Interleukin receptor-associated kinase (IRAK) family mediates signals downstream of various pathogen- and cytokine-responsive receptors [1,2]. IRAK proteins consist of four functionally and structurally related members (IRAK1–4). In the context of hematologic disorders, IRAK1 and IRAK4 are the most widely studied [3], and are both ubiquitously expressed [4]. Under normal cellular conditions, MyD88 is recruited to activated Toll-like receptors (TLRs) or Inter-leukin 1 receptor (IL1R) resulting in activation of IRAK4 and IRAK1 [5]. Activated IRAK1/4 proteins then bind TRAF6 mediating NF-κB signaling. Activating mutations of MyD88 or B cell receptor result in chronic IRAK4 phosphorylation and downstream pathway activation in human B cell lymphoma, particularly in the activated B cell–like (ABC) subset of diffuse large B cell lymphoma (DLBCL) [3,6]. Knockdown of MyD88, IRAK4, or IRAK1 abrogates NF-κB pathway activation and induces ABC DLBCL cell death [3]. Interestingly, IRAK4 catalytic function is necessary for maintaining the viability of DLBCL cells, whereas the catalytic function of IRAK1 is dispensable [3]. These critical observations strongly implicate the dependency of ABC DLBCL on IRAK4 function.
More recently, we have reported that IRAK1 exists in an activated state (e.g., constitutively phosphorylated on threonine-209) in a large subset of human myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) samples [7]. In addition, overexpression of TLR1/2/6 has been reported in MDS, and MDS-associated mutations of TLR2 correspond with increased IRAK1 activation [8]. MDS originates within the hematopoietic stem cell compartment and manifests into a multilineage erythro/myeloid disease [9]. Patients with MDS also have a proclivity to develop AML [9]. Knockdown of IRAK1 in MDS marrow cells and in a panel of MDS/AML cell lines resulted in cell cycle arrest, apoptosis, and impaired leukemic progenitor function. To further validate these findings, we treated cells with an IRAK1/4 Inhibitor. Consistent with the knockdown experiments, IRAK1/4 Inhibitor impaired MDS/AML cell viability and progenitor function, which also coincided with reduced levels of phosphorylated IRAK1, but not IRAK4.
Given the importance of the IRAK1/IRAK4 complex in human hematologic malignancies, we decided to investigate the role of IRAK4 in MDS. To discern differences between the expression of IRAK1 and IRAK4, published microarray data from MDS CD34+ cells were examined [10]. IRAK4 expression is extremely low (at the lower limit of detection) and not significantly different as compared with control CD34+ cells (p = 0.073; Figure 1). By comparison, IRAK1 is preferentially expressed in normal CD34+ cells and further overexpressed in a subset (~20%) of MDS patients (p = 0.036; Figure 1). To evaluate the contribution of IRAK1 versus IRAK4 in MDS cells functionally, we performed RNAi-mediated knockdown experiments. An MDS cell line (MDSL) transduced with shRNA targeting IRAK1 or IRAK4 were first evaluated for RNA and protein knockdown. As shown in Figures 1B and C, shIRAK1 clone #17 and shIRAK4 clone #65 resulted in approximately 90% knockdown of the respected targets; therefore, these shRNA clones were selected for further validation. As described extensively in our recent report [7], knockdown of IRAK1 in MDSL cells results in apoptosis (Figure 1D) and impaired progenitor function in methylcellulose (Figure 1E). In contrast, knockdown of IRAK4 did not contribute to significant cell death of MDS cells (Figure 1D). Furthermore, knockdown of IRAK4 in MDSL reduced progenitor function (p = 0.0013), but not as dramatically as seen with knockdown of IRAK1 (p = 0.0004; Figure 1E). Under stimulated conditions or in DLBCL, IRAK4 phosphorylates IRAK1. Interestingly, knockdown of IRAK4 in MDS cells negligibly affects phosphorylated IRAK1 levels (Figure 1F), suggesting that IRAK1 is activated by alternative mechanisms in MDS. These findings reveal differences in IRAK1 versus IRAK4 dependency in MDS.
Figure 1.
