Abstract
Rice is a typical silicon-accumulating plant. Silicon (Si), deposited as phytoliths during plant growth, has been shown to occlude organic carbon, which may prove to have significant effects on the biogeochemical sequestration of atmospheric CO2. This study evaluated the effects of silicate fertilization on plant Si uptake and carbon bio-sequestration in field trials on China’s paddy soils. The results showed (1) Increased Si concentrations in rice straw with increasing application rates of silicate fertilizer; (2) Strong positive correlations between phytolith contents and straw SiO2 contents and between phytolith contents and phytolith-occluded carbon (PhytOC) contents in rice straw; (3) Positive correlations between the phytolith production flux and either the above-ground net primary productivity (ANPP) or the PhytOC production rates; (4) Increased plant PhytOC storage with increasing application rates of silicate fertilizer. The average above-ground PhytOC production rates during China’s rice production are estimated at 0.94 × 106 tonnes CO2 yr−1 without silicate fertilizer additions. However, the potential exists to increase PhytOC levels to 1.16–2.17 × 106 tonnes CO2 yr−1 with silicate fertilizer additions. Therefore, providing silicate fertilizer during rice production may serve as an effective tool in improving atmospheric CO2 sequestration in global rice production areas.
Human activities and industrialization have led to increasingly higher levels of carbon dioxide (CO2) in the atmosphere, with the resultant negative effects on global climate systems1. Current estimates of CO2 emissions amounted to 36 billion tonnes for the calendar year 20132. These increasing concentrations of atmospheric CO2 have been implicated as major contributors to global climate change, hence, negatively impacting human and environmental health and safety3. Thus, sustainable methods for reducing and sequestering atmospheric CO2 are needed.
A promising biogeochemical means of reducing atmospheric CO2 is the occlusion of carbon within plant phytoliths, also known as silica phytoliths or plant opals. Silicon is stored in plants mainly in the form of phytoliths4. These silica phytoliths comprise noncrystalline silica minerals deposited within cells and cell walls of different plant organs when monosilicic acid [Si(OH)4], is taken up by plant roots and transported to the aboveground organs, where phytoliths form near evaporative surfaces by deposition and polymerization5,6. The silica phytoliths that are formed occlude some of the organic carbon that is extracted from atmospheric CO2 during photosynthesis, which is then deposited during plant growth7. When plant residues are returned to the soil, they gradually decompose releasing these phytoliths. However, Phytolith-occluded carbon (PhytOC) is relatively stable and can remain in the soil for long periods of time, being present in Tertiary and Late Cretaceous sediments7,8,9. This carbon fraction has been recognized as an important long-term terrestrial carbon (C) sink, sequestering about 1.5 billion tonnes of CO2 annually10.
Recently, phytolith-occluded carbon has been investigated in some plant species including grass11 and forest12 species, millet (Setaria italica)13, bamboo10, sugarcane (Saccharum sinensis)14 and rice (Oryza sativa)8. These previous trials have demonstrated the potential for long-term biogeochemical sequestration of atmospheric CO2 during plant phytolith production, with grass species’ phytoliths determined to be less degradable in soil, and over geological time, than phytoliths from leaves of forest species6,15.
Rice, a Si accumulating species, actively accumulates Si at tissue concentrations of 5% or more of net aboveground biomass16. In China, rice is a staple food crop, and China continues to lead the world in rice production, with outputs totaling 28% of the total global rice production in 201117. It is estimated that the rice growing area in China is 18.5% of the world’s total tillable land area. Therefore, due to the large production area, high above-ground net primary productivity (ANPP), and high Si accumulation, paddy rice cropping systems may play an important role, not only in terrestrial production of phytoliths8, but also in the sequestration of atmospheric CO2.
Plant phytoliths, returned to the soil via crop residue at the end of each season, contribute to the pools of phytoliths in the soil’s upper layers, becoming an important component of soil structural systems18; Si depletion is known to occur on traditional paddy rice soils due to a continuous monoculture of high-yielding varieties under intensive cultivation practices19. Desplanques et al.20 estimate that 270 kg ha−1 of biogenic Si is removed each year from rice cultivation and that without additional Si fertilizer inputs, it would take only five years to exhaust the soils’ dissolved Si supplies from continued rice production in Camargue, France.
Destruction of global soils has become an important issue with global soil health issues gaining international attention, and 2015 being named the International Year of Soils by the 68th United Nations General Assembly21. Agricultural soils are largely composed of silicate minerals, and many soils contain adequate amounts of total Si, but are naturally low in soluble (plant-available) Si. Crop removal of Si can exceed the slow weathering processes of silicate clay minerals resulting in reductions in the Si supplying capacity of soils. Additionally, crop management practices of applications of acidifying fertilizer materials may increase the levels of soluble silicon in soils, but the removal of silicon from soils without fertilizer replacements has been implicated in the destruction of our once productive global agricultural soils22,23,24. Therefore, soluble Si supplies may be a limiting factor for sustainable rice production in some regions24,25. Silicon amendments have been shown to decrease lodging, reduce stress, and increase yields during paddy rice production26,27. Therefore, application of Si fertilizers to degraded paddy soils is now recognized as a best management practice in increasing and sustaining rice yields, in some Southeast Asian areas26.
Although an increasing body of evidence has shown the long-term C sequestration potential of silica phytoliths within soils1,8,10,11,12,13,14,28, the majority of these trials have been conducted in native ecosystems, and not under high production agriculture. Huang et al. provided the first field evidence that storage of PhytOC, within intensively cultivated soils, could be increased by heavy mulching of bamboo and rice residues, post-phytolith production29. However, the aim of the current research was to determine if phytolith production in-planta could be increased from the additions of supplemental silicate fertilizers, thus affecting long-term storage of C during biomass accumulation in intensive rice crop production. Therefore, research is still lacking on the effects of silicate fertilization on phytolith formation and biogeochemical sequestration under China’s rice paddy production systems.
To evaluate the C occlusion within phytoliths during rice cropping as affected by silicate fertilizer additions, in this study, we took rice straw samples at five sites receiving silicate fertilizer additions in southern China at rice grain maturity in 2012. Using the phytolith content-biogenic silica content transfer function obtained from the samples, and by applying grassland11 formulas for phytolith production flux and above-ground net primary productivity (ANPP), we arrived at an estimated value for the production of phytoliths and PhytOC in China’s paddy field ecosystems.
Results
Soil available silicon contents
Post-harvest soil Si concentrations at the five sites tended to increase with increasing silicate fertilizer application rates (Fig. 1a). At Longhui (LH) and Lengshuitan (LST), soils receiving the highest silicate fertilizer application rates (Si1500 and Si3000) also had highest post-harvest Si levels. At Xiangyin (XY) and Qionghai (QH), soil Si concentrations tended to increase with increasing silicate fertilizer application rates. At Danzhou (DZ), positive effects of silicate fertilizer were seen at all application rates above 300 kg ha−1.
Figure 1. Effect of silicate fertilizer application rate on post-harvest soil available Si contents (a) and silica contents in rice straw (b) at the five sites.
