Abstract
Objective
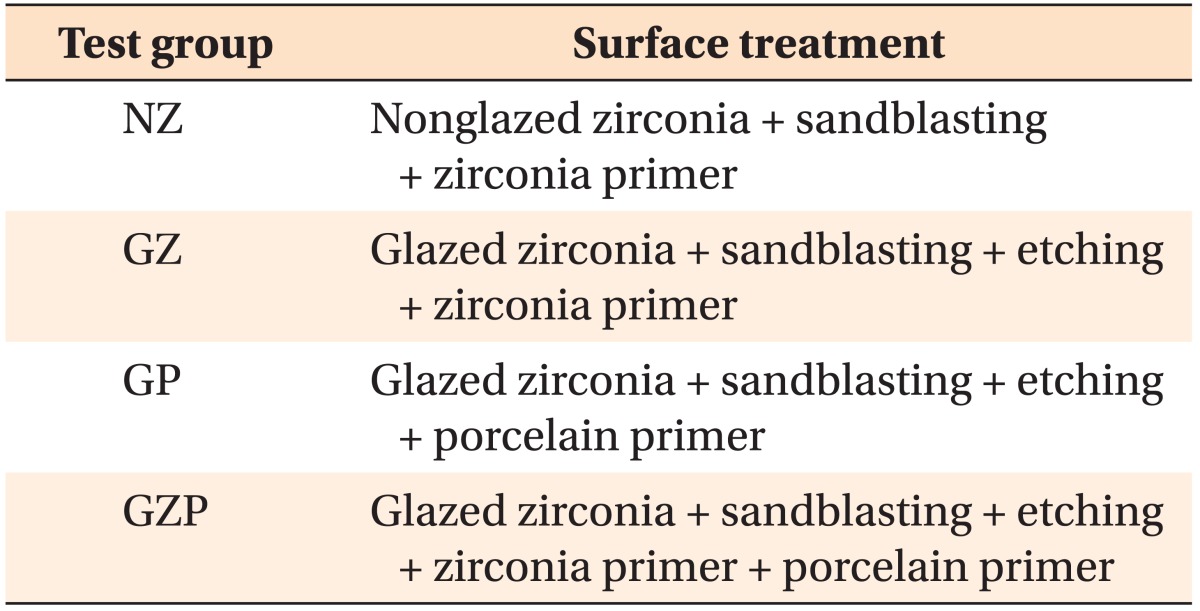
The aims of this study were to compare the shear bond strength between orthodontic metal brackets and glazed zirconia using different types of primer before applying resin cement and to determine which primer was more effective.
Methods
Zirconia blocks were milled and embedded in acrylic resin and randomly assigned to one of four groups: nonglazed zirconia with sandblasting and zirconia primer (NZ); glazed zirconia with sandblasting, etching, and zirconia primer (GZ); glazed zirconia with sandblasting, etching, and porcelain primer (GP); and glazed zirconia with sandblasting, etching, zirconia primer, and porcelain primer (GZP). A stainless steel metal bracket was bonded to each target surface with resin cement, and all specimens underwent thermal cycling. The shear bond strength of the specimens was measured by a universal testing machine. A scanning electron microscope, three-dimensional optical surface-profiler, and stereoscopic microscope were used to image the zirconia surfaces. The data were analyzed with one-way analyses of variance and the Fisher exact test.
Results
Group GZ showed significantly lower shear bond strength than did the other groups. No statistically significant differences were found among groups NZ, GP, and GZP. All specimens in group GZ showed adhesive failure between the zirconia and resin cement. In groups NZ and GP, bonding failed at the interface between the resin cement and bracket base or showed complex adhesive and cohesive failure.
Conclusions
Porcelain primer is the more appropriate choice for bonding a metal bracket to the surface of a full-contour glazed zirconia crown with resin cement.
Keywords: Bracket bonding, Adhesive, Glazed zirconia, Porcelain primer
INTRODUCTION
Zirconium dioxide (ZrO2), also called zirconia, is characterized by superior mechanical properties such as high flexural strength, relatively low elastic modulus, and high fracture toughness as compared to other ceramic materials.1,2 Yttria (Y2O3) is added to zirconia to improve its mechanical properties, and yttria tetragonal zirconia polycrystalline (Y-TZP) material is used in dentistry.
