Abstract
The aim of the present study was to evaluate the correlation between the positive expression rate of mutant p53 and the clinical characteristics of patients with oral squamous cell carcinoma (OSCC), as well as the effectiveness of intra-arterial chemotherapy. Expression of mutant p53 in tumor tissues was determined by immunohistochemical analysis of 51 OSCC patients, prior to and following intra-arterial chemotherapy. Prior to intra-arterial chemotherapy, mutant p53 positive rates in patients with higher pathological grades were significantly higher than those of the patients with lower pathological grades. The mutant p53 positive rate in patients with lymph node metastasis was 73% (19/26), which was significantly higher than that of the patients without lymph node metastasis (20%, 5/25). Mutant p53 was expressed in 17% (3/18) of clinical phase II patients, while 64% (21/33) of clinical phase III and IV patients exhibited positive expression of mutant p53 (P<0.05). The mutant p53 positive rate in chemotherapy non-responsive patients was 69% (11/16), which was significantly higher than that in the chemotherapy-responsive patients (37%, 13/35). Mutant p53 positive rates were not significantly correlated with age, gender or the location of the tumor. The mutant p53 positive rate prior to chemotherapy was 47% (24/51), and decreased to 18% (9/51) following chemotherapy. Expression of mutant p53 was decreased in all 13 (100%) chemotherapy-responsive patients, while only 5 (45%) chemotherapy non-responsive patients exhibited reduced expression levels of mutant p53 (P<0.05). In conclusion, mutant p53 has a significant role in the differentiation, development and treatment guidance of OSCC. Intra-arterial chemotherapy with 5-fluorourcil and carboplatin potentially exerts a therapeutic effect by reducing the expression of mutant p53.
Keywords: p53, intra-arterial chemotherapy, oral cancer, squamous cell, immunohistochemistry
Introduction
Oral squamous cell carcinoma (OSCC) is the most common tumor of the head and neck in India (1) and is the most prevalent oral malignancy worldwide, with ~274,300 cases diagnosed annually (2). Increasing attention has been paid to OSCC due to its high risk of malignancy and invasion, rapid metastasis and increasing occurrence. Although a variety of novel approaches have been proposed for the treatment of OSCC, surgery remains the most effective strategy (3), while radiotherapy and chemotherapy are recommended for malignancies. Chemotherapy has been widely applied as a significant component of comprehensive treatment for OSCC. Surgery complemented with neoadjuvant chemotherapy is the most common comprehensive treatment program for OSCC. Neoadjuvant chemotherapy may achieve short-term clinical remission of tumors and reduce the size of the primary tumor (4), thereby enhancing tumor resection rate, increasing the negative rate of the resection edge, inhibiting tumor metastasis (5), reducing postoperative tissue defects and improving the quality of life of patients, particularly those requiring oral and maxillofacial surgery.
Regional arterial infusion may achieve a high concentration of anticancer drugs surrounding the tumor, and maximize the apoptotic effect on tumor cells without apparent toxicity. Therefore, this treatment strategy provides a precise, simple, long-term, low-toxicity and highly effective method of drug administration (6). Eckardt et al (7), achieved marked clinical remission for patients with advanced head and neck squamous cell carcinoma by utilizing regional arterial infusion chemotherapy. A combination of 5-fluorourcil (5-Fu) and carboplatin has been demonstrated to be an effective treatment regimen for OSCC (8,9). Regional arterial infusion chemotherapy with 5-Fu and carboplatin for the treatment of OSCC has previously been utilized by our group, and achieved marked clinical efficacy (10,11).
Various studies have demonstrated that mutation of the p53 gene has a significant role in tumor development (12–17). When DNA is damaged by physical and/or chemical factors, p53 gene transcription is increased and wild-type p53 protein is concentrated, which results in arrest of the cell cycle at G1/S phase and apoptosis of cells with cancerous characteristics (18). When the p53 gene is mutated, mutant p53 protein loses its cancer inhibition function and promotes the transformation of normal cells to malignant cells. Mutant p53 is present in almost all types of human tumor (12,19–21), and is closely correlated with the development of OSCC. However, to the best of our knowledge, the effect of neoadjuvant chemotherapy on the expression of mutant p53 has not previously been investigated. Therefore, the present study aimed to determine the expression of mutant p53 in patients with OSCC prior to and following intra-arterial chemotherapy, in order to provide novel approaches for the treatment of OSCC.
