Etanercept is an inhibitor of the pro-inflammatory and immunoregulatory cytokine tumor necrosis factor-α (TNFα). It has been proved highly effective in the treatment of rheumatoid arthritis, psoriasis and ankylosing spondylitis, although several potentially severe side effects have been reported, including infections and allergic reactions. Recently, attention has been drawn to an increased risk of demyelinating diseases in patients treated with TNFα inhibitors [1]. Here, we present a case of a patient who developed an acute demyelinating spinal cord lesion while being treated with etanercept.
We report on a 55-year-old woman with psoriatic arthritis without history of neurological symptoms. She was on treatment with etanercept (50 mg weekly) for 6 months and did not take any other disease-modifying anti-rheumatic drugs when she presented with successively developing dysesthesia in the gluteal and genital region and in the lower extremities. Examination showed gait ataxia, reduced joint position and vibratory sense at distal lower extremities and urinary dysfunction. There were no clinical signs of infection and routine blood tests and CSF analysis were unremarkable. MRI revealed a singular 8 mm hyperintense lesion in the dorsal thoracic spine and no further lesions in the brain or spinal cord were detected (Fig. 1). Etanercept was discontinued and treatment with IV methylprednisolone (1000 mg/day for 5 days) initiated, resulting in rapid alleviation of symptoms. Skeletal scintigraphy showed no signs of active arthritis and the patient therefore was not re-started on a disease-modifying anti-rheumatic drug. At follow-up 8 months later, remission persisted and the patient had no neurological deficits. There were no clinical signs of psoriatic arthritis, although psoriatic skin changes recurred.
Figure 1:
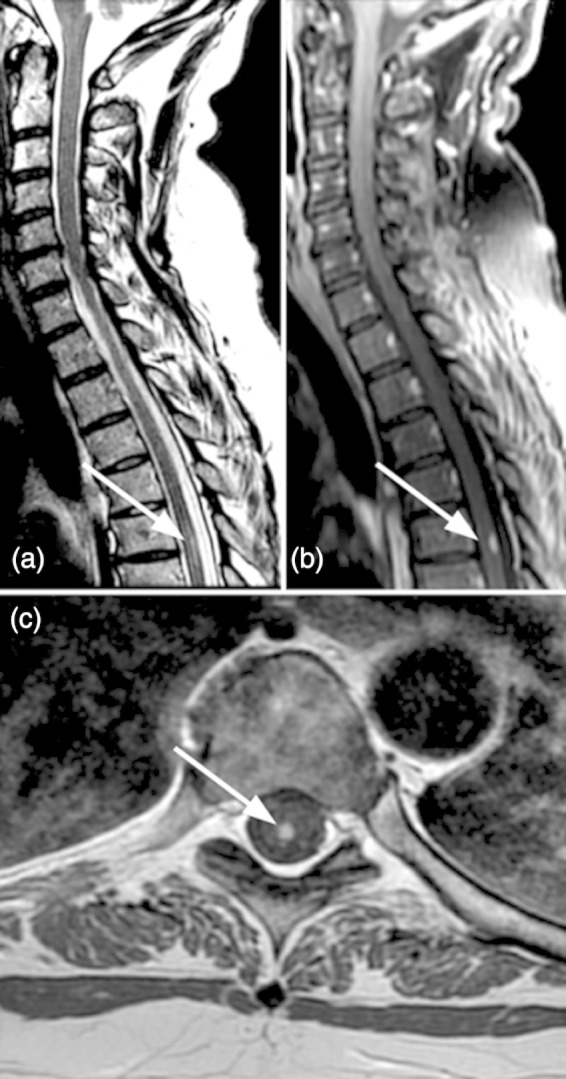
(a) Sagittal T2-weighted MRI shows a single posterior hyperintense demyelinating lesion of the cord at T5-6. (b) Sagittal CE T1 shows contrast enhancement of the lesion indicative of blood-spinal cord barrier disruption. (c): On axial CE T1 the lesion is located dorsomedially and limited to the posterior columns.
