Abstract
The red imported fire ant (RIFA) Solenopsis invicta Buren causes severe damage to humans and animals as well as the environment. Chemical treatment is the main strategy of RIFA management, which also is potentially toxic to the environment. Plant essential oils (EOs) are considered as potential substance that can be used to control insects. This study aimed to identify the chemical composition of camphor EO and investigate the insecticidal activity on RIFAs. The chemical composition of the EO was analyzed by gas chromatography/mass spectrometry and gas chromatography with flame ionization detection. Results revealed that 36.61% camphor and 30.05% cineole were the major components. The insecticidal activity of camphor EO was assessed against RIFA workers by conducting two different bioassays: fumigant toxicity and repellence. Fumigant toxicity assay results showed that the lethal dose (LC50) of the EO at 24 h was 1.67 and 4.28 μg/ml for minor and major workers, respectively; knockdown time (KT50) was 10.82 and 14.73 h. At 2.55 μg/ml, the highest average mortality of the ants was 84.89% after 72 h. Camphor EO exhibited fumigant toxicity against minor and major workers as indicated by the effects on attacking, feeding, and climbing behaviors. This EO was also strongly repellent to the two size workers of the colony as observed in their behavior against Tenebrio molitor treated with 5 µl EO. The fumigant toxicity and repellence of camphor EO against RIFA indicated that this substance could be a potential alternative for the development of eco-friendly products used to control pests.
Keywords: camphor essential oil, fumigant, repellent, Solenopsis invicta
Introduction
The red imported fire ant (RIFA) Solenopsis invicta is one of the most notoriously known agricultural insect pests in southern United States, Australia, Philippines, Taiwan, and China (Zhang et al. 2007). Numerous ant species exploit and colonize environments altered by human activity, and RIFAs are more aggressive than other ant species. In infested areas, RIFA colonies are commonly found in gardens, lawns, parks, school yards, golf courses, and roadsides (Brenda and Carl 2010). These ants reproduce and infest areas rapidly; for instance, they can be moved with grass sod or nursery stock; they can also be carried across long distances by soil-moving equipment.
RIFA colonies are destructive. These ants dominate their home ranges because of their large numbers and aggressiveness. They can even alter the composition of ecological communities in invaded areas, outcompeting and frequently eliminating the native fire ants (Brenda and Carl 2010). In cities, RIFA occasionally build colonies in electrical circuitry, causing shorted air conditioners; these ants may also inhabit telephone junction boxes, traffic and light control boxes, and transformers (Collins and Scheffrahn 2001). They even cause potholes in sidewalks or highways when a colony underneath these paths eventually dies out. In addition to structural damage, human health problems also arise, particularly from the stings of RIFAs. The presence of RIFA in crops or gardens threatens individuals because of stings, thereby impeding the manual harvesting of fruits and vegetables (Collins and Scheffrahn 2001). Fire ants sting repeatedly; with each sting, venom is injected into organism. RIFA venom contains high toxin concentrations that cause intense burning and itching; then a blister is formed and becomes a white pustule (Brenda and Carl 2010). Broken or scratched pustules can harbor secondary bacterial infections and may result in permanent scarring. In worse cases, severe allergic reactions can occur, resulting in anaphylactic shock and even death (Dowell et al. 1997).
Current methods used to control RIFAs are extremely costly and inefficient (Drees and Gold 2003). The conventional methods, such as chemical treatment of nests and surrounding areas, may result in environmental contamination. Moreover, the use of non-RIFA-specific agricultural insecticides in some places, such as nurseries, truck stops, and parks, have been considered as high-risk sites of RIFA invasion because RIFAs can easily travel in soil and potted plants, as well as vehicles may pose safety risks.
In recent years, a series of studies have shown that essential oils (EOs) derived from plants have proven to be toxic to different pests (Dugassa et al. 2009, Nerio et al. 2010, Kumar et al. 2012). EOs of plants are formed by complex mixes of compounds structurally different which can act synergistically increasing its action potential and efficacy (Berenbaum 1985). The fragrant camphor tree, Cinnamomum camphora (L.) J. Presl (Lauraceae), for extraction of its camphor EO rich in camphor, occurs naturally in Asian countries (Chen et al. 2013). Camphor EO has great commercial value to perfumery and cosmetic industry. Camphor exhibits a number of biological properties such as insecticidal, antimicrobial, antiviral, anticoccidial, antinociceptive, anticancer, and antitussive activities, in addition to its use as a skin penetration enhancer (Chen et al. 2013). However, to date, very few studies evaluated the activity of the EO of camphor against RIFA. Our previous study found that the EO of camphor has strong fumigant toxicity against S. invicta (Tang et al. 2013). Chen (2009) also investigated the repellency of camphor oil product in China against workers of RIFAs.