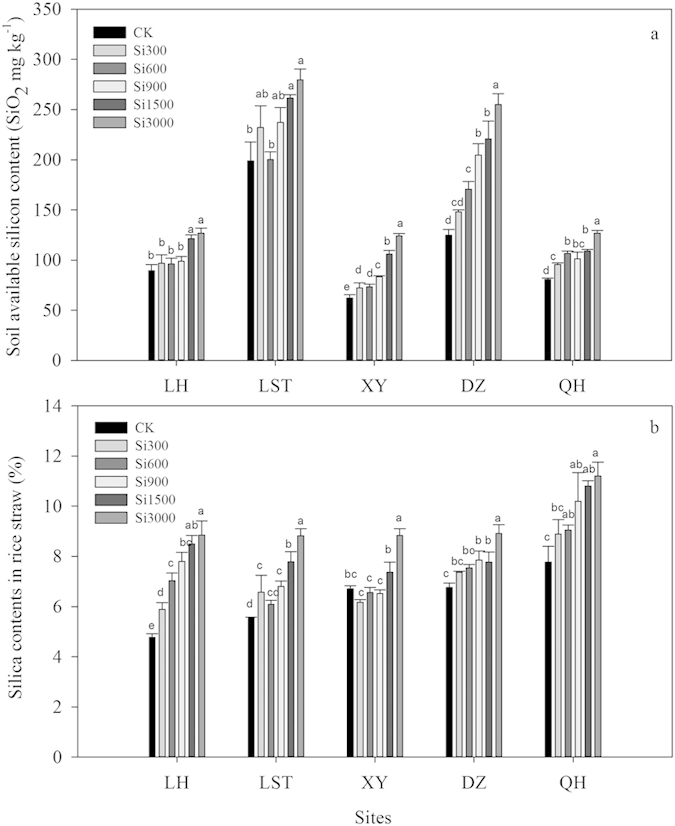
Data are means of three replicates. Mean values followed by different letters at the same site differ significantly at the (P < 0.05) level of significance.
Silica contents in rice straw
Silica concentrations in rice straw (leaf and stem) increased with increasing silicate fertilizer application rates (Fig. 1b). At LH, the silica concentrations of rice straw were 23%, 47%, 63%, 78% and 85% higher for the Si300, Si600, Si900, Si1500 and Si3000 treatments, consecutively, when compared with the non-treated control (CK), and 19%, 10%, 23%, 41% and 59% at LST, consecutively. At XY, DZ and QH sites, straw silica concentrations were highest at the Si3000 application rate. At DZ and QH, rice straw silica concentrations were higher than those of the controls at all rates above 600 kg ha−1 and 300 kg ha−1, respectively.
Phytolith and PhytOC contents in rice straw
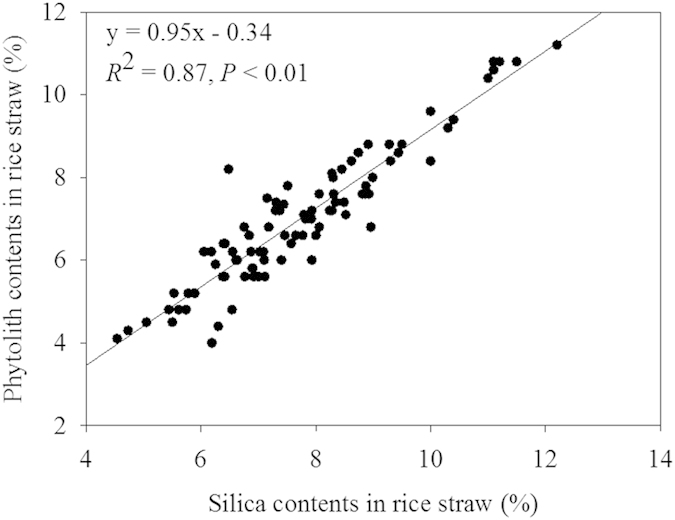
The percent phytolith contents in rice straw at the five sites ranged from 4.30% to 10.40% (Table 1), with the lowest levels recorded for the LH CK plots, and the highest levels recorded for the QH Si3000 plots. A trend was seen in increasing phytolith concentrations with increasing rates of silicate fertilizer applications (Table 1). There was also a significant correlation between the silica contents in rice straw and the phytolith contents in rice straw (Fig. 2).
Table 1. Experimental sites, Percentage of phytolith contents, percentage of carbon contents in phytolith and percentage of PhytOC content in straw.
| Site | Treatment | Percentage of phytolith contents (%) | Percent carbon contents in phytoliths (%) | Percent PhytOC in straw (%) |
|---|---|---|---|---|
| LH | CK | 4.30 ± 0.12d | 4.03 ± 0.40a | 0.17 ± 0.02b |
| Si300 | 4.97 ± 0.33cd | 3.33 ± 0.25ab | 0.17 ± 0.02b | |
| Si600 | 5.60 ± 0.46bc | 2.35 ± 0.19cd | 0.13 ± 0.01b | |
| Si900 | 6.73 ± 0.47ab | 2.11 ± 0.05d | 0.14 ± 0.01b | |
| Si1500 | 7.73 ± 0.47a | 2.14 ± 0.12d | 0.17 ± 0.02b | |
| Si3000 | 7.60 ± 0.40a | 3.06 ± 0.18bc | 0.23 ± 0.02a | |
| LST | CK | 4.93 ± 0.13cd | 3.09 ± 0.32ab | 0.15 ± 0.02c |
| Si300 | 5.47 ± 0.27c | 3.65 ± 0.13a | 0.20 ± 0.02b | |
| Si600 | 4.53 ± 0.35cd | 2.63 ± 0.19bc | 0.12 ± 0.02c | |
| Si900 | 5.60 ± 0.00c | 2.15 ± 0.04c | 0.12 ± 0.00c | |
| Si1500 | 6.73 ± 0.35b | 2.18 ± 0.13c | 0.15 ± 0.01c | |
| Si3000 | 8.20 ± 0.31a | 3.43 ± 0.06a | 0.28 ± 0.01a | |
| XY | CK | 6.53 ± 0.84b | 3.91 ± 0.27bc | 0.26 ± 0.05a |
| Si300 | 6.27 ± 0.07b | 4.59 ± 0.20a | 0.29 ± 0.02a | |
| Si600 | 6.00 ± 0.12b | 4.52 ± 0.14ab | 0.27 ± 0.01a | |
| Si900 | 6.30 ± 0.26b | 3.77 ± 0.27c | 0.24 ± 0.02a | |
| Si1500 | 6.27 ± 0.27b | 4.07 ± 0.09abc | 0.26 ± 0.02a | |
| Si3000 | 7.80 ± 0.31a | 3.97 ± 0.21abc | 0.31 ± 0.02a | |
| DZ | CK | 6.40 ± 0.12b | 2.11 ± 0.09e | 0.14 ± 0.00d |
| Si300 | 7.25 ± 0.05ab | 2.56 ± 0.04d | 0.19 ± 0.00c | |
| Si600 | 7.43 ± 0.20a | 2.97 ± 0.05c | 0.22 ± 0.01b | |
| Si900 | 7.33 ± 0.44ab | 3.96 ± 0.09a | 0.29 ± 0.01a | |
| Si1500 | 7.07 ± 0.26ab | 3.41 ± 0.14bc | 0.24 ± 0.00b | |
| Si3000 | 7.90 ± 0.59a | 3.04 ± 0.23bc | 0.24 ± 0.01b | |
| QH | CK | 6.93 ± 0.55b | 4.46 ± 0.10a | 0.31 ± 0.03b |
| Si300 | 8.53 ± 0.58ab | 4.47 ± 0.24a | 0.38 ± 0.01ab | |
| Si600 | 8.33 ± 0.37ab | 4.37 ± 0.16a | 0.37 ± 0.03ab | |
| Si900 | 9.60 ± 1.20a | 4.25 ± 0.54a | 0.42 ± 0.10ab | |
| Si1500 | 10.13 ± 0.37a | 4.38 ± 0.12a | 0.44 ± 0.01a | |
| Si3000 | 10.40 ± 0.61a | 4.41 ± 0.22a | 0.46 ± 0.01a |
Data are means of three replicates. Mean values followed by different letters in the same site are significantly different (P < 0.05).
