Abstract
This study intends to identify biomarkers that could refine the selection of patients with metastatic colorectal cancer (mCRC) for bevacizumab treatment. Pretreatment 36 plasma cytokines and angiogenic factors (CAFs) were first measured by protein microarray analysis in patients who received first-line bevacizumab-containing therapies (discovery cohort, n = 64), and further evaluated by enzyme-linked immunosorbent assay in patients treated on regimens with or without bevacizumab (validation cohort, n = 186). Factor levels were correlated with clinical outcomes, predictive values were assessed using a treatment by marker interaction term in the Cox model. Patients with lower pretreatment levels of hepatocyte growth factor (HGF) or VEGF-A121 gain much more benefit from bevacizumab treatment as measured by progression-free survival (PFS) and overall survival (OS), while angiopoietin-like 4 (ANGPTL4) levels negatively correlated with PFS and response rate following bevacizumab (all adjusted interaction P < 0.05). A baseline CAF signature combining these three markers has greater predictive ability than individual markers. Signature-negative patients showed impaired survival following bevacizumab treatment (PFS, 7.3 vs 7.0 months; hazard ratio [HR] 1.03; OS, 29.9 vs 21.1 months, HR 1.33) compared with signature-positive patients (PFS, 6.5 vs 11.9 months, HR 0.52; OS, 28.0 vs 55.3 months, HR 0.67). These promising results warrant further prospective studies.
Angiogenesis was a critical component in carcinogenesis and tumor growth, and therapies targeting tumor angiogenesis were major steps in the treatment of metastatic colorectal cancer (mCRC)1. Bevacizumab, the vascular endothelial growth factor-A (VEGF-A) monoclonal antibody, was the first proven target agent in the treatment of metastatic colorectal cancer (mCRC) and currently was a standard of care for mCRC patients in combination with first-line chemotherapy2,3,4. Despite the overall success of bevacizumab, clinical efficacy was variable and some individuals seemed to be resistant against it, resulting in rather modest gains in overall survival (OS) under bevacizumab-containing therapies5,6,7. Thus, the identification of predictive biomarkers will be urgently required in achieving the full therapeutic potential of bevacizumab.
Whereas mutations in the K-RAS oncogene well predicted resistance to epidermal growth factor receptor (EGFR) monoclonal antibodies in mCRC treatment8,9,10, equivalent reliable predictors of bevacizumab were currently lacking11,12. Possible baseline predictors of bevacizumab included circulating short VEGF-A isoforms and expression of VEGF receptors (VEGF receptor-1 [VEGFR1] or neuropilin 1 [NRP1]), either in plasma or in tumor tissues13,14. Beyond that, most biomarker studies were limited by the small sample size or the nonrandomized nature. The single-arm study design was unable to distinguish between markers associated with general prognosis for the cytotoxic chemotherapy component of the regimen and markers that predict for benefit afforded by bevacizumab. Furthermore, none of them has been consistently replicated across different studies and cancer types15,16,17,18. To overcome some of the deficiencies of earlier investigations, we simultaneously assessed a series of cytokines and angiogenic factors (CAFs) with a commercially available protein microarray which incorporated various markers known to be associated with the effect of bevacizumab in previous studies, first in pretreatment plasma of patients who received first-line bevacizumab-containing therapies from a single-center registry study, and next in patients treated on a regimen with or without bevacizumab. We also explored a “CAF index” that combined individual markers to better serve as a signature for predicting benefit from bevacizumab.
Methods
Patients and study design
This study has designed two cohorts of patients. The discovery cohort population was developed from data source in a single-center registry study, which was initiated in 2012 January for evaluating the efficacy and safety profile of bevacizumab combined with first-line chemotherapy in Chinese mCRC patients at Sun Yet-sen University Cancer Center. The recorded patients were assigned to treatment with backbone chemotherapies of FOLFIRI19 (45.6%), FOLFOX20 (34.9%), or XELOX21 (19.5%), in combination with bevacizumab 5 mg/kg every 2 weeks (5-FU-based regimens) or 7.5 mg/kg every 3 weeks (capecitabine-based regimens). The standardized data collection form which was adopted after each cycle of treatment, has recorded individual demographic characteristics, co-morbidities, laboratory data, dates and doses of bevacizumab therapy, treatment pattern, clinical effectiveness and adverse events, from the first cycle until completion of bevacizumab.
From the data source, a total of 178 histological proven locally advanced or metastatic CRC patients were screened for study eligibility. Enrolled patients should have received at least 3 cycles of first-line bevacizumab administration, have no prior chemotherapy for metastatic disease and with at least 6 months elapsed from completion of any adjuvant chemotherapy or radiotherapy. Patients were excluded if they had any surgery within one month from the collection of blood sample, brain metastases, or without adequate follow-up. Written informed consent was obtained from each patient regarding the use of plasma for this translational research.
A prespecified limit for hazard ratio of 0.54 was chosen to detect a difference in progression-free survival (PFS) of 6 months (7/13 months) or more, 64 events were required for a one-sided significance level of 10% and a statistical power of 80% (East Software; Cytel, Cambridge, MA). Hence 64 mCRC patients in Sun-Yet Sen University Cancer Center were recruited by systematic sampling from the registry. For the validation cohort, the sample size was chosen to maximize statistical power, thus this validation cohort utilized all patients recorded in the registry study, except for the patients chosen as the discovery cohort (hereinafter referred to as the bevacizumab group); with a random selection of a cross-section of mCRC patients (approximately 1:1) who received a combination treatment of chemotherapy but without bevacizumab at the Sun Yet-sen University Cancer Center by systematic sampling (hereinafter referred to as the control group).
Biomarker assessments and methodology
The pretreatment peripheral venous blood sample were first subjected to analysis with the RayBio® Human Angiogenesis Antibody Array 2 (RayBiotech Inc; Norcross, Georgia, USA) according to the manufacturer’s instructions22. To reduce variation between slices, all plasma samples were screened simultaneously and each unique target factor was assessed in quadruplicate. These factors were given alphabetically in Supplementary Table S1. The protein microarray utilized the sandwich-ELISA design principle. Briefly, samples were incubated with the arrays and then with a cocktail of biotinylated detection antibodies, followed by a streptavidin-conjugated fluor. Signals were visualized using a fluorescence laser scanner and saved digitally. The resulting value was the globally normalized densitometric value (GNDV) of the loci, the averaged GNDV value of the quadruplicates of each factor was referred to hereafter as the mean densitometric value (MDV), and was the value used for final interpretation of data in our analyses (details in supplementary experimental procedure and data analysis).
Promising candidate markers in the CAF profiling were further measured from the validation cohort by commercially available ELISA method per the manufacturers’ directions23. Antibodies against ANGPTL4 and HGF were purchased from R&D (Minneapolis, MN, USA), and anti-VEGF-A121 antibody was from Cusabio (Wuhan, Hubei, China). Samples were allocated onto the arrays using a randomized block design and analyzed in duplicate with no more than one prior freeze-thaw cycle and the mean value was used for final analysis. Out-of-range value was substituted with the median value of that analyte, and measurements below the detection level were conventionally assigned a value equal to half of the detection threshold. On retrospective review of computed tomography imaging, blood samples at the point of best response or disease progression (henceforth denoted as “at response” and “at progression”) were also measured by ELISA test. The study has been carried out in compliance with the Helsinki declaration, the protocol has been approved by the Institutional Review Board (IRB) and Human Ethics Committee of Sun Yet-sen University Cancer Center. Informed consent was obtained from all subjects involved in this study.
