Abstract
Background
Liver fibrosis is a sign of advanced liver disease and is often an indication for treatment. The current standard for diagnosing liver fibrosis and steatosis is biopsy, but noninvasive alternatives are available; one of the most common is transient elastography (FibroScan).
Objectives
The objective of this analysis was to assess the diagnostic accuracy and clinical utility of transient elastography alone for liver fibrosis and with controlled attenuation parameter (CAP) for steatosis in patients with hepatitis C virus, hepatitis B virus, nonalcoholic fatty liver disease, alcoholic liver disease, or cholestatic diseases. The analysis also aimed to compare the diagnostic accuracy of transient elastography with two alternative noninvasive technologies: FibroTest and acoustic force radiation impulse (ARFI).
Data Sources
Ovid MEDLINE, Ovid MEDLINE In-Process, Ovid Embase, and all EBM databases were searched for all studies published prior to October 2, 2014.
Review Methods
An overview of reviews was conducted using a systematic search and assessment approach. The results of the included systematic reviews were summarized, analyzed, and reported for outcomes related to diagnostic accuracy and clinical utility as a measure of impact on diagnoses, therapeutic decisions, and patient outcomes.
Results
Fourteen systematic reviews were included, summarizing more than 150 studies. The reviews demonstrated that transient elastography (with or without CAP) has good diagnostic accuracy compared to biopsy for the assessment of liver fibrosis and steatosis. Acoustic force radiation impulse and FibroTest were not superior to transient elastography.
Limitations
None of the included systematic reviews reported on the clinical utility of transient elastography.
Conclusions
Transient elastography (with or without CAP) offers a noninvasive alternative to biopsy for the assessment of liver fibrosis and steatosis, given its comparable diagnostic accuracy.
PLAIN LANGUAGE SUMMARY
The liver is the largest internal organ. It supports many bodily functions, including digestion and nutrient storage, as well as aiding the body's immune system. Liver fibrosis is the name used to describe a scarring that can indicate damage to the liver. Viral infections, excessive alcohol use and certain diseases can damage the liver. Consequences of liver damage can be serious, including cirrhosis and death. If liver damage is detected early, it can often be treated effectively. Doctors can test how healthy a patient's liver is by taking a tissue sample with a needle, but there are other ways to check liver health that don't require needles or tissue samples. One option is called transient elastography, a scan that measures how stiff the liver tissue is (the more stiff the tissue, the more damaged the liver). We reviewed the evidence to determine the accuracy of transient elastography. The results showed that transient elastography was about as accurate as taking a tissue sample.
BACKGROUND
Objective of Analysis
The objective of this analysis was to assess the diagnostic accuracy of transient elastography—alone for liver fibrosis and with controlled attenuation parameter (CAP) for steatosis—in patients with hepatitis C virus (HCV), hepatitis B virus (HBV), nonalcoholic fatty liver disease, alcoholic liver disease, or cholestatic diseases. The analysis also aimed to compare the diagnostic accuracy of transient elastography with two alternative noninvasive technologies: FibroTest and acoustic force radiation impulse (ARFI).
Clinical Need and Target Population
Description of Condition
The liver is the largest internal organ, and it performs many complex functions related to the management of blood, fat, cholesterol, vitamins, hormones, and toxins. (1) Liver fibrosis is the scarring of the liver as a result of the accumulation of excess connective tissues. (2) There are hundreds of known causes of liver fibrosis, including alcohol consumption, hepatitis virus infection, and genetic disorders. (1, 2) The accumulation of liver fibrosis is a progressive disease; the increased volume of fibrotic tissue is directly related to the decreased liver function. (1, 2)
Fatty tissue, known as steatosis, can also interfere with normal liver function. Furthermore, steatosis has been associated with the development of liver fibrosis in the absence of any other liver disease. (1)
If left untreated, liver fibrosis progresses over time and makes it increasingly difficult for the liver to function; it can also lead to a number of severe complications. (3) Since the liver interacts with so many other vital organs, such complications may include high blood pressure, renal failure, enlarged spleen, bleeding, and liver cancer. (1, 3)
The severity of fibrosis is typically classified by stage. The most common scoring system is the METAVIR system; it stages fibrosis from 0 to 4, where 0 is no fibrosis and 4 is cirrhosis of the liver. (4, 5)
Diseases of Interest: Prevalence and Incidence
Over 3 million Canadians have some measurable degree of liver disease. (1) The most common causes of morbidity and mortality due to liver disease in Canada are hepatitis B, hepatitis C, alcoholic liver disease (ALD), nonalcoholic fatty liver disease (NAFLD), cirrhosis, and hepatocellular cancer. (1)
Of the hundreds of potential causes of liver disease, the following were identified as the most relevant for this report, given their prevalence and the potential benefit of detecting and diagnosing liver fibrosis with the technology in question. These diseases of interest have a prevalence of between 4.5 per 100,000 Ontarians (NAFLD) and 25.5 per 100,000 Ontarians (ALD). (6)
Hepatitis
Hepatitis is a liver disease caused by a virus that attacks the liver. Hepatitis B and hepatitis C are both of interest given their current prevalence in Ontario and their expected increase in the near future.
People contract hepatitis C virus via contact with contaminated blood; it is most commonly transferred among drug users who share needles. (1) Patients can live with HCV for many years without showing signs or symptoms of disease. One study estimated that for patients with HCV, the probability of progression from one METAVIR stage to the next in a given year was approximately 0.11, with some variation depending on the stage the patient started at. (7) There are many types of HCV (different genotypes), some of which can be treated with medication (certain new medications have a cure rate as high as 90%). (1) If hepatitis C is treated early, progression of liver disease can be prevented, and some early stages of liver fibrosis may even be reversible.
Hepatitis B virus is very infectious and can be passed between individuals via blood or other bodily fluids. (1) Most people who become infected with HBV never feel ill and recover fully, but others may develop chronic hepatitis. (1) While only 5% of those infected go on to develop chronic disease, there are often no signs or symptoms until the liver is severely damaged. However, if HBV is caught early, it can be treated and liver damage prevented. (1)
It is estimated that at least 242,600 Canadians live with HCV, and that number is expected to climb by over 30,000 in the next 5 years; about 50% of cases are in Ontario. (8) Similarly, about 242,750 Canadians have HBV, and that number is expected to increase by approximately 50,000 in the next 10 years; Ontario currently manages approximately 42% of those cases. (8)
Nonalcoholic fatty liver disease
Excess fat distributes throughout the body and can have adverse effects on all organs, including the liver. Fatty liver, or steatosis, is diagnosed when there is a buildup of at least 5% of fat in the liver. (1) It can occur with no signs or symptoms, but it can put strain on the liver, which in turn may cause fibrosis and its related negative side effects. Because fatty liver is a progressive disease, early diagnosis can lead to targeted therapies to prevent further progression and possibly reverse fibrosis. (1)
NAFLD is anticipated to be the fastest-growing cause of liver disease because of the current obesity epidemic; it impacts an estimated 25% of Canadians, and 4.5 per 100,000 Ontarians. (6, 8)
Alcoholic liver disease
Chronic alcohol consumption has been associated with over 50% of chronic liver disease; consumption of 20 g to 30 g of alcohol per day can increase the risk of developing ALD. (9) ALD follows the typical progression from fibrosis to cirrhosis, but it is brought on by alcohol consumption. (9) In clinical practice, ALD may be underdiagnosed: patients may underestimate or underreport their alcoholic consumption, and they may present in complex and diverse ways. (9) Still, when ALD is diagnosed early, behaviour change can prevent further progression of disease and may reverse the early onset of liver fibrosis. (9)
In Ontario, ALD is the most prevalent liver disease, at 25.5 per 100,000; ALD incidence has been on the rise, from 11.7 to 15.5 per 100,000 between 2003 and 2012. (6)
Cholestatic liver diseases
Primary sclerosing cholangitis has an unknown pathology, but it is characterized by ongoing inflammation of the bile ducts and can lead to fibrosis development in the liver. (10) Some patients may show no signs or symptoms until the liver is severely damaged, while others may present with fatigue or jaundice. (10)
Primary biliary cirrhosis causes progressive degradation of the bile ducts; while the exact pathology is unknown, it is thought to be an autoimmune disease. (11) This degradation is often associated with fibrosis as the disease progresses, but thanks to increased access to diagnostic tools (e.g., laboratory tests), screening can now help capture patients before they are symptomatic, leading to improved management and slower progression to cirrhosis. (11)
Most patients diagnosed with primary sclerosing cholangitis are men (70%), but almost all patients diagnosed with primary biliary cirrhosis are women (90–95%). (11, 12) Primary sclerosing cholangitis has been estimated to affect approximately 6.3 people per 100,000 (in the United States); primary biliary cirrhosis is estimated at a slightly higher prevalence of 14.6 per 100,000 (in Norway). (11, 12)
Technology/Technique
The current standard for detecting, staging, and monitoring liver disease in Canada is liver biopsy. (1) There are many reasons to conduct a liver biopsy, including the following: to get information about the current status of a liver; to determine whether treatment is necessary; and to diagnose conditions that may require further monitoring, such as hepatocellular carcinoma. (13) Still, there have been numerous efforts to develop a noninvasive alternative to biopsy. These alternative technologies have had varying degrees of success depending on ease of use, ease of access, and perhaps most importantly, diagnostic accuracy. In Canada, the three most common technologies currently in use are liver biopsy, transient elastography, and FibroTest. (14)
Technologies of Interest: Description
Biopsy
Liver biopsy is the process by which a needle is inserted into the liver to retrieve a small amount of tissue and through visual inspection supported by microscope, the amount of fibrosis in the sample is used to estimate of the amount of fibrosis in the rest of the liver. (1) Biopsy provides important information about the degree and amount of liver damage by measuring fibrosis, steatosis, and necro-inflammation.(15)
Biopsy is still considered the best reference standard for the diagnosis of liver fibrosis, but there are limitations to this technique, including the resources required (biopsy is typically conducted in a hospital setting as an outpatient procedure) and complications for the patient (e.g., pain and in rare cases bleeding or accidental puncture of other organs). (1, 16) Liver biopsy has also been criticized for its sampling bias, in that it relies on only a very small sample (approximately 1/50,000 of an adult liver) to determine the status of the whole liver and can lead to false negatives and missed diagnoses. (16, 17) A 10% to 12% discordance in diagnosis has been shown to be due to variation in locations sampled, and to intra-observer error when a smaller (3 cm) sample is assessed rather than a larger one (4 cm). (18)
Given biopsy's limitations, caution is needed when comparing it with other diagnostic technologies. Mehta et al (19) estimated that under perfect conditions, a comparative diagnostic test couldn't be expected to achieve an area under the receiver operating characteristic (AUROC) curve of > 0.90 to diagnose liver fibrosis of METAVIR F ≥ 2 (where an AUROC of 1 is a perfect test). (19) Comparative technologies may be underestimated for effectiveness and could be inappropriately disregarded because they don't appear to measure up to the effectiveness of biopsy, which itself is flawed. (19) In fact, when the accuracy of biopsy is presumed to be 80%, a comparative technology with an AUROC of 0.76 actually represents an AUROC of 0.93 to 0.99 for diagnosing true disease.(19)
Transient Elastography
Transient elastography is sold under the brand name FibroScan (manufactured by EchoSens in France and distributed in Canada by KNS Canada Inc). TE provides a continuous measure of liver stiffness measured in kilopascals (kPa). (20) It is completely noninvasive, conducted using a probe with a tip about the size of a pen that is placed on the surface of the skin near the liver. TE can be provided by any trained person in an office or clinic environment, and the output reading is then interpreted by an appropriate health care provider (personal communication, KNS Canada Inc., November 2014).
Transient elastography does not directly measure liver fibrosis; it is a measure of stiffness that has been associated with the degree of fibrosis. (21, 22) While there are no official cut-offs to map TE values to METAVIR stages, some ranges have been developed, supported by academic research and clinical experience. These ranges have become part of the standard operation of TE and are summarized in a table provided by the manufacturer/distributor (personal communication, KNS Canada Inc., November 2014).
