Abstract
Background:
Picralima nitida seed extract (PNE) has aphrodisiac and contraceptive effect.
Aim:
To investigate the effect of PNE on reproductive hormones.
Materials and Methods:
The size and length of the combs of white leghorn day-old chicks treated with testosterone (0.5-1.5 mg/kg), cyproterone (3-30 mg/kg), or PNE (50-500 mg/kg) for 7 days, as well as cyproterone (10, and 30 mg/kg) on PNE-induced, and PNE (50-500 mg/kg) on testosterone-induced comb growth, were measured in the chick comb test. The effect of PNE the percentage change in an oviduct-chick weight ratio of Rhode Island Red layer day-old chicks treated with 17-β-estradiol (0.1-0.9 µg), PNE (30-300 mg/kg) or vehicle, for 6 days, was determined in the chick uterotrophic assay. Liver and kidney function was well lipid, and hematological profile tests were conducted to assess safety.
Results:
7-day treatment with PNE and testosterone increased significantly (P ≤ 0.01-0.001) while cyproterone significantly decreased (P ≤ 0.001) comb growth dose-dependently. Qualitatively, testosterone and PNE treatment resulted in relatively brighter red combs. Cyproterone caused significant inhibition (P ≤ 0.001) of both testosterone and PNE-induced comb growth. Co-administration of testosterone and PNE suppressed comb growth significantly (P ≤ 0.001). Administration of 17-β estradiol and PNE increased (P ≤ 0.001) oviduct-chick weight ratio dose-dependently. No significant changes were observed in assessing liver and kidney function, lipid profile, and hematological parameters.
Conclusion:
PNE exhibits both androgenic (partial testosterone agonist) and estrogenic activity. It has no detrimental effects on the blood, liver, and kidney tissue with prolonged use.
Keywords: Androgenic effect, estrogenic activity, libido, partial agonist, testosterone
INTRODUCTION
Reproductive hormones are chemical substances that regulate the reproductive process which include sexual behavior, mating, gametogenesis, embryonic and fetal development, gestation, parturition, and even lactation [1]. Earlier work by author (in the process of being published) on the assessment an ethanolic seed extract of Picralima nitida ([Stapf] Th. and H. durand) on reproductive and developmental indices arrived at a conclusion that acute administration of P. nitida extract enhances sexual behaviors in both males (aphrodisiac effect) and females possible by affecting reproductive hormones. Its chronic administration in females, however, reduces the chances of fertility, i.e. caused contraception, but had no teratogenic or abortifacient effect during pregnancy. The study also established the fact that in males, enhanced libido associated with an acute intake of the extract diminishes significantly with prolonged usage, with a significant reduction sperm count as well. The study further indicated safety as far as acute usage of the preparation was concerned (no observed adverse effect levels was lower than 1000 mg/kg and lethal dose beyond 2000 mg/kg in mice).
Per the findings, females seeking contraception might find the anti-fertility effects beneficial, but it is a form of reproductive toxicity to others seeking fertility or conception. Males who have reduced sexual abilities, or those who want to further enhance their sexual abilities who continue to take this preparation could develop low sperm count, and this could make them infertile. Caution is, therefore, needed during prolonged use of P. nitida preparations in the treatment of various ailments such as malaria, stomach problems, pneumonia, jaundice, measles, cough, typhoid fever, and gonorrhea [2]. Despite the widespread use of P. nitida traditionally, very limited data on its effects on reproductive hormones is available. What could be the fate of men and women using these for contraception as far as blood, liver and kidneys are concerned? These are some reasons why this study was carried out to assess the effects of the ethanolic seed extract P. nitida on reproductive hormones in both male and females to provide further explanation to earlier observations with regards to androgenic and estrogenic effects and to further ascertain its safety on prolonged usage.
MATERIAL AND METHODS
Duration of Study
This study was commenced in January 2013 and completed in March 2015 during which period experimental data were collected and analyzed.
Plant Materials
Fresh fruits of P. nitida were collected from the KNUST Botanical Gardens in January 2013. Its authenticity was confirmed by Dr. Kofi Annan, Department of Herbal Medicine, FPPS, KNUST, Ghana (Specimen voucher number: KNUST/HM1/2013/S054). The pods were cut open to collect the seed, which were then air dried.
