Abstract
Nanotechnology involves technology, science, and engineering in dimensions less than 100 nm. A virtually infinite number of potential nanoscale products can be produced from many different molecules and their combinations. The exponentially increasing number of nanoscale products will solve critical needs in engineering, science, and medicine. However, the virtually infinite number of potential nanotechnology products is a challenge for toxicologic pathologists. Because of their size, nanoparticulates can have therapeutic and toxic effects distinct from micron-sized particulates of the same composition. In the nanoscale, distinct intercellular and intracellular translocation pathways may provide a different distribution than that obtained by micron-sized particulates. Nanoparticulates interact with subcellular structures including microtubules, actin filaments, centrosomes, and chromatin; interactions that may be facilitated in the nanoscale. Features that distinguish nanoparticulates from fine particulates include increased surface area per unit mass and quantum effects. In addition, some nanotechnology products, including the fullerenes, have a novel and reactive surface. Augmented microscopic procedures including enhanced dark-field imaging, immunofluorescence, field-emission scanning electron microscopy, transmission electron microscopy, and confocal microscopy are useful when evaluating nanoparticulate toxicologic pathology. Thus, the pathology assessment is facilitated by understanding the unique features at the nanoscale and the tools that can assist in evaluating nanotoxicology studies.
Keywords: toxicologic pathology, mechanisms of toxicity, microscopy techniques, pharmaceutical development/products, risk identification, safety assessment, other
Introduction
Nanotechnology is defined by the National Nanotechnology Initiative as “the understanding and control of matter at dimensions between approximately 1 and 100 nm, where unique phenomena enable novel applications” (National Science and Technology Council Committee on Technology Subcommittee on Nanoscale Science Engineering and Technology 2011). This definition provides a useful and generally accepted framework for discussing the potential health effects of the products of nanotechnology. However, the definition of nanotechnology and the products of nanotechnology have potential regulatory significance and some of the specifics can become quite controversial. For example, the definition of the term nanomaterial resulted in a 46-page opinion paper (SCENIHR: Scientific Committee on Emerging and Newly Identified Health Risks 8 December 2010). There has even been controversy over the definition of the terms nanoparticle and nanoparticulate and how many dimensions must be less than 100 nm to meet some definitions (SCENIHR. 29 November 2007). For nanoscale products of nanotechnology with one or more dimensions less than 100 nm, we have sometimes used the term nanoparticulates (NPs) in part to avoid the controversy regarding the term nanoparticle and in part because occupational exposures to novel nanoscale materials were originally regulated as “particulates not otherwise regulated” or PNOR (OSHA 2006). Irrespective of semantics, nanotechnology and the products of nanotechnology are usually defined by size and not by chemical composition. Products of nanotechnology can also have more than one chemical component. This means that the number of potential nanotechnology products is virtually infinite. In addition, the study of nanotoxicology includes the study of a diverse group of particulates with at least one dimension in the scale of 1 to 100 nm (Figure 1). Many of these particulates are not engineered NPs. However, studies investigating the influence of nanoscale dimensions on toxicity do not always require engineered NPs.
Figure 1.

A few examples of environmental and occupational nanoparticulates (NPs). A, Diesel exhaust NPs. Bar is 40 nm. B, Cerium oxide NPs. Bar is 50 nm. C, Multiwalled carbon nanotubes. Bar is 200 nm.
Publications and products in the field of nanotechnology are rapidly increasing. Nanotechnology is largely the result of major scientific breakthroughs in the 1980s and 1990s that enabled chemical synthesis from the atom up and facilitated three-dimensional engineering in the nanoscale (Brauman 1991; Chen and Seeman 1991; Ebbesen and Ajayan 1992; Ghadiri et al. 1993; Haufler et al. 1990; Iijima 1991; Iijima and Ichihashi 1993; Kroto et al. 1985; Li and Mcgown 1994; Whitesides, Mathias, and Seto 1991). An exponential increase in nanotechnology publications began around the turn of the century. A PubMed search for papers published in the year 2000 using the search term nanotechnology recovered 51 publications; a search using the term nanomedicine recovered 2 publications, and a search using the term nanotoxicology did not recover a single publication. In contrast, a PubMed search of papers published in the year 2011 using the search term nanotechnology recovered 6,369 publications; a search using the term nanomedicine recovered 953 publications, a search using the term nanotoxicology recovered 214 references, a search of nanotoxicology and lung recovered 35 publications, but a search of nanotoxicology and pathology recovered only 17 publications in 2011. These searches demonstrate the massive increase in the scientific investigation of nanotechnology as well as the paucity of information regarding the safety of nanotechnology products, particularly the potential toxicity to tissues other than lung. Nanotoxicology research is urgently needed (Reich 2011).
Nanotechnology products have an expanding economic impact. Obviously, nanoscale engineering permits the creation of smaller devices. Other features of the nanoscale also have practical implications. Once size decreases into the range of chemical and physical phenomena, the fundamental properties of matter change. These fundamental properties include melting point, color, and surface reactivity (Scholl, Koh, and Dionne 2012; Volokitin et al. 1996). Therefore, control of size and shape in the nanoscale may allow the tuning of these fundamental properties. In addition, as the size of an object decreases, the surface area per unit mass increases. For applications dependent upon surface area, such as chemical catalysts, nanotechnology can greatly improve efficiency (Cuenya 2010). Such exciting properties are among the reasons that nanotechnology products are changing the future of many industries. The overall economic impact of products of nanotechnology and products enabled by nanotechnology is predicted to exceed 2 trillion dollars by 2015 (Lux Research Inc. 2009). The Project on Emerging Nanotechnologies maintains an inventory of consumer products, which indicates rapid expansion of nanotechnology consumer products already on the market (Woodrow Wilson International Center for Scholars and the Pew Charitable Trusts 2011).
The rapid growth in nanotechnology has major implications for toxicologic pathologists. Toxicologic pathologists are important contributors to the multidisciplinary team that is needed to assure the safe design of nanoscale products. This brief review will focus on three features of nanoscale products with important implications for toxicologic pathologists: (1) physical and chemical properties that change in nanoscale compounds, (2) biological interactions affected by nanoscaling, and (3) augmented microscopic evaluation techniques that facilitate the evaluation of morphologic changes induced by NPs. Additional information on the toxicologic pathology of NPs is available in a recent review and a book chapter (Hubbs et al. 2011; Hubbs et al. in press).
Physical and Chemical Properties That Change in Nanoscale Compounds
In the Nanoscale, Quantum Effects Predominate
As mentioned in the introduction, the physical and chemical properties of matter change in the nanoscale. These changes involve quantum effects that are not studied during traditional pathology training programs. In brief, as the size of a particulate approaches the nanoscale, an increasing percentage of the atoms in the material are at the particle surface. At a critical point, the fundamental properties of matter change (Kim et al. 2009; Scholl, Koh, and Dionne 2012; Volokitin et al. 1996). The properties that change include basic properties such a melting point and color but of greater importance to toxicologic pathologists are changes in aerodynamic behavior, potential increases in the physical size of each molecular component as the total number of molecular components decreases, an increase in the fraction of molecules on the surface of the particle, and changes in surface reactivity (Preining 1998; Roukes 2001; Scholl, Koh, and Dionne 2012; Volokitin et al. 1996). Quantum phenomena relate to the ability to change properties in the nanoscale by changing size and shape; but this also means that characterization of NPs is critical when reporting toxicity data so that the features responsible for toxicity are identified (Boverhof and David 2010; Chen et al. 2012; Landsiedel et al. 2009; Maynard et al. 2011; Oberdorster 2010; Porter et al. 2010; Warheit 2008). Understanding the quantum phenomena of the nanoscale is critical for understanding nanotoxicology and for the rational development of nanotechnology.
Novel Chemistry and Reactive Surfaces Contribute to the Toxicity of Some NPs
The synthesis of C60 (buckminsterfullerene, the buckyball), a symmetrical soccer ball-shaped NP comprised solely of carbon, resulted in the 1996 Nobel Prize in chemistry (Curl, Kroto, and Smalley 1997; Curl and Smalley 1988; Kroto 1997; Kroto et al. 1985; Smalley 1997). The discovery of C60 was followed by synthesis of nanoscale tubes comprising almost entirely carbon, although these carbon nanotubes generally had trace amounts of metal catalysts used in their production (Ebbesen and Ajayan 1992; Iijima 1991; Taylor and Walton 1993). The carbon that made up the carbon nanotubes was arranged into hexagons arranged in a sheet that formed a rolled tube in single-walled carbon nanotubes and multiple tubes in multiwalled carbon nanotubes (MWCNTs). These new NPs had both single and double bonds in the carbon rings but were not hydrocarbons since hydrogen was not a component. While buckminsterfullerene was originally described as an aromatic compound, most members of this new class of carbon compounds behaved like polyalkenes (Taylor and Walton 1993). The presence of multiple double bonds on the surface of the carbon nanotubes and C60 meant that these compounds had a novel, reactive, and potentially modifiable surface. Pathologists and toxicologists were largely uninvolved in early studies of this new class of unsaturated polycyclic carbon compounds devoid of hydrogen.
The neurotoxicity of C60 to the brains of fish and the pulmonary toxicity of single-walled carbon nanotubes were described more than a decade after the initial discovery of these novel carbon compounds (Oberdorster 2004; Shvedova et al. 2003, 2005). These early publications in nanotoxicology involved carbon NPs with a novel and reactive surface as described in the previous paragraph and were instrumental in the foundation of nanotoxicology (Kipen and Laskin 2005; Oberdorster et al. 2005; Oberdorster, Oberdorster, and Oberdorster 2005; Service 2004). The decreased toxicity of polystyrene-coated and acid-oxidized MWCNTs is consistent with the hypothesis that the reactive surface contributes to the toxicity of carbon nanotubes (Jain et al. 2011; Tabet et al. 2011). However, these modifications also change particle dimensions. Nevertheless, the importance of surface reactivity to nanotoxicity is supported by studies of ultrafine atmospheric and occupational particulates. Ultrafine particulates are not intentionally engineered from the atom up but do include particulates in the nanoscale. The existing data on the toxicity of ultrafines provide an excellent foundation for understanding the toxicity of many nanoscale particulates in the lung. The data from ultrafine particulates indicate that surface reactivity of poorly soluble particulates contributes to their toxicity (Oberdorster 2000; Oberdorster, Oberdorster, and Oberdorster 2005; Warheit et al. 2007; Warheit et al. 2006).
NPs Have Increased Surface Area per Unit Mass
As the particulate size decreases, the surface area per unit mass increases. When the toxicity of a particulate is dependent upon the surface reactivity of the particle, as in many particulates with low solubility, the pulmonary inflammatory response to particulate exposure correlates with surface area (Monteiller et al. 2007; Oberdorster 1996; Tran et al. 2000). This phenomenon can be attributed to an increase in the effective dose of the reactive surface. This is most easily demonstrated by studies of agglomerated particle toxicity in the lung. An agglomerate has an exposed surface area similar to a larger particulate of similar total mass (Figure 2). When a respirable agglomerate of carbon black is dispersed into its nanoscale components, the exposed surface area increases and the pulmonary toxicity is enhanced when compared with the parent agglomerate (Shvedova et al. 2007). Similarly, the toxicity of a number of nanoscale poorly soluble particulates is greater on a mass basis than fine particulates of comparable chemical composition and surface reactivity (Dankovic, Kuempel, and Wheeler 2007; Oberdorster 1996). In addition to an increased effective dose of exposure to a reactive surface, the small size of NPs of low solubility and toxicity may be particularly effective in escaping macrophage-mediated clearance, allowing migration to the interstitial tissue in the lung (International Life Sciences Institute 2000; Sager and Castranova 2009).
Figure 2.
The surface area of a micron-sized particulate, such as the one on the left, may be similar to the exposed surface area of an agglomerate of nanoparticulates (NPs) with comparable mass, such as the agglomerate in the middle. On the other hand, a dispersed group of NPs, such as those on the right, will have a much greater exposed surface area.
Unlike the situation with insoluble particulates, the dissolved dose of highly soluble particulates is usually dependent upon the overall mass of the deposited particulates and independent of the size of individual particulates. This is because the toxic dose is somewhat analogous to the dose of a pharmacologic agent that is highly soluble and easily enters the target tissue/tissues—the mass is the dose because the particle dissolves. However, the dissolution rate in mass per unit time for particulates of moderate solubility is usually enhanced by decreased particle size (Johnson 2012; Johnson and Swindell 1996; Ma, Levard, et al. 2012). This property is important in the design of drugs, and it is important in the toxicity of moderately soluble metals and mineral NPs. For these moderately soluble NPs in dilute solution, dissolution rate for a given mass increases as the size decreases (Ma, Levard, et al. 2012; Mudunkotuwa et al. 2012). Some, but not all, of the enhanced solubility of NPs can be attributed to the increased surface area to mass ratio (Erbs, Gilbert, and Penn 2008; Mudunkotuwa et al. 2012; Wang and Flanagan 2002).