Figure 2. Correlation between phytolith contents (%) and silica contents in rice straw (%) at the five sites tested (P < 0.01, n = 90).
The percentages of C contents in phytoliths ranged from 2.11% to 4.59% at the five sites tested and tended to decrease with increasing rice straw silica contents (Table 1). However, the PhytOC contents, based on percent straw dry weight, varied from 0.12% to 0.46% and tended to increase with increasing silicate fertilizer application rates (Table 1).
Estimated ANPP, phytolith production flux, phytolith production rate, PhytOC production flux, and PhytOC production rate
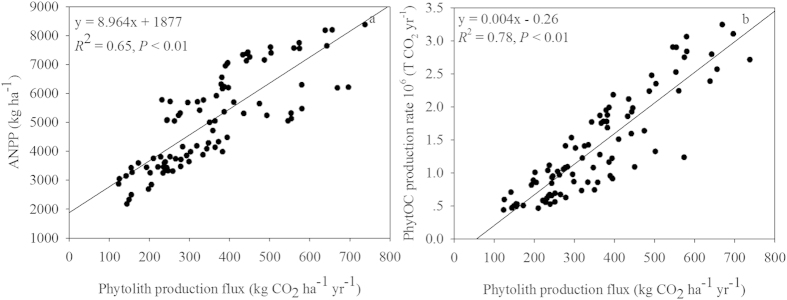
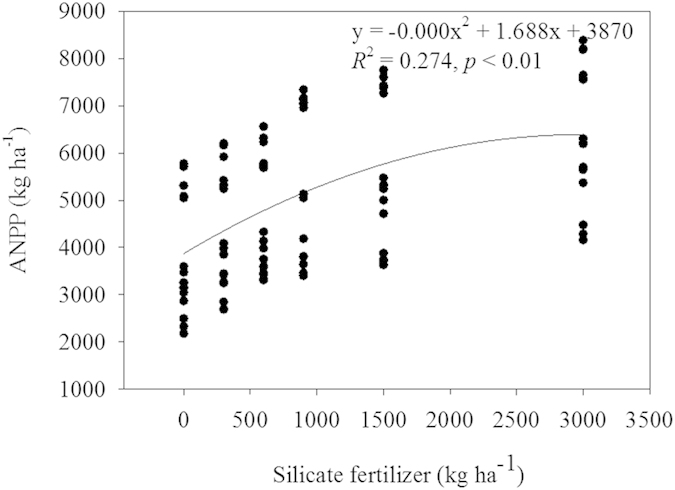
ANPP, phytolith production flux, phytolith production rate, PhytOC production flux and PhytOC production rate are shown in Table 2. ANPP at the five sites ranged from 2334.90 to 8256.27 kg ha−1 yr−1, with the lowest levels recorded for the DZ CK plots and the highest levels recorded for the LST Si3000 plots (Table 2). A trend was seen in increasing ANPP with increasing silicate fertilizer application rates (Fig. 3).
Table 2. The estimated ANPP, phytolith production flux, phytolith production rate, PhytOC production flux and PhytOC production rate per year in T of CO2.
| Site | Treatment | Straw dry weight (ANPP) (kg ha−1 yr−1)1 | Phytolith production flux (kg ha−1 yr−1)2 | Phytolith production rate (106 tonnes yr−1)3 | PhytOC production flux (kg CO2 ha−1 yr−1)4 | PhytOC production rate (106 tonnes CO2 yr−1)5 |
|---|---|---|---|---|---|---|
| LH | CK | 3021 ± 82e | 130 ± 6e | 3.91 ± 0.18e | 19.34 ± 2.58bc | 0.58 ± 0.08bc |
| Si300 | 3382 ± 55de | 168 ± 12de | 5.06 ± 0.37de | 20.75 ± 3.19bc | 0.62 ± 0.10bc | |
| Si600 | 3599 ± 86cd | 201 ± 15d | 6.06 ± 0.44de | 17.23 ± 1.13c | 0.52 ± 0.03c | |
| Si900 | 3933 ± 126cd | 266 ± 27c | 8.00 ± 0.81c | 20.50 ± 1.94bc | 0.62 ± 0.06bc | |
| Si1500 | 4531 ± 337b | 347 ± 7b | 10.45 ± 0.22b | 27.25 ± 1.27b | 0.82 ± 0.04b | |
| Si3000 | 5572 ± 103a | 424 ± 27a | 12.75 ± 0.79a | 47.74 ± 4.70a | 1.44 ± 0.14a | |
| LST | CK | 5069 ± 11f | 250 ± 6d | 7.53 ± 0.19d | 28.36 ± 3.26c | 0.85 ± 0.10c |
| Si300 | 5329 ± 51e | 292 ± 17d | 8.78 ± 0.51d | 39.20 ± 3.76b | 1.18 ± 0.11b | |
| Si600 | 5729 ± 27d | 260 ± 19d | 7.81 ± 0.57d | 25.29 ± 3.69c | 0.76 ± 0.11c | |
| Si900 | 7016 ± 32c | 393 ± 2c | 11.83 ± 0.05c | 30.91 ± 0.44bc | 0.93 ± 0.01bc | |
| Si1500 | 7540 ± 145b | 509 ± 36b | 15.31 ± 1.08b | 40.46 ± 2.27b | 1.22 ± 0.07b | |
| Si3000 | 8256 ± 63a | 677 ± 31a | 20.39 ± 0.92a | 85.00 ± 3.13a | 2.56 ± 0.09a | |
| XY | CK | 5597 ± 145e | 363 ± 36c | 10.94 ± 1.09c | 52.80 ± 9.01b | 1.59 ± 0.27b |
| Si300 | 6095 ± 89d | 382 ± 9c | 11.50 ± 0.26c | 64.38 ± 4.25b | 1.94 ± 0.13b | |
| Si600 | 6370 ± 98c | 382 ± 2c | 11.50 ± 0.07c | 63.36 ± 2.16b | 1.91 ± 0.06b | |
| Si900 | 7208 ± 65b | 454 ± 17b | 13.66 ± 0.50b | 63.01 ± 6.22b | 1.90 ± 0.19b | |
| Si1500 | 7405 ± 12ab | 464 ± 20b | 13.97 ± 0.59b | 69.34 ± 4.41ab | 2.09 ± 0.13ab | |
| Si3000 | 7592 ± 28a | 592 ± 25a | 17.83 ± 0.76a | 86.28 ± 5.90a | 2.60 ± 0.18a | |
| DZ | CK | 2335 ± 184e | 149 ± 3c | 4.49 ± 0.10c | 16.24 ± 0.29d | 0.49 ± 0.01d |
| Si300 | 2928 ± 68d | 212 ± 11b | 6.39 ± 0.33b | 30.82 ± 1.85b | 0.93 ± 0.06b | |
| Si600 | 3356 ± 320d | 249 ± 5b | 7.51 ± 0.14b | 31.20 ± 1.72b | 0.94 ± 0.05b | |
| Si900 | 3501 ± 307c | 257 ± 21b | 7.75 ± 0.62b | 24.22 ± 2.32c | 0.73 ± 0.07c | |
| Si1500 | 3694 ± 95b | 261 ± 11b | 7.86 ± 0.34b | 20.27 ± 1.42 cd | 0.61 ± 0.04 cd | |
| Si3000 | 4307 ± 44a | 341 ± 32a | 10.27 ± 0.97a | 37.58 ± 1.50d | 1.13 ± 0.05a | |
| QH | CK | 3708 ± 92e | 239 ± 22c | 7.20 ± 0.66c | 39.13 ± 3.99c | 1.18 ± 0.12c |
| Si300 | 3972 ± 166d | 339 ± 26c | 10.22 ± 0.78c | 55.28 ± 2.30bc | 1.66 ± 0.07bc | |
| Si600 | 4772 ± 37c | 346 ± 22c | 10.42 ± 0.66c | 55.66 ± 5.05bc | 1.68 ± 0.15bc | |
| Si900 | 5686 ± 72bc | 488 ± 62b | 14.68 ± 1.87b | 78.44 ± 8.03ab | 2.36 ± 0.54ab | |
| Si1500 | 5358 ± 33b | 542 ± 26ab | 16.32 ± 0.78b | 86.88 ± 3.76a | 2.62 ± 0.11a | |
| Si3000 | 6248 ± 93a | 648 ± 35a | 19.52 ± 1.06a | 104.24 ± 1.83a | 3.14 ± 0.06a |
Figure 3. Correlations between Si fertilizer rate (kg ha−1) and ANPP (kg ha−1) at the five sites tested (P < 0.01, n = 90).