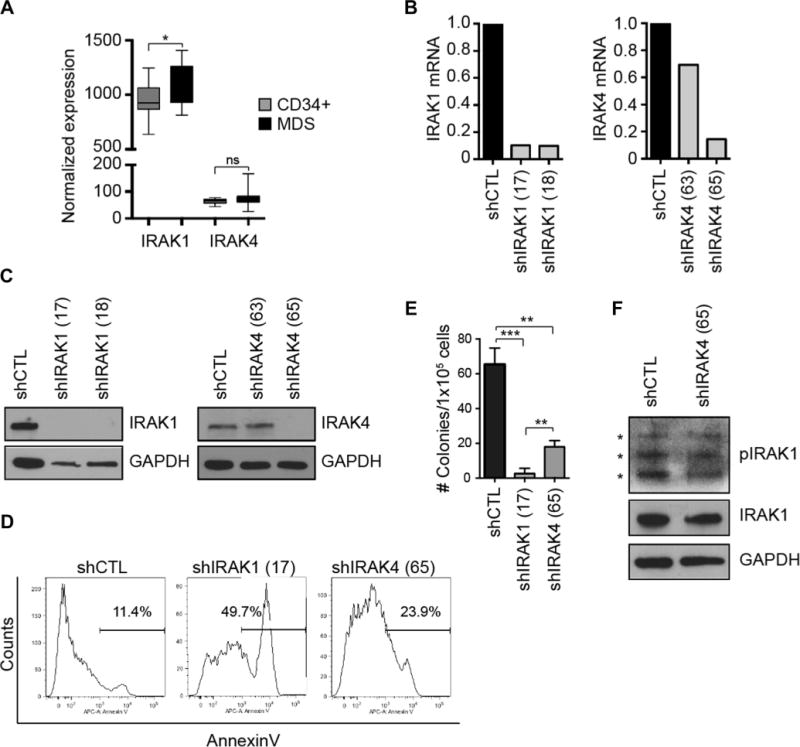
MDS cells depend on IRAK1, but less on IRAK4, for maintaining viability and progenitor function. (A) IRAK1 and IRAK4 expression is obtained from a gene expression study on CD34+ cells isolated from control and MDS marrows (IRAK1; p = 0.036) [10]. (B) IRAK1 and IRAK4 mRNA knockdown was confirmed in MDSL cells by quantitative real-time polymerase chain reaction in cells expressing a control (shCTL), shIRAK1 (clone #17 and #18), or shIRAK4 (clone #63 or #65) lentiviral vector. (C) IRAK1 and IRAK4 protein knockdown was confirmed in MDSL cells by immunoblotting cells expressing shCTL, shIRAK1, or shIRAK4 lentiviral vector. (D) Annexin V staining by flow cytometry in MDSL cells was measured after transduction with shIRAK1- or shIRAK4-expressing lentiviral vectors as described previously [7]. (E) MDSL cells transduced with lentiviral vectors containing either shIRAK1 or shIRAK4 were plated at 2 × 104 cells/mL in methylcellulose. Colonies were counted 11 days after plating. (F) MDSL cells transduced with lentiviral vectors containing shIRAK4 or shCTL (same lysates as in C) were analyzed for pIRAK1 (at threonine-209) and total IRAK1. ns = nonspecific.
IRAK1 and IRAK4 are related kinases within the innate immune pathway. However, their roles in myeloid versus lymphoid malignancies appear to be distinct. The work by Staudt and colleagues has clearly established a critical role of IRAK4 in DLBCL and the efficacy of targeting IRAK4 using an IRAK1/4 inhibitor [3]. We propose that IRAK1, but not IRAK4, is essential in the pathogenesis of MDS/AML, and that small molecules selectively targeting IRAK1 may be therapeutically beneficial in MDS/AML. In conclusion, the innate immune pathway involving IRAK1 and IRAK4 signaling is important in the pathogenesis of hematologic malignancies, but their individual contribution is lineage or disease specific, or both. As such, further research to discern the individual contribution of IRAK1 and IRAK4 to hematologic malignancies is warranted. Nevertheless, development of next generation IRAK inhibitors could be beneficial in both myeloid and lymphoid malignancies.
References
- 1.Flannery S, Bowie AG. The interleukin-1 receptor-associated kinases: critical regulators of innate immune signalling. Biochem Pharmacol. 2010;80:1981–1991. doi: 10.1016/j.bcp.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 2.Janssens S, Beyaert R. Functional diversity and regulation of different interleukin-1 receptor-associated kinase (IRAK) family members. Mol Cell. 2003;11:293–302. doi: 10.1016/s1097-2765(03)00053-4. [DOI] [PubMed] [Google Scholar]
- 3.Ngo VN, Young RM, Schmitz R, Jhavar S, Xiao W, Lim KH, Kohlhammer H, Xu W, Yang Y, Zhao H, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470:115–119. doi: 10.1038/nature09671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao Z, Henzel W, Gao X. IRAK: a kinase associated with the interleukin-1 receptor. Science. 1996;271:1128–1131. doi: 10.1126/science.271.5252.1128. [DOI] [PubMed] [Google Scholar]
- 5.Burns K, Martinon F, Esslinger C, et al. MyD88, an adapter protein involved in interleukin-1 signaling. J Biol Chem. 1998;273:12203–12209. doi: 10.1074/jbc.273.20.12203. [DOI] [PubMed] [Google Scholar]
- 6.Davis RE, Ngo VN, Lenz G, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463:88–92. doi: 10.1038/nature08638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhyasen GW, Bolanos L, Fang J, et al. Targeting IRAK1 as a Therapeutic Approach for Myelodysplastic Syndrome. Cancer Cell. 2013;24:90–104. doi: 10.1016/j.ccr.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei Y, Dimicoli S, Bueso-Ramos C, et al. Toll-like receptor alterations in myelodysplastic syndrome. Leukemia. 2013;27:1832–1840. doi: 10.1038/leu.2013.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corey SJ, Minden MD, Barber DL, Kantarjian H, Wang JC, Schimmer AD. Myelodysplastic syndromes: the complexity of stem-cell diseases. Nat Rev Cancer. 2007;7:118–129. doi: 10.1038/nrc2047. [DOI] [PubMed] [Google Scholar]
- 10.Pellagatti A, Cazzola M, Giagounidis A, et al. Deregulated gene expression pathways in myelodysplastic syndrome hematopoietic stem cells. Leukemia. 2010;24:756–764. doi: 10.1038/leu.2010.31. [DOI] [PubMed] [Google Scholar]


