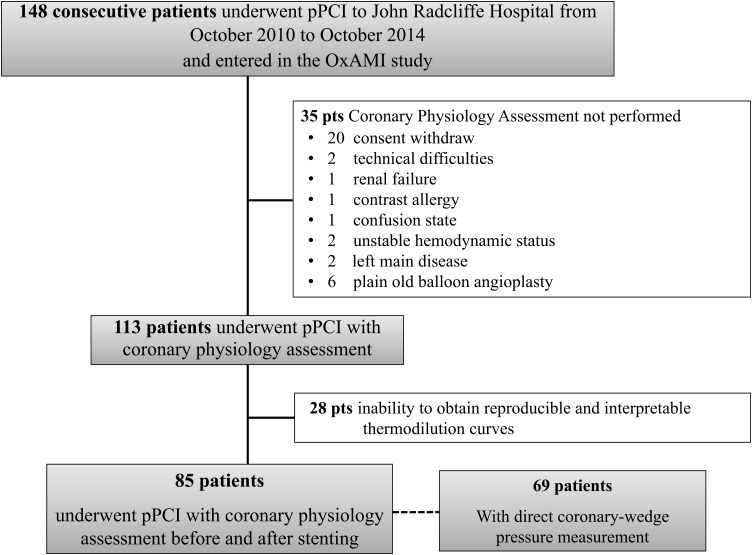
Patients with STEMI admitted to the Oxford Heart Centre for PPCI were prospectively considered for enrolment (Figure 1). ST elevation myocardial infarction was defined as the occurrence of ongoing chest pain for at least 30 min associated with ST-segment elevation >2 mm in at least two contiguous leads. Exclusion criteria were symptom duration >12 h, the presence of severe haemodynamic instability, severe left main disease, contraindications to adenosine infusion, plain old balloon angioplasty (POBA) performance without stent implantation, and inability to restore thrombolysis in myocardial infarction (TIMI) flow >2 before stenting. The local ethics committee approved the protocol and the study was conducted in accordance with the Declaration of Helsinki.
Figure 1.
Study flow chart.
This study population was recruited as part of the Oxford Acute Myocardial Infarction (Ox-AMI) study (REC number 10/H0408/24).
Primary percutaneous coronary intervention was performed according to the international guidelines. Patients were on dual antiplatelet therapy at the time of the procedure, usually loaded with 600 mg of clopidogrel in the ambulance and 300 mg of aspirin. Anticoagulation was achieved with unfractionated heparin (100 IU/kg to maintain an activated clotting time between 250 and 300 s) in combination with abciximab (0.25 mg/kg intravenous bolus ± 0.125 µg/kg/min intravenous continuous infusion for 12 h) or bivalirudin (0.75 mg/kg bolus followed by an infusion of 1.75 mg/kg/min for up 4 h after the procedure as clinically warranted). Abciximab was only used in combination with bivalirudin as a bailout strategy. Patients enrolled after January 2014 were anticoagulated with bivalirudin and loaded with ticagrelor 180 mg if self-presenters at the Acute and Emergency unit or reloaded with ticagrelor 180 mg at the end of the procedure if loaded with clopidogrel in the ambulance, reflecting changes in routine clinical practice in our institution. Thrombus aspiration was recommended, but undertaken at the operator's discretion.
Measurement of parameters of coronary physiology
After crossing the culprit lesion, coronary flow was initially restored by thrombus aspiration and/or balloon predilation. Before proceeding with stenting, the pressure wire (Certus, St Jude Medical, St Paul, MN, USA) was calibrated, equalized, and advanced towards the distal third of the infarcted related artery. After intracoronary injection of 250 µg of isosorbide dinitrate, the following baseline parameters were measured: mean aortic pressure (Pa), mean distal pressure (Pd), and mean transit time (mTt) calculated as the average three transit time measurements during three separate intracoronary injections of 3 mL of room temperature 0.9% saline solution. The same parameters were measured again after hyperaemia, induced by an intravenous infusion of adenosine at a rate of 140 µg/kg/min.
Fractional flow reserve (FFR), coronary flow reserve (CFR), and IMR were calculated as previously described (see Supplementary material online, Table S1).9 At this time, the initial procedural angioplasty wire was removed and the pressure wire used for stent deployment. In 69 patients over 85, coronary wedge pressure (Pw) was measured during stent balloon inflation and used to calculate the coronary wedge pressure corrected IMR (IMRcorrected) value according to the following formula: Pa × mTt × (Pd − Pw)/(Pa − Pw), with Pa, Pd, and mTt measured during hyperaemia.10
Postdilation was left to the operator's discretion. When the operator was satisfied with the procedural result, Pa, Pd, mTt, FFR, CFR, and IMR were measured again and variation in IMR after stenting (ΔIMR = IMRpost-stenting − IMRpre-stenting) was determined. Before completion of the procedure, the pressure wire was withdrawn back close to the guiding catheter to exclude artefact due to pressure drift.
In 28 cases, it was not possible to obtain pre-stenting reproducible and interpretable thermodilution curves. In all of these cases, TIMI flow was <3 and since unreliable values for CFR and IMR were obtained, these patients were excluded from the study cohort (Figure 1).
Angiographic and electrocardiographic analysis
Angiographic analysis was performed offline by two experienced operators blinded to coronary physiology indices—cases of disagreement were resolved by consensus.
Angiographic area at risk was assessed by the Bypass Angioplasty Revascularization Investigation (BARI) jeopardy score, as previously described.11
Pre-stenting residual stenosis was measured by two-dimensional quantitative coronary angiography (Medcon QCA software, Medcon Limited, Tel Aviv, Israel). Angiographic thrombus burden was graded from 0 to 5 by the thrombus score, as previously described12 (see Supplementary material online, Table S2).
The stent after its deployment was approximated to a cylinder and thus, stent volume was measured as π × (stent diameter/2)2 × stent length. In case of overlapping stents, the final stent volume was expressed as the sum of individual stent volumes. Stent volume tertiles were then calculated.
Pre-PPCI and final TIMI flow13 and post-procedural myocardial blush grade (MBG) were assessed.14 Angiographic distal embolization was defined as occurrence of distal filling defect with an abrupt ‘cut-off’ appearance in one or more peripheral coronary branches of the infarcted-related artery, distal to the PPCI site.15
A 12-lead electrocardiogram was recorded at admission and 60 min after PPCI in all patients and ST resolution (ΣSTR) calculated (see Supplementary material online). No reflow was defined as the combination of angiographic no reflow (final TIMI flow <3 or TIMI flow 3 with MBG <2) and/or incomplete post-procedural ΣSTR.16
Statistical analysis
All variables were expressed as mean and ±standard deviation (SD) or as median accompanied by interquartile range (IQR), as appropriate. In detail, parameters of coronary physiology (FFR, CFR, and IMR) before and after stenting are shown as median and IQR, as the Shapiro–Wilk test showed that the data were non-normally distributed. To allow the use of parametric techniques, base 10 logarithmic transformation (Log (x) or Log(x + k), with k being a constant in case of x presenting null or negative values) was applied.
Frequencies’ comparisons were made using χ2 test or Fisher's exact test, as appropriate. Post hoc analysis of the χ2 test was performed by assessment of adjusted standardized residuals. Comparisons between continuous variables were performed using T-test or analysis of variance with Scheffe's post hoc comparisons, as appropriate. Comparisons before and after stenting were performed by T-test for paired samples.
For multiple testing, the Benjamini and Hochberg method of Type 1 error control was applied and a P-value of <0.01 was considered significant.17 Correlations were assessed by Pearson's R coefficient.