Statistical analysis and CAF signature development
The categorical data in this report were summarized by frequencies and percentages, the continuous covariates by median, range, and numbers of observations. Parameters others than survival were compared using the Mann–Whitney test and the Chi-square-test. Correlations between biomarker levels and the following clinical parameters were assessed in current study: OS, defined as time between initiation of first-line treatment and death from any cause; PFS, defined as time between initiation of first-line treatment and disease progression or death; overall response rate (ORR), including partial response (PR) and complete response (CR) per Response Evaluation Criteria in Solid Tumors criteria version 1.1 (RECIST 1.1)
Upon microarray profiling, the CAF analytes were initially explored as continuous variables and subsequently categorized as binary variables (using the median MDV as cut-off). Univariate analysis was performed among CAFs according to their baseline MDVs, by Cox regression for PFS and OS, and by logistic regression for ORR. Because of the exploratory nature of this step, no adjustment for multiple testing was made.
Candidate analytes in the CAF profiling were subsequently subjected to ELISA test to validate the predictive worth. Hazard ratios (HRs) for PFS and OS, and odds ratios (ORs) for ORR were calculated according to baseline analytes levels (dichotomized at the optimal cut-off) and treatment arm. The interaction between treatment effects and analyte concentration was assessed by interaction Wald tests from Cox proportional hazards model (for PFS and OS) and logistic regression models (for ORR) to determine the predictive value. The maximum statistic approach was used to search for appropriate cut-off point. This procedure considers all possible values of the continuous covariate as potential cut-points. The optimal cut-point is the value of the continuous covariate that gives the maximum different degrees of benefit from bevacizumab (i.e. that with the smallest interaction P-value)24,25. Additional multivariate regression models that incorporated treatment arm, biomarker, the interaction between these two, and clinical prognostic variables were also run. We next established a CAF “index” containing these candidate markers with the goal of identifying groups of patients who experienced different degrees of benefit from bevacizumab. Similarly, interaction Cox hazards model was used to test the predictive value of the CAF signature.
The primary end-point was the PFS, with secondary end-point being OS and ORR for all analyses. Kaplan–Meier plots and log-rank tests were applied to illustrate and compare patients’ survival. Pairwise correlations among analytes were evaluated by Spearman’s rank test. To assess the dynamic changes of analytes levels between different time points, parameters were compared by paired-sample t test or Wilcoxon matched-samples rank sum test depending on the normality of the on-treatment changes. All statistical analyses were performed using SPSS 17.0 (SPSS, Chicago, IL, USA) and STATA 12.0 (Stata, College Station, TX, USA).
Result
Patients
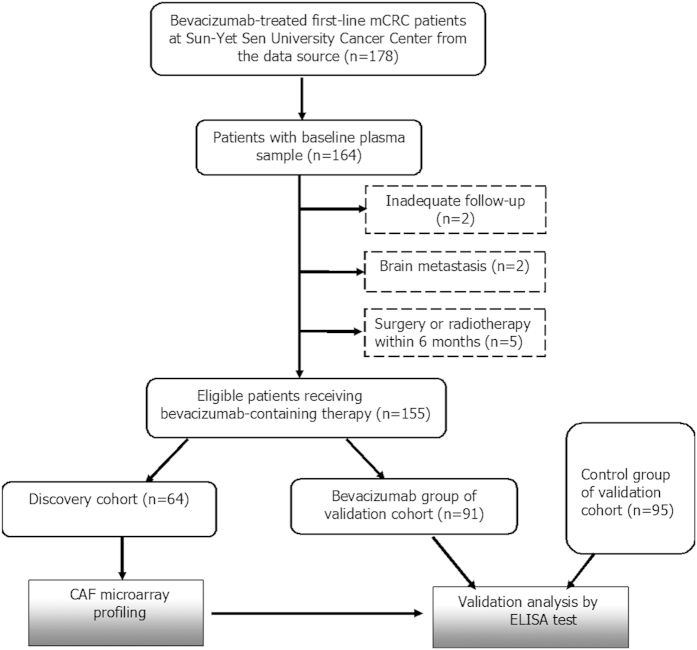
Among the 178 mCRC patients considered for this study from the registry data source, 155 patients were deemed eligible on the basis of inclusion and exclusion criteria from December 2011 to April 2014. Specifically, except for the 64 patients selected as the discovery cohort, the other 91 patients were chosen as the bevacizumab group of validation cohort. In addition, a cross-section of 95 patients treated at Sun Yat-sen University Cancer Center who had a similar chemotherapy but without bevacizumab were chosen as the control group of validation cohort (Fig. 1). Furthermore, 12 and 11 plasma samples acquired from the bevacizumab group were available at the time of tumor response and radiographic progression, respectively.
Figure 1. Study Patient Disposition; CAF, cytokine and angiogenic factor.
As of February 2015, after a median follow-up of 21.5 months (range, 1.5–40.6 months), median PFS, median OS, and ORR were 8.8 months (95% CI: 7.3–10.1 months), 24.6 months (21.7–27.4) and 45.2% in the discovery cohort population. Meanwhile, median PFS and OS were 10.5 months (9.1–11.8) and 26.6 months (20.6–32.5) in the bevacizumab group versus 6.9 months (5.7–8.1) and 24.5 months (20.5–28.4) in the control group, while ORR was 41.4% in bevacizumab-group and 31.0% in the control group.
The demographic, clinical, and pathologic characteristics of patients in the bevacizumab group were similar to those in the control group, as well as to patients incorporated in the discovery cohort (Table 1).
Table 1. Baseline demographics and clinical characteristics across different treatment groups.
| Clinical characteristics N (%) | Discovery Cohort | Validation Cohort |
||
|---|---|---|---|---|
| Bevacizumab group | Control group | P-valuea | ||
| No. of patients | 64 | 91 | 95 | — |
| Age at diagnosis | 52 (26–77) | 53 (21–76) | 55 (26–83) | 0.93 |
| Sex | 0.45 | |||
| male | 35 (56.5) | 59 (60.2) | 56 (63.6) | |
| female | 27 (43.5) | 39 (39.8) | 32 (36.4) | |
| Location of primary tumor | 0.54 | |||
| colon | 42 (67.7) | 62 (68.1) | 60 (63.2) | |
| rectum | 20 (32.3) | 29 (31.9) | 35 (36.8) | |
| ECOG performance status | 0.94 | |||
| 0–1 | 84 (95.5.) | 92 (97.9) | 84 (95.5) | |
| ≥2 | 4 (4.5) | 2 (2.1) | 4 (3.5) | |
| Pathology | 0.73 | |||
| moderately differentiated | 40 (65.6) | 55 (69.6) | 60 (73.2) | |
| poorly differentiated | 21 (34.4) | 24 (30.4) | 22 (26.8) | |
| Matestasis | 0.30 | |||
| single | 31 (50.0) | 46 (50.5) | 56 (58.9) | |
| multiple | 31 (50.0) | 45 (49.5) | 39 (41.1) | |
| Curative-intent matestasis resection | 6 (9.7) | 17 (18.7) | 19 (20.0) | 0.86 |
| Backbone chemotherapy | 0.28 | |||
| oxaliplatin-based | 36 (56.2) | 63 (70.0) | 57 (61.3) | |
| irinotecan-based | 28 (43.8) | 27 (30.0) | 36 (38.7) | |
| Prior adjuvant chemotherapy | 17 (27.4) | 30 (31.6) | 23 (25.8) | 0.42 |
| Recurrent diseaseb | 17 (27.4) | 23 (25.3) | 31 (32.6) | 0.33 |
| First-line duration of CT (median, mos) | 5 (3.0–18.0) | 4.5 (1.5- 25.0) | 3.8 (2.5–24.0) | 0.18 |
| Total duration of CT (median, mos) | 12 (3.0–22.0) | 9.5 (2.0–25.0) | 11.5 (2.5–26.0) | 0.60 |
| Second-line treatment | 23 (39.0) | 17 (23.3) | 14 (15.9) | 0.27 |
| Anti-EGFR treatment following first PD | 3 (4.8) | 22 (24.7) | 31 (32.6) | 0.26 |
| Maintenance treatmentc | 20 (32.3) | 10 (13.3) | 14 (15.6) | 0.83 |
aCompared patient characteristics between the bevacizumab group and the control group.
bPatients who had metastatic disease more than 6 months elapsed from adjuvant chemotherapy.
cMonotherapy of capecitabine or bevacizumab, or combined with both. Abbreviations: ECOG PS, eastern cooperative oncology group performance status; EGFR, epidermal growth factor receptor; CT, chemotherapy; mos, months. Statistical significance was set at 0.05 based on a two-sided test. P-values listed in bold were notable for possible association with clinical outcomes.
