Abstract
The aim of the present study was to investigate the generation of cell sheet-engineered bones used for the reconstruction of mandibular defects. Bone marrow stem cells (BMSCs) were cultured and induced to generate osteoblasts. Poly(lactic-co-glycolic acid) (PLGA) scaffolds were wrapped with or without cell sheets and then implanted into dogs with mandibular defects in the right side (experimental group) or the left side (control group), respectively. Subsequently, X-ray analyses, and hematoxylin and eosin staining were performed at various time points (at 4, 8, 12 or 16 weeks post-implantation; n=4 at each time point). The osteogenesis in the experimental group was significantly improved compared with that in the control group. At 16 weeks after implantation, numerous Haversian systems and a few lamellar bones were observed at the periphery. In the control group, the engineered bone (without BMSC sheets) presented fewer Haversian systems and no lamellar bones. The optical density of the fresh bone in the experimental group was significantly higher compared with that in the control group (P<0.05). In conclusion, tissue-engineered bone with the structure of lamellar bones can be generated using BMSC sheets and implantation of these bones had an improved effects compared with the control group. Cell sheet transplantation was found to enhance bone formation at the reconstruction site of the mandibular defects.
Keywords: bone marrow stromal stem cells, cell sheet, tissue-engineered bone, mandibular defect
Introduction
Bone loss is caused by various congenital and degenerative diseases, traumas or incorrect surgical procedures, leading to severe problems, particularly in elderly individuals (1). Tissue engineering is frequently used for the reconstruction of mandible defects. Yamato et al (2) have demonstrated a novel cell sheet engineering method for tissue regeneration, which uses temperature-responsive culture dishes (TRCDs). This technique allows for different types of cultured cells to be noninvasively harvested as intact sheets through simple temperature reduction, without the use of proteolytic enzymes (3). Using this method, the noninvasive transfer of these cell sheets can be achieved, while retaining the typical distributions of Na+/K+-ATPase, glucose transporter-1, sodium-glucose linked transporter-1, aquaporin-1, neutral endopeptidase and dipeptidylendopeptidase IV (4).
In the present study, scaffolds of poly(lactic-co-glycolic acid) (PLGA) were produced, and composited with recombination human bone morphogenetic protein-2 (rhBMP-2) and vascular endothelial growth factor (VEGF). In addition, bone marrow stem cells (BMSCs) were cultured in TRCDs to form BMSCs sheets. PLGA/BMP-2/VEGF wrapped with BMSCs sheets were implanted into dogs with mandibular defects. The aim of the present study was to investigate the effects of tissue-engineered bones that have the same structure as normal bones and can be used for the reconstruction of bones with mandible defects.
Materials and methods
Animals
In this study, 16 healthy, adult, male mongrel dogs (age, 14 months; weight, 18–23 kg) were used. All the animals were obtained from the Laboratory Animal Center of the Affiliated Hospital of Qingdao University (Qingdao, China) and treated under the same standard laboratory conditions. The study was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University.
Osteoinduction of BMSCs
Under general anesthesia (10 mg/kg ketamine and 5 mg/kg phenobarbital), 10 ml of bone marrow was collected from each dog using a disposable syringe and then transferred into a centrifuge tube containing 150 units heparin (Jiangsu Wanbang Biochemical Pharmaceutical Co., Ltd., Xuzhou, China). BMSCs were isolated by density gradient centrifugation (160 × g for 20 min at 4°C) and seeded into 50 ml culture flasks at a density of 1×107/ml. Next, the BMSCs were cultured in low-glucose Dulbecco's modified Eagle's medium (DMEM; Hyclone Laboratories, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS) and incubated continuously at 37°C in a saturated humidified atmosphere of 95% air and 5% CO2 (5). An inverted-phase contrast microscope (IX 50; Olympus Corporation, Tokyo, Japan) was used to observe the cells. When the BMSCs reached 80% confluence, the cells were detached with 0.25% trypsin (including 0.02% EDTA) and subcultured at a ratio of 1:2. Subsequently, the cells were cultured in a different medium in order to induce differentiation into osteoblasts. The medium used was changed from low-glucose DMEM to high-glucose DMEM, which was supplemented with 10% FBS and an osteogenesis-inducing reagent (50 µg/ml ascorbic acid, 10 mM β-glycerophosphate, and 10−4 mM dexamethasone) (6–8).