The use of zirconia in conservative aesthetic dentistry has become more common because of increasing aesthetic demands from patients and considerable development of computer-aided design (CAD) and computer-aided manufacturing (CAM) technologies, including scanners, CAD software, and network-connected machining centers.3 Such trends have widened the application of zirconia to anterior and posterior fixed prostheses, endodontic posts, implant abutments, and orthodontic brackets.4,5 In the early days, zirconia was simply a substitution of metal for metal-free restorations in fixed prostheses, and porcelain veneering was usually necessary because of the aesthetic limitations of zirconia. However, because the porcelain layer of a zirconia-core crown is too thin to withstand heavy posterior occlusal force, chipping or fracturing of veneering porcelain was frequently reported.6,7 In recent years, with the improvement in the natural tooth-like opacity of Y-TZP and techniques for precise machining and coloring of a zirconia block,8 clinical application of full-contour zirconia crowns has been actively pursued.9
With the increasing clinical application of zirconia and rising demand for adult orthodontic treatment, orthodontists may more frequently encounter zirconia surfaces on which orthodontic appliances must be bonded. Various surface treatments have been tested in order to determine the desirable bracket bonding strength to zirconia. Etching with hydrofluoric acid, sandblasting with Al2O3 particles with silanization, and etching with hot chemical solutions have been introduced, but these procedures have minimal effect on bonding strength.10,11,12,13,14,15,16,17
Various adhesive primers have been developed for "naked" zirconia. These primers enhance the adhesion between hydrophobic resin cements and oxide-based substrates. The anhydride group present in the 4-methacryloyloxyethyl trimellitate anhydride monomer18 and the phosphate ester group of the 10-methacryloyloxydecyl dihydrogen phosphate (MDP) monomer chemically bond to zirconia ceramics.18,19 However, these primers were invented for use with naked zirconia surfaces, i.e., for bonding between veneering ceramics and a zirconia core20 or resin cement and a zirconia surface without requiring glazing.
In clinical practice, the outer surface of a full-contour glazed zirconia crown is not made of naked zirconia. Zirconia crowns are generally glazed to improve their aesthetic appearance. Porcelain primer, a silane coupling agent, effectively creates a strong bond between resin cement and silica-based ceramics, including ceramic powder used for glazing, by increasing the wettability of the ceramic surface for bonding of the resin cement.21 Therefore, porcelain primer could be a desirable treatment option for glazed zirconia surfaces in dental clinics.
Many commercial zirconia primers are available, and porcelain primer could be used to treat glazed zirconia surfaces for bracket bonding. It is necessary to find the primer most suitable for use with zirconia that has been glazed to produce a highly aesthetic and glossy surface. The aims of this study were to compare the shear bond strength of different primers and determine if a zirconia primer or a conventional porcelain primer would provide the more effective surface treatment for orthodontic bracket bonding.
MATERIALS AND METHODS
Preparation of zirconia blocks and acrylic resin cylinders
Industrially manufactured Y-TZP ceramic blocks (Prettau Blocks; Zirkonzahn GmbH, Gais, Italy) were used in this study. After the design was completed with Rhinoceros software (Robert McNeel & Associates, Seattle, WA, USA), the zirconia blocks were CAD/CAM milled to an area of 8.0 × 8.0 mm2 and a thickness of 5.0 mm (n = 40). The blocks were then colored with a Zirkonzahn coloring kit (dipping each block in the coloring liquid for 3 min, according to manufacturer's instructions) and sintered in a furnace for zirconia (Zirkonofen 600; Zirkonzahn) by firing at 1,600℃ for 11 hours.
To prepare glazed zirconia blocks, a glazing liquid (INsitu Glaze Liquid, GC initial™; GC America Inc., Chicago, IL, USA) was applied with a brush on the sintered surface of each block before it was refired at 800℃ for 90 seconds in a porcelain furnace. This step was intended to reproduce the surface state delivered in a dental clinic. To ensure consistent surface preparation, all steps were completed according to the manufacturer's instructions by one dental technician.
A total of 40 zirconia blocks were embedded in acrylic resin (Ortho-Jet™; Lang Dental Manufacturing Co., Inc., Wheeling, IL, USA) with a diameter of 30.0 mm and a height of 15.0 mm for use in shear bonding strength test.
Surface roughening: alumina sandblasting with and without hydrofluoric acid etching
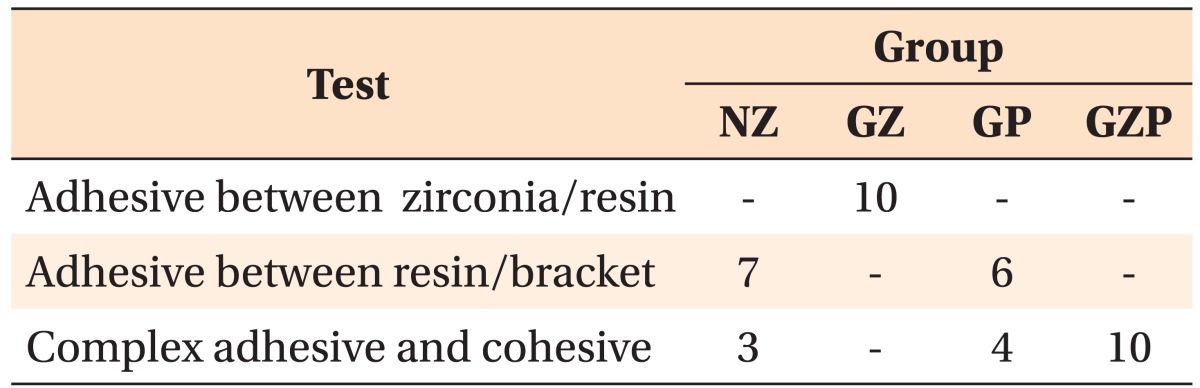
The specimens were randomly assigned to four groups of 10 specimens each: nonglazed zirconia treated with sandblasting and zirconia primer (NZ); glazed zirconia treated with sandblasting, etching, and zirconia primer (GZ); glazed zirconia treated with sandblasting, etching, and porcelain primer (GP); and glazed zirconia treated with sandblasting, etching, zirconia primer, and porcelain primer (GZP) (Table 1). Because hydrofluoricacid etching cannot change the surface structure of zirconia,14 only glazed specimens were etched.