Transient elastography ensures that if there is an error in reading, no output is provided. (20) This is advantageous in that only good-quality readings are reported, but when the technology was first developed, a large number of errors were associated with patients who had large amounts of visceral fat, preventing the TE waves from penetrating the liver as intended. (21) The manufacturer has since developed multiple probes to offer different options for technicians, substantially decreasing the proportion of the population in whom a reading is not possible. (20)
FibroScan also includes a newer technology known as controlled attenuation parameter (CAP) measurement, which can offer simultaneous measurement of steatosis. CAP is measured only if there is a valid TE measurement. (20) The ultrasound attenuation assesses steatosis of the liver by converting the amplitude of the ultrasound to waves expressed in decibels per metre (dB/m). (20)
FibroTest
A variety of blood serum tests have been developed to indicate concerns with the liver, (23) but FibroTest is the most commonly used across Canada. (14) It is an algorithm based on a panel that consists of several biomarkers (α2-macrogobulin, haptoglobulin, apolipoprotein A1, gammaglutamyl transpeptidase [GGT], total bilirubin, and alanine aminotransferase [ALT]) plus age and sex to predict the severity of liver fibrosis. (24) Similar to TE, the outputs are mapped to the METAVIR stages using tables. (24) FibroTest goes by the brand name FibroSure in the United States; for the purposes of this report, both are considered.
Other Technologies
Several other noninvasive imaging techniques have been evaluated for the assessment of liver fibrosis. After consultation with expert hepatologists in Ontario, ARFI was considered to be the most relevant comparative technology. Other technologies did not have enough dissemination pressure (such as shear wave) or were too cumbersome to access (such as magnetic resonance imaging).
ARFI has several potential advantages over TE, including the fact that it can be implemented on a standard ultrasound machine. (25) It may be more applicable for assessing complications beyond liver fibrosis (such as ascites), and the examined regions are numerous and managed by the operator. (25) While it is generally accepted that ARFI is probably equivalent to TE for diagnosing cirrhosis, there is some debate about its diagnostic effectiveness for less severe states. (25)
Ontario Context
There are currently 20 TE units in Ontario (personal communication, KNS Canada Inc., November 2014). Units are held largely in academic hospitals, except for one that is part of a mobile unit (personal communication, KNS Canada Inc., November 2014).
At the time of writing, patients with hepatitis C and hepatitis B could access treatment through the Ministry of Health and Long-Term Care Exceptional Access Program. Among other criteria, such as age and confounding patient factors, the Exceptional Access Program requires that patients present with liver fibrosis at METAVIR stage F ≥ 2, and will accept the results of either biopsy, TE, or FibroTest.
TE is not currently funded by the Ministry of Health and Long-Term Care; costs are absorbed by academic units or as an out-of-pocket expense for patients. (6) In fact, most Canadian provinces do not have a billing code for TE.
Canadian Context
A recent survey composed of Canadian gastroenterologists (64%), hepatologists (16%), infectious disease specialists (10%), and family and internal medicine specialists (10%) was conducted to determine which diagnostic tests they are using to assess liver fibrosis. (14) According to this survey, 46% of liver assessments are conducted using biopsy, 39% using TE, and 8% using FibroTest. (14) The rest are conducted using alternative technologies such as magnetic resonance elastography or other blood serum tests. (14)
Regulatory Status
TE is an approved Health Canada class 3 device (licence 80129).
EVIDENCE-BASED ANALYSIS
Research Questions
Clinical Utility
What is the clinical utility, with respect to the impact on diagnosis, therapeutic decision, or patient outcomes, of TE versus liver biopsy when used for the assessment of liver fibrosis in one or more of the disease areas of interest1?
What is the clinical utility, with respect to the impact on diagnosis, therapeutic decision or patient outcomes, of TE with CAP versus liver biopsy when used for the assessment of steatosis in one or more of the disease areas of interest1?
Diagnostic Accuracy
What is the diagnostic accuracy of TE versus liver biopsy for the assessment of liver fibrosis in one or more of the disease areas of interest1?
What is the diagnostic accuracy of TE versus FibroTest for the assessment of liver fibrosis in one or more of the disease areas of interest1?
What is the diagnostic accuracy of TE versus ARFI for the assessment of liver fibrosis in one or more of the disease areas of interest1?
What is the diagnostic accuracy of TE with CAP versus liver biopsy for the assessment of steatosis in one or more of the disease areas of interest1?
Research Methods
Literature Search
Search Strategy
A literature search was performed on October 2, 2014, using Ovid MEDLINE, Ovid MEDLINE In-Process and Other Non-Indexed Citations, Ovid Embase, and all EBM databases, for studies published prior to October 2, 2014. (Appendix 1 provides details of the search strategies.) Abstracts were reviewed by a single reviewer and, for those studies meeting the eligibility criteria, full-text articles were obtained. Reference lists and known health technology websites were also examined for any additional relevant studies not identified through the search.
Inclusion Criteria
English-language full-text publications
studies published prior to October 2, 2014
studies that examined TE for measuring fibrosis, with or without the addition of CAP to measure steatosis
studies that included patients with the following conditions: HCV, HBV, chronic hepatitis not specified, NAFLD, ALD, or cholestatic diseases
studies that compared TE to liver biopsy, FibroTest (or FibroSure), or ARFI
Exclusion Criteria
case reports, editorials, letters, comments, and conference abstracts
studies that measured organs other than the liver (e.g., spleen, heart, pancreas, lung, breast tissue)
studies that assessed the liver in populations other than those listed in the inclusion criteria (e.g., liver cancer, liver transplant, renal transplant, arthritis, psoriasis, bleeding disorders such as hemophilia, insulin resistance, chronic pancreatitis, Gaucher disease, cystic fibrosis, Crohn's disease, Wilson's disease, beta-thallasemia, asymptomatic healthy living donors, general population screening, pediatric liver diseases)
studies that compared technologies other than those listed in the inclusion criteria (e.g., magnetic resonance elastography, supersonic shear imaging, computed tomography, positron emission tomography, ultrasound, magnetic resonance imaging, and additional biomarkers such as aspartate aminotransferase to platelet ratio index and aspartate aminotransferase/alanine aminotransferase ratio)
studies that examined the use of TE to screen for disease (e.g., among the general population); monitor disease progression and regression after antiviral therapy; identify and triage patients who should access treatment; examine the prognostic value of liver stiffness in association to adverse patient outcomes; or examine TE in combination with other tests
studies where data on interventions and/or populations of interest could not be abstracted
Outcomes of Interest
-
clinical utility as measured by:
-
–
impact on diagnosis (e.g., test usefulness to clinicians for assessment)
-
–
impact on therapeutic decisions (e.g., change in patient management/treatment)
-
–
impact on patient outcomes: mortality, liver transplant, esophageal varices, development of liver cancer, other complications of cirrhosis
-
–
-
diagnostic accuracy as measured by:
-
–
sensitivity and specificity
-
–
receiver operating characteristic curve
-
–
secondary outcomes of interest, where available:
-
–
diagnostic odds ratio
diagnostic positive and negative likelihood ratios
Study Selection Process
An overview of reviews was selected as the most appropriate method for study selection to address the questions of interest. This decision was made a priori after scoping the topic, considering the volume of literature (specifically systematic reviews that addressed the questions of interest) and consulting with clinical experts regarding the potential harms and benefits of disseminating this technology in Ontario. The Cochrane overview of reviews protocol was consulted as a guide to the method applied in this report. (26)
Statistical Analysis
Statistical analyses are reported as originally presented in the included systematic reviews. Where data were available, additional calculations were conducted using Review Manager 1.3 and Meta-Disc 1.4. (27, 28) These additional calculations included diagnostic odds ratios and likelihood ratios and were calculated using random effects models with 95% confidence intervals (CIs).
A positive likelihood of > 10 is considered a threshold for a “rule-in” test, where the risk of missing a diagnosis for a person is undesirable. (29) Similarly, a negative likelihood ratio of < 0.1 is a threshold for a “rule-out” test, where the risk of wrongly diagnosing an individual with disease is undesirable. (29) For the purposes of this report, a balance between the two was preferred; while these thresholds were understood, they were not requirements to be met.
Quality of Evidence
The Assessment of Multiple Systematic Reviews (AMSTAR) measurement tool was used to assess the methodologic and reporting quality of systematic reviews (Appendix 2, Table A1). (30)
The quality of evidence for the body of literature of the individual studies was reported as conducted by each systematic review (as suggested by the Cochrane overview of reviews protocol). (26)
Results of Evidence-Based Analysis
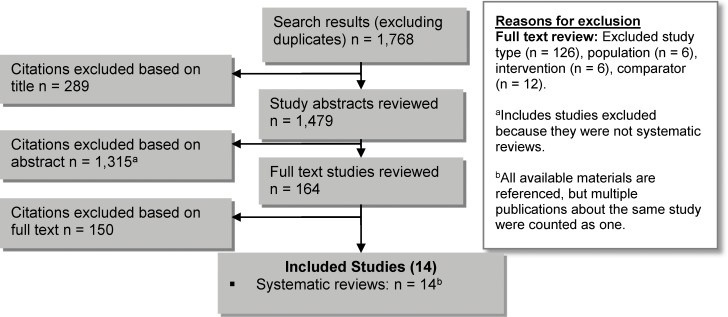
The database search yielded 1,768 citations published up to October 2, 2014 (with duplicates removed). Articles were excluded based on information in the title and abstract. The full texts of potentially relevant articles were obtained for further assessment. Figure 1 shows the breakdown of when and for what reason citations were excluded from the analysis.
Figure 1: Citation Flow Chart.
Fourteen systematic reviews met the inclusion criteria. The reference lists of the included studies and health technology assessment websites were hand-searched to identify other relevant studies, but no additional citations were included.
