Abstract
Background
Type 1 diabetes mellitus is caused by the autoimmune destruction of pancreatic beta (β) cells, resulting in severe insulin deficiency. Islet transplantation is a β-cell replacement therapeutic option that aims to restore glycemic control in patients with type 1 diabetes. The objective of this study was to determine the clinical effectiveness of islet transplantation in patients with type 1 diabetes, with or without kidney disease.
Methods
We conducted a systematic review of the literature on islet transplantation for type 1 diabetes, including relevant health technology assessments, systematic reviews, meta-analyses, and observational studies. We used a two-step process: first, we searched for systematic reviews and health technology assessments; second, we searched primary studies to update the chosen health technology assessment. The Assessment of Multiple Systematic Reviews measurement tool was used to examine the methodological quality of the systematic reviews and health technology assessments. We assessed the quality of the body of evidence and the risk of bias according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group criteria.
Results
Our searched yielded 1,354 citations. One health technology assessment, 11 additional observational studies to update the health technology assessment, one registry report, and four guidelines were included; the observational studies examined islet transplantation alone, islet-after-kidney transplantation, and simultaneous islet-kidney transplantation.
In general, low to very low quality of evidence exists for islet transplantation in patients with type 1 diabetes with difficult-to-control blood glucose levels, with or without kidney disease, for these outcomes: health-related quality of life, secondary complications of diabetes, glycemic control, and adverse events. However, high quality of evidence exists for the specific glycemic control outcome of insulin independence compared with intensive insulin therapy.
For patients without kidney disease, islet transplantation improves glycemic control and diabetic complications for patients with type 1 diabetes when compared with intensive insulin therapy. However, results for health-related quality of life outcomes were mixed, and adverse events were increased compared with intensive insulin therapy. For patients with type 1 diabetes with kidney disease, islet-after-kidney transplantation or simultaneous islet-kidney transplantation also improved glycemic control and secondary diabetic complications, although the evidence was more limited for this patient group. Compared with intensive insulin therapy, adverse events for islet-after-kidney transplantation or simultaneous islet-kidney transplantation were increased, but were in general less severe than with whole pancreas transplantation.
Conclusions
For patients with type 1 diabetes with difficult-to-control blood glucose levels, islet transplantation may be a beneficial β-cell replacement therapy to improve glycemic control and secondary complications of diabetes. However, there is uncertainty in the estimates of effectiveness because of the generally low to very low quality of evidence for all outcomes of interest.
PLAIN LANGUAGE SUMMARY
Type 1 diabetes mellitus is caused by the body attacking its own beta (β) cells in the pancreas. These cells are responsible for producing the hormone insulin, which allows for proper control of blood sugar levels. Some patients with type 1 diabetes cannot control their blood sugar levels, even with optimal medical management. For these patients, islet transplantation is a treatment option. The procedure restores β cells by infusing donor islets into the patient, typically through a vein in the liver.
This review examined the literature for studies on islet transplantation in patients with type 1 diabetes, with or without kidney disease. We considered these outcomes:
Control of blood sugar level
Quality of life
Secondary complications of diabetes
Safety
One health technology assessment, 11 clinical studies, one registry report, and four guidelines were found. All of these studies were observational studies where the patients were not randomized (randomly assigned to a treatment or control group). In addition, some studies did not have a control group (a group that receives no or an alternative treatment and that is used for comparison with the treatment group).
From these studies, we determined that islet transplantation can improve blood sugar control and may reduce diabetic complications for patients with type 1 diabetes, with or without kidney disease. Improvements in health-related quality of life can occur; however, the results were inconsistent. Compared with insulin therapy, there were more adverse (undesired) events with islet transplantation, but these were less severe than with pancreas transplantation. The body of evidence was generally considered to be of low to very low quality.
BACKGROUND
Objective of Analysis
The objective of this analysis was to determine the clinical effectiveness of islet transplantation for patients with type 1 diabetes mellitus, with or without kidney disease.
Clinical Need and Target Population
Diabetes mellitus is a chronic condition characterized by high blood glucose levels. It currently affects more than 3 million people in Canada. (1) Type 1 diabetes mellitus is a result of autoimmune destruction of the pancreatic beta (β) cells, causing severe insulin deficiency. Type 1 diabetes affects about 5% to 10% of patients with diabetes (2); daily intensive management of blood glucose levels, along with a balanced diet and physical activity, is essential for reducing the short- and long-term complications of the disease.
Patients with type 1 diabetes require lifelong insulin therapy to control their blood glucose levels. Insulin can be administered one of two ways:
Via a needle and syringe or an insulin pen that injects insulin under the skin
Through an insulin pump that connects a reservoir of insulin to a catheter inserted under the skin of the abdomen
However, despite optimal insulin treatment, some patients still experience frequent large and unpredictable fluctuations in their blood glucose levels. This rare form of severe diabetes is known as brittle (or labile) diabetes, and it affects about 3 in 1,000 people with type 1 diabetes. (3) Patients experience recurring episodes of hyperglycemia and hypoglycemia, hypoglycemic unawareness (the state when blood glucose levels decrease to dangerously low levels without any warning symptoms), and diabetic ketoacidosis (a potentially life-threatening complication that results in toxic high levels of ketones in the blood). For these patients, the unstable blood glucose levels lower quality of life, potentially lead to recurrent or prolonged hospitalization, and result in complications that may reduce their life expectancy. (3)
Although improvements have been made in the quality of diabetes care and insulin-delivery systems, they still fail to provide an effective treatment for some type 1 diabetes. As such, efforts to preserve and restore endogenous pancreatic function through β-cell replacement therapy offer an alternative treatment option for these patients.
Technology/Technique
Islet transplantation was first introduced in 1972 when it was found that it could cure chemical diabetes in rats. (4) In 1989, the first successful clinical islet transplantation was performed; however, insulin independence lasted only a month owing to inadequate immunosuppression and islet rejection. (5) The quest for insulin independence continued into the 1990s, and more than 450 attempts were made to treat type 1 diabetes with islet transplantation. However, the results were unpromising, with less than 10% maintaining insulin independence by 1 year. (6) It was not until the introduction of the landmark Edmonton Protocol in 2000 (7) that islet transplantation became a viable treatment option for patients with type 1 diabetes.
Evolution of Islet Transplantation
The evolution of islet transplantation has advanced considerably in recent years. The Edmonton Protocol, first published in 2000, suggested for the first time a steroid-free immunosuppression regimen for islet transplantation. (7) However, nowadays very few centres use the original Edmonton Protocol. Not only have immunosuppression protocols evolved to contain different cocktails of medications to optimize graft success and reduce rejection, but techniques in islet isolation and purification have also improved to increase islet yield and function. This makes it difficult to compare islet transplantation studies that have been published many years apart, as the literature quickly becomes outdated; transplantation success and outcomes are constantly being redefined with the advent of improved techniques and novel technologies.
Edmonton Protocol
The Edmonton study changed islet transplantation for the treatment of type 1 diabetes. All seven consecutive patients in the study achieved insulin independence at 1 year, an unprecedented outcome at the time, compared with previous results of less than 10%. (7) In the Edmonton study, only patients with brittle diabetes and life-threatening hypoglycemia were selected. The investigators also dissociated islet transplantation from kidney disease, and excluded patients who had end-stage renal disease or previous transplantation of the kidney or other solid organs. The inclusion and exclusion criteria for the Edmonton Protocol are presented in the Appendix 1.
There were four main approaches in the Edmonton Protocol that differed from previous islet transplantation protocols:
-
1.
Steroid-free immunosuppression regimen—Immunosuppression consisted of sirolimus, low-dose tacrolimus, and daclizumab. Previous regimens included glucocorticoids, which increase insulin resistance
-
2.
Sufficient number of viable islets from multiple donors—Pancreases from more than one donor (usually two to four) were used. More than 10,000 islet equivalents per kilogram were extracted and infused several weeks apart; previously the threshold had been 6,000 islet equivalents per kilogram. This increase in the total number of transplanted islets improved the likelihood of insulin independence after transplantation
-
3.
Islet isolation and purification—Non-human medium (e.g., fetal calf serum) was removed from the isolation and purification process to eliminate exposure to xenoproteins
-
4.
Short cold ischemic storage time—To optimize islet function, islets were transplanted immediately after the purification process. The Edmonton Protocol limited cold storage to less than 13 hours, including the islet-isolation process time, as it had been shown that storage beyond 12 hours reduces islet yield. (8) Previous methods cultured cells for several days before infusion
The key features that contributed to the success of the Edmonton Protocol, compared with earlier islet transplantation procedures, were the use of multiple donor pancreases to obtain large numbers of viable islets and the elimination of steroids from the post-transplantation immunosuppressive regimen. While the Edmonton Protocol provided a turning point for islet transplantation, challenges were encountered in its reproducibility, and follow-up at 5 years revealed graft function loss, with 90% of patients eventually returning to insulin therapy. (9) The multiple-donor approach decreased the feasibility of the procedure for some clinical islet transplantation centres owing to higher costs and a demand for increased islet availability. Also, post-purification transplantation was not possible for some centres because of technical limitations in islet preparation. Owing to some of these challenges, insulin independence rates at 1 year differed significantly between multiple clinical centres. To address these limitations, the Edmonton Protocol was modified over the years to improve upon the original protocol's efficacy and safety outcomes to achieve increased transplantation success.
Procedure
There are two methods or cell sources for β-cell replacement: islet allotransplantation and islet autotransplantation. Islet allotransplantation involves the harvesting of islets from pancreases of deceased organ donors; this procedure is used for patients with type 1 diabetes. In contrast, islet autotransplantation is performed after total pancreatectomy using islets extracted from the patient's own pancreas; islet autotransplantation is an option for patients with chronic pancreatitis to prevent diabetes or reduce the severity of diabetes after the removal of the pancreas. Three types of procedures exist for islet allotransplantation for type 1 diabetes: islet transplantation alone, islet-after-kidney transplantation, and simultaneous islet-kidney transplantation.
Nephropathy is one of the most common and serious complications in type 1 diabetes, occurring in 20–40% of patients with type 1 diabetes over a period of 25 years since the onset of their diabetes. (10, 11) Islet-after-kidney transplantation and simultaneous islet-kidney transplantation are considered for patients with kidney failure (uremia). Islet-after-kidney transplantation has the advantage of more favourable risk-benefit considerations as patients are already obligated to lifelong immunosuppression, compared with islet transplantation alone. (12) Clinical indications are not well-established for either transplantation.
Islet allotransplantation begins with pancreas selection from deceased donor(s), followed by the extraction, isolation, and purification of islets. The subsequent infusion of islets into the eligible recipient is the actual transplantation procedure. Donor selection has a significant impact on islet transplantation outcomes. Both the number of islets and their quality are affected by donor age and body mass index (and by ischemic cold storage time). (13) Older donors may provide an adequate islet yield; however, the islet function may be reduced. By contrast, young pancreas donors provide islets with superior function, but extracting the islets from the pancreas is difficult. (14)
Islet isolation requires considerable skill and experience. It is the most challenging aspect of islet transplantation preparation, and contributes to the wide variability in the success rates of various programs. This process involves enzymatic digestion and mechanical disruption to free the islets from the surrounding exocrine pancreatic tissue. Once isolated, islets are purified by density-gradient centrifugation. Isolated islets are counted and sized to determine the number of islet equivalents (an idealized islet with a diameter of 150 microns), and viability assessments are performed. Unlike in the original Edmonton Protocol, the culturing of islets now considers logistical benefits (e.g., preparation and potential transportation to different centres) and physiological benefits (e.g., to provide time to treat recipients with immunosuppressive and inflammatory drugs before islet transplantation).
To justify the risks associated with islet transplantation, patients usually selected for the procedure are (a) those with brittle type 1 diabetes mellitus with hypoglycemic unawareness and mild or no kidney disease, and (b) those with type 1 diabetes who have already received a kidney transplant and have prohibitive risks for whole-organ pancreas transplantation. Table 1 presents a summary of the current indications and exclusions for islet transplantation alone for type 1 diabetes. (15)
Table 1:
Indications and Contraindications for Islet Transplantation Alone
| Indications | Exclusions |
|---|---|
| Type 1 diabetes mellitus for > 5 y | Uncontrolled hypertension |
| > 18 y old | Severe cardiac disease |
| Negative stimulated C-peptide (< 0.3 ng/mL) | Macroalbuminuria |
| Hypoglycemic unawareness and glycemic lability (brittle diabetes, high variability in glucose levels) despite optimal insulin therapy | Glomerular filtration rate < 80 mL/min/1.73 m2 |
| Potential inability to comply with immunosuppression |
Source: Adapted from McCall and Shapiro. (15)
Compared with whole-organ pancreas transplantation, islet transplantation is less invasive for the recipient. The islets are infused via a percutaneous transhepatic catheter that has been guided into the portal vein of the liver. The recipients usually undergo one or two infusions depending on the total islet mass transplanted and the glycemic control and insulin requirements following the first infusion. Procedure-related complications include portal vein thrombosis (a blood clot that causes a blockage or narrowing of the portal vein), bleeding, and portal hypertension. Refinements in the infusion technique have reduced the rate of complications.
Islet transplantation recipients require treatment with immunosuppressive drugs to prevent rejection. Modifications have been made to the original Edmonton Protocol: the monoclonal antibody daclizumab has been replaced by thymoglobulin, basiliximab, or alemtuzumab. (16–18) Inhibitors of inflammatory factors (etanercept (19, 20) and infliximab (21)) have been introduced, and recently exenatide has been used to promote insulin secretion (22).
Close follow-up monitoring is required during the post-transplantation period. Graft function can be assessed by levels of HbA1c (glycosylated hemoglobin) and tests of oral glucose tolerance. Definitions of islet transplantation success vary; while the ultimate goal of islet transplantation is to achieve insulin independence, for patients with brittle type 1 diabetes with life-threatening hypoglycemia unawareness, reductions in these hypoglycemic events may significantly improve their quality of life even if insulin independence is not achieved. Thus, the frequency of hypoglycemic episodes and the decreased insulin dose requirements are important clinical outcomes for islet transplantation, in addition to the main outcome of insulin independence.
Current Limitations and Future Research
The two main limiting factors that prevent the widespread use of islet transplantation are the limited availability of donor pancreases for transplantation and the need for immunosuppressive therapy. The rate of deceased donation remains poor in Canada, and the majority of donated pancreases are not suitable for islet extraction. In addition, donor pancreases that cannot be used for whole-organ pancreas transplantation in Ontario are currently sent to Edmonton for islet transplantation and research. Alternative strategies have been explored to address the limitation of donors (23):
The use of a single donated pancreas
Living pancreas donors
Xenotransplantation (the transplantation of islets extracted from another species whose islets have close homology to human islets, such as pigs)
Stem cell–derived β cells, which have the potential to provide an unlimited supply of islet cells
The expansion of existing β cells that produce insulin and human pancreatic ductal cells
Transdifferentiation (the conversion of one cell type to another) of liver, bile duct, and exocrine pancreatic cells (24)
Immunoisolation represents an attractive approach to protect islets and prolong their graft survival after transplantation without immunosuppressive therapy. Islets are enclosed in a semipermeable immunoprotective capsule; nutrients and insulin may still be exchanged, but the islets are protected from the host's immune system. Methods of microencapsulation use biocompatible materials that also must allow for the vascularization and enervation of the graft, as a significant factor influencing islet survival and function is rapid and adequate revascularization.
In an attempt to improve revascularization, alternative sites for islet transplantation have been considered, apart from the portal vein and kidney capsule, which have been routine clinical practice; however, few of these alternative sites have proved feasible in a clinical setting. (25) This reflects the need for additional research for alternative strategies to improve the therapeutic benefit of islet transplantation, while highlighting the main theme that all of the above approaches come with their own challenges and likely will not be ready for widespread clinical use in the near future. As of March 31, 2015, there were over 70 ongoing trials of islet transplantation for type 1 diabetes registered on clinicaltrials.gov with the United States National Institutes of Health. (26)
Canadian and International Contexts
While pancreas transplantation for type 1 diabetes is available through clinical centres in Ontario, islet transplantation is currently not provided. Within Canada, the University of Alberta in Edmonton, the University of British Columbia in Vancouver, and McGill University in Montreal are the only centres that provide islet transplantation (fully funded) to patients with type 1 diabetes. Until 2013, Alberta supported islet transplantation for Ontario patients in Edmonton; however, this is no longer available. The Edmonton and Vancouver centres perform islet transplantation alone and islet-after-kidney transplantation, but do not currently perform simultaneous islet-kidney transplantation. The Canada Diabetes Association has recommended that islet transplantation be considered for a subset of patients with type 1 diabetes with preserved renal function, or who have undergone successful kidney transplantation but have persistent metabolic instability despite best efforts to optimize glycemic control. (27) Guidance from other organizations is summarized in Existing Guidelines for Technology.
Internationally, the Collaborative Islet Transplantation Registry compiles data from more than 40 islet transplantation centres in Canada, the United States, Europe, Australia, and Asia. (28) From 1999 to 2012, there were 516 recipients of islet transplantation in North America from participating islet transplantation centres, with 486 undergoing islet transplantation alone and 55 undergoing either islet-after-kidney transplantation or simultaneous islet-kidney transplantation. (29)
Islet transplantation is also funded in Australia and several countries in Europe, such as the United Kingdom, France, Italy, Sweden, and Switzerland.
Regulatory Status
In 2013, Health Canada released the second edition of Guidance Document for Cell, Tissue and Organ Establishments—Safety of Human Cells, Tissues and Organs for Transplantation. (30) This document serves to provide industry and health care professionals with guidance on how to comply with the governing statutes and regulations so that potential health risks to Canadian recipients of cells, tissues, and organs are minimized. Safety requirements are stated with respect to processing (e.g., donor screening, donor testing, donor suitability assessment, testing and measurements, preservation, quarantine, banking, and packaging and labelling); storage; recording keeping; distribution; importation; and error, accident, and adverse reaction investigation and reporting. Regulations regarding cells, tissues, and organs apply to all individuals and establishments that handle, process, distribute, or import human organs or minimally manipulated cells and tissues for transplantation in another individual in Canada. Since islet cells are considered biologicals, in addition to the information that is required of drugs, more detailed chemistry and manufacturing information are necessary; a drug submission must indicate that a biological is approved for sale in Canada. For adherence to the full regulations, one must also consult the most recent version of Cells, Tissues, and Organs for Transplantation and Assisted Reproduction (national standard), by the Canadian Standards Association, and the Food and Drugs Act.
Similarly, in the United States the Food and Drug Administration published in 2009 a guidance for industry titled Considerations for Allogeneic Pancreatic Islet Cell Products that describes its recommendations for those individuals involved in clinical studies of islet cells for the treatment of type 1 diabetes. (31) Within the guidance document, the Food and Drug Administration covers recommendations and suggestions on manufacturing quality and control considerations; preclinical considerations including goals of preclinical safety studies, animal models, and immunosuppressive regimens; clinical study protocols including design, eligibility criteria, study conduct, and study end points; data analysis plans; and follow-up. According to the Food and Drug Administration, islet cells for type 1 diabetes are also considered both a biological and drug product. An investigational new drug application is required to help establish the safety, purity, and potency of islets as a biological product.
EVIDENCE REVIEW
Research Questions
What is the effectiveness of islet transplantation for patients with type 1 diabetes mellitus?
What is the effectiveness of islet transplantation alone for patients with non-uremic type 1 diabetes?
What is the effectiveness of islet-after-kidney transplantation or simultaneous islet-kidney transplantation for patients with uremic type 1 diabetes?
Methods
Literature Search
A previous evidence-based analysis on islet transplantation was completed in 2003 by the Ontario Medical Advisory Secretariat. (32) The results of the analysis on effectiveness were inconclusive, and the author concluded that islet transplantation should be regarded as experimental until more consistent data were available. There was no corresponding recommendation by the Ontario Health Technology Advisory Committee since the analysis preceded the formation of that committee. The current analysis serves to update the previous analysis and to examine the new literature that has since been published on the topic.
Consulting with experts, the scope of the original evidence-based analysis was broadened to include all patients with type 1 diabetes who may benefit from the therapeutic option, including patients with type 1 diabetes who have kidney disease. Thus, the focus of this analysis was extended to include all types of islet transplantation, not just the islet-transplantation-alone procedure.