Table 1. Classification of study groups.
Alumina sandblasting was performed for 5 seconds at a pressure of 40 psi, with a mean particle size of 50 µm (PROPHYflex 3-2018; KaVo Dental GmbH, Dubai, United Arab Emirates). The distance of the sandblaster nozzle tip from the surface was about 5 mm. For specimens from the GZ, GP, and GZP groups, the sandblasted surface was etched with 9% hydrofluoric acid (Reliance Porc-Etch™; Reliance Orthodontic Products, Inc., Itasca, IL, USA) for 4 minutes.
Bracket bonding with primers and resin cement
After the surfaces of the specimens had been roughened, they were washed and dried thoroughly. One thin coat of zirconia primer (Zirconia Liner Premium; Sun Medical Co., Ltd., Moriyama, Japan) or porcelain primer (Reliance Porcelain Conditioner; Reliance Orthodontic Products, Inc.) was then applied to the surface of each specimen. A stainless steel metal bracket for the mandibular incisor (Tomy Inc., Tokyo, Japan) was bonded to the target surface of each specimen with resin cement (Transbond™ XT; 3M Unitek, Monrovia, CA, USA). After the excess resin around the bracket was removed, the resin was polymerized with a plasma arc curing light (Flipo, LOKKI S.A., Les Roches de Condrieu, France) at a light intensity of 1,100 mW/cm2 for 10 seconds.
All materials were used according to the manufacturers' instructions.
Shear-bond-strength test
All specimens underwent thermal cycling (from 5℃ to 55℃) for 2,000 cycles, with a dwell time of 30 seconds (KD-TCS30; Kwang-duk F.A., Seoul, Korea).22 After the procedure, all specimens were stored in distilled water at 37℃.
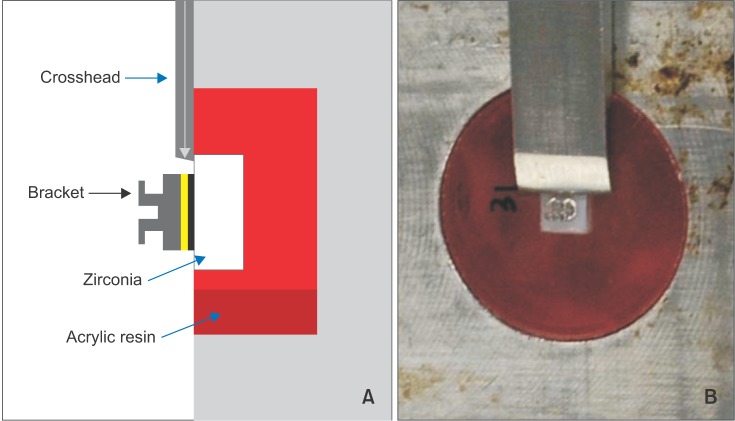
The shear bond strength of the specimens was measured by a universal testing machine (Model 3366; Instron® Co., Norwood, MA, USA) at a cross-head speed of 1 mm/min. The measured values were then divided by the area of the bracket base (9.81 mm2) and converted to units of MPa (Figure 1).
Figure 1. A, Schematic illustration of the shear-bond-strength test. B, Equipment for measuring shear bond strength.
Surface characterization
Scanning electron microscopy (SEM) (S-3000N; Hitachi, Tokyo, Japan) at a magnification of ×1,000 and an operating voltage of 15 kV was used to image zirconia surfaces before and after surface roughening. The roughened surfaces were analyzed with a three-dimensional optical surface profiler (NewView 6300; Zygo Co., Middlefield, CT, USA) based on noncontact scanning white-light interferometry to measure the surface roughness and evaluate the three-dimensional features on the surface. Failure-mode analysis was performed at a magnification of ×20 with a zoom stereoscopic microscope (JSZ-7TX; Samwon, Goyang, Korea) connected to a digital camera (EOS 1000D; Canon, Tokyo, Japan). The results were classified into three categories: adhesive failure between the zirconia and resin cement, adhesive failure between the resin cement and the bracket base, and complex adhesive and cohesive failure.
Statistical analysis
All preparation and testing procedures were performed by the same operator to eliminate interoperator variability. The data were submitted to one-way analyses of variance and multiple comparisons with the Tukey honest significant difference test (IBM SPSS Statistics software version 21.0; IBM Co., Armonk, NY, USA) to determine differences in shear bond strength between the specimen groups, depending on the type of primer used. The Fisher exact test was used to evaluate the relationship between surface treatment and failure modes. The level of statistical significance was set at p = 0.05 for all statistical analyses.