In this study, we identified the chemical composition of camphor EO and assessed the fumigant toxicity and repellence activity of camphor EO against the RIFA.
Materials and Methods
Essential Oil
Camphor EO was purchased from Kang Shen Natural Medicinal Oil Refinery, Jiangxi, China, and was stored in plastic bottles at 4°C until further use.
Chemicals
Compounds camphor and cineole were purchased from Jing Chun Chemical Reagent Co., Ltd., Shanghai, China. HPLC grade acetone was purchased from Guangzhou Chemical Reagent Factory.
Worker Ant
S. invicta colonies were directly obtained from nests on the suburb of Zengcheng, Guangdong Province. Workers were reared in plastic containers (50 cm in diameter by 20 cm in height) under the natural environment conditions (temperature 25 ± 2°C and a relative humidity of 60–80%). Workers were allowed free access to a test tube (25 mm by 200 mm), which was used as a water source, partially filled with 10% honey water and plugged with cotton. A Petri dish (8.5 cm by 1.5 cm) containing Tenebrio molitor was placed in containers as the food source. T. molitor were purchased from the insect–fish market in Guangzhou, fed with wheat bran, and kept in a dry indoor environment at 25 ± 2°C until use.
Gas Chromatography-Mass Spectrometry Analysis
Chemical composition of camphor EO was determined by a gas chromatography/mass spectroscopic detector (GC–MS) Agilent 6890N GC interfaced with a Agilent 5957C mass spectrometer fitted with a capillary column (HP-5MS, 30 m by 0.25 mm in internal diameter, 0.25 μm in film thickness). Helium was used as the carrier gas (flow rate = 1.0 ml/min). The injector temperature was 25°C, and the detector (or interface) temperature was 300°C. The injection volume of samples was 1.0 μl; the partition rate of the injected volume was 1:50. The temperature program was as follows: 50°C for 5.0 min, an increase of 10°C/min to 220°C, and then held at 220°C for 5.0 min, with a total run time of 40.0 min. The mass spectrum was obtained at an ionization voltage of (EI) 70 eV, mass range 50–500 m/z and detector voltage 1.5 V. The chemical constituents were quantified by gas chromatography with flame ionization detection (GC-FID). The samples were then analyzed on same Agilent instrument fitted with the same column and following the same temperature program as above. The amount of each constituent was determined by area normalization (%) and the concentrations were calculated from the GC peak areas. Analytes profile was characterized from their mass spectral data using National Institute of Standards and Technology (NIST05) and Wiley8 mass spectrometry libraries.
Fumigant Activity Bioassays
Lethal Dose
Fumigation assays for LC50 and LC90 were conducted in a glass cylinder (1 dm3) fitted with a screw cap and a cotton yarn (7 cm in length by 1.5 cm in width) was suspended from the center of the inner face of the cap. The vertical wall inside each glass cylinder was coated with fluon emulsion and died for 24 h to prevent the ants from escaping. Five concentrations (2.55, 5.10, 10.19, 15.29, and 25.48 μg/ml) of camphor EO and camphor cineole were applied separately to the yarn for the major workers (body length = 4.3–4.5 mm; head width = 1.0–1.1 mm) and minor workers (body length = 2.8–3.0 mm; head width = 0.6–0.7 mm). In the control glass cylinder, the cotton yarn was not applied with EO. Each group comprised 15 ants placed at the bottom and then put the lid on the glass cylinder. Each assay was replicated three times. The jars were tightly sealed and then kept in a room at 25 ± 2°C with relative humidity of 80%. The mortality of the workers was evaluated at 12, 24, 48, and 72 h after the bioassays were conducted to verify the increase in toxicity over time. Ants were considered dead when they remained immobile and did not respond to stimulation applied using a brush.
Knockdown Time
To evaluate the knockdown time (KT50) of the camphor EO, we used LC90 for each group in accordance with previous fumigant bioassays. In brief, the ants were divided into two groups, the treatment group (with camphor EO) and the control group (no EO). We employed the same experimental design and procedures for the RIFA exposure to oil as the bioassays used to determine LCs; in this experiment, LC90 was used. The ants were monitored for 24 h, and those turned over with their abdomen exposed were considered knocked down. The activity of camphor EO was determined by counting the knocked down ants after the bioassays were performed.