Independent predictors of ΔIMR, pre-stenting, and post-stenting IMR ≤40 were measured using the linear regression model and the multivariable binary logistic regression model, respectively (for further details, see Supplementary material online).
Statistical analysis was performed using SPSS 22.0 (SPSS, Inc., Chicago, IL, USA) and P-values <0.05 were considered statistically significant.
Results
Clinical and procedural characteristics
Eighty-five consecutive patients with STEMI were recruited in two sequential periods from October 2010 to October 2014. Clinical and procedural characteristics are summarized in Tables 1 and 2 and stratified according to post-stenting IMR (Tables 1 and 2). Stratification according to pre-stenting IMR is reported in Supplementary material online, Tables S3 and S4. The threshold for IMR >40, previously validated for post-stenting IMR, was adopted to delineate the groups both before and after stenting.
Table 1.
Clinical characteristics
| Whole cohort (85 patients) | Post-stent IMR ≤40 (57 patients) | Post-stent IMR >40 (28 patients) | P-values | |
|---|---|---|---|---|
| Male gender | 71 (83.5) | 47 (82.4) | 24 (85.7) | 0.70 |
| Age | 60.2 ± 10.3 | 58.5 ± 10.5 | 63.6 ± 9.0 | 0.03 |
| Hypertension | 42 (49.4) | 29 (50.9) | 13 (46.4) | 0.70 |
| Hypercholesterolaemia | 37 (43.5) | 27 (47.4) | 10 (35.7) | 0.31 |
| Diabetes mellitus | 31 (36.5) | 24 (42.1) | 7 (25.0) | 0.12 |
| Active smoker | 48 (56.5) | 33 (57.9) | 15 (53.6) | 0.71 |
| Family history of IHD | 43 (50.6) | 30 (52.6) | 13 | 0.59 |
| Previous cardiological history | 44 (51.8) | 32 (56.1) | 12 (42.8) | 0.25 |
| Pain to wire time | ||||
| <3 h | 44 (51.8) | 35 (61.4) | 9 (32.1) | 0.01 |
| ≥3 and <6 h | 24 (28.2) | 15 (26.3) | 9 (32.1) | |
| ≥6 h | 17 (20.0) | 7 (12.3) | 10 (35.8) | |
| Culprit vessel | ||||
| LAD | 38 (44.7) | 25 (43.8) | 13 (46.4) | 0.59 |
| LCx | 6 (7.1) | 3 (5.3) | 3 (10.7) | |
| RCA | 41 (48.2) | 29 (50.9) | 12 (42.9) | |
| TIMI flow at presentation | ||||
| 0 | 64 (75.3) | 41 (71.9) | 23 (82.1) | 0.23 |
| 1 | 4 (4.7) | 2 (3.5) | 2 (7.1) | |
| 2 | 10 (11.8) | 7 (12.3) | 3 (10.8) | |
| 3 | 7 (8.2) | 7 (12.3) | 0 (0.0) | |
| Vessel closed at presentation | 64 (75.3) | 41 (71.9) | 23 (82.1) | 0.36 |
| Thrombus score | ||||
| 0–1–2 | 14 (16.5) | 10 (17.5) | 4 (14.2) | <0.001 |
| 3 | 20 (23.5) | 16 (28.1) | 4 (14.2) | |
| 4 | 43 (50.6) | 31 (54.4) | 12 (43.0) | |
| 5 | 8 (9.4) | 0 (0.0) | 8 (28.6) | |
| Rentrop score | ||||
| 0 | 65 (76.5) | 43 (75.4) | 22 (78.7) | 0.85 |
| 1 | 11 (12.9) | 7 (12.3) | 4 (14.2) | |
| 2 | 8 (9.4) | 6 (10.5) | 2 (7.1) | |
| 3 | 1 (1.2) | 1 (1.8) | 0 (0.0) | |
| Number vessel disease | ||||
| 1 | 52 (61.2) | 36 (63.1) | 16 (57.2) | 0.69 |
| 2 | 19 (22.3) | 13 (22.8) | 6 (21.4) | |
| 3 | 14 (16.5) | 8 (14.1) | 6 (21.4) | |
| Syntax score | 8.0 (4.0–13.0) | 8.0 (4.0–11.0) | 8.5 (5.0–13.7) | 0.58 |
| BARI Jeopardy score | 31.0 (23.7–35.0) | 31.0 (25.0–35.5) | 29.7 (19.4–33.2) | 0.11 |
| Troponin peak (ng/mL) | 86.1 (34.2–223.9) | 51.5 (30.1–176.0) | 139.5 (43.9–284.6) | 0.01 |
| Troponin AUC | 144.2 (52.9–307.6) | 87.5 (31.6–210.3) | 246.5 (75.6–419.9) | 0.008 |
| Creatinine (µmol/mL) | 77.6 ± 27.1 | 75.2 ± 18.5 | 82.70 ± 39.7 | 0.23 |
| Periprocedural medications | ||||
| Aspirin | 85 (100.0) | 57 (100.0) | 28 (100.0) | 1.00 |
| Clopidogrel | 81 (95.3) | 56 (98.2) | 25 (92.6) | 0.07 |
| Ticagrelor | 4 (4.7) | 1 (1.8) | 3 (7.4) | 0.07 |
| Heparin | 39 (45.9) | 29 (50.9) | 10 (35.7) | 0.19 |
| Bivalirudin | 51 (60.0) | 31 (54.4) | 20 (71.4) | 0.13 |
| GPIIbIIIa inhibitors | ||||
| Total adopted | 39 (45.9) | 26 (45.6) | 13 (46.4) | 0.94 |
| Bailout | 10 (11.8) | 4 (7.0) | 6 (21.4) | 0.05 |
| GPIIbIIIa inhibitors + bivalirudin | 10 (11.8) | 4 (7.0) | 6 (21.4) | 0.05 |
| GPIIbIIIa inhibitors + heparin | 29 (34.1) | 22 (38.6) | 7 (25.0) | 0.05 |
Continuous normally distributed variables are presented as mean ± standard deviation. Continuous not-normally distributed variables are presented as median (interquartile range). Frequencies are expressed as number (percentage). Frequencies are compared by application of χ2 test or Fisher's exact test. Continuous normally distributed variables (e.g. age and creatinine) are compared by application of unpaired T-test. Continuous not-normally distributed variables are compared by application of unpaired T-test after logarithmic transformation.
BARI, Bypass Angioplasty Revascularization Investigation; GPIIbIIIa, glycoprotein IIbIIIa; IHD, ischaemic heart disease; IMR, index of microcirculatory resistance; LAD, left anterior descending; LCx, left circumflex; RCA, right coronary artery; TIMI, thrombolysis in myocardial infarction.
P-value <0.01 considered statistically significant. The values of P < 0.05 are denoted as bold numbers.
Table 2.