Baseline CAF profile
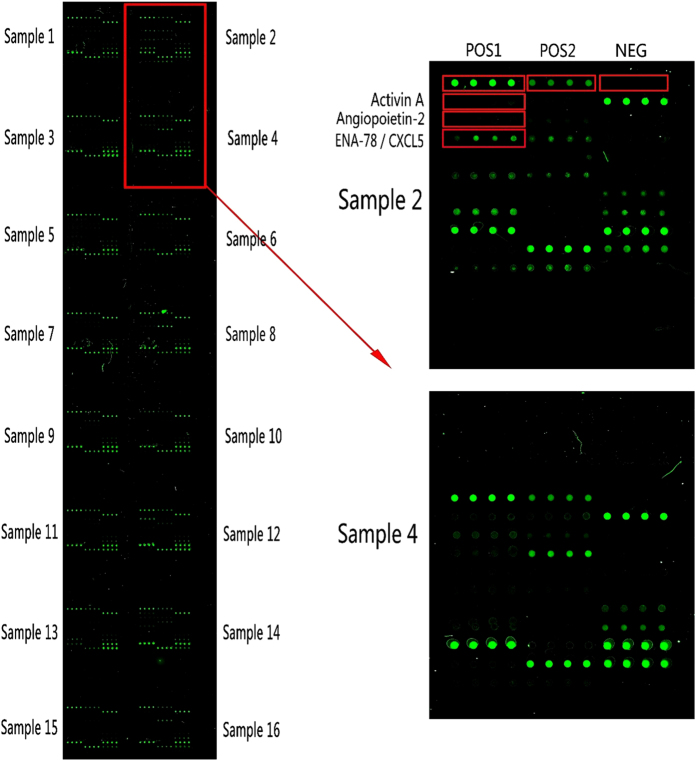
Figure 2 represents one of the microarray slices. The MDVs of bFGF, HB-EGF and TPO were below detection threshold in more than 20% patients and was not further analyzed for clinical parameter relevance.
Figure 2. A typical individual slice of protein microarray, using a panel of 36 cytokines from RayBio® Human Angiogesis Antibody Array 2, the images were captured using a GenePix® 4000A scanner, a total of 8 slices (2 slices for each patient) were processed to generate data for 64 patients.
POS1, positive control 1; POS2, positive control 2; NEG, negative control; ENA-78/CXCL5, neutrophil-activating peptide 78/chemokine (C-X-C motif) ligand 5.
In univariate analysis, PFS was longer in patients with lower MDVs of hepatocyte growth factor (HGF; HR 1.84, 95% CI 1.05–3.24, P = 0.034), VEGF-A121 (HR 1.85, 95% CI 1.11–3.08, P = 0.019), angiopoietin-like 4 (ANGPTL4; HR 1.64, 95% CI 0.97–2.79, P = 0.06) and groucho (GRO; HR 1.64; 95% CI 0.97–2.75, P = 0.077) than patients with high MDVs. While Activin-A (HR 0.47, 95% CI 0.27–0.82, P = 0.019) was inversely correlated with PFS (Table 2 and Fig. S1). OS was remarkably prolonged in patients with low MVD levels of ANGPTL4, HGF and Angioprotein-2 (Ang-2). Lower MDVs of ANGPTL4, HGF, VEGF121 and Ang-2 were correlated with better ORR. Therefore, ANPGLT4, HGF and VEGF-A121 correlated with all assessed outcome parameters when analyzed as both continuous (for each standard deviation change) and discrete variables (median as the cut-off), and were further evaluated by ELISA.
Table 2. CAFs with significant or borderline prognostic values in protein microarray profiling.
| Biomarkers | Progression-Free Survivalb |
Overall Survivalb |
Overall Response Rateb |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Median, mos | HR (95% CI) | P | Median, mos | HR (95% CI) | P | ORR (%) | OR (95% CI) | P | |
| Activin-A | |||||||||
| Low vs Higha | 6.8 vs 10.6 | 0.47 (0.27–0.82) | 0.007 | 21.1 v 31.7 | 0.54 (0.26–1.12) | 0.098 | 41.9 vs 48.4 | 1.30 (0.48–3.54) | 0.610 |
| Each SD change | — | 1.09 (0.77–1.53) | 0.637 | — | 0.74 (0.32–1.69) | 0.473 | — | 0.40 (0.07–2.19) | 0.293 |
| ANGPTL4 | |||||||||
| Low vs Higha | 10.5 vs 6.2 | 1.64 (0.97–2.79) | 0.061 | 32.0 vs 24.6 | 2.45 (1.07–5.61) | 0.034 | 67.2 vs 26.1 | 0.16 (0.05–0.55) | 0.004 |
| Each SD change | — | 1.24 (0.95–1.63) | 0.096 | — | 1.73 (1.22–2.49) | 0.002 | — | 0.13 (0.03–0.66) | 0.014 |
| Ang-2 | |||||||||
| Low vs Higha | 10.6 vs 7.1 | 1.24 (0.73–2.10) | 0.430 | 27.5 vs 20.7 | 1.88 (0.92–3.82) | 0.189 | 56.4 vs 34.8 | 0.30 (0.11–0.86) | 0.025 |
| Each SD change | — | 1.12 (0.84–1.49) | 0.439 | — | 1.40 (0.95–2.07) | 0.089 | — | 0.38 (0.13–1.17) | 0.092 |
| GRO | |||||||||
| Low vs Higha | 10.1 vs 7.1 | 1.64 (0.97- 2.75) | 0.077 | 27.5 vs 18.3 | 1.70 (0.85–3.40) | 0.134 | 58.1 vs 32.3 | 0.54 (0.32–1.17) | 0.073 |
| Each SD change | — | 1.02 (0.82–1.28) | 0.835 | — | 0.85 (0.55–1.34) | 0.490 | — | 1.13 (0.68–1.86) | 0.636 |
| HGF | |||||||||
| Low vs Higha | 12.1 vs 5.7 | 1.84 (1.05- 3.24) | 0.034 | 32.7 vs 19.0 | 2.62 (1.09–5.68) | 0.017 | 61.5 vs 28.6 | 0.25 (0.08–0.78) | 0.017 |
| Each SD change | — | 1.18 (0.96–1.44) | 0.091 | — | 1.34(1.01–1.80) | 0.047 | — | 0.28 (0.10–0.82) | 0.020 |
| VEGF-A121 | |||||||||
| Low vs Higha | 10.6 vs 6.1 | 1.85 (1.11–3.08) | 0.019 | 25.2 vs 20.7 | 1.46 (0.73–2.91) | 0.278 | 64.3 vs 35.7 | 0.34 (0.12–0.97) | 0.044 |
| Each SD change | — | 1.50 (1.16–1.94) | 0.002 | — | 1.17 (0.85–1.59) | 0.337 | — | 0.49 (0.18–1.30) | 0.152 |
aPatients were dichotomized according to the median mean densitometric value (MDV) of each molecule. High indicates above the median MDV; low indicates less than or equal to the MDV.
bCox regression analysis was performed to test different clinical outcomes between the lower and upper median MDV or between each standard deviation (SD) increase in MDV. Abbreviations: ORR, overall response rate; ANGPTL4, angiopoietin-like 4; HGF, hepatocyte growth factor; Ang-2, angiopoietin-2; VEGF-A121, isoform vascular endothelial growth factor-A 121. OR, odds ratio; 95% CI, 95% confidence interval. Statistical significance was set at 0.10 based on a two-sided test. Other footnotes as in Table 1.