Search Strategy
A literature search was performed on November 27, 2014, using Ovid MEDLINE, Ovid MEDLINE In-Process and Other Non-Indexed Citations, and EBM Reviews, for studies published from January 1, 2003, to November 27, 2014. (Appendix 2 provides details of the search strategies.) We used a two-step process: first, we searched the database for systematic reviews and health technology assessments; second, we searched primary studies to update the chosen health technology assessment. We excluded articles based on information in the title and abstract, and obtained full texts of potentially relevant articles for further assessment. A single reviewer reviewed the abstracts and, for those studies meeting the eligibility criteria, we obtained full-text articles. We also examined reference lists for any additional relevant studies not identified through the search.
Inclusion Criteria
English-language full-text publications
Studies published between January 1, 2003, and November 27, 2014
Randomized controlled trials, observational studies, systematic reviews, meta-analyses, and health technology assessments
Studies on islet (allo)transplantation (islet transplantation alone, islet-after-kidney transplantation, or simultaneous islet-kidney transplantation)
Studies in adults (age ≥ 18 years) with type 1 diabetes mellitus
Studies in uremic or non-uremic patients
Studies that reported an analysis of database or registry data
Exclusion Criteria
Studies comparing different immunosuppression protocols or methods for islet transplantations
Studies on stem cell–derived islet transplantation, islet xenotransplantation, microencapsulated islet transplantation, or autologous transplantation or autotransplantation
Editorials, case reports, or commentaries
Animal and in vitro studies
Studies where outcomes of interest could not be extracted
Outcomes of Interest
-
Glycemic control:
-
○
HbA1c levels
-
○
Hypoglycemia (events/unawareness)
-
○
Graft loss and insulin independence
-
○
Reduction in insulin dose requirements
-
○
C-peptide levels
-
○
-
Secondary complications of diabetes:
-
○
Cardiovascular disease and risk factors
-
○
Nephropathy
-
○
Retinopathy
-
○
Neuropathy
-
○
Adverse events (e.g., infection, mortality)
Health-related quality of life (e.g., generic, diabetes-specific)
Statistical Analysis
Meta-analysis was considered but could not be performed owing to the heterogeneity in patient populations, study design, and outcome measurements. Therefore, a narrative report of the results are provided by outcome.
Quality of Evidence
We used the Assessment of Multiple Systematic Reviews (AMSTAR) measurement tool to assess the methodological quality of systematic reviews. (33) The evidence quality assessment is presented in Appendix 3.
We examined the quality of the body of evidence for each outcome according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group criteria. (34) Using a step-wise, structural methodology, we determined the overall quality to be high, moderate, low, or very low.
Study design was the first consideration. The starting assumption was that randomized controlled trials are high quality, whereas observational studies are low quality. Five additional factors—risk of bias, inconsistency, indirectness, imprecision, and publication bias—were then taken into account. Limitations in these areas resulted in downgrading the quality of evidence. Finally, we considered three main factors that may raise the quality of evidence: the large magnitude of effect, the dose response gradient, and any residual confounding factors. (34) For more detailed information, please refer to the latest series of GRADE articles. (34)
As stated by the GRADE Working Group, the final quality score can be interpreted using the following definitions:
| High | High confidence in the effect estimate—the true effect lies close to the estimate of the effect |
| Moderate | Moderate confidence in the effect estimate—the true effect is likely to be close to the estimate of the effect, but may be substantially different |
| Low | Low confidence in the effect estimate—the true effect may be substantially different from the estimate of the effect |
| Very Low | Very low confidence in the effect estimate—the true effect is likely to be substantially different from the estimate of the effect |
Results
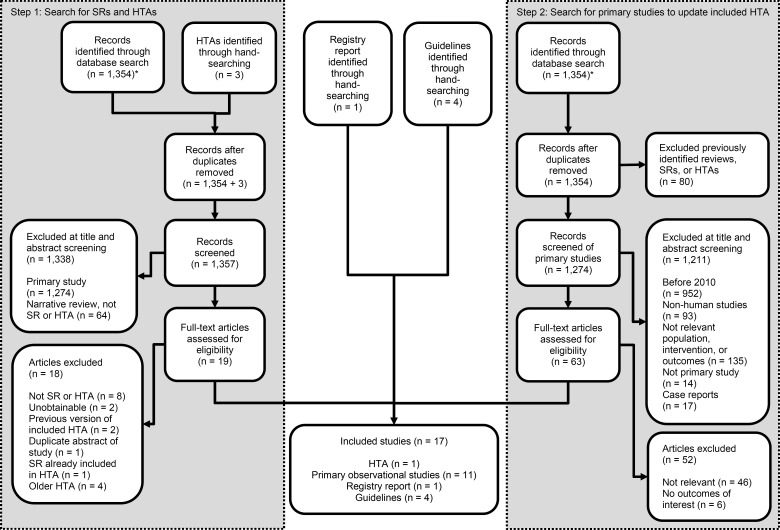
The database search yielded 1,354 citations published between January 1, 2003, and November 27, 2014 (with duplicates removed). Figure 1 shows the breakdown of when and for what reason citations were excluded from the analysis.
Figure 1: Citation Flow Chart.
Abbreviations: HTA, health technology assessment; SR, systematic review.
*Same initial database search containing 1,354 citations.
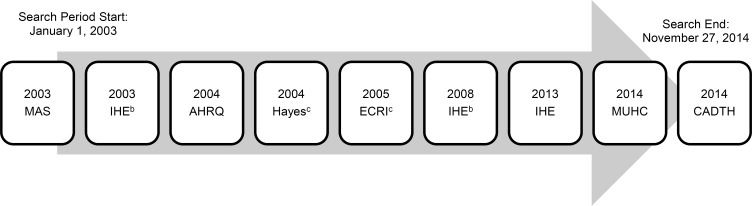
Six relevant health technology assessments (32, 35–39) and one systematic review (40) were found in the database search. The most recent health technology assessment in the search was a 2008 health technology assessment on islet transplantation by Alberta's Institute of Health Economics. (38) However, health technology assessment websites (the Canadian Agency for Drugs and Technologies in Health [CADTH] and the University of York's Centre for Reviews and Dissemination) were also hand-searched and an additional two recent health technology assessments were found from 2013 (41) and 2014 (42) as well as a rapid response by CADTH from 2014. (43) Figure 2 shows the timeline of the health technology assessments found through step one of the search.
Figure 2: Health Technology Assessments Found Through Database Search and Hand-Searching (2003–2014)a.
aNine health technology assessments were found: Medical Advisory Secretariat (MAS), 2003 (Health Quality Ontario (32)); Institute of Health Economics (IHE), 2003 (Guo et al (39)); Agency for Healthcare Research and Quality (AHRQ), 2004 (Piper et al (37)); Hayes Inc, 2004 (36); ECRI Institute, 2005 (35); IHE, 2008 (Guo et al (38)); IHE, 2013 (41); McGill University Health Centre (MUHC), 2014 (Xie et al (42)); Canadian Agency for Drugs and Technology in Health (CADTH), 2014. (43) The three most recent were found through hand-searching. The 2013 IHE health technology assessment was chosen for inclusion because of its high quality and relevant population and outcomes.
bPrevious versions (2003 and 2008) of the most recent 2013 IHE report.
cFull-text articles could not be obtained.
While the CADTH report (43) was the most recent article (published in December 2014), it was only a rapid response, which is a quick project that is limited in scope and not comprehensive, unlike a full health technology assessment. The second most recent health technology assessment was from McGill University Health Centre (42); however, the methodology and results were not as well-defined for clinical effectiveness because the report primarily focused on the health economics of islet transplantation. The third most recent health technology assessment was by the Institute of Health Economics, from 2013. (41) (The database search captured only the institute's two previous versions, from 2003 (39) and 2008. (38)) Both the CADTH (43) and McGill University Health Centre (42) reports referenced this 2013 Institute of Health Economics report, (41) which scored 10 out of 11 on AMSTAR. Therefore, based on its high quality, its recency, the relevance of its population and study outcomes, and its original analysis, the health technology assessment by the Institute of Health Economics (41) was chosen as the primary report for inclusion.
Step two of the database search involved searching for primary studies to update the 2013 Institute of Health Economics health technology assessment, (41) which had search end dates of November 2010 for published literature and April 2011 for grey literature. Additional studies that would not have been included in that report (i.e., those published in December 2010 and onward) were examined. We found an additional 11 relevant studies published since the Institute of Health Economics search was performed, and we included these in the analysis. We received automated MEDLINE alerts until March 23 for new citations that met the search criteria after the original November 27 search date; we reviewed these for relevancy, but none met the inclusion criteria. Table 2 summarizes the characteristics and findings of the three most recent health technology assessments.
Table 2:
Identified Health Technology Assessments on Islet Transplantation for Patients with Type 1 Diabetes Mellitus
| Author, Year | Search Dates | Inclusion Criteria | Outcomes of Interest | No. of Studies Included | Main Clinical Conclusions | AMSTAR Ratinga |
|---|---|---|---|---|---|---|
| IHE, 2013 (41) | 2006–2011 (for grey literature)and 2000–2010 (for published literature) |
|
|
|
|
10 |
| MUHC, 2014 (Xie et al (42)) | 2008–November 25, 2013 |
|
|
|
|
4 |
| CADTH, 2014 (rapid response) (43) | 2011–November 12, 2014 |
|
|
|
|
6 |
Abbreviations: AMSTAR, Assessment of Multiple Systematic Reviews; CADTH, Canadian Agency for Drugs and Technologies in Health; HRQOL, health-related quality of life; HTA, health technology assessment; IAK, islet-after-kidney transplantation; IHE, Institute for Health Economics; IT, islet transplantation; MUHC, McGill University Health Centre; PT, pancreas transplantation; RCT, randomized controlled trial; T1DM, type 1 diabetes mellitus.
Scored out of 11.
Study Characteristics
The 2013 Institute of Health Economics report included six observational comparative studies with eight publications, two systematic reviews (one of which was its previous 2008 health technology assessment), 13 case series with 20 publications, and nine safety-only case series studies. (41) In the health technology assessment, quality assessment was performed using the Downs and Black checklist (which includes the four domains of reporting, external validity, internal validity, and power) for the observational comparative studies, and the institute's case series quality assessment checklist was used for the case series studies. Many of the observational comparative and case series studies in the health technology assessment scored low in quality. A meta-analysis was not performed owing to heterogeneities in the study population, intervention, and outcome. Because of the prevalence of low-quality studies, none were excluded based on quality assessment. The authors did however exclude studies with less than 1 year of follow-up, case series that included fewer than 10 patients, and all case reports.
Tables 3 and 4 present the study characteristics of the included observational comparative and case series studies in the health technology assessment, along with the 11 additional studies that were found to update the results of the health technology assessment. Assessment of risk of bias for these 11 studies can be found in Tables A4 and A5 of Appendix 3.
Table 3:
Observational Comparative Studies on Islet Transplantation for Patients with Type 1 Diabetes Mellitus
| Author, Year | Country | N | Patient Criteria | Study Design (Prospective/Retrospective) | Intervention | Comparator | Main Outcomes | Follow-Up Length (y) |
|---|---|---|---|---|---|---|---|---|
| Studies included in the 2013 IHE report | ||||||||
| Fiorina et al, 2003 (44) | Italy | 241 | Diabetic patients with kidney transplants, C-peptide negative Exclusion criteria: previous stroke, major amputations, severe dilated cardiomyopathy, coronary artery disease |
Unclear | IAK or SIK | IIT or SPK | Glycemic control, diabetes complications | 5 |
| Frank et al, 2004 (45) | United States | 43 | Patients with highly labile type 1 diabetes complicated by repeated episodes of severe hypoglycemic unawareness | Retrospective | ITA or IAK | SPK or PAK | Glycemic control | 2.5 |
| Fiorina et al, 2005 (46) | Italy | 42 | Patients with type 1 diabetes and end-stage renal disease Exclusion criteria: severe hepatic dysfunction, major stroke with neurological inability, major amputation, severe dilated cardiomyopathy, severe coronary artery disease |
Retrospective | IAK | IIT | Glycemic control, diabetes complications | 3 |
| Fiorina et al, 2005 (10) | Italy | 234 | Patients with type 1 diabetes and end-stage renal disease Exclusion criteria: lymphoproliferative disease or neoplasm |
Prospective | IAK or SIK | IIT or SPK | Glycemic control, diabetes complications | 6 |
| Venturini et al, 2006 (47) | Italy | 20 | Patients with type 1 diabetes Exclusion criteria: cardiovascular, nephrological and psychological problems (not specified) |
Unclear | ITA | IIT | Glycemic control, diabetes complications | 1 |
| Gerber et al, 2008 (48) | Switzerland | 38 | Patients with type 1 diabetes and end-stage renal failure with need for dialysis | Retrospective | SIK | SPK | Glycemic control, severe hypoglycemia, diabetes complications | 5 |
| Warnock et al, 2008 (49) | United States | 42 | Patients with type 1 diabetes > 5 y in duration, aged 20–65 y, C-peptide negative, evidence of retinopathy and mild nephropathy Exclusion criteria: ischemic heart disease, previous transplantation, recurrent infections, malignancy (except basal or squamous skin cancer) |
Prospective | ITA | IIT | Glycemic control, diabetes complications | 5 |
| Vantyghem et al, 2009 (50) | France | 43 | ITA: patients with type 1 diabetes with hypoglycemia unawareness or diabetes lability, failure or refusal of subcutaneous insulin pump, aged 18–65 y, body mass index < 28 kg/m2, blood creatinine < 250 mg/dL, albuminuria < 300 mg/d, no desire for pregnancy IAK: patients ineligible for kidney-pancreas transplantation if creatinine blood level stable for at least 6 mo after kidney transplantation and steroid discontinuation |
Unclear | ITA or IAK | IIT | Glycemic control, severe hypoglycemia, diabetes complications | 3 |
| Additional recent studies | ||||||||
| Thompson et al, 2011 (51) | United States | 45 | Patients with type 1 diabetes (no other explicit criteria listed) | Prospective | ITA | IIT | Glycemic control, diabetes complications | 8 |
| Maffi et al, 2011 (52) | Italy | 66 | Patients with type 1 diabetes > 5 y in duration, aged 18–64 y, undetectable stimulated C-peptide, weight < 75 kg for males and < 70 kg for females, reduced hypoglycemic awareness, unstable metabolic control with severe hypoglycemia or ketoacidosis that required hospitalization despite intensive insulin management, progression of neuropathy and retinopathy, serum creatinine <1.5 mg/dL and urinary protein excretion < 300 mg/dL, cardiovascular disease excluding patient from pancreas transplantation | Unclear | ITA | PTA | Glycemic control | 1 |
| D'Addio et al, 2014 (53) | Italy | 22 | Patients with type 1 diabetes actively enrolled on islet transplantation waiting list Exclusion criteria: history of cerebrovascular disease and/or taking oral anticoagulant agent |
Prospective | ITA | IIT | Glycemic control, diabetes complications, health-related quality of life | 1.25 |
| Radosevich et al, 2013 (54) | United States | 75 | Patients with type 1 diabetes (no other explicit criteria were listed) | Prospective | ITA | IIT | Health-related quality of life | 5 |
Abbreviations: IAK, islet-after-kidney transplantation; IIT, intensive insulin therapy; ITA, islet transplantation alone; PAK, pancreas-after-kidney transplantation; PTA, pancreas transplantation alone; SIK, simultaneous islet-kidney transplantation; SPK, simultaneous pancreas-kidney transplantation.
