Abstract
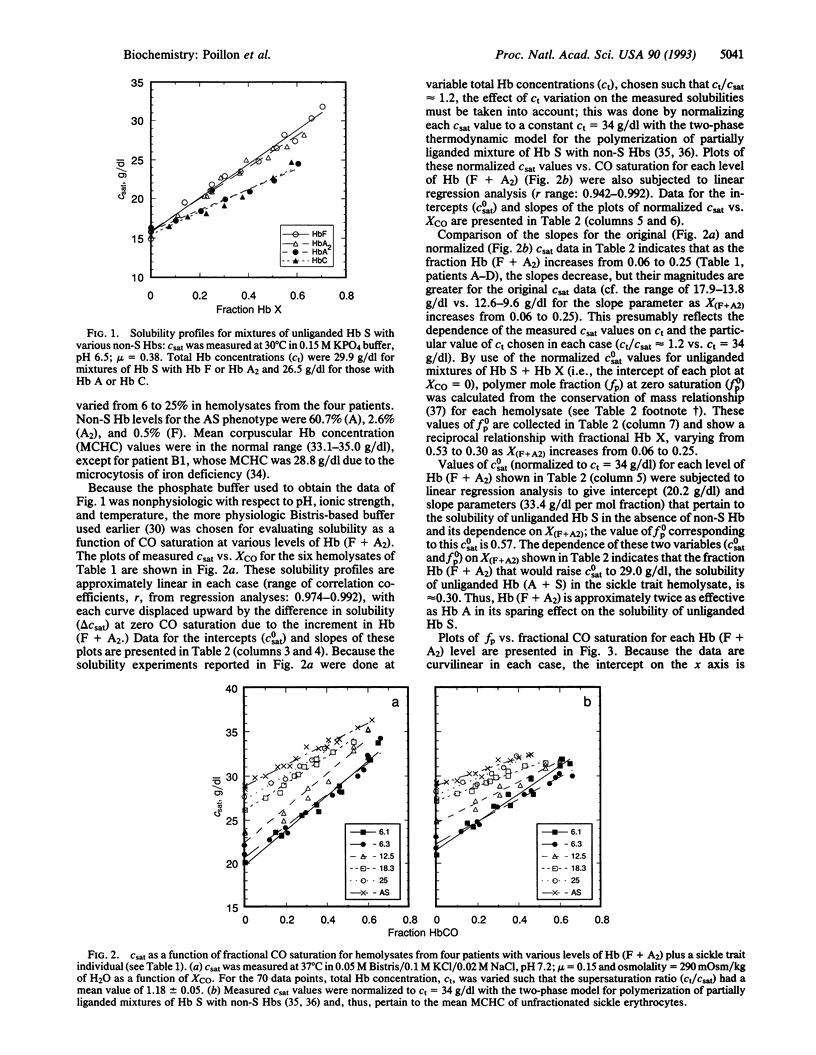
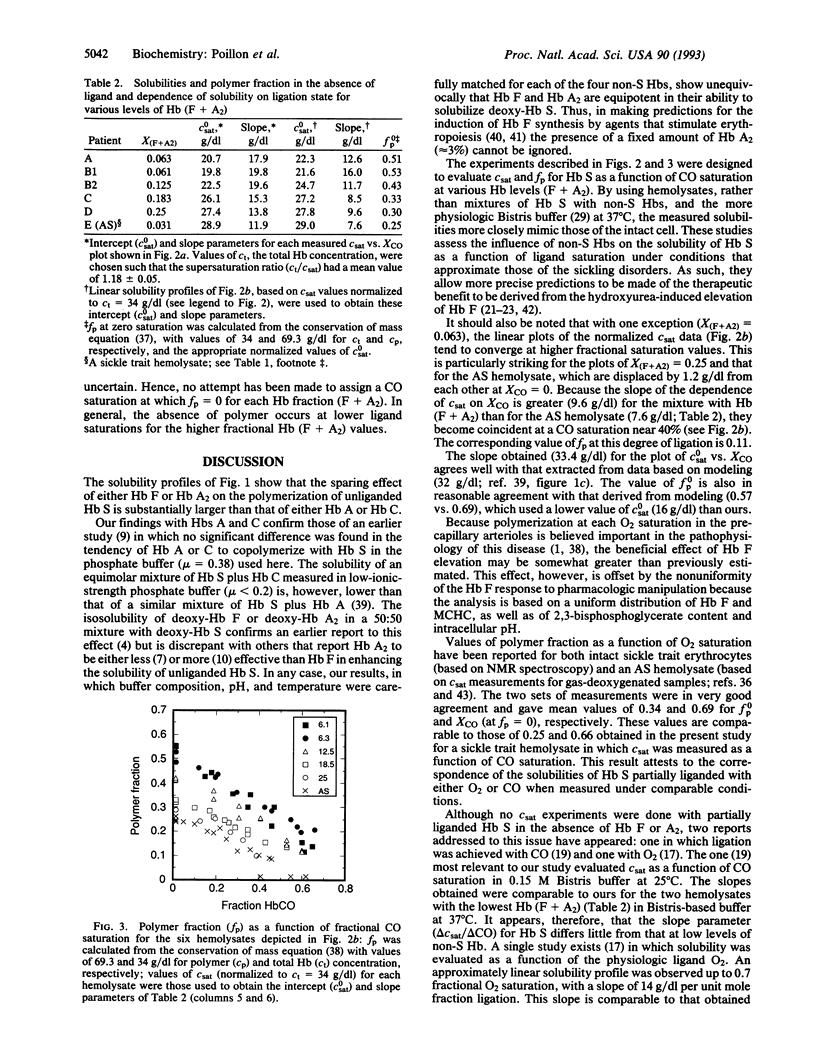
Recent interest in therapies for sickle cell anemia based on elevating fetal Hb has made accurate estimates of the sparing effect of fetal Hb (Hb F) and other non-sickle Hbs on sickle Hb (Hb S) polymerization essential. We have developed a technique, using HbCO as surrogate for HbO2, that enables us to assess the solubility of Hb S as a function of ligand saturation under conditions that mimic those of the sickling disorders. Equimolar mixtures of unliganded Hb S with Hb F or normal Hb A2 were isosoluble. Solubilities for equimolar mixtures with normal (Hb A) or abnormal (Hb C) Hbs were also identical but were lower than in the prior case. Thus, the sparing effect of both Hb F and Hb A2 should be considered in therapeutic strategies designed to modify Hb S polymerization. Hemolysates, stripped of 2,3-bisphosphoglycerate, from sickle cell disease patients with Hb (F + A2) levels varying from 6 to 25%, as well as from a sickle trait individual, were used to evaluate equilibrium solubility as a function of ligand saturation over the range of pathophysiologic interest (25-70%). Our results show that the sparing effect of Hb (F + A2) increases relative to that of Hb A as ligand saturation increases, and that in the absence of ligand, approximately 30% Hb (F + A2) is essentially isosoluble with the 60% Hb A of sickle trait. Although detailed knowledge of expected therapeutic benefits is confounded by the heterogeneity of Hb F distribution and other variables, these data should provide a framework for estimating likely clinical benefit from pharmacologic efforts to modulate globin gene expression.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BETKE K., MARTI H. R., SCHLICHT I. Estimation of small percentages of foetal haemoglobin. Nature. 1959 Dec 12;184(Suppl 24):1877–1878. doi: 10.1038/1841877a0. [DOI] [PubMed] [Google Scholar]
- Benesch R. E., Edalji R., Benesch R., Kwong S. Solubilization of hemoglobin S by other hemoglobins. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5130–5134. doi: 10.1073/pnas.77.9.5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertles J. F., Rabinowitz R., Döbler J. Hemoglobin interaction: modification of solid phase composition in the sickling phenomenon. Science. 1970 Jul 24;169(3943):375–377. doi: 10.1126/science.169.3943.375. [DOI] [PubMed] [Google Scholar]
- Bookchin R. M., Balazs T. Ionic strength dependence of the polymer solubilities of deoxyhemoglobin S + C and S + A mixtures. Blood. 1986 Apr;67(4):887–892. [PubMed] [Google Scholar]