Biomarker levels by ELISA test and correlations with clinical characteristics
The lower limits of detection for ANGPTL4, HGF and VEGF-A121 were 0.25 ng/mL, 0.2 ng/mL and 0.1 ng/mL, respectively. Pre-therapeutic plasma concentrations of ANGPTL4 ranged from 0.28 to 140.5 ng/mL (median: 2.4 ng/mL); HGF, 0.26 to 2.51 ng/mL (median: 0.8 ng/mL) and VEGF-A121, 0.12 to 18.5 ng/mL (median: 0.6 ng/mL). Assays were highly reproducible with intra-assay and inter-assay coefficients of variation (CV) for ANGPTL4 being 2.1% and 6.4%; HGF, 1.3% and 4.3%; VEGF-A121, 8.2% and 10.5%.
Weak association (correlation coefficients between 0.2 and 0.4) and very weak association (correlation coefficients between 0 and 0.19) were noted for 2 pairs of analytes: ANGPTL4-VEGF-A121 (0.33, P < 0.001), and HGF-VEGF-A121 (0.19, P = 0.011), respectively. There were no noteworthy correlations between baseline levels of ANGPTL4-HGF (0.01, P = 0.996). Baseline levels of these analytes were unbiased by demographic and clinical characteristics except that VEGF121 levels were slightly higher in patients who had primary tumor resected than those who had not (median, 0.87 v 0.59 ng/L; P = 0.045; Mann-Whitney U test) (Supplementary Table S2).
Predictive value of baseline factors on the benefits with bevacizumab by ELISA test
Table 3 shows the clinical outcomes by treatment separately for groups defined by low and high baseline levels. For disease progression, the HRs were <1 in most subcategories, indicating the superiority of bevacizumab-containing treatment over chemotherapy alone. Therefore, we searched for markers that identified groups of patients who experienced different degrees of benefit from bevacizumab, using cutoff values determined by maximum statistic approach.
Table 3. Correlations between baseline biomarker levels and clinical outcomes by ELISA test.
| Biomarker | Progression-Free Survivala |
Overall Survivala |
Overall Response Ratea |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median (mos) | HR (95% CI) | P | Pb | Median (mos) | HR (95% CI) | P | Pb | Median (%) | OR (95% CI) | P | Pb | |
| All patients | 6.9 v 10.5 | 0.60 (0.44–0.81) | 0.001 | — | 24.5 v 26.6 | 0.80 (0.56–1.15) | 0.230 | — | 31.0 v 41.4 | 1.58 (0.84–2.96) | 0.20 | — |
| ANGPTL4 | ||||||||||||
| Low | 6.5 v 12.2 | 0.58 (0.36–0.94) | 0.023 | 0.054 (0.041) | 37.9 v 27.5 | 0.96 (0.51–1.80) | 0.900 | 0.596 (0.980) | 10.7 v 48.8 | 8.00 (2.09–30.3) | 0.002 | 0.003 |
| High | 7.3 v 9.5 | 0.73 (0.49–1.08) | 0.115 | 25.7 v 28.5 | 0.84 (0.50–1.41) | 0.520 | 41.1 v 34.1 | 0.74 (0.33–1.69) | 0.476 | |||
| HGF | ||||||||||||
| Low | 7.0 v 11.7 | 0.52 (0.35–0.76) | 0.001 | 0.020 (0.039) | 26.0 v 55.3 | 0.42 (0.20–0.89) | 0.020 | 0.010 (0.026) | 28.8 v 49.0 | 2.37 (1.04–5.38) | 0.040 | 0.134 |
| High | 6.3 v 8.5 | 0.79 (0.49–1.27) | 0.323 | 34.5 v 21.1 | 1.19 (0.74–1.90) | 0.473 | 34.4 v 31.6 | 0.88 (0.32–2.40) | 0.804 | |||
| VEGF-A121 | ||||||||||||
| Low | 6.4 v 10.8 | 0.53 (0.36–0.76) | 0.001 | 0.023 (0.028) | 28.0 v 50.0 | 0.42 (0.21–0.84) | 0.011 | 0.034 (0.032) | 28.1 v 39.3 | 1.66 (0.77–3.60) | 0.136 | 0.141 |
| High | 7.8 v 7.8 | 0.90 (0.53–1.53) | 0.70 | 29.9 v 19.6 | 1.25 (0.77–2.02) | 0.370 | 37.0 v 46.2 | 1.46 (0.49–4.37) | 0.583 | |||
aPatients were stratified according to baseline marker levels (using corresponding optimal cutoff), clinical outcomes of control group versus bevacizumab group were compared using Cox proportional hazards model (for PFS and OS), and logistic regression models (for ORR). High indicates above the corresponding cutoff and low indicates less than or equal to the corresponding cutoff. The cutoff values for ANGPTL4, HGF and VEGF121 were 1.97 ng/ml, 0.88 ng/ml and 0.59 ng/ml, respectively.
bP-value for treatment-marker interaction assessed by interaction Wald Cox proportional hazards model (for PFS and OS), and interaction logistic regression models (for ORR). In parentheses, the interaction P-value was adjusted for known clinical prognostic variables (gender, age, performance status, primary tumor site, tumor grade, prior adjuvant chemotherapy, number of metastasis site, and curative-intent metastasis resection). Abbreviations: ELISA, enzyme-linked immunosorbent assay. Statistical significance was set at 0.05 based on a two-sided test. Other footnotes as in Table 1.