The included reviews were published between 2007 and 2014 and varied in their focus: some were more inclusive (any cause of liver disease and any diagnostic technology), while others were limited to only a few technologies or populations (Table 1). The reviews also ranged in quality, with AMSTAR scores from 3 to 10. Two reviews were written by the TE inventor. (31, 32)
Table 1:
Characteristics of Systematic Reviews
| Author, Year | Literature Search | Objective | Diagnostic Technology | Population | Conclusion | AMSTAR Score |
|---|---|---|---|---|---|---|
| Bota et al, 2013 (35) | Up to May 2012 | To assess the diagnostic performance of 2 specific technologies compared to biopsy for the diagnosis of liver fibrosis |
|
Chronic hepatitis; excluded post-liver transplant | ARFI has similar value to TE for significant fibrosis and cirrhosis | 7 |
| Chon et al, 2012 (36) | 2002 to March 2011 | To assess the diagnostic accuracy of TE to quantify liver fibrosis |
|
Chronic HBV | TE can be performed with good diagnostic accuracy in patients with chronic HBV | 8 |
| Friedrich-Rust et al, 2008 (37) | 2002 to April 2007 | To assess the diagnostic performance of TE for the diagnosis of liver fibrosis and patient factors that may impact the accuracy |
|
All causes of liver disease | TE is an excellent diagnostic tool for cirrhosis, but the underlying cause of liver disease impacts its accuracy for significant fibrosis | 8 |
| Friedrich-Rust et al, 2012 (38) | Up to October 2010 | To assess the diagnostic performance of ARFI for the diagnosis of liver fibrosis |
|
All causes of liver disease | ARFI has good diagnostic accuracy for staging liver fibrosis | 4 |
| Kwok et al, 2014 (39) | Up to June 2013 | To assess the diagnostic performance of 3 specific technologies compared to biopsy for NASH and liver fibrosis |
|
NAFLD | Noninvasive tests are good at excluding advanced cirrhosis and could be used as part of the initial assessment, but further evaluation of biomarkers are needed | 8 |
| Poynard et al, 2008 (32)a | 1991 to 2008 | To assess the diagnostic performance of any noninvasive technology for the assessment of liver fibrosis |
|
All causes of liver disease | There is no evidence to justify biopsy as a first-line estimate of liver fibrosis; biomarkers could be used as an alternative | 3 |
| Poynard et al, 2011 (31)a | February 2001 to December 2010 | To update the diagnostic accuracy evidence specific to hepatitis B from an earlier review (32) |
|
HBV | TE and FibroTest were the most validated assessments of fibrosis in patients with HBV, but there were questions as to the reliability of FibroScan (TE brand name) | 4 |
| Shaheen et al, 2007 (40) | January 1997 to October 2006 | To assess the diagnostic performance of 2 specific technologies compared to biopsy for the diagnosis of liver fibrosis |
|
HCV | TE and FibroTest have excellent utility for diagnosing HCV-related cirrhosis, but are less accurate for earlier stages of fibrosis; these tests are not ready to fully replace biopsy in the diagnosis of disease | 10 |
| Shi et al, 2014 (41) | Up to May 2013 | To assess the diagnostic performance of the CAP add-on to TE to assess steatosis |
|
All causes of steatosis | CAP has good sensitivity and specificity for diagnosing steatosis, but it has limited utility and should not be disseminated for widespread use | 9 |
| Steadman et al, 2013 (42, 43) | 2001 to June 2011 | To assess the diagnostic performance of TE and ARFI in adult liver disease, as well as TE in pediatric liver disease and other potential applications, such as use in examining breast tissue |
|
HCV, HBV, NAFLD, chronic liver disease, liver transplant patients | TE is an accurate diagnostic method for moderate fibrosis or cirrhosis; more studies are necessary to establish the effectiveness in pediatric patients and other potential applications | 10 |
| Stebbing et al, 2010 (44) | Not specified, but prior to February 2009 | To assess the diagnostic performance of TE for the diagnosis of liver fibrosis |
|
All causes of liver disease | More research is needed to improve the sensitivity and establish the clinical utility of TE | 6 |
| Talwalkar et al, 2007 (45) | Up to January 2007 | To assess the diagnostic performance of TE for the diagnosis of liver fibrosis |
|
All causes of liver disease | TE is a clinically useful test for the diagnosis of cirrhosis | 8 |
| Tsochatzis et al, 2011 (46) | Up to May 2009 | To assess the diagnostic performance of TE for the diagnosis of liver fibrosis |
|
All causes of liver disease | TE has good sensitivity and specificity for cirrhosis—less so for lesser degrees of fibrosis; it should be cautiously applied to regular clinical practice due to a lack of validated cut-offs for stiffness values | 9 |
| Tsochatzis et al, 2015 (47, 48) | 1998 to April 2012 | To assess the diagnostic performance of noninvasive liver tests to diagnose liver fibrosis, and the incremental cost-effectiveness of the tests in managing disease |
|
HCV, HBV, ALD, NAFLD | The most cost-effective strategies were: for HCV, treating all patients without assessment; for HBV, treating all patients who were hepatitis B e antigen–negative without assessment, but there was uncertainty for hepatitis B e antigen–positive patients; for ALD, biopsy was cost-effective under certain assumptions; for NAFLD, it was not possible to determine the cost-effectiveness of fibrosis testing | 9 |
Abbreviations: ALD, alcoholic liver disease; AMSTAR, Assessment of Multiple Systematic Reviews; ARFI, acoustic radiation force impulse; CAP, controlled attenuation parameter; CK-18, blood test of plasma cytokeratin-18 fragments; HBV, hepatitis B virus; HCV, hepatitis C virus; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; TE, transient elastography.
Poynard is the inventor of the FibroScan technology.
The reviews included 8 to 302 research studies depending on their focus, and represented a breadth of inclusion/exclusion criteria and different literature search dates. Approximately 150 unique studies that focused on the interventions of interest were included in the different reviews. Across all studies, patients with HCV made up the largest proportion of the populations, and patients with cholestatic disease the smallest. Ten of the 14 reviews conducted a quality assessment of the body of literature using some variation of the quality assessment tool for diagnostic accuracy studies (QUADAS) system (Appendix 2, Tables A2 and A3). (33, 34) The 14 included reviews are summarized in Tables 1 and 2.
Table 2:
Results From Systematic Reviews
| Author, Year | Studies, N | Sample Size, N | Sample Size by Liver Disease, N | Male, % | Mean Age, y | Mean BMI, kg/m2 | Quality of Evidencea |
|---|---|---|---|---|---|---|---|
| Bota et al, 2013 (35) | 13 (11 full texts, 2 abstracts) | 1,163 | HCV: 448 Nonspecified chronic hepatitis: 715 | NR | NR | NR | 80% of QUADAS-2 measures for good quality were met |
| Chon et al, 2012 (36) | 18 | 2,772 | Chronic HBV: 2,772 | 48.6 | 44.8 | 24b | Summary measure not calculable; the study reported that all studies fulfilled 10 to 14 of a possible 14 QUADAS measures for good quality |
| Friedrich-Rust et al, 2008 (37)b | 50 (15 full texts, 35 abstracts) | 11,275 | HCV: 2,216 HBV: 255 NAFLD: 1,188 ALD: 479 Cholestatic diseases: 315 Other hepatitis disease (e.g., HCV/HIV coinfection, or otherwise could not be determined): 6,822 | NR | 50.2 | 24.8 | 71% of QUADAS measures for good quality were met |
| Friedrich-Rust et al, 2012 (38) | 8 | 518 | HCV: 380 HBV: 51 NAFLD: 77 ALD: 4 Cholestatic diseases: 5 Autoimmune hepatitis: 1 | 49 | 51 $pL SD 13 | NR | NR |
| Kwok et al, 2014 (39) | 22 | 1,047c | NAFLD: 1,047 | NR | NR | NR | 98% of QUADAS measures for good quality were metc (limited to the 9 studies evaluating TE) |
| Poynard et al, 2008 (32) | 66d | NR | NR | NR | NR | NR | NR |
| Poynard et al, 2011 (31) | 18 | 2,714 | HBV: 2,714 | NR | 39.6b | NR | NR |
| Shaheen et al, 2007 (40) | 12 | 1,981 | HCV: 1,528 HCV coinfection with HIV, HBV, ALD, or transplant: 453 | 60 | 46 | NR | 98% of QUADAS measures for good quality were met |
| Shi et al, 2014 (41) | 9 | 1,771 | HCV: 821 HBV: 313 NAFLD: 421 ALD: 45 Hepatitis nonspecified: 67 Other: 104 | 63.9b | 48.3b | 26.1b | 86% of QUADAS measures for good quality were met |
| Steadman et al, 2013 (42, 43) | 64 | 6,028 | HCV: 1,089 HBV: 3,118 NAFLD: 873 Cholestatic disease: 150 Transplant: 371 Other hepatitis disease (e.g., HCV/HIV coinfection or otherwise could not be determined): 427 | 61.2b | 49.7b | NR | 97% of QUADAS measures for good quality were met (limited to the 57 studies evaluating TE) |
| Stebbing et al, 2010 (44) | 22 | 4,625 | HCV: 801 NAFLD: 97 Cholestatic disease: 150 Mixed populations including coinfection and otherwise could not be determined: 3,577 | 55.4b | 52.0b | 24.5b | NR |
| Talwalkar et al, 2007 (45) | 9 | 2,083 | HCV: 985 Other or otherwise could not be determined: 1,098 | 48.3b | 50.8b | 24.5b | Summary measure not calculable; the study reported that all studies fulfilled 10 to 14 of a possible 14 QUADAS measures for good quality |
| Tsochatzis et al, 2011 (46) | 40e | 7,661 | HCV: 4,353 HBV: 1,089 NAFLD: 168 ALD: 382 Transplant: 390 Various: 1,279 | NR | 50.3b | 24.6b | Summary measure was not calculable; the study reported that no study was free of risk of bias based on quality assessment using QUADAS |
| Tsochatzis et al, 2015 (47, 48) | 302e | NR | HCV: 162 studies HBV: 52 studies NAFLD: 49 studies ALD: 12 studies | NR | NR | NR | 55% of QUADAS-2 measures for good quality were met |
Abbreviations: ALD, alcoholic liver disease; HBV, hepatitis B virus; HCV, hepatitis C virus; NAFLD, nonalcoholic fatty liver disease; NR, not reported; QUADAS (34) and QUADAS-2 (33), quality assessment tool for diagnostic accuracy studies; SD, standard deviation; TE, transient elastography.
The body of evidence quality summary score was based on results reported by original reviews, summarized as a percentage of total possible measures for good quality. For example, if a body of literature consisted of 6 studies and was evaluated using QUADAS-2, a perfect score would be (6*7)/(6*7). Tables A2 and A3 summarize the quality assessments conducted by the reviews.
Mean of the means was calculated based on the information provided in the published review.
Modified QUADAS to a 13-item questionnaire by excluding the one question about whether the reference test results were blinded.
Poynard et al (32) summarized individual studies pulled from other published systematic reviews that are already accounted for in this report and was therefore excluded from further evaluation to minimize a known duplication bias.
Includes both full texts and abstracts; the number of each was not reported.
Clinical Utility
Clinical utility, the assessment of the impact of a diagnostic test on patient outcomes, was not evaluated in any of the systematic reviews identified.
Transient Elastography for the Assessment of Liver Fibrosis
No studies were identified that examined the clinical utility of transient elastography versus biopsy as a diagnostic test for liver fibrosis. However, since there is a well-established link between fibrosis stage and diagnosis, therapy, and patient outcomes for patients with viral hepatitis, the utility of TE could be presumed in this patient population because it could provide a noninvasive alternative to biopsy for diagnosing liver fibrosis if it were proven to have comparable diagnostic accuracy. (49)
CAP for the Assessment of Liver Steatosis
Only one review examined CAP. The review did not assess for clinical utility, but the authors noted that CAP was not yet available for the XL probe, which is designed for obese patients. (41) Since that review was published, the Echosens website has advertised that CAP is available for the XL probe. (20) However, uncertainty remains around the utility of steatosis assessment beyond clinical diagnosis alone; its impact on therapeutic decisions (and ultimately patient outcomes) is not clear given that treatment for NAFLD is management of lifestyle risk factors related to weight, and weight loss. (50) There is the potential for benefit in screening for steatosis in otherwise clinically healthy individuals, but that discussion is beyond the scope of this analysis.
Diagnostic Accuracy
Transient Elastography for the Assessment of Liver Fibrosis
Eleven reviews reported on the diagnostic accuracy of TE compared to liver biopsy for liver fibrosis. Of these, there was variation in the underlying cause of liver disease and the cut-off values of TE stiffness used to define METAVIR stages (Tables 3 and 4).
Table 3:
Diagnosing METAVIR Stages—TE Liver Stiffness Cut-off Values (kPa)
| Author, Year | Study Population | F ≥ 2 | F ≥ 3 | F = 4 |
|---|---|---|---|---|
| Bota et al, 2013 (35) | Multiple diseasesa | Mean 7.37b | NR | Mean 12.77b |
| Chon et al, 2012 (36) | Chronic HBV | Mean 7.8 | Mean 8.8 | Mean 11.7 |
| Friedrich-Rust et al, 2008 (37) | Multiple diseasesa | Mean 7.80 | Mean 10.61b | Mean 13.97b |
| Kwok et al, 2014 (39) | NAFLD | Mean 7.06b,c | Mean 9.36b,c | Mean 12.87b,c |
| Poynard et al, 2011 (31) | HBV | NR | NR | NR |
| Shaheen et al, 2007 (40) | HCV | Range 7.1–8.8 | NR | Range 12.5–14.8 |
| Steadman et al, 2013 (42, 43) | Multiple diseasesa | Mean 7.4 $pL 1.5 | Mean 9.9 $pL 2.4 | Mean 13.2 ± 3.5 |
| Stebbing et al, 2010 (44) | Multiple diseasesa | Mean 7.81 (95% CI 7.77–7.85) | NR | Mean 15.56 (95% CI 15.5–15.70) |
| HCV | Mean 8.44 ± 0.74 | NR | Mean 16.14 ± 4.26 | |
| HBV/HCV | Mean 8.62 ± 0.42 | NR | Mean 16.90 ± 5.20 | |
| HIV/HCV | Mean 6.39 ± 1.24 | NR | Mean 14.57 ± 0.05 | |
| Primary biliary cirrhosis | Mean 7.30 (1 study only) | NR | Mean 16.68 ± 0.82 | |
| NAFLD | Mean 7.47 ± 0.55 | NR | Mean 13.92 ± 2.48 | |
| Mixed patients | Mean 7.02 ± 1.03 | NR | Mean 15.45 ± 1.78 | |
| Transplant patients | Mean 8.59 ± 0.72 | NR | Mean 15.24 ± 5.77 | |
| Talwalkar et al, 2007 (45) | Multiple diseasesa | Range 4.0–8.8 | NR | Range 11.7–17.6 |
| Tsochatzis et al, 2011 (46) | Multiple diseasesa | Mean 7.3 ± 1.4 | Mean 10.2 ± 1.9 | Mean 15 ± 4.1 |
| HCV | Mean 7.6 (NR) | Mean 10.9 (NR) | Mean 15.3 (NR) | |
| HBV | Mean 7.0 (NR) | Mean 8.2 (NR) | Mean 11.3 (NR) | |
| Tsochatzis et al, 2015 (47, 48) | HCV | Range 5.2–10.1 | Range 8.6–15.4 | Range 9.2–17.3 |
| HBV | Range 6.3–8.9 | Range 7.3–10.7 | Range 9.4–16.0 | |
| NAFLD | NR | Range 7.5–10.4 | Range 10.3–17.5 | |
| ALD | NR | Range 11.0–12.5 | Range 11.4–25.8 |
Abbreviations: ALD, alcoholic liver disease; CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; NAFLD, nonalcoholic fatty liver disease; NR, not reported; TE, transient elastography.