The phytolith production flux and the phytolith production rate at the five sites ranged from 129.95 to 677.40 kg ha−1 yr−1 and 3.91 × 106 tonnes yr−1 to 20.39 × 106 tonnes yr−1, respectively (Table 2). The lowest rates were recorded for the LH CK plots, whereas, the highest were recorded for the LST Si3000 plots. The change in phytolith production flux and the phytolith production followed a similar rate-dependent trend as ANPP, and once again, the highest recorded levels at each site were from the Si3000 treated plots.
The PhytOC production flux and the PhytOC production rates, varying greatly from 16.24 (DZ CK plots) to 104.24 (QH Si3000 plots) kg CO2 ha−1 yr−1 and 0.49 × 106 (DZ CK plots) to 3.14 × 106 (QH Si3000 plots) tonnes CO2 yr−1, respectively, were always highest at the Si3000 rate at each site tested (Table 2).
Correlation analysis
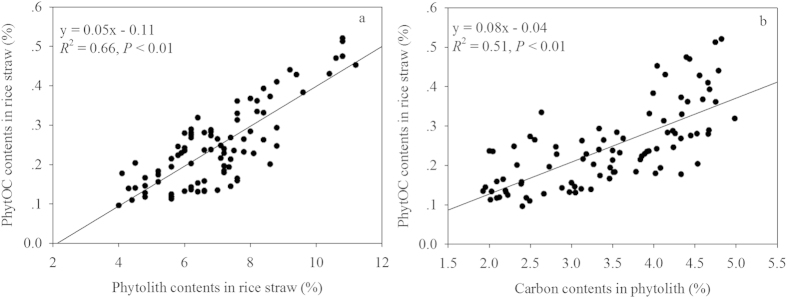
The relationships were examined among phytolith contents, carbon contents in phytolith, PhytOC contents in rice straw, ANPP, phytolith production flux and PhytOC production rate (Fig. 4). The results show that there are strong positive correlations between the contents of phytolith in rice straw and the PhytOC contents in rice straw (R2 = 0.66, P < 0.01) (Fig. 4a), and between the carbon contents of straw phytoliths and the PhytOC contents in rice straw (R2 = 0.51, P < 0.01) (Fig. 4b). In addition, phytolith production flux was positively correlated with either ANPP (Fig. 5a) or PhytOC production rate (Fig. 5b).
Figure 4. Correlations between phytolity contents in rice straw (%) and PhytOC contents in rice straw (%) (a) and carbon contents in phytolith (%) with PhytOC contents in rice straw (%) (b), at the five sites tested (P < 0.01, n = 90).
Figure 5. Correlations between ANPP and phytolith production flux (a) and PhytOC production rate with phytolith production flux (b) in rice straw at the five sites tested (P < 0.01, n = 90).
Discussion
Factors controlling rice growth and silicon uptake for phytolith production
Plants differ greatly in their ability to accumulate Si, with dry weight concentrations ranging from 0.02% to 32% SiO227,30. Paddy rice is categorized as a Si accumulating crop having straw contents of 10% to 20% SiO231. The differences in Si accumulation among plant species is closely tied to the ability of the plant’s root system to take up [Si(OH)4]27. However, variations in response to silicate fertilizer additions in rice cropping systems have been attributed to the soil’s native soluble Si content, soils with low to medium native soluble Si content exhibiting a greater response than soils with high native soluble Si content32. The variability in rice silica uptake in this trial attributed to the soluble silicon supplying capacity of each soil is shown in Fig. 1a. As this graph represents post-harvest soil levels, these values would include initial soil silicon levels, plus any silicate additions, minus plant removal. The QH site might be more efficient at supplying soluble Si to meet the plant’s needs during the 2012 rice cropping season. At the XY, DZ, and QH sites, the Si3000 silicate fertilizer treatment rate had higher residual levels than the other treatments. The LST site had the highest native soluble silicon levels as evidenced by the CK plots, but the fact that the highest rates of silicate fertilizer (Si1500 and Si3000) resulted in higher post-harvest soil soluble Si and also increased straw SiO2 levels over the CK plots (Fig. 1) suggests a more efficient soluble Si supplying capacity from the silicate fertilizer material than from native soil supplies. And, considering that in many areas of China under rice crop production systems, depletion of plant-available Si from soils has been linked to decreased rice yields25, we agree that the soil test critical levels at which silicate fertilizer additions are recommended need further research.
Plant tissue phytoliths perform a beneficial function by increasing structural rigidity and mechanical strength33,34,35,36. Silica phytoliths also enhance the plants’ resistance to biotic and abiotic stresses27,37. The beneficial effects of silicon nutrition in rice and other crop production systems, globally, are not new, but have been reported extensively25,33,38,39,40,41. In this study, rice straw Si uptake (Fig. 1b), and dry weights (Table 2) were increased by the different rates of silicate fertilizer addition, supporting the previously referenced research of the beneficial effects of Si nutrition on rice growth.
In the present study, the percentage of plant silica phytolith contents were increased with increasing application rates of silicate fertilizer, with the highest application rate (Si3000) consistently increasing the phytolith contents by 31.85 to 85.20% for all the five sites above control levels (Fig. 2, Table 1), and also increasing the PhytOC percentage in the straw for the five sites tested (Table 1). A recent study indicated that the additions of mulch with high phytolith contents would further increase bamboo soil PhytOC accumulation, providing effective long-time storage of organic C, and significant mitigation potential for climate change29. Their research supports the need for Si fertilization as they stated that the increased PhytOC storage in the top soil layer resulted from the accumulation of phytoliths rather than the concentrations of C in the phytoliths. They also mentioned the potential for Si fertilization to indirectly increase PhytOC storage through increased litter-fall. However, research on the effects of Si fertility in increasing PhytOC production was not evaluated in their trial. As such, soil applications of silicate fertilizers have the potential to not only supply Si for plant uptake and deposition in rice straw (Fig. 1) to meet production needs, but also increase the phytolith contents and the PhytOC contents in the straw (Table 1).
The role of rice in the global phytolith production and the potential of carbon occlusion within phytoliths.
