Abstract
Enzymes play key roles in fungal pathogenesis. Manipulation of enzyme expression or activity can significantly alter the infection process, and enzyme expression profiles can be a hallmark of disease. Hence, enzymes are worthy targets for better understanding pathogenesis and identifying new options for combatting fungal infections. Advances in genomics, proteomics, transcriptomics, and mass spectrometry have enabled the identification and characterization of new fungal enzymes. This review focuses on recent developments in the virulence-associated enzymes from Cryptococcus neoformans. The enzymatic suite of C. neoformans has evolved for environmental survival, but several of these enzymes play a dual role in colonizing the mammalian host. We also discuss new therapeutic and diagnostic strategies that could be based on the underlying enzymology.
INTRODUCTION
The facultative intracellular fungal pathogen Cryptococcus neoformans is the causative agent of cryptococcosis, a disease that primarily affects individuals with impaired immunity, such as those with advanced HIV infection (1, 2). C. neoformans is a ubiquitous environmental fungus associated with both pigeon guano and eucalyptus trees, and its environmental niche ranges from the tropical to the temperate (3). C. neoformans infection is acquired from the environment via inhalation, after which it forms a local infection in the lungs. This infection may be cleared, may be contained as a granuloma, or may disseminate from this initial site, leading to pneumonia and/or meningoencephalitis, the latter being uniformly fatal if untreated. Despite the availability of antifungal therapy, more than 650,000 people die each year from C. neoformans infection (1, 2, 4). The principal virulence factors of C. neoformans are a polysaccharide capsule, melanin production (5, 6), the ability to grow at body temperature (7), and the secretion of extracellular enzymes (7). These virulence factors confer a selective advantage to C. neoformans for both residing in the environment and in a mammalian host. Tightly controlled regulation leads to expression of enzymes required for fungal survival and host damage once inside its mammalian host (8).
Many enzymes contribute to the composite cryptococcal virulence phenotype. Dissection of the pathogenic role of these enzymes will enhance our understanding of cryptococcal pathogenic mechanisms and facilitate directed inhibitor development and/or vaccine discovery. We have included a table summarizing basic information regarding global C. neoformans enzymology (Table 1) and a schematic displaying localization of most of the highlighted enzymes discussed (Fig. 1). In this review, we discuss in detail the most important virulence-associated enzymes (Table 2), as well as additional target enzymes with potential for rational antifungal drug design (Table 3). We examine this information in the context of infection and analyze candidate target enzymes for drug inhibition and vaccine discovery.
TABLE 1.
Described enzymes in Cryptococcus neoformans
| Enzyme | Function(s)a | EC no. | Reference(s) |
|---|---|---|---|
| Localized on capsule and/or cell wall | |||
| 1,3-β-Glucan synthase | Involved in β-glucan synthesis | 2.4.1.34 | 135 |
| Acid phosphatase | Involved in fungal cell adhesion to host tissues, localized in lysosomes, and related to virulence (Table 2) | 3.1.3.2 | 106, 136, 137 |
| Cas1 glycosyltransferase | Participates in O-acetylation | 2.4.1.X | 138 |
| Chitin deacetylase | Involved in chitin metabolism | 3.5.1.41 | 139 |
| Chitin synthase | Involved in chitin synthesis | 2.4.1.16 | 140 |
| Chitinase | Involved in chitin degradation | 3.2.1.14 | 141 |
| Creatinine deaminase | Involved in arginine and proline metabolism | 3.5.4.21 | 142 |
| Esterase lipase | Catalyzes hydrolysis of fatty acids | 3.1.1.3 | 136 |
| GDP-mannose pyrophosphorylase | Involved in GDP-mannose synthesis | 2.7.7.13 | 143 |
| Glucan 1,3-β-glucosidase | Involved in glucan synthesis | 3.2.1.58 | 16 |
| Glucan 1,4-α-glucosidase | Involved in glucan synthesis | 3.2.1.3 | 16 |
| Gmt1 GDP-mannose | Transport of GDP-mannose | 2.7.7.22 | 144 |
| Lactonohydrolase | Deficient strains show larger capsule size and facilitated immune evasion | 3.1.1.15 | 37 |
| N-Acetylgalactosaminoglycan deacetylase | Involved in polysaccharide metabolism | 3.1.1.58 | 145 |
| Phosphoaminase | Involved in amino acid synthesis | 136 | |
| Phosphomannomutase | Involved in GDP-mannose synthesis | 5.4.2.8 | 143 |
| Phosphomannose isomerase | Involved in GDP-mannose synthesis | 5.3.1.8 | 143 |
| Uph1 ATPase | Required for vesicle acidification | 146 | |
| Uxs1 decarboxylase | Converts UDP-glucuronic acid to UDP-xylose | 147 | |
| α-1,3-Glucanase | Involved in glucan synthesis | 3.2.1.59 | 16 |
| α-Amylase | Hydrolyzes alpha bonds of several polysaccharides and involved in cell wall building | 3.2.1.1 | 148 |
| α-Glucosidase | Breaks down disaccharides to glucose and starch and involved in cell wall building | 3.2.1.20 | 136 |
| α-Mannosidase | Involved in cell building through mannose metabolism | 3.2.1.24 | 136 |
| α-Mannosyltransferase | Involved in polysaccharide metabolism | 2.4.1.132 | 38, 149 |
| β-Endoglucanase | Involved in cell wall formation | 3.2.1.4 | 148 |
| β-Glucosidase | Involved in cell wall formation | 3.2.1.21 | 136 |
| β-Glucuronidase | Involved in cell wall formation, catalyzing breakdown of complex carbohydrates | 3.2.1.31 | 136 |
| Secreted/released | |||
| Acyltransferase | Involved in food acquisition | 3.1.1.3 | 92 |
| Alkaline phosphatase | Involved in regulation of signaling cascades and several protein structure and localized in endoplasmic reticulum | 3.1.3.1 | 150 |
| Aspartyl protease | Involved in food acquisition | 3.4.23.X | 111 |
| Cellulase | Involved in polysaccharide degradation | 3.2.1.4 | 151 |
| DNase | DNA degradation and related to virulence (Table 2) | 3.1.21.1 | 79 |
| Metalloprotease | Catalyzes mechanism that involves a metal and related to virulence (Table 2) | 3.4.24.77 | 113, 152 |
| Phospholipase B | Similar to phospholipase C function, degrades cell membrane components, supports fungal attachment to host cells, localized on cell wall, and related to virulence (Table 2) | 3.1.1.5 | 91, 92 |
| Phospholipase C | Degrades cell membrane components, supports fungal attachment to host cells, and related to virulence (Table 2) | 3.1.4.11 | 93 |
| Protease | Performs proteolysis interfering with host defense response | 3.4.21.53 | 107, 108 |
| S2P endopeptidase | Performs proteolysis | 3.4.24.85 | 153 |
| Serine peptidase | Performs proteolysis, coordinating several physiological functions | 3.4.21.X | 152 |
| Superoxide dismutase | Catalyzes dismutation of toxic superoxide, converting superoxide to hydrogen peroxide and oxygen and related to virulence (Table 2) | 1.15.1.1 | 83–85 |
| Localized intracellularly | |||
| 2-Methylcitrate synthase | Converts acyl groups into alkyl groups on transfer | 2.3.3.5 | 154 |
| 3-β-Hydroxysteroid 3-dehydrogenase | Oxidizes a substrate by reduction reaction that transfers 1 or more hydrides to electron acceptor | 1.1.1.270 | 155 |
| 6-Phosphogluconate dehydrogenase | Involved in production of ribulose | 1.1.1.44 | 156, 157 |
| Acetate kinase | Catalyzes formation of acetyl-CoA | 2.7.2.1 | 158 |
| Aconitase | Catalyzes isomerization of citrate to isocitrate and involved in response to nitrosative stress | 4.2.1.3 | 159 |
| Adenylyl cyclase Cac1 | Converts ATP to cAMP | 4.6.1.1 | 160 |
| Alternative oxidase | Part of electron transport chain in mitochondria | 1.10.3.11 | 161 |
| Aminopeptidase | Catalyzes cleavage of amino acids from amino terminus of protein | 3.4.11.21 | 137 |
| C-9-methyltransferase | Involved in glycosphingolipid pathway | 2.1.1.129 | 127 |
| Can2 carbonic anhydrase | Responds directly to intracellular carbon oxide | 4.2.1.1 | 162, 163 |
| Casein kinase 1 | Dephosphorylation of Hog1 under stress conditions | 2.7.11.1 | 164 |
| Catalase | Protects cells from oxidative damage by reactive oxygen species | 1.11.1.6 | 137, 150 |
| Cytochrome c peroxidase | Takes reduced equivalents from cytochrome c and reduces hydrogen peroxide to water | 1.11.1.5 | 165 |
| Deacetylase | Removes acetyl groups from lysine in proteins and is localized in cell wall | 3.5.1.108 | 166 |
| Dolichyl-diphosphooligosaccharide-protein glycotransferase | Participates in N-glycan biosynthesis | 2.4.99.18 | 167 |
| Ferrochelatase | Catalyzes final step in heme biosynthesis from highly photoreactive porphyrins | 4.99.1.1 | 168 |
| Flippase | Participates in phospholipid translocation between membrane sides and localized in cell wall | 3.6.3.1 | 169, 170 |
| Glucose-6-phosphate dehydrogenase | Is in pentose phosphate pathway, maintaining the level of coenzyme NADPH | 1.1.1.49 | 171 |
| Glucose-phosphate isomerase | Catalyzes conversion of glucose-6-phosphate into fructose 6-phosphate | 5.3.1.9 | 172 |
| Glucosylceramide synthase | Involved in glucosylceramide synthesis, localized in cell wall, and related to virulence (Table 2) | 2.4.1.80 | 127, 128 |
| Glucuronyltransferase | Involved in biosynthetic pathway of O-acetylated mannan | 2.4.1.17 | 28 |
| Glutathione peroxidase | Protects cells from oxidative damage | 1.11.1.9 | 173 |
| Glyoxal oxidase | Copper metalloenzyme that catalyzes oxidation of aldehydes to corresponding carboxylic acids coupled to reduction of dioxygen to H2O2 | 1.2.1.23 | 148 |
| Homoisocitrate dehydrogenase | Participates in lysine biosynthesis | 1.1.1.87 | 115 |
| Homoserine kinase | Participates in glycine, serine, and threonine metabolism | 2.7.1.39 | 174 |
| Homoserine O-acetyltransferase | Participates in methionine and sulfur metabolism | 2.3.1.31 | 175 |
| Hyaluronic synthase | Involved in production of glycosaminoglycan at cell surface | 2.4.1.212 | 176 |
| Imidazole glycerol-phosphate dehydratase | Participates in histidine biosynthesis | 4.2.1.19 | 177 |
| IMP dehydrogenase | Participates in GTP biosynthesis | 1.1.1.205 | 178 |
| Inositol phosphotransferase 1 | Involved in glycosphingolipid pathway | 2.7.1.X | 127 |
| Inositol-phosphorylceramide synthase | Involved in glycosphingolipid pathway | 2.7.1.X | 179 |
| Ire1 kinase | Involved in cellular response to unfolded proteins | 2.7.11.1 | 180 |
| Isocitrate lyase | Catalyzes cleavage of isocitrate to succinate and glyoxylate | 4.1.3.1 | 181 |
| Laccase | Polyphenol oxidase and copper-containing oxidase enzyme, localized in cell wall, and related to virulence (Table 2) | 1.10.3.2 | 45, 46, 50 |
| Malate dehydrogenase | Catalyzes oxidation of malate to oxaloacetate | 1.1.1.37 | 182 |
| Mannitol-1-phosphate 5-dehydrogenase | Participates in fructose and mannose metabolism | 1.1.1.17 | 183, 184 |
| Mannose-1-phosphate guanylyltransferase (GDP) | Participates in fructose and mannose metabolism | 2.7.7.22 | 144 |
| Mannosyl phosphorylinositol ceramide synthase | Involved in glycosphingolipid pathway | 2.4.X.X | 127 |
| Mannosyltransferase | Participates in O-mannosylation of proteins and involved in cell wall integrity and morphogenesis | 2.4.1.109 | 185 |
| Myristoyl-CoA: protein N-myristoyltransferase | Catalyzes transfer of myristate from CoA to proteins | 2.3.1.97 | 116 |
| Pde1 phosphodiesterase | Modulates cAMP | 3.1.4.1 | 186 |
| Phosphoglucomutase | Participates in interconversion of glucose 1-phosphate and glucose 6-phosphate | 5.4.2.2 | 172 |
| Protein farnesyltransferase | Participates in formation of farnesyl protein and diphosphate | 2.5.1.58 | 187 |
| Rho1 GTPase | Involved in MAPK cascade | 3.6.5.2 | 188 |
| RNase III | Binds and cleaves double-stranded RNA | 3.1.26.3 | 189 |
| Saccharopine dehydrogenase | Participates in lysine metabolism | 1.5.1.10 | 190 |
| Sphingolipid methyltransferase 1 | Participates in methylation of glucosylceramide | 2.1.1.1 | 191 |
| Sterol 14α-demethylase | Involved in sterol metabolism | 1.14.13.7 | 192 |
| Sterol 24-C-methyltransferase | Involved in sterol metabolism | 1.15.1.1 | 193 |
| Thiol peroxidase | Reduces peroxides and inhibits hydrogen peroxide response | 1.11.1.7 | 194 |
| Thioredoxin reductase | Catalyzes reduction of thioredoxin | 1.8.1.9 | 195 |
| Threonine synthase | Participates in glycine, serine, and threonine metabolism | 4.2.3.1 | 174 |
| Thymidylate synthase | Catalyzes conversion of dUMP to deoxythymidine monophosphate | 2.1.1.45 | 196 |
| Transaldolase | Involved in pentose phosphate pathway | 2.2.1.2 | 159 |
| Trehalose-6-phosphate phosphatase | Participates in starch and sucrose metabolism | 3.1.3.12 | 197 |
| Trehalose-6-phosphate synthase | Participates in starch and sucrose metabolism | 2.4.1.15 | 197 |
| UDP-galactopyranose mutase | Catalyzes conversion of UDP-d-galactopyranose in UDP-d-galacto-1,4-furanose | 5.4.99.9 | 198 |
| UDP-glucose dehydrogenase | Participates in conversion of UDP-glucose to UDP-glucuronate, and formation of glycosaminoglycans | 1.1.1.22 | 199 |
| UDP-glucuronate decarboxylase | Participates in nucleotide sugar metabolism | 4.1.1.35 | 147 |
| Urease | Catalyzes hydrolysis of urea into carbono dioxide and ammonia and related to virulence (Table 2) | 3.5.1.5 | 74 |
| Xylosylphosphotransferase | Participates in O-glycosylation biosynthesis and related to virulence (Table 2) | 2.7.8.32 | 28, 31, 200 |
| Δ8 desaturase | Involved in glycosphingolipid pathway | 1.14.19.4 | 127 |
cAMP, cyclic AMP; MAPK, mitogen-activated protein kinase.