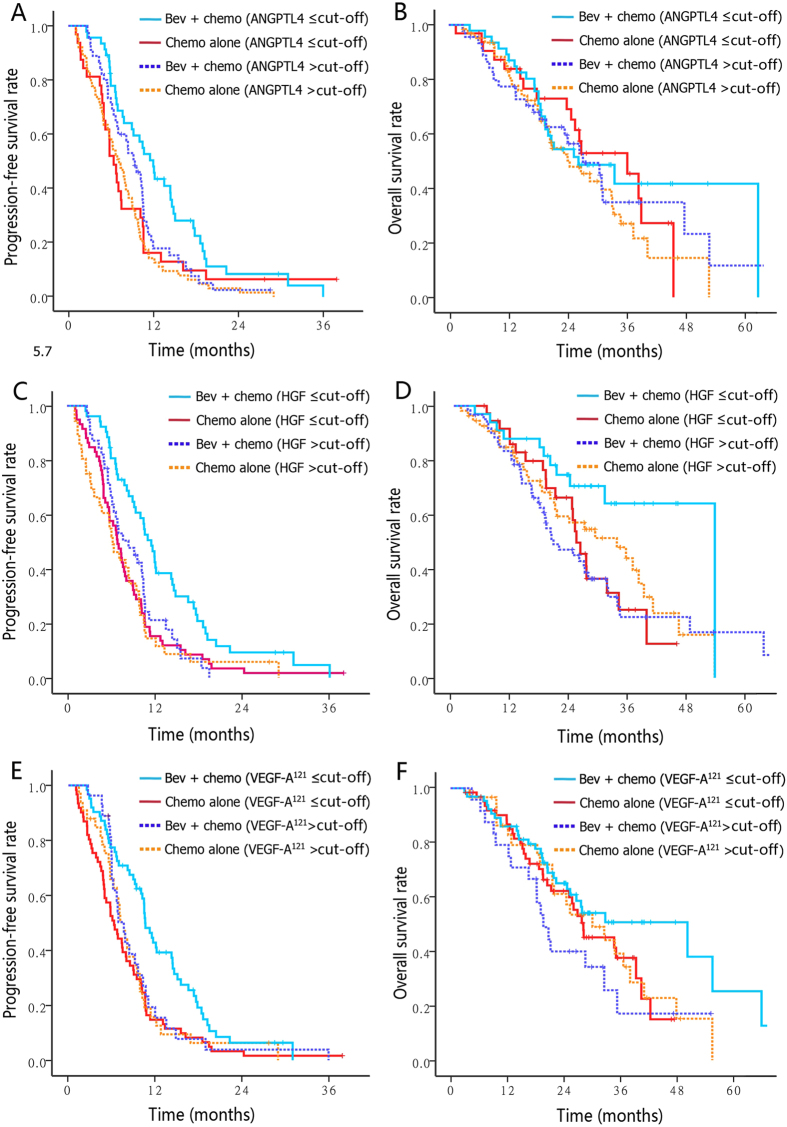
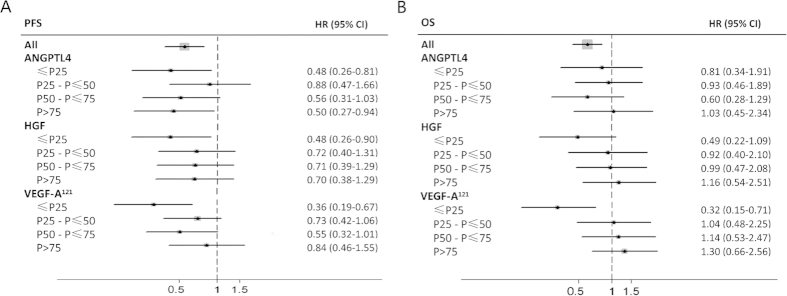
As a result, patients with lower baseline plasma levels of ANGPTL4 (i.e. lower than the median 1.97 ng/ml), HGF (0.88 ng/ml) or VEGF121 (0.59 ng/ml) were more sensitive to bevacizumab treatment as measured by PFS (for ANGPTL4, HR 0.58; 95% CI 0.36–0.94; for HGF, HR 0.52, 95% CI 0.35–0.76; for VEGF121, HR 0.52; 95% CI 0.36–0.76) than those with higher analytes levels (for ANGPTL4, HR 0.73; 95% CI 0.49–1.08, unadjusted P = 0.048 from interaction Cox Wald tests; for HGF, HR 0.79, 95% CI 0.49–1.27, unadjusted interaction P = 0.020; for VEGF121, HR 0.90; 95% CI 0.53–1.53, unadjusted interaction P = 0.023). Similarly, patients with lower baseline HGF or VEGF-A121 levels experienced remarkably larger benefit from bevacizumab in terms of OS than patients with higher analytes levels (for HGF, HR 0.42 versus 1.19, lower versus higher levels, unadjusted interaction P = 0.010; for HGF, HR 0.42 versus 1.25, lower versus higher levels, unadjusted interaction P = 0.034) (Table 3 and Fig. 3). With the addition of bevacizumab, response rates were only increased in patients with lower ANGPTL4 levels (48.8% vs 10.7%) rather than those with higher ANGPTL4 levels (34.1% vs 41.1%; unadjusted interaction P = 0.003). Likewise, patients who had lower HGF or VEGF121 levels also showed a trend toward improved ORR versus patients with higher levels, though the interaction test did not show enough power (Table 3). The P-values for treatment-marker interaction remained significant in multivariate models after adjusted for known clinical prognostic variables (gender, age, performance status, primary tumor site, tumor grade, prior adjuvant chemotherapy, number of metastasis site, and curative-intent metastasis resection) (Table 3). To identify trends that may not have been apparent at the binary split, analytes were further categorized and analyzed by quartile. The forest plots provided a clear trend indicating that the outcomes became poorer as the concentrations of these markers increased, patients with baseline VEGF121 or HGF concentrations in the lowest quartile obtained the most survival benefit from bevacizumab (Fig. 4).
Figure 3. The predictive value of candidate markers for progression-free survival (A, C, E) and overall survival (B, D, F) in ELISA test were presented by Kaplan–Meier curves stratified according to baseline marker levels (using corresponding optimal binary split) and treatment arms.
BEV, bevacizumab; chemo, chemotherapy.
Figure 4. Forest plots of hazard ratios (bevacizumab plus chemotherapy versus chemotherapy alone) for (A) PFS and (B) OS by each biomarker (categorised into quarters).
PFS, progression-free survival; OS, overall survival; HR, hazard ratio 95% CI, 95% confidence interval; ANGPTL4, angiopoietin-like 4; HGF, hepatocyte growth factor; VEGF-A121, isoform vascular endothelial growth factor-A121.
From baseline to the point of disease progression, plasma HGF level was significantly increased (mean concentration, 0.85 ng/mL to 1.15 ng/mL, P = 0.047). Meanwhile, ANGPTL4 increased and VEGF-A121 decreased in 9 of 11 patients, respectively. But the change did not reach statistical significance (3.0 to 8.2 ng/mL, P = 0.15 for ANGPTL4; 0.76 to 0.54 ng/ml, P = 0.07 for VEGF-A121). Nevertheless, none of these factors showed significant changes from baseline to best tumor response (ANGPTL4, 2.9 to 3.9 ng/mL, p = 0.16; HGF, 0.79 to 0.75 ng/mL, P = 0.60; VEGF-A121, 0.60 to 0.39 ng/mL, P = 0.26).
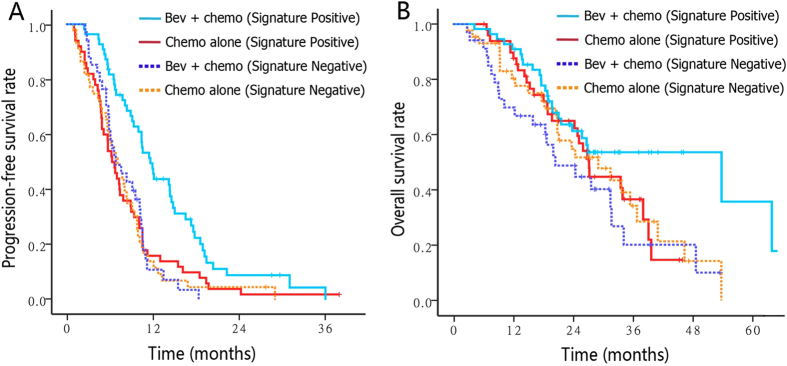
To create a “CAF index” from these candidate markers (ANGPTL4, HGF or VEGF121), a score of +1 was assigned for markers concentrations below the corresponding cut-off or 0 for those above the cut-off. Then the index for each patient was calculated by adding the score for each marker so that the final CAF index ranged from 0 to 3. We selected a CAF index value of 1 as the cut-off because the resulting groups were the most balanced (Half patients with CAF Index = 0 or 1 [signature negative] and half with CAF Index = 2 or 3 [signature positive]). Signature negative individuals showed no improvement in PFS following bevacizumab treatment (7.3 vs 7.0 months; HR, 1.03) compared to signature positive individuals (6.5 vs 11.9 months; HR, 0.52). With the addition of bevacizumab, median OS was only prolonged in patients with positive signature (28.0 vs 55.3 months; HR 0.67), while signature negative patients had an impaired OS following bevacizumab (29.9 vs 21.1; HR 1.33) (Fig. 5). The interaction between the CAF signature and the effect of bevacizumab was highly significant (interaction P = 0.001 for PFS; interaction P = 0.011 for OS).