In this study, ANPP values, estimated phytolith production flux, and PhytOC production flux values for rice straw at the five sites were all consistently higher with the Si3000 rate application when compared with all other treatments, and trends were seen in increasing levels with increasing rates of silicate fertilizer additions overall (Table 2). The above-ground phytolith production rate during rice production in China, based on the CK plot data from our five rice cropping sites, would amount to between 3.91 × 106 and 10.94 × 106 tonnes yr−1, with no silicate fertilizer additions. Currently, the total estimated area of rice production in China amounts to 30.1 × 106 ha42. If the variety of rice grown at XY, Jinyou277, were to be grown at all the rice paddy sites across China, this change alone has the potential to increase the above-ground PhytOC production and carbon sequestration rate to approximately 0.94 × 106 tonnes CO2 yr−1, with no additional silicate fertilizer inputs. However, the widespread use of only one grain variety can have devastating effects, due to genetic uniformity. In the United States during the years 1969 and 1970, 85% of US corn production acreage was affected by a Southern corn leaf blight (causal agent, Bipolarismaydis race T) epidemic, due to the extensive planting of a single hybrid corn (Zea mays L.) variety carrying the cms-T male sterility gene43. Therefore, a more viable option of increasing CO2 sequestration during rice paddy production is with silicate fertilizer additions. From our trial, CO2 sequestration could be increased, on the average, by 67.83%, or 0.16 to 2.17 × 106 tonnes CO2 yr−1 with silicate fertilizer additions.
Research on phytolith production and phytolith occluded carbon has recently received the attention of other researchers working with various crops. The PhytOC sequestration rates for millet13, wheat44, sugarcane14, bamboo10 and rice8 have been estimated at 0.03, 0.25, 0.36, 0.70 and 0.13 tonnes CO2 ha−1 yr−1, respectively. These results reveal how agricultural production activities can affect the overall global C balance. More interesting is the fact that all of the above-mentioned crops are known to be Si accumulators, and have been shown to benefit from silicon fertility45,46,47,48. So, the potential to expand CO2 sequestration beyond China’s paddy rice production, and to increase CO2 sequestration levels with silicate fertilizer additions, worldwide, is huge.
However, the need for our current focus on China’s rice paddy production systems is intimately tied to human food security49. Of particular concern are the multiple cropping indexes, which have been increasing recently in Southeast Asia and particularly in China during recent years. To the best of our knowledge, our results are the first field evidence showing that such agricultural management practices as silicate fertilizer additions during the rice growing season can potentially increase the storage of PhytOC, an important soil C fraction with long-term stability.
These effects of silicate fertilizer on PhytOC contents appear to be both direct and indirect, by improving PhytOC contents in rice straw and plant growth (Figs 3 and 4a, Table 1), which increased phytolith accumulation (Table 1). In previous studies, supplemental Si under heavy-nitrogen inputs affected improvements in rice yields and grain quality20. In this study, we determined that a significant positive correlation existed between the phytolith and silica contents of rice straw with application of silicate fertilizer (R2 = 0.87, P < 0.01; Fig. 2). Strong positive correlations were also exhibited between PhytOC content, based on straw dry weight, and phytolith or carbon content in phytolith (Fig. 4a,b). These results suggest that PhytOC concentrations based on dry weight are closely related to, not only phytolith content, but also the efficiency of C deposition during phytolith formation in rice plants following silicate fertilizer additions. Our results are in agreement with the previous work of Li et al.8 on rice, that the phytolith content and the PhytOC content based on dry weight correlated well. Although some researchers have suggested that the PhytOC contents of bamboo, wheat, sugarcane and millet are not directly tied to actual phytolith content10,13,14,41 factors such as variety, disease resistance, and nutrient requirements can play a role in phytolith accumulation during plant growth12,32,44. In this study, phytolith concentrations were increased with increasing Si fertilizer rate at the five different sites with each site having a different rice variety. However, the highest rate of silicate fertilizer additions proved to be the most effective at all the sites and for all the varieties tested (Table 1, Table 3).
Table 3. The basic information of the five sites tested.
| Site | Rice variety | Geographical conditions | Basal and topdressing fertilization | Transplanting and harvesting time, plant protection and irrigation | Total N (%) | Total P (%) | Available K (mg kg−1) | Organic carbon (g kg−1) | pH |
|---|---|---|---|---|---|---|---|---|---|
| LH | Tianyouhuazhan | Subtropical monsoon climate zone with effective accumulative temperature of 5081 °C, mean annual precipitation of 1340 mm and mean annual temperature of 16.1–17.1 °C. | Basal fertilizers were applied on 23rd July at a rate of 195 kg-0 kg-67.5 kg ha−1 (N-P2O5-K2O). N, P and K were applied in the forms of urea, calcium superphosphate and potassium chloride, respectively, with a basal to topdressing ratio of 7:0:3. Topdressing was done on 2nd August at tillering stage. | The sowing and harvesting time was on 22nd June and 19th October, respectively. Pesticides were sprayed on 12th August and 19th September to control leafroller, stem borer, planthoppers and sheath blight. Irrigation was applied on 30th July, 5th, 12th August, 10th September and 2nd October, respectively, to maintain a 5.0 cm water layer above ground. | 2.34 ± 0.00 | 0.35 ± 0.01 | 171.40 ± 1.78 | 27.02 ± 0.48 | 6.02 ± 0.03 |
| LST | T-you207 | Subtropical monsoon climate zone with its mean annual precipitation of 1354.6 mm and mean annual temperature of 16.7 °C. | Basal fertilizers were applied on 18th July at a rate of 450 kg ha−1 in the form of compound fertilizer (N: P2O5: K2O = 15:15:15). Topdressing fertilizers were applied at a rate of 180 kg ha−1 in the form of urea on 10th August 2012. | Rice was transplanted on 23rd July and harvested on 19th October. Pesticides were applied on 13th August, 24th August, 9th September and 19th September, respectively, to control leafroller, stem borer, planthoppers and sheath blight. Irrigation was applied on 18th August and 20th September, respectively, to maintain a 5.0 cm water layer above ground. | 2.59 ± 0.02 | 0.49 ± 0.02 | 160.48 ± 2.75 | 32.42 ± 0.66 | 6.02 ± 0.05 |
| XY | Jinyou277 | Subtropical monsoon climate zone with its effective accumulative temperature of 5081 °C, mean annual precipitation of 1340 mm and mean annual temperature of 16.1–17.1 °C. | Basal fertilization was applied on 14th July at a rate of 210 kg-375 kg-0 kg ha−1 (N-P2O5-K2O); nitrogen, phosphorus and potassium were applied in the forms of urea, calcium superphosphate and potassium chloride. Topdressing fertilizers were applied at the rates of 90 kg-0 kg-112.5 kg ha−1 (N-P2O5-K2O) at tillering stage on 23rd July. | Rice was transplanted on 15th July and harvested on 12nd November. Pesticides were applied on 5st August and 17th, 28th September to control leafroller, stem borer, planthoppers and sheath blight. Irrigation was made to rice on 21st, 27th July, 9th, 29th August, 15th, 27th September and 11st, 24th November, respectively. | 2.23 ± 0.03 | 0.50 ± 0.05 | 130.55 ± 0.41 | 37.62 ± 1.79 | 5.30 ± 0.01 |
| DZ | Bo II you 312 | Tropical monsoon climate zone with its mean annual precipitation of 1500–2000 mm, and mean annual temperature of 23.3 °C. | Basal fertilizers were applied on 22nd August at a rate of 1200 kg ha−1 in the form of compound fertilizer (N: P2O5: K2O = 15:15:15), while topdressing fertilizers were applied at a rate of 1200 kg ha−1 in the form of compound fertilizer (N: P2O5: K2O = 15:15:15) on 9th September 2012 at tillering stage. | Rice was transplanted on 12nd August and harvested on 19th November. Pesticides were applied on 27th August and 11th September to control leafroller, stem borer, planthoppers and sheath blight. Irrigation was made to rice on 22nd August, 27th August and 22nd September, respectively. | 1.00 ± 0.02 | 0.47 ± 0.01 | 71.78 ± 1.73 | 32.09 ± 1.25 | 5.28 ± 0.03 |