Biological Interactions Affected by Nanoscaling
NPs Can Pass through Biological Barriers
The ability of NPs to enter cells and pass through intercellular junctions also depends upon the size of the particulate. The endothelial lining of lymphatic capillaries has been described as discontinuous (Baluk et al. 2007), which may explain the ease with which the intercellular barriers of the lymphatics can be penetrated and the ability of phagocytic cells and large molecules to enter the lymphatic circulation. Importantly, lymphatic circulation is very important in systemic redistribution of particulates (Harmsen et al. 1987, 1985; Porter et al. 2010; Riviere 2009). Within the lymph node, particulates have intimate access to the immune system. Particulates that pass through filtering in the lymph nodes may subsequently enter the vasculature via the thoracic duct and possibly through additional lymphovenous communications (Fanous, Phillips, and Windsor 2007).
NPs have more rapid access to the lymphatics than do fine particulates. For micron-sized particulates deposited within the lung, lymph node translocation depends upon phagocytosis by macrophages and neutrophils that migrate to the lung-associated lymph nodes (Figure 3; Harmsen et al. 1987, 1985). Recent studies suggest that intracellular transport is not required for pulmonary migration of NPs with diameters less than approximately 34 nm (Choi et al. 2010; Kreyling et al. 2009). These small NPs translocate from the alveolar surface to the interstitium and then to the draining lymph node followed by appearance in the vasculature (Choi et al. 2010). This rapid translocation to the lymphatics is charge dependent and cationic NPs are excluded (Choi et al. 2010). While the NP lymphatic transport is obvious, the consequences of this transport are not fully understood. It remains to be determined whether their interaction with these microvascular tissues influences their normal function as it does with arterioles and venules. Lymphatic pumping, lymphatic contracture, and direction of lymphatic flow are all functions that could be influenced by NP interactions (Davis, Davis, Ku et al. 2009; Davis, Davis, Lane et al. 2009; Porter et al. 2010). Given the lymphatic contribution to volume and pressure homeostasis as well as particulate clearance from tissues and potential delivery to the vasculature, the consequences of NP lymphatic transport should be explored.
Figure 3.
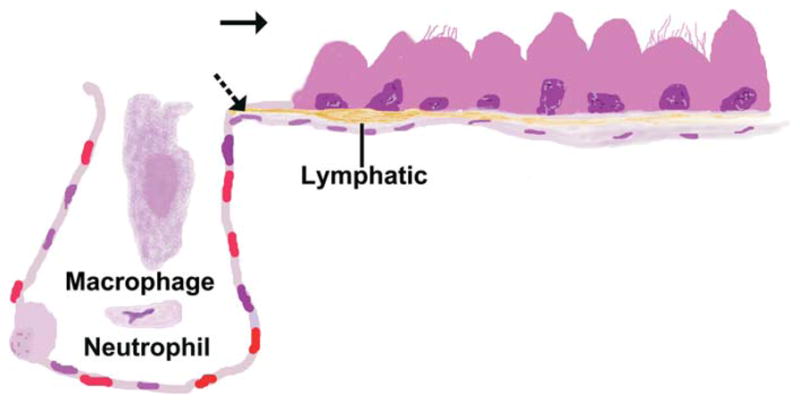
Drawing of the bronchioloalveolar junction illustrating pathways for particulates leaving the lung. Mucociliary clearance (solid arrow) is a pathway that can be used by nanoparticulates (NPs) and larger particulates. Lymphatic translocation (dashed arrows) is a pathway that removes particulates from the lung but redistributes them with the major redistribution site being the draining lymph nodes. Micron-sized particulates reach the lymphatics intracellularly after phagocytosis by macrophages and neutrophils. The smallest of NPs can enter the lymphatics without phagocytosis and can potentially pass through the lymph nodes to enter the vasculature.
Once in the systemic circulation, NPs may interact with the blood–brain barrier (BBB) or further translocate to specific neuronal or glial cells and potentially elicit a series of intracellular events leading to disruption of neuronal function. The BBB is formed by brain microvessel endothelial cells together with adjacent processes from astroglial cells. The BBB exhibits morphological features like tight junctions between endothelial cells, lack of fenestrations, and reduced pinocytic activity, which restrict the transfer of substances from the blood into the extracellular compartment of the brain (Kniesel and Wolburg 2000). The tight junctions in the BBB have a gap of only 4 to 6 nm and exert significant trans-endothelial electrical resistance to brain microvessel endothelial cells and thereby impede the penetration of substances such as oligonucleotides, antibodies, peptides, and proteins (Lo et al. 2001). Similar mechanisms may apply for translocation of NPs. Thus, NP translocation to the brain may occur through the endothelial cell membrane rather than via interendothelial junctions. An important factor that governs the penetration of the BBB by NPs is their electrostatic charge. Cationic charged molecules can potentially occupy anionic regions of the endothelium and increase endothelial cell permeability (Hardebo and Kahrstrom 1985; Nagy and Huttner 1983). Indeed, it has been shown that cationic NPs translocate more readily to the brain compared with anionic or neutral NPs (Fenart et al. 1999). NPs may also gain access to the brain by circumventing the BBB. Areas of the brain that are devoid of BBB, such as the area postrema and the posterior pituitary, can potentially serve as portals for the translocation of NPs.
NPs can also circumvent the BBB and enter the brain from the peripheral nervous system and the olfactory neurons. Neurons can transport NPs. Retrograde axonal transport of NPs is the basis for the commonly used technique of labeling ganglia of sensory nerves by instillation of nanoscale fluorescent beads into the nose or the trachea (Hunter and Dey 1998; Wu et al. 2008). NPs can also be transported in an anterograde or a perineuronal manner (Oberdorster, Elder, and Rinderknecht 2009). Since the olfactory nerves that extend to the lumen of the nose have axons that cross the cribriform plate and then synapse in the olfactory bulb of the brain, anterograde transport of NPs by olfactory nerves appears to be a particularly important pathway by which NPs or the components of their dissolution may gain access to the brain (Arvidson 1994; Dorman et al. 2002; Liu et al. 2012; Lucchini et al. 2011; Oberdörster et al. 2004; Tjalve and Henriksson 1999; Wang, Wang et al. 2011). Neuronal pathways can potentially be used for the delivery of nanopharmaceuticals as an intended part of therapy. These pathways can also provide unintended brain access after environmental and occupational exposures. Importantly, neurons are highly susceptible to oxidant-induced injury (Amicarelli et al. 2003; Hubbs et al. 2007). Since the distribution and regulation of many xenobiotic metabolizing systems is different in the brain than in other tissues (Haining and Nichols-Haining 2007; Meyer et al. 2007; Miksys and Tyndale 2004), an important consideration for nanopharmaceuticals is whether or not the brain will be able to metabolize xenobiotics delivered as NPs.
The respiratory tract is just one of the routes of potential NP exposure. Pathways for systemic absorption and distribution of orally administered NPs are not fully elucidated. However, a few recent studies have identified greater absorption of NPs than micron-sized particles of comparable toxicity following oral administration. This should be important since NPs are present in food. For example, amorphous silica NPs are present in food additives and nanoemulsions are increasingly used in the food industry (Dekkers et al. 2011; McClements and Rao 2011; Peters et al. 2012). In theory, NPs can be absorbed from the intestine using endocytic pathways or intercellular junctions in M-cells, enterocytes, and/or endothelial cells (Card et al. 2011). The lymphatic capillaries of the intestinal villi are the lacteals that play a role in lipid uptake and are a route for transport of particles from the gastrointestinal tract (Dixon 2010; Hussain, Jaitley, and Florence 2001). For nonionic and carboxylated polystyrene spheres in a size range from 100 nm to 3 microns, the lymphatic transport route appears to be important, since these particulates principally accumulate in the villi, mucosa-associated lymphoid tissue, lymph nodes, spleen, and liver after gavage administration (Jani et al. 1989). For polystyrene spheres in the size range from 50 to 1000 nm, gastrointestinal uptake increases with decreasing particle size and reaches 34% for the 50 nm NPs (Jani et al. 1989, 1990). Microparticle and NP carrier systems for delivery of orally administered pharmaceuticals have recently been reviewed (Hunter et al. 2012). A recent review on the safety of NPs in food concluded that existing studies are insufficient for adequate risk characterization (Card et al. 2011).
A few studies suggest that oral NP exposure can have effects that extend beyond the gastrointestinal tract. Orally administered, water-miscible 14C-fullerene (C60) has been shown to penetrate the BBB (Yamago et al. 1995). We have recently found that gastric NP exposure results in arteriolar dysfunction that is specific to the NP. In rats gavaged with 600 μg of nano CeO2 or MWCNT, coronary endothelium-dependent arteriolar dilation was impaired only in the MWCNT group (Nurkiewicz et al. 2012). The explanation for this differential NP effect may be due to a variety of mechanisms ranging from gastric damage, intestinal interactions or uptake, lymphatic mobility or translocation, or inherent NP characteristics, and these are currently under study.
In addition to respiratory and oral exposures, nanotechnology products are being administered parenterally in nanomedicine. In addition, the skin can be exposed to NPs in commercial products, nanomedicines, or through environmental or occupational exposures (Maynard et al. 2004; Teow et al. 2011). The interactions between the skin and inorganic NPs have been reviewed recently (Labouta and Schneider, 2013).
Understanding how NPs gain access to new tissues and subcellular compartments is important for understanding nanotoxicology in general. One means for reaching some tissue targets is avoiding phagocytosis. Avoiding recognition by phagocytic cells may prolong the circulation time of nanopharmaceutics until specific targets are reached. Techniques intended to reduce scavenging of NPs are known as stealth technology and can be part of nanopharmaceutical design (Cavadas, Gonzalez-Fernandez, and Franco 2011). The most frequent stealth technology is the use of poly(ethylene glycol) coating and additional stealth technologies are being developed (Knop et al. 2010; Nystrom and Wooley 2011).
NPs can use intracellular pathways that cannot be used by larger particles. Internalization of extracellular material by cells occurs by the general process known as endocytosis, which includes phagocytosis and pinocytosis. Phagocytic cells serve as particle scavengers but a relatively small percentage of cells are phagocytic. However, all cell types use endocytic pathways. Micron-sized particles are largely excluded by the size of the endocytic vesicles of three important endocytic pathways: (1) caveolin-mediated endocytosis, (2) clathrin-mediated endocytosis, and (3) clathrin-independent and caveolin-independent endocytosis (Conner and Schmid 2003). In addition, unlike the situation in phagocytosis, endolysosomal degradation is only one potential fate for material that is internalized by these three pathways (Cullen and Korswagen 2012; Steinberg et al. 2012). Not surprisingly, endocytic pathways contribute to the toxicity of many NPs (Singh et al. 2010; Xia et al. 2008; Zhang et al. 2009).
An understanding of endocytic pathways leading to internalization of NPs is critical to understanding nanopharmaceutical design as well as nanotoxicology. For example, caveolin-mediated endocytosis may facilitate NP transport into the cell, within the cell, and then exocytosis back out of the cell, a process that is sometimes known as transcytosis. Because such transcytosis pathways are capable of transporting NPs across cells, caveolin-mediated NP transport may be a means for traversing the BBB to directly access the brain (Gibbs-Flournoy et al. 2011; Kreuter 2013; Wagner et al. 2012; Wohlfart, Gelperina, and Kreuter 2012). Additional endocytic pathways can also be used for traversing the BBB (Bhaskar et al. 2010; Wagner et al. 2012). Within cells throughout the body, endocytic pathways can provide access to specific intracellular targets, for example, targeting the nucleus for gene therapy without viral vectors (Perez-Martinez et al. 2011; Zhang et al. 2011). The endocytic pathways are particularly useful for the intracellular delivery of nanopharmaceuticals because they can sometimes be selectively targeted (Canton and Battaglia 2012; Chen et al. 2008; Chen et al. 2011; Perez-Martinez et al. 2011; Vinogradov and Wei 2012; Wang, Byrne et al. 2011; Wang et al. 2006). To be effective in NP delivery to an intended intracellular or extracellular target, the NP must also be successfully released from the endocytic network (Chen et al. 2011; Martinez-Fong et al. 2012; Massignani et al. 2010; Sonawane, Szoka, and Verkman 2003; Vercauteren et al. 2012). For therapeutic NPs, the NP should be released prior to fusion of the endosome with the lysosome. This is because the lysosome may degrade the therapeutic agent or, if NP release involves lysosomal membrane permeability, release of the lysosomal enzymes may cause cell death. Thus, understanding the role of endocytic pathways in NP toxicology is central to understanding nanotoxicology and nanomedicine. The endocytic networks are often highly regulated and organized intracellular systems that are an area of intense research and the subject of several recent reviews (Abbey et al. 1999; Canton and Battaglia 2012; Conner and Schmid 2003; Martinez-Fong et al. 2012; McMahon and Boucrot 2011; Vercauteren et al. 2012; Vinogradov and Wei 2012; Wang, Tiruppathi et al. 2011).