Determination of Attacking and Feeding Behaviors of RIFAs on T. molitor
A total of 50 ants for each size workers of the colony (major workers, minor workers, and medium-sized workers, body length = 3.5–3.7 mm; head width = 0.8–0.9 mm) were placed in a glass cylinder rand fumigated for 30 min (Seo et al. 2009). The ants were starved for 2 d before the assays were conducted and 10.19 μg/ml of camphor EO was applied to the cotton yarn. To observe the attack and feeding behaviors on T. molitor, we placed one T. molitor of the same size in a Petri dish; the treated ants were then placed into the Petri dish. Each treatment was replicated three times. Only the control assay was not fumigated. All of the bioassays were performed at 25 ± 2°C and relative humidity of 75 ± 5%. The behaviors of RIFAs against T. molitor were observed at 1, 5, 10, 15, 20, 25, and 30 min; afterward, the rate of attack activity was calculated using the following equation:
Where A (%) is the attack rate, An is the number of major and minor RIFAs attacking T. molitor, and Tn is the total number of RIFAs. The feeding activities of medium-sized workers were observed and recorded at 24 and 48 h; the rate of feeding activity was then calculated as the percentage reduction in feeding behavior emergence or inhibition rate. Percentage inhibition rate was calculated as follows:
Where F is the feeding rate, Iw is the initial weight of T. molitor, and Rw is the remaining weight of T. molitor.
Determination of Climbing Behavior
To evaluate the climbing behavior of the fumigated ants, we selected and starved 50 medium-sized RIFA workers for 2 d. These ants were subsequently fumigated for 30 min in a glass cylinder. We employed the same experimental design, procedures of ant exposure to EO, and evaluations as previously described bioassays to determine the attack and feeding behaviors of RIFAs except the observation method. In this experiment, the ants were fumigated and then placed into a conical flask. Afterward, the conical flask was gently flipped with the mouth facing downward on a piece of paper for 3 s. The conical flask was then flipped gently back upward. The number of individuals present on the paper was recorded after 1, 30, 60, 90, 120, 150, and 180 min. The control ants were kept under the same conditions without fumigation. The rate of climbing activity was calculated using the following equation:
Where C is the climbing rate, Tn is the total number of RIFA, and Fn is the number of RIFA that fell on the paper.
Repellence Bioassay
The repellence of camphor EO on the two size workers of the colony was evaluated. Repellence bioassays were conducted using 50 ants in the presence of one T. molitor. The bioassays were performed in a Petri dish (9 mm by 9 cm) and the inner vertical wall of each Petri dish was coated with Fluon emulsion. Approximately 5 μl of camphor EO was applied on the body of T. molitor by using a 10 μl microsyringe (Hamilton). These ants were starved for 2 d and placed in the same Petri dish with the treated T. molitor. This procedure was performed three times. Camphor EO was not applied on the control T. molitor. The repellence of camphor EO was determined using the same observation step of attack and feeding behaviors on T. molitors as previous bioassays. The results of the attack behavior of major and minor RIFAs were recorded at 1, 2, 3, 4, 5, 6, 7, and 8h. The feeding activities of medium-sized workers were recorded after 24 and 48 h.
Statistical Analysis
Data obtained from each dose and time-response bioassay were subjected to regression analysis by probit to generate LC50, LC90, and KT50 (Finney 1971, SPSS 2012). A one-way analysis of variance was performed to compare the effects of exposure period in the fumigant and repellence bioassay. Data were expressed as the average number ± standard error and evaluated using Data Processing System statistics. Statistical analyses were performed using SPSS version 20.0 (SPSS 2012).
Results and Discussion
Chemical Composition of the EO
Camphor EO contains 18 compounds that account for 99.9% of the total oil compositions (Table 1). Camphor is the major component, accounting for 36.61% of the compounds, followed by cineole (30.02%), α-terpineol (5.22%), and safrole (5.15%). Camphor is also a main EO component in many aromatic plant species (Hammer Schmidt et al. 1993, Kamdem and Gage 1995, Tirillini et al. 1996, Juteau et al. 2002, Viljoen et al. 2003). Other studies have found that cineole accounts for 90% of EOs in plant species with the generic product eucalyptus oil; this compound is also commonly used as an insecticide and insect repellent (Boland et al. 1991). Considering that the insecticidal efficacy of EO is possibly dependent on monoterpene, we may characterize this EO to determine the oil components responsible for the insecticidal activity (Kumar et al. 2012). The amount of the major composition of camphor EO was moderate in this study compared with that in previous studies.
Table 1.