Procedural characteristics
| Whole cohort (85 patients) | Post-stent IMR ≤40 (57 patients) | Post-stent IMR >40 (28 patients) | P-values | |
|---|---|---|---|---|
| Thrombus aspiration | 69 (81.2) | 46 (80.7) | 23 (82.1) | 0.87 |
| Predilation | 85 (100.0) | 57 (100.0) | 28 (100.0) | 1.00 |
| Maximum balloon diameter (mm) | 2.5 ± 0.3 | 2.5 ± 0.3 | 2.4 ± 0.4 | 0.39 |
| Pre-stent 2D-QCA | ||||
| MLD (mm) | 1.30 ± 0.46 | 1.29 ± 0.46 | 1.30 ± 0.46 | 0.97 |
| %DS | 52.9 ± 14.4 | 53.3 ± 13.8 | 52.1 ± 15.7 | 0.71 |
| Lesion length (mm) | 15.3 (11.0–21.2) | 15.2 (11.5–23.3) | 16.4 (10.3–20.6) | 0.58 |
| DES | 76 (89.4) | 48 (84.2) | 28 (100.0) | 0.02 |
| Second generation | 74 (87.0) | 47 (82.4) | 27 (96.4) | 0.70 |
| PES | 2 (2.3) | 1 (1.7) | 1 (3.6) | 0.85 |
| EES | 72 (84.7) | 46 (80.7) | 26 (92.8) | |
| ZES | 2 (2.3) | 1 (1.7) | 1 (3.6) | |
| Number of stents | ||||
| 1 | 67 (78.8) | 46 (80.7) | 21 (75.0) | 0.83 |
| 2 | 13 (15.3) | 8 (14.0) | 5 (17.8) | |
| 3 | 5 (5.9) | 3 (5.3) | 2 (7.2) | |
| Stent length (mm) | 28.0 (20.0–48.0) | 24.0 (20.0–32.0) | 28.0 (20.0–47.0) | 0.55 |
| Stent diameter (mm) | 3.5 (3.0–4.0) | 3.5 (3.0–4.0) | 3.3 (3.0–4.0) | 0.29 |
| Stent volume (mm3) | ||||
| First tertile | 26 (30.6) | 18 (31.6) | 9 (32.1) | 0.21 |
| Second tertile | 31 (36.5) | 24 (42.1) | 7 (25.0) | |
| Third tertile | 28 (32.9) | 15 (26.3) | 12 (42.9) | |
| Postdilation | 57 (67.0) | 37 (64.9) | 20 (71.4) | 0.55 |
| Number of postdilations | 2.0 (1.0–3.0) | 2.0 (1.7–3.0) | 2.0 (1.0–4.5) | 0.67 |
| Maximum postdilation pressure (atm) | 16.3 ± 2.9 | 15.9 ± 2.7 | 16.9 ± 3.2 | 0.28 |
| Maximum balloon diameter (mm) | 4.0 (3.5–4.0) | 4.0 (3.5–4.0) | 3.5 (3.1–4.0) | 0.35 |
| Final TIMI flow | ||||
| 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | <0.001 |
| 1 | 3 (3.6) | 0 (0.0) | 3 (10.8) | |
| 2 | 6 (7.0) | 0 (0.0) | 6 (21.4) | |
| 3 | 76 (89.4) | 57 (100.0) | 19 (67.8) | |
| MBG | ||||
| 0–1 | 14 (16.5) | 0 (0.0) | 14 (50.0) | <0.001 |
| 2 | 23 (27.0) | 14 (24.6) | 9 (32.1) | |
| 3 | 48 (56.5) | 43 (75.4) | 5 (17.9) | |
| Incomplete ∑STR (<70%) | 15 (17.6) | 0 (0.0) | 15 (53.6) | <0.001 |
| Angiographic distal embolization | 13 (15.3) | 0 (0.0) | 13 (46.4) | <0.001 |
| Angiographic no reflow (TIMI <3 or TIMI 3 with MBG <2) | 18 (21.2) | 0 (0.0) | 18 (64.3) | <0.001 |
| Angiographic and ECG no reflow | 22 (25.9) | 0 (0.0) | 22 (78.6) | <0.001 |
Continuous normally distributed variables are presented as mean ± standard deviation. Continuous not-normally distributed variables are presented as median (interquartile range). Frequencies are expressed as number (percentage). Frequencies are compared by application of χ2 test or Fisher's exact test. Continuous variables normally distributed (e.g. maximum balloon diameter, MLD, and %DS) are compared by application of unpaired T-test. Continuous variables not-normally distributed are compared by application of unpaired T-test after logarithmic transformation.
DES, drug-eluting stent; ECG, electrocardiographic; IMR, index of microcirculatory resistance; MBG, myocardial blush grade; MLD, minimal lumen diameter; TIMI, thrombolysis in myocardial infarction; 2D-QCA, two-dimensional quantitative coronary angiography; %DS, percentage of diameter stenosis; ∑STR, ST resolution; PES, paclitaxel eluting stent; EES, everolimus eluting stent; ZES, zotarolimis eluting stent.
P-value <0.01 considered statistically significant. The values of P < 0.05 are denoted as bold numbers.
Fifty-one patients (60%) presented a pre-stenting IMR of >40. In this group, pain to wire time was longer, infarct size measured by troponin AUC was larger, and more patients had TIMI flow 0 at presentation. The remaining clinical characteristics were homogeneously distributed (see Supplementary material online, Tables S3 and S4).
After coronary stenting, 28 patients (32.9%) had an IMR of >40. These patients were more likely to have larger infarct size, a longer pain to wire time, a higher thrombotic burden, and worse indices of myocardial reperfusion compared with those patients with a final IMR of ≤40 (Tables 1 and 2).
Indices of coronary physiology
Parameters of coronary physiology before and after stenting, including mean Pa, mean Pd, mTt at both baseline and after hyperaemia induction, FFR, CFR, and IMR, are summarized in Table 3 and stratified according to a post-stenting IMR value of 40 (Table 3). Stratification according to pre-stenting IMR below or above 40 is presented in Supplementary material online, Table S5.
Table 3.