Some NPs have a much greater length than width, a feature known as a high aspect ratio. With some high aspect particulates, phagocytic cells may only partially internalize the particulate. This can lead to the release of lysosomal constituents into a phagolysosome that retains an opening to the extracellular milieu. This process, known as incomplete or frustrated phagocytosis, is believed to contribute to the toxicity of durable fibers (Donaldson et al. 2010; Kamp et al. 1992). Incomplete phagocytosis may explain the observed correlation between asbestos fiber dimensions and carcinogenicity (Stanton et al. 1981; Stanton and Layard 1978; Stanton et al. 1977). In addition, recent studies suggest that incomplete endocytosis may not be a process that is limited to phagocytosis. Endocytosis of nanotubes with curved tips proceeds irrespective of the length of the particulate, while high aspect ratio elliptical particles often undergo only partial membrane wrapping, with each of these defective endocytic processes causing incomplete endocytosis (Decuzzi and Ferrari 2008; Shi et al. 2011). Other recent studies suggest that MWCNTs, a form of high aspect ratio NPs, may directly penetrate cytoplasmic and nuclear membranes without an enclosing membrane (Cheng et al. 2009; Lacerda et al. 2012; Mercer et al. 2010; Nagai et al. 2011; Porter et al. 2010). This suggests a process that is distinct from phagocytosis and nonphagocytic endocytosis (Hubbs et al. 2011; Nagai et al. 2011). Alternatively, NPs may penetrate the phagocytic or the endocytic vacuole itself after internalization or may be released from the enclosing membrane due to an ionization state incompatible with the membrane (Nemmar et al. 2003; Xia et al. 2008).
Endocytic pathways and stealth technologies can potentially be used to give drugs access to intended targets such as subcellular organelles and difficult to reach target tissues, such as the brain or tumors. However, once these targets are reached, toxicity needs to be avoided and those toxic effects may be different from those in other sites. Successful degradation is important for all components of nanopharmaceuticals as well as for occupational or environmental NPs. All components of the NP, including any stealth and/or triggering components, need to be successfully detoxified if side-effects are to be minimized as has been demonstrated in recent studies of occupational and environmental NPs. Failure to degrade the NPs, resulting from occupational or environmental exposures, plays a critical role in chronic toxicity of these agents in the lung (Roller 2009). In addition, oxidative stress and inflammation play a critical role in the toxicity of inhaled NPs (Nurkiewicz et al. 2011).
While nanotoxicology studies have largely focused on the inhalation exposure route, the paucity of data on other exposure routes does not mean these routes are safe—these need to be studied. For example, unintended damage to the brain has been described after NP exposure through a variety of exposure routes, although neuronal transport after inhalation appears to be the most effective route for brain exposure (Calderon-Garciduenas et al. 2008; De Jong et al. 2008; Lasagna-Reeves et al. 2010; Oberdorster, Elder, and Rinderknecht 2009; Rahman et al. 2009). Intentional targeting of pathways that traverse the BBB should enhance exposure of the brain by parenteral or gastrointestinal exposure. Brain exposure is critical to some therapies, as in treatment of neurodegenerative disorders, but can be a concern because the central nervous system can have distinctive responses to cytotoxic particles. These responses include neuronal susceptibility to oxidative stress injury (Amicarelli et al. 2003); variation among neuronal populations in their susceptibility to oxidative stress injury (Hubbs et al. 2007; Surmeier et al. 2011); and the distinctive cell type-specific xenobiotic metabolizing capabilities within the central nervous system (Dutheil et al. 2009, 2010; Haining and Nichols-Haining 2007; Miksys and Tyndale 2004). In short, stealth technologies and harnessing of endocytic pathways are important for intentional selective targeting by NPs; but once the target is reached, the NP needs to be eliminated without extensive injury to essential cell populations.
Thus, NPs can potentially enter cells that are not phagocytic and within those cells may access subcellular compartments inaccessible to micron-sized particles. Some NPs may enter previously unreachable target tissue such as the brain through endocytic pathways, by avoiding phagocytosis, or through a combination of both. Some NPs may enter cells through diffusion or through unique energy-independent entry mechanisms. As a consequence, some NPs and fine particulates of comparable chemical composition may differ in their target cells, cytotoxic effects, and potency.
Nanosizing May Permit Interaction with Subcellular Structures
Many normal cellular structures are comparable in size to NPs. The similarity in size and shape can facilitate interactions between subcellular structures and NPs. For example, DNA complexes with carbon nanotubes in acellular conditions (Zhao et al. 2006; Zheng et al. 2003).
The histopathologic evaluation of an early single-walled carbon nanotube inhalation study included an observation of single-walled carbon nanotubes apparently interacting with chromatin in an abnormal mitotic figure (Shvedova et al. 2008). Subsequent investigations demonstrated interactions between single-walled carbon nanotubes and chromatin as well as with the centrosomes and microtubules of the mitotic spindles (Sargent, Hubbs et al. 2012; Sargent et al. 2009). Single-walled carbon nanotube exposure caused a significant disruption of the mitotic spindle apparatus that was predominantly multipolar (Sargent, Hubbs et al. 2012; Sargent et al. 2009). The three-dimensional reconstructed images of exposed cells were truly remarkable (Figure 4). Using an exposure calculated to represent the exposure dose per cell surface received by a worker exposed at the Permissible Exposure Limit for particles not otherwise specified, an exposure to 0.024 μg/cm2 single-walled carbon nanotubes, the aneuploidy rate was 35 ± 11% in normal primary human respiratory epithelial cells.
Figure 4.
Three-dimensional reconstruction of a dividing BEAS-2B cell containing single-walled carbon nanotubes. In the upper panel, the black single-walled carbon nanotubes are present in the cytoplasm and are associated with the microtubules (red), the DNA (blue), and the centrosomes (green). The cell should contain two centrosomes but at least four are clearly present in the upper panel. The DNA is organizing into three bundles instead of two. The lower panel (B) is an enlarged image showing the interaction between single-walled carbon nanotubes with the centrosome, microtubules, and DNA. Reprinted with permission from Sargent, Hubbs et al. 2012.
As with single-walled carbon nanotubes, thin MWCNTs can interact with microtubules and cause abnormalities of the mitotic spindles (Rodriguez-Fernandez et al. 2012; Sargent, Reynolds et al. 2012). However, the abnormal mitotic spindles produced by thinnest MWCNTs were largely multipolar spindles while abnormal spindles produced by thicker MWCNTs were largely monopolar (Rodriguez-Fernandez et al. 2012; Sargent, Reynolds et al. 2012). Multipolar mitotic spindles were also observed in HeLa cells exposed to heparin-coated, 10 nm, iron oxide NPs (Villanueva et al. 2009). Micronuclei have been seen in studies with a variety of NPs, but not all NPs interact with microtubules (Gonzalez, Decordier, and Kirsch-Volders 2010).
The structural similarity between single-walled carbon nanotubes and microtubules and their apparent interaction may contribute to the remarkable aneuploidy observed in exposed cells (Sargent et al. 2009). In addition to playing a critical role in mitosis, microtubules are also important cytoskeletal components with a variety of dynamic functions. Indeed, the 25 nm diameter of microtubules and the presence of sites known to interact with metals suggests the potential for interaction with many NPs (Gonzalez, Decordier, and Kirsch-Volders 2010). In vitro exposure to a high concentration (50 μg/ml) of iron oxide NPs can disrupt the normal structural microtubular network and increase the concentration of acetylated α-tubulin in human microvascular endothelial cells (Apopa et al. 2009). Single-walled carbon nanotubes have been observed in the midbody (Mangum et al. 2006; Sargent et al. 2009), a transient furrow composed of actin and tubulin filaments, which separates daughter cells (Straight and Field 2000). Actin filaments are ~7 nm in diameter and, like microtubules, actin filaments may interact with single-walled carbon nanotubes with resulting changes in cell structure and function (Holt et al. 2010). As with many features of NPs, interactions with subcellular structures should be understood to control toxicity and fully harness their commercial potential.
Microscopic Evaluation of Nanotoxicology Studies: Important Considerations
An important component of most in vivo nanotoxicology studies is a standard toxicologic histopathology evaluation (Crissman et al. 2004). Additional procedures may assist in finding the NPs in the initial sites of entry, in finding the NPs in sites of redistribution, in identifying cytopathologic changes, and in identifying damage to tissues that are routes of NP translocation including lymphatics, the vasculature, and nerves.
Sufficient magnification is important because at least one NP dimension will be less than 100 nm. Similarly, the depth of focus using a 100× objective is roughly 200 nm or 1/25th of a 5 μ section. In short, a pathologist who evaluates an entire section for NPs using a 100× objective and a single focal plane has evaluated roughly 4% of the potential information present in the section. At lower magnifications, important cytopathologic changes may be missed and at high magnification, multiple focal planes are needed. The histopathology findings may, therefore, depend in part upon what portions of the section were evaluated at high magnification and what procedures were used to augment the demonstration of NPs or NP-associated morphologic changes.
Several procedures can be used to augment the histopathologic evaluation of NP-associated changes. At sufficient magnification, many NPs can be demonstrated in tissue either because they block light or have a crystalline structure (Hubbs et al. 2011). In standard hematoxylin and eosin (H&E) sections, NPs that block light appear as black structures (Figure 5). A recent procedure, enhanced dark-field imaging, uses a focused light source that enters the section at an angle to produce a very high-resolution image (Vainrub, Pustovyy, and Vodyanoy 2006). Crystalline material in the section will deflect the light so that the crystalline structures appear as bright objects within the high-resolution dark-field image (Figure 6). Because many NPs have a crystalline composition, this enhances their detection within tissue sections (Ma, Mercer et al. 2012; McKinney et al. 2012; Mercer et al. 2011; Vainrub, Pustovyy, and Vodyanoy 2006; Weinkauf and Brehm-Stecher 2009). Enhanced dark-field imaging can be used in sections stained with standard histology stains including H&E, provided the slides are free of crystalline contaminants.
Figure 5.
Multiwalled carbon nanotubes in an alveolar macrophage (solid arrow) or in the interstitium (dashed arrow) in a standard hematoxylin and eosin (H&E)–stained microscopic section can be observed at high magnification because they block transmitted light. However, thin multiwalled carbon nanotubes block less light than thicker multiwalled carbon nanotubes and detection can be challenging. Bar is 20 μm.
Figure 6.
Titanium dioxide nanoparticles (arrows) from a commercial spray can easily be seen as bright objects in macrophages and the interstitium using enhanced dark-field imaging (McKinney et al. 2012). Bar is 20 μm.
Immunofluorescence staining can use antibodies which are directed to proteins that are specifically expressed on certain cells or subcellular structures. This can be used to demonstrate intracellular structures and thin cells that are not easily identified in standard H&E sections. For example, podoplanin is predominantly expressed in lymphatic endothelium (Schacht et al. 2003). Dual-label immunofluorescence for podoplanin and e-cadherin can, thus, be used to localize the pulmonary lymphatics (Battelli et al. 2011; Porter et al. 2010). If the same section is then illuminated with transmitted light, carbon nanotubes can be demonstrated within the merged image (Figure 7). The demonstration of lymphatics can be critical since some NPs are widely distributed in the lymphatics and the lymphatics are not easily demonstrated in H&E stained sections (Choi et al. 2010; Porter et al. 2010; Riviere 2009). In addition, fluorescence microscopy can be used for visualizing NPs in cells from animals that have been genetically engineered to express fluorescent tagged proteins (Hadjantonakis and Nagy 2001). Laser confocal microscopy allows serial optical sectioning and three-dimensional reconstruction of immunofluorescent images. The confocal microscope can also demonstrate NPs using fluorescent, reflective, or transmitted light, depending upon the features of the NP. For example, fluorescent NPs such as quantum dots can be imaged in the fluorescent microscope, while MWCNTs can be demonstrated using either transmitted light or reflected light (Gibbs-Flournoy et al. 2011; Porter et al. 2010). These features allow the localization of NPs within cells or even within nuclei (Porter et al. 2012; Figure 8).
Figure 7.
Indirect immunofluorescence staining for podoplanin (red) allows the demonstration of a peribronchiolar lymphatic while indirect immunofluorescence staining for e-cadherin (green) and direct staining of nuclear material with DAPI help visualize adjacent structures. By adding low level transmitted light, multiwalled carbon nanotubes can be seen within the section just beneath the lymphatic endothelium as outlined in the square on the right side of the image. The inset in the square on the left is an enlarged image that has been digitally enhanced by adding pixels for image smoothing to assist in demonstrating the multiwalled carbon nanotubes.