RESULTS
Effect of primers on shear bond strength of resin cement
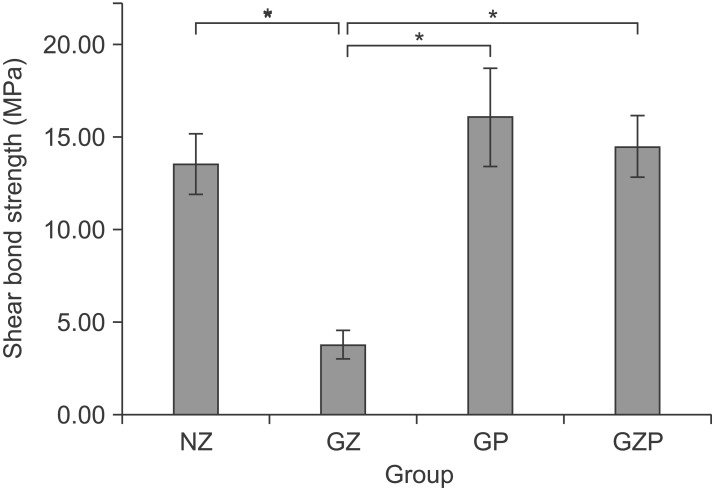
The mean shear bond strength (MPa) and standard deviations for each specimen group are presented in Figure 2. Group GZ, with zirconia primer on a glazed surface, showed significantly lower shear bond strength than the other groups (NZ, GP, and GZP; p < 0.05). There were no statistically significant differences among the NZ, GP, and GZP groups (p > 0.05).
Figure 2. Comparison of the values of shear bond strength. *Statistically significant (p < 0.05).
NZ, Nonglazed zirconia with sandblasting and zirconia primer; GZ, glazed zirconia with sandblasting, etching, and zirconia primer; GP, glazed zirconia with sandblasting, etching, and porcelain primer; GZP, glazed zirconia with sandblasting, etching, and both zirconia and porcelain primers.
Surface imaging of specimens
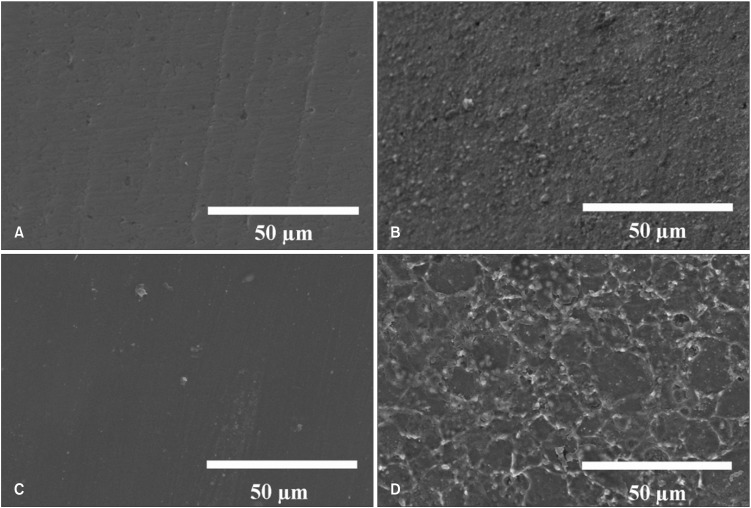
SEM images of the zirconia surfaces before and after roughening are presented in Figure 3. Figure 3A shows the slightly cut surface of zirconia (group NZ) that resulted from the cutting operation. In Figure 3C, the glazed zirconia (groups GZ, GP, and GZP) exhibited a plane surface with a few pits.
Figure 3. Scanning electron microscopy images of zirconia surface (magnification ×1,000). A, Nonglazed zirconia; B, nonglazed zirconia + sandblasting; C, glazed zirconia; and D, glazed zirconia + sandblasting and acid etching.
Sandblasting (and acid etching for glazed specimens) resulted in increased surface roughness (Figure 3). The surface of sandblasted and acid-etched glazed zirconia (Figure 3D) which had small or large pores (1-50 µm) was more roughened than that of sandblasted nonglazed zirconia (Figure 3B).