Preparation of the BMSC sheets
The differentiation-induced BMSCs were seeded in a TRCD (UpCell; Nunc, Thermo Scientific, Basingstoke, UK) and incubated at 37°C in an atmosphere of 95% air and 5% CO2 (2). After 7–10 days, the cells had spread over the entire TRCD. Next, the TRCD was placed at 20°C for 60 min. The BMSCs were then separated from the TRCD to be used as a cell sheet (9–11).
Preparation of the PLGA scaffold
The PLGA scaffold (aperture, 100–300 µm; porosity, 85%; molecular weight, 100,00; Shandong Institute of Medical Instruments, Jinan, China) was shaped into a gengon and a longitudinal groove was made in the gengon to prepare for vessels embedded. The scaffold was examined under scanning electron microscopy (SEM; JSM-840; JEOL, Ltd., Tokyo, Japan). Two growth factors, rhBMP-2 (0.1 µg/ml) and VEGF (5 µg/ml), were added into the PLGA scaffold by lyophilization (12). Following low-temperature plasma sterilization, the scaffold was stored at 4°C (13) The scaffold and gengon were purchased from the Shandong Institute of Medical Instruments (Jinan, China).
Construction of BMSC sheets/PLGA complex
The osteogenically induced BMSCs were detached from the culture flasks using 0.25% trypsin (including 0.02% EDTA) and seeded into the PLGA scaffolds at a density of 1×107/ml, using a disposable syringe. Scaffolds wrapped with or without two BMSC sheets at their surface were used. All the scaffolds were incubated continuously at 37°C in an atmosphere of 95% air and 5% CO2. After 3 days of incubation, these in vitro scaffolds were fixed in 2% glutaric dialdehyde and then characterized using SEM.
Implantation
The 16 dogs were divided into 4 groups (4 dogs per group). Under general anesthesia, the dogs were placed on the operating table in a supine position. The mandibular border was exposed through a 5-cm submandibular skin incision and the mandibular periosteum was carefully dissected. A defect with the same shape as the PLGA scaffold was made in the two sides of the dogs' mandible. Next, PLGA scaffolds wrapped with or without two BMSC sheets were separately implanted into the defects in the two sides. PLGA scaffolds wrapped with two BMSC sheets were implanted into the right side of the mandible, serving as the experimental side. PLGA scaffolds without BMSC sheets were implanted into the left side of the mandible, serving as the control side. The inferior alveolar neurovascular bundle was dissociated from the mandible and embedded in the longitudinal groove of the scaffold. The incision on the soft tissue was sutured to stabilise the scaffold. Each dog was injected with penicillin (400 IU/day) for 7 days after the implantation.
X-ray analyses
After anesthesia, the dogs were sacrificed at 4, 8, 12 and 16 weeks after surgery. General observation and X-ray examination were performed. The X-ray examination was performed under the same conditions for all dogs (voltage, 60 kV; current, 2.51 mA; time, 0.04 sec; distance, 1 m). In the present study, the optical density of the X-ray images, which is identified as the gray value of the images and indicates the degree of bone regeneration, was measured using the Image Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA) was measured and analyzed using the Image Pro Plus 6.0 software. Specimens (5×5 mm) were selected from the same part of the bone regeneration tissue at both sides of the mandible and were embedded in paraffin for preservation.