Aggregate results compiling multiple causes of liver diseases as reported in original studies; summary of included diseases provided in Table 2.
Calculated based on information provided in the published review.
Information is based on the M probe; this review noted that lower cut-offs were needed for the XL probe but did not provide further information.
Table 4:
Accuracy of TE Versus Biopsy for the Diagnosis of Liver Fibrosis, by METAVIR Stage
| Author, Year | Study Details | F ≥ 2 | F ≥ 3 | F = 4 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Population | Studies (Pts), N | Studies (Pts), N | SROC Curve (95% CI) | Sensitivity (95% CI) Specificity (95% CI) | Studies (Pts), N | SROC Curve (95% CI) | Sensitivity (95% CI) Specificity (95% CI) | Studies (Pts), N | SROC Curve (95% CI) | Sensitivity (95% CI) Specificity (95% CI) | |
| Bota et al, 2013 (35) | Multiple diseasesa | 13 (1,163) | 10 (1,016) | 0.87 (0.83–0.89) | 0.78 (0.72–0.83) 0.84 (0.75–0.90) | NR | NR | NR NR | 13 (1,163) | 0.93 (0.91–0.95) | 0.89 (0.80 0.94) 0.87 (0.82–0.91) |
| HCV | 4 (NR) | NR | NR | NR NR | NR | NR | NR NR | 4 (NR) | NR | 0.92 (0.78–0.97) 0.86 (0.82–0.90) | |
| Chon et al, 2012 (36) | Chronic HBV | 18 (2,772) | 12 (2,000) | 0.86 (0.86–0.86) | 74.3 (NR) 78.3 (NR) | 9 (1,792) | 0.89 (0.89–0.89) | 74.0 (NR) 63.8 (NR) | 16 (2,614) | 0.93 (0.93–0.93) | 84.6 (NR) 81.5 (NR) |
| Friedrich-Rust et al, 2008 (37) | Multiple diseasesa | 33 (4,992)b | 25 (3,685)b | Mean AUROC: 0.84 (0.82–0.86) | NR NR | 27 (3,946)b | Mean AUROC: 0.89 (0.88–0.91) | NR NR | 25 (4,557)b | Mean AUROC: 0.94 (0.93–0.95) | NR NR |
| HCV | NR | NR | Mean AUROC: 0.84 (0.80–0.89) | NR NR | NR | NR | NR NR | NR | NR | NR NR | |
| HCV and other liver conditions | NR | NR | Mean AUROC: 0.83 (0.80–0.86) | NR NR | NR | NR | NR NR | NR | NR | NR NR | |
| Non-HCV patients | NR | NR | Mean AUROC: 0.84 (0.81–0.87) | NR NR | NR | NR | NR NR | NR | NR | NR NR | |
| Kwok et al, 2014 (39) | NAFLD | 8 (854) M probe | 7 (800) | 0.83 (0.79–0.87)c | 0.79 (0.72–0.84) 0.75 (0.71–0.79) | 8 (854) | 0.89 (0.86–0.93)c | 0.85 (0.73–0.92) 0.85 (0.79–0.90) | 6 (639) | 0.96 (0.94–0.99)c | 0.92 (0.82–0.97) 0.92 (0.86–0.96) |
| 1 (193) XL probe | 1 (193) | 0.80 (NR) | NR NR | 1 (193) | 0.85 (NR) | NR NR | 1 (193) | 0.91 (NR) | NR NR | ||
| Poynard et al, 2011 (31) | HBV | 5 (618)b | 4 (NR) | 0.84 (0.78–0.89) | NR NR | NR | NR | NR NR | NR | 0.93 (0.87–0.99) | NR NR |
| Shaheen et al, 2007 (40) | HCV | 4 (546) | 4 (546) | 0.83 (0.01–1.00) | 0.64 (0.50–0.76) 0.87 (0.80–0.91) | NR | NR | NR NR | 3 (506) | 0.95 (0.87–0.99) | 0.86 (0.78–0.91) 0.93 (0.90–0.95) |
| Steadman et al, 2013 (42, 43) | Multiple diseasesa | 57 (9,415)b | 45 (NR) | 0.88 (0.84–0.90) | 0.80 (0.76–0.83) 0.81 (0.77–0.85) | 35 (NR) | 0.92 (0.89–0.94) | 0.84 (0.81–0.87) 0.87 (0.83–0.90) | 49 (NR) | 0.94 (0.91–0.96) | 0.86 (0.82–0.89) 0.89 (0.87–0.91) |
| HBV | 8 (1,092)b | 5 (710) | 0.81 (0.78–0.84) | 0.77 (0.68–0.84) 0.72 (0.55–0.85) | 4 (528)b | 0.89 (0.85–0.91) | 0.83 (0.75–0.88) 0.91 (0.75–0.86) | 8 (1,092)b | 0.86 (0.82–0.89) | 0.67 (0.57–0.75) 0.87 (0.83–0.91) | |
| HCV | 14 (3,118) | 13 (2,732)b | 0.89 (0.86–0.91) | 0.76 (0.61–0.86) 0.86 (0.77–0.92) | 8 (1,135)b | 0.92 (0.89–0.94) | 0.88 (0.84–0.92) 0.91 (0.83–0.96) | 12 (2,887)b | 0.94 (0.92–0.96) | 0.85 (0.77–0.91) 0.91 (0.87–0.93) | |
| NAFLD | 6 (684) | 5 (630) b | 0.78 (0.74–0.82) | 0.77 (0.70–0.83) 0.75 (0.70–0.79) | 6 (684) b | Not conducted due to heterogeneity | 4 (469) b | 0.96 (0.94–0.97) | 0.92 (0.77–0.98) 0.95 (0.88–0.98) | ||
| Cholestatic diseases | 2 (150) | 1 (95)b | NR | 0.8 0.9 | 2 (150)b | NR | Range: 0.6–0.9 Range: 0.9–1.0 | 1 (95)b | NR | 0.9 1.0 | |
| Stebbing et al, 2010 (44) | Multiple diseasesa | 22 (4,625) | 17 (3,066) | NR | 0.72 (0.71–0.72) 0.82 (0.82–0.83) | NR | NR | NR NR | 17 (4,052) | NR | 0.84 (0.84–0.85) 0.95 (0.94–0.95) |
| Talwalkar et al, 2007 (45) | Multiple diseasesa | 9 (2,083) | 7 (> 1,101)d | 0.87 (0.83–0.91)b | 0.70 (0.67–0.73) 0.84 (0.80–0.88) | NR | NR | NR NR | 9 (2,083) | 0.96 (0.94–0.98)b | 0.87 (0.84–0.90) 0.91 (0.89–0.92) |
| Tsochatzis et al, 2011 (46) | Multiple diseasesa | 40 (7,723) | 31 (5,919) | NR | 0.79 (0.74–0.82) 0.78 (0.72–0.83) | 24 (NR) | NR | 0.82 (0.78–0.86) 0.86 (0.82–0.89) | 30 (6,530) | NR | 0.83 (0.79–0.86) 0.89 (0.87–0.91) |
| HCV | 17 (4,353) | 14 (NR) | NR | 0.78 (0.71–0.84) 0.80 (0.71–0.86) | NR | NR | NR NR | 11 (NR) | NR | 0.83 (0.77–0.88) 0.90 (0.87–0.93) | |
| HBV | 10 (1,089) | 4 (NR) | NR | 0.84 (0.67–0.93) 0.78 (0.68–0.85) | NR | NR | NR NR | 6 (NR) | NR | 0.80 (0.61–0.91) 0.86 (0.82–0.94) | |
| Tsochatzis et al, 2015 (47, 48) | HCV | 37 (NR) | 37 (NR) | 0.87 (0.83–0.90)c | 0.79 (0.74–0.84) 0.83 (0.77–0.88) | 19 (NR) | 0.94 (0.92–0.96)c | 0.88 (0.82–0.92) 0.90 (0.85–0.93) | 36 (NR) | 0.96 (0.94–0.97)c | 0.89 (0.84–0.92) 0.91 (0.89–0.93) |
| HBV | 13 (NR) | 13 (NR) | 0.83 (0.76–0.90)c | 0.71 (0.62–0.78) 0.84 (0.74–0.91) | 13 (NR) | 0.86 (0.82–0.91)c | 0.69 (0.58–0.78) 0.84 (0.79–0.89) | 13 (NR) | 0.92 (0.89–0.96)c | 0.86 (0.79–0.91) 0.85 (0.78–0.89) | |
| NAFLD | 8 (NR) | NR | NR | NR NR | 8 (NR) | 0.83 (0.80–0.86)c | 0.82 (0.74–0.88) 0.84 (0.78–0.89) | 4 (NR) | 0.96 (0.94–0.99)c | 0.96 (0.83–0.99) 0.89 (0.85–0.92) | |
| ALD | 6 (NR)b | NR | NR | NR NR | 4 (NR) | 0.90 (0.83–0.97)c | 0.87 (0.64–0.96) 0.82 (0.67–0.91) | 6 (NR) | 0.90 (0.87–0.94)c | 0.86 (0.76–0.92) 0.83 (0.74–0.89) | |
Abbreviations: ALD, alcoholic liver disease; AUROC, area under the receiving operator characteristic; CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; NAFLD, nonalcoholic fatty liver disease; NR, not reported; Pts, patients; SROC, summary receiver operating characteristic; TE, transient elastography.
Aggregate results compiling multiple causes of liver diseases as reported in original studies; summary of included diseases provided in Table 2.
Calculated based on information provided in the published review.
Calculated based on the number of true positives/false positives/false negatives/true negatives information provided in the published review using random effects models; see Appendix 3 for a sample graph.
There was some uncertainty around the exact number of patients included due to conflicting information in a forest plot vs. a summary table; the author was contacted for clarification but did not reply.
The diagnostic accuracy of TE appeared to be greater overall for METAVIR stage 4 than for other stages, across all diseases (Table 4). However, after adjusting for the known errors related to using biopsy as the reference standard for diagnosing METAVIR F ≥ 2, this differential disappeared, since an AUROC of 0.76 could be considered equivalent to an AUROC of 0.93 to 0.99 for diagnosing true disease presence/absence. (19) When assessing diagnostic accuracy by individual disease, there did not appear to be a substantial difference for one disease over any of the others (Table 4).
Secondary Outcomes of Interest and Sensitivity Analyses
It was possible to report on the secondary outcomes of diagnostic odds ratios (DORs) and likelihood ratios for eight reviews. Some studies had already conducted these analyses, and others provided enough data for them to be conducted as part of the current review (Table 5). Additionally, eight systematic reviews conducted meta-regressions or other sensitivity analyses to identify potentially confounding factors (Table 6). None of the sensitivity analyses identified any specific factor affecting the study results or being a significant cause of heterogeneity, with one exception. Talwalkar et al (45) found that when pooled across all causes for liver disease, the cut-off values were a significant source of heterogeneity.