Coronary physiology indices
| Whole cohort (85 patients) | Post-stent IMR ≤40 (57 patients) | Post-stent IMR >40 (28 patients) | P-values | |
|---|---|---|---|---|
| Pre-stenting | ||||
| Baseline | ||||
| Pa (mmHg) | 88.0 (74.0–97.0) | 85.0 (73.0–94.0) | 93.0 (86.0–105.0) | 0.01 |
| Pd (mmHg) | 67.0 (58.0–83.0) | 64.0 (56.0–81.0) | 77.0 (61.0–94.0) | 0.02 |
| mTt (s) | 1.12 (0.65–1.59) | 0.89 (0.59–1.47) | 1.43 (1.02–1.87) | 0.002 |
| Pd/Pa | 0.83 (0.71–0.93) | 0.81 (0.70–0.92) | 0.85 (0.74–0.94) | 0.37 |
| Hyperaemia | ||||
| Pa (mmHg) | 83.0 (70.0–96.0) | 80.0 (69.0–94.0) | 84.0 (81.0–103.0) | 0.07 |
| Pd (mmHg) | 58.0 (48.0–73.0) | 55.0 (43.0–70.0) | 65.0 (53.0–82.0) | 0.01 |
| mTt (s) | 0.86 (0.49–1.38) | 0.74 (0.41–1.29) | 1.30 (0.67–1.54) | 0.02 |
| FFR | 0.74 (0.61–0.88) | 0.74 (0.57–0.84) | 0.77 (0.65–0.90) | 0.18 |
| IMR | 49.7 (29.4–78.4) | 40.1 (24.5–61.6) | 68.5 (46.4–101.4) | <0.001 |
| CFR | 1.20 (0.96–1.62) | 1.20 (0.87–1.76) | 1.16 (0.99–1.43) | 0.80 |
| Post-stenting | ||||
| Baseline | ||||
| Pa (mmHg) | 90.0 (79.0–105.0) | 86.0 (76.0–102.0) | 100.0 (88.0–110.0) | 0.02 |
| Pd (mmHg) | 87.0 (78.0–99.0) | 84.0 (75.0–93.0) | 96.0 (85.0 0.107.0) | 0.004 |
| mTt (s) | 0.66 (0.33–0.95) | 0.53 (0.30–0.74) | 1.09 (0.71–1.77) | 0.03 |
| Pd/Pa | 0.97 (0.93–1.00) | 0.95 (0.93–0.99) | 0.99 (0.96–1.00) | 0.03 |
| Hyperaemia | ||||
| Pa (mmHg) | 80.0 (69.0–91.0) | 77.0 (68.0–86.0) | 87.0 (74.0–100.0) | 0.01 |
| Pd (mmHg) | 75.0 (64.0–86.0) | 73.0 (62.0–80.0) | 83.0 (68.0–93.0) | 0.004 |
| mTt (s) | 0.43 (0.24–0.67) | 0.28 (0.22–0.44) | 0.85 (0.64–1.80) | <0.001 |
| FFR | 0.94 (0.90–0.98) | 0.93 (0.89–0.98) | 0.95 (0.91–0.99) | 0.29 |
| IMR | 29.2 (18.9–54.3) | 21.1 (15.5–29.4) | 65.9 (54.1–128.6) | <0.001 |
| CFR | 1.35 (1.10–2.00) | 1.51 (1.18–2.21) | 1.18 (0.89–1.56) | 0.003 |
| ΔIMR(post-stenting − pre-stenting) | −18.7 (−37.6 − 3.97) | −20.8 (−39.4 − 4.1) | −1.7 (−26.5 − 70.7) | <0.001 |
| Whole cohort (69 patients) | Post-stent IMR ≤40 (49 patients) | Post-stent IMR >40 (20 patients) | P-values | |
| Pre-stenting | ||||
| Coronary Pw (mmHg) | 20.0 (15.5–26.0) | 20.0 (14.0–24.0) | 22.0 (20.0–29.0) | <0.003 |
| IMRcorrected | 41.7 (25.0–67.4) | 31.0 (20.8–57.7) | 59.0 (40.5–86.5) | <0.001 |
| Post-stenting | ||||
| IMRcorrected | 27.9 (18.3–50.9) | 21.9 (15.0–28.9) | 64.0 (51.2–113.4) | <0.001 |
| ΔIMRcorrected | −6.6 (−25.7 − 5.3) | −12.9 (−29.2 − 1.2) | 3.53 (−15.7 − 63.5) | <0.001 |
All variables are continuous not-normally distributed variables and presented as median (interquartile range). Comparisons have been performed by application of unpaired T-test after logarithmic transformation.
CFR, coronary flow reserve; Pw, wedge pressure; FFR, fractional flow reserve; IMR, index of microcirculatory resistance; IMRcorrected, coronary wedge pressure corrected IMR; mTt, mean transit time; Pa, aortic pressure; Pd, distal pressure.
P-value <0.01 considered statistically significant. The values of P < 0.05 are denoted as bold numbers.
Patients with a post-stenting IMR of >40 presented with a significantly higher pre-stenting IMR value [68.5 (46.4–101.4) vs. 40.1 (24.5–61.6), P < 0.001]. Final CFR was lower in patients with post-stenting IMR >40 [1.18 (0.89 −1.56) vs. 1.51 (1.18–2.21), P: 0.003] and accordingly a significant relationship was observed between CFR and IMR in both pre-stenting (R coefficient: −0.35 P: 0.001) and post-stenting (R coefficient: −0.32; P: 0.003).
Notably, a significant correlation was observed between measured pre-stenting IMR and pre-stenting IMRcorrected (R: 0.95, P < 0.001; see Supplementary material online, Figure S1).
Change of coronary physiology after stenting
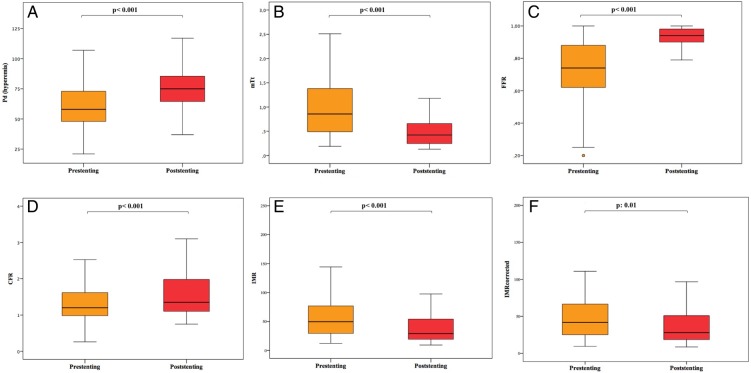
In the whole cohort of 85 patients, stenting was associated with an overall improvement of all the measured indices of coronary physiology (Figure 2). Mean Pd at hyperaemia was significantly improved after stenting from 58.0 (48.0–73.0) to 75.0 (64.0–86.0) mmHg, P < 0.001 (Figure 2A), and similarly hyperaemic mTt was reduced from 0.86 (0.49–1.38) to 0.43 (0.24–0.67) s (P < 0.001; Figure 2B). As expected, both FFR [from 0.74 (0.61–0.88) to 0.94 (0.90–0.98), P < 0.001] and CFR [from 1.20 (0.96–1.62) to 1.35 (1.10–2.00), P < 0.001] improved after stenting (Figure 2C and D). Interestingly, a significant IMR reduction [from 49.7 (29.4–78.4) to 29.2 (18.9–54.3), P < 0.001] was observed after stenting (Figure 2E), and this significant trend was confirmed also in IMRcorrected [from 41.7 (25.0–67.4) to 27.9 (18.3–50.9), P: 0.01] in the subgroup of 69 patients with coronary Pw measurement (Figure 2F).
Figure 2.
Evolution of coronary physiology indices after stenting in the whole patient cohort. Stenting in primary percutaneous coronary intervention is associated with an improvement in all indices of coronary physiology, both those strictly associated with the presence of the pre-procedural epicardial stenosis [mean distal pressure (Pd, A) and fractional flow reserve (FFR, C)], and those reflecting microvascular status [mean transit time (mTt, B), coronary flow reserve (CFR, D), and index of microcirculatory resistance measured and corrected (IMR and IMRcorrected, E and F)].
At multivariable logistic regression analysis, thrombotic burden [odds ratio (OR) 2.82; 95% CI 1.35–5.88, P: 0.006] and pre-stenting IMR >40 (OR 1.03; 95% CI 1.01–1.05, P: 0.007) were the best predictors of post-stenting IMR >40 (R2 for the model: 0.30, c-statistic 0.76; Table 4), whereas longer pain to wire time (OR 9.74; 95% CI 3.35–28.34, P < 0.001) and TIMI flow 0 at presentation (10.72; 95% CI 1.86–30.29, P: 0.008) were the only independent predictors of a pre-stenting IMR of >40 (R2 for the model: 0.48, c-statistic 0.83; see Supplementary material online, Table S6).
Table 4.