Experimental Animals
In the investigation of androgenic and estrogenic effects of PNE, White leghorn and Rhode Island Red layer day-old chicks, purchased from Akati Farms, Kumasi Ghana were used. For safety assessment, Sprague-Dawley rats (180-220 g) were used. All animals were maintained in the Animal House of the Department of Pharmacology, KNUST, housed in stainless steel cages (34 × 47 × 18 cm) with soft wood shavings as bedding. The chicks were fed ad libitum with chick starter mash and the rats with normal pelleted rat chow all obtained from Agricare Ltd, Kumasi, Ghana. They were kept under normal conditions of humidity (60-75%) and temperature (25 ± 3°C). All animals were handled humanely throughout the experiment as recommended by the Declaration of Helsinki and the Guiding Principles in the Care and Use of Animals.
Preparation of P. nitida Seed Extract (PNE)
The dried seeds of P. nitida were milled into powder. A 2.25 kg quantity of the powder was sequentially extracted with 70% ethanol by cold maceration technique for the two consecutive 72-h periods. The extract obtained was concentrated using a rotary evaporator (Rotavapor R-215, BUCHI Labortechnik AG, Flawil, Switzerland) at 60°C to yield a syrupy mass which was subsequently dried at 40°C, in a hot air oven. The solid mass obtained (279.9 g: Percentage yield 12.44), labeled as PNE, was reconstituted in normal saline for dosing in this study.
Drugs and Chemicals
17-β estradiol (Fortress Diagnostics Limited, UK), testosterone propionate (Jinling Pharmaceutical, China), cyproterone acetate (Bayer, Germany) were used in this study.
Androgenic effect of PNE
Chick comb test
The method described by Dorfman (1969) [3] was used with slight modifications to study the androgenic effect of PNE. Day old white leghorn chicks, after 14 days acclimatization to the experimental laboratory conditions, were randomly assigned to 10 treatment groups (n = 10). The length and height of the combs of each of the chicks in the various groups were measured and recorded. Doses were administered as follows for 7 days. Group 1, the control group, was treated orally with distilled water (vehicle), Groups 2-4 were treated intramuscularly with 0.5, 1.0, and 1.5 mg/kg testosterone propionate, respectively, Groups 5-7: Received orally 3, 10, and 30 mg/kg cyproterone acetate, respectively, whiles Groups 8-10 received, orally, 50, 100, 500 mg/kg PNE, respectively. The length and height of the combs of each chick were measured and recorded 24 h after the last drug administration.
Effect of cyproterone on testosterone and PNE-induced comb growth
To estimate the effects of cyproterone on PNE, 20 white leghorn chicks were randomly divided into four groups (n = 5) and treated as follows; chicks in Group 1 were treated with only testosterone (0.6 mg/kg; i.m) while Group 2 received testosterone (0.6 mg/kg; i.m) and cyproterone (10 mg/kg; p.o). Group 3 was treated with PNE (30 mg/kg; p.o) and Group 4 both cyproterone (10 mg/kg; p.o) and PNE (30 mg/kg; p.o). Doses of testosterone and PNE used in this study were estimated from the ED50 values from the chick comb test. 24 h after the last administration, change in comb growth (length and height) were measured.
Effect of PNE on testosterone-induced comb growth
To evaluate the effects of PNE on testosterone, 20 single comb white leghorn chicks were grouped into four (n = 5) and treated as follows; chicks in Group 1 were administered testosterone (0.6 mg/kg; i.m). Group 2 was administered testosterone (0.6 mg/kg; i.m) and PNE (50 mg/kg; p.o). Group 3 was administered testosterone (0.6 mg/kg; i.m) and PNE (100 mg/kg; p.o) while Group 4 received testosterone (0.6 mg/kg; i.m) and PNE (500 mg/kg). 24 h after the last administration, change in comb growth (length and height) were measured.
