Abstract
Background:
Intraventricular meningiomas constitute 2% of intracranial meningiomas, representing a challenging disease for neurosurgeons; we report our experience through a case series, emphasizing surgical approaches and results.
Methods:
Between 2009 and 2012, four patients underwent microsurgical resection in our department. Clinical and imaging findings, surgical approaches, outcomes, and follow-up were analyzed.
Results:
Four patients (three females and one male) were included and the signs of intracranial hypertension were the main clinical presentation in all cases. The parietal approach through intraparietal sulcus was performed in 3 cases and parieto-occipital interhemispheric surgical route in 1 case. Gross total resection was achieved in all the patients without additional deficits and without the aid of neuronavigation, intraoperative monitoring, and intraoperative magnetic resonance imaging.
Conclusion:
Gross total resection is the gold standard treatment for such tumors and the intraparietal sulcus approach is an excellent choice for most of the cases. Careful anatomical knowledge contributes to a safer procedure even in the absence of high tech equipment assistance.
Keywords: Lateral ventricle, meningioma, trigone
BACKGROUND
Intraventricular meningiomas are uncommon lesions which represent about 0.5–5% (average 2%) of all meningiomas. The most common location is atrioventricular.[6] A review of 500 cases by Criscuolo and Symon[2] revealed 10 cases of intraventricular meningiomas, accounts for 2% of the total incidence rate. About 80% were in lateral ventricle, 15% in the posterior third of the third ventricle, and 5% in the fourth ventricle. The incidence of ventricular meningiomas was higher in pediatric patients. Cushing and Eisenhardt reviewed 15 series in the literature, which arrived at a total of 298 meningiomas in children of which 9.4% were intraventricular. Surgical resection in these cases is difficult without adding new neurological deficits. Hence, we found in literature a morbidity rate of up to 42%.[3] Although, many approaches have been described in the literature for these tumors in order to provide a better exposure and facilitate a gross total resection. We present four patients in a case series of ventricular trigone's meningiomas operated in our department between 2009 and 2013. The objective is to perform a comparative analysis of our results with the literature and describe the most suitable approach for each case.
Objectives
Atrioventricular meningiomas are very rare lesions. We present 4 cases with a literature review of the surgical decision process, with an emphasis on approaches and management issues as well as outcomes. Also, we report the experience of our department with four meningiomas of ventricular trigone operated without neuronavigation, intraoperative monitoring, and intraoperative magnetic resonance imaging (MRI) and discuss the most suitable surgical approach based on literature.
METHODS
A retrospective review of four patients who underwent a surgical operation at a reference center in neurosurgery (Hospital of Restoration - Recife-PE) between April 2009 and March 2013 [Table 1]. All the lesions were confirmed to be atrioventricular meningiomas at the histological examination. Access to information in medical records was authorized by the Ethics Committee.
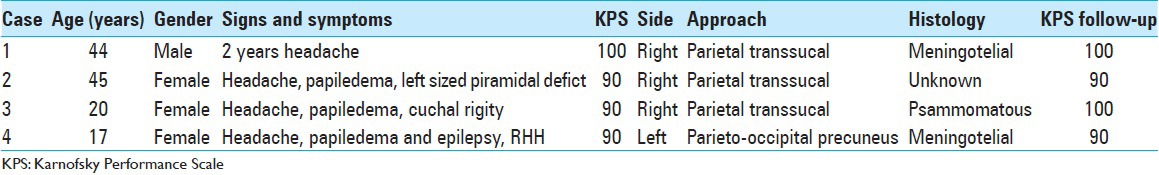
Table 1.