Figure 5. PFS (A) and OS (B) Kaplan–Meier plots of patients stratified by treatment arm based on three-marker signature index (positive versus negative).
The signature negative indicates CAF Index = 0 or 1; the signature positive indicates CAF Index = 2 or 3. A score of +1 was assigned for candidate marker (ANGPTL4, HGF or VEGF121) concentrations below the corresponding cut-off or 0 for those above the cut-off. Then the score of each marker was added for each patient. Bev, bevacizumab; Chemo, chemotherapy.
Discussion
This study aims to search for circulating biomarkers that could identify patients likely to benefit from bevacizumab. Finally, patients with either lower baseline ANGPTL4, HGF or VEGF-A121 levels experienced the most benefit from addition of bevacizumab to chemotherapy for PFS (42%, 48% and 47% reduction in HRs, respectively). The three-marker CAF index could further refine the selection of patients expected to benefit or lack of benefit from bevacizumab (interaction P = 0.001 for PFS; interaction P = 0.011 for OS).
Factors known to be associated with the effect of bevacizumab in prior studies were either not incorporated in our study (VEGF-A, vascular cell adhesion molecule 1 [VCAM-1], intercellular cell adhesion molecule-1 [ICAM-1], E-selection, matrix metalloproteinase-2 [MMP-2])16,26,27, or failed to predict benefit of bevacizumab (Angiopoietin-2, Insulin-like growth factor 1 [IGF-1], soluble VEGFR1, soluble VEGFR2, heparin-binding epidermal growth factor [HB-EGF], basic fibroblast growth factor [bFGF], IL-8, platelet-derived growth factor beta polypeptide b [PDGF-BB] and placental growth factor [PlGF])27,28,29, although ANG-2 potentially associated with survival (Table 2).
VEGF-A, the target of bevacizumab, was the intuitive candidate predictor for bevacizumab therapy30. Beyond its well-established prognostic value, the use of VEGF-A for antiangiogenic treatment outcome prediction remains uncertain31,32,33. Baseline levels of VEGF-A have fail to predict the effectiveness of bevacizumab in most investigations involving multiple cancer types, and the increased levels of VEGF-A was deemed to be indicator of poor prognosis of mCRC regardless of whether bevacizumab was part of the therapy26,34,35,36,37.
Recent data suggested that a modified ELISA with a preference to detect short VEGF-A isoforms might be more promising in discovering bioactive VEGF-A. These isoforms freely diffused over long distances, thereby provided more specific readout of tumor-secreted VEGF-A38,39. Several trials have revealed that patients with metastatic breast cancer, gastric cancer or pancreatic cancer who expressed high pretreatment levels of VEGF-A121 would exhibit improved PFS and/or OS after bevacizumab treatment, but the results have been inconsistent and failed to replicate in similar research for CRC, NSCLC and RCC16,39,40. In current study, patients with low baseline VEGF-A121 levels could derive more benefit from bevacizumab treatment as measured by PFS and OS relative to patients with high VEGF-A121 levels (Table 3; Figs 3 and 4), largely owing to the more complete blockage of the ligand at lower VEGF-A121 concentrations. These different results across studies suggested that the roles of VEGF-A121 may be context and cancer type-dependent. The phase III MERiDiAN trial which evaluated the impact of bevacizumab in metastatic breast cancer patients stratified for plasma VEGF-A121 will confirm the definitive predictive value of VEGF-A121.
HGF and its receptor, the MET tyrosine kinase, was frequently deregulated in cancer cells and promoted its proliferation, invasion and migration41,42, the expression of the HGF/c-Met in serum or tumor has been reported to play important roles in the progression and prognosis of solid tumors43,44,45. The predictive effect of baseline HGF level under VEGF-targeting therapies has not been implicated in mCRC before. With respect to other VEGFR tyrosine kinase inhibitors (TKIs), low baseline HGF level has been reported to correlate with better clinical outcomes in pazopanib-treated patients with renal cell carcinoma (RCC) or soft-tissue sarcoma28,46. Moreover, HGF has been reported to be a potent angiogenic factor via activated HGF/c-Met pathway and may augment the VEGF expression under hypoxia conditions47,48. On the contrary, VEGF on endothelial cells can lead to increased expression of HGF and subsequent activation of MET on tumor cells, thereby resulting in a paracrine feedback loop between tumor and vasculature49,50. Additionally, recent research has demonstrated that VEGF could negatively regulate HGF/c-MET axis through a MET/VEGFR2 heterocomplex, therefore suppress tumor invasion51. Hence, we hypothesized that in malignant phenotypes with enhanced HGF/c-Met signaling, the therapeutic VEGF blockade could restore and increased MET activity, thus overcame the inhibitory effects of bevacizumab. Above data suggested it might be able to select mCRC patients upfront who may likely to have primary bevacizumab resistance by assessing circulating HGF and VEGF-A121 levels. A subset of patients might benefit from treatment modalities that concurrently block VEGF and HGF/Met signaling51,52.
ANGPTL4 was identified in systemic circulation and share amino acid sequence similarity with the angiopoietin family (ANGs)53. Recent findings have revealed ANGPTL4 as a critical factor in tumor growth and progression in cancers54,55,56,57 and a potential therapeutic target58,59,60. Several new studies shown that ANGPTL4 might exert a VEGF-independent proangiogenic effect, meanwhile, impair VEGF-induced neovascularization59,61,62,63. Thus, we speculated that higher ANGPTL4 expression could represent an alternative tumor angiogenesis route which was resistant to VEGF inhibition, hence lead to a poor therapeutic effect of bevacizumab.
Peak levels at the emergence of radiographic disease progression was seen with HGF and VEGF-A121, indicated that upregulation of VEGF-A121 and HGF may act as a mechanism of adaptive resistance against bevacizumab, and may be used as early markers for disease progression after anti-VEGF therapy. The pairwise correlation between pretreatment VEGF-A121 and HGF levels, and correlation between ANGPTL4 and VEGF121 levels were rather weak. Further investigation may provide insights into the coregulation and counter regulation of these factors and their underlying biology.