Estrogenic Effect of PNE
Chick uterotrophic assay
This test is based on the principle that elevated levels of natural estrogens and phytoestrogens in female animals during the early stages of development, dose dependently, increases the uterine/body weight ratio [3-5]. Day old Rhode Island Red layer chicks were randomly assigned to seven groups (n = 6). Groups 1-3 were treated subcutaneously with 0.1, 0.3, or 0.9 µg of 17-β-estradiol twice daily. Groups 4-6 were treated orally with 30, 100, and 300 mg/kg of PNE, respectively. Group 7, the control group, was treated subcutaneously with 0.2 % v/v corn oil (vehicle) control. Dosing was done 12 h for 6 continuous days. During treatments, chicks were weighed every other day before feeding. On the 6thday of treatment, the chicks were weighed, euthanized with ether, dissected, and the oviduct was isolated; whiles carefully removing any attached connective tissue. The oviduct was immediately weighed. Weights were then normalized with the final body weight of the chick and expressed as a percentage using the formulas below.
Percentage oviduct-chick weight ratio = (oviduct weight [g] ÷ chick weight [g]) × 100
The percentage change in weight was then plotted against the log of the concentration of the various treatments to obtain log concentration-response curves.
Safety Assessment
Four groups of male Sprague-Dawley rats (n = 10) were used with Group I receiving distilled water only whiles Groups II-IV received 30, 100, and 300 mg/kg of PNE, respectively, for 14 days. On the last day of administration, blood samples obtained from the jugular vein were collected into tubes containing gel and clot activator (Channel MED, China) and centrifuged at 3,000 × g for 5 min, to obtain plasma for liver and kidney function and the lipid profile tests using the Vital Scientific Flexor Junior Chemistry Analyzer. Blood samples were also collected into ethylenediaminetetraacetic acid tubes for hematological analyzes using the Sysmex KX 21NTM Automated Hematoanalyzer.
Ethical Considerations
This study was conducted at the Department of Pharmacology, KNUST in compliance with: OECD Principles of Good Laboratory Practices ENV/MC/CHEM (98)17, EEC Good Laboratory Practices (90/18/EEC) and FDA Good Laboratory Practice Standards (Part 58 of 21 CFR). All experiments were approved by The Committee on Animal Research, Publication and Ethics (CARPE) with ethics reference number FPPS/PCOL/0017/2012.
Data Analysis
GraphPad Prism for Windows Version 5 (GraphPad Software, San Diego, USA) was used for all statistical analysis. Data were analyzed using one-way Analysis of Variance followed by Newman-Keuls post-hoc test for comparison between control and treatment groups. P ≤ 0.05 was taken to be statistically significant.
RESULTS
Chick Comb Test
7 days treatment with PNE (30-300 mg/kg) and testosterone (0.5-1.5 mg/kg) increased significantly (P ≤ 0.01-0.001), while cyproterone acetate significantly decreased (P ≤ 0.001) comb growth (size and length) in a dose-dependent manner; compared to the vehicle treated chicks [Figure 1]. The qualitative assessment revealed that chicks treated with testosterone (1.0, and 1.5 mg/kg) and all doses of PNE had relatively brighter red combs and wattle [Figure 2], and well-developed feathers. Using the comb length as the response, the ED50 of testosterone and PNE was estimated to be 0.6, and 27.37 mg/kg, respectively. The slope rate of growth was steep at low doses (50-100) mg/kg but very gentle at high doses (100-500) mg/kg. PNE exhibited partial agonist-like activity in the study [Figure 3]. Cyproterone acetate inhibited comb growth at all dose levels with the highest inhibition was observed at 30 mg/kg.
Figure 1.

Effect of Picralima nitida seed extract [PNE] (50-500 mg/kg), Testosterone [Test] (0.5-1.5 mg/kg), and Cyproterone acetate [Cyp] (3-30 mg/kg) on chick comb growth. Data expressed as mean± standard error of mean, n=10. Significant different from control: **P ≤ 0.01; ***P ≤ 0.001 (one way Analysis of Variance followed by Newman keuls post hoc test)
Figure 2.

Representative images of chick combs after 7 days of treatment with Picralima nitida seed extract (PNE) (50-500 mg/kg), testosterone (0.5-1.5 mg/kg), and cyproterone acetate (3-30 mg/ kg). Note the comb growth (and wattle) with associated with testosterone and PNE treatment; and the comb-growth inhibition with cyproterone treatment
Figure 3.

Log-dose-response of Picralima nitida seed extract (50-500 mg/kg), testosterone (0.5-1.5 mg/kg), and cyproterone acetate (3-30 mg/kg) on chick comb growth. Values plotted are mean ± standard error of mean, n = 10
Effect of Cyproterone on Testosterone and PNE-Induced Comb Growth
Cyproterone caused a significant inhibition of both testosterone (72.84 ± 5.39%; P ≤ 0.001) and PNE (81.57 ± 9.00%; P ≤ 0.001) induced comb growth. The appearance of wattle was also inhibited until the last day of treatment [Figure 4].