Chemical composition of camphor EO
| Composition | RRT (min) | Percentage | ||
|---|---|---|---|---|
| 1 | (1R)-(+)-α-pinene | 7.915 | 2.47 | |
| 2 | Camphene | 8.272 | 1.17 | |
| 3 | Sabinene | 8.898 | 1.92 | |
| 4 | β-Pinene | 8.963 | 1.31 | |
| 5 | 2-N-propylpyridine | 9.282 | 1.14 | |
| 6 | Cineole | 10.265 | 30.02 | |
| 8 | γ-terpinene | 10.697 | 0.50 | |
| 9 | Camphor | 12.55 | 36.61 | |
| 10 | DL-Isoborneol | 12.755 | 1.32 | |
| 11 | Terpinen-4-ol | 12.934 | 3.04 | |
| 12 | α-Terpineol | 13.182 | 5.22 | |
| 13 | Safrole | 14.646 | 5.15 | |
| 14 | α-Santalene | 16.391 | 2.73 | |
| 15 | α-Bergamotene | 16.558 | 0.89 | |
| 16 | 2-methyl-3-methylene -2-(4-methylpent -3-enyl)norbornane | 16.899 | 1.37 | |
| 17 | Cubebene | 17.212 | 0.44 | |
| 18 | Cis-nerolidol | 18.146 | 4.69 | |
RRT, relative retention time.
Bioassay
Fumigant toxicity was dependent on time and oil concentration used in the experiment. The mortality of ants was significantly different among the tested concentrations (F = 61.53, P < 0.0001) and sizes (F = 78.39, P < 0.0001). However, the mortality was not significantly different between camphor EO and the major chemical compounds of EO, namely, camphor and cineole (F = 1.42, P = 0.296).
The mortality of the ants is presented in Fig. 1. There were no workers found dead in the control groups. In all treatment groups, a noticeable variation of ant mortality was observed as the exposure interval was prolonged. A significant difference (F = 78.39, P < 0.0001) in the mortalities between minor and major workers was found; in particular, minor workers were more susceptible to camphor EO at 2.55 μg/ml concentration. An evident increase in mortality of workers was observed at different exposure times, in which the average mortality was 13.3–74.7% for the major workers and 20.0–100.0% for the minor workers. The highest average mortality percentage of the ants at 2.55 μg/ml was on 84.89% after 72 h.
Fig. 1.
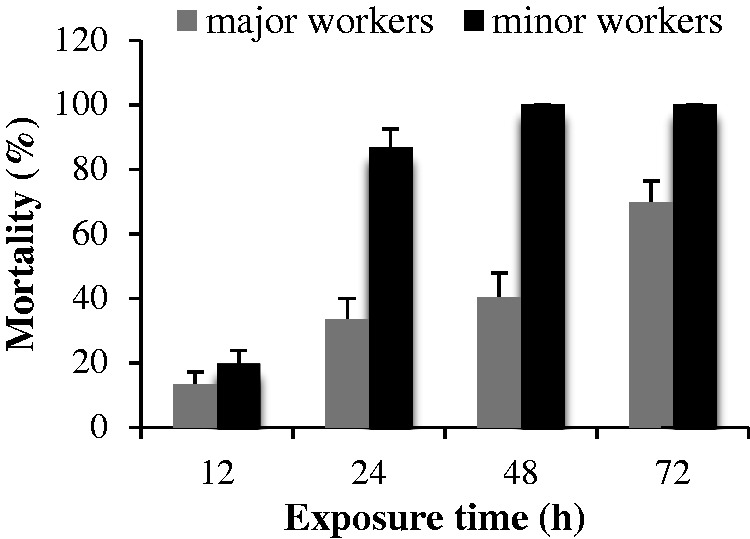
Mortality (%) of minor and major workers of RIFAs caused by the camphor EO at 2.55 μg/ml in the fumigation bioassay.
LC50 and LC90 of camphor EO and the major components (camphor and cineole) work on the major and minor RIFA workers are presented in Table 2. An increase in toxicity of these components was observed at all concentrations (2.55–25.48 μg/ml) in major and minor workers. After 24 h, the following values were, respectively, obtained for minor and major workers: LC50 of camphor EO, 1.67 and 4.28 μg/ml; KT50, 10.82 and 14.73 h; and LC90 of EO, 2.73 and 7.29 μg/ml. After camphor EO was applied, the minor workers displayed signs of intoxication. Cineole (36.61%; LC50 of 2.34 and 5.99 μg/ml) and Camphor (30.02%; LC50 of 1.91and 5.59 μg/ml) were also toxic to minor and major RIFA workers. Camphor EO exhibits a stronger toxicity than natural camphor possibly because of the presence of cineole, which contributes to camphor toxicity. Camphor EO displayed fumigant toxicity against the two sizes of RIFA workers. This result was observed in a greater effect on attack, feeding, and climbing behaviors of the fumigated ants than of the control ants (Figs. 2, 3, and 5). This result also indicated that camphor EO could reduce the attack and feeding rate of RIFAs compared with the control ants after fumigation. In addition, camphor EO elicited a higher inhibitory effect on the climbing ability of RIFAs, this ability of RIFAs to climb plants, soil, stones, and nest walls is crucial for survival. From Figs. 2 and 4, it is also evident that the feeding rate of ants in the control group was <60% after 48 h. This could be attributed to the amount of food they consume every day. We determined and compared the toxicity of camphor EO obtained in this study with that in previous studies (Phelan 1976, Rabl et al. 1997, Love et al. 2003). Although the overall knowledge on the toxicity of camphor EO to RIFAs is poor, several published studies show that it exhibits toxic effects against stored-product insects. For instance, contact toxicity, grain treatment, and repellence assays have been conducted to investigate the toxicity of camphor EO and its major component (camphor) to four beetles, namely, Sitophilus granarius, Sitophilus zeamais, Tribolium castaneum, and Prostephanus truncatus, after 24 h of exposure (Chen et al. 2013). Another report has indicated that pure camphor compound shows contact and fumigant activity against Sitophilus oryzae and Rhyzopertha dominica but does not affect Tr. castaneum after 24 h of exposure at 0.1 µl/720 ml (Rozman et al. 2006). It was demonstrated that the insecticidal activity of camphor EO against R. dominica and Si. zeamais is attributed to camphor and the combined effects of different components, but camphor alone does not affect rice weevil (Si. oryzae) at an LC50 > 100 µl/liter (Bekele and Hassanali 2001).