Table 5:
TE Versus Biopsy for the Diagnosis of Liver Fibrosis—Additional Analyses
| Author, Year | Diagnosis | Positive LR (95% CI) | Negative LR (95% CI) | DOR (95% CI) | |
|---|---|---|---|---|---|
| Liver Disease | Fibrosis Stage | ||||
| Bota et al, 2013 (35) | Multiple diseasesa | F ≥ 2 | 4.79 (2.92–7.88) | 0.26 (0.19–0.35) | 18.3 (8.8–38.1) |
| F = 4 | NR | NR | NR | ||
| Friedrich-Rust et al, 2008 (37) | Multiple diseasesa | F ≥ 2 | NR | NR | NR |
| Kwok et al, 2014 (39) | NAFLD | F ≥ 2 | 3.05 (2.58–3.61)b | 0.30 (0.23–0.40)b | 10.80 (7.61–15.34)b |
| F ≥ 3 | 5.00 (3.58–6.99)b | 0.26 (0.18–0.36)b | 20.29 (10.61–38.79)b | ||
| F = 4 | 9.58 (6.01–15.28)b | 0.12 (0.06–0.25)b | 94.22 (39.56–238.90)b | ||
| Shaheen et al, 2007 (40) | Multiple diseasesa | F ≥ 2 | NR | NR | 7.2 (3.2–16.2) |
| Steadman et al, 2013 (42, 43) | HBV | F ≥ 2 | 2.94 (1.28–6.79)b | 0.34 (0.27–0.44)b | 8.74 (5.22–14.63)b |
| F ≥ 3 | 4.12 (3.16–5.37)b | 0.23 (0.15–0.36)b | 19.23 (11.96–30.90)b | ||
| F = 4 | 5.16 (3.90–6.83)b | 0.42 (0.33–0.55)b | 13.52 (8.4–21.76)b | ||
| HCV | F ≥ 2 | 4.40 (2.00–9.67)b | 0.25 (0.16–0.41)b | 17.59 (8.59–36.02)b | |
| F ≥ 3 | 8.52 (4.91–14.79)b | 0.14 (0.11–0.19)b | 75.14 (33.0–171.09)b | ||
| F = 4 | 7.48 (4.43–12.62)b | 0.22 (0.16–0.30)b | 37.59 (22.20–63.64)b | ||
| NAFLD | F ≥ 2 | 3.03 (2.50–3.68)b | 0.32 (0.23–0.44)b | 9.88 (6.20–15.72)b | |
| F ≥ 3 | 5.11 (3.51–7.43)b | 0.25 (0.16–0.39)b | 20.28 (9.66–42.58)b | ||
| F = 4 | 14.76 (6.65–32.78)b | 0.13 (0.06–0.29)b | 113.85 (39.83–325.43)b | ||
| Talwalkar et al, 2007 (45) | Multiple diseasesa | F ≥ 2 | 4.2 (2.4–7.2) | 0.31 (0.22–0.43) | 15 (9.8–24.6) |
| F = 4 | 11.7 (7.9–17.1) | 0.14 (0.10–0.20) | 87 (60.0–127.9) | ||
| Tsochatzis et al, 2011 (46) | HCV | F ≥ 2 | NR | NR | 13.9 (8.5–22.8) |
| F = 4 | NR | NR | 46.5 (26.7–91.0) | ||
| HBV | F ≥ 2 | NR | NR | 17.9 (7.7–41.7) | |
| F = 4 | NR | NR | 34.3 (17.0–69.2) | ||
| Tsochatzis et al, 2015 (47, 48) | HCV | F ≥ 2 | 4.16 (3.04–5.69)b | 0.29 (0.24–0.35)b | 16.29 (10.05–26.40) |
| F ≥ 3 | 7.13 (5.49–9.26)b | 0.17 (0.11–0.24)b | 52.84 (29.83–93.61)b | ||
| F = 4 | 9.21 (7.67–11.06)b | 0.16 (0.12–0.21)b | 64.36 (47.56–87.09)b | ||
| HBV | F ≥ 2 | 3.65 (2.43–5.50)b | 0.38 (0.29–0.49)b | 12.43 (6.26–24.70)b | |
| F ≥ 3 | 4.21 (3.30–5.37)b | 0.38 (0.28–0.50)b | 13.64 (9.08–20.49)b | ||
| F = 4 | 5.03 (3.86–6.92)b | 0.19 (0.12–0.29)b | 32.89 (17.90–60.44) | ||
| NAFLD | F ≥ 2 | 3.66 (1.45–9.22)b | 0.17 (0.09–0.32)b | 24.64 (5.49–110.62)b | |
| F ≥ 3 | 3.16 (2.70–3.69)b | 0.29 (0.23–0.38)b | 11.19 (8.03–15.58)b | ||
| F = 4 | 8.02 (6.16–10.42)b | 0.08 (0.02–0.26)b | 114.83 (40.23–327.81)b | ||
| ALD | F ≥ 2 | NR | NR | NR | |
| F ≥ 3 | 4.71 (3.38–6.56)b | 0.17 (0.09–0.33)b | 31.68 (80.06)b | ||
| F = 4 | 4.93 (3.56–6.83)b | 0.20 (0.15–0.26)b | 25.34 (14.42–44.53)b | ||
Abbreviations: ALD, alcoholic liver disease; CI, confidence interval; DOR, diagnostic odds ratio; HBV, hepatitis B virus; HCV, hepatitis C virus; LR, likelihood ratio; NAFLD, nonalcoholic fatty liver disease; NR, not reported; TE, transient elastography.
Aggregate results compiling multiple causes of liver diseases as reported in original studies; summary of included diseases provided in Table 2.
Calculated based on the number of true positives/false positives/false negatives/true negatives information provided in the published review using random effects models; see Appendix 3 for a sample graph.
Table 6:
TE Versus Biopsy for the Diagnosis of Liver Fibrosis—Sensitivity Analyses
| Author, Year | Sensitivity Analyses | |||||||
|---|---|---|---|---|---|---|---|---|
| Country of Study | Publication Date | Sample Size | Cut-off Values | Biopsy Quality | Fibrosis Staging System | Patient Characteristics | Quality of Studies | |
| Bota et al, 2013 (35) | X | X | X | X | X | |||
| Friedrich-Rust et al, 2008 (37) | X | X | X | Xa | Xb | |||
| Kwok et al, 2014 (39) | Xc | Xc | Xc | |||||
| Shaheen et al, 2007 (40) | Xd | X | Xe | |||||
| Steadman et al, 2013 (42, 43) | X | X | X | X | ||||
| Talwalkar et al, 2007 (45) | X | X | Xf | |||||
| Tsochatzis et al, 2011 (46) | X | Xg | Xb | |||||
| Tsochatzis et al, 2015 (47, 48) | X | Xb | ||||||
Abbreviations: ALT, alanine aminotransferase; CI, confidence interval; HCV, hepatitis C virus; HIV, human immunodeficiency virus; SROC, summary receiver operating characteristic; TE, transient elastography.
Underlying cause of disease, age, body mass index, and gender were individually considered.
Comparison between published full-text papers and abstracts.
Defined a priori to examine cut-off values, quality of studies, body mass index, and blood serum ALT levels as potentiation causes of variation; however, due to limitations with the available data, sensitivity analyses could not/should not be conducted.
Results of the SROC were similar to the unadjusted analyses but with narrower confidence intervals: SROC 0.82 (95% CI 0.74–0.88).
Special HCV populations, such as those with a coinfection of HIV, were considered.
Underlying cause of liver disease.
Serum ALT levels.
Friedrich-Rust et al (37) found no differences based on proportion of biopsies or TE failure rates.
Poynard et al (31) adjusted for difference between advanced and nonadvanced fibrosis groups and found no significant impact on the results.
Shaheen et al (40) had planned to conduct a sensitivity analysis based on a publication's association with the manufacturer, but could not complete it since all included publications were funded by the company.
Talwalkar et al (45) had planned to conduct additional sensitivity analyses for patients with fibrosis F ≥ 2, but were unable to because of the limited number of studies available.
Transient Elastography Versus FibroTest for the Assessment of Liver Fibrosis
Three reviews evaluated both TE and FibroTest, but did so with some variation in the underlying cause of liver disease and the cut-off values of the included studies within the respective reviews of the FibroTest algorithm used to define the various METAVIR stages (Table 7).
Table 7:
FibroTest Algorithm—Liver Stiffness Cut–off Values, by METAVIR Stage
| Author, Year | Study Population | F ≥ 2 | F ≥ 3 | F = 4 |
|---|---|---|---|---|
| Poynard et al, 2011 (31) | HBV | NR | NR | NR |
| Shaheen et al, 2007 (40) | HCV | 0.58–0.60 | NR | 0.75 |
| Tsochatzis et al, 2015 (47, 48) | HCV | 0.32–0.53 | 0.32–0.67 | 0.56–0.74 |
| HBV | 0.40–0.48 | 0.31–0.42 | 0.58–0.74 | |
| NAFLD | NR | Low cut-off value: 0.30 High cut-off value: 0.57–0.70 | 0.57 | |
| ALD | NR | NR | Low cut-off value: 0.3 High cut-off value: 0.7 |
Abbreviations: ALD, alcoholic liver disease; HBV, hepatitis B virus; HCV, hepatitis C virus; NAFLD, nonalcoholic fatty liver disease; NR, not reported.
The three reviews offered different interpretations of their analyses (Table 8). Poynard (31) conducted a quantitative analysis comparing three tests and found no statistically significant difference between any of the tests. The other two reviews simply commented on the similarities between the results for FibroTest versus biopsy compared to the results for TE versus biopsy. All results were consistent in demonstrating that TE has greater sensitivity and specificity when used to diagnose cirrhosis (METAVIR stage F = 4) than earlier stages of fibrosis.
Table 8:
Accuracy of FibroTest Versus TE for the Diagnosis of Liver Fibrosis, by METAVIR Stage
| Author, Year | Study Details | F ≥ 2 | F ≥ 3 | F = 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Population | Studies (Patients), n | Studies (Patients), n | FibroTest vs. TE | Studies (Patients), n | FibroTest vs. TE | Studies (Patients), n | Comparison of FibroTest vs. TE | |
| Poynard et al, 2011 (31) | HBV | 8 (1,842) | 8 (1,842) | No significant difference among FibroTest, FibroScan, or HepaScorea FibroTest SROC curve 0.79 (95% CI 0.76–0.82) | NR | NR | 8 (1,842) | No significant difference among FibroTest, FibroScan, or HepaScorea FibroTest SROC curve 0.83 (95% CI 0.78–0.87) |
| Shaheen et al, 2007 (40) | HCV | 9 (1,679) | 8 (1,503) | SROC curve 0.81 (95% CI 0.78–0.84) | NR | NR | 2 (320) | SROC curve 0.90 (95% CI not calculable) |
| Tsochatzis et al, 2015 (47, 48) | HCV | 23 (NR) | 17 (NR) | Sensitivity 0.68 (0.58–0.77) Specificity 0.72 (0.70–0.77) | 9 (NR) | Sensitivity 0.73 (0.56–0.85) Specificity 0.69 (0.55–0.80) | 8 (NR) | Sensitivity 0.60 (0.43–0.76) Specificity 0.86 (0.81–0.91) |
| HBV | 6 (NR) | 6 (NR) | Sensitivity 0.66 (0.57–0.75) Specificity 0.80 (0.72–0.86) | 3 (NR) | Sensitivity 0.49 (0.01–0.99) Specificity 0.71 (0.53–0.84) | 6 (NR) | Sensitivity 0.74 (0.25–0.96) Specificity 0.90 (0.83–0.94) | |
| NAFLD | 4 (NR) | NR | NR | Low cut-offc 3 (NR) High cut-offc 4 (NR) | Low-cut offc Sensitivity 0.88 (0.68–0.99) Specificity 0.73 (0.56–0.85) High-cut offc Sensitivity 0.40 (0.24–0.58) Specificity 0.96 (0.91–0.98) | 1 (NR) | Sensitivity 0.74 (0.54–0.87) Specificity 0.92 (0.88–0.95) | |
| ALD | 1 (NR) | NR | NR | NR | NR | Low cut-offc 1 (NR) High cut-offc 1 (NR) | Low cut-offc Sensitivity 1.00 (0.95–0.100) Specificity 0.50 (0.42–0.58) High cut-offc Sensitivity 0.91 (0.82–0.96) Specificity 0.87 (0.81–0.91) | |
Abbreviations: ALD, alcoholic liver disease; CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; NAFLD, nonalcoholic fatty liver disease; NR, not reported; SROC, summary receiver operating characteristic; TE, transient elastography.