Figure 8.

This is a photomicrograph of an optical section of an alveolar macrophage in the lung of mouse exposed to multiwalled carbon nanotubes by inhalation. The lung has been stained using lamin B1 (green) to demonstrate the nuclear envelope and ethidium homodimer-1 to stain nucleic acids. Confocal microscopy and serial optical sectioning allowed clear demonstration of nuclear penetration. Bar = 2 μm. Reprinted with permission from Porter et al. 2012.
Transmission electron microscopy can provide useful information on intracellular localization of NPs. However, the small number of cells sampled, the narrow depth of field of transmission electron microscopy, and morphologic similarities between some NPs and normal biological structures can create enormous technical challenges for visualizing NPs using transmission electron microscopy. Importantly, the response to NP exposure can sometimes include inflammation and fibrosis (Mercer et al. 2011; Shvedova et al. 2005). Collagen fibers and granules of inflammatory cells must be distinguished from the NPs themselves, although both may be increased by exposure. Labeling of the NPs is extremely useful to assist in the unequivocal demonstration of NPs and clear identification of their ultrastructural appearance (Hubbs et al. 2011; Mercer et al. 2008). However, surface modification has recently been demonstrated to alter the toxicity of some NPs. Specifically, the addition of surface functional groups not only alters the functionality and reactivity of the surface, but additions can also alter the stability and dispersibility of NPs. For example, recent studies have shown that modification of the surface of a carbon nanotube does in fact decrease the bioactivity and in turn the toxicity of the carbon nanotube (Kim et al. 2010; Sager et al. 2012; Tabet et al. 2011). This suggests a limitation to the use of some labels. The technical difficulties of electron microscopy are accentuated at occupationally or environmentally relevant exposure concentrations because far fewer NPs are present within each cell and far fewer exposed cells are present in exposed tissues. Nevertheless, transmission electron microscopy can be extremely useful to detect cytopathology in cells that accumulate NPs, such as in macrophages responding to NPs that are phagocytized (Mercer et al. 2008). A caveat is that NPs enter many different cell types, not just phagocytic cells, as noted in the previous section of this review. This suggests that transmission electron microscopy can be most useful as part of a series of microscopic tools, rather than as the primary imaging tool in evaluating histopathologic changes in nanotoxicology studies.
Traditional scanning electron microscopy has insufficient resolution for most studies in the nanoscale. Field-emission scanning electron microscopy produces higher-resolution images than conventional scanning electron microscopy (Allen et al. 2008; Mercer et al. 2010; Pawley 1997). These field-emission scanning electron microscopy images provide excellent three-dimensional images of nanoscale materials within biological specimens. Additional recent imaging techniques for localizing NPs in tissue include coherent anti-Stokes Raman scattering microscopy, two-photon fluorescence microscopy, second harmonic generation microscopy, and combinations of these new techniques (Garrett et al. 2012).
In addition to the techniques for visualizing NPs in fixed tissue, several techniques can demonstrate NPs in vivo. Intravital fluorescence microscopy is an invasive technique that provides high-resolution in vivo fluorescence imaging of dynamic processes (Kuebler 2011; Pittet and Weissleder 2011). In studies of nanotechnology products, intravital fluorescence microscopy can visualize fluorescent tracers and fluorescent nanoparticles to directly document targeted organs and track dynamic changes in cells and tissues (Cheng et al. 2012; Nurkiewicz et al. 2009, 2011; Oh et al. 2007; Smith et al. 2010). A more sensitive in vitro technique is isolated, pressurized, and perfused microvessels. This approach isolates arterioles from any organ of interest, and in conjunction with bright-field or fluorescence microscopy, NP effects can be measured in terms of functional or biochemical outcomes (LeBlanc et al. 2009; LeBlanc et al. 2010). Near-infrared imaging is a form of in vivo fluorescence imaging that relies upon the low autofluorescence of living tissue at wavelengths greater than 750 nm (Rasmussen et al. 2009). Dyes or NPs that are excited at wavelengths greater than 750 nm can be imaged within tissue to provide data on in vivo distribution (le Masne de Chermont et al. 2007; Sevick-Muraca 2012). An existing dye that is approved for human use, indocyanine green, has been used off-label in human clinical near-infrared lymphangiography studies (Rasmussen et al. 2012; Rasmussen et al. 2009). Near-infrared in vivo imaging of NPs is also a technique used to study the tissue distribution of NPs after oral, parenteral, or inhalation exposure of laboratory animals (Choi et al. 2010; le Masne de Chermont et al. 2007; Lee et al. 2012). Some nanopharmaceuticals are actually designed for both imaging and therapeutic functions, which facilitate in vivo imaging using a variety of conventional imaging techniques (Chen, Gambhir, and Cheon 2011; Ng, Lovell, and Zheng 2011; Nystrom and Wooley 2011). Additional information on in vivo imaging of NPs is available in a recent review (Roller et al. 2011). Recent developments in the general field of small animal imaging can contribute to in vivo imaging during nanotoxicology studies and have also been reviewed recently (Kagadis et al. 2010; Koba et al. 2011; Loudos, Kagadis, and Psimadas 2011; Rowland and Cherry 2008; Wang and Hu 2012; Wessels et al. 2010).
Conclusions
The biological behavior of NPs is often fundamentally different than the biological behavior of larger particulates. In particular, there is a net difference in the expected location. Translocation between tissues is generally increased. The diversity of cells and tissues exposed is often greater than would be expected from exposure to larger particulates. Extracellular transport is more frequent than with larger particulates yet less well understood. Many NPs tend to circulate within the lymphatics, a feature which also distinguishes NPs from traditional pharmaceuticals. This too is poorly understood, and additional studies will be needed to identify the influence of NPs on lymphatic function. Finally, NPs can utilize intracellular transport methods that exclude larger particulates.
For pathologists, high-magnification and augmented microscopic imaging procedures are labor intensive but important for evaluating nanotechnology products. In addition, target tissues of particular importance for some nanotechnology products include the lymphatics and the central nervous system, tissues where subtle changes often require detailed evaluation. However, the ability to circulate in lymphatics and to potentially enter the central nervous system is not just a challenge for the pathologist; it is part of the excitement of nanomedicine. By intentionally targeting the central nervous system or lymphatics, some of the new nanoscale pharmaceuticals may be particularly useful for treating neurodegenerative conditions and metastatic carcinomas. In addition, engineered NPs can potentially be designed to answer some of the most important hypotheses in particle toxicology. Understanding the features that make some nanotechnology products hazardous and others much safer will allow the design of the nanotechnology products of the future.
Acknowledgments
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The contribution of Timothy R. Nurkiewicz was supported by NIH-R01-ES015022.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Abbey DE, Nishino N, McDonnell WF, Burchette RJ, Knutsen SF, Lawrence Beeson W, Yang JX. Long-term inhalable particles and other air pollutants related to mortality in nonsmokers. Am J Respir Crit Care Med. 1999;159:373–82. doi: 10.1164/ajrccm.159.2.9806020. [DOI] [PubMed] [Google Scholar]
- Allen TD, Rutherford SA, Murray S, Drummond SP, Goldberg MW, Kiseleva E. Scanning electron microscopy of nuclear structure. Methods Cell Biol. 2008;88:389–409. doi: 10.1016/S0091-679X(08)00420-2. [DOI] [PubMed] [Google Scholar]
- Amicarelli F, Colafarina S, Cattani F, Cimini A, Di Ilio C, Ceru MP, Miranda M. Scavenging system efficiencyis crucial for cellresistance to ROS-mediated methylglyoxal injury. Free Radic Biol Med. 2003;35:856–71. doi: 10.1016/s0891-5849(03)00438-6. [DOI] [PubMed] [Google Scholar]
- Apopa PL, Qian Y, Shao R, Guo NL, Schwegler-Berry D, Pacurari M, Porter D, Shi X, Vallyathan V, Castranova V, Flynn DC. Iron oxide nanoparticles induce human microvascular endothelial cell permeability through reactive oxygen species production and microtubule remodeling. Part Fibre Toxicol. 2009;6:1. doi: 10.1186/1743-8977-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidson B. A review of axonal transport of metals. Toxicology. 1994;88:1–14. doi: 10.1016/0300-483x(94)90107-4. [DOI] [PubMed] [Google Scholar]
- Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E, McDonald DM. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 2007;204:2349–62. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battelli LA, VC, Porter DW, Friend S, Schwegler-Berry D, Willard P, Hubbs AF. The use of e-cadherin immunofluorescence in pulmonary toxicologic pathology studies. Toxicol Sci (The Toxicologist) 2011;126:123. [Google Scholar]
- Bhaskar S, Tian F, Stoeger T, Kreyling W, de la Fuente JM, Grazu V, Borm P, Estrada G, Ntziachristos V, Razansky D. Multi-functional Nanocarriers for diagnostics, drug delivery and targeted treatment across blood-brain barrier: Perspectives on tracking and neuroimaging. Part Fibre Toxicol. 2010;7:3. doi: 10.1186/1743-8977-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boverhof DR, David RM. Nanomaterial characterization: Considerations and needs for hazard assessment and safety evaluation. Anal Bioanal Chem. 2010;396:953–61. doi: 10.1007/s00216-009-3103-3. [DOI] [PubMed] [Google Scholar]
- Brauman JI. Room at the bottom. Science. 1991;254:1277–1277. doi: 10.1126/science.254.5036.1277. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Solt AC, Henriquez-Roldan C, Torres-Jardon R, Nuse B, Herritt L, Villarreal-Calderon R, Osnaya N, Stone I, Garcia R, Brooks DM, Gonzalez-Maciel A, Reynoso-Robles R, Delgado-Chavez R, Reed W. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol Pathol. 2008;36:289–310. doi: 10.1177/0192623307313011. [DOI] [PubMed] [Google Scholar]
- Canton I, Battaglia G. Endocytosis at the nanoscale. Chem Soc Rev. 2012;41:2718–739. doi: 10.1039/c2cs15309b. [DOI] [PubMed] [Google Scholar]
- Card JW, Jonaitis TS, Tafazoli S, Magnuson BA. An appraisal of the published literature on the safety and toxicity of food-related nanomaterials. Crit Rev Toxicol. 2011;41:22–49. doi: 10.3109/10408444.2010.524636. [DOI] [PubMed] [Google Scholar]
- Cavadas M, Gonzalez-Fernandez A, Franco R. Pathogenmimetic stealth nanocarriers for drug delivery: A future possibility. Nanomedicine. 2011;7:730–43. doi: 10.1016/j.nano.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Chen BT, Schwegler-Berry D, McKinney W, Stone S, Cumpston JL, Friend S, Porter DW, Castranova V, Frazer DG. Multi-walled carbon nanotubes: Sampling criteria and aerosol characterization. Inhal Toxicol. 2012;24:798–820. doi: 10.3109/08958378.2012.720741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JH, Seeman NC. Synthesis from DNA of a molecule with the connectivity of a cube. Nature. 1991;350:631–33. doi: 10.1038/350631a0. [DOI] [PubMed] [Google Scholar]