- Bookchin R. M., Nagel R. L. Ligand-induced conformational dependence of hemoglobin in sickling interactios. J Mol Biol. 1971 Sep 14;60(2):263–270. doi: 10.1016/0022-2836(71)90292-0. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Noguchi C. T., Hofrichter J., Schechter G. P., Schechter A. N., Eaton W. A. Molecular and cellular pathogenesis of hemoglobin SC disease. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7527–7531. doi: 10.1073/pnas.79.23.7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro O., Hardy K. P., Winter W. P., Hornblower M., Meryman H. T. Freeze preservation of sickle erythrocytes. Am J Hematol. 1981;10(3):297–304. doi: 10.1002/ajh.2830100309. [DOI] [PubMed] [Google Scholar]
- Chang H., Ewert S. M., Bookchin R. M., Nagel R. L. Comparative evaluation of fifteen anti-sickling agents. Blood. 1983 Apr;61(4):693–704. [PubMed] [Google Scholar]
- Charache S., Dover G. J., Moore R. D., Eckert S., Ballas S. K., Koshy M., Milner P. F., Orringer E. P., Phillips G., Jr, Platt O. S. Hydroxyurea: effects on hemoglobin F production in patients with sickle cell anemia. Blood. 1992 May 15;79(10):2555–2565. [PubMed] [Google Scholar]
- Cheetham R. C., Huehns E. R., Rosemeyer M. A. Participation of haemoglobins A, F, A2 and C in polymerisation of haemoglobin S. J Mol Biol. 1979 Mar 25;129(1):45–61. doi: 10.1016/0022-2836(79)90058-5. [DOI] [PubMed] [Google Scholar]
- Chung L. L., Magdoff-Fairchild B. Extent of polymerization in partially liganded sickle hemoglobin. Arch Biochem Biophys. 1978 Aug;189(2):535–539. doi: 10.1016/0003-9861(78)90244-8. [DOI] [PubMed] [Google Scholar]
- Eaton W. A., Hofrichter J. Sickle cell hemoglobin polymerization. Adv Protein Chem. 1990;40:63–279. doi: 10.1016/s0065-3233(08)60287-9. [DOI] [PubMed] [Google Scholar]
- Franklin I. M., Rosemeyer M. A., Huehns E. R. Sickle cell disease: the proportion of liganded haemoglobin needed to prevent crises. Br J Haematol. 1983 Aug;54(4):579–587. doi: 10.1111/j.1365-2141.1983.tb02137.x. [DOI] [PubMed] [Google Scholar]
- Goldberg M. A., Brugnara C., Dover G. J., Schapira L., Charache S., Bunn H. F. Treatment of sickle cell anemia with hydroxyurea and erythropoietin. N Engl J Med. 1990 Aug 9;323(6):366–372. doi: 10.1056/NEJM199008093230602. [DOI] [PubMed] [Google Scholar]
- Goldberg M. A., Husson M. A., Bunn H. F. Participation of hemoglobins A and F in polymerization of sickle hemoglobin. J Biol Chem. 1977 May 25;252(10):3414–3421. [PubMed] [Google Scholar]
- Hofrichter J. Ligand binding and the gelation of sickle cell hemoglobin. J Mol Biol. 1979 Mar 5;128(3):335–369. doi: 10.1016/0022-2836(79)90092-5. [DOI] [PubMed] [Google Scholar]