| QH | Tecanzhan 25 | Tropical monsoon and marine humid climate zone with its mean annual precipitation of 2000 mm and mean annual temperature of 24 °C. | Basal fertilizers were applied on 15th June at a rate of 112.5 kg ha−1 in the form of urea. Topdressing fertilizers were applied at a rate of 150 kg ha−1 in the form of compound fertilizer (N: P2O5: K2O = 16:16:16) on 25th June 2012. | Rice was transplanted on 9th June and harvested on 15th September. Pesticides were applied on 1st July, and 15th July to control leafroller, stem borer, planthoppers and sheath blight. Irrigation was made to rice on 22nd June, 27th June and 22nd August, respectively. | 1.37 ± 0.02 | 0.33 ± 0.03 | 81.70 ± 3.87 | 19.52 ± 0.64 | 5.69 ± 0.01 |
With the increasing effects of global climate change on agriculture in the developing world50,51, fertility measures will undoubtedly become more and more important in sustaining and improving crop yields and grain quality. Therefore, in the future, silicate fertilizers may be targeted not only for their beneficial effects in increasing stress resistance and yields, but also for their indirect effects in increasing the rate of atmospheric CO2 sequestration. Moreover, PhytOC is very stable and can accumulate and remain in soils for hundreds and thousands of years after plant decomposition7,12. Relative to other forms of organic carbon, the PhytOC is considered to be an important part of the stable organic C fraction in soil. For example, in situ decomposition at Numundo sites in Australia showed PhytOC representing up to 82% of the total soil C was buried in soils up to a 2 m depth, whereas the concentration of the total C decreased markedly over a period of 2000 years7. PhytOC is highly resistant to decomposition when compared to other organic C components in the soil environment. By using a silicate fertilizer to increase rice growth and ANPP (Fig. 3), we may fully realize the long-term C sequestration and mitigation of global climate change potential. For the five sites receiving different application rates of silicate fertilizer, a strong and positive relationship existed both between ANPP and the phytolith production flux, and between the PhytOC production rate and phytolith production flux (Fig. 5). These results suggest that ANPP, at least for rice, is instrumental in determining the above-ground phytolith production flux and PhytOC production rate (Table 2). And, silicate fertilization practices may enhance ANPP and PhytOC production rate of rice plants. Improving the PhytOC production rate during rice production in China by optimizing Si supply (Si3000 application rate) during the growing period may provide a sustainable and important means for crop production to reduce atmospheric carbon buildup, thus decreasing CO2 induced global climate change.
Conclusions
Our study reveals that addition of silicate fertilizer affects PhytOC sequestration by improving PhytOC contents in rice straw and the plants growth; the average above-ground phytolith-occluded carbon (PhytOC) production rates at five of China’s paddy rice production areas are increased by the addition of silicate fertilizer. Our results are the first field evidence that there is a potential to increase the storage of PhytOC through such agricultural management practices as the addition of silicate fertilizer. Therefore, regulating Si supply during rice growth may serve as an effective tool in improving PhytOC production rate and play an important role in carbon bio-sequestration and global warming mitigation.
Methods
Experimental design
Field experiments were conducted simultaneously at 5 different paddy rice field sites across China during the 2012 growing season. The 5 selected sites included Longhui (LH, E110°51′19.2″, N27°07′45.3″, elevation 228.3 m), Lengshuitan (LST, E111°35′30.8″, N26°36′59.1″, elevation 142 m) and Xiangyin (XY, E112°52′9.5″, N28°36′27.4″, elevation 228.3 m) in Hunan povince, and Danzhou (DZ, E109°30′14.0″, N19°30′17.6″, elevation 135 m) and Qionghai (QH, E110°27′39.8″, N19°20′48.1″, elevation 60.0 m) in Hainan povince. All the consecutive trial plots remained consistent for treatment as experiments initiated in 2011. The basic information of the five sites is listed in Table 3.
There were six treatments based on silicate fertilizer rates, i.e. Control (CK), 300 (Si300), 600 (Si600), 900 (Si900), 1500 (Si1500) and 3000 (Si3000) kg ha−1 with 3 replicates of each treatment. In total, 18 plots (5 × 6 m each) were arranged in a randomized complete block design at each site. The silicate fertilizer was applied as the basal fertilizer one week prior to rice seedling transplant.
Soil and plant sampling
Soil samples were taken from the top layer (0–20 cm) from five randomly-selected positions within each plot after treatment application of silicate fertilizer. The soil samples were thoroughly mixed and sieved to pass a 2.0 mm mesh screen. A 1 kg subsample of soil was sealed in air-tight bags prior to laboratory submission. All soil samples were air dried prior to analysis for plant-available Si contents.
Plant subsamples were collected at rice grain maturity. Five rice traps (100 cm × 100 cm in size) were randomly placed on the rice floor of each plot during harvest. The samples were washed carefully with running water followed by repeated rinsings with distilled water. The cleaned samples were oven-dried to a constant weight at 80 °C for 72 h, then ground to pass a 0.15 mm sieve prior to analysis of silica content, phytolith content, and PhytOC content.
Chemical analysis
Plant-available Si in the soil was determined using the pH 4.0 acetate buffer solution method that includes a 40 °C for 5 h setting period followed by silicon molybdenum blue spectrophotometry analysis52.
Silica content in rice straw (leaf and stem) was determined by the colorimetric Si molybdenum blue method53. Briefly, 0.1 g pretreated plant samples are fused using the high-temperature alkaline fusion method then diluted to 50 ml with distilled water, followed by colorimetric molybdenum blue analysis53.
Phytolith extraction from leaf and stem tissue was accomplished using microwave digestion as described by Parr et al.54. This process was followed by Walkley-Black digestion55 to ensure removal of any potentially extraneous organic materials. The phytolith extraction solution was also used to examine for extraneous organic materials outside the phytolith cells by adding 0.8 mol L−1 potassium dichromate to the solution. If the color of the solution remained unchanged for 5 min, this was an indicator that any extraneous organic materials outside the phytoliths had been thoroughly removed. The phytoliths extracted were oven-dried at 75 °C for 24 h in a centrifuge tube of known weight. The samples were allowed to cool and then weighed with the tube weight subtracted to obtain the phytolith quantities.
Carbon content of phytoliths (PhytOC) was determined using a CNS (carbon, nitrogen, sulfur) Analyzer (Elementar Americas Inc., Mt. Laurel, NJ USA).
Straw dry weight was determined using the weight of five rice traps (100 cm × 100 cm in size) within each plot. The traps were randomly placed within each plot during harvest. The samples were oven-dried to a constant weight at 80 °C for 72 h, and then weighed to obtain the straw dry weight after removal of rice grains.
Construction of silica-phytolith content transfer function
The silica-phytolith content transfer function was constructed using the regression analysis method based on the determined silica and phytolith contents of the rice straw samples (Fig. 2). Silica content was converted to phytolith content using the following Equation:
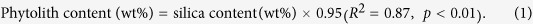 |
Estimation of phytolith and PhytOC production flux and rate
As the production of biogenic Si is primarily driven by plant Si concentration and ANPP56, the phytolith production flux of rice aboveground biomass was estimated using rice straw phytolith content data (Equation 1, above) and ANPP as shown below in Equation 2:
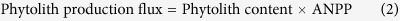 |
Where:
Phytolith production flux is the total phytolith production in rice aboveground biomass per ha per year (kg ha−1 yr−1).
