Abstract
Nervous system relapse of patients with advanced HER2–neu-positive breast cancer is an increasing problem, with one-third of women developing brain metastases. Standard therapies using steroids, surgery and radiotherapy do not provide a lasting response. We evaluated CPT-11 and bevacizumab, which can both cross the blood–brain barrier, as combination therapy to treat HER2–neu-positive breast cancer with brain metastases.
INTRODUCTION
Patients with breast cancer that overexpress HER2/neu have a poor prognosis, shorter relapse time and a low survival rate. Before the introduction of HER2-targeted therapy, HER2-positive breast cancer was treated with protocols similar to those employed for HER2-negative tumors. The development of the anti-HER2 monoclonal antibody, trastuzumab, led to clinical trials of trastuzumab in combination with systemic chemotherapy for the treatment of HER2-positive metastatic breast cancer. Nervous system relapse of patients with advanced HER2–neu-positive breast cancer is an increasing problem, with one-third of women developing brain metastases. However, once patients with HER2/neu breast cancer have brain metastases, they tend to fail all the standard chemotherapy regimens. This is a case study, extrapolating a treatment used in glioblastomas, namely CPT-11 and avastin, in this group of HER2/neu metastatic breast cancer patients.
CASE REPORT
Women who had had failed other treatments for HER2–neu-positive metastatic breast cancer were selected. There were four women (Patients 1–4, Table 1), with a median age of 42.5 years (range, 33–53). Prior treatment included surgery (n = 2), whole-brain radiation (n = 4), radiosurgery (n = 3) and lapatanib (n = 4). CPT-11 (350 mg/m2) and bevacizumab (10 mg/kg) were administered every 3 weeks for a 6-week cycle (three patients) or every 2 weeks of bevacizumab (10 mg/kg) and CPT-11 (125 mg/m2) for a 4-week cycle (one patient). We determined an expected chance of six-month survival [1] response, median time-to-progression (TTP) and median overall survival (OS: Kaplan–Meier method; Table 2).
Table 1:
Characteristics of the four patients
| Patient and type of breast cancer | Radiation dose | Chemotherapy | Number of brain metastases | Surgery |
|---|---|---|---|---|
| Patient 1 HER-2-/neu-positive stage IIA infiltrating breast cancer ER/PR negative |
After her right frontal metastasis was resected, she had whole-brain radiation. Subsequently, her other three brain metastases were resected, and she had stereotactic radiosurgery: right parietal (1500 cGy), right frontal (1500 cGy) and right temporal (1200 cGy). 4 months later, she had SRS again to all four cavities due to new growth at 1600 cGy. |
She was status post lumpectomy followed by four cycles of adjuvant Adriamycin and Cytoxan and then nine cycles of weekly Taxol. After her first recurrence, she was started on weekly Herceptin. She developed brain metastases and due to prior extensive radiation treatment, she was initially on Temodar + Lapatinib, but due to progression, and she was switched to Avastin and CPT11. |
Four | The right frontal metastasis was resected first and then the three others were subsequently resected. |
| Patient 2 HER-2/neu-positive, Invasive ductal carcinoma, Grade 3, ER/PR negative, associated with DCIS extensive nuclear grade III |
Whole-brain radiation (3750 cGy) + Lapatinib when she developed brain metastases. | Four cycles of Adriamycin + Cytoxan followed by mastectomy, then on weekly Taxol + Herceptin. After WBRT, she had Lapatinib + Herceptin. When this failed, she was on Avastin + CPT11. |
Numerous lesions | No surgery |
| Patient 3 HER-2-/neu-positive high-grade infiltrating carcinoma with lobular and ductal features, it was ER/PR negative, with high-grade DCIS with positive lymphovascular invasion |
Cyberknife radiosurgery to the resection cavity for a total dose of 2500 cGy. A brain MRI done 5 months later showed enhancement inferior tract margin of the surgery. The patient then had CyberKnife RS single-fraction boost to 1600 cGy. |
Neoadjuvant Taxotere plus Adriamycin for six cycles, and then she had a right mastectomy. She had recurrence and was then started on Herceptin and Xeloda, but then developed a brain metastasis. She was placed on Xeloda plus lapatanib for better penetration of the brain after her CyberKnife treatment. When she progressed, CPT11 + Avastin was administered with Herceptin. |
Large right cerebellar mass | Resected |
| Patient 4 HER-2-/neu- positive Grade 2 invasive breast cancer with lympho vascular invasion |
She had radiosurgery to left temporal lesions with 1800 cGy given to the lateral lesion and 1600 cGy to the medial lesion. The chiasm was treated, to 2000 cGy in five fractions. Two months later, she had a right medial temporal lesion as well as a new left cerebellar metastasis. There was also a scalp lesion that was noted in the right parietal area. She then received 2400 cGy. The left cerebellar lesion was treated with 2000 cGy. |
After lumpectomy, she received radiation and cyclophosphamide + methotrexate + 5-fluorouracil chemotherapy. She had recurrence 6 years later with metastases to the posterior fossa, leptomeninges, lung, mediastinum, bone and liver, and was status post multiple trials of chemotherapy and hormonal therapies, including Aromasin, Faslodex and Herceptin prior to being on CPT11 + Avastin. | Numerous including leptomeningeal disease | None |
ER, estrogen receptor; PR, partial response; SRS, stereotactic radio-surgery; DCIS, ductal carcinoma in situ; WBRT, whole-brain radiation.