Hematoxylin and eosin (H&E) staining
Tissue specimens were collected for H&E staining (Beyotime Institute of Biotechology, Haimen, Jiangsu, China) and observed under the inverted-phase contrast microscope. Three fields under 100× magnification were randomly selected for measurement. The initially repaired, the Haversian system and the new blood vessel areas were measured. Then the percentages of the new bone and new blood vessel areas were calculated in relation to the total, initially repaired area, and were then analyzed. All measurements and analyses were performed using the Image Pro Plus 6.0 software.
Bone hardness analysis
Bone tissue specimens were collected from the new bone and surrounding area, and fixed in a gypsum base. The gypsum base used in the present study was produced using ultra-anhydrite power by the lab of the Qingdao University. The ultra-anhydrite power (XSC-20) was purchased from Yuyao Xinshi Gypsum Products Co., Ltd. (Yuyao, China). The bone hardness was analyzed with a hardness tester (HXD-1000TMC/LCD; Taiming Optical Instrument Co., Ltd., Shanghai, China). The hardness of each specimen was independently determined three times (14).
Statistical analysis
Statistical analysis was performed using the SPSS version 16.0 software (SPSS, Inc., Chicago, IL, USA). All the data are presented as the mean ± standard deviation. Statistically significant differences were indicated by P<0.05 and were determined among the week 4, 8, 12 and 16 groups using analysis of variance and the least significant difference test. Differences (P<0.05) between the control and experimental groups were detected using t-test.
Results
Establishment of BMSC sheets-PLGA scaffolds complex
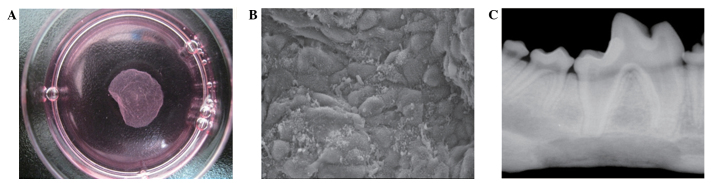
At 10 days after BMSCs were seeded, the cells spread over the entire bottom of the TRCD and were closely-arranged (Fig. 1A). The dishes were placed in a new incubator with a humidified atmosphere of 5% CO2 at 20°C and the cell sheets were detached from the edge of the dish (15). Approximately 60 min were required for complete cell sheet detachment.
Figure 1.
Cell sheets used in the present study. (A) Cell sheets and dish separated following placement in a temperature-responding culture dish at 20°C for 1 h. The differentiation-induced BMSCs were seeded in a temperature-responding culture dish and incubated at 37°C in an atmosphere of 95% air and 5% CO2. After 7–10 days, the cells had spread over the entire dish. Next, the BMSCs were separated from the dish and used as a cell sheet. (B) Scanning electron microscopy image of cell sheet adhered to the surface of the scaffold. The cells were secreted on the extracellular matrix. (C) A representative X-ray radiograph of the mandible in the experimental group at week 16 after implantation, performed under the following conditions: Voltage, 60 kV; current, 2.51 mA; time, 0.04 sec; and distance, 1 m. BMSCs, bone marrow stem cells.
As shown in Fig. 1B, the PLGA scaffolds had a porous three-dimensional structure (aperture, 100–300 µm), as observed using SEM. At 3 days after seeding, the BMSCs were well-adhered and adequately proliferated, and had extended on the surface and pores of the three-dimensional scaffold. Cell sheets were adhered to the surface of the scaffold and secreted on a large part of the extracellular matrix (Fig. 1B).
Implantation
All dogs were healthy during the experiments. In the experimental group, the outline of the scaffold was observed 4 weeks post-implantation, with fibrous connective tissues surrounding the complex. From 8 weeks post-implantation, absorption of the scaffolds was observed, while at 12 weeks, the majority of the defects were reconstructed by the new engineered bones. At 16 weeks post-implantation, the areas of new bones were larger than at 12 weeks. Compact bones were observed at the lingual of the mandible and the defects of the mandible had been completely reestablished with the new engineered bones. In the experimental group, substantial new bone was observed without a clear boundary between the newly formed tissue-engineered bones and the native bones. In the control group, the size of the new bone was significantly smaller compared with that in the experimental group. At 16 weeks, the percentage of the new bone area in relation to the initially repaired area in the experimental group was 90.5±2.3, significantly higher than that in the control group (79.3±3.4) (P<0.05).