Phytolith content is the content of phytoliths in rice aboveground biomass (wt%).
ANPP is aboveground net primary productivity of rice (kg ha−1 yr−1)41.
Phytolith production rate of rice aboveground biomass was estimated using the data from the phytolith production flux and the rice straw sampling area as shown in Equation 3, below:
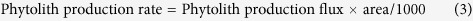 |
Where:
Phytolith production rate is total phytolith production of rice aboveground biomass per year (T yr−1),
Phytolith production flux is obtained from Equation (2) and,
Area is the area of rice planting (106 ha).
PhytOC production flux of rice aboveground biomass was determined using the phytolith production flux and PhytOC content of phytoliths data as shown in Equation 4, below:
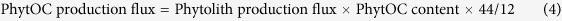 |
Where:
PhytOC production flux is the total PhytOC production in rice straw per area per year (kg CO2 ha−1 yr−1);
Phytolith production flux is obtained from Equation (2);
PhytOC content is the content of PhytOC in the phytoliths.
PhytOC production rate of rice aboveground biomass was obtained from the PhytOC production flux and rice area data as shown in Equation 5:
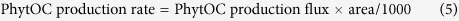 |
Where:
PhytOC production rate is the total PhytOC production of rice by rice straw (T CO2 yr −1);
PhytOC production flux is obtained from Equation (4) and,
Area is the area of rice planting (106 ha).
Statistical analysis
All the experimental data presented in this paper were statistically examined by one-way analysis of variance between the means of different treatments at the same rice growing site. Before all data analysis, the normality of data was tested by Shapiro-Wilk test according to a significance level at P < 0.05 under the null hypothesis that the population is normally distributed, Shapiro-Wilk tests indicated that most of the data met the normality test, while for the datasets that showed non-normality, a log-transformation was performed prior to ANOVA analysis using Duncan’s multiple range test. Similar test results were obtained when using either the original or transformed data, so, the authors opted to present the results using the original data. Statistical significance of the means of three replicates was compared at the 0.05 probability level using SAS (Version 8). All figures were drawn using SigmaPlot software (12.5) or Excel 2013.
Additional Information
How to cite this article: Song, A. et al. The potential for carbon bio-sequestration in China's paddy rice (Oryza sativa L.) as impacted by slag-based silicate fertilizer. Sci. Rep. 5, 17354; doi: 10.1038/srep17354 (2015).
Acknowledgments
This research was jointly supported by The National Science Foundation of China entitled “The quantitative study of influence of straw returning on silicon releasing in major types of paddy soils in South China” (Approved No. 41301310), The 12th Five-year Key Programs entitled “Techniques for Agricultural Use of Steel and Iron Slag: Research and Demonstration”, China (2013BAB03B02), National Nonprofit Institute Research Grant of CAAS (IARRP-2015-20), the foundation for the “The studies on the mechanism and the potential of phytolith carbon sequestration” from Institute of Agricultural Resources and Regional Planning of Chinese Academy of Agricultural Sciences and Tisco-Harsco Technologies co-operative project (2011–2013).
Footnotes
Author Contributions Y.L. designed the study and supervised the project. Y.L., F.F., Z.L. and M.P.B. selected the sampling sites. A.S., F.F. and D.N. carried out the sampling. A.S. and D.N. performed the experimental work. A.S. analyzed the data and wrote the manuscript. Y.L., F.F., and M.P.B. edited the manuscript. All authors discussed the results and contributed to the manuscript.
References
- Li B. et al. Lithological control on phytolith carbon sequestration in moso bamboo forests. Sci. Rep. 4, 5262 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon dioxide information analysis center. Glob. Carbon Proj. (2013). Available at: http://cdiac.ornl.gov/GCP/carbonbudget/2013/www.globalcarbonproject.org (Accessed: November 2013).
- Intergovernmental Panel on Climate Change (IPCC). Climate Change (2001): The Scientific Basis (Cambridge University Press, Cambridge) (2001). [Google Scholar]
- Umemura M. & Takenaka C. Biological cycle of silicon in moso bamboo (Phyllostachys pubescens) forests in central Japan. Ecol. Res. 29, 501–510 (2014). [Google Scholar]
- Rovner I. Plant opal phytolith analysis: major advances in archaeobotanical research. Adv. Archaeol. Method Theory. 6, 225–266 (1983). [Google Scholar]
- Piperno D. R. The production, deposition, and dissolution of phytoliths. Phytoliths: a comprehensive guide for archaeologists and paleoecologists. Piperno D. R. (ed) 5–22 (Rowman Altamira, 2006). [Google Scholar]
- Parr J. F. & Sullivan L. A. Soil carbon sequestration in phytoliths. Soil Biol. Biochem. 37, 117–124 (2005). [Google Scholar]
- Li Z., Song Z., Parr J. F. & Wang H. Occluded C in rice phytoliths: implications to biogeochemical carbon sequestration. Plant Soil. 370, 615–623 (2013). [Google Scholar]
- Prasad V., Strömberg C. A. E., Alimohammadian H. & Sahni A. Dinosaur coprolites and the early evolution of grasses and grazers. Science. 310, 1177–1180 (2005). [DOI] [PubMed] [Google Scholar]
- Parr J., Sullivan L., Chen B., Ye G. & Zheng W. Carbon bio-sequestration within the phytoliths of economic bamboo species. Glob. Chang. Biol. 16, 2661–2667 (2010). [Google Scholar]
- Song Z., Liu H., Si Y. & Yin Y. The production of phytoliths in China’s grasslands: implications to the biogeochemical sequestration of atmospheric CO2. Glob. Chang. Biol. 18, 3647–3653 (2012). [Google Scholar]
- Song Z., Liu H., Li B. & Yang X. The production of phytolith-occluded carbon in China’s forests: implications to biogeochemical carbon sequestration. Glob. Chang. Biol. 19, 2907–2915 (2013). [DOI] [PubMed] [Google Scholar]
- Zuo X. & Lü H. Carbon sequestration within millet phytoliths from dry-farming of crops in China. Chinese Sci. Bull. 56, 3451–3456 (2011). [Google Scholar]
- Parr J., Sullivan L. & Quirk R. Sugarcane phytoliths: encapsulation and sequestration of a long-lived carbon fraction. Sugar Tech. 11, 17–21 (2009). [Google Scholar]
- Song Z., Wang H., Strong P. J. & Guo F. Phytolith carbon sequestration in China’s croplands. Eur. J. Agron. 53, 10–15 (2014). [Google Scholar]
- Liang Y., Zhang W., Chen Q., Liu Y. & Ding R. Effect of exogenous silicon (Si) on H+-ATPase activity, phospholipids and fluidity of plasma membrane in leaves of salt-stressed barley (Hordeum vulgare L.). Environ. Exp. Bot. 57, 212–219 (2006). [Google Scholar]
- FAOSTAT Production Database. (2013). Available at: http://faostat.fao.org/default.aspx?lang=en. (Accessed: 26 May 2013).
- Struyf E., Smis A., Van Damme S., Meire P. & Conley D. J. The global biogeochemical silicon cycle. Silicon 1, 207–213 (2009). [Google Scholar]
- Desplanques V. et al. Silicon transfers in a rice field in Camargue (France). J. Geochem Explor. 88, 190–193 (2006). [Google Scholar]
- Yogendra N. D. et al. Effect of silicon on real time nitrogen management in a rice ecosystem. Afr. J. Agric. Res. 9, 831–840 (2014). [Google Scholar]
- Resolution adopted by the General Assembly on 20 December 2013. Sixty-eighth session, agenda item 25. 68/232. Word Soil Day and International Year of Soils. Available at: http://www.un.org/en/ga/search/view_doc.asp?symbol=A/RES/68/232 (Accessed: 7 February 2014).