- Hofrichter J., Ross P. D., Eaton W. A. Supersaturation in sickle cell hemoglobin solutions. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3035–3039. doi: 10.1073/pnas.73.9.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman T. H., Schroeder W. A., Brodie A. N., Mayson S. M., Jakway J. Microchromatography of hemoglobins. II. A simplified procedure for the determination of hemoglobin A2. J Lab Clin Med. 1975 Oct;86(4):700–702. [PubMed] [Google Scholar]
- Klug P. P., Lessin L. S., Radice P. Rheological aspects of sickle cell disease. Arch Intern Med. 1974 Apr;133(4):577–590. [PubMed] [Google Scholar]
- Magdoff-Fairchild B., Poillon W. N., Li T., Bertles J. F. Thermodynamic studies of polymerization of deoxygenated sickle cell hemoglobin. Proc Natl Acad Sci U S A. 1976 Apr;73(4):990–994. doi: 10.1073/pnas.73.4.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel R. L., Bookchin R. M., Johnson J., Labie D., Wajcman H., Isaac-Sodeye W. A., Honig G. R., Schilirò G., Crookston J. H., Matsutomo K. Structural bases of the inhibitory effects of hemoglobin F and hemoglobin A2 on the polymerization of hemoglobin S. Proc Natl Acad Sci U S A. 1979 Feb;76(2):670–672. doi: 10.1073/pnas.76.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi C. T., Rodgers G. P., Schechter A. N. Intracellular polymerization. Disease severity and therapeutic predictions. Ann N Y Acad Sci. 1989;565:75–82. doi: 10.1111/j.1749-6632.1989.tb24152.x. [DOI] [PubMed] [Google Scholar]
- Noguchi C. T., Rodgers G. P., Serjeant G., Schechter A. N. Levels of fetal hemoglobin necessary for treatment of sickle cell disease. N Engl J Med. 1988 Jan 14;318(2):96–99. doi: 10.1056/NEJM198801143180207. [DOI] [PubMed] [Google Scholar]
- Noguchi C. T., Schechter A. N. The intracellular polymerization of sickle hemoglobin and its relevance to sickle cell disease. Blood. 1981 Dec;58(6):1057–1068. [PubMed] [Google Scholar]
- Noguchi C. T., Torchia D. A., Schechter A. N. Intracellular polymerization of sickle hemoglobin. Effects of cell heterogeneity. J Clin Invest. 1983 Sep;72(3):846–852. doi: 10.1172/JCI111055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi C. T., Torchia D. A., Schechter A. N. Polymerization of hemoglobin in sickle trait erythrocytes and lysates. J Biol Chem. 1981 May 10;256(9):4168–4171. [PubMed] [Google Scholar]
- Noguchi S., Kubota Y., Shuin T., Miura T., Moriyama M., Sakuramoto T., Oshima H. [Intravesical instillation therapy of aclacinomycin-A (ACM) for superficial bladder tumor]. Hinyokika Kiyo. 1984 Sep;30(9):1153–1158. [PubMed] [Google Scholar]