Figure 4.

Effects of cyproterone [Cyp] (10 mg/kg) pre-treatment on testosterone propionate [TST] (0.6 mg/kg) and Picralima nitida seed extract [PNE] (30 mg/kg)-induced comb growth. Values plotted are mean ± standard error of mean, n=5. *** P ≤ 0.001 (one-way Analysis of Variancefollowed by Newman-Kuels post-hoc test)
Effect of PNE on Testosterone-Induced Comb Growth
Co-administration of testosterone and PNE suppressed comb growth significantly (70.05 ± 6.182%, P ≤ 0.001). All treated chicks developed light pink combs and wattles [Figure 5].
Figure 5.
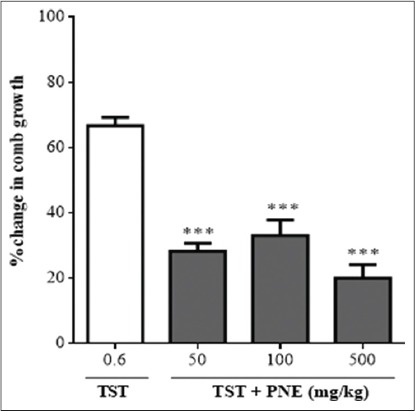
Effects of Picralima nitida seed extract [PNE] (50-500 mg/kg) on testosterone propionate [TST] (0.6 mg/kg)-induced comb growth. Values plotted are mean ± standard error of mean, n=5. *** P ≤ 0.001 (one-way Analysis of Variance followed by Newman-Kuels post-hoc test)
Chick Oviduct Test
A 6-day continuous administration of 17-β estradiol and PNE increased dose-dependently (P ≤ 0.001) the percentage oviduct-chick weight ratio [Figures 6 and 7]. The efficacy exhibited by both treatments were not significantly different, but 17-β estradiol was more potent at increasing oviduct weight compared to PNE as indicated in the estimated ED50’s (Estradiol: 0.25 µg, PNE: 1.5 mg/kg) [Figure 8]. However, no significant differences in body weights were observed [Figure 9].
Figure 6.

Effect of Picralima nitida seed extract [PNE] (30-300 mg/kg), and (b) 17- ß estradiol [E2] (0.1-0.9 µg) on chick uterine to body weight ratio. Values plotted are mean ± standard error of mean, n=5. Significant difference from control: ** P < 0.01; *** P < 0.001 (one-way Analysis of Variance followed by Newman-Keuls post hoc test)
Figure 7.

Dose-response curves of Picralima nitida seed extract [PNE] (30-300 mg/kg) and 17- ß oestradiol [E2] (0.1-0.9 9 µg) with respect to percentage change in uterine to body weight in the chick uterotrophic assay. Each point represents the mean ± standard error of mean, n=5
Figure 8.

Time-course curve of the effects of (a) Picralima nitida seed extract [ PNE] (30-300 mg/kg), and (b) 17- ß oestradiol [E2] (0.1-0.9 µg) on weight change. Values plotted are mean ± standard error of mean, n=6
Figure 9.

Effects of Picralima nitida seed extract [PNE], and 17- ß oestradiol [E2] on body weight changes in chick uterotrophic assay. Area under the curve (AUC) values plotted are mean ± standard error of mean, n=6. The lower and upper margins of the boxes represent 25thand 75thpercentiles with the extended arms representing the 10thand 90thpercentiles respectively. The median line is shown as the horizontal line within the box
Safety Assessment
No significant changes were observed in blood biochemical and hematological parameters when compared to control. All hematological parameters were in normal ranges in the control and tested animals. Both red blood cells (RBC) and white blood cells (WBC) were increased, but insignificant compared to control. At a lower dose of 30 mg/kg, cholesterol and triglyceride levels were slightly increased but levels decreased with higher doses. Aspartate transaminase (AST) and alanine transaminase (ALT) levels, although slightly increased were within the acceptable range. Blood urea and creatinine were also not significantly affected [Table 1].
Table 1.