Poynard et al (31) results for HepaScore were beyond the scope of this review and are not presented in this table.
Adjusted for variation in cut-off values for the diagnosis of different stages of fibrosis.
See Table 7.
Poynard et al (31) found no significant difference for the diagnosis of either advanced fibrosis or cirrhosis when AUROC curves were adjusted for the distribution of fibrosis stages using the difference between advanced and nonadvanced fibrosis for the standardization formula.
Tsochatzis et al (47, 48) conducted sensitivity analyses limited to full-text articles (they excluded results available from abstracts) and found that the specificity for FibroTest increased, but there was no difference based on patient ALT blood serum or cut-off values for TE.
Transient Elastography Versus ARFI for the Assessment of Liver Fibrosis
Four reviews identified evaluated both TE and ARFI, but did so with some variation in the underlying cause of liver disease and the ARFI cut-off values used to define METAVIR liver fibrosis stages (Table 9).
Table 9:
ARFI Liver Stiffness Values for the Diagnosis of Liver Fibrosis, by METAVIR Stage
| Author, Year | Study Population | F ≥ 2 | F ≥ 3 | F = 4 |
|---|---|---|---|---|
| Bota et al, 2013 (35) | Multiple diseasesa | Mean 1.30 ± 0.07 m/s | NR | Mean 1.80 ± 0.16 m/s |
| Friedrich-Rust et al, 2012 (38) | Multiple diseasesa | Mean 1.34 m/s | Mean 1.55 m/s | Mean 1.80 m/s |
| Steadman et al, 2013 (42, 43) | Multiple diseasesa | Mean 1.31 m/s | Mean 1.55 m/s | Mean 1.80 m/s |
| NAFLD | NR | Mean 4.24 m/s | NR | |
| Tsochatzis et al, 2015 (47, 48) | HCV | Range: 1.21–1.34 m/s | Range: 1.49–2.11 m/s | Range: 1.6–2.3 m/s |
| HBV | 1.33 m/s (1 study) | NR | NR | |
| NAFLD | NR | 4.2 m/s | NR |
Abbreviations: ARFI, acoustic radiation force impulse; HBV, hepatitis B virus; HCV, hepatitis C virus; NAFLD, nonalcoholic fatty liver disease; NR, not reported.
Aggregate results compiling multiple causes of liver diseases as reported in original studies; summary of included diseases provided in Table 2.
Bota et al, (35) Steadman et al, (42, 43) and Tsochatzis et al (47, 48) compared the results from meta-analyses of TE with those from meta-analyses of ARFI; Friedrich-Rust et al (38) limited their meta-analysis to studies that directly compared ARFI and TE. As summarized in Table 10, the results showed that TE and ARFI are similar, and in direct comparison, there was statistically significant evidence that ARFI was inferior to TE for the diagnostic evaluation of patients with significant fibrosis (F ≥ 2) or cirrhosis (F = 4).
Table 10:
Accuracy of ARFI Versus TE for the Diagnosis of Liver Fibrosis, by METAVIR Stage
| Author, Year | Study Details | F ≥ 2 | F ≥ 3 | F = 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Population | Studies (Patients), n | Studies (Patients), n | ARFI vs. TE | Studies (Patients), n | ARFI vs. TE | Studies (Patients), n | ARFI vs. TE | |
| Bota et al, 2013 (35) | Multiple diseasesa | 13 (1,163) | 10 (1,016) | Mean difference in rDOR 0.27 (95% CI −0.69 to 0.14) | NR | NR | 13 (1,163) | Mean difference in rDOR 0.12 (95% CI −0.29 to 0.52) |
| Friedrich-Rust et al, 2012 (38) | Multiple diseasesa | 4 (312) | NR (199) | Mean difference of SROC curve 0.05, P = 0.037 (ARFI inferior to TE) | NR (127) | Mean difference of SROC curve 0.04, P = 0.092 | NR (85) | Mean difference of SROC curve 0.04, P = 0.048 (ARFI inferior to TE) |
| Steadman et al, 2013 (42, 43) | Multiple diseasesa | 7 (616) | 4 (NR) | Mean differences of SROC curves 0.00b | 6 (NR) | Mean difference of SROC curves 0.05b SROC for ARFI vs. biopsy: 0.97 (95% CI 0.95–0.98) | 7 (616) | Mean differences of SROC curves 0.00b |
| Tsochatzis et al, 2015 (47, 48) | HCV | NR | 3 (NR) | Sensitivity 0.79 (0.75–0.83) Specificity 0.89 (0.84–0.93) | 4 (NR) | Sensitivity 0.85 (0.69–0.94) Specificity 0.89 (0.72–0.97) | 4 (NR) | Sensitivity 0.84 (0.72–0.91) Specificity 0.77 (0.50–0.92) |
| HBV | NR | 1 (NR) | Sensitivity 0.71 (0.59–0.80) Specificity 0.67 (0.30–0.90) | NR | NR | NR | NR | |
| NAFLD | NR | NR | NR | 1 (NR) | Sensitivity 0.90 (0.77–0.96) Specificity 0.90 (0.82–0.94) | NR | NR | |
Abbreviations: ARFI, acoustic radiation force impulse; CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; NAFLD, nonalcoholic fatty liver disease; NR, not reported; rDOR, relative diagnostic odds ratio; SROC, summary receiver operating characteristic; TE, transient elastography.
Aggregate results compiling multiple causes of liver diseases as reported in original studies; summary of included diseases provided in Table 2.
Calculated based on information provided in the published review; results for TE vs. biopsy are summarized in Table 4. The systematic review concluded that results were similar between TE and ARFI.
Bota et al (35) examined the reliability of TE versus ARFI, calculated from 8 of the 13 studies (5 limited their inclusion to only patients with valid results). The authors concluded that TE had three times more failures than ARFI (TE 6.6% versus ARFI 2.1%, P < 0.0001).
Kwok et al (39) also looked for studies that examined ARFI but were unable to conduct analyses as intended due to a paucity of literature and high heterogeneity.
CAP Versus Liver Biopsy for the Assessment of Liver Steatosis
One systematic review evaluated CAP for the diagnosis of hepatic steatosis in patients with various liver conditions. Mean cut-off values were used to diagnose the different stages of steatosis (Table 11).
Table 11:
Diagnosis of Histological Steatosis Stages
| Author, Year | Study Population | ≥ S1 | ≥ S2 | ≥ S3 |
|---|---|---|---|---|
| Shi et al, 2014 (41) | Multiple diseasesa | Mean 232.5 dB/mb | Mean 255 dB/mb | Mean 290 dB/mb |
Abbreviations: dB/m, decibels per metre.
Aggregate results compiling multiple causes of liver diseases as reported in original studies; summary of included diseases provided in Table 2.
Calculated based on information provided in the published review.
As summarized in Table 12, CAP had good diagnostic accuracy in the evaluation of patients with steatosis, with similar results regardless of stage.
Table 12:
Accuracy of CAP Versus Biopsy for the Diagnosis of Liver Steatosis, by Histological Steatosis Stage
| Author, Year | Study Details | Results | ||||
|---|---|---|---|---|---|---|
| Population | Steatosis Stage | Studies (Patients), n | AUROC (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | |
| Shi et al, 2014 (41) | Multiple diseasesa | ≥ S1 | 8 (NR) | 0.85 (0.81–0.88) | 0.78 (0.69–0.84) | 0.79 (0.68–0.86) |
| ≥ S2 | 7 (NR) | 0.88 (0.85–0.91) | 0.85 (0.75–0.92) | 0.79 (0.71–0.85) | ||
| ≥ S3 | 7 (NR) | 0.87 (0.84–0.90) | 0.83 (0.76–0.89) | 0.79 (0.68–0.87) | ||
Abbreviations: AUROC, area under the receiving operator characteristic; CAP, controlled attenuation parameter; CI, confidence interval; NR, not reported.
Aggregate results compiling multiple causes of liver diseases as reported in original studies; summary of included diseases provided in Table 2.
Secondary Outcomes
Shi et al (41) compared the accuracy of CAP versus biopsy, and determined that CAP was accurate for all steatosis stages (results statistically significant) (Table 13).
Table 13:
CAP Versus Biopsy for the Diagnosis of Steatosis—Secondary Outcomes
| Author, Year | Diagnosis | Positive LR (95% CI) | Negative LR (95% CI) | DOR (95% CI) | |
|---|---|---|---|---|---|
| Liver Disease | Steatosis Stage | ||||
| Shi et al, 2014 (41) | Multiple diseasesa | ≥ S1 | 3.61 (2.4–5.44) | 0.29 (0.20–0.40) | 12.63 (6.79–23.49) |
| ≥ S2 | 4 (NR) | 0.19 (NR) | 21.22 (10.67–42.21) | ||
| ≥ S3 | 4 (NR) | 0.19 (NR) | 18.74 (CI 10.18–34.52) | ||
Abbreviations: CAP, controlled attenuation parameter; CI, confidence interval; DOR, diagnostic odds ratio; LR, likelihood ratio; NR, not reported.
Aggregate results compiling multiple causes of liver diseases as reported in original studies; summary of included diseases provided in Table 2.
Shi et al (41) conducted additional analyses and determined the following:
When given an assumed prevalence of ≥ S1 at 47%, the positive predictive value was 0.77 (95% CI 0.71–0.83) and the negative predictive value was 0.76 (95% CI 0.70–0.82).
When given an assumed prevalence of ≥ S2 at 28.8%, the positive predictive value was 0.79 (95% CI 0.74–0.84) and the negative predictive value was 0.82 (95% CI 0.77–0.87).
When given an assumed prevalence of ≥ S3 at 11.3%, the positive predictive value was 0.78 (95% CI 0.72–0.84) and the negative predictive value was 0.81 (0.74–0.87).
Conclusions drawn from the results did not change following a sensitivity analysis excluding any studies that were available only in abstract form.
Shi et al (41) also conducted Fagan plot analyses and concluded that using a likelihood ratio of > 10 or < 0.1 as indicators of a strong association between a test and diagnosis, CAP was not a valuable diagnostic test in spite of its reasonably good sensitivity and specificity (Table 14).
Table 14:
Post-test Probabilities of Disease With CAP, Given Varying Pre-test Probabilitiesa
| CAP Steatosis Stage | Assumed Pre-test Probability | Positive Disease Results | Negative Disease Results | ||
|---|---|---|---|---|---|
| Positive LR (95% CI) | Post-test Probability of Disease | Negative LR (95% CI) | Post-test Probability of Disease | ||
| ≥ S1 | 25% | 3.61 (2.4–5.44) | 55% | 0.29 (0.20–0.40) | 9% |
| 50% | 78% | 22% | |||
| 75% | 92% | 46% | |||
| ≥ S2 | 25% | 4 (NR) | 58% | 0.3 (NR) | 6% |
| 50% | 80% | 16% | |||
| 75% | 92% | 37% | |||
| ≥ S3 | 25% | 4 (NR) | 57% | 0.3 (NR) | 7% |
| 50% | 80% | 17% | |||
| 75% | 92% | 39% | |||
Abbreviations: CAP, controlled attenuation parameter; CI, confidence interval; LR, likelihood ratio; NR, not reported.
Table data from the systematic review by Shi et al. (41)
CONCLUSIONS
There was evidence to support the diagnostic accuracy of transient elastography compared to liver biopsy for assessing liver fibrosis in the disease areas of interest.2
There was evidence that the diagnostic accuracy of FibroTest and acoustic force radiation impulse were not significantly different from transient elastography for assessing liver fibrosis in the disease areas of interest.2
There was evidence to support the diagnostic accuracy of controlled attenuation parameter compared to liver biopsy for assessing steatosis in the disease areas of interest.2
No evidence was found that assessed the clinical utility of transient elastography (with or without controlled attenuation parameter) versus biopsy, as measured by a change in clinical diagnosis, treatment, or patient outcomes. Beneficial impact could be presumed, given that the accuracy of TE is comparable to that of a biopsy and would have an impact as a noninvasive alternative to diagnose. The clinical utility of CAP is less certain given that treatment for this condition generally consists of providing advice about healthy behaviours.