Predictors of post-stenting index of microcirculatory resistance >40
| Univariable analysis |
Multivariable analysis |
|||
|---|---|---|---|---|
| OR (95% CI) | P-values | OR (95% CI) | P-values | |
| Age | 1.05 (1.01–1.11) | 0.03 | 1.04 (0.99–1.11) | 0.13 |
| Gender male | 0.78 (0.22–2.76) | 0.70 | — | — |
| Diabetes | 0.46 (0.17–1.25) | 0.13 | — | — |
| Hypertension | 0.84 (0.34–2.07) | 0.70 | — | — |
| Pain to wire time | 1.01 (1.01–1.03) | 0.05 | 1.38 (0.64–2.96) | 0.41 |
| Culprit vessel (LAD vs. non-LAD) | 1.11 (0.45–2.75) | 0.82 | — | — |
| BARI jeopardy score | 0.96 (0.90–1.01) | 0.14 | — | — |
| TIMI flow 0 at presentation | 1.78 (0.52–6.04) | 0.36 | — | — |
| MLD | 1.02 (0.38–2.75) | 0.97 | — | — |
| DS% | 0.99 (0.96–1.03) | 0.71 | — | — |
| Lesion length | 0.98 (0.92–1.04) | 0.45 | — | — |
| Thrombus score | 2.04 (1.12–3.71) | 0.02 | 2.82 (1.35–5.88) | 0.006 |
| Thrombus aspiration | 1.10 (0.34–3.54) | 0.87 | — | — |
| Stent volume | 1.00 (0.99–1.01) | 0.58 | — | — |
| Postdilation | 1.35 (0.5–3.61) | 0.55 | — | — |
| Upstream GPIIbIIIa inhibitors | 0.54 (0.21–1.36) | 0.19 | — | — |
| Pre-stent IMR >40 | 1.03 (1.01–1.04) | 0.001 | 1.03 (1.01–1.05) | 0.007 |
BARI, Bypass Angioplasty Revascularization Investigation; DS%, percentage diameter stenosis; GPIIbIIIa, glycoprotein IIbIIIa; IMR, index of microcirculatory resistance; LAD, left anterior descending; MLD, minimal lumen diameter; OR, odds ratio; 95% CI, 95% confidence interval. The values of P < 0.05 are denoted as bold numbers.
Interestingly, linear regression analysis of independent predictors of change in IMR, expressed as ΔIMR, identified the extent of jeopardized myocardium [standardized beta coefficient −0.26 (IMR unit/BARI score unit), P: 0.009], the thrombotic burden [standardized beta coefficient 0.24 (IMR unit/thrombus score unit), P: 0.01], stent volume [standardized beta coefficient 0.26 (IMR unit/mm3 of stent), P: 0.01], and pre-stenting IMR >40 [standardized beta coefficient: −0.34 (IMR unit), P: 0.001; R2 for the model: 0.38; Table 5]. The choice of anticoagulation strategy and the performance of thrombus aspiration appeared to have no impact on either the initial or the final IMR.
Table 5.
Predictors of changes in the index of microcirculatory resistance
| Univariable analysis |
Multivariable analysis |
|||
|---|---|---|---|---|
| ΔIMR(post-stenting − pre-stenting) Beta (IMR unit) |
P-value | ΔIMR(post-stenting − pre-stenting) Beta (IMR unit) |
P-value | |
| Age (per year) | 0.08 | 0.49 | 0.12 | 0.21 |
| Gender male | 0.15 | 0.18 | 0.05 | 0.62 |
| Diabetes | −0.23 | 0.03 | −0.08 | 0.36 |
| Pain to wire time (per h) | −0.21 | 0.06 | — | — |
| Culprit vessel (LAD vs. non-LAD) |
−0.21 | 0.06 | — | — |
| BARI jeopardy score (BARI score unit) | −0.22 | 0.04 | −0.26 | 0.009 |
| TIMI flow 0 at presentation | 0.03 | 0.76 | — | — |
| MLD (per mm) | 0.08 | 0.45 | — | — |
| DS% (per % unit of diameter stenosis) | −0.01 | 0.97 | — | — |
| Lesion length (per mm) | 0.03 | 0.81 | — | — |
| Thrombus score (per unit of thrombus score) | 0.27 | 0.01 | 0.24 | 0.01 |
| Thrombus aspiration | −0.10 | 0.54 | — | — |
| Stent volume (per mm3 of stent) | 0.33 | 0.002 | 0.26 | 0.01 |
| Postdilation | 0.11 | 0.32 | — | — |
| Upstream GPIIbIIIa inhibitors | −0.11 | 0.32 | — | — |
| Pre-stenting IMR >40 | −0.30 | 0.005 | −0.34 | 0.001 |
Correlation between dependent and independent variables expressed by standardized beta coefficient.
BARI, Bypass Angioplasty Revascularization Investigation; DS%, percentage of diameter stenosis; GPIIbIIIa, glycoprotein IIbIIIa; IMR, index of microcirculatory resistance; LAD, left anterior descending; MLD, minimal lumen diameter; TIMI, thrombolysis in myocardial infarction. The values of P < 0.05 are denoted as bold numbers.
Evolution of coronary physiology according to pre-stenting index of microcirculatory resistance and patterns of index of microcirculatory resistance changes after stenting
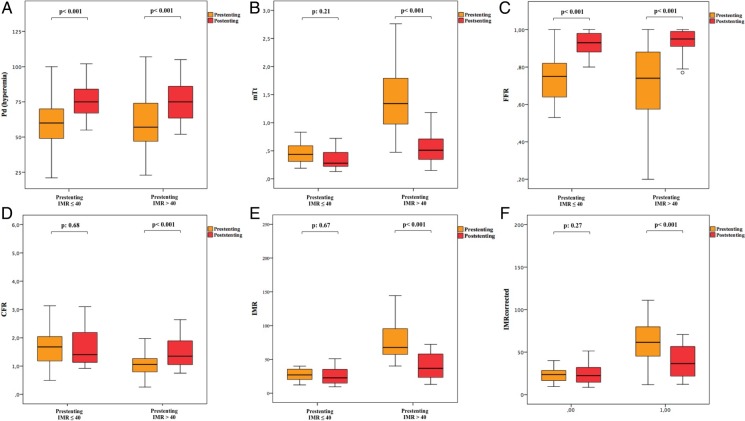
The change in coronary physiology indices between patients with pre-stenting IMR ≤40 and >40 was compared. A significant improvement in parameters directly affected by epicardial stenosis, such as hyperaemic Pd and FFR, was observed after stenting in both groups (Figure 3A and C). Conversely, a significant improvement in parameters directly reflecting microvascular function, such as hyperaemic mTt [from 1.34 (0.97–1.81) to 0.73 (0.50–1.12) s, P < 0.001], CFR [from 1.06 (0.79–1.28) to 1.35 (1.02–1.90), P < 0.001], and IMR [from 67.7 (56.2–95.8) to 36.7 (22.7–59.5), P < 0.001], was observed only in the group of patients with a pre-stenting IMR of >40, whereas no significant changes after stenting was observed in the group of patients with pre-stenting IMR ≤40 [mTt from 0.43 (0.31–0.60) to 0.28 (0.21–0.50), P: 0.21; CFR from 1.68 (1.18–2.04) to 1.40 (1.12–2.21), P: 0.68; IMR from 27.1 (19.8–35.8) to 22.7 (14.7–35.4), P: 0.67] (Figure 3B, D, and E).
Figure 3.
Evolution of indices of coronary physiology after stenting according to the pre-procedural microvasculature status, expressed by pre-stenting index of microcirculatory resistance. Evolution of indices of coronary physiology is influenced by the status of the coronary microvasculature before stenting. While indices mainly affected by the presence of epicardial stenosis [mean distal pressure (Pd, A) and fractional flow reserve (FFR, C)] improve irrespectively of pre-stenting index of microcirculatory resistance, a significant improvement in mean transit time (mTt, B), coronary flow reserve (CFR, D), and index of microcirculatory resistance measured and corrected (IMR and IMRcorrected, E and F) is observed after stenting only in patients with pre-stenting IMR >40.