Table 4:
Case Series Studies on Islet Transplantation for Patients with Type 1 Diabetes Mellitus
| Author, Year | N | Patient Population | Intervention | Immunosuppression Protocol | Main Outcome | Length of Follow-Up (y) |
|---|---|---|---|---|---|---|
| International | ||||||
| Shapiro et al, 2006 (55) | 36 | Patients with type 1 diabetes > 5 y in duration, aged 18–65 y, undetectable C-peptide levels, recurrent neuroglycopenia, reduced awareness of hypoglycemic episodes or severe glycemic lability Exclusion criteria: noncorrectable coronary artery disease, body mass index > 26 kg/m2, weight > 70 kg for women or 75 kg for men, insulin requirement ≤ 0.7 U/kg/d, HbA1c > 12%, inadequate renal reserve, creatinine clearance < 80 mL/min/1.73 m2, macroalbuminuria, presence of Epstein–Barr virus |
ITA | Induction: daclizumab Maintenance: sirolimus, tacrolimus (Edmonton Protocol) |
Glycemic control | 2 |
| Edmonton, Canada | ||||||
| Ryan et al, 2005 (9) | 65 | Patients with type 1 diabetes, with problematic hypoglycemia, labile diabetes, or progressive complications of diabetes Exclusion criteria: unstable coronary artery disease, active proliferative retinopathy, macroproteinuria ≥ 1 g/d, macroproteinuria < 1 g/d |
ITA | Induction: daclizumab Maintenance: sirolimus, tacrolimus (Edmonton Protocol) |
Glycemic control | 5 |
| Toso et al, 2007 (56) | 99 | Patients with type 1 diabetes (no other explicit criteria listed) | ITA | Not reported | HRQOL | 3 |
| Koh et al, 2007 (19) | 97 | Patients with type 1 diabetes, C-peptide negative, frequent hypoglycemia, hypoglycemia unawareness, severe glycemic lability | ITA | Induction: daclizumab Maintenance: sirolimus, tacrolimus (Edmonton Protocol, 83 patients) Induction: alemtuzumab Maintenance: sirolimus or tacrolimus, MMF (14 patients) |
Glycemic control | 3 |
| Milan, Italy | ||||||
| Maffi et al, 2007 (57) | 19 | Patients with type 1 diabetes > 5 y in duration, decreased awareness of hypoglycemia, metabolic instability, progressive chronic complications despite intensive insulin regimen Exclusion criteria: severe cardiovascular disease, progressive nephropathy, history of chronic infectious disease, malignancy |
ITA | Induction: daclizumab Maintenance: sirolimus, tacrolimus (Edmonton Protocol) |
Renal function, glycemic control | 2 |
| Fiorina et al, 2003 (58) | 36 | Patients with type 1 diabetes who underwent kidney transplantation, C-peptide negative | ITA, IAK, SIK | Induction: ATG Maintenance: cyclosporine, MMF, azathioprine, methylprednisolone |
Renal function | 7 |
| Fiorina et al, 2003 (44) | 34 | Patients with type 1 diabetes who underwent kidney transplantation, C-peptide negative Exclusion criteria: previous strokes, major amputations, severe dilated cardiomyopathy, coronary artery disease |
IAK | Induction: ATG Maintenance: cyclosporine, MMF, methylprednisolone |
Cardiovascular disease | 4 |
| Bertuzzi et al, 2002 (59) | 15 | Patients with type 1 diabetes who underwent kidney transplantation (no other explicit criteria listed) | IAK | Induction: ATG Maintenance: cyclosporine or tacrolimus, MMF, methylprednisolone |
Glycemic control | 1 |
| France and Switzerland | ||||||
| Benhamou et al, 2009 (60) | 20 | Adults with type 1 diabetes, negative basal and stimulated C-peptide, established kidney transplantation functioning > 6 mo | ITA or IAK | Induction: daclizumab Maintenance: sirolimus, tacrolimus (Edmonton Protocol) |
HRQOL | 1 |
| Badet et al, 2007 (61) | 10 | Patients with type 1 diabetes > 5 y duration, aged 18–65 y, frequent episodes of severe hypoglycemic despite intensive insulin therapy Exclusion criteria: kidney disease, liver and coagulation abnormalities, unstabilized ischemic diabetic retinopathy, poor cardiovascular prognosis, weight > 70 kg in women and > 75 kg in men, body mass index > 26 kg/m2, exogenous insulin requirement > 0.7 IU/kg/d or > 50 IU/d |
ITA | Induction: daclizumab Maintenance: sirolimus, tacrolimus (Edmonton Protocol) |
Glycemic control | 3 |
| Benhamou et al, 2001 (62) | 10 | Adults with type 1 diabetes, negative basal and stimulated C-peptide, established kidney transplantation functioning > 6 mo | IAK | Induction: basiliximab, methylprednisolone Maintenance: cyclosporine, MMF |
Glycemic control | 1 |
| aBorot et al, 2011 (63) | 19 | Patients with type 1 diabetes, undetectable C-peptide, functional kidney graft, creatinine clearance > 50 mL/min, proteinuria < 0.5 g/d, insulin requirement < 0.7 U/kg/d, body mass index < 27 kg/m2, weight < 80 kg for males and < 75 kg for females | IAK | Induction: daclizumab Maintenance: sirolimus, tacrolimus |
Glycemic control | 2 |
| United States | ||||||
| Froud et al, 2005 (21) | 16 | Patients with type 1 diabetes patients and hypoglycemic unawareness Exclusion criteria: renal dysfunction |
ITA | Induction: daclizumab Maintenance: sirolimus, tacrolimus (8 patients also received infliximab) |
Glycemic control | 3 |
| Tharavanij et al, 2008 (64) | 40 | Patients with type 1 diabetes who underwent islet transplantation | ITA or IAK | Induction: not reported Maintenance: sirolimus, tacrolimus, or MMF |
HRQOL | 6 |
| Leitao et al, 2008 (65) | 31 | Patients with type 1 diabetes (no other explicit criteria listed) | ITA or IAK | Not reported | Restoration of hypoglycemic awareness | 4 |
| Lee et al, 2005 (66) | 12 | Patients with type 1 diabetes, hypoglycemic unawareness, and diabetes-induced metabolic instability, unresponsive to exogenous insulin administration | ITA | Not reported | Retinopathy | 1 |
| Barshes et al, 2005 (67) | 10 | Patients with type 1 diabetes (no other explicit criteria listed) | ITA | Not reported | HRQOL | 1 |
| Turgeon et al, 2010 (68) | 12 | Patients with type 1 diabetes with onset prior to age 40 y, insulin-dependent > 5 y, hypoglycemic unawareness despite insulin management, body mass index < 26 kg/m2, preserved renal function Exclusion criteria: insulin resistance, significant comorbid conditions |
ITA | Induction: daclizumab Maintenance: sirolimus, tacrolimus (Edmonton Protocol, 8 patients) Induction: daclizumab Maintenance: tacrolimus, efalizumab, MMF (4 patients) |
Glycemic control | 3 |
| Gangemi et al, 2008 (69) | 10 | Patients with type 1 diabetes > 5 y in duration, hypoglycemic unawareness, metabolic lability with documented severe hypoglycemia, ketoacidosis despite insulin therapy Exclusion criteria: cardiac disease, history of nonadherence to prescribed regiments, body mass index > 26 kg/m2 or weight > 70 kg, creatinine clearance < 80 mL/min/1.73m2, insulin requirement > 0.7 IU/kg/d, HbA1c > 12% |
ITA | Induction: daclizumab Maintenance: sirolimus, tacrolimus (Edmonton Protocol, 4 patients) Induction: daclizumab Maintenance: sirolimus, tacrolimus, etanercept, exenatide (6 patients) |
Glycemic control | 1 |
| aDanielson et al, 2013 (70) | 15 | Patients with type 1 diabetes > 5 y in duration, aged 18–65 y, hypoglycemic unawareness despite optimal insulin management efforts Exclusion criteria: untreated cardiac, kidney or liver disease, hyperlipidemia, history of cancer or stroke, active infection, substance abuse, HbA1c > 12%, body mass index > 26 kg/m2, uncontrolled psychiatric disorder, use of corticosteroids or anticoagulants, pregnancy |
ITA | Induction: daclizumab Maintenance: sirolimus or MMF, tacrolimus (4 patients) Induction: daclizumab Maintenance: sirolimus, MMF, etanercept, exenatide (11 patients) |
Cardiovascular disease | 5 |
| Belgium | ||||||
| Keymeulen et al, 2006 (71) | 24 | Patients with type 1 diabetes, negative C-peptide, no kidney disease (no other explicit criteria listed) | ITA | Induction: ATG Maintenance: tacrolimus, MMF |
Glycemic control | 1 |
| France | ||||||
| Vantyghem et al, 2009 (72) | 14 | Patients with type 1 diabetes > 5 y in duration, aged 18–65 y, stimulated C-peptide < 0.2 ng/mL, hypoglycemia unawareness or metabolic lability Exclusion criteria: body mass index > 28 kg/m2, unstable arteriopathy or heart disease, active infection, previous transplantation, insulin requirements > 1.2 U/kg, creatinine clearance < 60 mL/min/1.73m2, urinary albumin excretion > 300 mg/d, malignancy, smoking, desire for pregnancy, psychiatric disorders, lack of compliance |
ITA | Induction: daclizumab Maintenance: tacrolimus, sirolimus (Edmonton Protocol) |
Glycemic control | 3 |
| aVantyghem et al, 2012 (73) | 23 | Same as in Vantyghem et al, 2009 (72) | ITA or IAK | Induction: daclizumab Maintenance: sirolimus, tacrolimus (Edmonton Protocol) |
Glycemic control | 3 |
| Australia | ||||||
| aO'Connell, 2013 (74) | 17 | Patients with type 1 diabetes > 5 y in duration, aged 18–65 y, severe hypoglycemia unawareness Exclusion criteria: diabetic nephropathy, renal impairment |
ITA | Induction: ATG Maintenance: tacrolimus and MMF, or sirolimus and MMF |
Glycemic control | 1 |
| Sweden | ||||||
| aHaggstrom et al, 2011 (75) | 11 | Patients with severe insulin-dependent diabetes (no other explicit criteria listed) | ITA | Not reported | HRQOL | Cross-sectional |
Abbreviations: ATG, antithymocyte globulin; GRAGIL, Groupe Rhin-Rhône-Alpes-Genève pour la Transplantation d'Ilots de Langerhans; HRQOL, health-related quality of life; IAK, islet-after-kidney transplantation; ITA, islet transplantation alone; MMF, mycophenolate mofetil.
Denotes additional recent study that was not included in the Institute of Health Economics 2013 health technology assessment. (41)
Source: Adapted from Institute of Health Economics, 2013. (41)
The observational comparative studies (a) compared islet transplantation to intensive insulin therapy or a waiting list, (b) had a crossover design of intensive insulin therapy to islet transplantation, or (c) used as “controls” pancreas transplantation or another islet transplantation procedure within the same islet transplantation centre. Owing to the nature of the intervention, no randomized controlled studies were found, with the highest quality of evidence being from the observational comparative studies. Follow-up varied from 1 to 8 years. As done in the health technology assessment by the Institute of Health Economics, the case series presented in Table 4 are categorized by their original country.
Since islet transplantation can be performed in uremic patients with type 1 diabetes as simultaneous islet-kidney transplantation or islet-after-kidney transplantation, or in non-uremic patients with type 1 diabetes as islet transplantation alone, the results are reported in separate patient populations depending on kidney disease status.
Glycemic Control
Non-uremic Patients
Five observational comparative studies examined islet transplantation alone for patients with type 1 diabetes without kidney disease. (47, 49, 51–53) Table 5 summarizes the glycemic control outcomes from the observational comparative studies. Four of the studies compared islet transplantation alone with intensive insulin therapy, (47, 49, 51, 53) whereas one study compared islet transplantation alone with pancreas transplantation alone (52) using data from a single study centre. Three of the studies noted significant changes in HbA1c levels in the group receiving islet transplantation alone compared with intensive insulin therapy, (49, 51, 53) with a fourth study showing marginally non-significant results. (47) Insulin independence was noted at 96% 3 months after islet transplantation alone in one study; however, ranges decreased to the 50% range when assessed at 5 years. (51) For the studies that assessed partial graft function, about 60% of patients maintained partial graft function at the end of the study. (49, 52)
Table 5:
Glycemic Outcomes for Patients with Type 1 Diabetes Mellitus, from Observational Comparative Studies
| Glycemic Control | |||||||
|---|---|---|---|---|---|---|---|
| Author, Year | Treatment | Immunosuppression Protocol | Graft Loss/Insulin Independence | HbA1c Levels (ng/mL) | Insulin Requirements (U/d) | C-Peptide (ng/mL) | Hypoglycemia |
| Non-uremic patients | |||||||
| Venturini et al, 2006 (47) | 10 ITA or 10 IIT | Induction: daclizumab Maintenance: sirolimus, tacrolimus (Edmonton Protocol) |
ITA: 7.95 ± 0.29 pre-transplantation vs. 7.50 ± 0.46 at 1 y (P = .06) IIT: 8.28 ± 0.36 pre-transplantation vs. 8.15 ± 0.22 at 1 y (NS) |
ITA: 31.1 ± 4.2 pre-transplantation vs. 20.3 ± 5.5 at 1 y (P = .06) IIT: 49.0 ± 3.51 pre-transplantation vs. 48.0 ± 4.05 at 1 y (NS) |
ITA: 0.20 ± 0.06 pre-transplantation vs. 0.84 ± 0.18 at 1 y (P < .01) IIT: 0.21 ± 0.11 pre-transplantation vs. 0.14 ± 0.08 at 1 y (NS) |
||
| Warnock et al, 2008 (49) | 31 ITA or 11 IIT | Induction: ATG Maintenance: sirolimus or MMF, tacrolimus |
16/25 patients (64%) insulin independent at end of follow-up | 6.6 ± 0.7 ITA vs. 7.5 ± 0.9 IIT (P < .01) | 33%–75% of pre-transplantation insulin doses due to partial graft function | ||
| Thompson et al, 2011 (51) | 32 ITA or 13 IIT | Induction: ATG or basiliximab Maintenance: sirolimus or MMF, tacrolimus |
Graft loss: 9/32 (28%) patients Insulin independence: 22/23 (96%) at 3 mo 12/23 (52%) at end of follow-up |
8.1% ± 1.2% pre- vs. 7.0% ± 0.7% post-transplantation (P = NR) 6.7 ± 0.2 ITA vs. 7.8 ± 0.3 IIT (P < .001) |
All 23 ITA patients maintained on immunosuppression had persistently detectable C-peptide | ||
| Maffi et al, 2011 (52) | 33 ITA or 33 PTA | Daclizumab induction, maintenance with sirolimus and tacrolimus (Edmonton Protocol, 22 patients) ATG induction, maintenance with sirolimus and MMF (11 patients) |
Early graft loss: 5/33 (15%) ITA vs. 7/33 (21%) PTA Partial graft function: 9/33 (27%) ITA Insulin independence: 19/33 (57%) ITA vs. 25/33 (76%) PTA |
||||
| D'Addio et al, 2014 (53) | 12 ITA or 12 IIT | Induction: daclizumab Maintenance: tacrolimus, sirolimus (Edmonton Protocol) |
Significant changes in ITA vs. IIT | Significant changes in ITA vs. IIT | |||
| Uremic patients | |||||||
| Gerber et al, 2008 (48) | 13 SIK or 25 SPK | Induction: daclizumab Maintenance: sirolimus, tacrolimus (Edmonton Protocol) |
Primary non-function: 2 SIK vs. 0 SPK Insulin independence at 1 y: 31% SIK vs. 96% SPK |
At baseline: SIK (n = 13) 8.1 ± 1.5 vs. SPK (n = 25) 8.7 ± 1.9 (NS) At 1 y: SIK (n = 13) 6.2 ± 0.8 vs. SPK (n = 25) 6.0 ± 0.6 (NS) At 2 y: SIK (n = 9) 6.3 ± 0.7 vs. SPK (n = 22) 5.7 ± 0.5 (P < .05) At 3 y: SIK (n = 8) 6.7 ± 1.0 vs. SPK (n = 15) 5.8 ± 0.4 (P < .05) At 4 y: SIK (n = 5) 6.2 ± 0.5 vs. SPK (n = 10) 5.5 ± 0.6 (NS) HbA1c at 5 y: SIK (n = 1) 5.7 vs. SPK (n = 3) 5.3 (P = NR) |
50% reduction in SIK group |
At end of follow-up: 1.005 ± 0.735 SIK vs. 2.505 ± 0.762 SPK (P = NR) |
Severe hypoglycemia pre-transplantation: 10/13 patients (77%) in SIK Severe hypoglycemia post-transplantation: 0 in both groups |
| Fiorina et al, 2003 (44) | 37 IAK/SIK or 162 SPK or 42 IIT | Induction: ATG Maintenance: cyclosporine, MMF, prednisone |
Successful vs. unsuccessful IAK/SIK: 24 patients vs. 13 patients |
Successful vs. unsuccessful IAK/SIK vs. SIK vs. SPK: Baseline: 8.3 ± 0.3 vs. 7.7 ± 0.6 vs. 11.2 ± 1.7 vs. 11.1 ± 2.3 At 1 y: 7.35 ± 0.29 vs. 7.96 ± 0.35 vs. 5.8 ± 0.8 vs. 8.9 ± 1.3 (NS) At 4 y: 7.33 ± 0.51 vs. 8.08 ± 0.43 vs. 6.0 ± 0.1 vs. 8.6 ± 0.4 (NS) At 7 y: 7.38 ± 0.35 vs. 8.26 ± 0.61 vs. 6.2 ± 0.2 vs. 8.7 ± 0.5 (NS) |
Successful vs. unsuccessful IAK/SIK: At 1 y: 19.1 ± 4.3 vs. 46.0 ± 6.2 (P < .01) At 4 y: 23.0 ± 5.3 vs. 51.8 ± 8.5 (P = .01) At 7 y: 17.8 ± 4.7 vs. 36.4 ± 9.7 (NS) |
Successful vs. unsuccessful IAK/SIK vs. SIK vs. SPK: Baseline: 0.15 ± 0.02 vs. 0.15 ± 0.03 vs. 0.11 ± 0.02 vs. 0.13 ± 0.03 At 1 y: 1.64 ± 0.25 vs. 0.39 ± 0.25 vs. 1.62 ± 0.15 vs. 0.21 ± 0.09 At 4 y: 1.09 ± 0.16 vs. 0.14 ± 0.02 vs. 1.43 ± 0.21 vs. 0.17 ± 0.05 At 7 y: 1.39 ± 0.49 vs 0.10 ± 0.01 vs. 1.39 ± 0.22 vs. 0.15 ± 0.04 |
|
| Fiorina et al, 2005 (10) | 17 IAK or 25 IIT | Induction ATG Maintenance: cyclosporine, MMF, prednisone |
Insulin independence > 3 mo: 12/17 patients (71%) |
For IAK: 7.7 ± 0.3 pre- vs. 7.7 ± 0.2 at 3 y post-transplantation (NS) For IIT: 8.6 ± 0.6 pre- vs. 8.1 ± 0.5 at 3 y post-transplantation (NS) NS differences between IAK and IIT |
In IAK: 25.2 ± 4.3 pre- vs. 17.3 ± 3.4 at 3 y post-transplantation (P < .05) In IIT: 32.1 ± 7.0 pre- vs. 35.1 ± 4.4 at 3 y post-transplantation (NS) In IAK vs. IIT: pre- and 3 y post-transplantation (P < .05) |
At 3 y: 1.7 ± 0.2 IAK vs. 0.3 ± 0.1 IIT (P < .01) | |
| Fiorina et al, 2005 (46) | 24 IAK/SIK, 166 SPK, 44 IIT | Induction: ATG Maintenance: cyclosporine, MMF, prednisone |
Insulin independence at 6 y: 0% IAK/SIK vs. 100% SPK |
Pre- vs. 6 y post-transplantation: In IAK/SIK: 7.4 ± 0.2 vs. 8.1 ± 0.3 at 6 y (P < .05) In SPK: 5.7 ± 0.1 vs. 5.8 ± 0.2 at 6 y (P < .05) In IIT: 8.0 ± 0.4 vs. 7.8 ± 0.2 at 6 y (NS) |
In successful IAK/SIK: 50% decrease from baseline at 2, 4, and 6 y |
In IAK/SIK: 1.6 ± 0.2 pre-transplantation vs. 1.1 ± 0.4 at 6 y (NS) |
|
| Mixed uremic and non-uremic patients | |||||||
| Frank et al, 2004 (45) | 9 ITA, 4 IAK, 25 SPK, 5 PAK | Induction: daclizumab Maintenance: sirolimus, tacrolimus (Edmonton Protocol) |
Graft loss: 5 patients ITA/IAK, 4 patients SPK/PAK Insulin independence at 2 y: 5 patients (42%) ITA/IAK vs. 25 patients (83%) SPK/PAK |
1 y: 6.3% ITA/IAK vs. 5.0% SPK/PAK (P ≤ .001) Average between insulin-independent patients and those requiring small doses of insulin For ITA/IAK: 5.6% vs. 6.65% (NS) |
During first 600 d post-transplantation: 1.7 ITA/IAK vs. 3.9 SPK/PAK (P < .001) Average between insulin-independent patients and those requiring small doses of insulin: for ITA/IAK: 2.3 vs 1.1 (NS) |
No hypoglycemic episodes in any ITA/IAK patients with graft function | |
| Vantyghem et al, 2009 (72) | 7 ITA, 6 IAK, 17 IIT | Induction: daclizumab Maintenance: sirolimus, tacrolimus (Edmonton Protocol) |
Insulin independence at 1 y ITA/IAK: 10 of 13 patients (77%) |
ITA/IAK vs. IIT: Baseline: 8.2 ± 1.1 vs. 8.4 ± 1.8 (NS) At 1 y: 6.1 ± 0.7 vs. 7.9 ± 1.0 (P < .0001) At 3 y: 6.6 ± 1.1 vs. 8.1 ± 1.3 (P < .01) |
ITA/IAK vs. IIT: Baseline: 46 ± 12 vs. 43 ± 18 (NS) At 1 y: 4.4 ± 8.5 vs. 43 ± 20 (P < .0001) At 3 y: 12 ± 16 vs. 46 ± 19 (P < .0001) |
At 3 mo: 1.5 ± 0.7 At 3 y: 11/13 patients (85%) > 0.2 |
Severe hypoglycemia (no. per week) ITA/IAK vs. IIT: Baseline: 2.6 ± 2.1 vs. 2.9 ± 2.2 (NS) At 1 y: 0.3 ± 0.5 vs. 1.6 ± 1.6 (P < .01) At 3 y: 0.7 ± 1.1 vs. 1.7 ± 1.8 (NS) |
Abbreviations: ATG, antithymocyte globulin; HbA1c, glycosylated hemoglobin; IAK, islet-after-kidney transplantation; IIT, intensive insulin therapy; ITA, islet transplantation alone; MMF, mycophenolate mofetil; NA, not available; NR, not reported; NS, non-significant; PAK, pancreas-after-kidney transplantation; PTA, pancreas transplantation alone; SIK, simultaneous islet-kidney transplantation; SPK, simultaneous pancreas-kidney transplantation.
Source: Adapted from Institute of Health Economics, 2013. (41)
Maffi et al (52) performed the only study that compared islet transplantation alone to pancreas transplantation alone, through a single-centre experience; they found that pancreas transplantation alone was associated with greater insulin independence (76%) than was islet transplantation alone (57%). Investigators found that early graft loss was similar in both groups; however, islet transplantation alone offered the benefit of partial graft function, which allowed for decreased insulin dose requirements. (52)
Table 6 presents the data for the observational case series studies on glycemic control. Insulin independence rates were variably reported in the observational case series studies, ranging from 30% to 70% at 1 year post-transplantation. In all but one study, HbA1c levels improved post–islet transplantation during the follow-up period; in the exception, patients returned to baseline levels over 3 years. (21) Insulin dose requirements in patients with partial graft function were also reduced in all studies that examined this outcome, suggesting that partial graft function still provided the benefits of glycemic control for patients. Improvements in hypoglycemia, measured from both hypoglycemic scores and the number of episodes, were seen in all the case series studies that examined that outcome.