Epidemiological, clinical, surgical approach and results for the four patients
REPORTS
Case 1
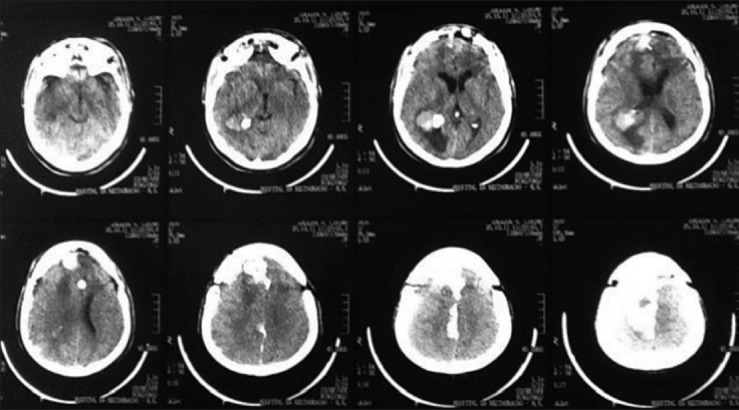
A 44-year-old male patient presented with morning headache and no other complains at the emergency room. Physical examination showed no neurological deficits. The computed tomography (CT) scan revealed a hyperdense lesion with homogeneous and intense contrast enhancement in the right ventricular trigone. The patient was submitted to surgery and placed supine with the head in neutral position fixed with Mayfield. A “C” shaped incision was performed and followed by a craniotomy of 6 cm lateral to the midline and 8 cm anterior to lambda. A dural opening with the base facing the superior sagital sinus was made. At this point, the intraparietal sulcus was identified running parallel and 3 cm lateral to the midline. Trans-sulcal dissection was performed toward the ventricular atrium's roof. A straight line direction is fundamental due to the fact that optical radiation fibers are laterally and inferiorly localized. Tumor debulking and resection was achieved without the brain retraction. In postoperative, no new deficits were added and the patient remains asymptomatic after a 3 years follow-up without signs of recurrence [Figures 1 and 2].
Figure 1.
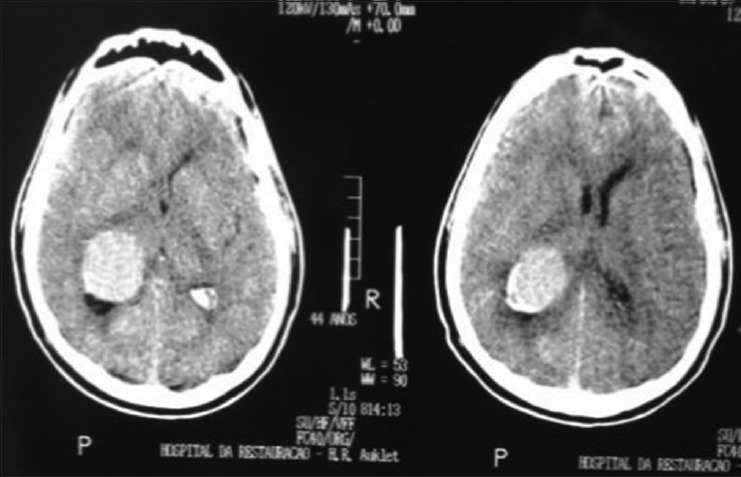
Head computed tomography (computed tomography scan showing hyperdense lesion in right ventricle's atrium)
Figure 2.
Postoperative computed tomography scan
Case 2
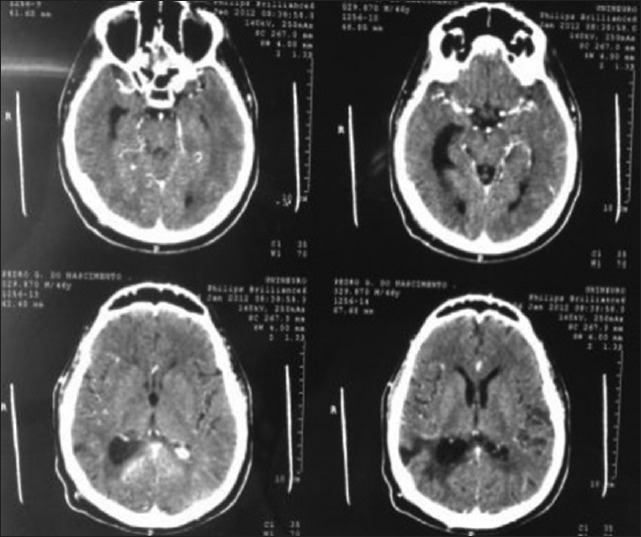
A 45-year-old female presented with a headache and difficulty walking. On examination, she had papilledema and a left sized pyramidal motor syndrome. CT scan revealed multiple meningiomas. The first approach was directed to the parasagittal lesion and the second surgery to the atrium tumor. She underwent the craniotomy with a parietal approach through the intraparietal sulcus. Complete resection of both lesions was achieved with the clinical improvement and no additional deficits [Figure 3].
Figure 3.
Preoperative axial T2 magnetic resonance imaging (axial T2 magnetic resonance imaging sequence showing multiple meningiomas with calcifications and perilesional vasogenic edema)
Axial T2 MRI sequence is showing multiple meningiomas with calcifications and perilesional vasogenic edema [Figures 4 and 5].