- Chen X, Gambhir SS, Cheon J. Theranostic nanomedicine. Acc Chem Res. 2011;44:841. doi: 10.1021/ar200231d. [DOI] [PubMed] [Google Scholar]
- Chen X, Kube DM, Cooper MJ, Davis PB. Cell surface nucleolin serves as receptor for DNA nanoparticles composed of pegylated polylysine and DNA. Mol Ther. 2008;16:333–42. doi: 10.1038/sj.mt.6300365. [DOI] [PubMed] [Google Scholar]
- Chen X, Shank S, Davis PB, Ziady AG. Nucleolin-mediated cellular trafficking of DNA nanoparticle is lipid raft and microtubule dependent and can be modulated by glucocorticoid. Mol Ther. 2011b;19:93–102. doi: 10.1038/mt.2010.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Muller KH, Koziol KK, Skepper JN, Midgley PA, Welland ME, Porter AE. Toxicity and imaging of multi-walled carbon nanotubes in human macrophage cells. Biomaterials. 2009;30:4152–60. doi: 10.1016/j.biomaterials.2009.04.019. [DOI] [PubMed] [Google Scholar]
- Cheng SH, Li FC, Souris JS, Yang CS, Tseng FG, Lee HS, Chen CT, Dong CY, Lo LW. Visualizing dynamics of sub-hepatic distribution of nanoparticles using intravital multiphoton fluorescence microscopy. ACS Nano. 2012;6:4122–31. doi: 10.1021/nn300558p. [DOI] [PubMed] [Google Scholar]
- Choi HS, Ashitate Y, Lee JH, Kim SH, Matsui A, Insin N, Bawendi MG, Semmler-Behnke M, Frangioni JV, Tsuda A. Rapid translocation of nanoparticles from the lung airspaces to the body. Nat Biotechnol. 2010;28:1300–03. doi: 10.1038/nbt.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- Crissman JW, Goodman DG, Hildebrandt PK, Maronpot RR, Prater DA, Riley JH, Seaman WJ, Thake DC. Best practices guideline: Toxicologic histopathology. Toxicol Pathol. 2004;32:126–31. doi: 10.1080/01926230490268756. [DOI] [PubMed] [Google Scholar]
- Cuenya BR. Synthesis and catalytic properties of metal nanoparticles: Size, shape, support, composition, and oxidation state effects. Thin Solid Films. 2010;518:3127–50. [Google Scholar]
- Cullen PJ, Korswagen HC. Sorting nexins provide diversity for retromer-dependent trafficking events. Nat Cell Biol. 2012;14:29–37. doi: 10.1038/ncb2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curl RF, Kroto HW, Smalley RE. Nobel prize in chemistry for 1996. S Afr J Chem-S-Afr T. 1997;50:102–105. [Google Scholar]
- Curl RF, Smalley RE. Probing c60. Science. 1988;242:1017–22. doi: 10.1126/science.242.4881.1017. [DOI] [PubMed] [Google Scholar]
- Dankovic D, Kuempel E, Wheeler M. An approach to risk assessment for TiO2. Inhal Toxicol. 2007;19(Suppl 1):205–12. doi: 10.1080/08958370701497754. [DOI] [PubMed] [Google Scholar]
- Davis MJ, Davis AM, Ku CW, Gashev AA. Myogenic constriction and dilation of isolated lymphatic vessels. Am J Physiol Heart Circ Physiol. 2009a;296:H293–302. doi: 10.1152/ajpheart.01040.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MJ, Davis AM, Lane MM, Ku CW, Gashev AA. Rate-sensitive contractile responses of lymphatic vessels to circumferential stretch. J Physiol. 2009b;587:165–82. doi: 10.1113/jphysiol.2008.162438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decuzzi P, Ferrari M. The receptor-mediated endocytosis of nonspherical particles. Biophys J. 2008;94:3790–97. doi: 10.1529/biophysj.107.120238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong WH, Hagens WI, Krystek P, Burger MC, Sips AJ, Geertsma RE. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials. 2008;29:1912–19. doi: 10.1016/j.biomaterials.2007.12.037. [DOI] [PubMed] [Google Scholar]
- Dekkers S, Krystek P, Peters RJ, Lankveld DP, Bokkers BG, van Hoeven-Arentzen PH, Bouwmeester H, Oomen AG. Presence and risks of nanosilica in food products. Nanotoxicology. 2011;5:393–405. doi: 10.3109/17435390.2010.519836. [DOI] [PubMed] [Google Scholar]
- Dixon JB. Mechanisms of chylomicron uptake into lacteals. Ann N Y Acad Sci. 2010;1207(Suppl 1):E52–57. doi: 10.1111/j.1749-6632.2010.05716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson K, Murphy FA, Duffin R, Poland CA. Asbestos, carbon nanotubes and the pleural mesothelium: A review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part Fibre Toxicol. 2010;7:5. doi: 10.1186/1743-8977-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman DC, Brenneman KA, McElveen AM, Lynch SE, Roberts KC, Wong BA. Olfactory transport: A direct route of delivery of inhaled manganese phosphate to the rat brain. J Toxicol Environ Health A. 2002;65:1493–511. doi: 10.1080/00984100290071630. [DOI] [PubMed] [Google Scholar]
- Dutheil F, Dauchy S, Diry M, Sazdovitch V, Cloarec O, Mellottee L, Bieche I, Ingelman-Sundberg M, Flinois JP, de Waziers I, Beaune P, Decleves X, Duyckaerts C, Loriot MA. Xenobiotic-metabolizing enzymes and transporters in the normal human brain: Regional and cellular mapping as a basis for putative roles in cerebral function. Drug Metab Dispos. 2009;37:1528–38. doi: 10.1124/dmd.109.027011. [DOI] [PubMed] [Google Scholar]
- Dutheil F, Jacob A, Dauchy S, Beaune P, Scherrmann JM, Decleves X, Loriot MA. ABC transporters and cytochromes P450 in the human central nervous system: Influence on brain pharmacokinetics and contribution to neurodegenerative disorders. Expet Opin Drug metabo Toxicol. 2010;6:1161–74. doi: 10.1517/17425255.2010.510832. [DOI] [PubMed] [Google Scholar]
- Ebbesen TW, Ajayan PM. Large-scale synthesis of carbon nanotubes. Nature. 1992;358:220–22. [Google Scholar]
- Erbs JJ, Gilbert B, Penn RL. Influence of size on reductive dissolution of six-line ferrihydrite. J Phys Chem C. 2008;112:12127–33. [Google Scholar]
- Fanous MY, Phillips AJ, Windsor JA. Mesenteric lymph: The bridge to future management of critical illness. JOP. 2007;8:374–99. [PubMed] [Google Scholar]
- Fenart L, Casanova A, Dehouck B, Duhem C, Slupek S, Cecchelli R, Betbeder D. Evaluation of effect of charge and lipid coating on ability of 60-nm nanoparticles to cross an in vitro model of the blood-brain barrier. J Pharmacol Exp Ther. 1999;291:1017–22. [PubMed] [Google Scholar]
- Garrett NL, Lalatsa A, Uchegbu I, Schatzlein A, Moger J. Exploring uptake mechanisms of oral nanomedicines using multimodal nonlinear optical microscopy. J Biophoton. 2012;5:458–68. doi: 10.1002/jbio.201200006. [DOI] [PubMed] [Google Scholar]
- Ghadiri MR, Granja JR, Milligan RA, Mcree DE, Khazanovich N. Self-assembling organic nanotubes based on a cyclic peptide architecture. Nature. 1993;366:324–327. doi: 10.1038/366324a0. [DOI] [PubMed] [Google Scholar]
- Gibbs-Flournoy EA, Bromberg PA, Hofer TP, Samet JM, Zucker RM. Darkfield-confocal microscopy detection of nanoscale particle internalization by human lung cells. Part Fibre Toxicol. 2011;8:2. doi: 10.1186/1743-8977-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez L, Decordier I, Kirsch-Volders M. Induction of chromosome malsegregation by nanomaterials. Biochem Soc Trans. 2010;38:1691–7. doi: 10.1042/BST0381691. [DOI] [PubMed] [Google Scholar]
- Hadjantonakis AK, Nagy A. The color of mice: In the light of GFP-variant reporters. Histochem Cell Biol. 2001;115:49–58. doi: 10.1007/s004180000233. [DOI] [PubMed] [Google Scholar]
- Haining RL, Nichols-Haining M. Cytochrome P450-catalyzed pathways in human brain: Metabolism meets pharmacology or old drugs with new mechanism of action? Pharmacol Ther. 2007;113:537–45. doi: 10.1016/j.pharmthera.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Hardebo JE, Kahrstrom J. Endothelial negative surface charge areas and blood-brain barrier function. Acta Physiol Scand. 1985;125:495–99. doi: 10.1111/j.1748-1716.1985.tb07746.x. [DOI] [PubMed] [Google Scholar]
- Harmsen AG, Mason MJ, Muggenburg BA, Gillett NA, Jarpe MA, Bice DE. Migration of neutrophils from lung to tracheobronchial lymph node. J Leukoc Biol. 1987;41:95–103. doi: 10.1002/jlb.41.2.95. [DOI] [PubMed] [Google Scholar]
- Harmsen AG, Muggenburg BA, Snipes MB, Bice DE. The role of macrophages in particle translocation from lungs to lymph nodes. Science. 1985;230:1277–80. doi: 10.1126/science.4071052. [DOI] [PubMed] [Google Scholar]
- Haufler RE, Conceicao J, Chibante LPF, Chai Y, Byrne NE, Flanagan S, Haley MM, Obrien SC, Pan C, Xiao Z, Billups WE, Ciufolini MA, Hauge RH, Margrave JL, Wilson LJ, Curl RF, Smalley RE. Efficient production of C60 (Buckminsterfullerene), C60h36, and the solvated Buckide ion. J Phys Chem-Us. 1990;94:8634–36. [Google Scholar]
- Holt BD, Short PA, Rape AD, Wang YL, Islam MF, Dahl KN. Carbon nanotubes reorganize actin structures in cells and ex vivo. ACS Nano. 2010;4:4872–78. doi: 10.1021/nn101151x. [DOI] [PubMed] [Google Scholar]
- Hubbs AF, Benkovic SA, Miller DB, O’Callaghan JP, Battelli L, Schwegler-Berry D, Ma Q. Vacuolar leukoencephalopathy with widespread astrogliosis in mice lacking transcription factor Nrf2. Am J Pathol. 2007;170:2068–76. doi: 10.2353/ajpath.2007.060898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbs AF, Mercer RR, Benkovic SA, Harkema J, Sriram K, Schwegler-Berry D, Goravanahally MP, Nurkiewicz TR, Castranova V, Sargent LM. Nanotoxicology—a pathologist’s perspective. Toxicol Pathol. 2011;39:301–24. doi: 10.1177/0192623310390705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbs AF, Porter DW, Mercer RRVC, Sargent LM, Sriram K. Nanoparticulates. In: Haschek WM, Rousseaux CG, Wallig MA, Ochoa R, Bolon B, editors. Haschek and Rousseaux’s Handbook of Toxicologic Pathology. Elsevier; in press. [Google Scholar]
- Hunter AC, Elsom J, Wibroe PP, Moghimi SM. Polymeric particulate technologies for oral drug delivery and targeting: A pathophysiological perspective. Maturitas. 2012;73:5–18. doi: 10.1016/j.maturitas.2012.05.014. [DOI] [PubMed] [Google Scholar]
- Hunter DD, Dey RD. Identification and neuropeptide content of trigeminal neurons innervating the rat nasal epithelium. Neuroscience. 1998;83:591–99. doi: 10.1016/s0306-4522(97)00324-2. [DOI] [PubMed] [Google Scholar]
- Hussain N, Jaitley V, Florence AT. Recent advances in the understanding of uptake of microparticulates across the gastrointestinal lymphatics. Adv Drug Deliv Rev. 2001;50:107–42. doi: 10.1016/s0169-409x(01)00152-1. [DOI] [PubMed] [Google Scholar]
- Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354:56–58. [Google Scholar]
- Iijima S, Ichihashi T. Single-shell carbon nanotubes of 1-nm diameter. Nature. 1993;363:603–605. [Google Scholar]
- International Life Sciences Institute. The relevance of the rat lung response to particle overload for human risk assessment: A workshop consensus report. ILSI Risk Science Institute Workshop Participants. Inhal Toxicol. 2000;12:1–17. doi: 10.1080/08958370050029725. [DOI] [PubMed] [Google Scholar]
- Jani P, Halbert GW, Langridge J, Florence AT. The uptake and translocation of latex nanospheres and microspheres after oral administration to rats. J Pharm Pharmacol. 1989;41:809–12. doi: 10.1111/j.2042-7158.1989.tb06377.x. [DOI] [PubMed] [Google Scholar]
- Jani P, Halbert GW, Langridge J, Florence AT. Nanoparticle uptake by the rat gastrointestinal mucosa: Quantitation and particle size dependency. J Pharm Pharmacol. 1990;42:821–26. doi: 10.1111/j.2042-7158.1990.tb07033.x. [DOI] [PubMed] [Google Scholar]
- Jain S, Thakare VS, Das M, Godugu C, Jain AK, Mathur R, Chuttani K, Mishra AK. Toxicity of multiwalled carbon nanotubes with end defects critically depends on their functionalization density. Chem Res Toxicol. 2011;24:2028–39. doi: 10.1021/tx2003728. [DOI] [PubMed] [Google Scholar]
- Johnson KC. Comparison of methods for predicting dissolution and the theoretical implications of particle-size-dependent solubility. J Pharm Sci. 2012;101:681–89. doi: 10.1002/jps.22778. [DOI] [PubMed] [Google Scholar]