Glossary
LIST OF ABBREVIATIONS
- ALD
Alcoholic liver disease
- ALT
Alanine aminotransferase
- AMSTAR
Assessment of Multiple Systematic Reviews
- ARFI
Acoustic radiation force impulse
- AUROC
Area under the receiver operating characteristic
- CAP
Controlled attenuation parameter
- CI
Confidence interval
- GGT
Gamma-glutamyl transpeptidase
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- NAFLD
Nonalcoholic fatty liver disease
- QUADAS
Quality assessment for diagnostic accuracy studies
- TE
Transient elastography
APPENDICES
Appendix 1: Literature Search Strategies
Search date: October 02, 2014
Librarians: Caroline Higgins
Databases searched: Ovid MEDLINE, Ovid MEDLINE In-Process, Embase, All EBM Databases (see below)
Database: EBM Reviews - Cochrane Database of Systematic Reviews <2005 to August 2014>, EBM Reviews - ACP Journal Club <1991 to September 2014>, EBM Reviews - Database of Abstracts of Reviews of Effects <3rd Quarter 2014>, EBM Reviews - Cochrane Central Register of Controlled Trials <August 2014>, EBM Reviews - Cochrane Methodology Register <3rd Quarter 2012>, EBM Reviews - Health Technology Assessment <3rd Quarter 2014>, EBM Reviews - NHS Economic Evaluation Database <3rd Quarter 2014>, Embase <1980 to 2014 Week 39>, Ovid MEDLINE(R) <1946 to September Week 4 2014>, Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations <October 02, 2014>
Search Strategy:
| # | Searches | Results |
|---|---|---|
| 1 | Hepatitis/ or exp Hepatitis B/ or exp Hepatitis C/ | 269370 |
| 2 | exp Hepatitis, Chronic/ use mesz,acp,cctr,coch,clcmr,dare,clhta,cleed | 35562 |
| 3 | Chronic Hepatitis/ use emez | 21595 |
| 4 | exp Cholestasis, Intrahepatic/ use mesz,acp,cctr,coch,clcmr,dare,clhta,cleed | 11309 |
| 5 | Intrahepatic Cholestasis/ use emez | 3957 |
| 6 | Cholangitis, Sclerosing/ use mesz,acp,cctr,coch,clcmr,dare,clhta,cleed | 3047 |
| 7 | Primary Sclerosing Cholangitis/ use emez | 4933 |
| 8 | exp Fatty Liver/ or exp Liver Cirrhosis/ | 230593 |
| 9 | ((hepatiti* adj5 (B or C)) or (HIV adj3 (hepatiti* or HCV or coinfection* or coinfection*)) or ((liver or hepat*) adj5 (fat* or chronic or fibros* or steatos* or stiffness or cirrhos* or cholesta* or non-alcoholic or nonalcoholic or alcoholic)) or NAFLD or NASH or ALD or steatohepatiti* or (biliary adj5 cirrhos*) or (chronic adj5 cholesta*) or (sclerosing adj5 cholangi*) or PSC).ti,ab. | 526335 |
| 10 | or/1–9 | 682259 |
| 11 | Elasticity Imaging Techniques/ use mesz,acp,cctr,coch,clcmr,dare,clhta,cleed | 3652 |
| 12 | Elastography/ use emez | 5906 |
| 13 | (elastogra* or elastomet* or sonoelastogra*or ultraso* or acoustic radiation force impulse* or ARFI or vibro-acoustograph* or (elasticity adj3 imag*) or ((attenuat* or CAP) adj3 (parameter or measur* or difference or ultraso* or US)) or liver stiffness measurement*).ti,ab. | 19730 |
| 14 | FibroScan.mp. | 2119 |
| 15 | or/11–14 | 23274 |
| 16 | 10 and 15 | 5517 |
| 17 | Case Reports/ or Comment.pt. or Editorial.pt. or Letter.pt. or Congressess.pt. | 4216476 |
| 18 | Case Report/ or Comment/ or Editorial/ or Letter/ or conference abstract.pt. | 7515847 |
| 19 | or/17–18 | 7535819 |
| 20 | 16 not 19 | 3405 |
| 21 | limit 20 to english language [Limit not valid in CDSR, ACP Journal Club, DARE, CLCMR; records were retained] | 3016 |
| 22 | remove duplicates from 21 | 1768 |
Appendix 2: Evidence Quality Assessment
Table A1:
AMSTAR Scores of Included Systematic Reviews
| Author, Year | AMSTAR Scorea | (1) Provided Study Design | (2) Duplicate Study Selection and Data Extraction | (3) Broad Literature Search | (4) Considered Status of Publication | (5) Listed Excluded Studies | (6) Provided Characteristics of Studies | (7) Assessed Scientific Quality | (8) Considered Quality in Report | (9) Methods to Combine Appropriate | (10) Assessed Publication Bias | (11) Stated Conflict of Interest |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bota et al, 2013 (35) | 7 | Yes | Nob | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Noc |
| Chon et al, 2012 (36) | 8 | Yes | Nod | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Noc |
| Friedrich-Rust et al, 2008 (37) | 8 | Yes | Nod | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NR | NR |
| Friedrich-Rust et al, 2012 (38) | 4 | Yes | NR | Yes | No | No | Yes | No | No | Yes | NR | Noc |
| Kwok et al, 2014 (39) | 8 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | NR | Noc |
| Poynard et al, 2008 (32) | 3 | Yes | NR | Yes | No | No | No | No | No | Yes | NR | Noc |
| Poynard et al, 2011 (31) | 4 | Yes | NR | Yes | No | No | Yes | No | No | Yes | NR | Noc |
| Shaheen et al, 2007 (40) | 10 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Noc |
| Shi et al, 2014 (41) | 9 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Noc |
| Steadman et al, 2013 (42, 43) | 10 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Noc |
| Stebbing et al, 2010 (44) | 6 | Yes | NR | Yes | Yes | No | Yes | No | Yes | Yes | NR | Noc |
| Talwalkar et al, 2007 (45) | 8 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | NR | NR |
| Tsochatzis et al, 2011 (46) | 9 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Noc |
| Tsochatzis et al, 2014 (47, 48) | 9 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yese | Noc |
Abbreviation: AMSTAR, Assessment of Multiple Systematic Reviews; NR, not reported.
Maximum possible score is 11. Details of AMSTAR score are described in Shea et al. (51)
The review reported duplicate study selection, but it did not report if there was also duplicate data extraction.
The review reported conflict of interest for the authors but not for the individual studies.
The review did not report duplicate study selection, but it did report duplicate data extraction.
Many of the analyses included only 1 study, which was a potential source of bias and a limitation of the body of evidence.
Table A2:
Summary of Studies With “Yes” Responses, QUADAS
| Author, Year | Total Studies | (1) Representative Patient Sample | (2) Selection Criteria Clearly Described | (3) Adequate Reference Standard | (4) Acceptable Delay Between Tests | (5) Complete Verification of Diagnosis | (6) No Differential Verification | (7) No Incorporation Bias | (8) Adequate Index Test Description | (9) Adequate Reference Test Description | (10) Blinding for Index Test Results | (11) Blinding for Reference Test Results | (12) Clinical Data Available as in Practice | (13) Uninterpretable Test Results Reported | (14) Withdrawals Explained |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Friedrich-Rust et al, 2008 (37) | 50 | 50 | 21 | 29 | 50 | 36 | 50 | 50 | 50 | 15 | 7 | 15 | 49 | 26 | 50 |
| Kwok et al, 2014 (39) | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 8 | 8 | 9 | NRa | 9 | 9 | 9 |
| Shaheen et al, 2007 (40) | 12b | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Shi et al, 2014 (41) | 9 | 9 | 9 | 9 | 7 | 9 | 9 | 9 | 9 | 9 | 2 | 6 | 9 | 7 | 5 |
| Steadman et al, 2013 (42, 43) | 55 | 55 | 55 | 55 | 55 | 55 | 55 | 55 | 53 | 49 | 48 | 48 | 55 | 53 | 54 |
Abbreviations: NR, not reported; QUADAS (34), quality assessment tool for diagnostic accuracy studies.
Modified the QUADAS questionnaire and did not conduct evaluation for question 11.
Details not provided, but it was possible to determine that there 164 out of a possible 168.
Table A3:
Summary of Studies With “Low Risk” Responses, QUADAS-2
| Author, Year | Total Studies | Risk of Bias | Applicability Concerns | |||||
|---|---|---|---|---|---|---|---|---|
| (1) Patient Selection | (2) Index Test | (3) Reference Standard | (4) Flow and Timing | (5) Patient Selection | (6) Index Test | (7) Reference Standard | ||
| Bota et al, 2013 (35) | 13 | 10 | 11 | 10 | 6 | 12 | 13 | 11 |
| Tsochatzis et al, 2015 (47, 48)a | 275 | 113 | 182 | 76 | 241 | 50 | 267 | 125 |
Abbreviations: QUADAS-2 (33), quality assessment tool for diagnostic accuracy studies, version 2.
Summary limited to studies that evaluated the populations and interventions of interest for this report.
Appendix 3: Additional Analyses
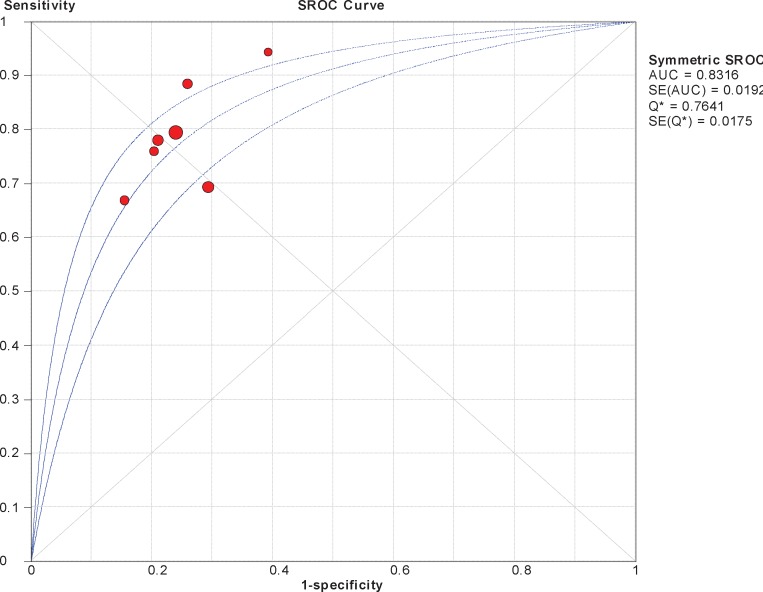
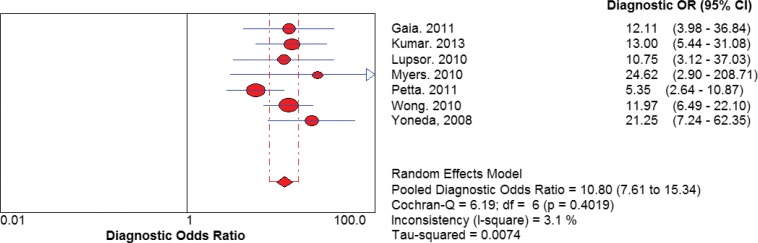
Where summary receiver operating characteristic curves and diagnostic odds ratios were calculated based on the number of true positives/false positives/false negatives/true negatives provided in the published reviews, random effects models were used as illustrated in the sample analyses below.
Figure A1: Sample SROC Curve, TE Versus Biopsy for F ≥ 2, Kwok et al (39).
Abbreviations: AUC, area under the curve; SE, standard error; SROC, summary receiver operating characteristic; TE, transient elastography.
Figure A2: Sample DOR Plot, TE Versus Biopsy for F ≥ 2, Kwok et al (39).
Abbreviations: CI, confidence interval; DOR, diagnostic odds ratio; OR, odds ratio; TE, transient elastography.
Footnotes
Liver disease areas of interest (see inclusion/exclusion criteria): HCV, HBV, NAFLD, ALD, and cholestatic diseases.
Liver disease areas of interest (see inclusion/exclusion criteria for more detail): HCV, HBV, NAFLD, ALD and cholestatic diseases
About Health Quality Ontario
Health Quality Ontario is the provincial advisor on the quality of health care. We are motivated by a single-minded purpose: Better health for all Ontarians.