Effect of Picralima nitida on some hematological and biochemical parameters in Sprague-Dawley rats

DISCUSSION
P. nitida is used extensively across countries in Africa for the treatment of an appreciable number of ailments [6]. In view of the fact that among the population using the plant product is males and females of childbearing age, this study aimed at investigating the effect of the plant on reproductive hormones and also its safety for use. Earlier work by authors and also by other workers has demonstrated adverse effects of the PNE on male reproduction [7]; and its contraceptive capability in females on chronic usage. The effect of the PNE on male chick comb growth and female chick oviduct were assessed.
Comb growth in male chicks is highly androgen dependant. These chicks demonstrate an exaggerated response to induction or elevation of androgens (particularly testosterone) in their system by the expression of male secondary sex characteristics as revealed by changes in the comb, wattle, and ear lobe [8]. This makes the chick comb growth model ideal for screening androgens. Testosterone’s androgenic effects have been attributed to its principal metabolite, 5a-dihydrotestosterone, known to have a fivefold affinity for the androgen receptor than testosterone in both mammals and Aves [8-10]. PNE activity was comparable to testosterone. Cyproterone’s ability to block the androgenic activity of PNE indicates that PNE acts by directly or indirectly stimulating the avian androgen receptor.
The ability of PNE to stimulate comb growth may corroborate with our earlier observations of enhanced mating and mounting behaviors in treated rodents as well as its purported traditional usage as an aphrodisiac [6,11]. In general, there is an association between sexual behavior in male (and in females) and elevated serum testosterone levels [12]. Indeed, in conditions of diminished libido such as in hypogonadism, or menopause, there is enhanced sexual desire on testosterone administration [13]. In our previous study, we reported that PNE affects mating behaviors in rats. Subsequently in this study, we have demonstrated that PNE affects reproductive hormones principally testosterone and estradiol. Aphrodisiacs, according to Sandroni’s (2001) [14], can be classified based on their ability to either increase libido sexual pleasure or potency. Because PNE alters the levels or activity of specific sex hormones, it can be said to be an aphrodisiac that increases libido.
Co-administration of testosterone and PNE, however, suppressed comb growth significantly suggesting that PNE could be a partial agonist on androgen receptors, characteristic of dose-response curves of testosterone and PNE obtained [Figure 1]. The partial agonist activity observed in this study may explain why the subacute use of PNE led to a reversal of the acute aphrodisiac effects as well as alterations in male the reproductive parameters such as sperm count. Estrogens have been shown to block the activation of the androgen receptors by testosterone [15]. PNE demonstrates significant estrogenic activity. The partial agonist activity exhibited by PNE could be due to antagonism by estrogenic activity or the presence of estrogenic elements in PNE.
Androgens are known for their ability to stimulate erythropoeisis and anemia is associated with androgen deprivation [16]. In all the hematological assessments, PNE increased levels (although not statistically significant) of hemoglobin, hematocrit, mean corpuscular hemoglobin (MCH), MCH concentration, as well as RBC. This could possibly account for the reddening of the combs.
In previous studies, PNE prolongs estrous phase in rats in vivo and possessed uterotonic effects in vitro [17]. Using the chick uterotrophic assay, we have demonstrated that PNE has uterotrophic effects which were comparable to estradiol. The chick uterotrophic assay is based on the principle that elevated levels of natural estrogens and phytoestrogens in female animals during the early stages of development, dose-dependently, increase the uterine/body weight ratio [3-5]. The uterus responds to estrogens in two-ways. An initial response would be an increase in weight due to water imbibition. This response is followed by a weight gain due to tissue growth. The results of these studies indicated that PNE either directly or indirectly enhance the activity of estradiol and or gonadotrophins, luteinizing (LH), and follicle-stimulating hormone.
Exhibiting androgenic and estrogenic activity for an extract is not unusual because of the presence of a myriad of components; whereas some components may contribute to the overall androgenic effect, others may have inhibitory effects. The phenolic components of the crude soy extract, for example, have been demonstrated to have an affinity for both estrogen and androgen receptors - although the components have a relatively lower affinity than the cognate agonist [18].