- Johnson KC, Swindell AC. Guidance in the setting of drug particle size specifications to minimize variability in absorption. Pharmaceut Res. 1996;13:1795–98. doi: 10.1023/a:1016068705255. [DOI] [PubMed] [Google Scholar]
- Kagadis GC, Loudos G, Katsanos K, Langer SG, Nikiforidis GC. In vivo small animal imaging: Current status and future prospects. Med Phy. 2010;37:6421–42. doi: 10.1118/1.3515456. [DOI] [PubMed] [Google Scholar]
- Kamp DW, Graceffa P, Pryor WA, Weitzman SA. The role of free radicals in asbestos-induced diseases. Free Radic Biol Med. 1992;12:293–315. doi: 10.1016/0891-5849(92)90117-y. [DOI] [PubMed] [Google Scholar]
- Kim JE, Lim HT, Minai-Tehrani A, Kwon JT, Shin JY, Woo CG, Choi M, Baek J, Jeong DH, Ha YC, Chae CH, Song KS, Ahn KH, Lee JH, Sung HJ, Yu IJ, Beck GR, Jr, Cho MH. Toxicity and clearance of intratracheally administered multiwalled carbon nanotubes from murine lung. J Toxicol Environ Health A. 2010;73:1530–43. doi: 10.1080/15287394.2010.511578. [DOI] [PubMed] [Google Scholar]
- Kim WY, Choi YC, Min SK, Cho Y, Kim KS. Application of quantum chemistry to nanotechnology: Electron and spin transport in molecular devices. Chem Soc Rev. 2009;38:2319–33. doi: 10.1039/b820003c. [DOI] [PubMed] [Google Scholar]
- Kipen HM, Laskin DL. Smaller is not always better: Nanotechnology yields nanotoxicology. Am J Physiol Lung Cell Mol Physiol. 2005;289:L696–97. doi: 10.1152/ajplung.00277.2005. [DOI] [PubMed] [Google Scholar]
- Kniesel U, Wolburg H. Tight junctions of the blood-brain barrier. Cell Mol Neurobiol. 2000;20:57–76. doi: 10.1023/A:1006995910836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop K, Hoogenboom R, Fischer D, Schubert US. Poly(ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew Chem Int Ed Engl. 2010;49:6288–308. doi: 10.1002/anie.200902672. [DOI] [PubMed] [Google Scholar]
- Koba W, Kim K, Lipton ML, Jelicks L, Das B, Herbst L, Fine E. Imaging devices for use in small animals. Semin Nucl Med. 2011;41:151–65. doi: 10.1053/j.semnuclmed.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Kreuter J. Mechanism of polymeric nanoparticle-based drug transport across the blood-brain barrier (BBB) J Microencapsul. 2013;30:49–54. doi: 10.3109/02652048.2012.692491. [DOI] [PubMed] [Google Scholar]
- Kreyling WG, Semmler-Behnke M, Seitz J, Scymczak W, Wenk A, Mayer P, Takenaka S, Oberdorster G. Size dependence of the translocation of inhaled iridium and carbon nanoparticle aggregates from the lung of rats to the blood and secondary target organs. Inhal Toxicol. 2009;21(Suppl1):55–60. doi: 10.1080/08958370902942517. [DOI] [PubMed] [Google Scholar]
- Kroto H. Symmetry, space, stars, and C-60 (Nobel lecture) Angew Chem Int Edit. 1997;36:1579–93. [Google Scholar]
- Kroto HW, Heath JR, O’Brien SC, Curl RF, Smalley RE. C60: Buckminsterfullerene. Nature. 1985;318:162–63. [Google Scholar]
- Kuebler WM. Real-time imaging assessment of pulmonary vascular responses. Proc Am Thorac Soc. 2011;8:458–65. doi: 10.1513/pats.201101-005MW. [DOI] [PubMed] [Google Scholar]
- Labouta HI, Schneider M. Interaction of inorganic nanoparticles with the skin barrier: Current status and critical review. Nanomedicine. 2013;9:39–54. doi: 10.1016/j.nano.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Lacerda L, Russier J, Pastorin G, Herrero MA, Venturelli E, Dumortier H, Al-Jamal KT, Prato M, Kostarelos K, Bianco A. Translocation mechanisms of chemically functionalised carbon nanotubes across plasma membranes. Biomaterials. 2012;33:3334–43. doi: 10.1016/j.biomaterials.2012.01.024. [DOI] [PubMed] [Google Scholar]
- Landsiedel R, Kapp MD, Schulz M, Wiench K, Oesch F. Genotoxicity investigations on nanomaterials: Methods, preparation and characterization of test material, potential artifacts and limitations–many questions, some answers. Mutat Res. 2009;681:241–58. doi: 10.1016/j.mrrev.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Lasagna-Reeves C, Gonzalez-Romero D, Barria MA, Olmedo I, Clos A, Sadagopa Ramanujam VM, Urayama A, Vergara L, Kogan MJ, Soto C. Bioaccumulation and toxicity of gold nanoparticles after repeated administration in mice. Biochem Biophys Res Commun. 2010;393:649–55. doi: 10.1016/j.bbrc.2010.02.046. [DOI] [PubMed] [Google Scholar]
- LeBlanc AJ, Cumpston JL, Chen BT, Frazer D, Castranova V, Nurkiewicz TR. Nanoparticle inhalation impairs endothelium-dependent vasodilation in subepicardial arterioles. J Toxicol Environ Health A. 2009;72:1576–84. doi: 10.1080/15287390903232467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc AJ, Moseley AM, Chen BT, Frazer D, Castranova V, Nurkiewicz TR. Nanoparticle inhalation impairs coronary micro-vascular reactivity via a local reactive oxygen species-dependent mechanism. Cardiovasc Toxicol. 2010;10:27–36. doi: 10.1007/s12012-009-9060-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CM, Jeong HJ, Yun KN, Kim DW, Sohn MH, Lee JK, Jeong J, Lim ST. Optical imaging to trace near infrared fluorescent zinc oxide nanoparticles following oral exposure. Int J Nanomedicine. 2012;7:3203–09. doi: 10.2147/IJN.S32828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- le Masne de Chermont Q, Chaneac C, Seguin J, Pelle F, Maitrejean S, Jolivet JP, Gourier D, Bessodes M, Scherman D. Nanoprobes with near-infrared persistent luminescence for in vivo imaging. Proc Natl Acad Sci U S A. 2007;104:9266–71. doi: 10.1073/pnas.0702427104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Mcgown LB. Molecular Nanotube Aggregates of beta-cyclodextrins and gamma-cyclodextrins linked by diphenylhexatrienes. Science. 1994;264:249–51. doi: 10.1126/science.264.5156.249. [DOI] [PubMed] [Google Scholar]
- Liu Q, Shen Y, Chen J, Gao X, Feng C, Wang L, Zhang Q, Jiang X. Nose-to-brain transport pathways of wheat germ agglutinin conjugated PEG-PLA nanoparticles. Pharm Res. 2012;29:546–58. doi: 10.1007/s11095-011-0641-0. [DOI] [PubMed] [Google Scholar]
- Lo EH, Singhal AB, Torchilin VP, Abbott NJ. Drug delivery to damaged brain. Brain research. 2001;38:140–48. doi: 10.1016/s0165-0173(01)00083-2. [DOI] [PubMed] [Google Scholar]
- Loudos G, Kagadis GC, Psimadas D. Current status and future perspectives of in vivo small animal imaging using radiolabeled nanoparticles. Eur J Radiol. 2011;78:287–95. doi: 10.1016/j.ejrad.2010.06.025. [DOI] [PubMed] [Google Scholar]
- Lucchini RG, Dorman DC, Elder A, Veronesi B. Neurological impacts from inhalation of pollutants and the nose-brain connection. Neurotoxicology. 2011;33:838–41. doi: 10.1016/j.neuro.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux Research Inc. The Recession’s Ripple Effect on Nanotech. Boston, MA: 2009. [Google Scholar]
- Ma JY, Mercer RR, Barger M, Schwegler-Berry D, Scabilloni J, Ma JK, Castranova V. Induction of pulmonary fibrosis by cerium oxide nanoparticles. Toxicol Appl Pharmacol. 2012;262:255–64. doi: 10.1016/j.taap.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma R, Levard C, Marinakos SM, Cheng Y, Liu J, Michel FM, Brown GE, Lowry GV. Size-controlled dissolution of organic-coated silver nanoparticles. Environ Sci Technol. 2012;46:752–59. doi: 10.1021/es201686j. [DOI] [PubMed] [Google Scholar]
- Mangum JB, Turpin EA, Antao-Menezes A, Cesta MF, Bermudez E, Bonner JC. Single-walled carbon nanotube (SWCNT)-induced interstitial fibrosis in the lungs of rats is associated with increased levels of PDGF mRNA and the formation of unique intercellular carbon structures that bridge alveolar macrophages in situ. Part Fibre Toxicol. 2006;3:15. doi: 10.1186/1743-8977-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Fong D, Bannon MJ, Trudeau LE, Gonzalez-Barrios JA, Arango-Rodriguez ML, Hernandez-Chan NG, Reyes-Corona D, Armendariz-Borunda J, Navarro-Quiroga I. NTS-Polyplex: A potential nanocarrier for neurotrophic therapy of Parkinson’s disease. Nanomedicine. 2012;8:1052–69. doi: 10.1016/j.nano.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massignani M, Canton I, Sun T, Hearnden V, Macneil S, Blanazs A, Armes SP, Lewis A, Battaglia G. Enhanced fluorescence imaging of live cells by effective cytosolic delivery of probes. PLoS One. 2010;5:e10459. doi: 10.1371/journal.pone.0010459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard AD, Baron PA, Foley M, Shvedova AA, Kisin ER, Castranova V. Exposure to carbon nanotube material: Aerosol release during the handling of unrefined single-walled carbon nanotube material. J Toxicol Environ Health A. 2004;67:87–107. doi: 10.1080/15287390490253688. [DOI] [PubMed] [Google Scholar]
- Maynard AD, Warheit DB, Philbert MA. The new toxicology of sophisticated materials: Nanotoxicology and beyond. Toxicol Sci. 2011;120(Suppl 1):S109–29. doi: 10.1093/toxsci/kfq372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClements DJ, Rao J. Food-grade nanoemulsions: Formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit Rev Food Sci Nutr. 2011;51:285–330. doi: 10.1080/10408398.2011.559558. [DOI] [PubMed] [Google Scholar]
- McKinney W, Jackson M, Sager TM, Reynolds JS, Chen BT, Afshari A, Krajnak K, Waugh S, Johnson C, Mercer RR, Frazer DG, Thomas TA, Castranova V. Pulmonary and cardiovascular responses of rats to inhalation of a commercial antimicrobial spray containing titanium dioxide nanoparticles. Inhal Toxicol. 2012;24:447–57. doi: 10.3109/08958378.2012.685111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2011;12:517–33. doi: 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- Mercer RR, Hubbs AF, Scabilloni JF, Wang L, Battelli LA, Friend S, Castranova V, Porter DW. Pulmonary fibrotic response to aspiration of multi-walled carbon nanotubes. Part Fibre Toxicol. 2011;8:21. doi: 10.1186/1743-8977-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer RR, Hubbs AF, Scabilloni JF, Wang L, Battelli LA, Schwegler-Berry D, Castranova V, Porter DW. Distribution and persistence of pleural penetrations by multi-walled carbon nanotubes. Part Fibre Toxicol. 2010;7:28. doi: 10.1186/1743-8977-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer RR, Scabilloni J, Wang L, Kisin E, Murray AR, Schwegler-Berry D, Shvedova AA, Castranova V. Alteration of deposition pattern and pulmonary response as a result of improved dispersion of aspirated single-walled carbon nanotubes in a mouse model. Am J Physiol Lung Cell Mol Physiol. 2008;294:L87–97. doi: 10.1152/ajplung.00186.2007. [DOI] [PubMed] [Google Scholar]
- Meyer RP, Gehlhaus M, Knoth R, Volk B. Expression and function of cytochrome p450 in brain drug metabolism. Curr Drug Metabol. 2007;8:297–306. doi: 10.2174/138920007780655478. [DOI] [PubMed] [Google Scholar]
- Miksys S, Tyndale RF. The unique regulation of brain cytochrome P450 2 (CYP2) family enzymes by drugs and genetics. Drug Metabol Rev. 2004;36:313–33. doi: 10.1081/dmr-120034149. [DOI] [PubMed] [Google Scholar]
- Monteiller C, Tran L, MacNee W, Faux S, Jones A, Miller B, Donaldson K. The pro-inflammatory effects of low-toxicity low-solubility particles, nanoparticles and fine particles, on epithelial cells in vitro: The role of surface area. Occup Environ Med. 2007;64:609–15. doi: 10.1136/oem.2005.024802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudunkotuwa IA, Rupasinghe T, Wu CM, Grassian VH. Dissolution of ZnO nanoparticles at circumneutral pH: A study of size effects in the presence and absence of citric acid. Langmuir. 2012;28:396–403. doi: 10.1021/la203542x. [DOI] [PubMed] [Google Scholar]