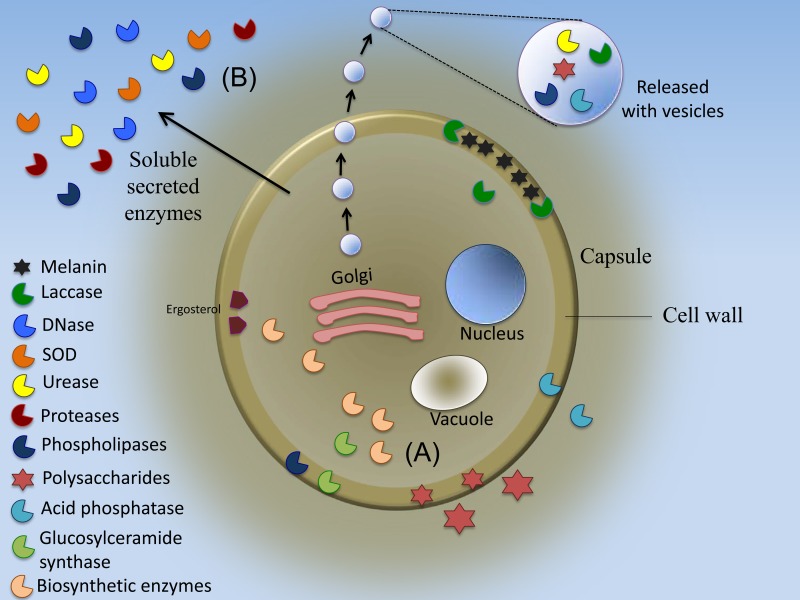
FIG 1.
Enzymes are crucial for fungal pathogenesis and can alter the infection process. These enzymes are potential targets for new antifungal agents. (A) Some pathogenesis-related enzymes are retained to be active inside the cell body, while others are secreted. Some, like laccase, are both retained and secreted. (B) Of those released, some are secreted using traditional secretion systems, while others are included as cargo in extracellular vesicles.
TABLE 2.
Enzymes related to the virulence in Cryptococcus neoformans
| Enzyme | Comment(s) | Reference(s) |
|---|---|---|
| Acid phosphatase | Deficient strains show affected virulence in mouse and Galleria mellonella models of infection | 106 |
| DNase | Acts in degrading host DNA and supplies C. neoformans with nucleotides | 79 |
| Glucosylceramide synthase | Required for virulence in murine model of infection | 127, 128 |
| Laccase | Deficient strains show decreased virulence in survival studies with rabbit and mouse models of infection | 59 |
| Mannosyltransferase | Required for virulence in murine model of infection | 185 |
| Metalloprotease | Deficient strains unable to cross endothelium in in vitro model of human blood-brain barrier and is required for invasion of central nervous system | 113 |
| Phospholipase B | Required in invasion of host tissue and dissemination in murine model | 95 |
| Phospholipase C | Shown to be important for several virulence phenotypes | 101, 102 |
| Superoxide dismutase | Attenuated growth of deficient strains within macrophages | 89 |
| Urease | Deficient strains less virulent than wild-type strain in mouse model of infection and is involved in fungal escape from lung to cross blood-brain barrier | 76 |
| Xylosylphosphotransferase | Deficient strains manifest reduced growth in lung tissue in mouse model of infection | 30 |
TABLE 3.
Possible target enzymes for rational antifungal drug design
| Enzyme(s) | Comment(s) | Reference(s) |
|---|---|---|
| 14α-Demethylase | A critical enzyme in sterol assembly | 119 |
| Glucosylceramide synthase | Glucosylceramide plays critical role in pathogenicity of C. neoformans | 127, 128 |
| Laccase | Melanization aids virulence | 60, 63, 64, 65 |
| Myristoyltransferase | Myristoylation inhibition is fatal for C. neoformans | 116, 117 |
| Phosphoribosylaminoimidazole carboxylase | Mutants that cannot synthesize adenine have reduced virulence | 114 |
| Pyrophosphorylase and cytosine-specific permease | Enzymes are basis of C. neoformans flucytosine resistance | 201, 202 |
| Sterol synthesis enzymes | Sterol synthesis enzyme mutants show resistance to fluconazole and amphotericin | 122–124 |
POLYSACCHARIDE CAPSULE
C. neoformans is the only fungal pathogen with a polysaccharide capsule, an outermost polysaccharide structure located just outside the cell wall. The two major polysaccharide capsule constituents are glucuronoxylomannan (GXM) and glucuroxylomannogalactan (GXMGal) (9–11). GXM is the major component of C. neoformans, a compound of α-1,3-linked mannose residues with xylosyl and glucuronyl side groups (12), whereas GXMGal is made of α-1,6-linked galactose residues with xylose, mannose, and glucuronic acid (13). The capsule also contains nonpolysaccharide components, such as mannoprotein (MP) (10, 14, 15), although these MP components may represent transient components destined for cellular export.
The role of capsule in environmental growth is unknown, although speculations have been made that the capsule protects the fungus from desiccation or acts as a food source (16). During mammalian infection, the capsule participates in resisting phagocytosis and modulating the immune response (17–21). Not only protective against phagocytosis in both mammalian and lepidopteran hosts (22, 23), the capsule also protects the fungus after ingestion by serving as a free radical sink that can shield the cell from oxidative bursts (24). Hence, while the capsule is not part of the enzymatic microbial arsenal, the machinery responsible for capsule synthesis and assembly does directly contribute to cryptococcal virulence. The primary structures of GXM and GXMGal subunits have been defined, but the mechanisms of subunit assembly into >106-Da branched structures have not (25, 26). The degree of branching and conformation of polysaccharides imply an elaborate assembly and regulatory enzymatic machinery (27).
The subunits of GXM and GXMGal are large glycans that require several glycosyltransferases for synthesis. Both xylosyltransferase and glucuronyltransferase activities are involved in capsular polysaccharide biosynthesis (28–31). A xylosyltransferase, Cxt1, was the first glycosyltransferase identified with a defined role in capsule synthesis (31). It is a large transmembrane protein with β-1,2-xylosyltransferase activity (31), and deletion of the corresponding gene (CXT1) decreased capsular β-1,2-xylose linkages and fungal growth in the lung in a mouse model of infection (30).
Several acapsular mutants were obtained through identification of rough colonies. This type of screen identified four genes required for capsule formation: CAP10, CAP59, CAP60, and CAP64. Although these genes are not essential, their mutation does confer defects in growth and in mouse models of infection (17, 32–35). Cells from these mutant strains lacked or produced extremely reduced capsule, but these mutations did not correlate with enzymatic deficiency in UDP-glucose dehydrogenase, UDP-glucuronate decarboxylase, UDP-glucuronyl:acceptor transferase, UDP-xylosyl:acceptor transferase, or lipid-linked oligosaccharide biosynthetic pathways. CAP10 is a putative xylosyltransferase gene, and cap10Δ mutants show a pleiotropic phenotype, which includes enlarged cell size, smaller extracellular vesicles, and affected expression of some virulence factors (36). CAP10 therefore is required for both capsule formation and other aspects of fungal virulence.
Capsular lactonohydrolase also affects multiple capsule-related phenotypes (37). A strain lacking lactonohydrolase (lhc1Δ) produced capsules with a larger size and altered branching, density, and solvation compared to the parental strain. These capsular structure alterations increased virulence in murine infection (37). Taken together, these results suggest that lactone may be involved in cross-linking of the capsule.
α-1,3-Mannosyltransferase (encoded by CMT1) synthesizes the mannose backbone of GXM and thus plays a crucial role in capsule synthesis. However, α-1,3-mannosyltransferase activity is more involved in in serotype A capsule biosynthesis than in the serotype D C. neoformans (38, 39). Serotypes A and D represent two of the four C. neoformans serotypes: C. neoformans var. neoformans (serotypes A and D) and C. neoformans var. gattii (serotypes B and C), which can be distinguished according to their growth differences on diagnostic media (40). The strain-specific capsule synthesis differences, such as the role of CMT1, show the importance of studying multiple strain backgrounds.
Much remains to be learned about the enzymatic machinery involved in capsule synthesis, including enzyme localization and kinetics. Detailed studies of capsule structure and the enzymatic machinery involved are critical for a better understanding of the function of the capsule production and regulation.