Table 2.
LC50 and LC90 of camphor EO, cineole, and camphor for minor and major RIF workers after 24 h fumigating exposurea
| Chemical constituents | Nb | Species | LC50 (95% CI)c | LC90 (95% CI)c | βd | χ2 | Р |
|---|---|---|---|---|---|---|---|
| Camphor EO | 270 | Minor | 1.67(1.32–2.90) | 2.73(2.36–3.91) | 6.023 | 0.184 | 0.980 |
| 270 | Major | 4.28(3.94–4.63) | 7.29(6.55–8.39) | 5.534 | 4.516 | 0.211 | |
| Cineole | 270 | Minor | 2.34(2.05–2.54) | 3.53(3.18–4.38) | 7.194 | 0.776 | 0.855 |
| 270 | Major | 5.99(5.50–6.70) | 7.70(6.83–9.70) | 11.744 | 0.466 | 0.792 | |
| Camphor | 270 | Minor | 1.91(0.87–2.22) | 2.97(2.69–4.22) | 6.708 | 0.217 | 0.975 |
| 270 | Major | 5.59(4.27–7.08) | 11.06(8.52–17.83) | 4.331 | 4.930 | 0.186 |
a LC50 and LC90 were not determined for the species due to the low toxicity (mortality of <30%) observed after 24 h of exposure.
b Number of insects.
c LC: lethal concentration in μg of EO/ml.
d Slope of the curve.
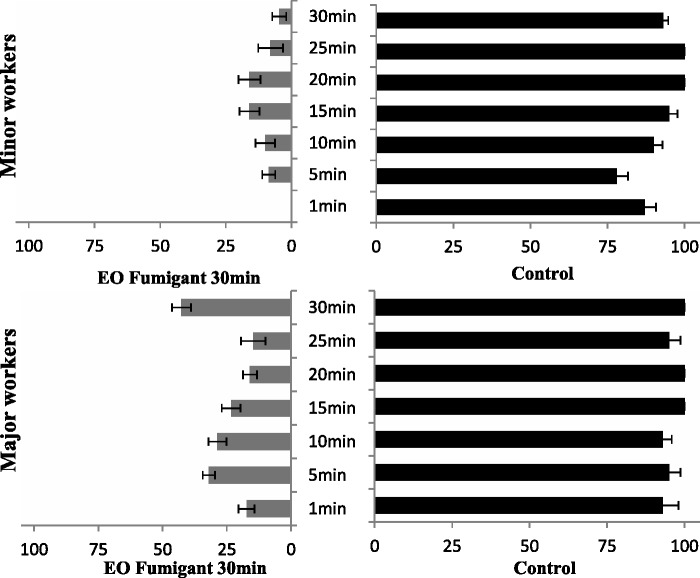
Fig. 2.