Who We Are
We are a scientifically rigorous group with diverse areas of expertise. We strive for complete objectivity, and look at things from a vantage point that allows us to see the forest and the trees. We work in partnership with health care providers and organizations across the system, and engage with patients themselves, to help initiate substantial and sustainable change to the province's complex health system.
What We Do
We define the meaning of quality as it pertains to health care, and provide strategic advice so all the parts of the system can improve. We also analyze virtually all aspects of Ontario's health care. This includes looking at the overall health of Ontarians, how well different areas of the system are working together, and most importantly, patient experience. We then produce comprehensive, objective reports based on data, facts and the voice of patients, caregivers and those who work each day in the health system. As well, we make recommendations on how to improve care using the best evidence. Finally, we support large scale quality improvements by working with our partners to facilitate ways for health care providers to learn from each other and share innovative approaches.
Why It Matters
We recognize that, as a system, we have much to be proud of, but also that it often falls short of being the best it can be. Plus certain vulnerable segments of the population are not receiving acceptable levels of attention. Our intent at Health Quality Ontario is to continuously improve the quality of health care in this province regardless of who you are or where you live. We are driven by the desire to make the system better, and by the inarguable fact that better has no limit.
REFERENCES
- (1).Canadian Liver Foundation. Liver.ca [Internet]. Markham (ON): Canadian Liver Foundation; 2014. [updated 2015; cited 2015 January 28]. Available from: http://www.liver.ca/. [Google Scholar]
- (2).Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005; 115(2): 209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Diseases and Conditions: Cirrhosis [Internet]. Rochester (MN): Mayo Clinic; 2014. [updated 2014; cited 2015 January 28]. Available from: http://www.mayoclinic.org/diseases-conditions/cirrhosis/basics/definition/con-20031617. [Google Scholar]
- (4).Strader DB, Wright T, Thomas DL, Seeff LB, American Association for the Study of Liver D. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39(4): 1147–71. [DOI] [PubMed] [Google Scholar]
- (5).Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996; 24(2): 289–93. [DOI] [PubMed] [Google Scholar]
- (6).Thavorn K, Coyle D. An economic analysis of transient elastography and controlled attenuation paramter in diagnosing liver fibrosis and steatosis in Ontario. Ont Health Technol Assess Ser [Internet]. In press. [PMC free article] [PubMed]
- (7).Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008; 48(2): 418–31. [DOI] [PubMed] [Google Scholar]
- (8).Canadian Liver Foundation. Liver disease in Canada: a crisis in the making. Markham (ON): Canadian Liver Foundation; 2013. [Google Scholar]
- (9).Mueller S, Seitz HK, Rausch V. Non-invasive diagnosis of alcoholic liver disease. World J Gastroenterol. 2014; 20(40): 14626–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Lee YM, Kaplan MM, Gheorghe L, American College of G. [Guidelines for the diagnosis and therapy of primary sclerosing cholangitis. Guidelines of the American College of Gastroenterology 2002]. Rom J Gastroenterol. 2002;11(4): 346–50. [PubMed] [Google Scholar]
- (11).Lee Y-M, Lopez MJ. Primary Biliary Cirrhosis (PBC) [Internet]. Bethesda (MD): American College of Gastroenterology; 2004. [updated January 2010; cited 2014 October 2]. Available from: http://patients.gi.org/topics/primary-biliary-cirrhosis-pbc/. [Google Scholar]
- (12).Lee Y-M, Lopez MJ. Primary Sclerosing Cholangitis (PSC) [Internet]. Bethesda (MD): American College of Gastroenterology; 2004. [updated January 2010; cited 2014 October 2]. Available from: http://patients.gi.org/topics/primary-sclerosing-cholangitis-psc/. [Google Scholar]
- (13).Ghany MG, Strader DB, Thomas DL, Seeff LB, American Association for the Study of Liver D. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4): 1335–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Sebastiani G, Ghali P, Wong P, Klein MB, Deschenes M, Myers RP. Physicians’ practices for diagnosing liver fibrosis in chronic liver diseases: a nationwide, Canadian survey. Can J Gastroenterol Hepatol. 2014; 28(1): 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Moreno S, Garcia-Samaniego J, Moreno A, Ortega E, Pineda JA, del Romero J, et al. Noninvasive diagnosis of liver fibrosis in patients with HIV infection and HCV/HBV coinfection. J Viral Hepat. 2009; 16(4): 249–58. [DOI] [PubMed] [Google Scholar]
- (16).Alswat KA, Mumtaz K, Jafri W. Liver biopsy for histological assessment: the case in favor. Saudi J Gastroenterol. 2010; 16(2): 133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Adeyi O, Fischer SE, Guindi M. Liver allograft pathology: approach to interpretation of needle biopsies with clinicopathological correlation. J Clin Pathol. 2010; 63(1): 47–74. [DOI] [PubMed] [Google Scholar]
- (18).Sebastiani G, Alberti A. How far is noninvasive assessment of liver fibrosis from replacing liver biopsy in hepatitis C? J Viral Hepat. 2011;18(Suppl 2): 18–32. [DOI] [PubMed] [Google Scholar]
- (19).Mehta SH, Lau B, Afdhal NH, Thomas DL. Exceeding the limits of liver histology markers. J Hepatol. 2009; 50(1): 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).FibroScan [Internet]. France: Echosens; 2015. [updated 2015; cited 2015 March 30]. Available from: http://www.fibroscan.com/en/expertise. [Google Scholar]
- (21).de Ledinghen V, Vergniol J. Transient elastography (FibroScan). Gastroenterol Clin Biol. 2008; 32(6 Suppl 1): 58–67. [DOI] [PubMed] [Google Scholar]
- (22).Pang JX, Zimmer S, Niu S, Crotty P, Tracey J, Pradhan F, et al. Liver stiffness by transient elastography predicts liver-related complications and mortality in patients with chronic liver disease. PLoS One. 2014; 9(4): e95776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Grigorescu M. Noninvasive biochemical markers of liver fibrosis. J Gastrointestin Liver Dis. 2006; 15(2): 149–59. [PubMed] [Google Scholar]
- (24).BioPredictive. Practice of FibroTest for Hepatitis C [Internet]. Paris, France: BioPredictive; 2014. [updated 2014; cited 2015 January 29]. Available from: http://www.biopredictive.com/intl/physician/fibrotest-for-hcv/. [Google Scholar]
- (25).Berzigotti A, Castera L. Update on ultrasound imaging of liver fibrosis. J Hepatol. 2013; 59(1): 180–2. [DOI] [PubMed] [Google Scholar]
- (26).Becker L, Oxman A. Cochrane handbook for systematic reviews of interventions [Internet]. London (UK): Cochrane Collaboration; 2011. Chapter 22, Overviews of reviews [cited 2015 January 28]. Available from: www.cochrane-handbook.org. [Google Scholar]
- (27).Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014. [Google Scholar]
- (28).Zamora J, Abraira V, Muriel A, Khan KS, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol 2006; 6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Akobeng AK. Understanding diagnostic tests 2: likelihood ratios, pre- and post-test probabilities and their use in clinical practice. Acta Paediatr. 2007; 96(4): 487–91. [DOI] [PubMed] [Google Scholar]
- (30).Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007; 7(10): 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Poynard T, Ngo Y, Munteanu M, Thabut D, Ratziu V. Noninvasive markers of hepatic fibrosis in chronic hepatitis B. Curr Hepat Rep. 2011; 10(2): 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Poynard T, Morra R, Ingiliz P, Imbert-Bismut F, Thabut D, Messous D, et al. Assessment of liver fibrosis: noninvasive means. Saudi J Gastroenterol. 2008; 14(4): 163–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011; 155(8): 529–36. [DOI] [PubMed] [Google Scholar]
- (34).Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003; 3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Bota S, Herkner H, Sporea I, Salzl P, Sirli R, Neghina AM, et al. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int. 2013; 33(8): 1138–47. [DOI] [PubMed] [Google Scholar]
- (36).Chon YE, Choi EH, Song KJ, Park JY, Kimdo Y, Han KH, et al. Performance of transient elastography for the staging of liver fibrosis in patients with chronic hepatitis B: a meta-analysis. PLoS One. 2012; 7(9): e44930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Friedrich-Rust M, Ong M, Martens S, Sarrazin C, Bojunga J, Zeuzem S, et al. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008; 134(4): 960–74.e8. [DOI] [PubMed] [Google Scholar]
- (38).Friedrich-Rust M, Nierhoff J, Lupsor M, Sporea I, Fierbinteanu-Braticevici C, Strobel D, et al. Performance of acoustic radiation force impulse imaging for the staging of liver fibrosis: a pooled meta-analysis. J Viral Hepat. 2012; 19(2): e212–E9. [DOI] [PubMed] [Google Scholar]
- (39).Kwok R, Tse YK, Wong GLH, Ha Y, Lee AU, Ngu MC, et al. Systematic review with meta-analysis: Non-invasive assessment of non-alcoholic fatty liver disease: the role of transient elastography and plasma cytokeratin-18 fragments. Aliment Pharmacol Ther. 2014; 39(3): 254–69. [DOI] [PubMed] [Google Scholar]
- (40).Shaheen AAM, Wan AF, Myers RP. FibroTest and FibroScan for the prediction of hepatitis C-related fibrosis: a systematic review of diagnostic test accuracy. Am J Gastroenterol. 2007; 102(11): 2589–600. [DOI] [PubMed] [Google Scholar]
- (41).Shi KQ, Tang JZ, Zhu XL, Ying L, Li DW, Gao J, et al. Controlled attenuation parameter for the detection of steatosis severity in chronic liver disease: a meta-analysis of diagnostic accuracy. J Gastroenterol Hepatol (Australia). 2014; 29(6): 1149–58. [DOI] [PubMed] [Google Scholar]
- (42).Steadman R, Leggett L, Lorenzetti D, Noseworthy T, Rose S, Sutherland L, et al. A health technology assessment of transient elastography in liver disease. Edmonton (AB): Alberta Health; 2012. 180 p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Steadman R, Myers RP, Leggett L, Lorenzetti D, Noseworthy T, Rose S, et al. A health technology assessment of transient elastography in adult liver disease. Can J Gastroenterol. 2013; 27(3): 149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Stebbing J, Farouk L, Panos G, Anderson M, Jiao LR, Mandalia S, et al. A meta-analysis of transient elastography for the detection of hepatic fibrosis. J Clin Gastroenterol. 2010; 44(3): 214–9. [DOI] [PubMed] [Google Scholar]
- (45).Talwalkar JA, Kurtz DM, Schoenleber SJ, West CP, Montori VM. Ultrasound-based transient elastography for the detection of hepatic fibrosis: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2007; 5(10): 1214–20. [DOI] [PubMed] [Google Scholar]
- (46).Tsochatzis EA, Gurusamy KS, Ntaoula S, Cholongitas E, Davidson BR, Burroughs AK. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J Hepatol. 2011; 54(4): 650–9. [DOI] [PubMed] [Google Scholar]
- (47).Tsochatzis EA, Crossan C, Longworth L, Gurusamy K, Rodriguez-Peralvarez M, Mantzoukis K, et al. Cost-effectiveness of noninvasive liver fibrosis tests for treatment decisions in patients with chronic hepatitis C. Hepatology. 2014; 60(3): 832–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Crossan C, Tsochatzis E, Longworth L, Gurusamy K, Davidson B, Rodriguez-Peralvarez M, et al. Cost-effectiveness of non-invasive methods for assessment and monitoring of liver fibrosis and cirrhosis in patients with chronic liver disease: systematic review and economic evaluation. Health Technol Assess. 2015; 19(9): 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Schunemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008; 336(7653): 1106–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Tolman KG, Dalpiaz AS. Treatment of non-alcoholic fatty liver disease. Ther Clin Risk Manag. 2007; 3(6): 1153–63. [PMC free article] [PubMed] [Google Scholar]
- (51).Palomaki GE, Melillo S, Neveux L, Douglas MP, Dotson WD, Janssens AC, et al. Use of genomic profiling to assess risk for cardiovascular disease and identify individualized prevention strategies–a targeted evidence-based review. Genetics in medicine : official journal of the American College of Medical Genetics. 2010; 12(12): 772–84. [DOI] [PubMed] [Google Scholar]