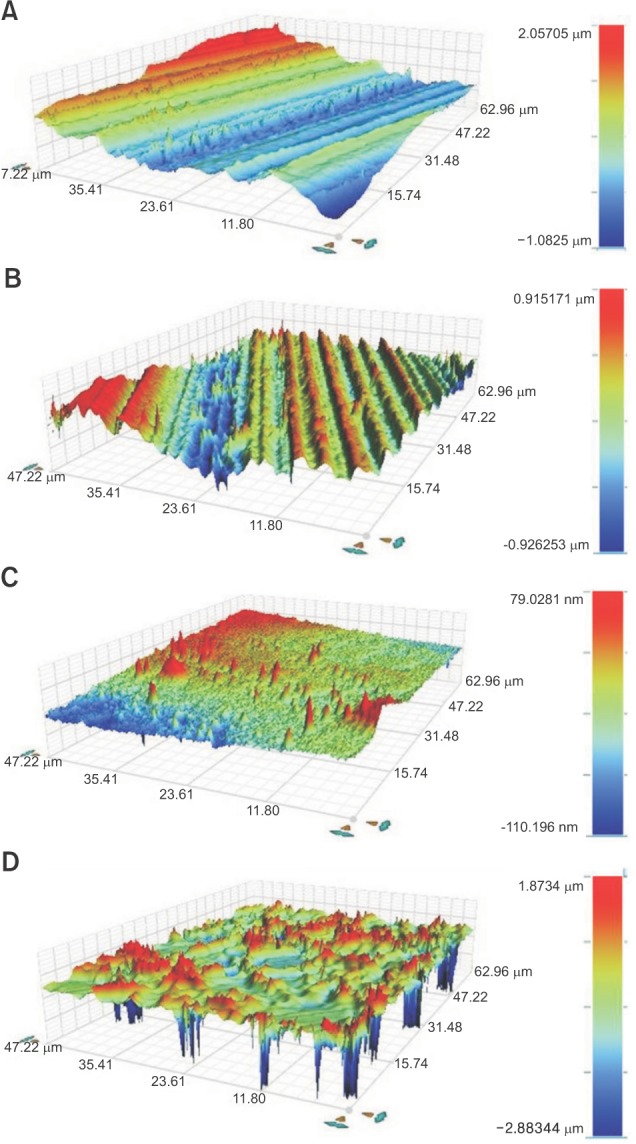
Figure 4 shows three-dimensional optical profiler images corresponding to the SEM images. Each color represents the height of each spot. Compared with the glazed specimens (Figure 4C), the sandblasted and etched surfaces had pits of various depths (Figure 4D). The roughness average (Ra) of nonglazed zirconia was significantly increased after sandblasting treatment (from 0.104 ± 0.03 to 0.165 ± 0.02, p < 0.05). The Ra of glazed zirconia was also significantly increased after sandblasting and etching (from 0.01 ± 0.01 to 0.209 ± 0.03, p < 0.05).
Figure 4. Three-dimensional optical profiler images of zirconia surface (magnification ×100). A, Nonglazed zirconia; B, nonglazed zirconia + sandblasting; C, glazed zirconia; and D, glazed zirconia + sandblasting and acid etching. The right side of each figure includes a colorimeter band representing the height of each spot as different color.
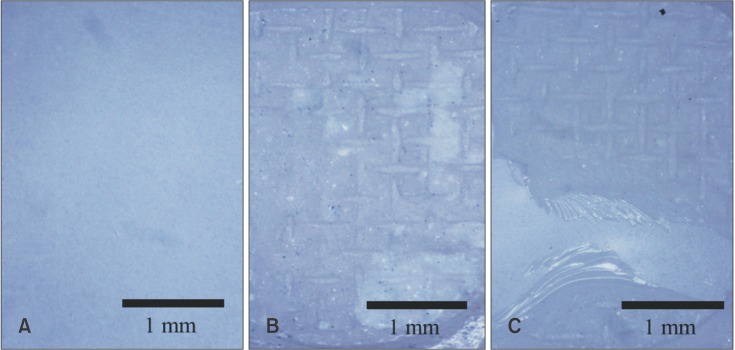
Failure-mode analysis
Failure-mode analysis was performed with a stereoscopic microscope. Images representing each failure mode are shown in Figure 5, and the results (n = 40) are shown in Table 2.
Figure 5. Stereoscopic micrographs of debonded surfaces (magnification ×16). A, Adhesive failure between zirconia and resin cement; B, adhesive failure between resin cement and bracket base; C, complex adhesive and cohesive failure.
Table 2. Failure mode after shear bond strength test.
Refer to Table 1 for the definition for each group.
All specimens (n = 10) in group GZ showed adhesive failure between the zirconia and resin cement. In groups NZ and GP, bonding failed at the interface between the resin cement and the bracket base or showed complex adhesive and cohesive failure. The specimens in group GZP, which were treated with a zirconia primer followed by a porcelain primer, all showed complex adhesive and cohesive failure (Table 2). Upon analysis with the Fisher exact test, groups GZ and GZP were statistically distinct from the others (p < 0.05). In other words, all 10 samples in the GZ group showed adhesive failure between zirconia and resin, and all 10 samples in the GZP group showed complex adhesive and cohesive failure. However, samples in the NZ and GP groups showed a statistically similar failure mode (p > 0.05).
DISCUSSION
In this study, the shear bond strengths of various zirconia blocks were measured to determine whether a recently commercialized zirconia primer or a conventional porcelain primer was more suitable for bonding orthodontic brackets to a full-contour zirconia crown. Previous studies have shown that porcelain primer successfully increases the shear bonding strength of bracket-resin cement complex bonded to glazed porcelain surfaces that have been etched and sandblasted.23,24,25 However, other studies have reported opposite results, showing that porcelain primer has no significant effect on bonding strength on glazed porcelain surfaces that have been etched and sandblasted.26
In the present study, unprimed samples with a glazed zirconia surface subjected to sandblasting and etching were excluded because of the results of a preliminary study. The preliminary study tested bracket shear bonding strength on unprimed glazed, sandblasted, etched zirconia samples as well as on samples in the NZ, GZ, GP, and GZP groups after incubation in distilled water at 37℃ for 24 h. Unprimed samples showed a relatively lower bonding strength than primed samples (10.3 and 15.2 Mpa, respectively; p < 0.05). For this reason, the group of samples subjected only to sandblasting and etching, with no primer, was excluded, and the other four groups were subjected to further thermocycling tests in this study.