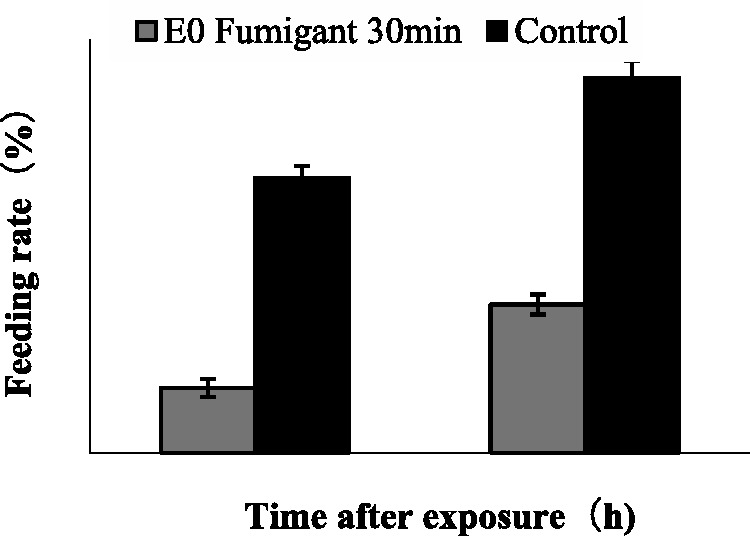
Feeding rate of medium-sized workers of RIFAs 30 min after fumigation treatment with camphor EO at 10.19 μg/ml on T. molitor.
Fig. 3.
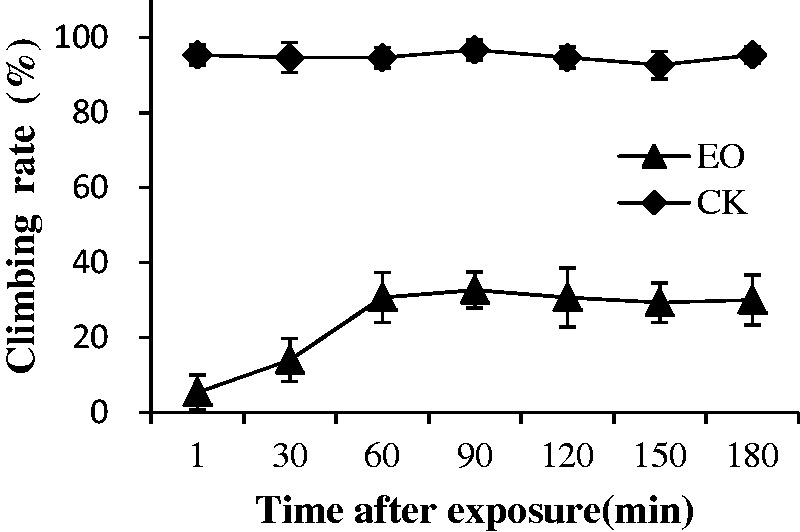
Climbing rate of medium-sized workers of RIFAs 30 min after fumigation treatment with camphor EO at 10.19 μg/ml tube (Phelan et al.).
Fig. 5.
Attack rate of minor and major workers of RIFAs fumigation treatment 30 min with camphor EO at 10.19 μg/ml along the time on T. molitor.
Fig. 4.
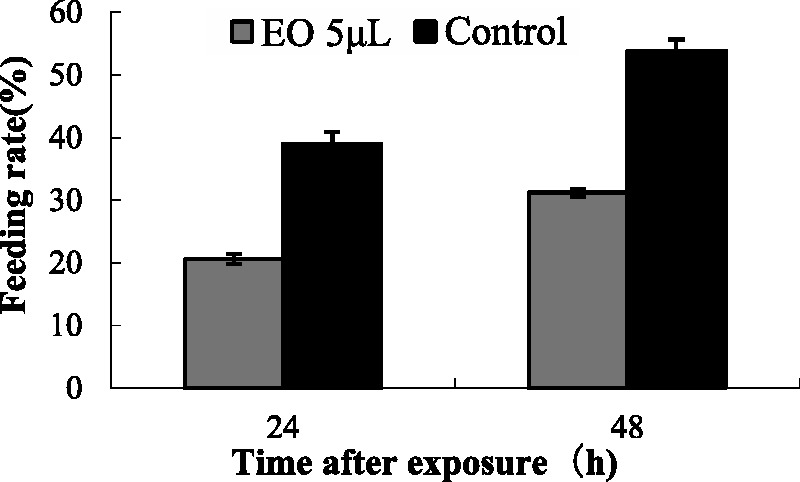
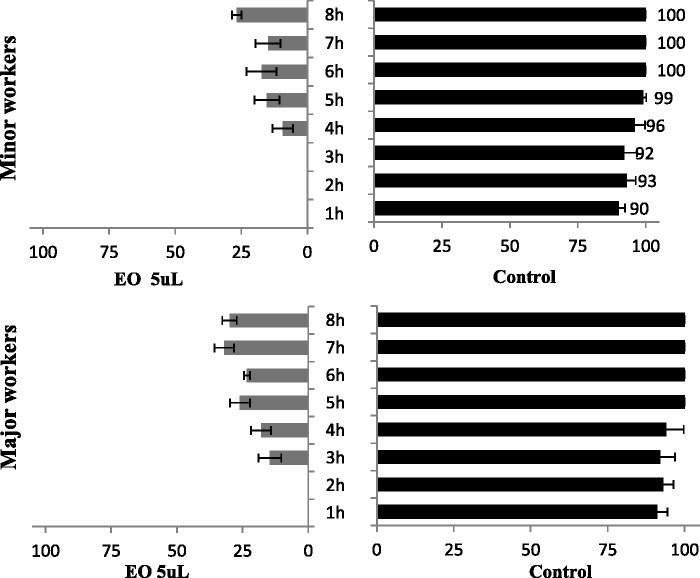
Feeding rate of medium-sized workers of RIFAs on T. molitor treated with camphor EO at 5 µl/T. molitor.