Table 6:
Glycemic Outcomes for Patients with Type 1 Diabetes Mellitus, from Observational Case Series Studies
| Glycemic Control | |||||
|---|---|---|---|---|---|
| Author, Year | Graft Loss/Insulin Independence | HbA1c Levels | Insulin Requirements | C-Peptide Levels | Hypoglycemia |
| Non-uremic patients | |||||
| Shapiro et al, 2006 (55) |
Insulin independence: Any time during the study: 21/36 pts (58%) 1 y: 16/36 pts (44%) 2 y: 5/36 pts (14%) Graft function at 1 y: Partial: 10/36 patients (28%) Complete loss: 10/36 patients (28%) |
Reduced in pts with insulin independence or partial graft function over 2 y | Reduced in pts with insulin independence or partial graft function over 2 y | Detectable (≥ 3 ng/mL) in 70% of patients at 2 y | Full protection in insulin-independent group Partial function group had marked benefit in glycemic control compared with baseline |
| Ryan et al, 2005 (9) |
Insulin independence: 5 y: 7.5% Median duration: 15 mo (6.2–25.5 mo) Graft survival: 82% at 5 y |
Significantly reduced in pts off insulin and on insulin with persisting C-peptide vs. pts with lost graft function | Significantly decreased post-transplantation in pts on insulin but still had persistent C-peptide secretion Significantly increased post-transplantation in pts who lost islet function |
Significantly lower in pts on insulin vs. off insulin: Baseline: 0.49 ± 0.05 vs. 0.86 ± 0.05 nmol/L (P < .001) Post-stimulation: 0.93 ± 0.08 vs. 1.62 ± 0.07 nmol/L (P < .001) |
Some hypoglycemia episodes occurred with the use of insulin; HYPOa scores significantly improved for up to 4 y |
| Koh et al, 2010 (19) |
Insulin independence: 13/85 pts (15.3%) for > 4 wk after infusion from single donor Median duration: 18.1 mo (12.1–24.9 mo) |
Reduction in pts who received insulin/heparin infusion vs. those who did not: 80.1 ± 4.3% vs. 54.2 ± 2.8% (P < .001) | |||
| Froud et al, 2005 (21) |
Insulin independence: Any time during the study: 14/16 pts (88%) 1 y: 11/16 pts (69%) 1.5 y: 6/16 pts (37%) 2 y: 5/16 pts (31%) |
Returned to normal in 8 insulin-independent pts over 3 y | 32.7 ± 11.2 U/d pre- vs. 12.6 ± 5.4 U/d post-transplantation (8 pts) | Detectable in all pts while on immunosuppression | No severe hypoglycemia |
| Maffi et al, 2007 (57) |
Insulin independence: 1 y: 8/19 pts (42%) 2 y: 7/8 pts (88%) |
Pre- vs. post-transplantation: 1 y: 8.6 ± 0.03% vs. 6.8 ± 0.2% (P < .001) 2 y: 8.6 ± 0.03% vs. 6.4 ± 0.2% (P < .02) |
Pre- vs. post-transplantation 1 y: 0.01 ± 0.01 vs. 0.46 ± 0.07 nmol/L (P < .001) 2 y: 0.01 ± 0.01 vs. 0.50 ± 0.03 nmol/L (P < .001) |
No severe hypoglycemia post-transplantation, even with insulin therapy | |
| Badet et al, 2007 (61) |
Insulin independence: 1 mo: 8/10 pts (80%) 6 mo: 6/10 pts (60%) 1 y: 3/10 pts (30%) |
8.58 ± 0.47% pre- vs. 6.65 ± 0.17% at 1 y post-transplantation (P < .002); improved in all pts | 30.5 ± 2.8 U/d pre-transplantation vs. 7.8 ± 3.3 U/d at 1 y post-transplantation (P < .001) | Basal levels maintained at 1.19 ± 0.22 ng/mL at 1 y (P < .001 vs. pre-transplantation) 0.5 ng/mL in 80% of patients at 1 y |
No. episodes per month: 18 ± 4 pre-transplantation, 2 (1 pt) at 6 mo, 4 (1 pt) and 20 (1 pt) at 1 y |
| Turgeon et al, 2010 (68) |
Insulin independence: 2/8 pts (25%) after one infusion 6/8 pts (75%) after completion |
Decreased 0.2 to 1.6% from baseline in 10 pts (< 6.5% in 8 pts) | Fasting: Edmonton Protocol 0.89 ± 0.34 mg/mL (0.7–1.5 mg/mL) vs. efalizumab 1.43 ± 0.46 mg/mL (0.8–1.9 mg/mL) Stimulated: Edmonton Protocol 1.96 ± 1.44 mg/mL (0.8–5.0 mg/mL) vs. efalizumab 1.22 ± 1.27 mg/mL (1.1–4.0 mg/mL) |
||
| Gangemi et al, 2008 (69) |
Insulin independence: Any time during the study: all pts (100%) At 15 mo: 4 in Edmonton Protocol, 4 in Edmonton Protocol + etanercept |
7.2 ± 1.1% pre- vs. 5.9 ± 0.4% at 15 mo post-transplantation (P = .001) Edmonton Protocol: 6.5 ± 0.6% pre-transplantation vs. 5.6 ± 0.5% at 15 mo Edmonton Protocol + etanercept: 7.8 ± 1.1% pre-transplantation vs. 5.8 ± 0.3% at 15 mo |
0 severe episodes during 12 mo follow-up Mild hypoglycemia in 2 pts |
||
| Keymeulen et al, 2006 (71) |
Insulin independence: 1 y: 10/24 pts (42%) |
< 6% in 10 insulin-independent pts at 1 y (P < 0.01) | Significantly lower at 1 y in 8 insulin-dependent pts (P < .01) | ≥ 0.5 ng/mL in 18 pts at 1 y | No severe episodes in 18 pts with C-peptide ≥ 0.5 ng/mL |
| Vantyghem et al, 2009 (72) |
Insulin independence: Any time during the study: 14/14 pts (100%) 1 y: 10 pts (71%) 3.3 y (2.8–4.0 y): 8/14 pts (57%) |
Pts with optimal primary graft function (n = 9): pre-transplantation 8.3% (7.3–8.6%) vs. 5.8% (5.4–6.5%) at 2 y (P < .05 vs. suboptimal and vs. pre-transplantation) vs. 6.2% (5.6–6.7%) at 3.3 y (P < .05 vs. suboptimal and vs. pre-transplantation) | Pts with optimal primary graft function (nmol/L): pre-transplantation 0 (0–0) vs. 0.5 (0.4–0.6) at 2 y (P < .05 vs. pre-transplantation) vs. 0.5 (0.43–0.6) at 3.3 y (P < .05 vs. pre-transplantation and vs. suboptimal) | ||
| Danielson et al, 2013 (70) |
Insulin independence Any time during study: 15/15 patients (100%) At end of follow-up: 11/15 patients (73%) |
7.2% pre- vs. 5.9% at 1 y (P < .001) | Insulin-dependent patients had large declines compared with pre-transplantation doses | No severe hypoglycemic events during follow-up | |
| O'Connell et al, 2013 (74) |
Insulin independence: 9/17 patients (53%) |
Mean 8.3 ± 2.0% pre- vs. 6.5 ± 1.3% post-transplantation at 1 y (P < .001) 14/17 patients (82%) < 7.0% |
Reduction of > 1% within 1 mo, sustained until 1 y | Detectable C-peptide in all patients | Absence of hypoglycemia in all patients at 1 y |
| Uremic patients | |||||
| Benhamou et al, 2001 (62) |
Insulin independence: 2/10 patients (20%) at 1 y Partial function: 5/10 patients (50%) at 1 y |
Pre- vs. post-transplantation at 1 y: 8.6 ± 1.6% vs. 6.0 ± 0.4% (5 pts with functioning graft) |
Decrease in insulin requirements (in 3 patients who were C-peptide positive) | > 0.5 ng/mL in all pts immediately post-transplantation At 1 y: 5 pts remained > 0.5 ng/mL |
|
| Borot et al, 2011 (63) |
Insulin independence: 10/15 patients (67%) No patients with primary graft non-function |
HbA1c < 7% Pre-transplantation: 7 patients (33%) 1 y: 12 patients (80%) 2 y: 11 patients (73%) |
Daily requirements reduced by 60% at 1 y and 2 y vs. pre-transplantation (P < .001) | No. mild to moderate hypoglycemic events: 9 (4–16) pre- vs. 0 (0–2) 1 y post-transplantation | |
| Bertuzzi et al, 2002 (59) |
Insulin independence: 6 mo: 50% 1 y: 5 pts (33%) 2 y: 2 pts (13%) |
Mean HbA1c pre- vs. post-transplantation: 8.4% vs. 6.8% (P < .01) 10 pts < 7.0% at 1 y |
Reduced more than 50% of pre-transplantation doses | > 0.17 nmol/L during 1 y | |
| Fiorina et al, 2003 (76) | NS changes during follow-up | Lower in successful IAK than unsuccessful IAK (P < .05) | Higher in successful IAK than unsuccessful IAK (P < .05) | ||
| Mixed non-uremic and uremic | |||||
| Leitao et al, 2008 (65) | Insulin-independent patients: 0 Proportion of patients with hypoglycemic unawareness: 87% pre- vs. 13% post-transplantation (P <. 001) Clarke hypoglycemic score: 5.29 ± 1.51 pre- vs. 1.35 ± 1.92 post-transplantation (P < .001) |
||||
| Vantyghem et al, 2012 (73) |
Insulin independence: 3 y: 10/23 patients (43%) Optimal graft function: in 5/23 patients (22%) at 3 y Partial function: 3 y: 19/23 patients (82%) |
8.3% (7.3–9.0%) pre- vs. 6.7% (5.9–7.7%) at 3 y post-transplantation (P < .01) | 0.63 IU/kg/d (0.40–0.75 IU/kg/d) pre- vs. 0 IU/kg/d (0– 0.28 IU/kg/d) at 3 y post-transplantation (P < .01) | Detectable in 19/23 patients (82%) at 3 y | Percentage time spent in hypoglycemia state (< 3 mmol/L): 5 (1–8) pre- vs. 0 (0–2) at 3 y post-transplantation (P < .05) |
| Fiorina et al, 2003 (58) |
Insulin independence: Any time during the study: 12/36 patients (33%) Mean duration: 21.5 ± 4.2 mo In successful islet transplantation group: 23 pts at 1 y, 21 pts at 2 y, 12 pts at 4 y |
7.8 ± 0.2% pre- vs. 6 mo post-transplantation 7.2 ± 0.2% (P < .01) NS differences between successful and unsuccessful groups during follow-up |
Reduction in successful islet transplantation group at 1, 2, and 4 y (P < .05) | Increase in successful islet transplantation group (P < .01) | |
Abbreviations: HbA1c, glycosylated hemoglobin; IAK, islet-after-kidney transplantation; NS, non-significant; pts, patients.
HYPO score is a composite hypoglycemic score that is based on the frequency, severity, and degree of unawareness of the hypoglycemia.
Source: Adapted from Institute of Health Economics, 2013. (41)
Uremic Patients
Four comparative observational studies were found on uremic patients with type 1 diabetes. Simultaneous islet-kidney transplantation was compared with simultaneous pancreas-kidney transplantation in one study by Gerber et al, (48) and Fiorina et al investigated in three study populations islet-after-kidney transplantation/simultaneous islet-kidney transplantation compared with simultaneous pancreas-kidney transplantation or intensive insulin therapy. (10, 44, 46) Insulin independence at follow-up was higher in the simultaneous pancreas-kidney transplantation group compared with islet transplantation (Table 5):
31% in simultaneous islet-kidney transplantation versus 96% in simultaneous pancreas-kidney transplantation was found by Gerber et al at 1 year (48)
0% for islet-after-kidney transplantation or simultaneous islet-kidney transplantation compared with 100% in simultaneous pancreas-kidney transplantation at 6 years in Fiorina et al's study (46)
However, despite the lower insulin independence rates for islet transplantation, in general the studies found that most patients still maintained partial graft function, which allowed for improved glycemic control compared with their status prior to islet transplantation. Both Gerber et al and Fiorina et al found a 50% reduction in insulin requirements in the simultaneous islet-kidney transplantation/islet-after-kidney transplantation groups and significantly improved HbA1c and C-peptide levels. (10, 44, 46, 48)
Four case series studies examined glycemic control in patients with type 1 diabetes with uremia. (44, 59, 62, 63) Insulin independence rates were noted at 20% (62), 33% (59), and 67% (63) at 1 year in three of the studies (Table 6). Despite the lower rates for insulin independence for these patients, studies that examined HbA1c and C-peptide levels found general significant improvement, along with decreased insulin requirements. (44, 59, 62, 63)
Both Uremic and Non-uremic Patients
Two observational comparative studies on islet transplantation were found that included both uremic and non-uremic patients with type 1 diabetes. (45, 72) Frank et al examined islet transplantation alone/islet-after-kidney transplantation (ITA/IAK) versus simultaneous pancreas-kidney transplantation/pancreas-after-kidney transplantation (SPK/PAK) using the Edmonton Protocol and found insulin independence was 42% for the ITA/IAK group versus 83% for the SPK/PAK group. (45) Despite the gap in insulin independence rates, patients who underwent ITA/IAK still showed significant improvement in HbA1c and C-peptide levels at least 1 year post–islet transplantation, with no severe hypoglycemic events observed during the post–ITA/IAK follow-up period. (45)
Vantyghem et al examined ITA/IAK versus intensive insulin therapy. (72) In their study, 77% of patients remained insulin independent at 1 year, and significant insulin requirement reductions and changes in HbA1c levels were noted both at 1- and 3-year follow-ups. Significant reductions in the number of hypoglycemic events were seen up to 2 years in the ITA/IAK group; however, the results were non-significant at 3 years compared with the intensive insulin therapy group. (72)
Secondary Complications of Diabetes
Outcomes for retinopathy, nephropathy, neuropathy, and cardiovascular disease and their risk factors were examined for islet transplantation for uremic and non-uremic patients. Table 7 summarizes the results for the observational comparative studies, while Table 8 shows data for non-uremic patients in the observational case series studies.
Table 7:
Islet Transplantation and Secondary Complications of Diabetes for Patients with Type 1 Diabetes Mellitus, from Observational Comparative Studies
| Secondary Complications from Diabetes | ||||||
|---|---|---|---|---|---|---|
| Author, Year | Treatment | Immunosuppression Protocol | Cardiovascular Disease and Risk Factors | Retinopathy | Nephropathy | Neuropathy |
| Non-uremic patients | ||||||
| Venturini et al, 2006 (47) | 10 ITA or 10 IIT | Daclizumab, sirolimus, tacrolimus (Edmonton Protocol) | NS changes in blood pressure, cholesterol, or triglycerides in either group pre-transplantation vs. at 1 y |
Increased blood flow velocity of central retinal artery in ITA only: Peak systolic: 6.09 ± 0.46 vs. 10.12 ± 1.20 cm/s (P = .01) End diastolic: 1.65 ± 0.07 vs. 2.99 ± 0.48 cm/s (P = .02) Increased blood flow velocity of central retinal vein in ITA only: Maximum: 3.12 ± 0.28 vs. 6.12 ± 1.00 cm/s (P = .01) Minimum: 1.86 ± 0.22 vs. 4.14 ± 0.56 cm/s (P = .003) |
||
| Warnock et al, 2008 (49) | 31 ITA or 11 IIT | ATG, sirolimus or MMF, tacrolimus |
Progression of retinopathy: 0/51 (0%) eyes ITA vs. 10/82 (12%) eyes IIT (P < .01) |
Decline in eGFR (mL/min/mo): 0.12 ± 0.7 ITA vs. 0.45 ± 0.7 IIT (P = .1) |
Nerve conduction velocity: 47.2 ± 4.5 to 47.7 ± 3.5 m/s ITA vs. 47.8 ± 5.3 to 47.1 ± 5.3 m/s IIT (NS) |
|
| Thompson et al, 2011 (51) | 32 ITA or 13 IIT | ATG, MMF, tacrolimus |
Systolic blood pressure: 122 ± 7 mm Hg ITA vs. 130 ± 10 mm Hg IIT (P < .001) Diastolic blood pressure: 70 ± 4 mm Hg vs. 73 ± 5 mm Hg (NS) |
Progression of retinopathy: 0/51 (0%) eyes ITA vs. 10/82 (12%) eyes IIT (P < .01) |
Decline in eGFR (mL/min/y) At 2 y: 1.42 ± 0.98 ITA vs. 4.79 ± 2.35 IIT (P < .0001) At 3 y: 1.40 ± 1.08 ITA vs. 3.55 ± 2.02 IIT (P < .0001) |
Fairly stable nerve conduction velocity in both groups (P = .07) |
| D'Addio et al, 2014 (53) | 12 ITA or 12 IIT | Daclizumab, tacrolimus, sirolimus (Edmonton Protocol) |
No signs of cardiovascular disease in either group Total cholesterol: 183.5 ± 12.5 mg/dL ITA vs. 172.5 ± 11.0 IIT (NS) HDL cholesterol: 58.9 ± 9.9 mg/dL ITA vs. 54.3 ± 10.5 mg/dL IIT (NS) Triglycerides: 64.1 ± 18.8 mg/dL ITA vs. 97.0 ± 37.7 mg/dL IIT (NS) |
Creatinine: 1.0 ± 0.2 mg/dL ITA vs. 0.8 ± 0.1 mg/dL IIT (NS) | NS changes in cerebral morphology and cerebral volume | |
| Uremic patients | ||||||
| Gerber et al, 2008 (48) | 13 SIK or 25 SPK | Daclizumab, sirolimus, tacrolimus (Edmonton Protocol) | Cardiovascular disease: NS changes in blood pressure, triglycerides, total cholesterol, or HDL and LDL cholesterol levels pre- and post-transplantation between groups | NS changes in eGFR between groups | ||
| Fiorina et al, 2003 (44) | 37 IAK/SIK or 162 SPK or 42 IIT | Induction ATG Maintenance cyclosporine, MMF, prednisone |
Cardiovascular death at 1, 4, and 7 y: 100%, 100%, 90% in successful IAK/SIK 84%, 75%, 45% in unsuccessful IAK/SIK (P = .02) 18% in IAK/SIK overall (similar to IIT group at 19%) 5% in successful IAK/SIK (similar to SPK group) NS changes in hypertension rate, blood pressure between successful vs. unsuccessful IAK/SIK group |
Urinary albumin excretion (successful IAK/SIK vs. unsuccessful IAK/SIK) 1 patient vs. 6 patients (P < .05) NS kidney rejection rate, creatinine, aldosterone, renin, and dosage of furosemide between successful vs. unsuccessful IAK/SIK group |
||
| Fiorina et al, 2005 (46) | 17 IAK or 25 IIT | Induction ATG Maintenance cyclosporine, MMF, prednisone |
Ejection fraction in IAK: 68.2 ± 3.5% at baseline to 74.9 ± 2.1% at 3 y post-transplantation (P < .05) NS ejection fraction changes in IIT Peaking filling rate in end diastolic volume/s in IAK: 3.87 ± 0.25 at baseline to 4.20 ± 0.37 at 3 y post-transplantation (P < .05) NS peak filling rate changes in IIT Time to peak filling rate remained stable in IAK but worsened in IIT (P < .05) NS changes in systolic or diastolic blood pressure, cholesterol, or triglycerides in either group pre- vs. post-transplantation, or between groups |
NS changes in creatinine in either group pre- or post-transplantation, or between groups | ||
| Fiorina et al, 2005 (10) | 24 IAK/SIK or 166 SPK or 44 IIT | Induction ATG Maintenance cyclosporine, MMF, prednisone |
Triglycerides pre- vs. 6 y post-transplantation: NS changes in successful IAK/SIK or IIT SPK: 157 ± 9 mg/dL vs. 105 ± 5 mg/dL (P <. 01) Total cholesterol pre- vs. 6 y post-transplantation: NS changes in successful IAK/SIK or IIT SPK: 182 ± 6 mg/dL vs. 210 ± 5 mg/dL (P < .01) Systolic blood pressure: NS changes in successful IAK/SIK and SPK group IIT: 146 ± 4 mm Hg vs. 144 ± 4 mm Hg (P < .05) Diastolic blood pressure pre- vs. 6 y post-transplantation NS changes in all groups |
Creatinine pre- vs. 6 y post-transplantation: NS changes for successful IAK/SIK or SPK For IIT: 1.58 ± 0.08 mg/dL vs. 2.78 ± 0.44 mg/dL (P < .01) Urinary albumin excretion pre- vs. post-transplantation: NS changes for successful IAK/SIK or SPK For IIT: 31.4 ± 9.0 mg/dL vs. 82.9 ± 33.6 mg/dL (P < .05) Renal arterial resistance index pre- vs. 6 y post-transplantation: Successful IAK/SIK: 0.72 ± 0.02 mg/dL vs. 0.69 ± 0.02 (P < .05) SPK: 0.74 ± 0.01 mg/dL vs. 0.68 ± 0.01 mg/dL (P < .01) NS changes for IIT |
||
| Mixed uremic and non-uremic patients | ||||||
| Frank et al, 2004 (45) | 9 ITA, 4 IAK or 25 SPK, 5 PAK | Creatinine clearance: average loss of 16.5 mL/min in ITA/IAK group | ||||
Abbreviations: ATG, antithymocyte globulin; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; IAK, islet-after-kidney transplantation; IIT, intensive insulin therapy; ITA, islet transplantation alone; LDL, low-density lipoprotein; MMF, mycophenolate mofetil; NS, non-significant; PAK, pancreas-after-kidney transplantation; SIK, simultaneous islet-kidney transplantation; SPK, simultaneous pancreas-kidney transplantation.