- Nagai H, Okazaki Y, Chew SH, Misawa N, Yamashita Y, Akatsuka S, Ishihara T, Yamashita K, Yoshikawa Y, Yasui H, Jiang L, Ohara H, Takahashi T, Ichihara G, Kostarelos K, Miyata Y, Shinohara H, Toyokuni S. Diameter and rigidity of multiwalled carbon nanotubes are critical factors in mesothelial injury and carcinogenesis. Proc Natl Acad Sci U S A. 2011;108:E1330–38. doi: 10.1073/pnas.1110013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z, PH, Huttner I. Charge-related alterations of the cerebral endothelium. Lab Invest. 1983;49:662–71. [PubMed] [Google Scholar]
- National Science and Technology Council Committee on Technology Subcommittee on Nanoscale Science Engineering and Technology; National Science and Technology Council, editor. The National Nanotechnology Initiative, Supplement to the President’s FY2012 Budget. National Nanotechnology Coordination Office; Arlinton, VA: 2011. [Accessed February 15, 2012]. p. 50. http://www.nano.gov/sites/default/files/pub_resource/nni_2012_budget_supplement.pdf. [Google Scholar]
- Nemmar A, Hoylaerts MF, Hoet PH, Vermylen J, Nemery B. Size effect of intratracheally instilled particles on pulmonary inflammation and vascular thrombosis. Toxicol Appl Pharmacol. 2003;186:38–45. doi: 10.1016/s0041-008x(02)00024-8. [DOI] [PubMed] [Google Scholar]
- Ng KK, Lovell JF, Zheng G. Lipoprotein-inspired nanoparticles for cancer theranostics. Acc Chem Res. 2011;44:1105–13. doi: 10.1021/ar200017e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurkiewicz TR, Porter DW, Hubbs AF, Stone S, Chen BT, Frazer DG, Boegehold MA, Castranova V. Pulmonary nanoparticle exposure disrupts systemic microvascular nitric oxide signaling. Toxicol Sci. 2009;110:191–203. doi: 10.1093/toxsci/kfp051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurkiewicz TR, Porter DW, Hubbs AF, Stone S, Moseley AM, Cumpston JL, Goodwill AG, Frisbee SJ, Perrotta PL, Brock RW, Frisbee JC, Boegehold MA, Frazer DG, Chen BT, Castranova V Committee, H. E. I. H. R. Pulmonary particulate matter and systemic microvascular dysfunction. Res Rep Health Eff Inst. 2011:3–48. [PubMed] [Google Scholar]
- Nurkiewicz TR, Stapleton PG, Minarchick VC, Chen BT, Cumpston J, McKinney W, Frazer D, VC, Nurkiewicz TR. Thinking outside the lung: Alternate routes of nanoparticle exposure. Toxicol Sci (The Toxicologist) 2012;126:197. [Google Scholar]
- Nystrom AM, Wooley KL. The importance of chemistry in creating well-defined nanoscopic embedded therapeutics: Devices capable of the dual functions of imaging and therapy. Accounts Chem Res. 2011;44:969–78. doi: 10.1021/ar200097k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdorster G. Significance of particle parameters in the evaluation of exposure-dose-response relationships of inhaled particles. Inhal Toxicol. 1996;8(Suppl):73–89. [PubMed] [Google Scholar]
- Oberdorster G. Toxicology of ultrafine particles: In vivo studies. Philos Transact R Soc London Series A Math Phy Eng Sci. 2000;358:2719–39. [Google Scholar]
- Oberdorster E. Manufactured nanomaterials (fullerenes, C60) induce oxidative stress in the brain of juvenile largemouth bass. Environ Health Perspect. 2004;112:1058–62. doi: 10.1289/ehp.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdorster G. Safety assessment for nanotechnology and nanomedicine: Concepts of nanotoxicology. J Intern Med. 2010;267:89–105. doi: 10.1111/j.1365-2796.2009.02187.x. [DOI] [PubMed] [Google Scholar]
- Oberdorster G, Elder A, Rinderknecht A. Nanoparticles and the brain: Cause for concern? J Nanosci Nanotechnol. 2009;9:4996–5007. doi: 10.1166/jnn.2009.gr02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdorster G, Maynard A, Donaldson K, Castranova V, Fitzpatrick J, Ausman K, Carter J, Karn B, Kreyling W, Lai D, Olin S, Monteiro-Riviere N, Warheit D, Yang H. Principles for characterizing the potential human health effects from exposure to nanomaterials: Elements of a screening strategy. Part Fibre Toxicol. 2005a;2:8. doi: 10.1186/1743-8977-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113:823–39. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, Cox C. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol. 2004;16:437–45. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- Oh P, Borgstrom P, Witkiewicz H, Li Y, Borgstrom BJ, Chrastina A, Iwata K, Zinn KR, Baldwin R, Testa JE, Schnitzer JE. Live dynamic imaging of caveolae pumping targeted antibody rapidly and specifically across endothelium in the lung. Nat Biotechnol. 2007;25:327–37. doi: 10.1038/nbt1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSHA. Toxic and Hazardous Substances. US Department of Labor, Occupational Safety & Health Administration; 2006. 29 CFR—Occupational Safety and Health Regulations (OSHA Standards) Section 1910. 1000 Table Z-1. [Google Scholar]
- Pawley J. The development of field-emission scanning electron microscopy for imaging biological surfaces. Scanning. 1997;19:324–36. [PubMed] [Google Scholar]
- Perez-Martinez FC, Guerra J, Posadas I, Cena V. Barriers to non-viral vector-mediated gene delivery in the nervous system. Pharm Res. 2011;28:1843–58. doi: 10.1007/s11095-010-0364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R, Kramer E, Oomen AG, Rivera ZE, Oegema G, Tromp PC, Fokkink R, Rietveld A, Marvin HJ, Weigel S, Peijnenburg AA, Bouwmeester H. Presence of nano-sized silica during in vitro digestion of foods containing silica as a food additive. ACS Nano. 2012;6:2441–51. doi: 10.1021/nn204728k. [DOI] [PubMed] [Google Scholar]
- Pittet MJ, Weissleder R. Intravital imaging. Cell. 2011;147:983–91. doi: 10.1016/j.cell.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter DW, Hubbs AF, Chen TB, McKinney W, Mercer RR, Wolfarth MG, Battelli L, Wu N, Sriram K, Leonard S, Andrew ME, Willard P, Tsuruoka T, Endo M, Tsukada T, Munekane F, Frazer DG, Castranova V. Acute pulmonary dose-responses to inhaled multi-walled carbon nanotubes. Nanotoxicology. 2012 doi: 10.3109/17435390.2012.719649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter DW, Hubbs AF, Mercer RR, Wu N, Wolfarth MG, Sriram K, Leonard S, Battelli L, Schwegler-Berry D, Friend S, Andrew M, Chen BT, Tsuruoka S, Endo M, Castranova V. Mouse pulmonary dose- and time course-responses induced by exposure to multi-walled carbon nanotubes. Toxicology. 2010;269:136–47. doi: 10.1016/j.tox.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Preining O. The physical nature of very, very small particles and its impact on their behaviour. J Aerosol Sci. 1998;29:481–95. [Google Scholar]
- Rahman MF, Wang J, Patterson TA, Saini UT, Robinson BL, Newport GD, Murdock RC, Schlager JJ, Hussain SM, Ali SF. Expression of genes related to oxidative stress in the mouse brain after exposure to silver-25 nanoparticles. Toxicol Lett. 2009;187:15–21. doi: 10.1016/j.toxlet.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Rasmussen JC, Kwon S, Sevick-Muraca EM, Cormier JN. The role of lymphatics in cancer as assessed by near-infrared fluorescence imaging. Ann Biomed Eng. 2012;40:408–21. doi: 10.1007/s10439-011-0476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen JC, Tan IC, Marshall MV, Fife CE, Sevick-Muraca EM. Lymphatic imaging in humans with near-infrared fluorescence. Curr Opin Biotechnol. 2009;20:74–82. doi: 10.1016/j.copbio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich ES. Nano rules fall foul of data gap. Nature. 2011;480:160–61. doi: 10.1038/480160a. [DOI] [PubMed] [Google Scholar]
- Riviere JE. Pharmacokinetics of nanomaterials: An overview of carbon nanotubes, fullerenes and quantum dots. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1:26–34. doi: 10.1002/wnan.24. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Fernandez L, Valiente R, Gonzalez J, Villegas JC, Fanarraga ML. Multiwalled carbon nanotubes display microtubule biomimetic properties in vivo, enhancing microtubule assembly and stabilization. ACS Nano. 2012;6:6614–25. doi: 10.1021/nn302222m. [DOI] [PubMed] [Google Scholar]
- Roller J, Laschke MW, Tschernig T, Schramm R, Veith NT, Thorlacius H, Menger MD. How to detect a dwarf: In vivo imaging of nanoparticles in the lung. Nanomedicine. 2011;7:753–62. doi: 10.1016/j.nano.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Roller M. Carcinogenicity of inhaled nanoparticles. Inhal Toxicol. 2009;21:144–57. doi: 10.1080/08958370902942541. [DOI] [PubMed] [Google Scholar]
- Roukes M. Plenty of room, indeed. Sci Am. 2001;285:48–51. 54–7. doi: 10.1038/scientificamerican0901-48. [DOI] [PubMed] [Google Scholar]
- Rowland DJ, Cherry SR. Small-animal preclinical nuclear medicine instrumentation and methodology. Semin Nucl Med. 2008;38:209–22. doi: 10.1053/j.semnuclmed.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Sager TM, Castranova V. Surface area of particle administered versus mass in determining the pulmonary toxicity of ultrafine and fine carbon black: Comparison to ultrafine titanium dioxide. Part Fibre Toxicol. 2009;6:15. doi: 10.1186/1743-8977-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager TM, Wolfarth M, Porter D, Wu N, Hamilton R, Holian A, Castranova V. Activation of the NLRP3 inflammasome correlates with the pulmonary bioactivity of multiwalled carbon nanotubes. Toxicol Sci (The Toxicologist) 2012;126:145. abstract. [Google Scholar]
- Sargent LM, Hubbs AF, Young SH, Kashon ML, Dinu CZ, Salisbury JL, Benkovic SA, Lowry DT, Murray AR, Kisin ER, Siegrist KJ, Battelli L, Mastovich J, Sturgeon JL, Bunker KL, Shvedova AA, Reynolds SH. Single-walled carbon nanotube-induced mitotic disruption. Mutation Research. 2012;745:28–37. doi: 10.1016/j.mrgentox.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent LM, Reynolds SH, Lowry D, Kashon ML, Benkovic SA, Salisbury JL, Hubbs AF, Keane MJ, Mastovich J, Bunker KL, Sturgeon J, Cena L, Dinu CZ. Genotoxicity of multi-walled carbon nanotubes at occupationally relevant doses. Canc Res. 2012;72:Abstract nr 5464. doi: 10.1186/1743-8977-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent LM, Shvedova AA, Hubbs AF, Salisbury JL, Benkovic SA, Kashon ML, Lowry DT, Murray AR, Kisin ER, Friend S, McKinstry KT, Battelli L, Reynolds SH. Induction of aneuploidy by single-walled carbon nanotubes. Environ Mol Mutagen. 2009;50:708–17. doi: 10.1002/em.20529. [DOI] [PubMed] [Google Scholar]
- SCENIHR., S. C. o. E. a. N. I. H. R. E. European Commission. Health & Consumer Protection DG; Brussels: Nov 29, 2007. The Scientific Aspects of the Existing and Proposed Definitions Relating to Products of Nanoscience and Nanotechnologies; p. 22. [Google Scholar]
- SCENIHR: Scientific Committee on Emerging and Newly Identified Health Risks. Scientific Basis for the Definition of the Term “nanomaterial”. European Commission. Directorate-General for Heatlh and Consumers; Brussels: Dec 8, 2010. p. 46. [Google Scholar]
- Schacht V, Ramirez MI, Hong YK, Hirakawa S, Feng D, Harvey N, Williams M, Dvorak AM, Dvorak HF, Oliver G, Detmar M. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 2003;22:3546–56. doi: 10.1093/emboj/cdg342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl JA, Koh AL, Dionne JA. Quantum plasmon resonances of individual metallic nanoparticles. Nature. 2012;483:421–27. doi: 10.1038/nature10904. [DOI] [PubMed] [Google Scholar]
- Service RF. Nanotoxicology: Nanotechnology grows up. Science. 2004;304:1732–34. doi: 10.1126/science.304.5678.1732. [DOI] [PubMed] [Google Scholar]
- Sevick-Muraca EM. Translation of near-infrared fluorescence imaging technologies: Emerging clinical applications. Annu Rev Med. 2012;63:217–31. doi: 10.1146/annurev-med-070910-083323. [DOI] [PubMed] [Google Scholar]