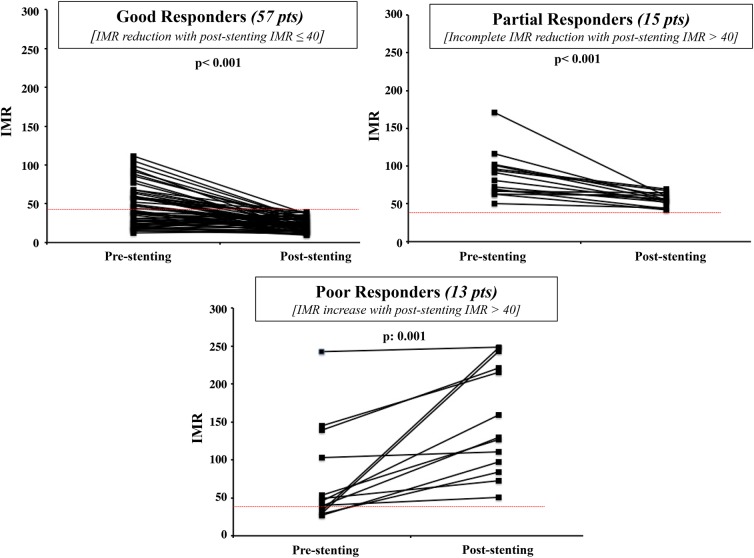
Three different patterns of IMR evolution after stenting were arbitrarily categorized (Figure 4): Good responders (n = 57 patients, 67% of the whole cohort) defined as patients with a final post-stenting IMR of ≤40 [IMR changed from 40.1 (24.5–61.6) to 22.1 (15.5–29.9), P < 0.001 Figure 4A]; partial responders (n = 15 patients, 17.6% of the whole cohort) were those with a pre-stenting IMR of >40 and a post-stenting IMR still of >40 [IMR changed from 80.6 (66.4–100.9) to 55.5 (51.4–61.2), P < 0.001; Figure 4B]; poor responders (n = 13 patients, 15.4% of the whole cohort) were patients with an increase in IMR and a final IMR of >40 after implantation of the stent [IMR changed from 45.7 (31.1–120.9) to 129.3 (90.9–231.8), P: 0.001; Figure 4C].
Figure 4.
Patterns of index of microcirculatory resistance (IMR) response to stenting in primary percutaneous coronary intervention patients. The graph shows the three possible patterns of index of microcirculatory resistance responses after stenting. Good responders presenting a final favourable IMR of ≤40, partial responders presenting a significant reduction in index of microcirculatory resistance, but incomplete since the final value will be still >40, and poor responders with a significant increase in index of microcirculatory resistance after stenting, ending with a final value of >40.
Interestingly, review of the clinical/procedural characteristics of these patient groups demonstrated that poor responders presented with a significantly higher thrombotic burden and underwent a higher volume of stent implantation with a high incidence of angiographic distal embolization, compared with both good and partial responders (Table 6). Conversely, partial responders had significantly longer pain to wire time and thus higher pre-stenting IMR, compared with good responders (Table 6). Ultimately, 82.3% (28/34) of patients presenting with a pre-stenting IMR of ≤40 and 56.9% (29/51) of those with a pre-stenting IMR of >40 resulted to be good responders to stenting.
Table 6.
Differences among good responders (post-stenting IMR ≤40), incomplete responders (post-stenting IMR >40, though reduction), and poor responders (post-stenting IMR >40 with an increase)
| Good responders (57 patients) | Partial responders (15 patients) | Poor responders (13 patients) | Overall P-values | |
|---|---|---|---|---|
| Pre-stenting IMR | 40.1 (24.5–61.6)a,b | 80.6 (66.4–100.9)b | 45.7 (31.1–120.9)a | 0.001 |
| Pre-stenting IMR ≤40 | 28 (49.1)c | 0 (0.0)c | 6 (46.1) | 0.002 |
| ΔIMR(post-stenting − pre-senting) | −20.8 (−39.4 − 4.1)b | −26.4 (−42.1 − 10.9)a | 70.8 (17.1 − 101.7)a,b | <0.001 |
| Thrombus aspiration | 46 (80.7) | 12 (80.0) | 11 (84.6) | 0.94 |
| GPIIbIIIa inhibitors | 26 (45.6) | 7 (46.7) | 6 (46.1) | 0.40 |
| Pain to wire time | ||||
| <3 h | 35 (61.4)c | 3 (20.0) | 6 (46.1) | 0.005 |
| ≥3 and <6 h | 15 (26.3) | 4 (26.7) | 5 (38.5) | |
| ≥6 h | 7 (12.3) | 8 (53.3)c | 2 (15.4) | |
| Thrombus score | ||||
| 0–1–2 | 6 (17.6) | 3 (20.0) | 1 (7.7) | <0.001 |
| 3 | 8 (23.5) | 2 (13.3) | 2 (15.4) | |
| 4 | 17 (50.0) | 9 (60.0) | 3 (23.1) | |
| 5 | 3 (8.9) | 1 (6.7) | 7 (53.8)c | |
| Stent volume | ||||
| First tertile | 17 (29.8) | 6 (40.0) | 3 (23.1) | 0.03 |
| Second tertile | 24 (42.1) | 6 (40.0) | 1 (7.7) | |
| Third tertile | 16 (28.1) | 3 (20.0) | 9 (69.2)c | |
| Angiographic distal embolization | (0.0)c | 0 (0.0) | 13.0 (100.0)c | <0.001 |
GPIIbIIIa, glycoprotein IIbIIIa; ΔIMR, variation in IMR; IMR, index of microcirculatory resistance.
a,bSignificant comparison at post-analysis test for ANOVA.
cCells with statistical significance after analysis of adjusted standardized residuals for χ2 test. The values of P < 0.05 are denoted as bold numbers.
Discussion
This study demonstrates that the implantation of a coronary stent in patients presenting with STEMI usually leads to an improvement in microvascular function and a reduction in the measured IMR. However, one-third of patients in this study population had evidence of impaired microvascular function at completion of the revascularization procedure with an IMR of >40 after stent implantation. In some patients, this suboptimal outcome reflected an incomplete normalization of an elevated IMR, and these patients were more commonly late clinical presenters with longer ischaemic times. However, in another subgroup of patients with a final IMR of >40, an increase in IMR was observed after stent implantation, which was associated with a larger thrombotic burden and/or higher implanted stent volume.
Complete and timely restoration of myocardial perfusion is the main aim in the treatment of STEMI patients and coronary stenting is regarded as optimal therapy for most patients. This study shows that when coronary microvascular function is measured invasively in STEMI, implantation of a coronary stent lowers the IMR of <40 in approximately half of the patients presenting for treatment. This improvement in the parameters of coronary physiology after stenting included an increase in mean Pd and FFR, parameters known to be directly affected by the presence of an epicardial coronary stenosis, and an improvement in parameters reflecting microcirculatory status and myocardial reperfusion. However, despite optimal stent implantation, many STEMI patients still have significant microvascular obstruction and/or dysfunction. This series demonstrates that measurement of IMR, immediately after coronary flow has been resumed, is possible in most STEMI patients, but we were unable to measure a numerical IMR in some patients who were potentially eligible for the study. Ultimately, these patients had to be excluded from our subsequent analysis and consequently, the observed study cohort probably underestimates the absolute extent of persistent microvascular dysfunction as this could approach 50% in an ‘all-comers’ STEMI population.