Source: Adapted from Institute of Health Economics, 2013. (41)
Table 8:
Islet Transplantation and Secondary Complications of Diabetes for Patients with Type 1 Diabetes Mellitus, from Observational Case Series Studies
| Author, Year | Secondary Complications of Diabetes |
|---|---|
| Non-uremic patients | |
| Ryan et al, 2005 (9) |
Retinopathy: Deterioration of retinopathy in 4/47 patients (9%) Neuropathy: No change in peripheral neuropathy |
| Lee et al, 2005 (66) |
Retinopathy: No progression when compared with pre-transplantation measures in all 8 patients, improvement in 1 patient No significant correlation between changes in HbA1c values and retinopathic changes Neuropathy: Improvement or stabilization of diabetic neuropathy in 50% of 8 patients |
| Maffi et al, 2007 (57) |
Nephropathy: 17/19 patients (89%) normal creatinine, creatinine clearance, and urinary protein excretion pre- and post-transplantation 2/19 patients (11%) progressed to end-stage renal disease |
| Danielson et al, 2013 (70) |
Cardiovascular: Decrease in common carotid intima-media thickness at 12 mo (P = .006) NS changes in internal carotid artery thickness at 12 or 50 mo NS changes in systolic or diastolic blood pressure, triglycerides, and total cholesterol Nephropathy: NS changes in urine albumin-to-creatinine ratio or eGFR |
| O'Connell et al, 2013 (74) |
Nephropathy: Mean eGFR 77 ± 21 mL/min pre- vs. 67 ± 18 mL/min at 12 mo post-transplantation (P = .051) Mean creatinine 75 ± 11 µmol/L pre- vs. 86 ± 16 µmol/L at 12 mo post-transplantation (P < .05) |
| Uremic patients | |
| Borot et al, 2011 (63) |
Nephropathy: NS changes in creatinine and creatinine clearance pre- vs. post-transplantation |
| Fiorina et al, 2003 (58) |
Cardiovascular: Lower systolic blood pressure in successful islet transplantation group at 4 y (P = .01) NS differences in diastolic blood pressure between groups |
| Fiorina et al, 2003 (76) |
Cardiovascular: Higher cardiovascular death rate in unsuccessful IAK (4/13 pts) vs. successful IAK (1/21 pts) (P = .04) Lower intima-media thickness in unsuccessful IAK vs. successful IAK (P = .03) NS changes in arterial blood pressure Nephropathy: NS changes in creatinine levels during follow-up |
Abbreviation: eGFR, estimated glomerular filtration rate; HbA1c, glycosylated hemoglobin; IAK, islet-after-kidney transplantation; NS, non-significant.
Source: Adapted from Institute of Health Economics, 2013. (41)
Non-uremic Patients
Four of the observational comparative studies examined secondary complications for non-uremic patients with type 1 diabetes. (47, 49, 51, 53) The most common cardiovascular disease outcomes were surrogate cardiovascular risk factors such as elevated blood pressure, cholesterol, and triglycerides. Non-significant changes were found in two of the studies, (47, 53) with another noting significant changes in systolic blood pressure, but not diastolic. (51)
In three studies, improvements in retinopathy were observed in the islet-transplantation-alone group compared with the group receiving intensive insulin therapy. (47, 49, 51) Increased blood flow velocity of the central retinal artery was found in the islet-transplantation-alone group in another study. (47) Nephropathy, as measured by estimated glomerular filtration rate, was examined in two studies, with conflicting results. (49, 51) The three studies that examined neuropathy found no significant changes in nerve conduction (49, 51) or in cerebral morphology or volume. (53)
Most case series did not report any long-term diabetic complications; only two studies reported data for retinopathy and neuropathy for non-uremic patients. (9, 66) Deterioration in eye disease was found in one study, (9) and another study observed no progression in retinopathy while one patient showed improvement. (66) The results for diabetic neuropathy for the case series studies were similarly conflicting, with one study noting no change in peripheral neuropathy (9) and another suggesting improvement or stabilization of diabetic neuropathy. (66) Renal changes were inconsistent for estimated glomerular filtration rate and creatinine. (57, 70, 74)
Uremic Patients
Non-significant changes in blood pressure, cholesterol, and triglycerides were found post–islet transplantation compared with baseline in one study. (46) Another study that followed up patients in the long term found that triglycerides were significantly lower at 2 and 4 years’ follow-up, but not at 6 years. (10) Conversely, significant changes in cholesterol were generally not found.
One study reported on urinary albumin excretion for kidney function, comparing the successful case of islet-after-kidney transplantation/simultaneous islet-kidney transplantation (specific procedure not reported) with the six unsuccessful cases of islet-after-kidney transplantation/simultaneous islet-kidney transplantation. This comparison showed significant improvement in the patient in whom islet-after-kidney transplantation/simultaneous islet-kidney transplantation was successful; however, based on the small number of patients compared, results should be interpreted with caution. (44)
Neuropathy was not reported in any of the observational comparative studies, and no observational case series studies were found that assessed neuropathy for uremic patients. Cardiovascular death was found to be significant between successful and unsuccessful islet transplantation groups in one case series study. (58) Significant changes in nephropathy were not found in the case series studies. (63, 76)
Adverse Events
Adverse events from the observational comparative studies can be found in Table 9. Table 10 summarizes the adverse events reported in the case series studies. Adverse events that were reported were mainly bleeding, portal vein thrombosis, and impaired renal or liver function.
Table 9:
Safety of Islet Transplantation in Patients with Type 1 Diabetes Mellitus, from Observational Comparative Studies
| Immunosuppression Regimen | Adverse Outcome | |||
|---|---|---|---|---|
| Author, Year | Intervention | Control | Intervention | Control |
| Non-uremic patients | ||||
| Warnock et al, 2008 (49) | 31 ITA | 42 IIT |
Death: 0 Skin cancer: 1/31 (3%) Fatigue: 2/31 (6%) CMV: 1/31 (3%) |
Death: 0 Skin cancer: NR Fatigue: NR CMV: NR |
| Maffi et al, 2011 (52) | 33 ITA | 33 PTA |
Hospitalization: 16/33 (48%)a Transfusion: 2/33 (6%)a Re-laparotomy: 0/33 (0%)a Thrombosis: 3/33 (9%) Bleeding: 12/33 (36%) CMV reactivation: 2/33 (6%)a Other infection: 2/33 (6%) Worsening kidney function: 5/33 (15%) Other medical complication: 1/33 (3%) |
Hospitalization: 19/33 (58%)a Transfusion: 14/33 (42%)a Re-laparotomy: 18/33 (55%)a Thrombosis: 13/33 (39%) Bleeding: 5/33 (15%) CMV reactivation: 21/33 (64%)a Other infection: 5/33 (15%) Worsening kidney function: 4/33 (12%) Other medical complication: 2/33 (6%) |
| Uremic patients | ||||
| Gerber et al, 2008 (48) | 13 SIK | 25 SPK |
Death: 1 (not related) Intraperitoneal bleeding: 2/13 (15%) (no surgery) Laparotomy: 0a Infection: 0 Complications with islets: 3/13 (23%) |
Death: 0 Intraperitoneal bleeding: 2/25 (8%) (surgery required) Laparotomy: 10/25 (40%)a Infection: 2/25 (8%) Complications with pancreas: 12/25 (48%) |
| Mixed uremic and non-uremic patients | ||||
| Frank et al, 2004 (45) | 9 ITA 4 IAK |
25 SPK 5 PAK |
Death: 0 Hepatic steatosis: 3/13 (23%) (3 ITA, 0 IAK) Post-transplant surgery: 1/13 (8%) (0 ITA, 1 IAK) Mouth ulcer: 10/13 (77%)a (9 ITA, 1 IAK) Peripheral edema: 7/13 (54%) CMV: 0 Transfusion: 1/13 (8%)a (0 ITA, 1 IAK) Abscess drainage: 0 Malignancy: 1/13 (8%) (in situ squamous cell) |
Death: 1 (unknown cause) Hepatic steatosis: NR Post-transplant surgery: 7/30 (23%) (5 SPK, 2 PAK) Mouth ulcer: 0a Peripheral edema: NR CMV: 3/30 (10%) (3 SPK, 0 PAK) Transfusion: 13/30 (43%)a (10 SPK, 3 PAK) Abscess drainage: 3/30 (10%) (2 SPK, 1 PAK) Malignancy: NR |
| Vantyghem et al, 2009 (50) | 7 ITA 6 IAK |
17 IIT |
No. adverse events per patient Major: 18/13 Minor: 50/13 |
No. adverse events per patient Major: 13/17 Minor: 8/17 |
Abbreviations: CMV, cytomegalovirus; IAK, islet-after-kidney transplantation; IIT, intensive insulin therapy; ITA, islet transplantation alone; NR, not reported; PAK, pancreas-after-kidney transplantation; PTA, pancreas transplantation alone; SIK, simultaneous islet-kidney transplantation; SPK, simultaneous pancreas-kidney transplantation.
Denotes statistical significance.
Source: Adapted from Institute of Health Economics, 2013. (41)
Table 10:
Safety of Islet Transplantation in Patients with Type 1 Diabetes Mellitus, from Observational Case Series Studies
| Type of Adverse Event | |||||
|---|---|---|---|---|---|
| Author, Year | N | Intervention | Death | Hepatic or Renal | Other |
| Non-uremic patients | |||||
| Shapiro et al, 2006 (55) | 36 | ITA | Death: 0 |
PVT: partial branch vein occlusion in 2/36 patients (6%) Complete thrombosis of portal vein: 0 |
23/38 (61%) serious adverse events related to study therapy Intraperitoneal bleeding: 7/77 infusions (9%) No lymphoproliferative disease, cancer, opportunistic infections |
| Ryan et al, 2005 (9) | 65 | ITA | Death: 1 patient (unrelated) |
PVT: segmental branch thrombosis in 5/65 patients (8%) Liver abnormalities: AST increased to > 2.5 times the ULN in 55% of procedures and > 5 times the ULN in 23% of procedures Hepatic steatosis: 8/36 patients (22%) |
Intraperitoneal bleeding: 15/65 patients (23%) CMV seroconversion: 2/43 patients (5%) Cancer: 1/65 patients (2%) (thyroid) Most common adverse events: mouth ulcer, diarrhea, acne, edema |
| Froud et al, 2006 (84) | 16 | ITA | Death: 0 |
PVT: 0 Liver abnormality: fatty liver 1/13 patients (8%) Proteinuria: 100% Macroalbuminuria: 10/16 patients (6%) |
Intraperitoneal bleeding: 2/34 procedures (6%) Most common adverse events: leukopenia, neutropenia, hyperlipidemia, mouth ulcer, peripheral edema |
| Badet et al, 2007 (61) | 10 | ITA | Death: 0 |
PVT: segmental branch in 1/10 patients (10%) Liver abnormality: transient liver transaminases in 1/10 patients (10%) |
Intraperitoneal bleeding: 1/10 patients (10%) |
| Keymeulen et al, 2006 (71) | 24 | ITA | Death: 0 |
PVT: 0 Liver abnormality: ALT increased in 8/24 patients (33%) |
Intraperitoneal bleeding: 0 CMV hepatitis: 1/24 patients (4%) Cerebellar ataxia: 1/24 patients (4%) Other common adverse events: fever, heartburn, leukopenia |
| Turgeon et al, 2010 (68) | 12 | ITA | Death: 0 | Liver abnormality: ALT and AST higher in Edmonton Protocol immunosuppression group | Common adverse events: mouth ulcer, diarrhea, leukopenia, anemia No cancers or opportunistic infections |
| Gangemi et al, 2008 (69) | 10 | ITA | Death: 0 | PVT: 0 | Bleeding: 2 (11% of infusions, 20% of patients) Abdominal hysterectomy: 1/10 patients (10%) Common adverse events: weight loss, anemia |
| Vantyghem et al, 2009 (72) | 14 | ITA | Death: 0 |
PVT: 0 Liver abnormality: liver enzyme elevated in 3/14 patients (21%) |
Bleeding: 0 Other common adverse events: diarrhea, leukopenia, anemia |
| Villinger et al, 2005 (85) | 67 | ITA | Death: 0 | PVT: 5 events/132 procedures (4%) | Bleeding: 18 events/132 procedures (14%) in 17/67 patients (25%) |
| Barshes et al, 2005 (86) | 11 | ITA |
PVT: 0 Liver abnormality: elevated ALT in 11/11 patients (100%) |
||
| Yakubovich et al, 2007 (87) | 23 | ITA | CMV infection: 3/23 patients (13%) | ||
| Del Olmo Garcia et al, 2011 (80) | 18 | ITA |
Ovarian cysts: 10/18 patients (56%) Oligomenorrhea or amenorrhea: 5/18 patients (28%) Surgery for gynecological abnormalities: 8/18 patients (44%) |
||
| Takita et al, 2012 (77) | 9 | ITA |
16 adverse events in all 9 patients 12 procedure related Adverse events within 10 days’ post-transplantation: 5/16 (31%) related to infusion procedure Adverse events within 50 days’ post-transplantation: 7/16 (44%) related to immunosuppression therapy |
||
| Senior et al, 2007 (88) | 41 | ITA | Significant changes in microalbuminuria and macroalbuminuria status changes pre- vs. post-transplantation (P < .001) | ||
| Leitao et al, 2009 (89) | 35 | ITA | Stable eGFR during follow-up Microalbuminuria: 6/30 patients (20%) |
||
| Alfadhli et al, 2009 (79) | 57 | ITA | Ovarian cysts: 33/57 patients (58%) | ||
| Gillard et al, 2009 (78) | 23 | ITA | Graves hyperthyroidism: 4/13 (31%) who discontinued immunosuppression | ||
| Danielson et al, 2013 (70) | 15 | ITA | Death: 1 (sepsis, unknown origin) | Cancer: 1/15 patients (7%) (local breast cancer) | |
| O'Connell et al, 2013 (74) | 17 | ITA | Death: 0 | PVT: 1/17 patients (6%) |
Bleeding: 3/17 patients (18%) (2 patients required transfusion) Lymphocytopenia: 7/17 patients (41%) Colitis: 1/17 patients (6%) Transient anemia: 8/17 patients (47%) (1 patient required transfusion) |
| Uremic patients | |||||
| Eckhard et al, 2002 (81) | 48 | 14 IAK, 34 SIK | CMV infection: 29/48 patients (60%) | ||
| Bertuzzi et al, 2002 (59) | 15 | IAK | Death: 0 | Bleeding: 2/15 patients (13%) (1 hemothorax, 1 hemoperitoneum) | |
| Benhamou et al, 2001 (62) | 10 | IAK | Death: 0 |
PVT: 0 Liver abnormality: transient and reversible liver enzyme increase in some patients |
Bleeding: peri-hepatic hematoma in 3/10 patients (30%) |
| Borot et al, 2011 (63) | 19 | IAK | Death: 0 | Renal dysfunction: 2/15 patients (13%) |
Bleeding: 2/15 patients (13%) Mouth ulceration: 1/15 patients (7%) |
| Mixed non-uremic and uremic | |||||
| Hafiz et al, 2005 (82) | 26 | 16 ITA, 4 IAK | Death: 0 |
PVT: 0 Liver abnormality: elevated ALT and AST in 26/26 patients (100%) |
Bleeding: 3/26 patients (12%) Other common adverse events: leukopenia, anemia, mouth ulceration, diarrhea |
| Venturini et al, 2010 (83) | 35 | 30 IAK, 5 ITA | Liver focal fatty changes: 10/30 patients (33%) IAK, 2/5 patients (40%) ITA | ||
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; CMV, cytomegalovirus; eGFR, estimated glomerular filtration rate; IAK, islet-after-kidney transplantation; ITA, islet transplantation alone; PVT, portal vein thrombosis; SIK, simultaneous islet-kidney transplantation; ULN, upper limit of normal range.
Source: Adapted from Institute of Health Economics, 2013. (41)
Non-uremic Patients
One observational comparative study that compared islet transplantation with intensive insulin therapy did not report any procedure-related adverse events. (49) One study that compared islet transplantation alone with pancreas transplantation alone noted procedure-related adverse events such as hospitalization, thrombosis, and bleeding as the most common adverse events. (52) Cytomegalovirus reactivation, blood transfusion, thrombosis, and re-laparotomy were observed in the same study in more patients in the group receiving pancreas transplantation alone than in the group receiving islet transplantation alone. (52)
From the case series, the most common procedure-related adverse events were acute intraperitoneal bleeding and (partial) portal vein thrombosis. Transient elevation of liver enzymes was common, along with hepatic steatosis. Because of adverse effects from the medications, patients in some of the studies switched immunosuppression regimens—from the original Edmonton Protocol that included sirolimus and tacrolimus to the alternative, mycophenolate mofetil.
The timing of adverse events was examined in one study. (77) Investigators found that 31% of adverse events that were related to the infusion procedure occurred within 10 days’ post-transplantation; similarly, 44% of adverse events occurred within 50 days’ post-transplantation and were related to the immunosuppression protocol. (77) Few studies have reported on more long-term and rare adverse events, such as cancer and other disorders. Immunosuppression withdrawal in one study led to Graves hyperthyroidism in 4 of 13 patients (31%). (78)
Two studies focused on gynecological adverse outcomes for islet transplantation. (79, 80) Ovarian cysts developed in 56%–58% of female patients. (79, 80) Disrupted menstrual cycles (oligomenorrhea and amenorrhea) were found in 28% of patients. Surgery was required for these gynecological abnormalities in 44% of female patients. (80) No deaths related to islet transplantation were noted in any of the observational studies.
Uremic Patients
One observational comparative study showed a significantly higher frequency of procedure-related adverse events following simultaneous pancreas-kidney transplantation compared with simultaneous islet-kidney transplantation; 40% of the patients who underwent simultaneous pancreas-kidney transplantation required surgery for their complications. (48) The three other comparative observational studies did not report any procedure- or immunosuppression-related adverse events. (10, 44, 46)
Of the observational case series studies, two did not report any immunosuppression-related adverse events, (59, 62) whereas one noted cytomegalovirus infection in 60% of patients. (81) Intraperitoneal bleeding was the most common procedure-related event (59, 62, 63), and transient elevated liver enzymes were found in one study. (62)
Both Uremic and Non-uremic Patients
Two observational comparative studies examined adverse events for mixed uremic and non-uremic patients. (45, 50) Intraperitoneal bleeding, hepatic steatosis, and peripheral edema were the most common adverse events found. No deaths occurred in the islet transplantation groups. (45, 50) The observational case series studies noted local liver fatty changes, bleeding, elevated liver enzymes, and other minor adverse events such as mouth ulcerations, anemia, leukopenia, neutropenia, and leg edema. (82, 83)
Health-Related Quality of Life
The 2013 Institute of Health Economics health technology assessment (41) included one systematic review by Speight et al (40) that analyzed patient-reported outcomes for both islet transplantation and pancreas transplantation and included studies of all types of procedures that addressed these outcomes. Table 11 summarizes the characteristics and results of the systematic review. Because of the small sample sizes and lack of sensitivity of some outcome measures to detect meaningful changes, the systematic review could not conclusively address the impact of islet or pancreas transplantation on quality of life. It was also noted by the authors that no transplantation-specific measures were used in any of the studies; nor was patient satisfaction ever assessed. The authors of the systematic review concluded that qualitative research was generally lacking regarding the impact of islet transplantation on health-related quality of life.