- Brown T. H. & Mahler R. L. Effects of phosphorus and acidity on levels of silica extracted from a Palouse silt loam. Soil Sci. Soc. Amer. J. 51, 674–677 (1987). [Google Scholar]
- Bronick C. J. & Lal R. Soil structure and management: a review. Geoderma. 124, 3–22 (2005). [Google Scholar]
- Meena V. D. et al. A Case for silicon fertilization to improve crop yields in tropical soils. Proc. Natl. Acad. Sci. India Sect. B: Biol. Sci. 84, 505–518 (2014). [Google Scholar]
- Savant N. K., Snyder G. H. & Datnoff L. E. Silicon management and sustainable rice production. Adv. Agron. 58, 151–199 (1997a). [Google Scholar]
- Liang Y. C., Ma T. S., Li F. J. & Feng Y. J. Silicon availability and response of rice and wheat to silicon in calcareous soils. Commun. Soil Sci. Plant Anal. 25, 2285–2297 (1994). [Google Scholar]
- Ma J. F. & Takahashi E. Silicon uptake and accumulation in plants; Functions of silicon in plant growth. Soil, Fertilizer, and Plant Silicon Research in Japan. 1st edn. Ma J. F. & Takahashi E. (eds) Ch. 6, 73–90, Ch. 7, 118-119 (Elsevier Science, Amsterdam, 2002). [Google Scholar]
- Li Y. F. et al. Long-term management effects on soil organic carbon pools and chemical composition in Moso bamboo (Phyllostachys pubescens) forests in subtropical China. Forest Ecol. Manage. 303, 121–130 (2013). [Google Scholar]
- Huang Z. T. et al. Long-term intensive management increased carbon occluded in phytolith (PhytOC) in bamboo forest soils. Sci. Rep. 4, 3602, 10.1038/srep03602 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E. Silicon. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 641–664 (1999). [DOI] [PubMed] [Google Scholar]
- Takahashi E. Silica as a nutrient to the rice plant. Japan Agr. Res. Quart 3, 1–4 (1968). [Google Scholar]
- Narayanaswamy C. & Prakash N. B. Calibration and categorization of plant available silicon in rice soils of South India. J. Plant Nutr. 32, 1237–1254 (2009). [Google Scholar]
- Namaganda M., Lye K. A., Friebe B. & Heun M. Leaf anatomical characteristics of Ugandan species of Festuca L. (Poaceae). S. Afr. J. Bot. 75, 52–59 (2009). [Google Scholar]
- Ngoc Nguyen M., Dultz S. & Guggenberger G. Effects of pretreatment and solution chemistry on solubility of ricestraw phytoliths. J. Plant Nutr. Soil Sci. 177, 349–359 (2014). [Google Scholar]
- Savant N. K., Datnoff L. E. & Snyder , G. H. Depletion of plant-available silicon in soils: A possible cause of declining rice yields 1. Commun. Soil Sci. Plant Anal. 28, 1245–1252 (1997b). [Google Scholar]
- Ma J., Nishimura K. & Takahashi E. Effect of silicon on the growth of rice plant at different growth stages. Soil Sci. Plant Nutr. 35, 347–356 (1989). [Google Scholar]
- Mohseni V. G. & Sabbagh S. K. The ameliorative effects of silicon element on improvement of plants tolerance to diseases. Sci. Agri. 8, 80–85 (2014). [Google Scholar]
- Yeo A. R. et al. Silicon reduces sodium uptake in rice (Oryza sativa L.) in saline conditions and this is accounted for by a reduction in the transpirational bypass flow. Plant Cell Environ. 22, 559–565 (1999). [Google Scholar]
- Hossain M. T. et al. Growth promotion and an increase in cell wall extensibility by silicon in rice and some other Poaceae seedlings. J. Plant Res. 115, 23–27 (2002). [DOI] [PubMed] [Google Scholar]
- Sheng L. Y. Agriculture. National Bureau of Statistics of China (NBSC). China Statistical Yearbook. Sheng L. Y. et al. (eds) Ch. 13, 449–451 (China Statistics Press, Beijing, 2012). [Google Scholar]
- Parr J. F. & Sullivan L. A. Phytolith occluded carbon and silica variability in wheat cultivars. Plant Soil. 342, 165–171 (2011). [Google Scholar]
- Sheng L. Y. Agriculture. National Bureau of Statistics of China (NBSC). China Statistical Yearbook. Sheng L. Y. et al. (eds) Ch. 13, 449–451 (China Statistics Press, Beijing, 2012). [Google Scholar]
- Levings C. S. The Texas cytoplasm of maize: cytoplasmic male sterility and disease susceptibility. Science. 250, 942–947 (1990). [DOI] [PubMed] [Google Scholar]
- Parr J. F. & Sullivan L. A. Phytolith occluded carbon and silica variability in wheat cultivars. Plant Soil. 342, 165–171 (2011). [Google Scholar]
- Meyer J. H. & Keeping M. G. Review of research into the role of silicon for sugarcane production. Proc. S. Afr. SugarTechno. l Assoc. 74, 29–40 (2000). [Google Scholar]
- Deepak S. et al. Involvement of silicon in pearl millet resistance to downy mildew disease and its interplay with cell wall proline/hydroxyproline-rich glycoproteins. Australas Plant Pathol. 37, 498–504 (2008). [Google Scholar]
- Provance-Bowley M. C., Heckman J. R. & Durner E. F. Calcium silicate suppresses powdery mildew and increases yield of field grown wheat. Soil Sci. Soc. America J. 74, 1652–1661 (2010). [Google Scholar]
- Ueda K. & Ueda S. Effect of silicic acid on bamboo growth. Bull.Kyoto Univ. Forests. 33, 79–99 (In Japanese with English Summary) (1961). [Google Scholar]
- Mohanty S., Wassmann R., Nelson A., Moya P. & Jagadish S. V. K. The importance of rice for food and nutritional security. Rice and climate change: significance for food security and vulnerability. Mohanty S. et al. (eds) 1–9 (International Rice Research Institute, Copenhagen 2013). [Google Scholar]
- Rosenzweig C. & Parry M. L. Potential impact of climate change on world food supply. Nature, 367, 133–138 (1994). [Google Scholar]
- Parry M. L., Rosenzweig C., Iglesias A., Livermore M. & Fischer G. Effects of climate change on global food production under SRES emissions and socio-economic scenarios. Global Environ. Change, 14, 53–67 (2004). [Google Scholar]
- Bao S. D. Analysis of silicon, iron, aluminum and other elements in soil. Analytical Methods of Soil and Agro-chemistry. 3rd edn. Bao S. D. et al. (eds) 233–236 (Chinese Agricultural Press, Beijing, 2000). [Google Scholar]
- Dai W. M. et al. Rapid Determination of Silicon Content in Rice. Rice Sci. 12, 145–147 (2005). [Google Scholar]
- Parr J., Dolic V., Lancaster G. & Boyd W. E. A microwave digestion method for the extraction of phytoliths from herbarium specimens. Rev. Palaeobot. Palynol. 116, 203–212 (2001). [Google Scholar]
- Walkley A. & Black I. A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 37, 29–38 (1934). [Google Scholar]
- Blecker S. W., McCulley R. L., Chadwick O. A. & Kelly E. F. Biologic cycling of silica across a grassland bioclimosequence. Glob. Biogeochem. Cycles 20, GB3023. http://dx.doi.org/10.1029/2006GB002690 (2006).