Figure 4.
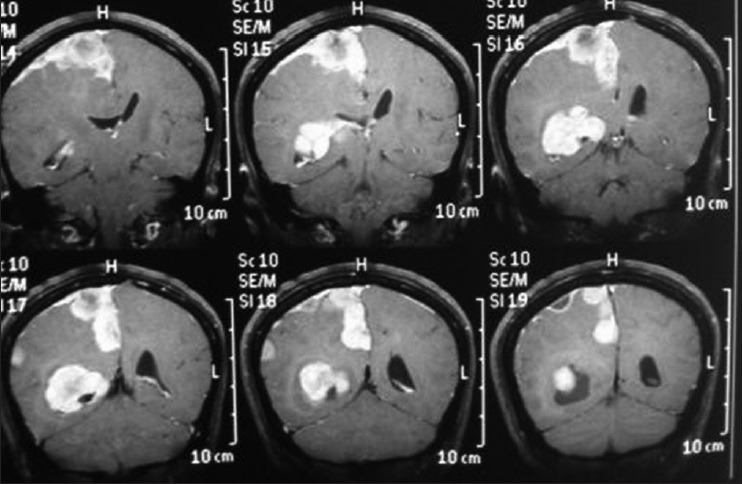
Coronal T1 magnetic resonance imaging sequence
Figure 5.
Postoperative computed tomography scan (first surgery for frontal lesion) - the atrium meningioma remains
Case 3
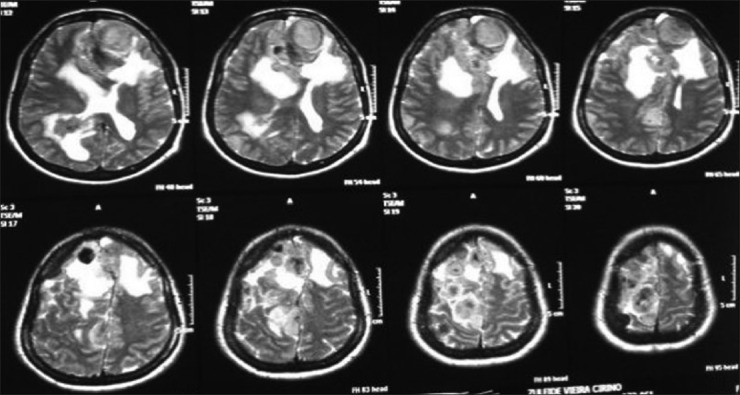
A 20-year-old female patient presented with a headache and vomiting. On examination, she had neck stiffness and papilledema. CT scan showed a large tumor in the right ventricular atrium. The same surgical approach was used and a total resection of the lesion was achieved. She was discharged in 3 days after the surgery without additional deficits and no recurrence after 2 years follow-up [Figures 6-9].
Figure 6.
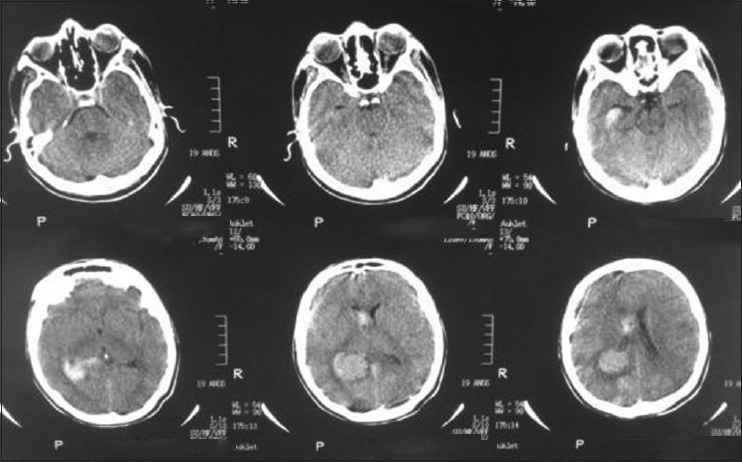
Preoperative computed tomography scan
Figure 9.
Postoperative computed tomography scan
Figure 7.