Our study first reported the impact of pre-therapeutic ANGPTL4 and HGF concentrations on the benefit of bevacizumab, and the upregulation of VEGF-A121 and HGF may be used as early markers for disease progression after anti-VEGF therapy. Furthermore, combining multiple angiogenic biomarkers to generate predictive signatures is a highly promising strategy. We need to acknowledge the inherent bias within this retrospective study, and the exploratory results from relative small sample size should be interpreted with caution. Ideally, plasma markers offer the ability to carry out continuous assessments over time, are easy to standardize and reproduce and may be more relevant to the metastatic tumor being treated than to the tissue-based measurements in archival primary tumors. Nevertheless, the type of plasma analyzed, the response of non-neoplastic, host tissue to the tumor, and other preanalytic factors, such as the number of freeze–thaw cycles could affect the circulating cytokine levels13,18. Moreover, angiogenesis is a complex and highly adaptive biologic process, the microarray system used in current study only assess part of the CAFs, multiple other factors could also play essential roles during angiogenesis13. A better understanding of the underlying cellular and molecular mechanisms of ANGPTL4, HGF, and VEGF-A121 will reveal the potential therapeutic value of these factors. Above all, these promising results warrants further prospective study to confirm their values in order to customize combined chemotherapeutic and anti-angiogenic treatment.
Additional Information
How to cite this article: Bai, L. et al. A plasma cytokine and angiogenic factor (CAF) analysis for selection of bevacizumab therapy in patients with metastatic colorectal cancer. Sci. Rep. 5, 17717; doi: 10.1038/srep17717 (2015).
Supplementary Material
Acknowledgments
All experiments and analyses were performed at Aleasci Immune. Lab (Aleasci; Guangzhou, Guangdong, China) where laboratory scientists were blinded to clinical outcome and treatment group. LN.F. performed the protein array experiments and enzyme-linked immunosorbent assays with help from WN.X. Shanghai Roche Pharmaceuticals Limited have provided grant support and scientific advice especially from Jun-ming Song and Gang Cheng. We acknowledge the invaluable contributions of the patients who participated in this study, their families, and the Sun Yat-sen University Cancer Center GI Oncology group.
Footnotes
Author Contributions W.F.H., R.H.X. and Y.H.L. conceived of the study and participated in its design and coordination. L.B. and F.W. drafted the manuscript. C.L. and Y.J. collected the plasma samples. M.Z.Q., H.Y.L. and Z.Q.W. performed the statistical analyses and interpretation. D.S.Z., D.S.W. and D.L.C. prepared all figures and Tables. All authors read, edited, and approved the final manuscript.
References
- Ellis L. M. & Hicklin D. J. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer 8, 579 (2008). [DOI] [PubMed] [Google Scholar]
- Strickler J. H. & Hurwitz H. I. Bevacizumab-based therapies in the first-line treatment of metastatic colorectal cancer. Oncologist 17, 513 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Z. Z. et al. Efficacy and safety of bevacizumab plus chemotherapy in Chinese patients with metastatic colorectal cancer: a randomized phase III ARTIST trial. Chin J Cancer 30, 682 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L. et al. Clinical outcomes of Chinese patients with metastatic colorectal cancer receiving first-line bevacizumab-containing treatment. Med Oncol 32, 469 (2015). [DOI] [PubMed] [Google Scholar]
- Macedo L. T., Da C. L. A. & Sasse A. D. Addition of bevacizumab to first-line chemotherapy in advanced colorectal cancer: a systematic review and meta-analysis, with emphasis on chemotherapy subgroups. Bmc Cancer 12, 89 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loupakis F. et al. Magnitude of benefit of the addition of bevacizumab to first-line chemotherapy for metastatic colorectal cancer: meta-analysis of randomized clinical trials. J Exp Clin Cancer Res 29, 58 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch S., Spithoff K., Rumble R. B. & Maroun J. Bevacizumab combined with chemotherapy for patients with advanced colorectal cancer: a systematic review. Ann Oncol 21, 1152 (2010). [DOI] [PubMed] [Google Scholar]
- Fornaro L. et al. FOLFOXIRI in combination with panitumumab as first-line treatment in quadruple wild-type (KRAS, NRAS, HRAS, BRAF) metastatic colorectal cancer patients: a phase II trial by the Gruppo Oncologico Nord Ovest (GONO). Ann Oncol 24, 2062 (2013). [DOI] [PubMed] [Google Scholar]
- De Roock W. et al. Association of KRAS p.G13D mutation with outcome in patients with chemotherapy-refractory metastatic colorectal cancer treated with cetuximab. JAMA 304, 1812 (2010). [DOI] [PubMed] [Google Scholar]
- Wang F. et al. Right- and left-sided colorectal cancers respond differently to cetuximab. Chin J Cancer 34, 24 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H. Y. & Xu R. H. Predictive and prognostic biomarkers with therapeutic targets in advanced colorectal cancer. World J Gastroenterol 20, 3858 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacker S. A. & Achen M. G. The VEGF signaling pathway in cancer: the road ahead. Chin J Cancer 32, 297 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrechts D., Lenz H. J., de Haas S., Carmeliet P. & Scherer S. J. Markers of response for the antiangiogenic agent bevacizumab. J Clin Oncol 31, 1219 (2013). [DOI] [PubMed] [Google Scholar]
- Murukesh N., Dive C. & Jayson G. C. Biomarkers of angiogenesis and their role in the development of VEGF inhibitors. Br J Cancer 102, 8 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugo H. S. Inhibiting angiogenesis in breast cancer: the beginning of the end or the end of the beginning? J Clin Oncol 30, 898 (2012). [DOI] [PubMed] [Google Scholar]
- Van Cutsem E. et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J Clin Oncol 30, 2119 (2012). [DOI] [PubMed] [Google Scholar]
- Jubb A. M. et al. Impact of exploratory biomarkers on the treatment effect of bevacizumab in metastatic breast cancer. Clin Cancer Res 17, 372 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopetz S. “Right drug for the right patient”: hurdles and the path forward in colorectal cancer. Am Soc Clin Oncol Educ Book (2013). doi: 10.1200/EdBook_AM.2013.33.e115. [DOI] [PubMed] [Google Scholar]
- Yukawa N. et al. [Modified FOLFIRI (l-LV, 5-fluorouracil and irinotecan) therapy for Japanese patients with metastatic colorectal cancer]. Gan To Kagaku Ryoho 37, 1291 (2010). [PubMed] [Google Scholar]
- Ajima H. et al. Clinical and economic evaluation of first-line therapy with FOLFIRI or modified FOLFOX6 for metastatic colorectal cancer. Jpn J Clin Oncol 40, 634 (2010). [DOI] [PubMed] [Google Scholar]
- Saltz L. B. et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 26, 2013 (2008). [DOI] [PubMed] [Google Scholar]
- Kocaoemer A., Kern S., Kluter H. & Bieback K. Human AB serum and thrombin-activated platelet-rich plasma are suitable alternatives to fetal calf serum for the expansion of mesenchymal stem cells from adipose tissue. Stem Cells 25, 1270 (2007). [DOI] [PubMed] [Google Scholar]
- Hanrahan E. O. et al. Distinct patterns of cytokine and angiogenic factor modulation and markers of benefit for vandetanib and/or chemotherapy in patients with non-small-cell lung cancer. J Clin Oncol 28, 193 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman D. G., Lausen B., Sauerbrei W. & Schumacher M. Dangers of using “optimal” cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst 86, 829 (1994). [DOI] [PubMed] [Google Scholar]
- Giancarlo R., Scaturro D. & Utro F. Computational cluster validation for microarray data analysis: experimental assessment of Clest, Consensus Clustering, Figure of Merit, Gap Statistics and Model Explorer. Bmc Bioinformatics 9, 462 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde P. S. et al. Predictive impact of circulating vascular endothelial growth factor in four phase III trials evaluating bevacizumab. Clin Cancer Res 19, 929 (2013). [DOI] [PubMed] [Google Scholar]