MELANIN SYNTHESIS
Melanin formation protects C. neoformans from oxidative damage as well as from both heat and cold (41, 42). Melanin is synthesized on 2,3- or 3,4-diphenol substrates by a phenoloxidase and accumulates in the C. neoformans cell wall (43, 44). The melanin-synthesizing enzyme has two classical laccase characteristics: a glycosylated copper-containing protein with the ability to oxidize diphenolic substrates and the ability to produce decarboxy dopachrome (45, 46). C. neoformans melanin synthesis occurs only in the presence of exogenous dihydroxyphenols, since no known C. neoformans endogenous substrate exists. Several diphenols can serve as the substrates for pigment synthesis by C. neoformans laccase (47), such as the substrates consisting of para- and ortho-diphenols, monophenols, l-dopa, and esculin, indicating that the enzyme has broad specificity and the ability to generate pigments from different compounds (47–53). Iron increases laccase activity, but hydrogen peroxide has no effect on enzymatic activity, despite the antioxidant properties of melanin (54).
The genes LAC1 and LAC2 encode two laccases, but a single deletion in LAC1 is able to prevent melanin production (55–58). Lac1 localizes in the cell wall, while Lac2 is cytoplasmic, but Lac2 can localize to the cell wall in the absence of Lac1 (55). lac1Δ mutants are easily identified as white colonies when cultivated on catecholamine-containing media (59). The lac1Δ mutant shows decreased virulence in survival studies with rabbit infection (59), corroborating the important role in the fungal virulence (5, 46). In addition to its cell wall localization, laccase is packaged into extracellular vesicles, a nontraditional mechanism of secretion, and can therefore mediate damage away from the laccase-producing fungal cell (Fig. 1).
Melanin is considered a powerful antioxidant, since it may protect cryptococcal cells against oxygen- and nitrogen-derived oxidants of the type made by host effector cells (5, 60–62). In addition to its capacity to absorb free radical fluxes, melanin can also contribute to acquired resistance against to the antifungals amphotericin B and caspofungin, since nonmelanized cryptococcal cells are more susceptible than melanized cells to amphotericin B and caspofugin. Moreover, killing assays demonstrated that addition of melanin particles to amphotericin B or caspofungin significantly reduces their toxicities against C. neoformans (63–65). Thus, melanin and laccase are considered promising targets for drugs against C. neoformans infection.
EXTRACELLULAR ENZYMES
As nature's “recyclers,” environmental fungi secrete a number of degradative enzymes to breakdown macromolecules and obtain nutrients in the environment (7, 66–69). C. neoformans is no exception and releases a number of lipases, proteases, and DNases. However, during the infection process, the same degradative enzymes contribute to virulence by destroying tissues, promoting fungal survival, and interfering with effective immune responses.
Urease is almost universally expressed by C. neoformans isolates. In the environment, C. neoformans is often isolated from avian excreta (70, 71). To survive and grow on this medium, the fungus must metabolize creatinine, xanthines, and uric acid. High urease activity may benefit the fungus under these conditions (72–74), as the enzyme catalyzes the hydrolysis of urea to ammonia and carbamate. Urease is considered a major cryptococcal virulence factor (75). A urease knockout (URE1) strain of C. neoformans was significantly less virulent than the wild-type strain in a mouse model of infection (76). Urease plays a role in fungal escape from the lung to cross the blood-brain barrier but is not required for fungal growth once inside the brain (76). Urease production varies among clinical isolates; however, the vast majority (99.6%) demonstrate some level of urease activity (74, 77, 78). Nevertheless, occasional urease-negative variants have been isolated in clinical isolates (77), suggesting that this enzyme can be dispensable, provided that there are compensatory virulence mechanisms.
Extracellular DNase is produced by C. neoformans in high quantities (79). This DNase may degrade host DNA secreted by neutrophils as part of the innate immune response (80) and additionally may supply C. neoformans with nucleotides. A survey of several yeast species, including C. neoformans, suggests a correlation between urease activity and extracellular DNase production (79). DNase activity is stronger in clinical strains than in environmental strains, further suggesting DNase may play a role as a virulence factor (81).
Superoxide dismutases (SODs) convert superoxide to hydrogen peroxide and oxygen (82). Two SODs have been described in C. neoformans (83–88). SOD contributes to virulence of C. neoformans by facilitating growth within macrophages (89), through a mechanism that is likely to involve protection of the fungus against superoxide generated by host immune response (2). In this regard, melanin and SOD may stimulate complementary defenses for the C. neoformans cells' protection against oxidative damage. SOD production is regulated by temperature, with increases in expression at 37°C compared to 25°C. Thus, increased SOD production at body temperatures may protect the fungus against oxidizing agents produced from host effector cells (90).
Phospholipases degrade cell membrane phospholipids in an enzyme-dependent mechanism. C. neoformans extracellular supernatants contain phospholipase B, phospholipase C, lysophospholipase, and acyltransferase (91–93), and phospholipase activity supports fungal attachment to host cells (94). Phospholipase B promotes fungal invasion of host tissue (95) and hydrolyzes phospholipids in lung surfactant and the plasma membrane (92, 96). Moreover, it contributes to fungal survival by maintaining cell wall integrity (97) and provides nutrients that can be used as sole carbon sources by C. neoformans during the infection (98, 99). As described above, it has also been localized to the cell wall (97), and its transport to the plasma membrane and cell wall is N-glycan dependent (100). Phospholipase C is crucial for several virulence phenotypes (melanin production, growth at 37°C, phospholipase B secretion, and antifungal drug resistance) and is also involved in homeostasis regulation, cell separation following cytokinesis, and cell wall integrity (101, 102).
Phosphatases remove a phosphate group from their substrates and play important roles in regulating protein structure and signaling cascades (103, 104). A secreted acid phosphatase is involved in fungal cell adhesion to host tissues, suggesting an important role in establishing infection (105). Acid phosphatase is encoded by the gene APH1 in C. neoformans. In both wax worm and murine models of cryptococcosis, aph1Δ strain-infected animals survived longer than those in the wild-type-infected model (106), demonstrating the importance of this enzyme during infection.
Proteases break down proteins and are considered important virulence factors, contributing to tissue invasion, colonization, and alteration of the host defense response. Protease activity in C. neoformans cultures has been reported by several investigators (107–111). Proteases play important roles in host cell penetration and virulence of C. neoformans (112). Recently, a metalloprotease was identified by proteomic analyses of the extracellular proteins from C. neoformans and found to be required for invasion of the central nervous system in murine infection of C. neoformans (113). Moreover, the metalloprotease knockout (mpr1Δ) strain was unable to cross the endothelium in an in vitro model of the human blood-brain barrier (113).
DRUG DESIGN AND RESISTANCE
Definition of enzymatic pathways can provide crucial targets for antimicrobial drug design. One way to identify targets is to identify unique metabolic requirements for cryptococcal growth and/or virulence. An example of this is the C. neoformans phosphoribosylaminoimidazole carboxylase gene (ADE2). Mutants with mutations in this gene lack an enzyme required for adenine synthesis and thus have reduced virulence compared to the wild-type strain (114). This observation suggests potential for rational drug design utilizing differences in adenine synthesis pathways between host and pathogen (as first suggested in reference 7). Several candidate enzymes in C. neoformans have been studied regarding fungal amino acid synthesis (e.g., homocitrate synthase, homoisocitrate dehydrogenase, α-aminoadipate reductase, saccharopine reductase, and saccharopine dehydrogenase) (115). However, comparisons between C. neoformans var. neoformans and C. neoformans var. gattii have shown that candidate targets do not necessarily translate across Cryptococcus species. Saccharopine reductase, an enzyme involved in lysine synthesis, was not detected in C. neoformans var. gattii but was detected in C. neoformans var. neoformans. This C. neoformans var. gattii strain was able to grow even in the absence of lysine (115), indicating that further research to identify enzymes essential across all Cryptococcus species is required.
Another essential process for C. neoformans is protein myristoylation. C. neoformans myristoyltransferase catalyzes the transfer of myristate from coenzyme A (CoA) to the amino-terminal glycine residue of a subset of cellular proteins, and this enzyme is essential for C. neoformans viability (116, 117). N-Myristoyl proteins and myristoylation inhibition by the myristic acid analog 4-oxatetradecanoic acid are crucial for this organism (118). Thus, therapies directed at myristoylation may also be a possible target for rational antifungal drug design.
In some cases, an antifungal target is well defined, but multiple enzymes involved in target synthesis provide several inhibitory strategies. Sterols and their synthetic pathways are major antifungal targets in many fungi, but resistance leads to difficulties in patient treatment. Fluconazole-resistant strains require a 10-fold-higher drug concentration to inhibit sterol 14α-demethylation (119), rendering the drug clinically unfeasible. The molecular basis for differential enzyme function has been identified in several clinical C. neoformans strains (120). One documented fluconazole- and amphotericin-resistant C. neoformans patient isolate showed reduced relative sterol content and a defect in δ-8-isomerase, depleted ergosterol, and accumulated aberrant δ-8-double-bonded ergosterol precursors (121, 122), suggesting the ability to form membrane pores due to aggregation and formation of amphotericin-ergosterol complexes. Another study evaluating fluconazole- and amphotericin-resistant isolates observed reduced ergosterol content in the isolates, as well as reduced sensitivity of P450 14α-demethylase to inhibition by fluconazole, and a defect in sterol Δ8-Δ7 isomerase (123). Another C. neoformans strain with defective sterol Δ8-Δ7 isomerase was discovered in an amphotericin B-resistant isolate from an AIDS patient (124). These mutations in sterol synthesis enzymes explain resistance evolution and generate targets to fight it with. This information can also help in rational drug design methodologies.
Identification of key virulence-related enzymes is yet another route toward finding an effective drug target. Glycosphingolipids are essential to regulate survival and/or replication of C. neoformans in the phagolysosome, as well as in the extracellular environment of the host (125–127). Glucosylceramide plays critical role in pathogenicity of C. neoformans, since glucosylceramide synthase (Gcs1) is required for virulence in the murine model of infection (128). gcs1Δ mutants corroborate the crucial role of the glycosphingolipid synthesis in regulation of this considerable aspect of C. neoformans virulence (127). Thus, the glycosphingolipid pathway may also be a reasonable target for antifungal therapies.
Laccase has been considered a drug target in C. neoformans because melanization is critical to virulence. Inhibition of fungal melanization in murine infection using the herbicide glyphosate prolonged average mouse survival. Glyphosate is an inhibitor of both the shikimate acid pathway and l-dopa polymerization (129). Thus, therapies directed at melanization may also be a potential target for antifungal drug design.
Occasionally, a drug proven to work on one microbial pathogen will also be effective against another. This appears to be the case with several viral medications. Drugs such as indinavir and oseltamivir inhibit human immunodeficiency virus (HIV) protease or influenza virus neuraminidase, respectively, and demonstrate the impact an enzymatic inhibitor can have in the clinic (130, 131). The use of protease inhibitors has shown positive effects on C. neoformans and Candida albicans infections, where drug treatment was associated with inhibition of fungal growth and proliferation in vitro (132, 133). These are likely inhibiting the fungal proteases, both cell associated and as part of the fungal secretome.
CONCLUSION
Recent advances in genomics, proteomics, transcriptomics, and mass spectrometry have facilitated the identification and characterization of new fungal enzymes, including those specific to both fungi and C. neoformans. These enzymes are required for many important biological processes, including growth and infection. The importance of the secretome in cryptococcal pathogenesis is apparent from the fact that strain differences in secreted enzymes correlate with their virulence (134). Nonetheless, important questions remain. Future research on cryptococcal enzymology will not only identify new enzymes and their roles during infection but also pinpoint enzymatic targets for the development of antifungal agents.