The seed extract of P. nitida has been shown to possess alkaloids that have opioid binding activity [19,20]. Opioids could modulate gonadotrophin, LH release and LH regulates testosterone and estrogen secretion by the gonads. Actions of opioids on the endocrine system are largely mediated through the Mu (µ) receptors [21,22]. Interestingly, more than five alkaloids isolated from PNE, have Mu receptor binding affinity [20]. Subsequently by influencing LH levels by PNE can possess estrogenic and androgenic effects. Indeed, chronic opioid administration tends to decrease serum testosterone and LH [23] which may explain why enhanced libido with acute administration dwindles with chronic use.
Safety assessment on PNE indicated its safety for use as far as its effect on blood; the liver and kidneys were concerned. Administration of herbal products has the propensity to cause significant changes in the structure, function, metabolic transformations, and concentration of biomolecules and enzymes. Such changes may lead to pathological and/or clinical effects [24]. Assessment of hematological parameters helps to determine the damaging effect of xenobiotics on blood. The non-significant increase in WBC number may probably be due to normal immune responses to foreign bodies. Furthermore, the insignificant changes in RBC count, hemoglobin and hematocrit suggest that PNE is unlikely to cause anemia. It could be that the extract has the potential to stimulate erythropoietin release in the kidney, which is the humoral regulator of red blood cell formation. This confirms previous studies on the hematological effects of P. nitida saponin extracts [25].
There were no significant changes in levels of cholesterol, triglycerides (TAG), low-density lipoprotein (LDL), and high-density lipoprotein (HDL), to be attributed to the drug treatments. Cholesterol levels could decrease during the treatment period because there is a possibility of drugs to causes general damage, blockage of an enzyme system for steroidogenesis in the ovary and the capacity of the liver to store cholesterol due to general damage [26]. Elevation of cholesterol, TAG, and LDL, and a decrease in HDL would increase the risk of cardiovascular disorders [27-29].
In the liver function test, ALT, alkaline phosphates (ALP), and total proteins (albumin and globulins) did not change significantly, while AST was slightly elevated than those in the control group. ALT and AST are liver associated enzymes that are indirect measures of liver homeostasis [30]. Hepatocellular injury leading to the permeability of intracellular enzymes into the bloodstream is accompanied by elevated ALT and AST [31]. AST is also present in red cells, cardiac, and skeletal muscles, therefore, not specific to the liver [32,33]. Thus, the increment in AST observed cannot be attributed to hepatocellular damage as it is also associated with other tissues. Thus, PNE is not hepatotoxic at the dose levels used. Increased in serum ALP is associated with liver disease caused by intra or extra hepatic cholestatis and some destruction of the hepatic cell membrane, as well as extrahepatic and intra hepatic bile duct obstruction [34]. The kidneys were also not affected as control blood urea nitrogen (BUN), and creatinine levels did not change significantly with treatments. BUN and creatinine are used to evaluate kidney function; to help diagnose kidney disease, and to monitor acute or chronic kidney dysfunction or failure. Elevated of these in blood suggests impaired kidney function which could be acute or chronic kidney disease, damage, or failure [35].
CONCLUSION