The repellence of camphor EO to RIFAs was presented in Figs. 4 and 6. No significant differences were observed in the percentage of individuals in the Petri dish treatment in terms of exposure time (F = 11.34; P = 0.002) and members of RIFA colonies (F = 11.12; P = 0.012). Camphor EO elicited high repellence for minor and major workers. Approximately 70% of the attack rate of major and minor RIFAs was also reduced. This repellence has also been observed in other insects. As a major component of the EO of aromatic plants, camphor exhibits repellence against Anopheles culicifacies, Culex quinquefasciatus, Anopheles. gambiae, and Anopheles funestus (Ansari and Razdan 1995, Seyoum et al. 2002, 2003). As RIFAs can easily travel in potted plants and vehicles, as well as other places such as nurseries, parks, and truck stops, insect repellent activities of natural substances could be considered as a practical alternative for the control of RIFAs.
Fig. 6.
Repellence of the camphor EO to minor and major workers of RIFAs along the time on T. molitor treated with EO at 5 µl/T. molitor.
Conclusions
The results obtained in this study demonstrated that camphor EO was biologically effective against RIFAs. Fumigation and repellence assay results also showed significant detrimental effects on the ants. Positive results indicated that camphor EO could be potentially used to develop eco-friendly RIFA control products, in which fumigation and repellence application are employed as delivery methods. As an economically viable product, camphor EO is a good alternative for harmful chemical insecticides used to control RIFAs.
Acknowledgments
We acknowledge the technical support provided by Mr. Yuanyuan Yang (South China Agricultural University, China) for GC–MS and GC-FID analyses and Prof. Yongyue Lu (Entomological Key Laboratory of Guangdong Province, China) for the identification of the ants. This research was supported by the Natural pesticide and Chemical Biology, Ministry of Education, China.
References Cited
- Ansari M. A., Razdan R. K. 1995. Relative efficacy of various oils in repelling mosquitoes. Indian J. Malariol. 32: 104–111. [PubMed] [Google Scholar]
- Bekele J., Hassanali A. 2001. Blend effects in the toxicity of the essential oil constituents of Ocimum kilimandscharicum and Ocimum kenyense (Labiateae) on two post-harvest insect pests. Phytochemistry 57: 385–391. [DOI] [PubMed] [Google Scholar]
- Berenbaum M. 1985. Allelochemical interactions in plants. Recent Adv. Phytochem. 19: 139–169. [Google Scholar]
- Boland D. J., Brophy J. J., House A.P.N. 1991. Eucalyptus leaf oils: use, chemistry, distillation and marketing. Inkata Press, Melbourne, Australia. [Google Scholar]
- Brenda D., Carl. D. 2010. Invasive species in the Sonoran desert region. Invaders. (http://www.desertmuseum.org/invaders). [Google Scholar]
- Chen J. 2009. Repellency of an over-the-counter essential oil product in China against workers of red imported fire ants. J. Agric. Food Chem. 57: 618–622. [DOI] [PubMed] [Google Scholar]
- Chen W. Y., Vermaak I., Viljoen A. 2013. Camphor—a fumigant during the black death and a coveted fragrant wood in ancient Egypt and Babylon—a review. Molecules 18: 5434–5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins L., Scheffrahn R. H. 2001. Red imported fire ant, Solenopsis invicta. Entomol. Plant Pathol. 3: 11–48. [Google Scholar]
- Dowell R. V., Gilbert A., Sorenson J. 1997. Red imported fire ant found in California. Calif. Plant Pest Dis. Rep. 16: 50–55. [Google Scholar]
- Drees B. M., Gold R. E. 2003. Development of integrated pest management programs for the red imported fire ant (Hymenoptera: Formicidae). J. Entomol. Sci. 38:170–180. [Google Scholar]
- Dugassa S., Medhin G., Balkew M., Seyoum A., Gebre-Michael T. 2009. Field investigation on the repellent activity of some aromatic plants by traditional means against Anopheles arabiensis and A. pharoensis (Diptera: Culicidae) around Koka, central Ethiopia. Acta Trop. 112: 38–42. [DOI] [PubMed] [Google Scholar]