- Perrine S. P., Ginder G. D., Faller D. V., Dover G. H., Ikuta T., Witkowska H. E., Cai S. P., Vichinsky E. P., Olivieri N. F. A short-term trial of butyrate to stimulate fetal-globin-gene expression in the beta-globin disorders. N Engl J Med. 1993 Jan 14;328(2):81–86. doi: 10.1056/NEJM199301143280202. [DOI] [PubMed] [Google Scholar]
- Poillon W. N., Bertles J. F. Deoxygenated sickle hemoglobin. Effects of lyotropic salts on its solubility. J Biol Chem. 1979 May 10;254(9):3462–3467. [PubMed] [Google Scholar]
- Poillon W. N., Kim B. C. 2,3-Diphosphoglycerate and intracellular pH as interdependent determinants of the physiologic solubility of deoxyhemoglobin S. Blood. 1990 Sep 1;76(5):1028–1036. [PubMed] [Google Scholar]
- Poillon W. N. Noncovalent inhibitors of sickle hemoglobin gelation: effects of aliphatic alcohols, amides, and ureas. Biochemistry. 1980 Jul 8;19(14):3194–3199. doi: 10.1021/bi00555a014. [DOI] [PubMed] [Google Scholar]
- Poillon W. N. Noncovalent inhibitors of sickle hemoglobin gelation: effects of aryl-substituted alanines. Biochemistry. 1982 Mar 16;21(6):1400–1406. doi: 10.1021/bi00535a046. [DOI] [PubMed] [Google Scholar]
- Rodgers G. P., Dover G. J., Noguchi C. T., Schechter A. N., Nienhuis A. W. Hematologic responses of patients with sickle cell disease to treatment with hydroxyurea. N Engl J Med. 1990 Apr 12;322(15):1037–1045. doi: 10.1056/NEJM199004123221504. [DOI] [PubMed] [Google Scholar]
- Rodgers G. P., Dover G. J., Uyesaka N., Noguchi C. T., Schechter A. N., Nienhuis A. W. Augmentation by erythropoietin of the fetal-hemoglobin response to hydroxyurea in sickle cell disease. N Engl J Med. 1993 Jan 14;328(2):73–80. doi: 10.1056/NEJM199301143280201. [DOI] [PubMed] [Google Scholar]
- Ross P. D., Hofrichter J., Eaton W. A. Thermodynamics of gelation of sickle cell deoxyhemoglobin. J Mol Biol. 1977 Sep 15;115(2):111–134. doi: 10.1016/0022-2836(77)90093-6. [DOI] [PubMed] [Google Scholar]
- Salvati A. M., Tentori L. Determination of aberrant hemoglobin derivatives in human blood. Methods Enzymol. 1981;76:715–731. doi: 10.1016/0076-6879(81)76153-6. [DOI] [PubMed] [Google Scholar]
- Sunshine H. R., Hofrichter J., Eaton W. A. Gelation of sickle cell hemoglobin in mixtures with normal adult and fetal hemoglobins. J Mol Biol. 1979 Oct 9;133(4):435–467. doi: 10.1016/0022-2836(79)90402-9. [DOI] [PubMed] [Google Scholar]
- Sunshine H. R., Hofrichter J., Ferrone F. A., Eaton W. A. Oxygen binding by sickle cell hemoglobin polymers. J Mol Biol. 1982 Jun 25;158(2):251–273. doi: 10.1016/0022-2836(82)90432-6. [DOI] [PubMed] [Google Scholar]