Table 11:
Systemic Review on Patient-Reported Outcomes of Islet and Pancreas Transplantation in Patients with Type 1 Diabetes Mellitus
| Objectives | Included Studies | Outcome Measures | Results |
|---|---|---|---|
|
12 case series: 9 ITA, 2 IAK, 2 PAK, 1 PTA | Generic questionnaires, e.g., SF-36 Diabetes-specific questionnaires, e.g., DQOL |
|
Abbreviations: SF-36, 36-Item Short Form Health Survey; DQOL; Diabetes Quality of Life Survey; IAK, islet-after-kidney transplantation; ITA, islet transplantation alone; PAK, pancreas-after-kidney transplantation; PTA, pancreas transplantation alone.
Source: Adapted from Institute of Health Economics, 2013. (41)
Uremic Patients
Only two of the studies in the systematic review included uremic patients with type 1 diabetes. (60, 90) One study noted that all Diabetes Quality of Life Survey subscales improved significantly at 12 months, with the Impact and Worry subscales persisting at 24 months. However, when examined at 36 months, all scores returned to baseline levels. (90) In the other study, no improvement in the results for the Diabetes Quality of Life Survey or 36-Item Short Form Health Survey (SF-36) was noted for the patients who underwent islet-after-kidney transplantation. (60)
Non-uremic Patients
In addition to the nine studies included in the systematic review, three additional studies were found that examined health-related quality of life. One study by Haggstrom et al surveyed 11 islet transplant recipients about their fear of hypoglycemia, and it used the 36-Item Short Form Health Survey and Swedish Hypoglycemic Fear Survey to investigate health-related quality of life. (75) Authors also examined patients’ social life situation in relation to their fear of hypoglycemia. While the results for health-related quality of life were lower than in the normal population, changes in fear of hypoglycemia suggested an improvement for the patients who had undergone islet transplantation. Patients felt they experienced improved control over their social life situations. It was noted that pre-transplantation, patients “struggled for control of social life situations”; post-transplantation, patients “regained power and control” of these situations. (75)
Another study by Radosevich et al compared 41 patients with type 1 diabetes who were screened for islet transplantation alone with 27 patients who had undergone that procedure. (54) Islet transplantation alone was found to be related to reductions in behaviours adopted to avoid hypoglycemia (P < .001) and attenuation in concerns about hypoglycemic episodes (P < .001). Health status among the patients who had undergone islet transplantation alone was also found to have improved, according to scores on the Euro Quality of Life scale (P = .002) and the Beck Depression Inventory scale (P = .003). Non-significant changes were found between groups for the 36-Item Short Form Health Survey and the Diabetes Distress Scale. The authors concluded that there are socio-emotional benefits related to islet transplantation alone that may be independent of islet graft function.
Although the study by D'Addio et al focused on homeostatic abnormalities and cerebral metabolism, the authors also reported on Profile of Mood states for islet transplantation alone versus intensive insulin therapy. (53) Significant improvements were found for the depression/dejection and confusion/bewilderment domains for islet transplantation alone; non-significant results were found for the other domains such as tension/anxiety, anger/hostility, vigour/activity, and fatigue/inertia.
Evidence From Registry Data
One registry report was found through hand-searching: the most recent (eighth) annual report from the Collaborative Islet Transplantation Registry. (29) Since 1999, this registry has been collecting comprehensive data on islet transplantation activity, recipient and donor characteristics, pancreas procurement, islet processing, infusion characteristics, immunosuppression medications, graft function, and adverse events. Tables 12 to 14 summarize clinical effectiveness and safety data from 1999 to 2014 from the Collaborative Islet Transplantation Registry's eighth annual report, published in December 2014. (29) In total, 864 islet transplantations were performed during that time, with 686 being islet transplantation alone (79%) and 178 being islet-after-kidney transplantation/simultaneous islet-kidney transplantation (21%). About 50% of patients received two infusions, and 30% and 20% of patients received single and triple infusions, respectively. Very rarely did patients receive four to six infusions.
Table 12:
Collaborative Islet Transplant Registry Data on Clinical Effectiveness
| Clinical Effectiveness | Patients (%) by Years | Patients (%) by IT Type | ||||
|---|---|---|---|---|---|---|
| 1999–2002 | 2003–2006 | 2007–2010 | 2011–2014 | ITA | IAK/SIK | |
| Insulin independence after last IT infusion | 1 y: 49 5 y: 20 |
1 y: 51 5 y: 28 |
1 y: 64 5 y: 5 |
1 y: 30 5 y: NA |
1 y: 49 5 y: 18 |
1 y: 43 5 y: 28 |
| Positive C-peptide (fasting C-peptide ≥ 0.3 ng/mL) after last IT infusion | 1 y: 72 5 y: 40 |
1 y: 83 5 y: 52 |
1 y: 85 5 y: 25 |
1 y: 83 5 y: NA |
1 y: 81 5 y: 44 |
1 y: 77 5 y: 49 |
| Severe hypoglycemia episode after last IT infusion | 1 y: 2 5 y: 11 |
1 y: 6 5 y: 15 |
1 y: 8 5 y: 25 |
1 y: 17 5 y: NA |
1 y: 6 5 y: 15 |
1 y: 5 5 y: 9 |
Abbreviations: IAK, islet-after-kidney transplantation; IT, islet transplantation; ITA, islet transplantation alone; NA, not available; SIK, simultaneous islet-kidney transplantation.
Source: Adapted from Collaborative Islet Transplant Registry, 2014. (29)
Table 14:
Collaborative Islet Transplant Registry Data on Serious Adverse Events in Year 1 After the First Transfusion
| Serious Adverse Event | Patients (%) by Year | Patients (%) by IT Type | ||||
|---|---|---|---|---|---|---|
| 1999–2002 | 2003–2006 | 2007–2010 | 2011–2014 | ITA | IAK/SIK | |
| Any serious AE | 38.3 | 41.0 | 31.7 | 15.6 | 32.1 | 38.2 |
| AEs related to infusion | 18.7 | 21.4 | 13.5 | 8.4 | 16.2 | 16.9 |
| AEs related to immunosuppression | 19.6 | 25.1 | 20.9 | 7.1 | 19.4 | 19.7 |
| AEs related to neither | 12.4 | 9.6 | 12.2 | 5.8 | 8.7 | 16.3 |
Abbreviation: AE, adverse event; IAK, islet-after-kidney transplantation; IT, islet transplantation; ITA, islet transplantation alone; SIK, simultaneous islet-kidney transplantation.
Source: Adapted from Collaborative Islet Transplant Registry, 2014. (29)
Table 13:
Collaborative Islet Transplant Registry Data on Serious Adverse Events in 30 Days After the First Infusion
| Serious Adverse Event | Patients (%) by Years | Patients (%) by IT Type | ||||
|---|---|---|---|---|---|---|
| 1999–2002 | 2003–2006 | 2007–2010 | 2011–2014 | ITA | IAK/SIK | |
| Any serious AE | 15.3 | 19.9 | 16.5 | 9.1 | 16.0 | 15.7 |
| AEs related to infusion | 11.0 | 12.9 | 7.8 | 4.5 | 9.6 | 9.6 |
| AEs related to immunosuppression | 2.9 | 9.6 | 11.3 | 4.5 | 7.9 | 6.2 |
| AEs related to neither | 2.4 | 1.8 | 2.2 | 1.9 | 1.6 | 3.9 |
Abbreviation: AE, adverse event; IAK, islet-after-kidney transplantation; IT, islet transplantation; ITA, islet transplantation alone; SIK, simultaneous islet-kidney transplantation.
Source: Adapted from Collaborative Islet Transplant Registry, 2014. (29)
Immunosuppression protocols over the past 5 years have also shifted from induction with interleukin-2 receptor antagonists only (for prophylaxis against acute rejection) to combinations that include T-cell depletion (elimination of T cells that play an important role in immune responses) and inhibition of tumour necrosis factor α (an inflammatory cytokine). (29)
Collaborative Islet Transplantation Registry data show that insulin independence (i.e., full graft function) is greater than 50% at 1 year post-transplantation for islet transplantation alone, with success rates increasing slightly from the original 1999 to 2002 period. However, about half of these patients do not maintain full graft function at 5 years post-transplantation. Partial or full graft function is maintained in about 75% of patients post-transplantation, again with a reduction of about half in terms of graft function when measured at the 5-year mark. (29) Owing to the low numbers of islet transplantation recipients analyzed, the values for the most recent time periods (2011–2014) only contain about two years of data (2011 and 2012) and are easily influenced by small changes in patient numbers within the group studies. Data for the clinical effectiveness of islet transplantation by time period (but not by islet transplantation type) at the 5-year mark for the most recent time period of 2011 to 2014 were not available owing to incomplete follow-up data.
There were a total of 592 serious adverse events that were reported from 1999 to 2014, with 29% categorized as life threatening and 52% requiring in-patient hospitalization. (29) Serious adverse events were attributed to being related to the infusion procedure or immunosuppression protocol or neither of the above, and included disorders of the following systems or conditions: blood and lymphatic, cardiac, endocrine, eye, gastrointestinal, hepatobiliary, immune system, infections, metabolism and nutrition, musculoskeletal and connective tissue, neoplasms, nervous, psychiatric, renal and urinary, respiratory, reproductive, vascular, and skin. Death was rare, occurring in about 1% of patients. Of the serious adverse events, 82% resolved with no residual effects. Rates of serious adverse events increased from 30 days to 1 year after the first infusion, regardless of year or type of islet transplantation, and were similar regardless of whether the serious adverse event was related to the infusion procedure or to the immunosuppression protocol used. A general trend of decreased serious adverse events is present, suggesting improvement in islet transplantation safety through the evolution of the surgical procedure and the immunosuppression protocols. Incomplete follow-up during the most recent time period of 2011 to 2014 influenced the data.
Limitations and Discussion
Patient Population
Some of the comparison studies were unmatched in patient baseline characteristics, causing selection bias that can affect the results between study groups. In comparing pancreas transplantation with islet transplantation, donor characteristics have been noted to be different; for example, certain donors for islet transplantation may be much older or have a higher body mass index, which would usually not be considered in pancreas transplantation. This difference resulted from the fact that pancreases used for islet transplantation can be those rejected for use in pancreas transplantation, indicating higher donor quality in the pancreas transplantation group.
Patient selection was also stringently applied in some studies, in some cases with less than 10% of patients initially referred being selected for inclusion. Patient selection also varied between studies, although the majority of studies applied the selection criteria developed by the initial Edmonton group. Owing to the patient population and the safety concerns, studies typically contained a small number of patients, limiting the confidence in the studies’ results. The selection criteria for islet-after-kidney transplantation and for simultaneous islet-kidney transplantation were more variable since indications for these islet transplantation procedures are less established.
Study Design
No randomized controlled studies existed for islet transplantation since the intervention and clinical outcomes make this approach not ethically justifiable; the majority of the evidence was derived from single-centre case series studies. Observational comparative studies existed, and these typically involved a type of islet transplantation procedure compared with either waiting list or regular medical therapy (e.g., intensive insulin therapy). These studies employed either a single crossover design or a comparison with historically matched patients who had received other types of islet transplantation or pancreas transplantation in the same centre. In some studies, it was unclear from the reported methods whether the study design was prospective or retrospective.
Case series studies reported pre-transplantation and post-transplantation outcomes for islet transplantation, and the implicit comparison within these studies was to pre-transplantation medical management. However, without a comparator group that had been matched and controlled for, there were potentially confounding factors and biases that might have influenced the study results. Case reports also offered another form of low-quality non-controlled observational evidence but were excluded in this review. Thus, because of the inherent design of the islet transplantation studies found, the body of evidence is limited, with uncertainty in the estimates of effect owing to the generally low to very low evidence.
Follow-up for islet transplantation studies was also limited: only a few studies examined outcomes past 5 years, with most studies having follow-up durations of 1 to 2 years. The 2013 Institute of Health Economics health technology assessment excluded studies that had a follow-up period of less than 1 year. Longer-duration studies with adequate follow-up are especially important since islet transplantation is still considered experimental in many countries, compared with pancreas transplantation, which is well-documented as the standard β-cell replacement therapy option for select eligible patients with type 1 diabetes. The long-term effects of islet transplantation, such as immunosuppressive agents and islet safety and function, have not been well determined.
Study Outcomes
Success for islet transplantation may be measured by outcomes other than insulin independence, although this is the most common indicator of graft success. One of the indications for islet transplantation is patients with brittle type 1 diabetes mellitus, defined as experiencing glycemic unawareness and labile glucose levels that significantly impact day-to-day functions, despite optimal medical management. Post-transplantation, patients may become insulin independent for months, but gradually graft function deteriorates causing patients to once again require insulin. This was apparent in the results of the observational studies for islet transplantation, as well as Collaborative Islet Transplantation Registry data.
Although graft function may not lead to insulin independence in all patients, the outcomes of frequency of hypoglycemic events and insulin dose requirements are also particularly important to patients. (91) Partial graft function can lead to insulin dose reductions for patients and relieve them of the hypoglycemic unawareness symptoms that they previously experienced. This represents improved glycemic control, even if patients are still insulin dependent. Glycemic control can also be measured in different ways, such as HbA1c and C-peptide values.
From the studies, there is general consensus within the limited evidence that islet transplantation can improve health-related quality of life for patients with type 1 diabetes. These studies have examined both generic and diabetes-specific health-related quality of life measures, although the results have not been consistent in all domains of the measurement tools or between different measurement tools. It has been observed that the patient outcomes that are measured in these health-related quality of life tools may not be able to capture the full extent of the impact of islet transplantation. None of the studies on health-related quality of life focused on any transplantation-specific measures for islet transplantation, which might help elucidate the transplantation-specific aspect of islet transplantation on health-related quality of life. These measures have not yet been administered in the islet transplantation or pancreas transplantation field. Patient satisfaction has not been evaluated either, and overall data on health-related quality of life for islet transplantation are lacking, given the few studies that have addressed this outcome.
For islet transplantation safety, the adverse events that have been documented (a) varied in severity and (b) can be attributed to either the islet transplantation procedure or the lifelong immunosuppression regimens that are required post-transplantation. It is important to consider that most adverse events are not serious and do not result in any downstream sequelae. For example, elevations in liver enzymes, while considered an adverse event, are transient in nature and do not have any clinical impact on patients in the long term. Procedure-related adverse events have also improved with time, and complications are now more manageable. Death related to the islet transplantation procedure is very rare, and the complications and risks are much higher in pancreas transplantation because of that procedure's more invasive nature. Immunosuppression-related adverse events are not uncommon in islet transplantation; however, it has been noted that the rates do not differ from those for other solid organ transplantations. Differences in patients’ pharmacokinetic tolerability can also lead to immunosuppression regimen changes between patients. The withdrawal of immunosuppression regimens or alterations in immunosuppressive agents is a particular transition point where immunosuppression-related adverse events may occur.
Collaborative Islet Transplantation Registry data (28) may serve as an important international resource to monitor the general trends and results of islet transplantation. Data monitoring processes allow for the comprehensive collection of patient and procedure characteristics and procedure outcomes in a standardized manner that can be uniformly analyzed. However, currently not all active clinical islet transplantation centres participate and contribute data to the registry. To address the uncertainty that results from the gaps and limitations in the evidence, more comparative studies that include larger patient populations and longer follow-up durations are required.
CONCLUSIONS
Islet transplantation offers an alternative for patients with type 1 diabetes who have brittle diabetes with difficult-to-control blood glucose levels or hypoglycemic unawareness despite optimal insulin therapy. Treatment depends on patient eligibility and is influenced by donor islet availability, technical limitations of the procedure, and immunosuppression protocols.
In non-uremic patients, compared with (a) status prior to islet transplantation or (b) intensive insulin therapy, islet transplantation alone generally:
Improves glycemic control (GRADE: low to high)
Improves secondary complications for diabetes (GRADE: very low to low)
Increases procedure-related and immunosuppression-related adverse events (GRADE: low)
Improves health-related quality of life (GRADE: very low)
In uremic patients, compared with (a) status prior to islet transplantation or (b) intensive insulin therapy, simultaneous islet-kidney transplantation and islet-after-kidney transplantation generally:
Improve glycemic control (GRADE: low to high)
Improve secondary complications for diabetes (GRADE: low)
Increase procedure-related and immunosuppression-related adverse events (GRADE: low)
Improve health-related quality of life (GRADE: very low)
Additional long-term comparative studies are required for a better understanding of the continuing effects of transplanted islets and the immunosuppression protocols used.
EXISTING GUIDELINES FOR TECHNOLOGY
The CADTH rapid response (43) also reported on relevant clinical practice guidelines published since 2011 for the use of islet transplantation in patients with unstable type 1 diabetes. Investigators found two relevant clinical practice guidelines: one from the Canadian Diabetes Association (27) and another from the Spanish National Health System. (92) Hand-searching for additional guidelines for islet transplantation for patients with type 1 diabetes was undertaken, and guidelines were found from the American Diabetes Association (93) and the National Institute for Health and Clinical Excellence (now the National Institute for Health and Care Excellence, or NICE). (94) While many diabetes mellitus guidelines exist, the vast majority did not contain any specific recommendations on islet transplantation for type 1 diabetes.
The American Diabetes Association guideline was published as a position statement that contained graded levels of recommendations and was not captured by the CADTH report. Similarly, the 2008 NICE guideline was not included as it fell outside of CADTH's search dates. The NICE guideline was the oldest included guideline, having been published more than 6 years earlier, and its evidence base should be met with caution given the changes within the field of islet transplantation. As stated within the NICE guideline: “immunosuppressive regimens and technology for harvesting islet cells continue to evolve.”
Table 15 lists the statements from the guidance documents that were found.
Table 15:
Guidance on Islet Transplantation for Patients with Type 1 Diabetes Mellitus
| Author | Country | Statements |
|---|---|---|
| Canadian Diabetes Association, 2013 (Paty et al (27)) | Canada | Individuals with type 1 diabetes with preserved renal function, or who have undergone successful kidney transplantation but have persistent metabolic instability characterized by severe glycemic lability and/or severe hypoglycemia despite best efforts to optimize glycemic control, may be considered for pancreas or islet allotransplantation (Grade D recommendation: [expert] consensus) |
| American Diabetes Association, 2014 (Chiang et al (93)) | United States | Consider referral to research centers for protocolized islet cell transplantation in patients with type 1 diabetes and debilitating complications of diabetes who are interested in research possibilities and fit the criteria for the research protocol (Grade E recommendation: expert consensus or clinical experience) |
| Spanish National Health System, 2012 (Working Group (92)) | Spain | Nowadays, islet transplantation is only recommended in the context of controlled trials (Grade C recommendation: a body of scientific evidence consisting of studies rated as 2+ [well-conducted case-control or cohort studies with low risk of bias and a moderate probability of establishing a causal relationship], directly applicable to the target population of the guide and demonstrating overall consistency of results; or extrapolated evidence from studies rated as 2++ [high-quality systematic reviews of case-control or cohort studies; cohort or case-control studies with very low risk of bias and with high probability to establish a causal relationship]) |
| National Institute for Health and Clinical Excellencea, 2008 (94) | United Kingdom |
|
Now the National Institute for Health and Care Excellence, or NICE.
Glossary
GLOSSARY
- Beta (β) cells
β Cells are one of the four major types of cells present in pancreatic islets. They are the most abundant cell type within pancreatic islets, and their primary function is to produce, store, and secrete the hormone insulin.
- Brittle (or labile) diabetes
Brittle diabetes is a severe form of diabetes that is particularly difficult to control because of large fluctuations in glucose levels. These changes are frequent, and typically rapid and unpredictable, affecting patients’ quality of life. It may lead to hospitalization and cause additional complications over time.