Our results showed that blocks in group GZ, with a glazed surface and a zirconia primer, had significantly lower shear bond strength than did blocks in other groups. In previous studies, however, roughening of a zirconia surface followed by application of zirconia primer improved the shear bond strength.27,28 Therefore, when the glazing layer of a full-contour zirconia crown (often encountered in an orthodontic clinic) is not removed by conventional roughening, one cannot expect the zirconia primer to work as intended.
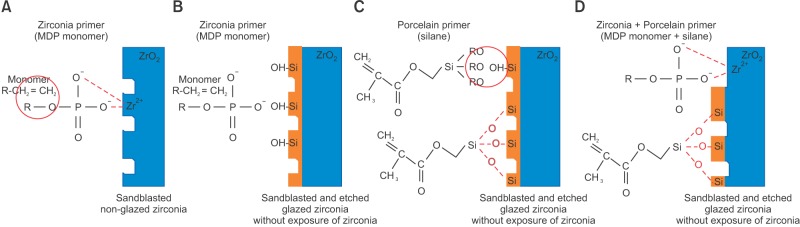
Chemical interaction models of each experimental group are shown in Figure 6. Zirconia Liner Premium, the zirconia primer used in this study, is composed mainly of methyl methacrylate (97%); the balance comprises 3% MDP and 0.01% 4-methoxyphenol. The MDP monomer effectively forms a bond between resin cement and zirconia, enabling the phosphate ester group (R-PO42-) of the MDP to directly bond to positively charged metal ions (such as Zr4+, Al3+, and Ti4+) in metal oxides including those of zirconium, chromium, nickel, aluminum, tin, and titanium (Figure 6A).29
Figure 6. Chemical interaction models between primer and various zirconia surfaces. A, Nonglazed zirconia with zirconia primer; B, glazed and roughened zirconia with zirconia primer, showing mismatched interaction between zirconia primer (MDP) and the glazed surface; C, glazed and roughened zirconia with porcelain primer; and D, glazed and roughened zirconia with both primers.
The porcelain primer, a silane coupling agent, effectively creates a strong bond between resin cement and silica-based ceramics by increasing the wettability of the ceramic surface for the resin cement.21 Silane monomer commonly has one backbone end containing a vinylic carbon-carbon double bond (-CH=CH2), while the other end has relatively rapidly hydrolyzable alkoxy groups (-O-CH3). Once activated by hydrolyzation with water, the alkoxy groups form hydrophilic labile silanol groups (-Si-OH), which combine -Si-OH in a silica surface (SiO2). Glazing surfaces generally include silica with minor amounts of various metal oxides. Therefore, it is reasonable that silane agents may attach to glazed surfaces with hydrophobic siloxane bonds (-Si-O-Si-, Figure 6C).
In this study, the bonding strength of the glazing layer could have been affected by the silane coupling agent because of glazing that remained on the surface after conventional roughening. For this reason, using a zirconia primer on the glazed surface would have been inappropriate (Figure 6B). The porcelain primer, however, could provide an acceptable shear bond strength between the glazed surface and the resin cement. In other words, the zirconia primer works only with nonglazed zirconia surfaces, and a porcelain primer is required when orthodontic brackets are bonded to a glazed zirconia surface.
While specimens in groups NZ and GP showed either adhesive failure between the resin cement and bracket base or complex adhesive and cohesive failure, specimens in group GZ all showed adhesive failure between the zirconia and resin cement. This is consistent with the results of the shear-bond-strength test. The adhesive failure in group GZ indicated failure of bonding at the interface between the glazed zirconia surface and the zirconia primer combined with resin cement. In the other groups, the adhesive failure between the resin cement and the bracket indicated a stable bonding interface between the zirconia surface and resin cement, which had a stronger tension resistance than did the interface between the resin cement and the bracket base.
Because the aesthetic appearance of a prosthesis is less important on the lingual side than on the buccal side, the glazing layer on the lingual side may be thin or absent, and the zirconia surface can be exposed during conventional roughening. In such a situation, an operator must either choose a single surface type in order to obtain acceptable bonding with a single primer or know whether two different primers can be used at the same time on two surface types without lowering their adhesion ability (Figure 6D).
In this study, specimens in group GZP were roughened with sandblasting and acid etching; zirconia primer, followed by porcelain primer, was then applied to the surface. As compared with groups NZ and GP, which mostly showed adhesive failure between the resin cement and the bracket base, the frequency of complex adhesive and cohesive failure increased when zirconia primer and porcelain primer were used together. However, the mean shear bond strength of group GZP was far above 6-9 MPa, which is known as the minimum shear bond strength that can withstand the force applied during orthodontic movement.30,31 Several specimens in group GZP showed even higher bond strengths than specimens that used a single primer. Therefore, when part of the glazed layer appears to have been removed by roughening, using a zirconia primer and porcelain primer together is an alternative way to obtain acceptable bond strength between two surfaces.