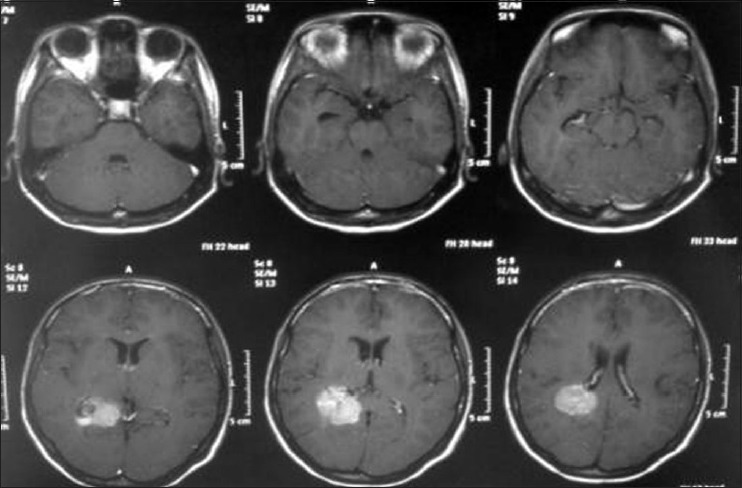
Axial T1 magnetic resonance imaging sequence with gadolineum
Figure 8.
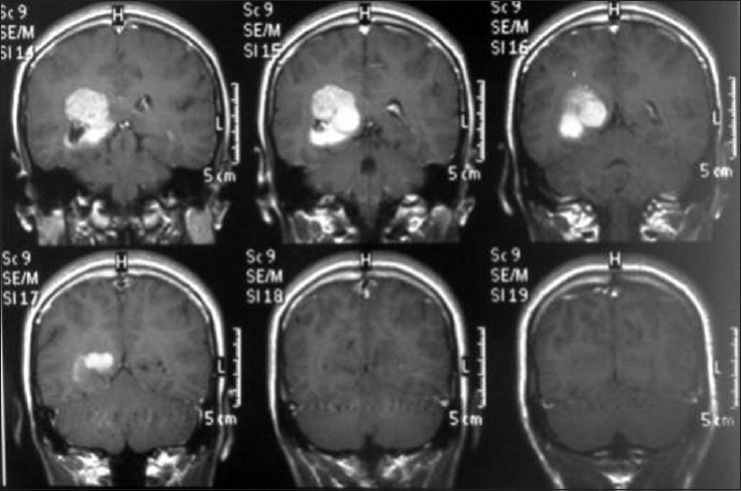
Coronal T1 magnetic resonance imaging with gadolineum
Case 4
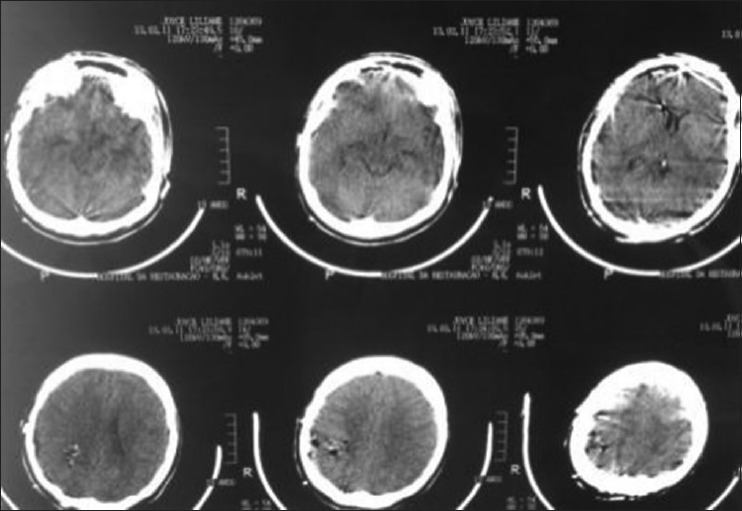
A 17-year-old female patient presented with a headache associated with the blurred vision and two previous seizures. On examination, right homonymous hemianopsia and papilledema were evident. CT scan showed a hyperdense lesion in the left ventricular trigone and underlying vasogenic edema. She underwent a surgical operation and the parieto-occipital interhemispheric precuneus approach (paraesplenial) was elected due to the fact that the tumor was in contact with the medial surface of the brain. In this case, the patient was operated on ¾ prone with the head parallel to the ground. Tumor side (left side) down facilitated resection aided by gravity. Gross total resection was obtained without the additional deficits. The patient remained with right homonymous hemianopia postoperatively [Figures 10-13].
Figure 10.