ADDENDUM IN PROOF
There are, of course, many enzymes involved in signaling cascades, most of which were not discussed in this review. One such enzyme is vital to stress response in C. neoformans and other pathogenic fungi and thus merits a well-deserved mention: the calcium-dependent phosphatase calcineurin (W. J. Steinbach, J. L. Reedy, R. A. Cramer, Jr., J. R. Perfect, J. Heitman, Nat Rev Microbiol 5:418–430, 2008). This enzyme is required for growth in a mammalian host and therefore is necessary to cause disease (A. Odom, S. Muir, E. Lim, D. L. Toffaletti, J. Perfect, J. Heitman, EMBO J 16:2576–2589, 1997). Studies utilizing calcineurin inhibitors for invasive disease in animal models have shown promising results, and this work is now moving into translational stages (D. P. Kontoyiannis, R. E. Lewis, B. D. Alexander, O. Lortholary, F. Dromer, K. L. Gupta, G. T. John, R. del Busto, G. B. Klintmalm, J. Somani, G. M. Lyon, K. Pursell, V. Stosor, P. Munoz, A. P. Limaye, A. C. Kalil, T. L. Pruett, J. Garcia-Diaz, A. Humar, S. Houston, A. A. House, D. Wray, S. Orloff, L. A. Dowdy, R. A. Fisher, J. Heitman, N. D. Albert, M. M. Wagener, N. Singh, Antimicrob Agents Chemother 52:735–738, 2008, http://dx.doi.org/10.1128/AAC.00990-07). Other enzymes involved in stress responses may similarly be identified and targeted in the future.
REFERENCES
- 1.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TA. 2009. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 2.Heitman J, Kozel TR, Kwon-Chung J, Perfect JR, Casadevall A. 2011. Cryptococcus: from human pathogen to model yeast. ASM Press, Washington, DC. [Google Scholar]
- 3.Nielsen K, De Obaldia AL, Heitman J. 2007. Cryptococcus neoformans mates on pigeon guano: implications for the realized ecological niche and globalization. Eukaryot Cell 6:949–959. doi: 10.1128/EC.00097-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchell TG, Perfect JR. 1995. Cryptococcosis in the era of AIDS—100 years after the discovery of Cryptococcus neoformans. Clin Microbiol Rev 8:515–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williamson PR. 1997. Laccase and melanin in the pathogenesis of Cryptococcus neoformans. Front Biosci 2:e99–e107. [DOI] [PubMed] [Google Scholar]
- 6.Casadevall A, Rosas AL, Nosanchuk JD. 2000. Melanin and virulence in Cryptococcus neoformans. Curr Opin Microbiol 3:354–358. doi: 10.1016/S1369-5274(00)00103-X. [DOI] [PubMed] [Google Scholar]
- 7.Casadevall A, Perfect JR. 1998. Cryptococcus neoformans. ASM Press, Washington, DC. [Google Scholar]
- 8.Kronstad J, Saikia S, Nielson ED, Kretschmer M, Jung W, Hu G, Geddes JM, Griffiths EJ, Choi J, Cadieux B, Caza M, Attarian R. 2012. Adaptation of Cryptococcus neoformans to mammalian hosts: integrated regulation of metabolism and virulence. Eukaryot Cell 11:109–118. doi: 10.1128/EC.05273-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cherniak R, Reiss E, Turner SH. 1982. A galactoxylomannan antigen of Cryptococcus neoformans serotype A. Carbohydr Res 103:239–250. doi: 10.1016/S0008-6215(00)80686-2. [DOI] [Google Scholar]
- 10.Cherniak R, Sundstrom JB. 1994. Polysaccharide antigens of the capsule of Cryptococcus neoformans. Infect Immun 62:1507–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bose I, Reese AJ, Ory JJ, Janbon G, Doering TL. 2003. A yeast under cover: the capsule of Cryptococcus neoformans. Eukaryot Cell 2:655–663. doi: 10.1128/EC.2.4.655-663.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kozel TR, Levitz SM, Dromer F, Gates MA, Thorkildson P, Janbon G. 2003. Antigenic and biological characteristics of mutant strains of Cryptococcus neoformans lacking capsular O acetylation or xylosyl side chains. Infect Immun 71:2868–2875. doi: 10.1128/IAI.71.5.2868-2875.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heiss C, Klutts JS, Wang Z, Doering TL, Azadi P. 2009. The structure of Cryptococcus neoformans galactoxylomannan contains beta-d-glucuronic acid. Carbohydr Res 344:915–920. doi: 10.1016/j.carres.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jesus MD, Nicola AM, Chow SK, Lee IR, Nong S, Specht CA, Levitz SM, Casadevall A. 2010. Glucuronoxylomannan, galactoxylomannan, and mannoprotein occupy spatially separate and discrete regions in the capsule of Cryptococcus neoformans. Virulence 1:500–508. doi: 10.4161/viru.1.6.13451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodrigues ML, Nimrichter L. 2012. In good company: association between fungal glycans generates molecular complexes with unique functions. Frontiers Microbiol 3:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Meara TR, Alspaugh JA. 2012. The Cryptococcus neoformans capsule: a sword and a shield. Clin Microbiol Rev 25:387–408. doi: 10.1128/CMR.00001-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang YC, Kwon-Chung KJ. 1994. Complementation of a capsule-deficient mutation of Cryptococcus neoformans restores its virulence. Mol Cell Biol 14:4912–4919. doi: 10.1128/MCB.14.7.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodrigues ML, Alviano CS, Travassos LR. 1999. Pathogenicity of Cryptococcus neoformans: virulence factors and immunological mechanisms. Microbes Infect 1:293–301. doi: 10.1016/S1286-4579(99)80025-2. [DOI] [PubMed] [Google Scholar]
- 19.Kozel TR, Pfrommer GS, Guerlain AS, Highison BA, Highison GJ. 1988. Role of the capsule in phagocytosis of Cryptococcus neoformans. Rev Infect Dis 10(Suppl 2):S436–S439. doi: 10.1093/cid/10.Supplement_2.S436. [DOI] [PubMed] [Google Scholar]
- 20.Pericolini E, Cenci E, Monari C, De Jesus M, Bistoni F, Casadevall A, Vecchiarelli A. 2006. Cryptococcus neoformans capsular polysaccharide component galactoxylomannan induces apoptosis of human T-cells through activation of caspase-8. Cell Microbiol 8:267–275. doi: 10.1111/j.1462-5822.2005.00619.x. [DOI] [PubMed] [Google Scholar]
- 21.Vecchiarelli A, Monari C. 2012. Capsular material of Cryptococcus neoformans: virulence and much more. Mycopathologia 173:375–386. doi: 10.1007/s11046-011-9513-8. [DOI] [PubMed] [Google Scholar]
- 22.Trevijano-Contador N, Herrero-Fernandez I, Garcia-Barbazan I, Scorzoni L, Rueda C, Rossi SA, Garcia-Rodas R, Zaragoza O. 2015. Cryptococcus neoformans induces antimicrobial responses and behaves as a facultative intracellular pathogen in the non mammalian model Galleria mellonella. Virulence 6:66–74. doi: 10.4161/21505594.2014.986412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alvarez M, Burn T, Luo Y, Pirofski LA, Casadevall A. 2009. The outcome of Cryptococcus neoformans intracellular pathogenesis in human monocytes. BMC Microbiol 9:51. doi: 10.1186/1471-2180-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaragoza O, Rodrigues ML, De Jesus M, Frases S, Dadachova E, Casadevall A. 2009. The capsule of the fungal pathogen Cryptococcus neoformans. Adv Appl Microbiol 68:133–216. doi: 10.1016/S0065-2164(09)01204-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frases S, Pontes B, Nimrichter L, Viana NB, Rodrigues ML, Casadevall A. 2009. Capsule of Cryptococcus neoformans grows by enlargement of polysaccharide molecules. Proc Natl Acad Sci U S A 106:1228–1233. doi: 10.1073/pnas.0808995106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McFadden DC, De Jesus M, Casadevall A. 2006. The physical properties of the capsular polysaccharides from Cryptococcus neoformans suggest features for capsule construction. J Biol Chem 281:1868–1875. doi: 10.1074/jbc.M509465200. [DOI] [PubMed] [Google Scholar]
- 27.Cordero RJ, Frases S, Guimaraes AJ, Rivera J, Casadevall A. 2011. Evidence for branching in cryptococcal capsular polysaccharides and consequences on its biological activity. Mol Microbiol 79:1101–1117. doi: 10.1111/j.1365-2958.2010.07511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White CW, Cherniak R, Jacobson ES. 1990. Side group addition by xylosyltransferase and glucuronyltransferase in biosynthesis of capsular polysaccharide in Cryptococcus neoformans. J Med Vet Mycol 28:289–301. doi: 10.1080/02681219080000381. [DOI] [PubMed] [Google Scholar]
- 29.Castle SA, Owuor EA, Thompson SH, Garnsey MR, Klutts JS, Doering TL, Levery SB. 2008. β1,2-Xylosyltransferase Cxt1p is solely responsible for xylose incorporation into Cryptococcus neoformans glycosphingolipids. Eukaryot Cell 7:1611–1615. doi: 10.1128/EC.00458-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klutts JS, Doering TL. 2008. Cryptococcal xylosyltransferase 1 (Cxt1p) from Cryptococcus neoformans plays a direct role in the synthesis of capsule polysaccharides. J Biol Chem 283:14327–14334. doi: 10.1074/jbc.M708927200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klutts JS, Levery SB, Doering TL. 2007. A beta-1,2-xylosyltransferase from Cryptococcus neoformans defines a new family of glycosyltransferases. J Biol Chem 282:17890–17899. doi: 10.1074/jbc.M701941200. [DOI] [PubMed] [Google Scholar]
- 32.Chang YC, Kwon-Chung KJ. 1999. Isolation, characterization, and localization of a capsule-associated gene, CAP10, of Cryptococcus neoformans. J Bacteriol 181:5636–5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang YC, Penoyer LA, KwonChung KJ. 1996. The second capsule gene of Cryptococcus neoformans, CAP64, is essential for virulence. Infect Immun 64:1977–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang YC, Kwon-Chung KJ. 1998. Isolation of the third capsule-associated gene, CAP60, required for virulence in Cryptococcus neoformans. Infect Immun 66:2230–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacobson ES, Tingler MJ. 1994. Strains of Cryptococcus neoformans with defined capsular phenotypes. J Med Vet Mycol 32:401–404. doi: 10.1080/02681219480000531. [DOI] [PubMed] [Google Scholar]
- 36.Tefsen B, Grijpstra J, Ordonez S, Lammers M, van Die I, de Cock H. 2014. Deletion of the CAP10 gene of Cryptococcus neoformans results in a pleiotropic phenotype with changes in expression of virulence factors. Res Microbiol 165:399–410. doi: 10.1016/j.resmic.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Park YD, Shin S, Panepinto J, Ramos J, Qiu J, Frases S, Albuquerque P, Cordero RJ, Zhang N, Himmelreich U, Beenhouwer D, Bennett JE, Casadevall A, Williamson PR. 2014. A role for LHC1 in higher order structure and complement binding of the Cryptococcus neoformans capsule. PLoS Pathog 10:e1004037. doi: 10.1371/journal.ppat.1004037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doering TL. 1999. A unique alpha-1,3 mannosyltransferase of the pathogenic fungus Cryptococcus neoformans. J Bacteriol 181:5482–5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sommer U, Liu H, Doering TL. 2003. An alpha-1,3-mannosyltransferase of Cryptococcus neoformans. J Biol Chem 278:47724–47730. doi: 10.1074/jbc.M307223200. [DOI] [PubMed] [Google Scholar]