As shown in Table I, the bone trabecula and OD of the new bone increased gradually between weeks 4 and 16 post-implantation. The ODs in the experimental group were significantly higher compared with those in the control group. Furthermore, the ODs of bones in the experimental group at 16 weeks (Fig. 1C) after implantation were higher in all the new bones, but remained lower than those of the normal mandible. Higher optical density values demonstrated by X-ray are associated with the more mineral contents in the bone. In addition, the optical density values also reflect the maturity of the new bone indirectly.
Table I.
Optical density detected at 4, 8, 12 and 16 weeks after surgery.
| Optical density at different times after surgery | ||||
|---|---|---|---|---|
| Groups | 4 weeks | 8 weeks | 12 weeks | 16 weeks |
| Experimental | 0.319±0.001a | 0.362±0.020a | 0.378±0.009a | 0.616±0.044a |
| Control | 0.231±0.005 | 0.256±0.020 | 0.326±0.019 | 0.572±0.042 |
P<0.05, vs. control group.
Bone hardness analysis
As shown in Fig. 2, bone hardness values in the control and experimental groups were not detectably increased at 4 and 8 weeks after implantation (P>0.05). By contrast, at 12 and 16 weeks after implantation, the values of bone hardness in the experimental group were significantly higher compared with the values in the control group (P<0.05). The bone hardness at 12 and 16 weeks after implantation were increased when compared with the values at 4 and 8 weeks after implantation, but were lower compared with the values of hardness of the adjacent normal bones (data not shown).
Figure 2.
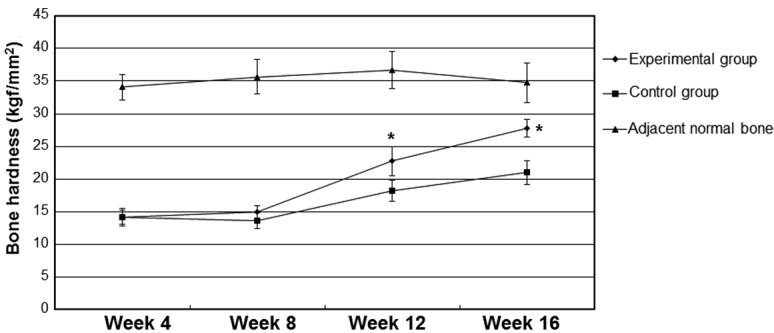
Changes in the bone hardness following implantation. Bone tissue specimens were selected from the new bone and surrounding area, and were fixed in a gypsum base. The bone hardness of each specimen was independently determined three times. *P<0.05 vs. the control group.
Histological examination and histomorphological analysis
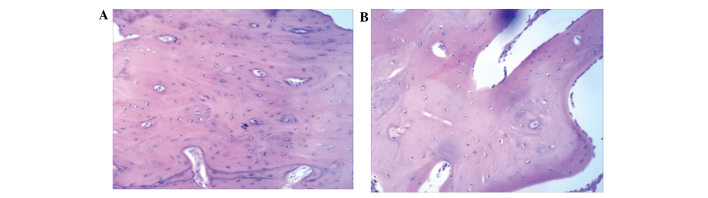
As shown in Fig. 3A, at 16 weeks after implantation, thick bone trabecula was found to be connected in the experimental side. Several Haversian bone systems and a few lamellar bones were observed at the surrounding region. Bone trabecula and red bone marrow were observed at the center of the new engineered bone (Fig. 3A). In the control side, a large number of blood vessels was observed (Fig. 3B). The control group had fewer Haversian bone systems compared with the experimental group, and no lamellar bones were identified (Fig. 3B). These results suggest that tissue-engineered bones with the structure of lamellar bones can be generated using BMSC sheets.