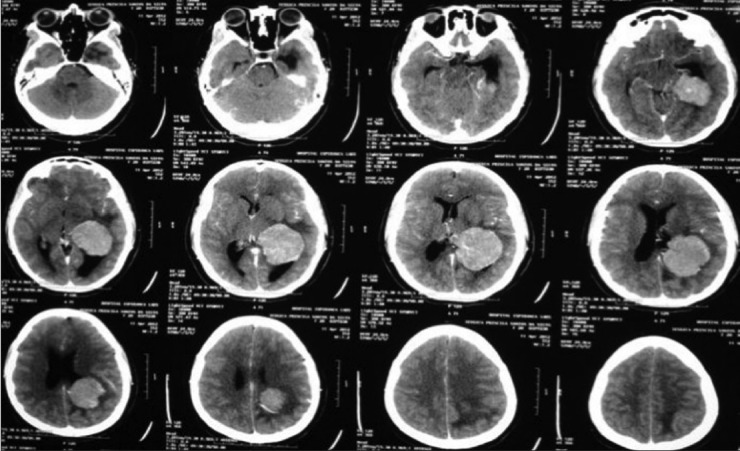
Preoperative tomography scan
Figure 13.
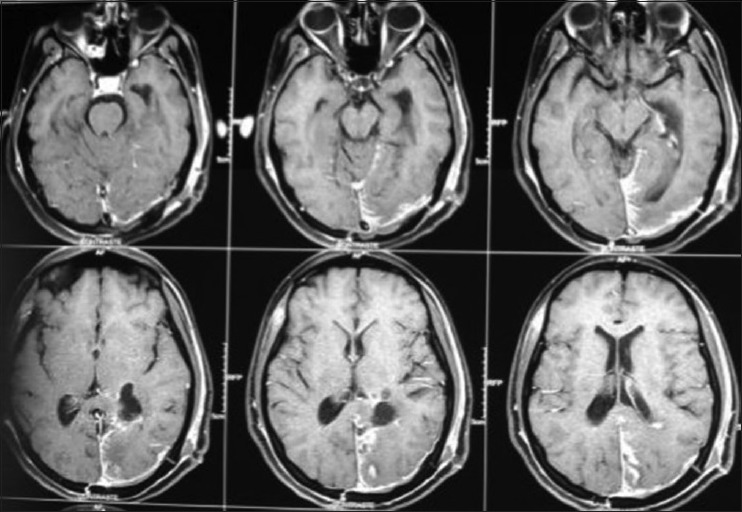
Postoperative magnetic resonance imaging
Figure 11.
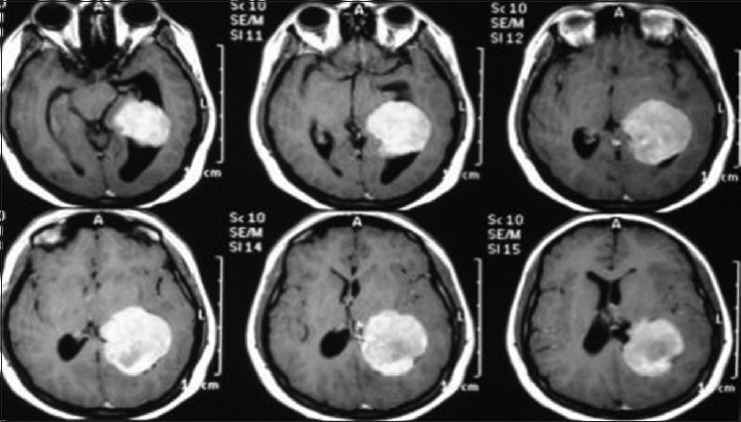
Axial T1 magnetic resonance imaging sequence with gadolineum
Figure 12.
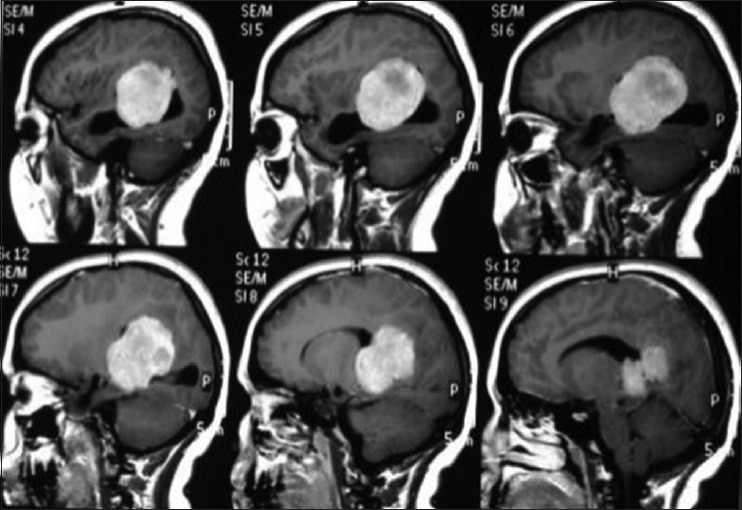
Preoperative sagital T1 magnetic resonance imaging with gadolineum sequence
RESULTS
In this case series, all the patients had large lesions (>3 cm) with an average age of 31.5 and female sex prevalence. Contrary to what we found in literature, there is a predominance of lesions on the right side (three). Signs or symptoms of intracranial hypertension occurred in four patients. A headache was reported in all the patients and three had papilledema on physical examination. Only one patient had visual field disturbance (homonymous hemianopsia). Also, only one patient presented a prior pyramidal deficit syndrome and another one, a history of seizures.