The ethanolic seed extract of P. nitida exhibits both androgenic (partial testosterone agonist) and estrogenic activity. It has no detrimental effects on the blood, liver, and kidney tissue with prolonged use.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Tortora GJ, Derrickson BH. Principles of Anatomy and Physiology. 13th ed. New Jersey: John Wiley and Sons; 2011. p. 1344. [Google Scholar]
- 2.Erharuyi O, Falodun A, Langer P. Medicinal uses, phytochemistry and pharmacology of Picralima nitida (Apocynaceae) in tropical diseases: A review. Asian Pac J Trop Med. 2014;7:1–8. doi: 10.1016/S1995-7645(13)60182-0. [DOI] [PubMed] [Google Scholar]
- 3.Dorfman RI. Methods in Hormone Research: Bioassay. 2A. New York: Academic Press; 1969. [Google Scholar]
- 4.Tullner WW, Hertz R. The effect of 17-alpha-hydroxy-11-desoxycorticosterone on estrogen-stimulated chick oviduct growth. Endocrinology. 1956;58:282–3. doi: 10.1210/endo-58-2-282. [DOI] [PubMed] [Google Scholar]
- 5.Lerner LJ, Holthaus FJ, Jr, Thompson CR. A non-steroidal estrogen antiagonist 1-(p-2-diethylaminoethoxyphenyl)-1-phenyl-2-p-methoxyphenyl ethanol. Endocrinology. 1958;63:295–318. doi: 10.1210/endo-63-3-295. [DOI] [PubMed] [Google Scholar]
- 6.Ayensu ES. Medicinal Plants of West Africa. 1st ed. Michigan: Reference Publications Inc; 1978. p. 330. [Google Scholar]
- 7.Solomon IP, Ekandem GJ, Oyebadejo SA, Okon EA. Chronic oral consumption of ethanolic extract of Picralima nitida (Akuamma) seed induced histopathological changes on the testes of adult wistar rats. Int J Pharm Res Allied Sci. 2014;3:71–7. [Google Scholar]
- 8.Dubé JY, Tremblay RR. Androgen binding proteins in cock’s tissues: Properties of ear lobe protein and determination of binding sites in head appendages and other tissues. Endocrinology. 1974;95:1105–12. doi: 10.1210/endo-95-4-1105. [DOI] [PubMed] [Google Scholar]
- 9.Dubé JY, Tremblay RR, Lesage R, Verret G. In vivo uptake and metabolism of testosterone by the head appendages of the cock. Mol Cell Endocrinol. 1975;2:213–20. doi: 10.1016/0303-7207(75)90007-6. [DOI] [PubMed] [Google Scholar]
- 10.Saartok T, Dahlberg E, Gustafsson JA. Relative binding affinity of anabolic-androgenic steroids: Comparison of the binding to the androgen receptors in skeletal muscle and in prostate, as well as to sex hormone-binding globulin. Endocrinology. 1984;114:2100–6. doi: 10.1210/endo-114-6-2100. [DOI] [PubMed] [Google Scholar]
- 11.Adjanohoun EJ, Aboubakar N, Dramane K, Ebot ME, Ekpere JA, et al. Traditional Medicine and Pharmacopoeia: Contribution to Ethnobotanical and Floristic Studies in Cameroon. 1st ed. Porto-Novo (Benin): STRC/OUA; 1996. p. 641. [Google Scholar]
- 12.Davis SR, Tran J. Testosterone influences libido and well-being in women. Trends Endocrinol Metab. 2001;12:33–7. doi: 10.1016/s1043-2760(00)00333-7. [DOI] [PubMed] [Google Scholar]
- 13.Hellstrom WJ, Paduch D, Donatucci CF. Importance of hypogonadism and testosterone replacement therapy in current urologic practice: A review. Int Urol Nephrol. 2012;44:61–70. doi: 10.1007/s11255-010-9879-4. [DOI] [PubMed] [Google Scholar]
- 14.Sandroni P. Aphrodisiacs past and present: A historical review. Clin Auton Res. 2001;11:303–7. doi: 10.1007/BF02332975. [DOI] [PubMed] [Google Scholar]
- 15.Kemppainen JA, Langley E, Wong CI, Bobseine K, Kelce WR, Wilson EM. Distinguishing androgen receptor agonists and antagonists: Distinct mechanisms of activation by medroxyprogesterone acetate and dihydrotestosterone. Mol Endocrinol. 1999;13:440–54. doi: 10.1210/mend.13.3.0255. [DOI] [PubMed] [Google Scholar]
- 16.Strum SB, McDermed JE, Scholz MC, Johnson H, Tisman G. Anaemia associated with androgen deprivation in patients with prostate cancer receiving combined hormone blockade. Br J Urol. 1997;79:933–41. doi: 10.1046/j.1464-410x.1997.00234.x. [DOI] [PubMed] [Google Scholar]
- 17.Mbegbu EC, Omoja VU, Ekere OS, Okoye CN, Uchendu CN. Effects of ethanolic fruit extract of Picralima nitida (Stapf) on fertility of pregnant rats. Comp Clin Pathol. 2014;24:269–73. [Google Scholar]