Figure 3.
Histological examination of new bones in the (A) experimental and (B) control groups. Tissue specimens were collected for hematoxylin and eosin staining and were observed under an inverted phase contrast microscope.
Discussion
BMSCs can be induced to generate various types of cells, including osteoblast and fat cells (16). Due to this property, BMSCs have been used in numerous fields. In the experiments of the present study, differentiation of dog BMSCs into osteoblasts was induced in vitro, and then the osteoblasts were implanted into porous biodegradable scaffold materials. In the cell sheets obtained, the BMSCs were arranged compactly, similar to their arrangement in normal bones. Subsequently, porous scaffold materials were wrapped with the cell sheets composed of osteoblasts.
Scaffolds are able to provide cells with an environment required for their differentiation, defining the ultimate shape of the engineered cartilage. They must be biocompatible, biodegradable, highly porous, mechanically strong and malleable (17). In the present study, PLGA scaffolds were used, which are biocompatible and have been approved by the Food and Drug Administration for certain human clinical applications (18). These were three-dimensional porous scaffolds with a pole diameter of 300–400 µm, which was beneficial to the growth of cells and blood vessels. In addition, their 80% porosity was beneficial to the growth of osteoblasts (19). The PLGA scaffolds used in the current study resembled inverted trapezia and had a groove on the back side in order to accommodate mandible nerves and vessels. This not only ensures the stability of the complex, but also provides large pieces of tissue-engineered bone with blood supply.
In the current study, after 16 weeks of the scaffold-BMSC sheet complex implantation, the mandibular border defect was almost completely repaired and the characteristics of the new engineered bone were similar to those of the normal mandible in the experimental groups; however, this was not achieved in the control group. The new engineered bone had a Haversian system structure, with thick bone trabecula and red bone marrow. The hardness of the new engineered bone was similar to that of the normal surrounding mandible in the experimental groups; however, these characteristics were not observed in the control group. The current study demonstrated a novel method for the reconstruction of large mandibular defects.
In conclusion, through the combination of a PLGA scaffold and BMSC sheets, engineered bone was successfully generated and the mandibular defect was reconstructed with a significant effect. Cell sheet transplantation was found to enhance bone formation at the reconstruction of mandibular defect.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (no. 30872896) and the Natural Science Foundation of Shandong Province of China (no. Y2008C7).
References
- 1.d'Aquino R, De Rosa A, Lanza V, Tirino V, Laino L, Graziano A, Desiderio V, Laino G, Papaccio G. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur Cell Mater. 2009;18:75–83. doi: 10.22203/ecm.v018a07. [DOI] [PubMed] [Google Scholar]
- 2.Yamato M, Akiyama Y, Kobayashi J, Yang J, Kikuchi A, Okano T. Temperature-responsive cell culture surfaces for regenerative medicine with cell sheet engineering. Prog Polym Sci. 2007;32:1123–1133. doi: 10.1016/j.progpolymsci.2007.06.002. [DOI] [Google Scholar]
- 3.Sekiya S, Shimizu T, Yamato M, Kikuchi A, Okano T. Bioengineered cardiac cell sheet grafts have intrinsic angiogenic potential. Biochem Biophys Res Commun. 2006;341:573–582. doi: 10.1016/j.bbrc.2005.12.217. [DOI] [PubMed] [Google Scholar]