- Shi X, von dem Bussche A, Hurt RH, Kane AB, Gao H. Cell entry of one-dimensional nanomaterials occurs by tip recognition and rotation. Nat Nanotechnol. 2011;6:714–19. doi: 10.1038/nnano.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvedova AA, Castranova V, Kisin ER, Schwegler-Berry D, Murray AR, Gandelsman VZ, Maynard A, Baron P. Exposure to carbon nanotube material: Assessment of nanotube cytotoxicity using human keratinocyte cells. J Toxicol Environ Health A. 2003;66:1909–26. doi: 10.1080/713853956. [DOI] [PubMed] [Google Scholar]
- Shvedova AA, Kisin E, Murray AR, Johnson VJ, Gorelik O, Arepalli S, Hubbs AF, Mercer RR, Keohavong P, Sussman N, Jin J, Yin J, Stone S, Chen BT, Deye G, Maynard A, Castranova V, Baron PA, Kagan VE. Inhalation vs. aspiration of single-walled carbon nanotubes in C57BL/6 mice: Inflammation, fibrosis, oxidative stress, and mutagenesis. Am J Physiol Lung Cell Mol Physiol. 2008;295:L552–65. doi: 10.1152/ajplung.90287.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvedova AA, Kisin ER, Mercer R, Murray AR, Johnson VJ, Potapovich AI, Tyurina YY, Gorelik O, Arepalli S, Schwegler-Berry D, Hubbs AF, Antonini J, Evans DE, Ku BK, Ramsey D, Maynard A, Kagan VE, Castranova V, Baron P. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am J Physiol Lung Cell Mol Physiol. 2005;289:L698–708. doi: 10.1152/ajplung.00084.2005. [DOI] [PubMed] [Google Scholar]
- Shvedova AA, Sager TARM, Kisin E, Porter DW, Leonard SS, Schwegler-Berry D, Robinson VA, Castranova V. Critical issues in the evaluation of possible adverse pulmonary effects resulting from airborne nanoaprticles. In: Monteiro-Riviere NA, Tran CL, editors. Nanotoxicology: Characterization, Dosing and Health Effects. Informa Healthcare USA, Inc; New York, NY: 2007. pp. 225–36. [Google Scholar]
- Singh S, Kumar A, Karakoti A, Seal S, Self WT. Unveiling the mechanism of uptake and sub-cellular distribution of cerium oxide nanoparticles. Mol Biosyst. 2010;6:1813–20. doi: 10.1039/c0mb00014k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley RE. Discovering the fullerenes (Nobel lecture) Angew Chem Int Edit. 1997;36:1595–601. [Google Scholar]
- Smith BR, Cheng Z, De A, Rosenberg J, Gambhir SS. Dynamic visualization of RGD-quantum dot binding to tumor neovasculature and extravasation in multiple living mouse models using intravital microscopy. Small. 2010;6:2222–29. doi: 10.1002/smll.201001022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonawane ND, Szoka FC, Jr, Verkman AS. Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine-DNA polyplexes. J Biol Chem. 2003;278:44826–31. doi: 10.1074/jbc.M308643200. [DOI] [PubMed] [Google Scholar]
- Stanton MF, Layard M, Tegeris A, Miller E, May M, Morgan E, Smith A. Relation of particle dimension to carcinogenicity in amphibole asbestoses and other fibrous minerals. J Natl Cancer Inst. 1981;67:965–75. [PubMed] [Google Scholar]
- Stanton MF, Laynard M, Tegeris A, Miller E, May M, Kent E. Carcinogenicity of fibrous glass: Pleural response in the rat in relation to fiber dimension. J Natl Cancer Inst. 1977;58:587–603. doi: 10.1093/jnci/58.3.587. [DOI] [PubMed] [Google Scholar]
- Stanton MF, Layard MW. Carcinogenicity of natural and man-made fibers. Adv Clin Oncol. 1978;1:181–87. [Google Scholar]
- Steinberg F, Heesom KJ, Bass MD, Cullen PJ. SNX17 protects integrins from degradation by sorting between lysosomal and recycling pathways. J Cell Biol. 2012;197:219–30. doi: 10.1083/jcb.201111121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straight AF, Field CM. Microtubules, membranes and cytokinesis. Curr Biol. 2000;10:R760–70. doi: 10.1016/s0960-9822(00)00746-6. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Guzman JN, Sanchez-Padilla J, Goldberg JA. The origins of oxidant stress in Parkinson’s disease and therapeutic strategies. Antioxid Redox Signal. 2011;14:1289–301. doi: 10.1089/ars.2010.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabet L, Bussy C, Setyan A, Simon-Deckers A, Rossi MJ, Boczkowski J, Lanone S. Coating carbon nanotubes with a polystyrene-based polymer protects against pulmonary toxicity. Part Fibre Toxicol. 2011;8:3. doi: 10.1186/1743-8977-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R, Walton DRM. The chemistry of fullerenes. Nature. 1993;363:685–93. [Google Scholar]
- Teow Y, Asharani PV, Hande MP, Valiyaveettil S. Health impact and safety of engineered nanomaterials. Chem Commun (Camb) 2011;47:7025–38. doi: 10.1039/c0cc05271j. [DOI] [PubMed] [Google Scholar]
- Tjalve H, Henriksson J. Uptake of metals in the brain via olfactory pathways. Neurotoxicology. 1999;20:181–95. [PubMed] [Google Scholar]
- Tran CL, Buchanan D, Cullen RT, Searl A, Jones AD, Donaldson K. Inhalation of poorly soluble particles. II. Influence Of particle surface area on inflammation and clearance. Inhal Toxicol. 2000;12:1113–26. doi: 10.1080/08958370050166796. [DOI] [PubMed] [Google Scholar]
- Vainrub A, Pustovyy O, Vodyanoy V. Resolution of 90 nm (lambda/5) in an optical transmission microscope with an annular condenser. Opt Lett. 2006;31:2855–57. doi: 10.1364/ol.31.002855. [DOI] [PubMed] [Google Scholar]
- Vercauteren D, Rejman J, Martens TF, Demeester J, De Smedt SC, Braeckmans K. On the cellular processing of non-viral nanomedicines for nucleic acid delivery: Mechanisms and methods. J Control Release. 2012;161:566–81. doi: 10.1016/j.jconrel.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Villanueva A, Canete M, Roca AG, Calero M, Veintemillas-Verdaguer S, Serna CJ, del Morales MP, Miranda R. The influence of surface functionalization on the enhanced internalization of magnetic nanoparticles in cancer cells. Nanotechnology. 2009;20:115103. doi: 10.1088/0957-4484/20/11/115103. [DOI] [PubMed] [Google Scholar]
- Vinogradov S, Wei X. Cancer stem cells and drug resistance: The potential of nanomedicine. Nanomedicine (Lond) 2012;7:597–615. doi: 10.2217/nnm.12.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volokitin Y, Sinzig J, deJongh LJ, Schmid G, Vargaftik MN, Moiseev II. Quantum-size effects in the thermodynamic properties of metallic nanoparticles. Nature. 1996;384:621–23. [Google Scholar]
- Wagner S, Zensi A, Wien SL, Tschickardt SE, Maier W, Vogel T, Worek F, Pietrzik CU, Kreuter J, von Briesen H. Uptake mechanism of ApoE-modified nanoparticles on brain capillary endothelial cells as a blood-brain barrier model. PLoS One. 2012;7:e32568. doi: 10.1371/journal.pone.0032568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Byrne JD, Napier ME, DeSimone JM. More effective nanomedicines through particle design. Small. 2011a;7:1919–31. doi: 10.1002/smll.201100442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Flanagan DR. General solution for diffusion-controlled dissolution of spherical particles. 2. Evaluation of experimental data. J Pharm Sci. 2002;91:534–42. doi: 10.1002/jps.10039. [DOI] [PubMed] [Google Scholar]
- Wang LV, Hu S. Photoacoustic tomography: In vivo imaging from organelles to organs. Science. 2012;335:1458–62. doi: 10.1126/science.1216210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Gao S, Ye WH, Yoon HS, Yang YY. Co-delivery of drugs and DNA from cationic core-shell nanoparticles self-assembled from a biodegradable copolymer. Nature materials. 2006;5:791–96. doi: 10.1038/nmat1737. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang B, Zhu MT, Li M, Wang HJ, Wang M, Ouyang H, Chai ZF, Feng WY, Zhao YL. Microglial activation, recruitment and phagocytosis as linked phenomena in ferric oxide nanoparticle exposure. Toxicol Lett. 2011b;205:26–37. doi: 10.1016/j.toxlet.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Wang Z, Tiruppathi C, Cho J, Minshall RD, Malik AB. Delivery of nanoparticle: Complexed drugs across the vascular endothelial barrier via caveolae. IUBMB life. 2011c;63:659–67. doi: 10.1002/iub.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warheit DB. How meaningful are the results of nanotoxicity studies in the absence of adequate material characterization? Toxicol Sci. 2008;101:183–85. doi: 10.1093/toxsci/kfm279. [DOI] [PubMed] [Google Scholar]
- Warheit DB, Webb TR, Colvin VL, Reed KL, Sayes CM. Pulmonary bioassay studies with nanoscale and fine-quartz particles in rats: Toxicity is not dependent upon particle size but on surface characteristics. Toxicol Sci. 2007;95:270–80. doi: 10.1093/toxsci/kfl128. [DOI] [PubMed] [Google Scholar]
- Warheit DB, Webb TR, Sayes CM, Colvin VL, Reed KL. Pulmonary instillation studies with nanoscale tio2 rods and dots in rats: Toxicity is not dependent upon particle size and surface area. Toxicol Sci. 2006;91:227–36. doi: 10.1093/toxsci/kfj140. [DOI] [PubMed] [Google Scholar]
- Weinkauf H, Brehm-Stecher BF. Enhanced dark field microscopy for rapid artifact-free detection of nanoparticle binding to Candida albicans cells and hyphae. Biotechnol J. 2009;4:871–79. doi: 10.1002/biot.200800358. [DOI] [PubMed] [Google Scholar]
- Wessels JT, Yamauchi K, Hoffman RM, Wouters FS. Advances in cellular, subcellular, and nanoscale imaging in vitro and in vivo. Cytometry. 2010;77:667–76. doi: 10.1002/cyto.a.20931. [DOI] [PubMed] [Google Scholar]
- Whitesides GM, Mathias JP, Seto CT. Molecular self-assembly and nanochemistry: A chemical strategy for the synthesis of nanostructures. Science. 1991;254:1312–19. doi: 10.1126/science.1962191. [DOI] [PubMed] [Google Scholar]
- Wohlfart S, Gelperina S, Kreuter J. Transport of drugs across the blood-brain barrier by nanoparticles. J Control Release. 2012;161:264–73. doi: 10.1016/j.jconrel.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Woodrow Wilson International Center for Scholars and the Pew Charitable Trusts. Analysis, Consumer Products Inventory. The Project on Emerging Technologies; 2011. [Accessed August 24 2012]. http://www.nanotechproject.org/inventories/consumer/analysis_draft/ [Google Scholar]
- Wu ZX, Barker JS, Batchelor TP, Dey RD. Interleukin (IL)-1 regulates ozone-enhanced tracheal smooth muscle responsiveness by increasing substance P (SP) production in intrinsic airway neurons of ferret. Respir Physiol Neurobiol. 2008;164:300–11. doi: 10.1016/j.resp.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia T, Kovochich M, Liong M, Zink JI, Nel AE. Cationic polystyrene nanosphere toxicity depends on cell-specific endocytic and mitochondrial injury pathways. ACS Nano. 2008;2:85–96. doi: 10.1021/nn700256c. [DOI] [PubMed] [Google Scholar]
- Yamago S, Tokuyama H, Nakamura E, Kikuchi K, Kananishi S, Sueki K, Nakahara H, Enomoto S, Ambe F. In vivo biological behavior of a water-miscible fullerene: 14C labeling, absorption, distribution, excretion and acute toxicity. Chem Biol. 1995;2:385–89. doi: 10.1016/1074-5521(95)90219-8. [DOI] [PubMed] [Google Scholar]
- Zhang LW, Yang J, Barron AR, Monteiro-Riviere NA. Endocytic mechanisms and toxicity of a functionalized fullerene in human cells. Toxicol Lett. 2009;191:149–57. doi: 10.1016/j.toxlet.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Zhang W, Zhang Y, Lobler M, Schmitz KP, Ahmad A, Pyykko I, Zou J. Nuclear entry of hyperbranched polylysine nanoparticles into cochlear cells. Int J Nanomedicine. 2011;6:535–46. doi: 10.2147/IJN.S16973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Gao Y, Brook MA, Li Y. Wrapping single-walled carbon nanotubes with long single-stranded DNA molecules produced by rolling circle amplification. Chem Commun (Camb) 2006:3582–84. doi: 10.1039/b606518j. [DOI] [PubMed] [Google Scholar]
- Zheng M, Jagota A, Strano MS, Santos AP, Barone P, Chou SG, Diner BA, Dresselhaus MS, McLean RS, Onoa GB, Samsonidze GG, Semke ED, Usrey M, Walls DJ. Structure-based carbon nanotube sorting by sequence-dependent DNA assembly. Science. 2003;302:1545–48. doi: 10.1126/science.1091911. [DOI] [PubMed] [Google Scholar]