Our results contrast with previous studies investigating IMR responses after stenting in elective stable patients. Aarnoudse et al. and Yong et al. demonstrated that IMR was independent of epicardial stenosis when coronary Pw was taken into account, and that IMRcorrected was unchanged after stenting.10,18 Even if the accuracy of coronary Pw and collateral flow index has been debated in STEMI patients,19 our larger study showed a strict relationship between measured and IMRcorrected and a reduction in IMR after stenting in this group of patients. This change in IMR was independent of residual epicardial stenosis before stenting, being independent of FFR, percentage diameter stenosis, minimal lumen diameter, and lesion length (see Supplementary material online, Tables S4 and S5). Obvious differences in the study populations are likely to explain these apparently conflicting observations. In elective patients, the IMR is lower, and the microcirculation is likely to be healthier and will not change after stent deployment unless relevant distal embolization occurs. In contrast, STEMI patients have a compromised microcirculation on presentation, with limited flow in the infarct zone and consequent impairment of both functional and structural integrity of the coronary microvasculature.2 This is reflected by higher values of measured IMR and IMRcorrected in STEMI than in stable patients. Moreover, multivariable analysis demonstrates that the only relevant predictors of pre-stenting IMR >40 were pain to wire time and TIMI flow at presentation reflecting the relationship between IMR and the evolving microvascular dysfunction in STEMI.20
Our results provide only minimal insights into the actual mechanisms accounting for IMR reduction after stenting in PPCI. However, we hypothesize that in these clinical circumstances, the increase in coronary flow after relief of the occlusion would probably lead to an increased perfusion pressure and epicardial conductance (reflected by Pd and FFR). Since microvascular tone depends on the upstream driving pressure, a mechanical downstream relaxation of coronary and microvascular tone, consequent to the release of vasoactive factors, with consequent reduction in microvascular resistance can be hypothesized.21 This pressure dependence of microvascular resistance has indeed been already described in the experimental setting, with an increase in vessel diameter and a decrease in microvascular resistance consequent to an increase in coronary pressure after relief of an epicardial stenosis.22
A reduction in IMR after stenting, however, is not universal. Multivariable analysis showed that thrombotic burden with an OR of 2.82 and high pre-stenting IMR with an OR of 1.03 were the two main predictors of an unfavourable IMR of >40 at the end of the procedure. These results were confirmed and supplemented by the linear regression analysis detecting the extent of jeopardized myocardium, thrombotic burden, volume of stent implanted, and the pre-stenting IMR as predictors of the degree of IMR change at completion of the procedure. Consequently, it is possible to surmise that the STEMI patients most likely to benefit from stenting (and therefore most likely to have a significant reduction in IMR after stenting) were those with higher pre-stenting IMR, with a larger area of myocardium at risk, a lower thrombotic burden, and requiring implantation of a smaller stent to cover the disease.
Our data reflect previous literature describing a significant relationship between stent volume, e.g. plaque burden, and thrombotic burden with distal embolization.23 Similarly, a larger area at risk implies a larger area of potentially salvageable coronary microvasculature with consequent potential higher reduction of microvascular resistance after revascularization compared with treating a smaller region of myocardium at risk.24
We have observed two principal mechanisms that result in a raised final IMR. The first is probably related principally to the late clinical presentation of the patient. Our data suggest that patients with an initial IMR of >40 and a pain to wire time of >6 h were at risk of a high final IMR. This is consistent with data demonstrating the relationship between time to reperfusion and the occurrence of myocardial haemorrhage seen as a marker of severe of microvascular injury.25 Currently, there are no proven particular treatment options for these late-presenting patients, but it is possible to speculate that this could include novel therapies directed specifically at the microvasculature and/or myocardial repair.
The second mechanism, which resulted in a raised final IMR, relates principally to distal embolization of the athero-thrombotic material. Importantly, in this group of patients, IMR was ≤40 after predilation and/or thrombectomy alone. A high final IMR after stenting was consequent to a high thrombotic burden requiring a large stent volume (upper tertile of stent volume, e.g. stent volume >336 mm3; see Supplementary material online, Figure S2). These data support the notion that stenting is a procedural step significantly associated with distal embolization.26 These observations suggest that this group of patients with extensive thrombus could benefit from adjunctive strategies, e.g. further thrombus aspiration and/or GPIIbIIIa inhibitors or perhaps a deferred stenting strategy.27
In conclusion, our data provide insights into the heterogeneity of the response of microvasculature to stenting, allowing speculation that using IMR prior to stent implantation, in combination with other clinical and angiographic variables, could potentially facilitate the triage of STEMI patients for consideration of novel therapies.
Limitations
In our study, we collected detailed coronary physiology data in a large cohort of patients undergoing PPCI; in this emergency setting, it is inevitable that some data will be unobtainable. This limitation will affect any flow-dependant technique including use of the Doppler wire. Theoretically, it could lead to selection bias, but it is not unreasonable to assume that patients with pre-stenting TIMI flow <3 would also have had a high pre-stenting IMR.
Only macroscopic angiographic distal embolization was considered in the present study. Our data do not take into account the inevitable role of microembolization that might contribute to explain the incomplete reduction in IMR observed in partial responders to stenting.
Prospective validation of the prognostic value of IMR >40 is awaited and currently, this arbitrary threshold has previously only been described after completion of the interventional procedure.8
Angiography is inferior to intravascular ultrasound or optical coherence tomography in the assessment of thrombotic burden and stent volume, particularly in case of overlapping stents when a detailed measurement of the stent area would allow an accurate definition of final stent volume.
Finally, in consideration of multiple comparisons testing, there is potential for a type 1 error. However, the consistency of the relationships and highly significant P-values between indices of coronary physiology and multiple important clinical variables suggest the relevance of our findings.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre. Funding to pay the Open Access publication charges for this article was provided by a donation from Oxford Radcliffe Hospitals Charitable fund no 0468.
Conflict of interest: A.P.B. has received an unrestricted research funding from Boston Scientific.
Acknowledgements
The authors thank all the OxAMI research nureses and all the members of the Coronary Care Unit and the Catheterization Laboratory team at the John Radcliffe Hospital Oxford for their invaluable help, support, and patience.