Table 2:
Patient outcomes on the CPT11–Avastin combination
| Patient | Duration of Treatment | Time to progression | Overall survival | Karnofsky performance score at start of CPT-11–Avastin | Extra-cranial metastases | Rades score [9] | Predicted chance of 6-month survival (%) |
|---|---|---|---|---|---|---|---|
| 1 | 21.5 months | 21 months | 26 months | 60 | No | 7 | 10 |
| 2 | 10 months | 11.5 months | 14.75 months | 60 | Yes | 4 | 10 |
| 3 | 6 months | 6.75 months | 7.5 months | 50 | Yes | 4 | 10 |
| 4 | 4 months | Clinical deterioration at 5.75 months, without radiographic or CSF evidence of progression | 6.5 months | 40 | Yes | 4 | 10 |
All of the patients had both clinical and MRI radiographic response to treatment (Figs 1–4). Median TTP was 8.5 months and median OS was 11 months. The predicted chance of six-month survival for each of the four patients was only 10% [1]. Our observed median TTP and median OS are an objective measure of a successful response to this salvage treatment strategy. All of the patients died within six months of stopping their treatment (range 1–6 months, median = 2 months). Hematological grade 3/4 toxicities were only that of lymphopenia (2/4). Treatment-related non-hematologic grade 3/4 toxicities were: fatigue (2/4), alopecia (1/4) and diarrhea (1/4).
Figure 2:
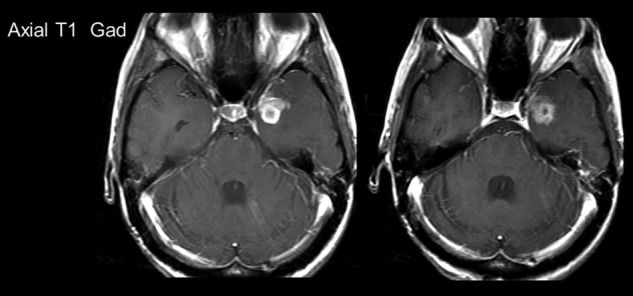
Patient 2: MRI scans post contrast shown pre- and post-treatment. Pre- and post-treatment scans were taken ∼3 months apart.
Figure 3:
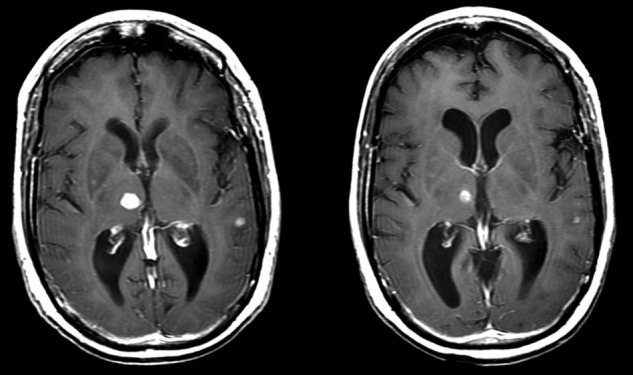
Patient 3: MRI scans post contrast shown pre- and post-treatment, note scans were taken ∼3 months apart.
Figure 1:
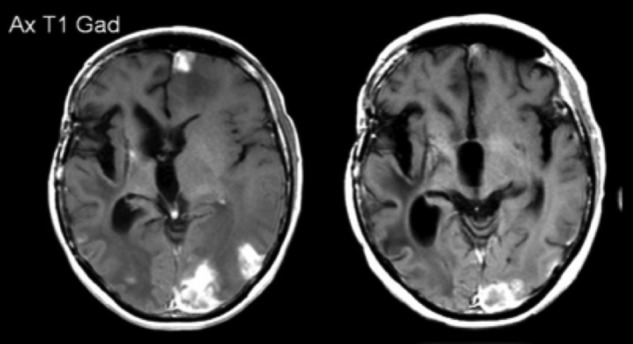
Patient 1: MRI scans post contrast shown pre and post-treatment. Pre- and post-treatment scans were taken ∼3 months apart.