- 18.Beck V, Unterrieder E, Krenn L, Kubelka W, Jungbauer A. Comparison of hormonal activity (estrogen, androgen and progestin) of standardized plant extracts for large scale use in hormone replacement therapy. J Steroid Biochem Mol Biol. 2003;84:259–68. doi: 10.1016/s0960-0760(03)00034-7. [DOI] [PubMed] [Google Scholar]
- 19.Duwiejua M, Woode E, Obiri DD. Pseudo-akuammigine, an alkaloid from Picralima nitida seeds, has anti-inflammatory and analgesic actions in rats. J Ethnopharmacol. 2002;81:73–9. doi: 10.1016/s0378-8741(02)00058-2. [DOI] [PubMed] [Google Scholar]
- 20.Menzies JR, Paterson SJ, Duwiejua M, Corbett AD. Opioid activity of alkaloids extracted from Picralima nitida (fam Apocynaceae) Eur J Pharmacol. 1998;29(350):101–8. doi: 10.1016/s0014-2999(98)00232-5. [DOI] [PubMed] [Google Scholar]
- 21.Maggi R, Dondi D, Rovati GE, Martini L, Piva F, Limonta P. Binding characteristics of hypothalamic mu opioid receptors throughout the estrous cycle in the rat. Neuroendocrinology. 1993;58:366–72. doi: 10.1159/000126564. [DOI] [PubMed] [Google Scholar]
- 22.Gabriel SM, Simpkins JW, Kalra SP. Modulation of endogenous opioid influence on luteinizing hormone secretion by progesterone and estrogen. Endocrinology. 1983;113:1806–11. doi: 10.1210/endo-113-5-1806. [DOI] [PubMed] [Google Scholar]
- 23.Yilmaz B, Konar V, Kutlu S, Sandal S, Canpolat S, Gezen MR, et al. Influence of chronic morphine exposure on serum LH, FSH, testosterone levels, and body and testicular weights in the developing male rat. Arch Androl. 1999;43:189–96. doi: 10.1080/014850199262481. [DOI] [PubMed] [Google Scholar]
- 24.Murray RK, Harper HA. Harper’s Biochemistry. Stamford: Appleton and Lange; 2000. [Google Scholar]
- 25.Unakalamba B, Ozougwu J, Ejere VC. Preliminary evaluation of the haematological effects of Picralima nitida saponin extracts on Rattus novergicus. Int J Biol Biol Sci. 2013;2:28–32. [Google Scholar]
- 26.Ganeshwade RM. Effect of dimethoate on the level of cholesterol in freshwater Puntius ticto (Ham) Sci Res Rep. 2012;2:26–9. [Google Scholar]
- 27.Peng SK, Morin RJ. Biological Effects of Cholesterol Oxides. 1st ed. Florida: CRC Press LLC; 1991. p. 224. [Google Scholar]
- 28.Massaro EJ. Handbook of Human Toxicology. 1st ed. Florida: CRC Press LLC; 1997. p. 1111. [Google Scholar]
- 29.Rame JE. Chronic heart failure: A reversible metabolic syndrome? Circulation. 2012;125:2809–11. doi: 10.1161/CIRCULATIONAHA.112.108316. [DOI] [PubMed] [Google Scholar]
- 30.Hyder MA, Hasan M, Mohieldein AH. Comparative levels of ALT, AST, ALP and GGT in liver associated diseases. Eur J Exp Biol. 2013;3:280–4. [Google Scholar]
- 31.Ni H, Soe HH, Htet A. Determinants of abnormal liver function tests in diabetes patients in Myanmar. Int J Diabetes Res. 2012;1:36–41. [Google Scholar]
- 32.Obici S, Otobone FJ, da Silva Sela VR, Ishida K, da Silva JC, Nakamura CV, et al. Preliminary toxicity study of dichloromethane extract of Kielmeyera coriacea stems in mice and rats. J Ethnopharmacol. 2008;115:131–9. doi: 10.1016/j.jep.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 33.Koffuor GA, Boye A, Ofori-Amoah J, Kyei S, Nouoma CK, Debrah AP, et al. Evaluating muco-suppressant, anti-tussive and safety profile of Polyscias fruticosa (L.). Harms (Araliaceae) in asthma management. Br J Med Med Res. 2015;10:1–11. [Google Scholar]
- 34.Koffuor GA, Woode E, Obirikorang C, Asiamah E. Toxicity evaluation of a polyherbal antihypertensive mixture in Ghana. J Pharm Allied Health Sci. 2011;1:34–48. [Google Scholar]
- 35.Lab Test Online, ©2001 - 2015 by American Association for Clinical Chemistry. [Last assessed on 2015 Sep 14]. Available from: https://www.labtestsonline.org/understanding/analytes/bun/tab/test/