- 4.Kushida A, Yamato M, Isoi Y, Kikuchi A, Okano T. A noninvasive transfer system for polarized renal tubule epithelial cell sheets using temperature-responsive culture dishes. Eur Cell Mater. 2005;10:23–30. doi: 10.22203/ecm.v010a03. discussion 23–30. [DOI] [PubMed] [Google Scholar]
- 5.Bi W, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Sox9 is required for cartilage formation. Nat Genet. 1999;22:85–89. doi: 10.1038/8792. [DOI] [PubMed] [Google Scholar]
- 6.Vortkamp A, Pathi S, Peretti GM, Caruso EM, Zaleske DJ, Tabin CJ. Recapitulation of signals regulating embryonic bone formation during postnatal growth and in fracture repair. Mech Dev. 1998;71:65–76. doi: 10.1016/S0925-4773(97)00203-7. [DOI] [PubMed] [Google Scholar]
- 7.Ferguson CM, Miclau T, Hu D, Alpern E, Helms JA. Common molecular pathways in skeletal morphogenesis and repair. Ann NY Acad Sci. 1998;857:33–42. doi: 10.1111/j.1749-6632.1998.tb10105.x. [DOI] [PubMed] [Google Scholar]
- 8.Vortkamp A, Lee K, Lanske B, Segre GV, Kronenberg HM, Tabin CJ. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science. 1996;273:613–622. doi: 10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- 9.Hayashida Y, Nishida K, Yamato M, Watanabe K, Maeda N, Watanabe H, Kikuchi A, Okano T, Tano Y. Ocular surface reconstruction using autologous rabbit oral mucosal epithelial sheets fabricated ex vivo on a temperature-responsive culture surface. Invest Ophthalmol Vis Sci. 2005;46:1632–1639. doi: 10.1167/iovs.04-0813. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu T, Yamato M, Kikuchi A, Okano T. Cell sheet engineering for myocardial tissue reconstruction. Biomaterials. 2003;24:2309–2316. doi: 10.1016/S0142-9612(03)00110-8. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura A, Akahane M, Shigematsu H, Tadokoro M, Morita Y, Ohgushi H, Dohi Y, Imamura T, Tanaka Y. Cell sheet transplantation of cultured mesenchymal stem cells enhances bone formation in a rat nonunion model. Bone. 2010;46:418–424. doi: 10.1016/j.bone.2009.08.048. [DOI] [PubMed] [Google Scholar]
- 12.Zhao YH, Yang Q, Xia Q, Peng J, Lu SB, Guo QY, Ma XL, Xu BS, Hu YC, Zhao B, et al. In vitro cartilage production using an extracellular matrix-derived scaffold and bone marrow-derived mesenchymal stem cells. Chin Med J (Engl) 2013;126:3130–3137. [PubMed] [Google Scholar]
- 13.Galiano RD, Tepper OM, Peb CR, Bhatt KA, Callaghan M, Bastidas N, Bunting S, Steinmetz HG, Gurtner GC. Topical vasular endothelial growth factor acceleretes diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow derived cells. Am J Pathol. 2004;164:1935–1947. doi: 10.1016/S0002-9440(10)63754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang LJ, Jin Y. Immunohistochemical observations on bone morphogenetic protein in normal and abnormal conditions. Clin Orthop Relat Res. 1990;257:249–256. [PubMed] [Google Scholar]
- 15.Hata H, Bär A, Dorfman S, Vukadinovic Z, Sawa Y, Haverich A, Hilfiker A. Engineering a novel three-dimensional contractile myocardial patch with cell sheets and decellularised matrix. Eur J Cardiothorac Surg. 2010;38:450–455. doi: 10.1016/j.ejcts.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: Revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Ran W, Wang GL, Jing XD. Biocompatibility of new bone tissue engineering scaffolds in vivo. Hua Xi Kou Qiang Yi Xue Za Zhi. 2009;27:447–450. (In Chinese) [PubMed] [Google Scholar]
- 18.Chen G, Sato T, Ushida T, Ochiai N, Tateishi T. Tissue engineering of cartilage using a hybrid scaffold of synthetic polymer and collagen. Tissue Eng. 2004;10:323–330. doi: 10.1089/107632704323061681. [DOI] [PubMed] [Google Scholar]
- 19.Xu WF, Liao XL. Evaluation of cell compatibility for porous 3D CF/PLA/CS composites scaffold in vitro. Ying Yong Hua Xue. 2011;28:214–218. (In Chinese) [Google Scholar]