- 40.Bennett JE, Kwonchung KJ, Howard DH. 1977. Epidemiologic differences among serotypes of Cryptococcus neoformans. Am J Epidemiol 105:582–586. [DOI] [PubMed] [Google Scholar]
- 41.Rosas AL, Casadevall A. 1997. Melanization affects susceptibility of Cryptococcus neoformans to heat and cold. FEMS Microbiol Lett 153:265–272. doi: 10.1016/S0378-1097(97)00239-5. [DOI] [PubMed] [Google Scholar]
- 42.Khajo A, Bryan RA, Friedman M, Burger RM, Levitsky Y, Casadevall A, Magliozzo RS, Dadachova E. 2011. Protection of melanized Cryptococcus neoformans from lethal dose gamma irradiation involves changes in melanin's chemical structure and paramagnetism. PLoS One 6:e25092. doi: 10.1371/journal.pone.0025092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shaw CE, Kapica L. 1972. Production of diagnostic pigment by phenoloxidase activity of Cryptococcus neoformans. Appl Microbiol 24:824–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Aisen P, Casadevall A. 1996. Melanin, melanin “ghosts,” and melanin composition in Cryptococcus neoformans. Infect Immun 64:2420–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williamson PR. 1994. Biochemical and molecular characterization of the diphenol oxidase of Cryptococcus neoformans—identification as a laccase. J Bacteriol 176:656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ikeda R, Shinoda T, Morita T, Jacobson ES. 1993. Characterization of a phenol oxidase from Cryptococcus neoformans var. neoformans. Microbiol Immunol 37:759–764. doi: 10.1111/j.1348-0421.1993.tb01702.x. [DOI] [PubMed] [Google Scholar]
- 47.Chaskes S, Tyndall RL. 1975. Pigment production by Cryptococcus neoformans from para- and ortho-diphenols: effect of the nitrogen source. J Clin Microbiol 1:509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Edberg SC, Chaskes SJ, Alture-Werber E, Singer JM. 1980. Esculin-based medium for isolation and identification of Cryptococcus neoformans. J Clin Microbiol 12:332–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwon-Chung KJ, Tom WK, Costa JL. 1983. Utilization of indole compounds by Cryptococcus neoformans to produce a melanin-like pigment. J Clin Microbiol 18:1419–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Polacheck I, Hearing VJ, Kwon-Chung KJ. 1982. Biochemical studies of phenoloxidase and utilization of catecholamines in Cryptococcus neoformans. J Bacteriol 150:1212–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Polacheck I, Platt Y, Aronovitch J. 1990. Catecholamines and virulence of Cryptococcus neoformans. Infect Immun 58:2919–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strachan AA, Yu RJ, Blank F. 1971. Pigment production of Cryptococcus neoformans grown with extracts of Guizotia abyssinica. Appl Microbiol 22:478–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang HS, Zeimis RT, Roberts GD. 1977. Evaluation of a caffeic acid-ferric citrate test for rapid identification of Cryptococcus neoformans. J Clin Microbiol 6:445–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jacobson ES, Compton GM. 1996. Discordant regulation of phenoloxidase and capsular polysaccharide in Cryptococcus neoformans. J Med Vet Mycol 34:289–291. doi: 10.1080/02681219680000491. [DOI] [PubMed] [Google Scholar]
- 55.Missall TA, Moran JM, Corbett JA, Lodge JK. 2005. Distinct stress responses of two functional laccases in Cryptococcus neoformans are revealed in the absence of the thiol-specific antioxidant Tsa1. Eukaryot Cell 4:202–208. doi: 10.1128/EC.4.1.202-208.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pukkila-Worley R, Gerrald QD, Kraus PR, Boily MJ, Davis MJ, Giles SS, Cox GM, Heitman J, Alspaugh JA. 2005. Transcriptional network of multiple capsule and melanin genes governed by the Cryptococcus neoformans cyclic AMP cascade. Eukaryot Cell 4:190–201. doi: 10.1128/EC.4.1.190-201.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu X, Williamson PR. 2004. Role of laccase in the biology and virulence of Cryptococcus neoformans. FEMS Yeast Res 5:1–10. doi: 10.1016/j.femsyr.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 58.Zhu XD, Gibbons J, Garcia-Rivera J, Casadevall A, Williamson PR. 2001. Laccase of Cryptococcus neoformans is a cell wall-associated virulence factor. Infect Immun 69:5589–5596. doi: 10.1128/IAI.69.9.5589-5596.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salas SD, Bennett JE, Kwon-Chung KJ, Perfect JR, Williamson PR. 1996. Effect of the laccase gene CNLAC1, on virulence of Cryptococcus neoformans. J Exp Med 184:377–386. doi: 10.1084/jem.184.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Y, Casadevall A. 1994. Susceptibility of melanized and nonmelanized Cryptococcus neoformans to nitrogen- and oxygen-derived oxidants. Infect Immun 62:3004–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Y, Aisen P, Casadevall A. 1995. Cryptococcus neoformans melanin and virulence: mechanism of action. Infect Immun 63:3131–3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jacobson ES, Tinnell SB. 1993. Antioxidant function of fungal melanin. J Bacteriol 175:7102–7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Duin D, Casadevall A, Nosanchuk JD. 2002. Melanization of Cryptococcus neoformans and Histoplasma capsulatum reduces their susceptibilities to amphotericin B and caspofungin. Antimicrob Agents Chemother 46:3394–3400. doi: 10.1128/AAC.46.11.3394-3400.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ikeda R, Sugita T, Jacobson ES, Shinoda T. 2003. Effects of melanin upon susceptibility of Cryptococcus to antifungals. Microbiol Immunol 47:271–277. doi: 10.1111/j.1348-0421.2003.tb03395.x. [DOI] [PubMed] [Google Scholar]
- 65.Wang YL, Casadevall A. 1994. Growth of Cryptococcus neoformans in presence of l-dopa decreases its susceptibility to amphotericin B. Antimicrob Agents Chemother 38:2648–2650. doi: 10.1128/AAC.38.11.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Almeida FB, Cerqueira FM, Silva Rdo N, Ulhoa CJ, Lima AL. 2007. Mycoparasitism studies of Trichoderma harzianum strains against Rhizoctonia solani: evaluation of coiling and hydrolytic enzyme production. Biotechnol Lett 29:1189–1193. doi: 10.1007/s10529-007-9372-z. [DOI] [PubMed] [Google Scholar]
- 67.Dos Reis Almeida FB, de Oliveira LL, de Sousa MV, Barreira MCR, Hanna ES. 2010. Paracoccin from Paracoccidioides brasiliensis; purification through affinity with chitin and identification of N-acetyl-beta-d-glucosaminidase activity. Yeast 27:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dos Reis Almeida FB, Carvalho FC, Mariano VS, Alegre ACP, Silva RD, Hanna ES, Roque-Barreira MC. 2011. Influence of N-glycosylation on the morphogenesis and growth of Paracoccidioides brasiliensis and on the biological activities of yeast proteins. PLoS One 6:e29216. doi: 10.1371/journal.pone.0029216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dos Reis Almeida FB, Pigosso LL, de Lima Damasio AR, Monteiro VN, de Almeida Soares CM, Silva RN, Roque-Barreira MC. 2014. alpha-(1,4)-Amylase, but not alpha- and beta-(1,3)-glucanases, may be responsible for the impaired growth and morphogenesis of Paracoccidioides brasiliensis induced by N-glycosylation inhibition. Yeast 31:1–11. doi: 10.1002/yea.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Partridge BM, Winner HI. 1965. Cryptococcus neoformans in bird droppings in London. Lancet i:1060–1061. [DOI] [PubMed] [Google Scholar]
- 71.Walter JE, Yee RB. 1968. Factors that determine the growth of Cryptococcus neoformans in avian excreta. Am J Epidemiol 88:445–450. [DOI] [PubMed] [Google Scholar]
- 72.Kwon-Chung KJ, Wickes BL, Booth JL, Vishniac HS, Bennett JE. 1987. Urease inhibition by EDTA in the two varieties of Cryptococcus neoformans. Infect Immun 55:1751–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vogel RA. 1969. Primary isolation medium for Cryptococcus neoformans. Appl Microbiol 18:1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zimmer BL, Roberts GD. 1979. Rapid selective urease test for presumptive identification of Cryptococcus neoformans. J Clin Microbiol 10:380–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cox GM, Mukherjee J, Cole GT, Casadevall A, Perfect JR. 2000. Urease as a virulence factor in experimental cryptococcosis. Infect Immun 68:443–448. doi: 10.1128/IAI.68.2.443-448.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Olszewski MA, Noverr MC, Chen GH, Toews GB, Cox GM, Perfect JR, Huffnagle GB. 2004. Urease expression by Cryptococcus neoformans promotes microvascular sequestration, thereby enhancing central nervous system invasion. Am J Pathol 164:1761–1771. doi: 10.1016/S0002-9440(10)63734-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bava AJ, Negroni R, Bianchi M. 1993. Cryptococcosis produced by a urease negative strain of Cryptococcus neoformans. J Med Vet Mycol 31:87–89. doi: 10.1080/02681219380000091. [DOI] [PubMed] [Google Scholar]
- 78.Ruane PJ, Walker LJ, George WL. 1988. Disseminated infection caused by urease-negative Cryptococcus neoformans. J Clin Microbiol 26:2224–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cazin J Jr, Kozel TR, Lupan DM, Burt WR. 1969. Extracellular deoxyribonuclease production by yeasts. J Bacteriol 100:760–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rocha JD, Nascimento MT, Decote-Ricardo D, Corte-Real S, Morrot A, Heise N, Nunes MP, Previato JO, Mendonca-Previato L, DosReis GA, Saraiva EM, Freire-de-Lima CG. 2015. Capsular polysaccharides from Cryptococcus neoformans modulate production of neutrophil extracellular traps (NETs) by human neutrophils. Sci Rep 5:8008. doi: 10.1038/srep08008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sanchez M, Colom F. 2010. Extracellular DNase activity of Cryptococcus neoformans and Cryptococcus gattii. Rev Iberoam Micol 27:10–13. doi: 10.1016/j.riam.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 82.Fridovich I. 1995. Superoxide radical and superoxide dismutases. Annu Rev Biochem 64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- 83.Hamilton AJ, Holdom MD. 1997. Biochemical comparison of the Cu,Zn superoxide dismutases of Cryptococcus neoformans var. neoformans and Cryptococcus neoformans var. gattii. Infect Immun 65:488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tesfa-Selase F, Hay RJ. 1995. Superoxide dismutase of Cryptococcus neoformans: purification and characterization. J Med Vet Mycol 33:253–259. doi: 10.1080/02681219580000511. [DOI] [PubMed] [Google Scholar]