Figure 4:
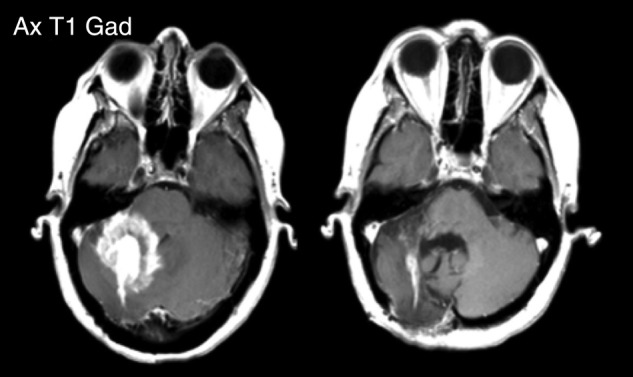
Patient 4: MRI scans post contrast shown pre- and post-treatment. Pre- and post-treatment scans were taken ∼5 months apart.
DISCUSSION
In terms of cancers, lung cancer and then breast cancer are the main causes of death in women. According to the American Cancer Society, ∼1.3 million women are diagnosed with breast cancer per year globally, and ∼465 000 will die from this cancer. Breast cancer has become a chronic disease, and metastasis to the brain is very difficult to treat, especially in the Her2/neu patient population.
HER2/neu oncogene (c-erbB-2) is a 185 kDa is a tyrosine kinase, which belongs to the family of epidermal growth factor receptor proteins [2, 3]. A worse histological grade is associated with tumor overexpression of HER2/neu, and this is found in ∼30% of patients with breast cancer. This in turn leads to poor survival and more resistance to the usual chemotherapeutic regimens used in the treatment of metastatic breast cancer [4, 5]. Experimental models indicate that neutralizing antibodies against epidermal growth factor receptor or HER2/neu cause decreased angiogenesis secondary to vascular endothelial growth factor gene suppression [6].
On 22 February 2008, the US Food and Drug Administration approved bevacizumab (Avastin; Genentech) plus paclitaxel as first-line therapy in patients with metastatic breast cancer [7]. A multicenter phase III randomized trial of adjuvant therapy for patients with HER2-positive node-positive or high-risk node-negative breast cancer comparing chemotherapy plus trastuzumab with chemotherapy plus trastuzumab plus bevacizumab, otherwise known as the BETH trial, is currently underway, but no longer recruiting participants. The combination of avastin and CPT-11 has been established in the treatment of glioblastoma [8, 9]. Since the combination of avastin and CPT-11 has been used in treating glioblastoma, this treatment was tried in four women as outlined above with metastatic breast cancer to the brain, who had failed various treatment options.
This case series illustrates that the combination of CPT-11 and bevacizumab yielded a high radiographic response for the treatment of refractory brain metastases from HER2–neu-positive breast cancer. The regimen was well tolerated with improvement of time to progression and survival. A prospective study is warranted.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1.Rades D, Dziggel L, Segedin B, Oblak I, Nagy V, Marita A, et al. A simple survival score for patients with brain metastases from breast cancer. Strahlenther Onkol 2013;189:664–7. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto T, Ikawa S, Akiyama T, Semba K, Nomura N, Miyajima N, et al. Similarity of protein encoded by the human c-erbB2 gene to epidermal growth factor receptor. Nature 1986;319:230–34. [DOI] [PubMed] [Google Scholar]
- 3.Bargmann CI, Hung MC, Wienberg RA. The neu oncogene encodes an epidermal growth factor receptor-related protein. Nature 1986;319:226–30. [DOI] [PubMed] [Google Scholar]
- 4.Revillion F, Bonneterre J, Peyrat JP. ERBB2 oncogene in human breast cancer and its clinical significance. Eur J Cancer 1998;34:791–808. [DOI] [PubMed] [Google Scholar]
- 5.Baselga J, Seidman AD, Rosen PP, Norton L. HER2 overexpression and paclitaxel sensitivity in breast cancer: therapeutic implications. Oncology 1997;11:43–48. [PubMed] [Google Scholar]
- 6.Petit AM, Rak J, Hung MC, Rockwell P, Goldstein N, Fendly B, et al. Neutralizing antibodies against epidermal growth factor and erbB-2/neu receptor tyrosine kinases down-regulate vascular endothelial growth factor production by tumor cells in vitro and in vivo. Angiogenic implications for signal transduction therapy of solid tumors. Am J Pathol 1997;151:1523–30. [PMC free article] [PubMed] [Google Scholar]
- 7.Alvarez RH, Valero V, Hortobagyi GN. Emerging targeted therapies for breast cancer. Clin Oncol 2010;28:3366–79. [DOI] [PubMed] [Google Scholar]
- 8.Chen W, Delaloye S, Silverman DH, Geist C, Czernin J, Sayre J, et al. Predicting treatment response of malignant gliomas to bevacizumab and irinotecan by imaging proliferation with [18F] fluorothymidine positron emission tomography: a pilot study. J Clin Oncol 2007;25:4714–21. [DOI] [PubMed] [Google Scholar]
- 9.Vredenburgh JJ, Desjardins AHJE, Marcello J, Reardon DA, Quinn JA, Rich JN, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 2007;25:4722–29. [DOI] [PubMed] [Google Scholar]