With regards to the surgical approach, 3 cases were approached through a parietal route along the intraparietal sulcus and one by a parieto-occipital interhemispheric precuneus approach. All the patients had tumor gross total resection without the additional morbidity and improvement of preoperative Karnofsky Performance Scale was achieved in 2 cases.
DISCUSSION
Intraventricular meningiomas are uncommon lesions accounting for 0.5–2% of intracranial meningiomas and their commonest location is in the ventricular trigone.
Among intraventricular meningiomas, 77–90% is found in the atrium ventricular region. They also represent 9.8–14% of all ventricular tumors and 20% of all tumors inside the lateral ventricle. The first atrial ventricular meningioma was described by Schaw et al. (1853)[14] as a hard and fibrous tumor of the lateral ventricle found at the autopsy of a patient with epilepsy and language disorder. MacDowall et al. (1881)[11] and Dreifus et al. (1923)[6] reported similar cases years later. The first surgical operation was performed by Cushing and Eisenhardt in 1916[3] and the patient remained free of deficits for 21 years. In Cushing and Eisenhardt[3] monograph published in 1938 about meningiomas, there were already 2 additional cases of atrial ventricular meningiomas. In 1965, Delandsheer et al. (1965)[5] published 175 cases of meningiomas of the lateral ventricle. More recently, Criscuolo and Symons (1986)[2] reported 400 intraventricular meningiomas. Nakamura et al.[12] reviewed 532 atrial ventricular meningiomas and observed that 414 (77.8%) occurred in the lateral ventricle, 15.6% in the third ventricle and 6.6% in the fourth ventricle. With regards to gender, there is a slight female predominance. Thus, it is postulated that this lesion is generated from the stroma or by choroidal plexus cells of arachnoid remnants, which is carried along with the choroidal plexus during the invagination process of the ventricular system.[13] Slow growth and compensatory mechanisms of accommodation occur due to the presence of a fluid cavity and so the tumors can reach the large volumes before first symptoms and proper diagnosis.[13]
These tumors are located deep in the brain, with surrounding intact brain tissue near vital ventricular structures and are vascularized by the anterior and posterior lateral choroidal artery. Lenticulostriate arteries and also participates in tumor's irrigation[3] while venous drainage of the trigone is made by the medial and lateral atrial veins. They tend to push the choroidal plexus medially and inferiorly. Most often, an unspecific clinical syndrome is found. Symptoms of intracranial hypertension and visual disturbances occur in 40–70% of cases.[6] Due to the fact that optical radiations always have lateral and inferior location than the trigone, they are only damaged in quite larger lesions.[7]
Atrioventricular meningiomas are resistant to the nonsurgical treatment. Radiosurgery is reserved only for the lesions smaller than 2 cm and can be used for residual or recurrent.[12] Surgery with complete excision of the lesion is the “Gold Standard” treatment.[4] Resection demands a considerable risk of morbidity and complications. Hence, in order to achieve the lesion, it is necessary to dissect white fibers and perform a cortex incision. Although, it could serve as injury conductor to the white fibers relating to speech and visual pathways, particularly optical radiation.[14] Visual fibers originating from lateral geniculate nucleus pass over the roof and the lateral wall of the temporal horn as well as on the inferolateral aspect of the atrium.[2]
With the objective of maximum tumor resection with less surgical morbidity, the anatomical knowledge through craniometric points and surgical safe corridors through the brain are essential, especially in developing countries like ours where technological assistance features such as neuronavigation and intraoperative monitoring, are not available at all hospitals due to the high cost. This craniometric knowledge should be taught and still practiced during residency, thus enabling the future neurosurgeon confidence and security needed to obtain good results even in the absence of high tech equipment.