- 85.Giles SS, Batinic-Haberle I, Perfect JR, Cox GM. 2005. Cryptococcus neoformans mitochondrial superoxide dismutase: an essential link between antioxidant function and high-temperature growth. Eukaryot Cell 4:46–54. doi: 10.1128/EC.4.1.46-54.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Narasipura SD, Ault JG, Behr MJ, Chaturvedi V, Chaturvedi S. 2003. Characterization of Cu,Zn superoxide dismutase (SOD1) gene knock-out mutant of Cryptococcus neoformans var. gattii: role in biology and virulence. Mol Microbiol 47:1681–1694. [DOI] [PubMed] [Google Scholar]
- 87.Narasipura SD, Chaturvedi V, Chaturvedi S. 2005. Characterization of Cryptococcus neoformans variety gattii SOD2 reveals distinct roles of the two superoxide dismutases in fungal biology and virulence. Mol Microbiol 55:1782–1800. doi: 10.1111/j.1365-2958.2005.04503.x. [DOI] [PubMed] [Google Scholar]
- 88.Siafakas AR, Wright LC, Sorrell TC, Djordjevic JT. 2006. Lipid rafts in Cryptococcus neoformans concentrate the virulence determinants phospholipase B1 and Cu/Zn superoxide dismutase. Eukaryot Cell 5:488–498. doi: 10.1128/EC.5.3.488-498.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cox GM, Harrison TS, McDade HC, Taborda CP, Heinrich G, Casadevall A, Perfect JR. 2003. Superoxide dismutase influences the virulence of Cryptococcus neoformans by affecting growth within macrophages. Infect Immun 71:173–180. doi: 10.1128/IAI.71.1.173-180.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jacobson ES, Jenkins ND, Todd JM. 1994. Relationship between superoxide-dismutase and melanin in a pathogenic fungus. Infect Immun 62:4085–4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen SC, Muller M, Zhou JZ, Wright LC, Sorrell TC. 1997. Phospholipase activity in Cryptococcus neoformans: a new virulence factor? J Infect Dis 175:414–420. doi: 10.1093/infdis/175.2.414. [DOI] [PubMed] [Google Scholar]
- 92.Chen SCA, Wright LC, Santangelo RT, Muller M, Moran VR, Kuchel PW, Sorrell TC. 1997. Identification of extracellular phospholipase B, lysophospholipase, and acyltransferase produced by Cryptococcus neoformans. Infect Immun 65:405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Henry J, Guillotte A, Luberto C, Del Poeta M. 2011. Characterization of inositol phospho-sphingolipid-phospholipase C 1 (Isc1) in Cryptococcus neoformans reveals unique biochemical features. FEBS Lett 585:635–640. doi: 10.1016/j.febslet.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Barrett-Bee K, Hayes Y, Wilson RG, Ryley JF. 1985. A comparison of phospholipase activity, cellular adherence and pathogenicity of yeasts. J Gen Microbiol 131:1217–1221. [DOI] [PubMed] [Google Scholar]
- 95.Santangelo R, Zoellner H, Sorrell T, Wilson C, Donald C, Djordjevic J, Shounan Y, Wright L. 2004. Role of extracellular phospholipases and mononuclear phagocytes in dissemination of cryptococcosis in a murine model. Infect Immun 72:2229–2239. doi: 10.1128/IAI.72.4.2229-2239.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen SC, Wright LC, Golding JC, Sorrell TC. 2000. Purification and characterization of secretory phospholipase B, lysophospholipase and lysophospholipase/transacylase from a virulent strain of the pathogenic fungus Cryptococcus neoformans. Biochem J 347:431–439. doi: 10.1042/bj3470431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Siafakas AR, Sorrell TC, Wright LC, Wilson C, Larsen M, Boadle R, Williamson PR, Djordjevic JT. 2007. Cell wall-linked cryptococcal phospholipase B1 is a source of secreted enzyme and a determinant of cell wall integrity. J Biol Chem 282:37508–37514. doi: 10.1074/jbc.M707913200. [DOI] [PubMed] [Google Scholar]
- 98.Wright LC, Santangelo RM, Ganendren R, Payne J, Djordjevic JT, Sorrell TC. 2007. Cryptococcal lipid metabolism: phospholipase B1 is implicated in transcellular metabolism of macrophage-derived lipids. Eukaryot Cell 6:37–47. doi: 10.1128/EC.00262-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Noverr MC, Cox GM, Perfect JR, Huffnagle GB. 2003. Role of PLB1 in pulmonary inflammation and cryptococcal eicosanoid production. Infect Immun 71:1538–1547. doi: 10.1128/IAI.71.3.1538-1547.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Turner KM, Wright LC, Sorrell TC, Djordjevic JT. 2006. N-linked glycosylation sites affect secretion of cryptococcal phospholipase B1, irrespective of glycosylphosphatidylinositol anchoring. Biochim Biophys Acta 1760:1569–1579. doi: 10.1016/j.bbagen.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 101.Chayakulkeeree M, Sorrell TC, Siafakas AR, Wilson CF, Pantarat N, Gerik KJ, Boadle R, Djordjevic JT. 2008. Role and mechanism of phosphatidylinositol-specific phospholipase C in survival and virulence of Cryptococcus neoformans. Mol Microbiol 69:809–826. doi: 10.1111/j.1365-2958.2008.06310.x. [DOI] [PubMed] [Google Scholar]
- 102.Lev S, Desmarini D, Li C, Chayakulkeeree M, Traven A, Sorrell TC, Djordjevic JT. 2013. Phospholipase C of Cryptococcus neoformans regulates homeostasis and virulence by providing inositol trisphosphate as a substrate for Arg1 kinase. Infect Immun 81:1245–1255. doi: 10.1128/IAI.01421-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bauman AL, Scott JD. 2002. Kinase- and phosphatase-anchoring proteins: harnessing the dynamic duo. Nat Cell Biol 4:E203–E206. doi: 10.1038/ncb0802-e203. [DOI] [PubMed] [Google Scholar]
- 104.McConnell JL, Wadzinski BE. 2009. Targeting protein serine/threonine phosphatases for drug development. Mol Pharmacol 75:1249–1261. doi: 10.1124/mol.108.053140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Collopy-Junior I, Esteves FF, Nimrichter L, Rodrigues ML, Alviano CS, Meyer-Fernandes JR. 2006. An ectophosphatase activity in Cryptococcus neoformans. FEMS Yeast Res 6:1010–1017. doi: 10.1111/j.1567-1364.2006.00105.x. [DOI] [PubMed] [Google Scholar]
- 106.Lev S, Crossett B, Cha SY, Desmarini D, Li C, Chayakulkeeree M, Wilson CF, Williamson PR, Sorrell TC, Djordjevic JT. 2014. Identification of Aph1, a phosphate-regulated, secreted, and vacuolar acid phosphatase in Cryptococcus neoformans. mBio 5:e01649-14. doi: 10.1128/mBio.01649-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brueske CH. 1986. Proteolytic activity of a clinical isolate of Cryptococcus neoformans. J Clin Microbiol 23:631–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen LC, Blank ES, Casadevall A. 1996. Extracellular proteinase activity of Cryptococcus neoformans. Clin Diagn Lab Immunol 3:570–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ruma-Haynes P, Brownlee AG, Sorrell TC. 2000. A rapid method for detecting extracellular proteinase activity in Cryptococcus neoformans and a survey of 63 isolates. J Med Microbiol 49:733–737. doi: 10.1099/0022-1317-49-8-733. [DOI] [PubMed] [Google Scholar]
- 110.Il Yoo J, Lee YS, Song CY, Kim BS. 2004. Purification and characterization of a 43-kilodalton extracellular serine proteinase from Cryptococcus neoformans. J Clin Microbiol 42:722–726. doi: 10.1128/JCM.42.2.722-726.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pinti M, Orsi CF, Gibellini L, Esposito R, Cossarizza A, Blasi E, Peppoloni S, Mussini C. 2007. Identification and characterization of an aspartyl protease from Cryptococcus neoformans. FEBS Lett 581:3882–3886. doi: 10.1016/j.febslet.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 112.Chen LC, Pirofski LA, Casadevall A. 1997. Extracellular proteins of Cryptococcus neoformans and host antibody response. Infect Immun 65:2599–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vu K, Tham R, Uhrig JP, Thompson GR III, Na Pombejra S, Jamklang M, Bautos JM, Gelli A. 2014. Invasion of the central nervous system by Cryptococcus neoformans requires a secreted fungal metalloprotease. mBio 5:e01101-14. doi: 10.1128/mBio.01101-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Perfect JR, Toffaletti DL, Rude TH. 1993. The gene encoding phosphoribosylaminoimidazole carboxylase (Ade2) is essential for growth of Cryptococcus neoformans in cerebrospinal fluid. Infect Immun 61:4446–4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Garrad RC, Bhattacharjee JK. 1992. Lysine biosynthesis in selected pathogenic fungi—characterization of lysine auxotrophs and the cloned Lys1 gene of Candida albicans. J Bacteriol 174:7379–7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lodge JK, Johnson RL, Weinberg RA, Gordon JI. 1994. Comparison of myristoyl-CoA:protein N-myristoyltransferases from three pathogenic fungi: Cryptococcus neoformans, Histoplasma capsulatum, and Candida albicans. J Biol Chem 269:2996–3009. [PubMed] [Google Scholar]
- 117.Lodge JK, Jackson-Machelski E, Toffaletti DL, Perfect JR, Gordon JI. 1994. Targeted gene replacement demonstrates that myristoyl-CoA/protein N-myristoyltransferase is essential for viability of Cryptococcus neoformans. Proc Natl Acad Sci U S A 91:12008–12012. doi: 10.1073/pnas.91.25.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Langner CA, Lodge JK, Travis SJ, Caldwell JE, Lu TB, Li Q, Bryant ML, Devadas B, Gokel GW, Kobayashi GS, Gordon JI. 1992. 4-Oxatetradecanoic acid is fungicidal for Cryptococcus neoformans and inhibits replication of human immunodeficiency virus I. J Biol Chem 267:17159–17169. [PubMed] [Google Scholar]
- 119.Lamb DC, Corran A, Baldwin BC, Kwon-Chung J, Kelly SL. 1995. Resistant P45051A1 activity in azole antifungal tolerant Cryptococcus neoformans from AIDS patients. FEBS Lett 368:326–330. doi: 10.1016/0014-5793(95)00684-2. [DOI] [PubMed] [Google Scholar]
- 120.Bozzette SA, Larsen RA, Chiu J, Leal MAE, Jacobsen J, Rothman P, Robinson P, Gilbert G, Mccutchan JA, Tilles J, Leedom JM, Richman DD. 1991. A placebo-controlled trial of maintenance therapy with fluconazole after treatment of cryptococcal meningitis in the acquired immunodeficiency syndrome. N Engl J Med 324:580–584. doi: 10.1056/NEJM199102283240902. [DOI] [PubMed] [Google Scholar]
- 121.Anonymous. 1980. Garlic in cryptococcal meningitis: a preliminary report of 21 cases. Chin Med J (Engl) 93:123–126. [PubMed] [Google Scholar]
- 122.Haynes MP, Chong PLG, Buckley HR, Pieringer RA. 1996. Fluorescence studies on the molecular action of amphotericin B on susceptible and resistant fungal cells. Biochemistry 35:7983–7992. doi: 10.1021/bi952910c. [DOI] [PubMed] [Google Scholar]
- 123.Venkateswarlu K, Taylor M, Manning NJ, Rinaldi MG, Kelly SL. 1997. Fluconazole tolerance in clinical isolates of Cryptococcus neoformans. Antimicrob Agents Chemother 41:748–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kelly SL, Lamb DC, Taylor M, Corran AJ, Baldwin BC, Powderly WG. 1994. Resistance to amphotericin B associated with defective sterol delta 8→7 isomerase in a Cryptococcus neoformans strain from an AIDS patient. FEMS Microbiol Lett 122:39–42. doi: 10.1111/j.1574-6968.1994.tb07140.x. [DOI] [PubMed] [Google Scholar]