References
- 1.The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). 2014 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2014;35:2541–2619. [DOI] [PubMed] [Google Scholar]
- 2.Niccoli G, Burzotta F, Galiuto L, Crea F. Myocardial no-reflow in humans. J Am Coll Cardiol 2009;54:281–292. [DOI] [PubMed] [Google Scholar]
- 3.Stone GW, Maehara A, Witzenbichler B, Godlewski J, Parise H, Dambrink JH, Ochala A, Carlton TW, Cristea E, Wolff SD, Brener SJ, Chowdhary S, El-Omar M, Neunteufl T, Metzger DC, Karwoski T, Dizon JM, Mehran R, Gibson CM. Intracoronary abciximab and aspiration thrombectomy in patients with large anterior myocardial infarction: the INFUSE-AMI randomized trial. JAMA 2012;307:1817–1826. [DOI] [PubMed] [Google Scholar]
- 4.Jolly SS, Cairns JA, Yusuf S, Meeks B, Pogue J, Rokoss MJ, Kedev S, Thabane L, Stankovic G, Moreno R, Gershlick A, Chowdhary S, Lavi S, Niemelä K, Steg PG, Bernat I, Xu Y, Cantor WJ, Overgaard CB, Naber CK, Cheema AN, Welsh RC, Bertrand OF, Avezum A, Bhindi R, Pancholy S, Rao SV, Natarajan MK, ten Berg JM, Shestakovska O, Gao P, Widimsky P, Džavík V. Randomized trial of primary PCI with or without routine manual thrombectomy. N Engl J Med 2015;372:1389–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fearon WF, Balsam LB, Farouque HM, Caffarelli AD, Robbins RC, Fitzgerald PJ, Yock PG, Yeung AC. Novel index for invasively assessing the coronary microcirculation. Circulation 2003;107:3129–3132. [DOI] [PubMed] [Google Scholar]
- 6.Fearon WF, Shah M, Ng M, Brinton T, Wilson A, Tremmel JA, Schnittger I, Lee DP, Vagelos RH, Fitzgerald PJ, Yock PG, Yeung AC. Predictive value of the index of microcirculatory resistance in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol 2008;51:560–565. [DOI] [PubMed] [Google Scholar]
- 7.Ng MK, Yong AS, Ho M, Shah MG, Chawantanpipat C, O'Connell R, Keech A, Kritharides L, Fearon WF. The index of microcirculatory resistance predicts myocardial infarction related to percutaneous coronary intervention. Circ Cardiovasc Interv 2012;5:515–522. [DOI] [PubMed] [Google Scholar]
- 8.Fearon WF, Low AF, Yong AS, McGeoch R, Berry C, Shah MG, Ho MY, Kim HS, Loh JP, Oldroyd KG. Prognostic value of the index of microcirculatory resistance measured after primary percutaneous coronary intervention. Circulation 2013;127:2436–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuculi F, De Maria GL, Meier P, Dall'Armellina E, De Caterina AR, Channon KM, Prendergast BD, Choudhury RC, Forfar JC, Kharbanda RK, Banning AB. Impact of microvascular obstruction on the assessment of coronary flow reserve, index of microcirculatory resistance, and fractional flow reserve after ST-elevation myocardial infarction. J Am Coll Cardiol 2014;64:1894–1904. [DOI] [PubMed] [Google Scholar]
- 10.Aarnoudse W, Fearon WF, Manoharan G, Geven M, van de Vosse F, Rutten M, De Bruyne B, Pijls NH. Epicardial stenosis severity does not affect minimal microcirculatory resistance. Circulation 2004;110:2137–2142. [DOI] [PubMed] [Google Scholar]
- 11.Graham MM, Faris PD, Ghali WA, Galbraith PD, Norris CM, Badry JT, Mitchell LB, Curtis MJ, Knudtson ML. Validation of three myocardial jeopardy scores in a population-based cardiac catheterization cohort. Am Heart J 2001;142:254–261. [DOI] [PubMed] [Google Scholar]
- 12.Sianos G, Papafaklis MI, Serruys PW. Angiographic thrombus burden classification in patients with ST-segment elevation myocardial infarction treated with percutaneous coronary intervention. J Invasive Cardiol 2010;22:6B–14B. [PubMed] [Google Scholar]
- 13.TIMI Study Group. The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase I findings. N Engl J Med 1985;312:932–936. [DOI] [PubMed] [Google Scholar]
- 14.van't Hof AW, Liem A, Suryapranata H, Hoorntje JC, de Boer MJ, Zijlstra F. Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: myocardial blush grade. Zwolle Myocardial Infarction Study Group. Circulation 1998;97:2302–2306. [DOI] [PubMed] [Google Scholar]
- 15.Napodano M, Peluso D, Marra MP, Frigo AC, Tarantini G, Buja P, Gasparetto V, Fraccaro C, Isabella G, Razzolini R, Iliceto S. Time-dependent detrimental effects of distal embolization on myocardium and microvasculature during primary percutaneous coronary intervention. JACC Cardiovasc Interv 2012;5:1170–1177. [DOI] [PubMed] [Google Scholar]
- 16.Porto I, De Maria GL, Leone AM, Dato I, D'Amario D, Burzotta F, Niccoli G, Trani C, Biasucci LM, Bolognese L, Crea F. Endothelial progenitor cells, microvascular obstruction, and left ventricular remodeling in patients with ST elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol 2013;112:782–791. [DOI] [PubMed] [Google Scholar]
- 17.Keselman HJ, Cribbie R, Holland B. Controlling the rate of Type I error over a large set of statistical tests. Br J Math Stat Psychol 2002;55(Pt1):27–39. [DOI] [PubMed] [Google Scholar]
- 18.Yong AS, Ho M, Shah MG, Ng MK, Fearon WF. Coronary microcirculatory resistance is independent of epicardial stenosis. Circ Cardiovasc Interv 2012;5:103–108, S1–2. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto K, Ito H, Iwakura K, Shintani Y, Masuyama T, Hori M, Kawano S, Higashino Y, Fujii K. Pressure-derived collateral flow index as a parameter of microvascular dysfunction in acute myocardial infarction. J Am Coll Cardiol 2001;38:1383–1389. [DOI] [PubMed] [Google Scholar]
- 20.Tarantini G, Cacciavillani L, Corbetti F, Ramondo A, Marra MP, Bacchiega E, Napodano M, Bilato C, Razzolini R, Iliceto S. Duration of ischemia is a major determinant of transmurality and severe microvascular obstruction after primary angioplasty: a study performed with contrast-enhanced magnetic resonance. J Am Coll Cardiol 2005;46:1229–1235. [DOI] [PubMed] [Google Scholar]
- 21.Verhoeff BJ, Siebes M, Meuwissen M, Atasever B, Voskuil M, de Winter RJ, Koch KT, Tijssen JG, Spaan JA, Piek JJ. Influence of percutaneous coronary intervention on coronary microvascular resistance index. Circulation 2005;111:76–82. [DOI] [PubMed] [Google Scholar]
- 22.Spaan JA, Cornelissen AJ, Chan C, Dankelman J, Yin FC. Dynamics of flow, resistance, and intramural vascular volume in canine coronary circulation. Am J Physiol Heart Circ Physiol 2000;278:383–403. [DOI] [PubMed] [Google Scholar]
- 23.Napodano M, Dariol G, Al Mamary AH, Marra MP, Tarantini G, D'Amico G, Frigo AC, Buja P, Razzolini R, Iliceto S. Thrombus burden and myocardial damage during primary percutaneous coronary intervention. Am J Cardiol 2014;113:1449–1456. [DOI] [PubMed] [Google Scholar]
- 24.Leone AM, De Caterina AR, Basile E, Gardi A, Laezza D, Mazzari MA, Mongiardo R, Kharbanda R, Cuculi F, Porto I, Niccoli G, Burzotta F, Trani C, Banning AP, Rebuzzi AG, Crea F. Influence of the amount of myocardium subtended by a stenosis on fractional flow reserve. Circ Cardiovasc Interv 2013;6:29–36. [DOI] [PubMed] [Google Scholar]
- 25.Wong CK, Bucciarelli-Ducci C. Q waves and failed ST resolution: will intra-myocardial haemorrhage be a concern in reperfusing ‘late presenting’ STEMIs? Int J Cardiol 2015;182:203–210. [DOI] [PubMed] [Google Scholar]
- 26.Okamura A, Ito H, Iwakura K, Kurotobi T, Koyama Y, Date M, Higuchi Y, Inoue K, Fujii K. Clinical implications of distal embolization during coronary interventional procedures in patients with acute myocardial infarction: quantitative study with Doppler guidewire. JACC Cardiovasc Interv 2008;1:268–276. [DOI] [PubMed] [Google Scholar]
- 27.Carrick D, Oldroyd KG, McEntegart M, Haig C, Petrie MC, Eteiba H, Hood S, Owens C, Watkins S, Layland J, Lindsay M, Peat E, Rae A, Behan M, Sood A, Hillis WS, Mordi I, Mahrous A, Ahmed N, Wilson R, Lasalle L, Genereux P, Ford I, Berry C. A randomized trial of deferred stenting versus immediate stenting to prevent no- or slow-reflow in acute ST-segment elevation myocardial infarction (DEFER-STEMI). J Am Coll Cardiol 2014;63:2088–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]