- C-peptide
C-peptide is a short protein (connecting peptide) that is the by-product of insulin production. The amount of C-peptide in the blood indicates how much insulin is being produced. C-peptide does not affect the blood glucose levels and may be used to determine the cause of low blood glucose.
- Glucagon
Glucagon is a peptide hormone that is produced by the alpha (α) cells of the pancreas and raises the concentration of blood glucose. It opposes the effects of insulin and, together with insulin, helps control blood glucose levels.
- HbA1c
Glycosylated hemoglobin (or HbA1c) is formed by hemoglobin's exposure to blood glucose. HbA1c levels are not affected by daily fluctuations in blood glucose levels and indicate how well-controlled blood glucose levels have been over a long period of time, such as the previous 2 to 3 months.
- Insulin
Insulin is a peptide hormone that is produced by the β cells of the pancreas and allows cells to use glucose for energy. Insulin helps regulate the metabolism of glucose and other nutrients. Injectable manufactured insulin is used by patients with type 1 diabetes to replace the insufficient levels of natural insulin because of β cell degeneration.
- Pancreatic islets
The islets of Langerhans, or pancreatic islets, are tiny clusters of 3,000–4,000 cells within the pancreas. Islets contain four major types of cells, including the hormone-producing α and β cells. Islets account for about 1–2% of the mass of the pancreas.
- Uremia
Uremia occurs when there is an accumulation in the blood of excessive amounts of urea and other waste products that are normally excreted through the kidney into urine. Toxicity occurs when high levels are reached. Uremia is a serious complication of chronic kidney disease or acute rental failure.
APPENDICES
Appendix 1: Inclusion and Exclusion Criteria of the Original Edmonton Protocol
Table A1:
Indications and Contraindications for Islet Transplantation in the Original Edmonton Protocol
| Indications | Contraindications |
|---|---|
| Age between 18–65 y | Children (< 18 y), elderly (> 65 y) |
| Type 1 diabetes mellitus > 5 y | Obesity and insulin resistance: patients with high body weight (> 90 kg), obesity (BMI > 28 kg/m2 or BMI > 30 kg/m2), or with high insulin requirements (> 1 U/kg/d) |
| Undetected stimulated C-peptide (< 0.48 ng/mL) | Blood HbA1c level > 12% |
| Severe hypoglycemia | Severe kidney dysfunction (creatinine > 200 µmol/L or other parameters) |
| Hypoglycemia unawareness | Infection, neoplasia |
| Glycemic lability (brittle diabetes, high variability in glucose levels despite exogenous insulin therapy) | Psychiatric disease, cognitive impairment, non-compliance |
| Smoking, alcohol use, drug use | |
| Taking systemic steroids | |
| Young women who wish to become pregnant |
Abbreviations: BMI, body mass index; HbA1c, glycosylated hemoglobin.
Source: Data from Shapiro et al, 2000. (7)
Appendix 2: Literature Search Strategies
Database: EBM Reviews—Cochrane Central Register of Controlled Trials <October 2014>, EBM Reviews—Cochrane Database of Systematic Reviews <2005 to October 2014>, EBM Reviews—Database of Abstracts of Reviews of Effects <4th Quarter 2014>, EBM Reviews - Health Technology Assessment <4th Quarter 2014>, All Ovid MEDLINE(R) <1946 to November 27, 2014>
Search Strategy:
____________________
| 1 | Diabetes Mellitus/ or exp Diabetes Mellitus, Type 1/ (160932) |
| 2 | (T1DM or T1D or IDDM or (diabet* adj3 (juvenile* or brittle or unstable or labile or insulin depend* or sudden onset or auto?immune or type 1 or type I))).ti,ab. (70487) |
| 3 | or/1–2 (184236) |
| 4 | Islets of Langerhans Transplantation/ (7893) |
| 5 | ((transplant* or allo?transplant*) adj3 (islet* or island*)).ti,ab. (6069) |
| 6 | or/4–5 (9395) |
| 7 | 3 and 6 (3810) |
| 8 | exp animals/ not humans.sh. (4101083) |
| 9 | 7 not 8 (2766) |
| 10 | (Comment or Editorial or Letter or Congresses).pt. (1478717) |
| 11 | 9 not 10 (2584) |
| 12 | limit 11 to (english language and yr=“2003 -Current”) [Limit not valid in CDSR, DARE; records were retained] (1495) |
| 13 | remove duplicates from 12 (1356) |
Appendix 3: Evidence Quality Assessment
Table A2:
AMSTAR Scores of Identified Health Technology Assessments and Systematic Reviews
| Author, Year | AMSTAR Scorea | (1) Provided Study Design | (2) Duplicate Study Selection | (3) Broad Literature Search | (4) Considered Status of Publication | (5) Listed Excluded Studies | (6) Provided Characteristics of Studies | (7) Assessed Scientific Quality | (8) Considered Quality in Report | (9) Methods to Combine Appropriate | (10) Assessed Publication Bias | (11) Stated Conflict of Interest |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AHRQ, 2004 (Piper et al (37)) | 7 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| CADTH, 2014b (43) | 6 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| IHE, 2013 (41) | 10 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Speight et al, 2010 (40) | 6 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| MUHC, 2014 (Xie et al (42)) | 4 | ✓ | ✓ | ✓ | ✓ |
Abbreviations: AHRQ, Agency for Healthcare Research and Quality; AMSTAR, Assessment of Multiple Systematic Reviews; CADTH, Canadian Agency for Drugs and Technologies in Health; IHE, Institute of Health Economics; MUHC, McGill University Health Centre.
Maximum possible score is 11. Details of AMSTAR score are described in Shea et al. (33)
Rapid response.
Table A3:
GRADE Evidence Profile for Islet Transplantation
| No. of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Glycemic control (non-uremic patients) | |||||||
|
Graft loss/insulin independence 15 (observational): 3 comparative 12 noncomparative |
Serious limitations (−1)a | No serious limitations | No serious limitations | Serious limitations (−1)b | Undetected | Large magnitude of effect (+2) | ⊕⊕⊕⊕ Highc |
|
HbA1c 15 (observational): 4 comparative 11 noncomparative |
Serious limitations (−1)a | No serious limitations | No serious limitations | Serious limitations (−1)b | Undetected | None | ⊕⊕ Low |
|
Insulin requirements 11 (observational): 3 comparative 8 noncomparative |
Serious limitations (−1)a | No serious limitations | No serious limitations | Serious limitations (−1)b | Undetected | None | ⊕⊕ Low |
|
C-peptide 11 (observational): 2 comparative 9 noncomparative |
Serious limitations (−1)a | No serious limitations | No serious limitations | Serious limitations (−1)b | Undetected | None | ⊕⊕ Low |
|
Hypoglycemia 9 (observational): 9 noncomparative |
Serious limitations (−1)a | No serious limitations | No serious limitations | Serious limitations (−1)b | Undetected | None | ⊕⊕ Low |
| Glycemic control (uremic patients) | |||||||
|
Graft loss/insulin independence 7 (observational): 4 comparative 3 noncomparative |
Serious limitations (−1)a | No serious limitations | No serious limitations | Serious limitations (−1)b | Undetected | Large magnitude of effect (+2) | ⊕⊕⊕⊕ Highc |
|
HbA1c 8 (observational): 4 comparative 4 noncomparative |
Serious limitations (−1)a | Serious limitations (−1)d | No serious limitations | Serious limitations (−1)b | Undetected | None | ⊕ Very Low |
|
Insulin requirements 8 (observational): 4 comparative 4 noncomparative |
Serious limitations (−1)a | No serious limitations | No serious limitations | Serious limitations (−1)b | Undetected | None | ⊕⊕ Low |
|
C-peptide 7 (observational): 4 comparative 3 noncomparative |
Serious limitations (−1)a | No serious limitations | No serious limitations | Serious limitations (−1)b | Undetected | None | ⊕⊕ Low |
|
Hypoglycemia 2 (observational): 1 comparative 1 noncomparative |
Serious limitations (−1)a | No serious limitations | No serious limitations | Serious limitations (−1)b | Undetected | None | ⊕⊕ Low |
| Secondary complications of diabetes (non-uremic patients) | |||||||
|
Cardiovascular disease 4 (observational): 3 comparative 1 noncomparative |
Serious limitations (−1)a | Serious limitations (−1)d | Serious limitations (−1)e | Serious limitations (−1)b | Undetected | None | ⊕ Very Low |
|
Retinopathy 5 (observational): 3 comparative 2 noncomparative |
Serious limitations (−1)a | No serious limitations | No serious limitations | Serious limitations (−1)b | Undetected | None | ⊕⊕ Low |
|
Nephropathy 6 (observational): 3 comparative 3 noncomparative |
Serious limitations (−1)a | Serious limitations (−1)d | No serious limitations | Serious limitations (−1)b | Undetected | None | ⊕ Very Low |
|
Neuropathy 5 (observational): 3 comparative 2 noncomparative |
Serious limitations (−1)a | Serious limitations (−1)d | No serious limitations | Serious limitations (−1)b | Undetected | None | ⊕ Very Low |
| Secondary complications of diabetes (uremic patients) | |||||||
|
Cardiovascular risk factors 6 (observational): 4 comparative 2 noncomparative |
Serious limitations (−1)a | No serious limitations | Serious limitations (−1)e | Serious limitations (−1)b | Undetected | None | ⊕⊕ Low |
|
Nephropathy 6 (observational): 4 comparative 2 noncomparative |
Serious limitations (−1)a | No serious limitations | No serious limitations | Serious limitations (−1)b | Undetected | None | ⊕⊕ Low |
| Health-related quality of life (non-uremic patients) | |||||||
| 12 (observational): 2 comparative 10 noncomparative |
Serious limitations (−1)a | Serious limitations (−1)a | Serious limitations (−1)f | Serious limitations (−1)b | Undetected | None | ⊕ Very Low |
| Health-related quality of life (uremic patients) | |||||||
| 2 (observational): 2 noncomparative |
Serious limitations (−1)a | Serious limitations (−1)a | Serious limitations (−1)f | Serious limitations (−1)b | Undetected | None | ⊕ Very Low |
| Adverse events (non-uremic patients) | |||||||
| 21 (observational): 2 comparative 19 noncomparative |
Serious limitations (−1)a | Serious limitations (−1)a | No serious limitations | Serious limitations (−1)b | Undetected | None | ⊕⊕ Low |
| Adverse events (uremic patients) | |||||||
| 5 (observational): 1 comparative 4 noncomparative |
Serious limitations (−1)a | Serious limitations (−1)a | No serious limitations | Serious limitations (−1)b | Undetected | None | ⊕⊕ Low |
Abbreviations: GRADE, Grading of Recommendations Assessment, Development, and Evaluation; HbA1c, glycosylated hemoglobin.
Most studies were of low quality owing to their study design. Noncomparative studies introduce bias and possible confounding. Follow-up period was usually less than 3 years, with few studies assessing outcomes of interest in the long term. Observational studies start at low quality according to GRADE.
Most studies had small sample sizes (included < 50 patients total).
Evidence upgraded because of large magnitude of effect when compared with insulin. Insulin independence is not possible for patients who are on intensive insulin therapy.
Inconsistencies in results.
Studies included surrogate outcomes for cardiovascular disease.
Studies included both generic and disease-specific scales. Scales are not sensitive to transplantation-specific outcomes.
Table A4:
Risk of Bias Among Observational Comparative Studies on Islet Transplantation for Type 1 Diabetes Mellitus
| Author, Year | Appropriate Eligibility Criteria | Appropriate Measurement of Exposure | Appropriate Measurement of Outcome | Adequate Control for Confounding | Complete Follow-Up |
|---|---|---|---|---|---|
| Maffi et al, 2011 (52) | Limitationsa | No limitations | Limitationsb | Limitationse | No limitations |
| Thompson et al, 2011 (51) | No limitations | No limitations | Limitationsb,c | Limitationse | No limitations |
| D'Addio et al, 2014 (53) | No limitations | No limitations | Limitationsb | Limitationse | Limitationsf |
| Radosevich et al, 2013 (54) | No limitations | No limitations | Limitationsb,d | No limitations | No limitations |
Not clear if study was retrospective or prospective. Patient characteristics were not clearly described.
No mention of blinding assessment of outcomes.
Retinopathy scale used was not as sensitive as gold standard.
Self-reported outcomes in questionnaire.
Unclear if patients were consecutive.
Adverse events not clearly reported.
Table A5:
Risk of Bias Among Other Observational Studies on Islet Transplantation for Type 1 Diabetes Mellitus
| Author, Year | Appropriate Eligibility Criteria | Appropriate Measurement of Exposure | Appropriate Measurement of Outcome | Adequate Control for Confounding | Complete Follow-Up |
|---|---|---|---|---|---|
| Borot et al, 2011 (63) | No limitations | No limitations | No limitations | Limitationsc,d | No limitations |
| Del Olmo Garcia et al, 2011 (80) | Limitationsa | No limitations | No limitations | Limitationsc,d | No limitations |
| Takita et al, 2012 (77) | Limitationsa | No limitations | No limitations | Limitationsc,d | No limitations |
| Haggstrom et al, 2011 (75) | Limitationsa | No limitations | Limitationsb | Limitationse | No limitations |
| Danielson et al, 2013 (70) | No limitations | No limitations | No limitations | Limitationsc | Limitationsf |
| Vantyghem et al, 2012 (73) | No limitations | No limitations | No limitations | Limitationsc | No limitations |
| O'Connell et al, 2013 (74) | No limitations | No limitations | No limitations | Limitationsc,d | No limitations |
Patient characteristics were not clearly described.
Instruments used were the generic 36-Item Short Form Health Survey and the Swedish version of the Hypoglycemic Fear Survey.
Studies were case series designs and lacked internal study controls.
Unclear if patients were consecutive.
Study was a cross-sectional survey comparing pre- and post-islet transplantation.
Significant number of patients lost to follow-up.
REFERENCES
- (1).Canadian Diabetes Association, Diabète Québec. Diabetes: Canada at the tipping point [Internet]. Toronto (ON): The Association; 2015. [cited 2015 Feb 6]. Available from: http://www.diabetes.ca/CDA/media/documents/publications-and-newsletters/advocacy-reports/canada-at-the-tipping-point-english.pdf [Google Scholar]
- (2).Types of diabetes [Internet]. Toronto (ON): Canadian Diabetes Association; 2015. [cited 2015 Feb 4]. Available from: http://www.diabetes.ca/about-diabetes/types-of-diabetes [Google Scholar]
- (3).Brittle diabetes (labile diabetes) [Internet]. United Kingdom: Diabetes.co.uk; 2015. [cited 2015 Feb 5]. Available from: http://www.diabetes.co.uk/brittle-diabetes.html [Google Scholar]
- (4).Ballinger WF, Lacy PE. Transplantation of intact pancreatic islets in rats. Surgery. 1972; 72(2): 175–86. [PubMed] [Google Scholar]
- (5).Scharp DW, Lacy PE, Santiago JV, McCullough CS, Weide LG, Falqui L, et al. Insulin independence after islet transplantation into type I diabetic patient. Diabetes. 1990; 39(4): 515–8. [DOI] [PubMed] [Google Scholar]
- (6).Brendel MHB, Schulz A, Bretzel R. International islet transplant registry report. Giessen, Germany: University of Giessen; 2001. [Google Scholar]
- (7).Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000; 343(4): 230–8. [DOI] [PubMed] [Google Scholar]
- (8).White SA, James RF, Swift SM, Kimber RM, Nicholson ML. Human islet cell transplantation—future prospects. Diabet Med. 2001; 18(2): 78–103. [DOI] [PubMed] [Google Scholar]
- (9).Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005; 54(7): 2060–9. [DOI] [PubMed] [Google Scholar]
- (10).Fiorina P, Venturini M, Folli F, Losio C, Maffi P, Placidi C, et al. Natural history of kidney graft survival, hypertrophy, and vascular function in end-stage renal disease type 1 diabetic kidney-transplanted patients: beneficial impact of pancreas and successful islet cotransplantation. Diabetes Care. 2005; 28(6): 1303–10. [DOI] [PubMed] [Google Scholar]
- (11).Luan FL, Samaniego M. Transplantation in diabetic kidney failure patients: modalities, outcomes, and clinical management. Semin Dial. 2010; 23(2): 198–205. [DOI] [PubMed] [Google Scholar]
- (12).Deng S, Markmann JF, Rickels M, Yeh H, Kim JI, Lian MM, et al. Islet alone versus islet after kidney transplantation: metabolic outcomes and islet graft survival. Transplantation. 2009; 88(6): 820–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Hilling DE, Bouwman E, Terpstra OT, Marang-van de Mheen PJ. Effects of donor-, pancreas-, and isolation-related variables on human islet isolation outcome: a systematic review. Cell Transplant. 2014; 23(8): 921–8. [DOI] [PubMed] [Google Scholar]
- (14).Ricordi C, Alejandro R, Zeng Y, Tzakis A, Casavilla A, Jaffe R, et al. Human islet isolation and purification from pediatric-age donors. Transplant Proc. 1991; 23(1 Pt 1): 783–4. [PMC free article] [PubMed] [Google Scholar]
- (15).McCall M, Shapiro AM. Islet cell transplantation. Semin Pediatr Surg. 2014; 23(2): 83–90. [DOI] [PubMed] [Google Scholar]
- (16).Matsumoto S, Takita M, Chaussabel D, Noguchi H, Shimoda M, Sugimoto K, et al. Improving efficacy of clinical islet transplantation with iodixanol-based islet purification, thymoglobulin induction, and blockage of IL-1beta and TNF-alpha. Cell Transplant. 2011; 20(10): 1641–7. [DOI] [PubMed] [Google Scholar]
- (17).Froud T, Baidal DA, Faradji R, Cure P, Mineo D, Selvaggi G, et al. Islet transplantation with alemtuzumab induction and calcineurin-free maintenance immunosuppression results in improved short- and long-term outcomes. Transplantation. 2008; 86(12): 1695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Anazawa T, Saito T, Goto M, Kenmochi T, Uemoto S, Itoh T, et al. Long-term outcomes of clinical transplantation of pancreatic islets with uncontrolled donors after cardiac death: a multicenter experience in Japan. Transplant Proc. 2014; 46(6): 1980–4. [DOI] [PubMed] [Google Scholar]
- (19).Koh A, Senior P, Salam A, Kin T, Imes S, Dinyari P, et al. Insulin-heparin infusions peritransplant substantially improve single-donor clinical islet transplant success. Transplantation. 2010; 89(4): 465–71. [DOI] [PubMed] [Google Scholar]
- (20).Hering BJ, Kandaswamy R, Ansite JD, Eckman PM, Nakano M, Sawada T, et al. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA. 2005; 293(7): 830–5. Erratum in: JAMA. 2005. Apr 6; 293(13): 1594. [DOI] [PubMed] [Google Scholar]
- (21).Froud T, Ricordi C, Baidal DA, Hafiz MM, Ponte G, Cure P, et al. Islet transplantation in type 1 diabetes mellitus using cultured islets and steroid-free immunosuppression: Miami experience. Am J Transplant. 2005; 5(8): 2037–46. [DOI] [PubMed] [Google Scholar]
- (22).Ghofaili KA, Fung M, Ao Z, Meloche M, Shapiro RJ, Warnock GL, et al. Effect of exenatide on beta cell function after islet transplantation in type 1 diabetes. Transplantation. 2007; 83(1): 24–8. [DOI] [PubMed] [Google Scholar]
- (23).Shapiro AM. Islet transplantation in type 1 diabetes: ongoing challenges, refined procedures, and long-term outcome. Rev Diabet Stud. 2012; 9(4): 385–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Hatziavramidis DT, Karatzas TM, Chrousos GP. Pancreatic islet cell transplantation: an update. Ann Biomed Eng. 2013; 41(3): 469–76. [DOI] [PubMed] [Google Scholar]