- 125.Luberto C, Toffaletti DL, Wills EA, Tucker SC, Casadevall A, Perfect JR, Hannun YA, Del Poeta M. 2001. Roles for inositol-phosphoryl ceramide synthase 1 (IPC1) in pathogenesis of C. neoformans. Genes Dev 15:201–212. doi: 10.1101/gad.856001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shea JM, Kechichian TB, Luberto C, Del Poeta M. 2006. The cryptococcal enzyme inositol phosphosphingolipid-phospholipase C confers resistance to the antifungal effects of macrophages and promotes fungal dissemination to the central nervous system. Infect Immun 74:5977–5988. doi: 10.1128/IAI.00768-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Del Poeta M, Nimrichter L, Rodrigues ML, Luberto C. 2014. Synthesis and biological properties of fungal glucosylceramide. PLoS Pathog 10:e1003832. doi: 10.1371/journal.ppat.1003832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rittershaus PC, Kechichian TB, Allegood JC, Merrill AH, Hennig M, Luberto C, Del Poeta M. 2006. Glucosylceramide synthase is an essential regulator of pathogenicity of Cryptococcus neoformans. J Clin Invest 116:1651–1659. doi: 10.1172/JCI27890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nosanchuk JD, Ovalle R, Casadevall A. 2001. Glyphosate inhibits melanization of Cryptococcus neoformans and prolongs survival of mice after systemic infection. J Infect Dis 183:1093–1099. doi: 10.1086/319272. [DOI] [PubMed] [Google Scholar]
- 130.De Clercq E. 2013. The nucleoside reverse transcriptase inhibitors, nonnucleoside reverse transcriptase inhibitors, and protease inhibitors in the treatment of HIV infections (AIDS). Adv Pharmacol 67:317–358. doi: 10.1016/B978-0-12-405880-4.00009-3. [DOI] [PubMed] [Google Scholar]
- 131.Loregian A, Mercorelli B, Nannetti G, Compagnin C, Palu G. 2014. Antiviral strategies against influenza virus: towards new therapeutic approaches. Cell Mol Life Sci 71:3659–3683. doi: 10.1007/s00018-014-1615-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cassone A, De Bernardis F, Torosantucci A, Tacconelli E, Tumbarello M, Cauda R. 1999. In vitro and in vivo anticandidal activity of human immunodeficiency virus protease inhibitors. J Infect Dis 180:448–453. doi: 10.1086/314871. [DOI] [PubMed] [Google Scholar]
- 133.Blasi E, Colombari B, Orsi CF, Pinti M, Troiano L, Cossarizza A, Esposito R, Peppoloni S, Mussini C, Neglia R. 2004. The human immunodeficiency virus (HIV) protease inhibitor indinavir directly affects the opportunistic fungal pathogen Cryptococcus neoformans. FEMS Immunol Med Microbiol 42:187–195. doi: 10.1016/j.femsim.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 134.Campbell LT, Chen C, Ferdous J, Padula MP, Harry E, Hofer M, Campbell IL, Carter DA. 2015. Cryptococcus strains with different pathogenic potential have diverse protein secretomes. Eukaryot Cell 14:554–563. doi: 10.1128/EC.00052-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Maligie MA, Selitrennikoff CP. 2005. Cryptococcus neoformans resistance to echinocandins: (1,3)beta-glucan synthase activity is sensitive to echinocandins. Antimicrob Agents Chemother 49:2851–2856. doi: 10.1128/AAC.49.7.2851-2856.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Casal M, Linares MJ. 1983. Contribution to the study of the enzymatic profiles of yeast organisms with medical interest. Mycopathologia 81:155–159. doi: 10.1007/BF00436820. [DOI] [PubMed] [Google Scholar]
- 137.Mason DL, Wilson CL. 1979. Cytochemical and biochemical identification of lysosomes in Cryptococcus neoformans. Mycopathologia 68:183–190. doi: 10.1007/BF00578528. [DOI] [PubMed] [Google Scholar]
- 138.Janbon G, Himmelreich U, Moyrand F, Improvisi L, Dromer F. 2001. Cas1p is a membrane protein necessary for the O-acetylation of the Cryptococcus neoformans capsular polysaccharide. Mol Microbiol 42:453–467. doi: 10.1046/j.1365-2958.2001.02651.x. [DOI] [PubMed] [Google Scholar]
- 139.Baker LG, Specht CA, Donlin MJ, Lodge JK. 2007. Chitosan, the deacetylated form of chitin, is necessary for cell wall integrity in Cryptococcus neoformans. Eukaryot Cell 6:855–867. doi: 10.1128/EC.00399-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Banks IR, Specht CA, Donlin MJ, Gerik KJ, Levitz SM, Lodge JK. 2005. A chitin synthase and its regulator protein are critical for chitosan production and growth of the fungal pathogen Cryptococcus neoformans. Eukaryot Cell 4:1902–1912. doi: 10.1128/EC.4.11.1902-1912.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Baker LG, Specht CA, Lodge JK. 2009. Chitinases are essential for sexual development but not vegetative growth in Cryptococcus neoformans. Eukaryot Cell 8:1692–1705. doi: 10.1128/EC.00227-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Polacheck I, Kwon-Chung KJ. 1980. Creatinine metabolism in Cryptococcus neoformans and Cryptococcus bacillisporus. J Bacteriol 142:15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wills EA, Roberts IS, Del Poeta M, Rivera J, Casadevall A, Cox GM, Perfect JR. 2001. Identification and characterization of the Cryptococcus neoformans phosphomannose isomerase-encoding gene, MAN1, and its impact on pathogenicity. Mol Microbiol 40:610–620. doi: 10.1046/j.1365-2958.2001.02401.x. [DOI] [PubMed] [Google Scholar]
- 144.Cottrell TR, Griffith CL, Liu H, Nenninger AA, Doering TL. 2007. The pathogenic fungus Cryptococcus neoformans expresses two functional GDP-mannose transporters with distinct expression patterns and roles in capsule synthesis. Eukaryot Cell 6:776–785. doi: 10.1128/EC.00015-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Biondo C, Beninati C, Bombaci M, Messina L, Mancuso G, Midiri A, Galbo R, Teti G. 2003. Induction of T helper type 1 responses by a polysaccharide deacetylase from Cryptococcus neoformans. Infect Immun 71:5412–5417. doi: 10.1128/IAI.71.9.5412-5417.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Erickson T, Liu L, Gueyikian A, Zhu XD, Gibbons J, Williamson PR. 2001. Multiple virulence factors of Cryptococcus neoformans are dependent on VPH1. Mol Microbiol 42:1121–1131. doi: 10.1046/j.1365-2958.2001.02712.x. [DOI] [PubMed] [Google Scholar]
- 147.Bar-Peled M, Griffith CL, Doering TL. 2001. Functional cloning and characterization of a UDP-glucuronic acid decarboxylase: the pathogenic fungus Cryptococcus neoformans elucidates UDP-xylose synthesis. Proc Natl Acad Sci U S A 98:12003–12008. doi: 10.1073/pnas.211229198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Levitz SM, Specht CA. 2006. The molecular basis for the immunogenicity of Cryptococcus neoformans mannoproteins. FEMS Yeast Res 6:513–524. doi: 10.1111/j.1567-1364.2006.00071.x. [DOI] [PubMed] [Google Scholar]
- 149.White CW, Jacobson ES. 1993. Mannosyl transfer in Cryptococcus neoformans. Can J Microbiol 39:129–133. doi: 10.1139/m93-019. [DOI] [PubMed] [Google Scholar]
- 150.Fiskin AM, Zalles MC, Garrison RG. 1990. Electron cytochemical studies of Cryptococcus neoformans grown on uric acid and related sources of nitrogen. J Med Vet Mycol 28:197–207. doi: 10.1080/02681219080000261. [DOI] [PubMed] [Google Scholar]
- 151.Biondo C, Mancuso G, Midiri A, Bombaci M, Messina L, Beninati C, Teti G. 2006. Identification of major proteins secreted by Cryptococcus neoformans. FEMS Yeast Res 6:645–651. doi: 10.1111/j.1567-1364.2006.00043.x. [DOI] [PubMed] [Google Scholar]
- 152.Eigenheer RA, Jin Lee Y, Blumwald E, Phinney BS, Gelli A. 2007. Extracellular glycosylphosphatidylinositol-anchored mannoproteins and proteases of Cryptococcus neoformans. FEMS Yeast Res 7:499–510. doi: 10.1111/j.1567-1364.2006.00198.x. [DOI] [PubMed] [Google Scholar]
- 153.Bien CM, Chang YC, Nes WD, Kwon-Chung KJ, Espenshade PJ. 2009. Cryptococcus neoformans site-2 protease is required for virulence and survival in the presence of azole drugs. Mol Microbiol 74:672–690. doi: 10.1111/j.1365-2958.2009.06895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Miyakoshi S, Uchiyama H, Someya T, Satoh T, Tabuchi T. 1987. Distribution of the methylcitric acid cycle and beta-oxidation pathway for propionate catabolism in fungi. Agric Biol Chem 51:2381–2387. doi: 10.1271/bbb1961.51.2381. [DOI] [Google Scholar]
- 155.Vanden Bossche H, Marichal P, Le Jeune L, Coene MC, Gorrens J, Cools W. 1993. Effects of itraconazole on cytochrome P-450-dependent sterol 14 alpha-demethylation and reduction of 3-ketosteroids in Cryptococcus neoformans. Antimicrob Agents Chemother 37:2101–2105. doi: 10.1128/AAC.37.10.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Niehaus WG, Richardson SB, Wolz RL. 1996. Slow-binding inhibition of 6-phosphogluconate dehydrogenase by zinc ion. Arch Biochem Biophys 333:333–337. doi: 10.1006/abbi.1996.0399. [DOI] [PubMed] [Google Scholar]
- 157.Niehaus WG, White RH, Richardson SB, Bourne A, Ray WK. 1995. Polyethylene sulfonate: a tight-binding inhibitor of 6-phosphogluconate dehydrogenase of Cryptococcus neoformans. Arch Biochem Biophys 324:325–330. doi: 10.1006/abbi.1995.0045. [DOI] [PubMed] [Google Scholar]
- 158.Thaker TM, Tanabe M, Fowler ML, Preininger AM, Ingram-Smith C, Smith KS, Iverson TM. 2013. Crystal structures of acetate kinases from the eukaryotic pathogens Entamoeba histolytica and Cryptococcus neoformans. J Struct Biol 181:185–189. doi: 10.1016/j.jsb.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Missall TA, Pusateri ME, Donlin MJ, Chambers KT, Corbett JA, Lodge JK. 2006. Posttranslational, translational, and transcriptional responses to nitric oxide stress in Cryptococcus neoformans: implications for virulence. Eukaryot Cell 5:518–529. doi: 10.1128/EC.5.3.518-529.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Alspaugh JA, Pukkila-Worley R, Harashima T, Cavallo LM, Funnell D, Cox GM, Perfect JR, Kronstad JW, Heitman J. 2002. Adenylyl cyclase functions downstream of the G alpha protein Gpa1 and controls mating and pathogenicity of Cryptococcus neoformans. Eukaryot Cell 1:75–84. doi: 10.1128/EC.1.1.75-84.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Akhter S, McDade HC, Gorlach JM, Heinrich G, Cox GM, Perfect JR. 2003. Role of alternative oxidase gene in pathogenesis of Cryptococcus neoformans. Infect Immun 71:5794–5802. doi: 10.1128/IAI.71.10.5794-5802.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bahn YS, Cox GM, Perfect JR, Heitman J. 2005. Carbonic anhydrase and CO2 sensing during Cryptococcus neoformans growth, differentiation, and virulence. Curr Biol 15:2013–2020. doi: 10.1016/j.cub.2005.09.047. [DOI] [PubMed] [Google Scholar]