- Liu Y. et al. Correlation of angiogenic biomarker signatures with clinical outcomes in metastatic colorectal cancer patients receiving capecitabine, oxaliplatin, and bevacizumab. Cancer Med 2, 234 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleijfer S. et al. Cytokine and angiogenic factors associated with efficacy and toxicity of pazopanib in advanced soft-tissue sarcoma: an EORTC-STBSG study. Br J Cancer 107, 639 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati A., Gray R., Sandler A. B., Schiller J. H. & Johnson D. H. Cell adhesion molecules, vascular endothelial growth factor, and basic fibroblast growth factor in patients with non-small cell lung cancer treated with chemotherapy with or without bevacizumab–an Eastern Cooperative Oncology Group Study. Clin Cancer Res 14, 1407 (2008). [DOI] [PubMed] [Google Scholar]
- Jubb A. M. et al. Impact of vascular endothelial growth factor-A expression, thrombospondin-2 expression, and microvessel density on the treatment effect of bevacizumab in metastatic colorectal cancer. J Clin Oncol 24, 217 (2006). [DOI] [PubMed] [Google Scholar]
- Yang J. C. et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 349, 427 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rini B. I. et al. Antitumor activity and biomarker analysis of sunitinib in patients with bevacizumab-refractory metastatic renal cell carcinoma. J Clin Oncol 26, 3743 (2008). [DOI] [PubMed] [Google Scholar]
- Hanrahan E. O. et al. Baseline vascular endothelial growth factor concentration as a potential predictive marker of benefit from vandetanib in non-small cell lung cancer. Clin Cancer Res 15, 3600 (2009). [DOI] [PubMed] [Google Scholar]
- Kopetz S. et al. Phase II trial of infusional fluorouracil, irinotecan, and bevacizumab for metastatic colorectal cancer: efficacy and circulating angiogenic biomarkers associated with therapeutic resistance. J Clin Oncol 28, 453 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D. O. et al. Association between VEGF splice isoforms and progression-free survival in metastatic colorectal cancer patients treated with bevacizumab. Clin Cancer Res 18, 6384 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles D. W. et al. Biomarker results from the AVADO phase 3 trial of first-line bevacizumab plus docetaxel for HER2-negative metastatic breast cancer. Br J Cancer 108, 1052 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider B. P. et al. Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J Clin Oncol 26, 4672 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugo H. S. Inhibiting angiogenesis in breast cancer: the beginning of the end or the end of the beginning? J Clin Oncol 30, 898 (2012). [DOI] [PubMed] [Google Scholar]
- Miles D. W. et al. Biomarker results from the AVADO phase 3 trial of first-line bevacizumab plus docetaxel for HER2-negative metastatic breast cancer. Br J Cancer 108, 1052 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles D. W. et al. Biomarker results from the AVADO phase 3 trial of first-line bevacizumab plus docetaxel for HER2-negative metastatic breast cancer. Br J Cancer 108, 1052 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastaldi S. et al. Met signaling regulates growth, repopulating potential and basal cell-fate commitment of mammary luminal progenitors: implications for basal-like breast cancer. Oncogene 32, 1428 (2013). [DOI] [PubMed] [Google Scholar]
- Rocci A. et al. MET dysregulation is a hallmark of aggressive disease in multiple myeloma patients. Br J Haematol 164, 841 (2014). [DOI] [PubMed] [Google Scholar]
- Canadas I. et al. High circulating hepatocyte growth factor levels associate with epithelial to mesenchymal transition and poor outcome in small cell lung cancer patients. Oncotarget 5, 5246 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hida Y. et al. Clinical significance of hepatocyte growth factor and c-Met expression in extrahepatic biliary tract cancers. Oncol REP 6, 1051 (1999). [DOI] [PubMed] [Google Scholar]
- Noguchi E., Saito N., Kobayashi M. & Kameoka S. Clinical significance of hepatocyte growth factor/c-Met expression in the assessment of gastric cancer progression. Mol Med Rep 11, 3423 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojaei F. et al. HGF/c-Met acts as an alternative angiogenic pathway in sunitinib-resistant tumors. Cancer Res 70, 10090 (2010). [DOI] [PubMed] [Google Scholar]
- Sulpice E. et al. Cross-talk between the VEGF-A and HGF signalling pathways in endothelial cells. Biol Cell 101, 525 (2009). [DOI] [PubMed] [Google Scholar]
- Gerritsen M. E., Tomlinson J. E., Zlot C., Ziman M. & Hwang S. Using gene expression profiling to identify the molecular basis of the synergistic actions of hepatocyte growth factor and vascular endothelial growth factor in human endothelial cells. Br J Pharmacol 140, 595 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgia R. et al. Phase I dose-escalation study of onartuzumab as a single agent and in combination with bevacizumab in patients with advanced solid malignancies. Clin Cancer Res 20, 1666 (2014). [DOI] [PubMed] [Google Scholar]
- Bendell J. C. et al. Treatment rationale and study design for a randomized, double-blind, placebo-controlled phase II study evaluating onartuzumab (MetMAb) in combination with bevacizumab plus mFOLFOX-6 in patients with previously untreated metastatic colorectal cancer. Clin Colorectal Cancer 12, 218 (2013). [DOI] [PubMed] [Google Scholar]
- Lu K. V. et al. VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell 22, 21 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Q. et al. Cabozantinib suppresses tumor growth and metastasis in hepatocellular carcinoma by a dual blockade of VEGFR2 and MET. Clin Cancer Res 20, 2959 (2014). [DOI] [PubMed] [Google Scholar]
- Khong T. L. et al. Identification of the angiogenic gene signature induced by EGF and hypoxia in colorectal cancer. Bmc Cancer 13, 518 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaup A. et al. Angiopoietin-like 4 prevents metastasis through inhibition of vascular permeability and tumor cell motility and invasiveness. Proc Natl Acad Sci USA 103, 18721 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P. et al. Angiopoietin-like 4 protein elevates the prosurvival intracellular O2(-):H2O2 ratio and confers anoikis resistance to tumors. Cancer Cell 19, 401 (2011). [DOI] [PubMed] [Google Scholar]
- Kim S. H. et al. ANGPTL4 induction by prostaglandin E2 under hypoxic conditions promotes colorectal cancer progression. Cancer Res 71, 7010 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hato T., Tabata M. & Oike Y. The role of angiopoietin-like proteins in angiogenesis and metabolism. Trends Cardiovasc Med 18, 6 (2008). [DOI] [PubMed] [Google Scholar]
- Oike Y., Akao M., Kubota Y. & Suda T. Angiopoietin-like proteins: potential new targets for metabolic syndrome therapy. Trends Mol Med 11, 473 (2005). [DOI] [PubMed] [Google Scholar]
- Kim S. H. et al. ANGPTL4 induction by prostaglandin E2 under hypoxic conditions promotes colorectal cancer progression. Cancer Res 71, 7010 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. et al. Hypoxia-inducible factor 1 alpha-activated angiopoietin-like protein 4 contributes to tumor metastasis via vascular cell adhesion molecule-1/integrin beta1 signaling in human hepatocellular carcinoma. Hepatology 54, 910 (2011). [DOI] [PubMed] [Google Scholar]
- Le Jan S. et al. Angiopoietin-like 4 is a proangiogenic factor produced during ischemia and in conventional renal cell carcinoma. Am J Pathol 162 1521 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y. et al. Inhibition of angiogenesis and vascular leakiness by angiopoietin-related protein 4. Cancer Res 63, 6651 (2003). [PubMed] [Google Scholar]
- Urano T. et al. Angiopoietin-related growth factor enhances blood flow via activation of the ERK1/2-eNOS-NO pathway in a mouse hind-limb ischemia model. Arterioscler Thromb Vasc Biol 28, 827 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.