We conclude that using a primer for silica-based ceramics is an effective way to pretreat a glazed zirconia surface so that clinically acceptable bond strength can be obtained. Further studies using various types of brackets and adhesive materials would be helpful for studying their bonding attachments to zirconia restorations.
CONCLUSION
The purpose of this study was to compare the shear bond strength of resin cement to a glazed zirconia surface using different primers. The results are summarized as follows:
1. The shear bond strength of group GZ (glazed zirconia surface with zirconia primer) was significantly lower than that of other groups (p < 0.05).
2. There was no statistically significant difference between the shear bond strength of a nonglazed zirconia surface with zirconia primer (group NZ) and that of a glazed surface with porcelain primer (group GP) (p > 0.05).
3. Concurrent use of zirconia primer and porcelain primer on a zirconia surface (group GZP) did not lower the shear bond strength compared with use of a single primer on a zirconia surface (groups NZ and GP) (p > 0.05).
Based on our results, we conclude that zirconia primer is an unsuitable choice for bonding a metal bracket to the surface of a full-contour glazed zirconia crown with resin cement. In such situations, the use of porcelain primer is more appropriate.
Footnotes
The authors report no commercial, proprietary, or financial interest in the products or companies described in this article.
References
- 1.Manicone PF, Rossi Iommetti P, Raffaelli L. An overview of zirconia ceramics: basic properties and clinical applications. J Dent. 2007;35:819–826. doi: 10.1016/j.jdent.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Piconi C, Maccauro G. Zirconia as a ceramic biomaterial. Biomaterials. 1999;20:1–25. doi: 10.1016/s0142-9612(98)00010-6. [DOI] [PubMed] [Google Scholar]
- 3.Cortellini D, Valenti M, Canale A. The metal-free approach to restorative treatment planning. Eur J Esthet Dent. 2006;1:230–247. [PubMed] [Google Scholar]
- 4.Bertolini Mde M, Kempen J, Lourenço EJ, Telles Dde M. The use of CAD/CAM technology to fabricate a custom ceramic implant abutment: a clinical report. J Prosthet Dent. 2014;111:362–366. doi: 10.1016/j.prosdent.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 5.Keith O, Kusy RP, Whitley JQ. Zirconia brackets: an evaluation of morphology and coefficients of friction. Am J Orthod Dentofacial Orthop. 1994;106:605–614. doi: 10.1016/S0889-5406(94)70085-0. [DOI] [PubMed] [Google Scholar]
- 6.Agustín-Panadero R, Román-Rodríguez JL, Ferreiroa A, Solá-Ruíz MF, Fons-Font A. Zirconia in fixed prosthesis. A literature review. J Clin Exp Dent. 2014;6:e66–e73. doi: 10.4317/jced.51304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ortorp A, Kihl ML, Carlsson GE. A 3-year retrospective and clinical follow-up study of zirconia single crowns performed in a private practice. J Dent. 2009;37:731–736. doi: 10.1016/j.jdent.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Miyazaki T, Nakamura T, Matsumura H, Ban S, Kobayashi T. Current status of zirconia restoration. J Prosthodont Res. 2013;57:236–261. doi: 10.1016/j.jpor.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Guess PC, Zavanelli RA, Silva NR, Bonfante EA, Coelho PG, Thompson VP. Monolithic CAD/CAM lithium disilicate versus veneered Y-TZP crowns: comparison of failure modes and reliability after fatigue. Int J Prosthodont. 2010;23:434–442. [PubMed] [Google Scholar]
- 10.Foxton RM, Cavalcanti AN, Nakajima M, Pilecki P, Sherriff M, Melo L, et al. Durability of resin cement bond to aluminium oxide and zirconia ceramics after air abrasion and laser treatment. J Prosthodont. 2011;20:84–92. doi: 10.1111/j.1532-849X.2010.00678.x. [DOI] [PubMed] [Google Scholar]
- 11.Kitayama S, Nikaido T, Takahashi R, Zhu L, Ikeda M, Foxton RM, et al. Effect of primer treatment on bonding of resin cements to zirconia ceramic. Dent Mater. 2010;26:426–432. doi: 10.1016/j.dental.2009.11.159. [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi K, Fujishima A, Manabe A, Kuriyama S, Hotta Y, Tamaki Y, et al. Combination treatment of tribochemical treatment and phosphoric acid ester monomer of zirconia ceramics enhances the bonding durability of resin-based luting cements. Dent Mater J. 2010;29:316–323. doi: 10.4012/dmj.2009-099. [DOI] [PubMed] [Google Scholar]