Our case highlights myelitis as a rare side-effect of etanercept that should prompt discontinuation of the drug and consideration of immunotherapy. It is well known that TNFα inhibitors can increase the number of exacerbations and gadolinium-enhancing lesions in patients with multiple sclerosis (MS) and they are accordingly contraindicated in patients with a history of a demyelinating disorder [2, 3]. More recently, peripheral and central demyelinating diseases have been reported in patients naïve of demyelinating events that were treated with TNFα inhibitors [1, 4]. Observed disorders ranged from Guillain-Barré syndrome and chronic inflammatory demyelinating polyneuropathy (CIDP) to retrobulbar neuritis, demyelinating (MS-like) encephalitis and transverse myelitis. Symptoms partially or fully resolved in the majority of patients after discontinuation of TNFα inhibitors and glucocorticoid treatment [1, 4]. Arguments in favour of a true association between TNFα inhibition and occurrence of demyelination in our case include the temporal association between etanercept treatment and manifestation of symptoms and improvement of symptoms upon etanercept discontinuation. A causal link is moreover supported by the report of similar disorders after exposure to TNFα inhibitors and positive re-challenge phenomena described in several cases [4, 5]. Without rechallenge, our patient remained without neurological deficits after follow-up of 8 months. Previous studies furthermore suggest that inhibition of TNFα induces complex alterations of immune homeostasis that are not restricted to suppression of pro-inflammatory actions (effective in the treatment of rheumatic diseases) but may also include promotion of autoimmune mechanisms [4]. The latter is in line with observations of stimulation of autoantibody production induced by treatment with TNFα inhibitors [6]. In summary, these observations strongly suggest a causal role of TNFα inhibition in the pathogenesis of myelitis in our patient, although there is no definite proof without a positive re-challenge phenomenon.
Taken together, our report demonstrates a rare but important side effect of etanercept treatment. Clinicians thus need to consider demyelinating diseases as differential diagnosis in patients with TNFα inhibitor treatment that present with new neurological deficits. In these patients, a discontinuation of etanercept treatment and IV glucocorticoid treatment is warranted.
CONFLICT OF INTEREST STATEMENT
The authors report no conflict of interest.
REFERENCES
- 1.Seror R, Richez C, Sordet C, Rist S, Gossec L, Direz G, et al. Pattern of demyelination occurring during anti-TNF-α therapy: a French national survey. Rheumatology (Oxford). 2013;52:868–874. doi: 10.1093/rheumatology/kes375. [DOI] [PubMed] [Google Scholar]
- 2.The Lenercept Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group. TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study. Neurology. 1999;53:457–465. [PubMed] [Google Scholar]
- 3.Van Oosten BW, Barkhof F, Truyen L, Boringa JB, Bertelsmann FW, von Blomberg BME, et al. Increased MRI activity and immune activation in two multiple sclerosis patients treated with the monoclonal anti-tumor necrosis factor antibody cA2. Neurology. 1996;47:1531–1534. doi: 10.1212/wnl.47.6.1531. [DOI] [PubMed] [Google Scholar]
- 4.Solomon AJ, Spain RI, Kruer MC, Bourdette D. Inflammatory neurological disease in patients treated with tumor necrosis factor alpha inhibitors. Mult Scler. 2011;17:1472–1487. doi: 10.1177/1352458511412996. [DOI] [PubMed] [Google Scholar]
- 5.Tristano AG. Neurological adverse events associated with anti-tumor necrosis factor α treatment. J Neurol. 2010;257:1421–1431. doi: 10.1007/s00415-010-5591-7. [DOI] [PubMed] [Google Scholar]
- 6.Alessandri C, Scrivo R, Spinelli FR, Ceccarelli F, Magrini L, Priori R, et al. Autoantibody production in anti-TNF-alpha-treated patients. Ann NY Acad Sci. 2007;1110:319–329. doi: 10.1196/annals.1423.034. [DOI] [PubMed] [Google Scholar]