- Finney D. J. 1971. Probit analysis, 3rd ed Cambridge University Press, London. [Google Scholar]
- Hammer Schmidt F. J., Clark A. M., Soliman F. M., El-Kashoury E. S., Abd El-Kawy M. M., El-Fishawy A. M. 1993. Chemical composition and antimicrobial activity of essential oils of Jasonia candicans and J. montana. Planta Med. 59: 68–70. [DOI] [PubMed] [Google Scholar]
- Juteau F., Masotti V., Bessière J. M., Dherbomez M., Viano J. 2002. Antibacterial and antioxidant activities of Artemisia annua essential oil. Fitoterapia 73: 532–535. [DOI] [PubMed] [Google Scholar]
- Kamdem D. P., Gage D. A. 1995. Chemical composition of essential oil from the root bark of Sassafras albidum. Planta Med. 61: 574–575. [DOI] [PubMed] [Google Scholar]
- Kumar P., Mishra S., Malik A., Satya S. 2012. Compositional analysis and insecticidal activity of Eucalyptus globulus (family: Myrtaceae) essential oil against housefly (Musca domestica). Acta Trop. 122: 212–218. [DOI] [PubMed] [Google Scholar]
- Love J. N., Sammon M., Smereck J. 2003. Are one or two dangerous? Camphor exposure in toddlers. J. Emerg. Med. 27: 49–54. [DOI] [PubMed] [Google Scholar]
- Nerio L. S., Jesus O. V., Stanshenko E. 2010. Repellent activity of essential oils: a review. Bioresour. Technol. 101: 372–378. [DOI] [PubMed] [Google Scholar]
- Phelan W. J. 1976. Camphor poisoning: over-the-counter dangers. Pediatrics 57: 428–431. [PubMed] [Google Scholar]
- Rabl W., Katzgraber F., Steinlechner M., 1997. Camphor ingestion for abortion (case report). Forensic Sci. Int. 89: 137–140. [DOI] [PubMed] [Google Scholar]
- Rozman V., Kalinovic I., Korunic Z. 2006. Toxicity of naturally occurring compounds of Lamiaceae and Lauraceae to three stored-product insects. J. Stored Prod. Res. 43: 349–355. [Google Scholar]
- Seo S. M., Kim J., Lee S. G., Shin C. H., Shin S. C., Park I. K. 2009. Fumigant antitermitic activity of plant essential oils and components from ajowan (Trachyspermum ammi), allspice (Pimenta dioica), caraway (Carumcarvi), dill (Anethum graveolens), geranium (Pelargonium graveolens), and litsea (Litsea cubeba) oils against Japanese termite (Reticulitermes speratus Kolbe). J. Agric. Food. Chem. 57: 6596–6602. [DOI] [PubMed] [Google Scholar]
- Seyoum A., Palsson K., Kung’a S., Kabiru E. W., Killeen G. F., Hassanali A., Knols B. G. 2002. Traditional use of mosquito-repellent plants in western Kenya and their evaluation insemi-field experimental huts against Anopheles gambiae: ethnobotanical studies and application by thermal expulsion and direct burning. Trans. R. Soc. Trop. Med. Hyg. 96: 225–231. [DOI] [PubMed] [Google Scholar]
- Seyoum A., Killeen G. F., Kabiru E. W., Knolls B. G. J., Hassanali A. 2003. Field efficacy of thermally expelled or live potted repellent plants against African malaria vectors in western Kenya . Trop. Med. Int. Health 8: 1005–1011. [DOI] [PubMed] [Google Scholar]
- SPSS. 2012. Statistical product and service solution, system user’s guide, version 17.5, International Business Machine Corporation (IBM). [Google Scholar]
- Tang L., Sun Y. Y., Zhang Q. P. 2013. Fumigant activity of eight plant essential oils against workers of red imported fire ant, Solenopsis invicta. Sociobiology 60:35–40. [Google Scholar]
- Tirillini B., Velasquez E. R., Pellegrino R. 1996. Chemical composition and antimicrobial activity of essential oil of Piper angustifolium. Planta Med. 62: 372–373. [DOI] [PubMed] [Google Scholar]
- Viljoen A., van Vuuren S., Ernst E., Klepser M., Demirci B., Baser H., van Wyk B. 2003. Osmitopsis astericoides (Asteraceae)—the antimicrobial activity and essential oil composition of a Cape-Dutch remedy. J. Ethnopharmacol. 88: 137–143. [DOI] [PubMed] [Google Scholar]
- Zhang R. Z., Li Y. C., Liu N., Porter S. D. 2007. An overview of the red imported fire ant (Hymenoptera: Formicidae) in mainland China. Fla. Entomol. 90: 723–731. [Google Scholar]