- 13.Ozcan M, Vallittu PK. Effect of surface conditioning methods on the bond strength of luting cement to ceramics. Dent Mater. 2003;19:725–731. doi: 10.1016/s0109-5641(03)00019-8. [DOI] [PubMed] [Google Scholar]
- 14.Borges GA, Sophr AM, de Goes MF, Sobrinho LC, Chan DC. Effect of etching and airborne particle abrasion on the microstructure of different dental ceramics. J Prosthet Dent. 2003;89:479–488. doi: 10.1016/s0022-3913(02)52704-9. [DOI] [PubMed] [Google Scholar]
- 15.Rüttermann S, Fries L, Raab WH, Janda R. The effect of different bonding techniques on ceramic/resin shear bond strength. J Adhes Dent. 2008;10:197–203. [PubMed] [Google Scholar]
- 16.Ernst CP, Cohnen U, Stender E, Willershausen B. In vitro retentive strength of zirconium oxide ceramic crowns using different luting agents. J Prosthet Dent. 2005;93:551–558. doi: 10.1016/j.prosdent.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Xie H, Chen C, Dai W, Chen G, Zhang F. In vitro short-term bonding performance of zirconia treated with hot acid etching and primer conditioning etching and primer conditioning. Dent Mater J. 2013;32:928–938. doi: 10.4012/dmj.2013-010. [DOI] [PubMed] [Google Scholar]
- 18.Kumbuloglu O, Lassila LV, User A, Vallittu PK. Bonding of resin composite luting cements to zirconium oxide by two air-particle abrasion methods. Oper Dent. 2006;31:248–255. doi: 10.2341/05-22. [DOI] [PubMed] [Google Scholar]
- 19.Wolfart M, Lehmann F, Wolfart S, Kern M. Durability of the resin bond strength to zirconia ceramic after using different surface conditioning methods. Dent Mater. 2007;23:45–50. doi: 10.1016/j.dental.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 20.Korkmaz FM, Bagis B, Turgut S, Ates SM, Ayaz EA. Effect of surface treatments on the bond strength of veneering ceramic to zirconia. J Appl Biomater Funct Mater. 2015;13:17–27. doi: 10.5301/jabfm.5000195. [DOI] [PubMed] [Google Scholar]
- 21.Debnath S, Wunder SL, McCool JI, Baran GR. Silane treatment effects on glass/resin interfacial shear strengths. Dent Mater. 2003;19:441–448. doi: 10.1016/s0109-5641(02)00089-1. [DOI] [PubMed] [Google Scholar]
- 22.Ando F, Komori A, Kojima I. Tensile bond strength of light-cured resin-reinforced glass ionomer cement with delayed light exposure. Odontology. 2001;89:45–48. doi: 10.1007/s10266-001-8184-1. [DOI] [PubMed] [Google Scholar]
- 23.Türkkahraman H, Küçükesmen HC. Porcelain surface-conditioning techniques and the shear bond strength of ceramic brackets. Eur J Orthod. 2006;28:440–443. doi: 10.1093/ejo/cjl026. [DOI] [PubMed] [Google Scholar]
- 24.Lee HS, Kim JS, Yoo SH. A comparative study of the shear bond strength and adhesive failure pattern of metal brackets bonded on natural teeth and porcelain teeth. J Korean Acad Pediatr Dent. 2008;35:195–204. [Google Scholar]
- 25.Kocadereli I, Canay S, Akça K. Tensile bond strength of ceramic orthodontic brackets bonded to porcelain surfaces. Am J Orthod Dentofacial Orthop. 2001;119:617–620. doi: 10.1067/mod.2001.113655. [DOI] [PubMed] [Google Scholar]
- 26.Schmage P, Nergiz I, Herrmann W, Ozcan M. Influence of various surface-conditioning methods on the bond strength of metal brackets to ceramic surfaces. Am J Orthod Dentofacial Orthop. 2003;123:540–546. doi: 10.1067/mod.2003.S0889540602569110. [DOI] [PubMed] [Google Scholar]
- 27.Qeblawi DM, Muñoz CA, Brewer JD, Monaco EA., Jr The effect of zirconia surface treatment on flexural strength and shear bond strength to a resin cement. J Prosthet Dent. 2010;103:210–220. doi: 10.1016/S0022-3913(10)60033-9. [DOI] [PubMed] [Google Scholar]
- 28.Tsuo Y, Yoshida K, Atsuta M. Effects of alumina-blasting and adhesive primers on bonding between resin luting agent and zirconia ceramics. Dent Mater J. 2006;25:669–674. doi: 10.4012/dmj.25.669. [DOI] [PubMed] [Google Scholar]
- 29.Yoshida K, Tsuo Y, Atsuta M. Bonding of dual-cured resin cement to zirconia ceramic using phosphate acid ester monomer and zirconate coupler. J Biomed Mater Res B Appl Biomater. 2006;77:28–33. doi: 10.1002/jbm.b.30424. [DOI] [PubMed] [Google Scholar]
- 30.Lopez JI. Retentive shear strengths of various bonding attachment bases. Am J Orthod. 1980;77:669–678. doi: 10.1016/0002-9416(80)90158-x. [DOI] [PubMed] [Google Scholar]
- 31.McCarthy MF, Hondrum SO. Mechanical and bond strength properties of light-cured and chemically cured glass ionomer cements. Am J Orthod Dentofacial Orthop. 1994;105:135–141. doi: 10.1016/S0889-5406(94)70109-1. [DOI] [PubMed] [Google Scholar]