Different approaches to these tumors were described in the literature making it a difficult task for neurosurgeons to elect the best for each case. On this note, one must consider that the aim must be a good exposure of the lesion and also an early visualization of the arterial pedicle. The main purpose is to accomplish complete removal with minimal or no damage to surrounding brain tissue. The principal approaches in literature are: Superior parietal transcortical approach (trans-sulcal), temporal approach (middle temporal gyrus), parieto-occipital interhemispheric approach (paresplenial), subtemporal, transtentorial supracerebellar, contralateral transfalcine, and posterior transcallosal approach. Cramer et al. (1960)[1] was the first to devise the posterior parieto-occipital approach and Fornari et al.[8] systematized it in his published series in 1981. The parietal transcortical approach through superior parietal lobule is one of the most preferred routes over the transtemporal access, especially in the dominant hemisphere.[8] A corticectomy is made along the medial to intraparietal sulcus, ±3 cm to 4 cm from the interhemisferic fissure, high enough to avoid optical radiations and posterior enough to avoid the language area. The cortical incision is made preferably parallel to the path of the fibers in order to minimize the visual symptoms. For tumors of medium and small size, located within the limits of the wall of the lateral ventricle, with or without ventricular dilatation, transcortical through superior parietal lobe in the nondominant hemisphere is more suitable. For small and medium lesions in the dominant hemisphere, parietal transcortical approach through the superior parietal lobule seems more appropriate. In asymptomatic patients with small lesions, efforts must be made to preserve the eloquent cortex and fibers.[7] Other authors reinforced the use of parietal trans-sulcal approach through the intraparietal sulcus, arguing for a more direct route to the trigone, reducing the route to the lesion.[11] This was our choice in the majority of presented cases. Some argued that this approach increases the risk of injury to white fibers and sulcal vessels. An alternative would be the transtemporal approach through the middle of inferior temporal gyrus, which is particularly suitable for lesions located in the anterolaterally portion of trigone or in the posterior third of the temporal horn.[3,7] Olivecrona et al.[13] was the first to suggest an access through the posterior portion of the middle temporal gyrus. This route allows rapid identification of choroidal vessels and the presence of hydrocephalus or a temporal horn dilation which facilitates the resection.[6] Nevertheless, there is a high risk of injury to optical radiation and language cortex.[2] The subtemporal approach reduces these complications risk.[6] However, one could have more complications relating to brain retraction, (p.ex. injury to the inferior anastomotic vein of Labbé).
Kempe and Blaylock (1976)[9] described the interhemispheric-transcallosal approach which was used by some authors for the resection of tumors in the left trigone with excellent results.
Suitable for small lesions of the midline and not requires the strong hemispheric retraction, this approach offers a quick access to branches of the posterior choroidal artery and medial atrial vein. Yasargil et al. (1996)[15] described the parieto-occipital interhemispheric precuneus approach (paraesplenial), which proved itself a good option, with short route for lesions that project into the medial wall of the trigone. The patient must be positioned with the head parallel to the ground, performed an incision at the level of precuneus, lying approximately 2 cm from trigone's medial wall. This approach avoids the optical radiation injuries and disorders in cortical function. This route is preferably suitable for medium and small lesions with medial projection. Despite, it has the following disadvantages: Narrow surgical corridor, narrow angle of attack, and difficulty to access the choroidal vessels and need for brain retraction.[10]
To avoid the brain retraction, it is necessary to carry out debulking and piecemeal resection.
Thereafter, the tumor capsule is deflected, exposing the arterial pedicle, and giving the possibility to coagulation. The ultrasonic aspirator is extremely useful. It is imperatively rigorous for hemostasis to leave an external ventricular drainage postoperatively to prevent an acute hydrocephalus.[7] The most commonly described complications are cerebral edema, intraventricular hemorrhage, subdural and epidural hematomas, and additional focal neurological deficits.[3] The risk of seizures postoperatively is greater in transcortical.[3] Mortality reported in most studies is quite a variable ranging between 0% and 42%.