- (25).Cantarelli E, Piemonti L. Alternative transplantation sites for pancreatic islet grafts. Curr Diab Rep. 2011; 11(5): 364–74. [DOI] [PubMed] [Google Scholar]
- (26).ClinicalTrials.gov [Internet]. Bethesda (MD): United States National Institutes of Health; 2015. [updated 2015 Mar 27; cited 2015 Feb 27]. Available from: https://clinicaltrials.gov/ [Google Scholar]
- (27).Paty BW, Koh A, Senior P. Canadian Diabetes Association 2013 clinical practice guidelines for the prevention and management of diabetes in Canada: pancreas and islet transplantation. Can J Diabetes. 2013; 37 Suppl 1:S94–6. [DOI] [PubMed] [Google Scholar]
- (28).Collaborative Islet Transplant Registry [Internet]. Rockville (MD): National Institute of Diabetes and Digestive and Kidney Diseases, Juvenile Diabetes Research Foundation; 2015. [cited 2015 Jan 15]. Available from: http://www.citregistry.org/ [Google Scholar]
- (29).Collaborative Islet Transplant Registry Collaborating Center. CITR Eight Annual Report [Internet]. Rockville (MD): The Registry; 2014. [cited 2015 Feb 3]. Available from: https://web.emmes.com/study/isl/reports/20150213_CITR_Eighth_Annual_Report_FINAL.pdf [Google Scholar]
- (30).Guidance document for cell, tissue and organ establishments—safety of human cells, tissues and organs for transplantation [Internet]. Ottawa (ON): Health Canada; 2015. [updated 2013 Aug 26; cited 2015. Feb 3]. Available from: http://www.hc-sc.gc.ca/dhp-mps/brgtherap/reg-init/cell/cto_gd_ld-eng.php [Google Scholar]
- (31).Guidance for industry: considerations for allogeneic pancreatic islet cell products [Internet]. Rockville (MD): United States Food and Drug Administration; 2009. [cited 2015 Feb 6]. Available from: http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/UCM182441.pdf [Google Scholar]
- (32).Health Quality Ontario. Islet transplantation: an evidence-based analysis. Ont Health Technol Assess Ser. 2003;3(4): 1–45. [PMC free article] [PubMed] [Google Scholar]
- (33).Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007; 7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011; 64(4): 380–2. [DOI] [PubMed] [Google Scholar]
- (35).ECRI. Islet cell transplantation for the treatment of type 1 diabetes [Internet]. Plymouth Meeting (PA): ECRI; 2005. [cited 2015 Feb 9]. Available from: http://www.ecri.org.uk/ [Google Scholar]
- (36).Hayes Inc. Islet cell transplantation for the treatment of type 1 diabetes [Internet]. Lansdale (PA): Hayes, Inc.; 2004. [cited 2015 Feb 9]. Available from: http://www.hayesinc.com [Google Scholar]
- (37).Piper M, Seidenfeld J, Aronson N. Islet transplantation in patients with type 1 diabetes mellitus [Internet]. Rockville (MD): Agency for Healthcare Research and Quality; 2004. [cited 2015 Jan 7]. Available from: http://archive.ahrq.gov/downloads/pub/evidence/pdf/islet/islet.pdf [Google Scholar]
- (38).Guo B, Corabian P, Harstall C. Islet transplantation for the treatment of type 1 diabetes—an update [Internet]. Edmonton (AB): Institute of Health Economics; 2008. [cited 2015 Feb 9]. Available from: http://www.ihe.ca/publications/islet-transplantation-for-the-treatment-of-type-1-diabetes-an-update [Google Scholar]
- (39).Guo B, Harstall C, Corabian P. Islet cell transplantation for the treatment of non-uremic type 1 diabetic patients with severe hypoglycemia [Internet]. Edmonton (AB): Alberta Heritage Foundation for Medical Research; 2003. [cited 2015 Feb 9]. Available from: http://www.ihe.ca/ [Google Scholar]
- (40).Speight J, Reaney MD, Woodcock AJ, Smith RM, Shaw JA. Patient-reported outcomes following islet cell or pancreas transplantation (alone or after kidney) in type 1 diabetes: a systematic review. Diabet Med. 2010; 27(7): 812–22. [DOI] [PubMed] [Google Scholar]
- (41).Institute of Health Economics. Islet transplantation for the treatment of type 1 diabetes [Internet]. Edmonton (AB): The Institute; 2013. [cited 2015 Jan 20]. Available from: http://www.ihe.ca/publications/islet-transplantation-for-the-treatment-of-type-1-diabetes [PubMed] [Google Scholar]
- (42).Xie X, Rich B, Dendukuri N. Islet transplantation in patients with type 1 diabetes mellitus [Internet]. Montreal (QC): Technology Assessment Unit (TAU) of the McGill University Health Centre (MUHC); 2014. [cited 2015 Jan 26]. Available from: http://www.mcgill.ca/tau/files/tau/muhc_tau_2014_66_islet_transplantation.pdf [Google Scholar]
- (43).Canadian Agency for Drugs and Technologies in Health. Islet cell transplantation in patients with unstable diabetes: a review of clinical- and cost-effectiveness and guidelines [Internet]. Ottawa (ON): The Agency; 2014. [cited 2015 Jan 27]. Available from: http://www.cadth.ca/media/pdf/htis/dec-2014/RC0614_Islet%20Cell%20Transplantation_Final.pdf [Google Scholar]
- (44).Fiorina P, Folli F, Maffi P, Placidi C, Venturini M, Finzi G, et al. Islet transplantation improves vascular diabetic complications in patients with diabetes who underwent kidney transplantation: a comparison between kidney-pancreas and kidney-alone transplantation. Transplantation. 2003; 75(8): 1296–301. [DOI] [PubMed] [Google Scholar]
- (45).Frank A, Deng S, Huang X, Velidedeoglu E, Bae YS, Liu C, et al. Transplantation for type I diabetes: comparison of vascularized whole-organ pancreas with isolated pancreatic islets. Ann Surg. 2004; 240(4): 631–40; discussion 40–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Fiorina P, Gremizzi C, Maffi P, Caldara R, Tavano D, Monti L, et al. Islet transplantation is associated with an improvement of cardiovascular function in type 1 diabetic kidney transplant patients. Diabetes Care. 2005; 28(6): 1358–65. [DOI] [PubMed] [Google Scholar]
- (47).Venturini M, Fiorina P, Maffi P, Losio C, Vergani A, Secchi A, et al. Early increase of retinal arterial and venous blood flow velocities at color Doppler imaging in brittle type 1 diabetes after islet transplant alone. Transplantation. 2006; 81(9): 1274–7. [DOI] [PubMed] [Google Scholar]
- (48).Gerber PA, Pavlicek V, Demartines N, Zuellig R, Pfammatter T, Wuthrich R, et al. Simultaneous islet-kidney vs pancreas-kidney transplantation in type 1 diabetes mellitus: a 5 year single centre follow-up. Diabetologia. 2008; 51(1): 110–9. [DOI] [PubMed] [Google Scholar]
- (49).Warnock GL, Thompson DM, Meloche RM, Shapiro RJ, Ao Z, Keown P, et al. A multi-year analysis of islet transplantation compared with intensive medical therapy on progression of complications in type 1 diabetes. Transplantation. 2008; 86(12): 1762–6. [DOI] [PubMed] [Google Scholar]
- (50).Vantyghem MC, Marcelli-Tourvieille S, Fermon C, Duhamel A, Raverdy V, Arnalsteen L, et al. Intraperitoneal insulin infusion versus islet transplantation: comparative study in patients with type 1 diabetes. Transplantation. 2009; 87(1): 66–71. [DOI] [PubMed] [Google Scholar]
- (51).Thompson DM, Meloche M, Ao Z, Paty B, Keown P, Shapiro RJ, et al. Reduced progression of diabetic microvascular complications with islet cell transplantation compared with intensive medical therapy. Transplantation. 2011; 91(3): 373–8. [DOI] [PubMed] [Google Scholar]
- (52).Maffi P, Scavini M, Socci C, Piemonti L, Caldara R, Gremizzi C, et al. Risks and benefits of transplantation in the cure of type 1 diabetes: whole pancreas versus islet transplantation. A single center study. Rev Diabet Stud. 2011; 8(1): 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).D'Addio F, Maffi P, Vezzulli P, Vergani A, Mello A, Bassi R, et al. Islet transplantation stabilizes hemostatic abnormalities and cerebral metabolism in individuals with type 1 diabetes. Diabetes Care. 2014; 37(1): 267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Radosevich DM, Jevne R, Bellin M, Kandaswamy R, Sutherland DE, Hering BJ. Comprehensive health assessment and five-yr follow-up of allogeneic islet transplant recipients. Clin Transplant. 2013; 27(6): E715–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006; 355(13): 1318–30. [DOI] [PubMed] [Google Scholar]
- (56).Toso C, Shapiro AM, Bowker S, Dinyari P, Paty B, Ryan EA, et al. Quality of life after islet transplant: impact of the number of islet infusions and metabolic outcome. Transplantation. 2007; 84(5): 664–6. [DOI] [PubMed] [Google Scholar]
- (57).Maffi P, Bertuzzi F, De Taddeo F, Magistretti P, Nano R, Fiorina P, et al. Kidney function after islet transplant alone in type 1 diabetes: impact of immunosuppressive therapy on progression of diabetic nephropathy. Diabetes Care. 2007; 30(5): 1150–5. [DOI] [PubMed] [Google Scholar]
- (58).Fiorina P, Folli F, Zerbini G, Maffi P, Gremizzi C, Di Carlo V, et al. Islet transplantation is associated with improvement of renal function among uremic patients with type I diabetes mellitus and kidney transplants. J Am Soc Nephrol. 2003; 14(8): 2150–8. [DOI] [PubMed] [Google Scholar]
- (59).Bertuzzi F, Grohovaz F, Maffi P, Caumo A, Aldrighetti L, Nano R, et al. Successful transplantation of human islets in recipients bearing a kidney graft. Diabetologia. 2002; 45(1): 77–84. [DOI] [PubMed] [Google Scholar]
- (60).Benhamou PY, Milliat-Guittard L, Wojtusciszyn A, Kessler L, Toso C, Baertschiger R, et al. Quality of life after islet transplantation: data from the GRAGIL 1 and 2 trials. Diabet Med. 2009; 26(6): 617–21. [DOI] [PubMed] [Google Scholar]
- (61).Badet L, Benhamou PY, Wojtusciszyn A, Baertschiger R, Milliat-Guittard L, Kessler L, et al. Expectations and strategies regarding islet transplantation: metabolic data from the GRAGIL 2 trial. Transplantation. 2007; 84(1): 89–96. [DOI] [PubMed] [Google Scholar]
- (62).Benhamou PY, Oberholzer J, Toso C, Kessler L, Penfornis A, Bayle F, et al. Human islet transplantation network for the treatment of type I diabetes: first data from the Swiss-French GRAGIL consortium (1999–2000). Groupe de Recherche Rhin Rhjne Alpes Geneve pour la transplantation d'Ilots de Langerhans. Diabetologia. 2001; 44(7): 859–64. [DOI] [PubMed] [Google Scholar]
- (63).Borot S, Niclauss N, Wojtusciszyn A, Brault C, Demuylder-Mischler S, Muller Y, et al. Impact of the number of infusions on 2-year results of islet-after-kidney transplantation in the GRAGIL network. Transplantation. 2011; 92(9): 1031–8. [DOI] [PubMed] [Google Scholar]
- (64).Tharavanij T, Betancourt A, Messinger S, Cure P, Leitao CB, Baidal DA, et al. Improved long-term health-related quality of life after islet transplantation. Transplantation. 2008; 86(9): 1161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Leitao CB, Tharavanij T, Cure P, Pileggi A, Baidal DA, Ricordi C, et al. Restoration of hypoglycemia awareness after islet transplantation. Diabetes Care. 2008; 31(11): 2113–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Lee TC, Barshes NR, O'Mahony CA, Nguyen L, Brunicardi FC, Ricordi C, et al. The effect of pancreatic islet transplantation on progression of diabetic retinopathy and neuropathy. Transplant Proc. 2005; 37(5): 2263–5. [DOI] [PubMed] [Google Scholar]
- (67).Barshes NR, Vanatta JM, Mote A, Lee TC, Schock AP, Balkrishnan R, et al. Health-related quality of life after pancreatic islet transplantation: a longitudinal study. Transplantation. 2005; 79(12): 1727–30. [DOI] [PubMed] [Google Scholar]
- (68).Turgeon NA, Avila JG, Cano JA, Hutchinson JJ, Badell IR, Page AJ, et al. Experience with a novel efalizumab-based immunosuppressive regimen to facilitate single donor islet cell transplantation. Am J Transplant. 2010; 10(9): 2082–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Gangemi A, Salehi P, Hatipoglu B, Martellotto J, Barbaro B, Kuechle JB, et al. Islet transplantation for brittle type 1 diabetes: the UIC protocol. Am J Transplant. 2008; 8(6): 1250–61. [DOI] [PubMed] [Google Scholar]
- (70).Danielson KK, Hatipoglu B, Kinzer K, Kaplan B, Martellotto J, Qi M, et al. Reduction in carotid intima-media thickness after pancreatic islet transplantation in patients with type 1 diabetes. Diabetes Care. 2013; 36(2): 450–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Keymeulen B, Gillard P, Mathieu C, Movahedi B, Maleux G, Delvaux G, et al. Correlation between beta cell mass and glycemic control in type 1 diabetic recipients of islet cell graft. Proc Natl Acad Sci U S A. 2006; 103(46): 17444–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Vantyghem MC, Kerr-Conte J, Arnalsteen L, Sergent G, Defrance F, Gmyr V, et al. Primary graft function, metabolic control, and graft survival after islet transplantation. Diabetes Care. 2009; 32(8): 1473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Vantyghem MC, Raverdy V, Balavoine AS, Defrance F, Caiazzo R, Arnalsteen L, et al. Continuous glucose monitoring after islet transplantation in type 1 diabetes: an excellent graft function (β-score greater than 7) is required to abrogate hyperglycemia, whereas a minimal function is necessary to suppress severe hypoglycemia (β-score greater than 3). J Clin Endocrinol Metab. 2012; 97(11): E2078–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).O'Connell PJ, Holmes-Walker DJ, Goodman D, Hawthorne WJ, Loudovaris T, Gunton JE, et al. Multicenter Australian trial of islet transplantation: improving accessibility and outcomes. Am J Transplant. 2013; 13(7): 1850–8. [DOI] [PubMed] [Google Scholar]
- (75).Haggstrom E, Rehnman M, Gunningberg L. Quality of life and social life situation in islet transplanted patients: time for a change in outcome measures? Int J Organ Transplant Med. 2011;2(3): 117–25. [PMC free article] [PubMed] [Google Scholar]
- (76).Fiorina P, Folli F, Bertuzzi F, Maffi P, Finzi G, Venturini M, et al. Long-term beneficial effect of islet transplantation on diabetic macro-/microangiopathy in type 1 diabetic kidney-transplanted patients. Diabetes Care. 2003; 26(4): 1129–36. [DOI] [PubMed] [Google Scholar]
- (77).Takita M, Matsumoto S, Noguchi H, Shimoda M, Ikemoto T, Chujo D, et al. Adverse events in clinical islet transplantation: one institutional experience. Cell Transplant. 2012; 21(2–3): 547–51. [DOI] [PubMed] [Google Scholar]
- (78).Gillard P, Huurman V, Van der Auwera B, Decallonne B, Poppe K, Roep BO, et al. Graves hyperthyroidism after stopping immunosuppressive therapy in type 1 diabetic islet cell recipients with pretransplant TPO autoantibodies. Diabetes Care. 2009; 32(10): 1817–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Alfadhli E, Koh A, Albaker W, Bhargava R, Ackerman T, McDonald C, et al. High prevalence of ovarian cysts in premenopausal women receiving sirolimus and tacrolimus after clinical islet transplantation. Transpl Int. 2009; 22(6): 622–5. [DOI] [PubMed] [Google Scholar]
- (80).Del Olmo Garcia MI, Lauriola V, Aracena AG, Messinger S, Corrales A, Ricordi C, et al. Alterations of the female reproductive system in islet recipient receiving immunosuppression. Cell Transplant. 2011; 20(10): 1649–51. [DOI] [PubMed] [Google Scholar]
- (81).Eckhard M, Martin I, Eich T, Weimer R, Zinn S, Bretzel RG, et al. Incidence of cytomegalovirus infections after immunosuppression induction in clinical islet transplantation and impact on graft function. Transplant Proc. 2002; 34(5): 1922–4. [DOI] [PubMed] [Google Scholar]
- (82).Hafiz MM, Faradji RN, Froud T, Pileggi A, Baidal DA, Cure P, et al. Immunosuppression and procedure-related complications in 26 patients with type 1 diabetes mellitus receiving allogeneic islet cell transplantation. Transplantation. 2005; 80(12): 1718–28. [DOI] [PubMed] [Google Scholar]
- (83).Venturini M, Angeli E, Maffi P, Losio C, Pozzi P, Paties C, et al. Liver focal fatty changes at ultrasound after islet transplantation: an early sign of altered graft function? Diabet Med. 2010;27(8): 960–4. [DOI] [PubMed] [Google Scholar]
- (84).Froud T, Baidal DA, Ponte G, Ferreira JV, Ricordi C, Alejandro R. Resolution of neurotoxicity and beta-cell toxicity in an islet transplant recipient following substitution of tacrolimus with MMF. Cell Transplant. 2006; 15(7): 613–20. [DOI] [PubMed] [Google Scholar]
- (85).Villiger P, Ryan EA, Owen R, O'Kelly K, Oberholzer J, Al Saif F, et al. Prevention of bleeding after islet transplantation: lessons learned from a multivariate analysis of 132 cases at a single institution. Am J Transplant. 2005; 5(12): 2992–8. [DOI] [PubMed] [Google Scholar]
- (86).Barshes NR, Lee TC, Goodpastor SE, Balkrishnan R, Schock AP, Mote A, et al. Transaminitis after pancreatic islet transplantation. J Am Coll Surg. 2005; 200(3): 353–61. [DOI] [PubMed] [Google Scholar]
- (87).Yakubovich N, Fung MA, Thompson DM. Three cases of cytomegalovirus infection following pancreatic islet transplantation. Transplant Proc. 2007; 39(5): 1599–603. [DOI] [PubMed] [Google Scholar]
- (88).Senior PA, Zeman M, Paty BW, Ryan EA, Shapiro AM. Changes in renal function after clinical islet transplantation: four-year observational study. Am J Transplant. 2007; 7(1): 91–8. [DOI] [PubMed] [Google Scholar]
- (89).Leitao CB, Cure P, Messinger S, Pileggi A, Lenz O, Froud T, et al. Stable renal function after islet transplantation: importance of patient selection and aggressive clinical management. Transplantation. 2009; 87(5): 681–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Cure P, Pileggi A, Froud T, Messinger S, Faradji RN, Baidal DA, et al. Improved metabolic control and quality of life in seven patients with type 1 diabetes following islet after kidney transplantation. Transplantation. 2008; 85(6): 801–12. [DOI] [PubMed] [Google Scholar]
- (91).Vanstone M, Rewegan A, Brundisini F, Dejean D, Giacomini M. Patient perspectives on quality of life with uncontrolled type 1 diabetes mellitus: a systematic review and qualitative meta-synthesis. Ont Health Technol Assess Ser [Internet]. 2015. Sep; 15(17): 1–29. Available from: http://www.hqontario.ca/evidence/publications-and-ohtac-recommendations/ontario-health-technology-assessment-series/fev-pancreas-islet-transplantation. [PMC free article] [PubMed] [Google Scholar]
- (92).Working Group of the Clinical Practice Guideline on Diabetes Mellitus Type 1. Clinical practice guidelines for diabetes mellitus type 1 [Internet]. Zaragoza, Spain: Quality Plan for the National Health Service of the Ministry of Health and Social Policy, Agency for Health Technology Assessment from the Basque Country—Osteba; 2012. [cited 2015 Feb 9]. Available from: http://www.guiasalud.es/GPC/GPC_513_Diabetes_1_Osteba_compl_en.pdf [Google Scholar]
- (93).Chiang JL, Kirkman MS, Laffel LM, Peters AL. Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care. 2014; 37(7): 2034–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).National Institute for Health and Clinical Excellence. Allogeneic pancreatic islet cell transplantation for type 1 diabetes mellitus [Internet]. London, United Kingdom: The Institute; 2008. [cited 2015 Feb 5]. Available from: http://www.nice.org.uk/guidance/ipg257 [Google Scholar]