- 163.Mogensen EG, Janbon G, Chaloupka J, Steegborn C, Fu MS, Moyrand F, Klengel T, Pearson DS, Geeves MA, Buck J, Levin LR, Muhlschlegel FA. 2006. Cryptococcus neoformans senses CO2 through the carbonic anhydrase Can2 and the adenylyl cyclase Cac1. Eukaryot Cell 5:103–111. doi: 10.1128/EC.5.1.103-111.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang YN, Liu TB, Patel S, Jiang LH, Xue CY. 2011. The casein kinase I protein Cck1 regulates multiple signaling pathways and is essential for cell integrity and fungal virulence in Cryptococcus neoformans. Eukaryot Cell 10:1455–1464. doi: 10.1128/EC.05207-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Giles SS, Perfect JR, Cox GM. 2005. Cytochrome c peroxidase contributes to the antioxidant defense of Cryptococcus neoformans. Fungal Genet Biol 42:20–29. doi: 10.1016/j.fgb.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 166.Biondo C, Beninati C, Delfino D, Oggioni M, Mancuso G, Midiri A, Bombaci M, Tomaselli G, Teti G. 2002. Identification and cloning of a cryptococcal deacetylase that produces protective immune responses. Infect Immun 70:2383–2391. doi: 10.1128/IAI.70.5.2383-2391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kelleher DJ, Banerjee S, Cura AJ, Samuelson J, Gilmore R. 2007. Dolichol-linked oligosaccharide selection by the oligosaccharyltransferase in protist and fungal organisms. J Cell Biol 177:29–37. doi: 10.1083/jcb.200611079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Idnurm A, Heitman J. 2010. Ferrochelatase is a conserved downstream target of the blue light-sensing white collar complex in fungi. Microbiology 156:2393–2407. doi: 10.1099/mic.0.039222-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hu GG, Kronstad JW. 2010. A putative P-type ATPase, Apt1, is involved in stress tolerance and virulence in Cryptococcus neoformans. Eukaryot Cell 9:74–83. doi: 10.1128/EC.00289-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rizzo J, Oliveira DL, Joffe LS, Hu G, Gazos-Lopes F, Fonseca FL, Almeida IC, Frases S, Kronstad JW, Rodrigues ML. 2014. Role of the Apt1 protein in polysaccharide secretion by Cryptococcus neoformans. Eukaryot Cell 13:715–726. doi: 10.1128/EC.00273-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Niehaus WG, Mallett TC. 1994. Purification and characterization of glucose-6-phosphate dehydrogenase from Cryptococcus neoformans identification as “nothing dehydrogenase”. Arch Biochem Biophys 313:304–309. [DOI] [PubMed] [Google Scholar]
- 172.Safrin RE, Lancaster LA, Davis CE, Braude AI. 1986. Differentiation of Cryptococcus neoformans serotypes by isoenzyme electrophoresis. Am J Clin Pathol 86:204–208. [DOI] [PubMed] [Google Scholar]
- 173.Missall TA, Cherry-Harris JF, Lodge JK. 2005. Two glutathione peroxidases in the fungal pathogen Cryptococcus neoformans are expressed in the presence of specific substrates. Microbiology 151:2573–2581. doi: 10.1099/mic.0.28132-0. [DOI] [PubMed] [Google Scholar]
- 174.Kingsbury JM, McCusker JH. 2008. Threonine biosynthetic genes are essential in Cryptococcus neoformans. Microbiology 154:2767–2775. doi: 10.1099/mic.0.2008/019729-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nazi I, Scott A, Sham A, Rossi L, Williamson PR, Kronstad JW, Wright GD. 2007. Role of homoserine transacetylase as a new target for antifungal agents. Antimicrob Agents Chemother 51:1731–1736. doi: 10.1128/AAC.01400-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Jong A, Wu CH, Chen HM, Luo F, Kwon-Chung KJ, Chang YC, LaMunyon CW, Plaas A, Huang SH. 2007. Identification and characterization of CPS1 as a hyaluronic acid synthase contributing to the pathogenesis of Cryptococcus neoformans infection. Eukaryot Cell 6:1486–1496. doi: 10.1128/EC.00120-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Parker AR, Moore TD, Edman JC, Schwab JM, Davisson VJ. 1994. Cloning, sequence analysis and expression of the gene encoding imidazole glycerol phosphate dehydratase in Cryptococcus neoformans. Gene 145:135–138. doi: 10.1016/0378-1119(94)90336-0. [DOI] [PubMed] [Google Scholar]
- 178.Morrow CA, Stamp A, Valkov E, Kobe B, Fraser JA. 2010. Crystallization and preliminary X-ray analysis of mycophenolic acid-resistant and mycophenolic acid-sensitive forms of IMP dehydrogenase from the human fungal pathogen Cryptococcus. Acta Crystallogr Sect F Struct Biol Cryst Commun 66:1104–1107. doi: 10.1107/S1744309110031659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Heung LJ, Luberto C, Plowden A, Hannun YA, Del Poeta M. 2004. The sphingolipid pathway regulates Pkc1 through the formation of diacylglycerol in Cryptococcus neoformans. J Biol Chem 279:21144–21153. doi: 10.1074/jbc.M312995200. [DOI] [PubMed] [Google Scholar]
- 180.Cheon SA, Jung KW, Chen YL, Heitman J, Bahn YS, Kang HA. 2011. Unique evolution of the UPR pathway with a novel bZIP transcription factor, Hxl1, for controlling pathogenicity of Cryptococcus neoformans. PLoS Pathog 7:e1002177. doi: 10.1371/journal.ppat.1002177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rude TH, Toffaletti DL, Cox GM, Perfect JR. 2002. Relationship of the glyoxylate pathway to the pathogenesis of Cryptococcus neoformans. Infect Immun 70:5684–5694. doi: 10.1128/IAI.70.10.5684-5694.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mahmoud YA, el Souod SM, Niehaus WG. 1995. Purification and characterization of malate dehydrogenase from Cryptococcus neoformans. Arch Biochem Biophys 322:69–75. doi: 10.1006/abbi.1995.1437. [DOI] [PubMed] [Google Scholar]
- 183.Perfect JR, Rude TH, Wong B, Flynn T, Chaturvedi V, Niehaus W. 1996. Identification of a Cryptococcus neoformans gene that directs expression of the cryptic Saccharomyces cerevisiae mannitol dehydrogenase gene. J Bacteriol 178:5257–5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Suvarna K, Bartiss A, Wong B. 2000. Mannitol-1-phosphate dehydrogenase from Cryptococcus neoformans is a zinc-containing long-chain alcohol/polyol dehydrogenase. Microbiology 146:2705–2713. doi: 10.1099/00221287-146-10-2705. [DOI] [PubMed] [Google Scholar]
- 185.Olson GM, Fox DS, Wang P, Alspaugh JA, Buchanan KL. 2007. Role of protein O-mannosyltransferase Pmt4 in the morphogenesis and virulence of Cryptococcus neoformans. Eukaryot Cell 6:222–234. doi: 10.1128/EC.00182-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hicks JK, Bahn YS, Heitman J. 2005. Pde1 phosphodiesterase modulates cyclic AMP levels through a protein kinase A-mediated negative feedback loop in Cryptococcus neoformans. Eukaryot Cell 4:1971–1981. doi: 10.1128/EC.4.12.1971-1981.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hast MA, Nichols CB, Armstrong SM, Kelly SM, Hellinga HW, Alspaugh JA, Beese LS. 2011. Structures of Cryptococcus neoformans protein farnesyltransferase reveal strategies for developing inhibitors that target fungal pathogens. J Biol Chem 286:35149–35162. doi: 10.1074/jbc.M111.250506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gerik KJ, Donlin MJ, Soto CE, Banks AM, Banks IR, Maligie MA, Selitrennikoff CP, Lodge JK. 2005. Cell wall integrity is dependent on the PKC1 signal transduction pathway in Cryptococcus neoformans. Mol Microbiol 58:393–408. doi: 10.1111/j.1365-2958.2005.04843.x. [DOI] [PubMed] [Google Scholar]
- 189.Jin LH, Kryukov K, Suzuki Y, Imanishi T, Ikeo K, Gojobori T. 2009. The evolutionary study of small RNA-directed gene silencing pathways by investigating RNase III enzymes. Gene 435:1–8. doi: 10.1016/j.gene.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 190.Kingsbury JM, Yang Z, Ganous TM, Cox GM, McCusker JH. 2004. Novel chimeric spermidine synthase-saccharopine dehydrogenase gene (SPE3-LYS9) in the human pathogen Cryptococcus neoformans. Eukaryot Cell 3:752–763. doi: 10.1128/EC.3.3.752-763.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ternes P, Sperling P, Albrecht S, Franke S, Cregg JM, Warnecke D, Heinz E. 2006. Identification of fungal sphingolipid C9-methyltransferases by phylogenetic profiling. J Biol Chem 281:5582–5592. [DOI] [PubMed] [Google Scholar]
- 192.Zhu J, Lu J, Zhou Y, Li Y, Cheng J, Zheng C. 2006. Design, synthesis, and antifungal activities in vitro of novel tetrahydroisoquinoline compounds based on the structure of lanosterol 14alpha-demethylase (CYP51) of fungi. Bioorg Med Chem Lett 16:5285–5289. doi: 10.1016/j.bmcl.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 193.Nes WD, Zhou W, Ganapathy K, Liu J, Vatsyayan R, Chamala S, Hernandez K, Miranda M. 2009. Sterol 24-C-methyltransferase: an enzymatic target for the disruption of ergosterol biosynthesis and homeostasis in Cryptococcus neoformans. Arch Biochem Biophys 481:210–218. doi: 10.1016/j.abb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 194.Missall TA, Pusateri ME, Lodge JK. 2004. Thiol peroxidase is critical for virulence and resistance to nitric oxide and peroxide in the fungal pathogen, Cryptococcus neoformans. Mol Microbiol 51:1447–1458. doi: 10.1111/j.1365-2958.2004.03921.x. [DOI] [PubMed] [Google Scholar]
- 195.Missall TA, Lodge JK. 2005. Thioredoxin reductase is essential for viability in the fungal pathogen Cryptococcus neoformans. Eukaryot Cell 4:487–489. doi: 10.1128/EC.4.2.487-489.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Livi LL, Edman U, Schneider GP, Greene PJ, Santi DV. 1994. Cloning, expression and characterization of thymidylate synthase from Cryptococcus neoformans. Gene 150:221–226. doi: 10.1016/0378-1119(94)90430-8. [DOI] [PubMed] [Google Scholar]
- 197.Petzold EW, Himmelreich U, Mylonakis E, Rude T, Toffaletti D, Cox GM, Miller JL, Perfect JR. 2006. Characterization and regulation of the trehalose synthesis pathway and its importance in the pathogenicity of Cryptococcus neoformans. Infect Immun 74:5877–5887. doi: 10.1128/IAI.00624-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Beverley SM, Owens KL, Showalter M, Griffith CL, Doering TL, Jones VC, McNeil MR. 2005. Eukaryotic UDP-galactopyranose mutase (GLF gene) in microbial and metazoal pathogens. Eukaryot Cell 4:1147–1154. doi: 10.1128/EC.4.6.1147-1154.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Jacobson ES. 1987. Cryptococcal UDP-glucose dehydrogenase: enzymic control of capsular biosynthesis. J Med Vet Mycol 25:131–135. doi: 10.1080/02681218780000201. [DOI] [PubMed] [Google Scholar]
- 200.Reilly MC, Levery SB, Castle SA, Klutts JS, Doering TL. 2009. A novel xylosylphosphotransferase activity discovered in Cryptococcus neoformans. J Biol Chem 284:36118–36127. doi: 10.1074/jbc.M109.056226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Block ER, Jennings AE, Bennett JE. 1973. 5-Fluorocytosine resistance in Cryptococcus neoformans. Antimicrob Agents Chemother 3:649–656. doi: 10.1128/AAC.3.6.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Schwarz P, Janbon G, Dromer F, Lortholary O, Dannaoui E. 2007. Combination of amphotericin B with flucytosine is active in vitro against flucytosine-resistant isolates of Cryptococcus neoformans. Antimicrob Agents Chemother 51:383–385. doi: 10.1128/AAC.00446-06. [DOI] [PMC free article] [PubMed] [Google Scholar]