CONCLUSION
Careful surgical planning and knowledge of anatomy is essential for a good outcome, especially in the absence of high tech tools. We consider the parietal approach through the intraparietal sulcus as the best choice for virtually all cases, considering that it provides a straight line pathway to lateral ventricle's trigone, in the shortest route and avoids the neurological morbidity to the patient. This approach was performed successfully in 3 out of 4 cases without the aid of neuronavigation, intraoperative monitoring, and intraoperative MRI. The parieto-occipital interhemispheric transprecuneus approach is an alternative choice, especially for those cases with a medial projection of the lesion.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Contributor Information
Igor Faquini, Email: ifaquini@yahoo.com.br.
Ricardo Brandão Fonseca, Email: ricardoneurocirurgia@uol.com.br.
Sérgio Luís Vale de Melo, Email: sergiovmelo@gmail.com.
Herika Negri, Email: hnegri@gmail.com.
Eduardo Vieira, Email: evcj2005@gmail.com.
Tammy Saboia, Email: tammyoliveira@gmail.com.
Hildo Azevedo-Filho, Email: azevedoh@uol.com.br.
REFERENCES
- 1.Cramer F. The intraventricular meningiomas: a note of the neurologic determinants governing the surgical approach. Arch Neurol. 1960;3:98. [Google Scholar]
- 2.Criscuolo GR, Symon L. Intraventricular meningioma. A review of 10 cases of the National Hospital, Queen Square (1974-1985) with reference to the literature. Acta neurochirurgica. 1986;83:83–91. doi: 10.1007/BF01402383. [DOI] [PubMed] [Google Scholar]
- 3.Cushing H, Eisenhardt L. Springfield, Illinois: Charles C. Thomas; 1938. Meningiomas: their classification, regional behaviour, life history and surgical end results. [Google Scholar]
- 4.D’Angelo VA, Galarza M, Catapano D, Monte V, Bisceglia M, Carosi I. Lat-eral ventricle tumors: surgical strategies according to tumor origin and de-velopment - a series of 72 cases. Neurosurgery. 2005;56(Suppl 1):S36–S45. doi: 10.1227/01.neu.0000144778.37256.ef. [DOI] [PubMed] [Google Scholar]
- 5.Delandsheer JM. Meningiomas of the Lateral Ventricle. Neuro-Chirurgie. 1965;11:3–83. [PubMed] [Google Scholar]
- 6.Dreifus W. Ueber endothelium des plexus choroideus. Beitr Path Anat. 1923;71:667–673. [Google Scholar]
- 7.Ellenbogen RG. Transcortical surgery for lateral ventricular tumors. Neurosurg Focus. 2001;10:1–13. doi: 10.3171/foc.2001.10.6.3. [DOI] [PubMed] [Google Scholar]
- 8.Fornari M, Savoiardo M, Morello G, Solero L. Meningiomas of the lateral ventricles. Neuroradiological and surgical considerations in 18 cases. J Neurosurg. 1981;54:64–74. doi: 10.3171/jns.1981.54.1.0064. [DOI] [PubMed] [Google Scholar]
- 9.Kempe LG, Blaylock R. Lateral-trigonal intraventricular tumors. A new operative approach. Acta neurochirurgica. 1976;35:233–42. doi: 10.1007/BF01406119. [DOI] [PubMed] [Google Scholar]
- 10.Liu M, Liu Y, Zhu S, Li X. Intraventricular meningiomas: a report of 25 cases. Neurosurg Rev. 2006;29:36–40. doi: 10.1007/s10143-005-0418-1. [DOI] [PubMed] [Google Scholar]
- 11.MacDowall TW. Large calcareous tumor involving chiefly the inner and middle portions of the left temporosphenoidal lobe and pressing upon the left crus and optic thalamus (brief communication) Edinburgh Med J. 1881;26:1088. [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura M, Roser F, Bundschuh O, Vorkapic P, Samii M. Intraventricular meningiomas: a review of 16 cases with reference to the literature. Surg Neurol. 2003;59:491–504. doi: 10.1016/s0090-3019(03)00082-x. [DOI] [PubMed] [Google Scholar]
- 13.Olivecrona H, Tönnis W. Handbuch der neurochirurgie. Vol. 4. Berlin, Heidelberg, New York: Springer; 1967. pp. 175–77. [Google Scholar]
- 14.Schaw A. Fibrous tumour in the lateral ventricle of the brain. Boney deposits in the arachnoid membrane of the right hemisphere. Trans Pathol Soc London. 1853;5:18–21. [Google Scholar]
- 15.Yasargil MG. Parieto-occipital interhemispheric approach. In: Yasargil MG, editor. Microneurosurgery. IVB. New York: Thieme; 1996. pp. 56–7. [Google Scholar]