Patients and methods
Patients and samples
A total of 51 patients with OSCC diagnosed at the First Affiliated Hospital of Chongqing Medical University (Chonqing, China) between 2001 and 2011 were included in the present study. The following criteria were required for the patients to be included in the present study: i) All patients had a complete clinical history; ii) all patients were recently diagnosed with OSCC by biopsy; iii) biopsy specimens were maintained and available; and iv) chemotherapy or other treatments had not been applied prior to diagnosis. Following diagnosis, an arterial chemotherapy pump was implanted by superficial temporal artery retrograde intubation. Carboplatin (300 mg/m2; Qilu Pharmaceutical Co., Ltd., Jinan, China) on the first day, and 5-Fu (500 mg/m2; Shanghai Xudong Haipu Pharmaceutical Industry Co., Ltd., Shanghai, China) from the first day to the fifth day, were delivered by arterial infusion. Measurable lesions were determined by computed tomography (CT) and magnetic resonance imaging (MRI). Chemotherapeutic efficacy was divided into: Complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD), according to World Health Organization (WHO) criteria (22). All patients received two cycles of treatment prior to the evaluation of therapeutic efficacy. CR and PR were considered to be responsive, while SD and PD were considered to be non-responsive. Following two cycles of chemotherapy, primary cancer resection and radical neck dissection surgery were performed, primary cancer and neck metastases were determined by pathological examination. Tissue specimens were fixed with 10% formalin (Biyuntian Biotech Co., Ltd., Shanghai, China), embedded in paraffin (Biyuntian Biotech Co., Ltd.) and sectioned into 4-µm slices prior to hematoxylin-eosin (Biyuntian Biotech Co., Ltd.) and immunohistochemical staining.
Clinical data
Clinical data, including age, gender, symptoms, disease duration, surgical records (resection range and involvement of the surrounding tissues) and imaging data, were obtained from the patients' original medical records. The average age of the 51 patients with OSCC (male, 37; female, 14) was 55.5±10.6 years. Complete resection of the tumors was performed for all cases. Based on the location of the tumor, there were 15 cases of buccal cancer, 14 cases of mouth floor cancer, 12 cases of tongue cancer, 5 cases of palate cancer and 5 cases of gum cancer. According to the WHO histological classification of OSCC (23), there were 19 well-differentiated, 21 moderately-differentiated and 11 poorly-differentiated cases of squamous cell carcinoma. Post-operative pathological examination confirmed that 26 cases possessed cervical lymph node metastasis, while the remaining 25 cases did not. Union for International Cancer Control (UICC) clinical phase classification (24) revealed that there were 18 cases of phase II, 27 cases of phase III and 6 cases of phase IV cancer. A total of 35 cases were responsive to chemotherapy, while 16 cases were non-responsive. The present study was approved by the Medical Ethics Committee at the First Affiliated Hospital of Chongqing Medical University. All patients provided written informed consent prior to undergoing chemotherapy.
Immunohistochemistry
Tissue paraffin blocks of each specimen were divided into 4-µm sections. The sections were then dewaxed by placing in pure xylene (Biyuntian Biotech Co., Ltd.) at 60°C for 10 min, followed by xylene at room temperature for 3 min. Subsequently, the sections were dehydrated in 100, 95, 80 and 75% alcohol (Biyuntian Biotech Co., Ltd.) (3 min at each concentration). Sections were then rinsed with distilled water for 3 min until they were clean and transparent. The endogenous peroxidase activity was blocked by placing the sections in 3% hydrogen peroxide (Biyuntian Biotech Co., Ltd.) for 10 min, followed by one rinse with distilled water and three rinses with phosphate-buffered saline (PBS; Biyuntian Biotech Co., Ltd.). Immunohistochemical staining of mutant p53 was performed by incubating the sections with mouse anti-human mutant p53 monoclonal antibody (cat. no. 0010–2; 1:200; Fuzhou Maixin Biotech., Co., Ltd., Fuzhou, China) overnight at 4°C, biotinylated rabbit anti-mouse immunoglobulin G (cat. no. 9710; 1:10; Fuzhou Maixin Biotech., Co., Ltd.) for 30 min at room temperature and streptavidin-horseradish peroxidase and diaminobenzidine solution (Zymed, San Francisco, CA, USA) for 30 min at room temperature. The sections were stained with hematoxylin and mounted with neutral gum (Biyuntian Biotech Co., Ltd.). Cells with brown particles in the nucleus were considered to be mutant p53 positive. Based on the number of mutant p53-positive cells, the specimens were classified into the following categories: Mutant p53-negative, no brown particles observed; weakly positive (+), <30% cells were mutant p53-positive; moderately positive (++), 30–70% cells were mutant p53-positive; and strongly positive (+++), >70% cells were mutant p53-positive.
Statistical analysis
The χ2 test was performed for statistical analysis using SPSS 10.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Correlations between mutant p53 expression and clinicopathological features of OSCC
Mutant p53 was expressed mainly in the nucleus of tumor cells in OSCC specimens, and thus the nuclei were stained brown (Figs. 1–3). Mutant p53 was not expressed in well-differentiated OSCC. Expression of mutant p53 occurred in 55% of moderately-differentiated OSCC cases and 100% of poorly-differentiated OSCC specimens (P<0.05). The mutant p53-positive rate in patients with lymph node metastasis was 73% (19/26), which was significantly higher than that in the patients without lymph node metastasis (20%, 5/25; P<0.05). Mutant p53 was expressed in 17% (3/18) of clinical phase II patients, while 64% (21/33) of clinical phase III and IV patients had positive expression of mutant p53 (P<0.05). The mutant p53 positive rate in chemotherapy non-responsive patients was 69% (11/16), which was significantly higher than that in the chemotherapy-responsive patients (37%, 13/35; P<0.05). The mutant p53 positive rate was not significantly correlated with age, gender or the location of the tumor (P>0.05; Table I).
Figure 1.
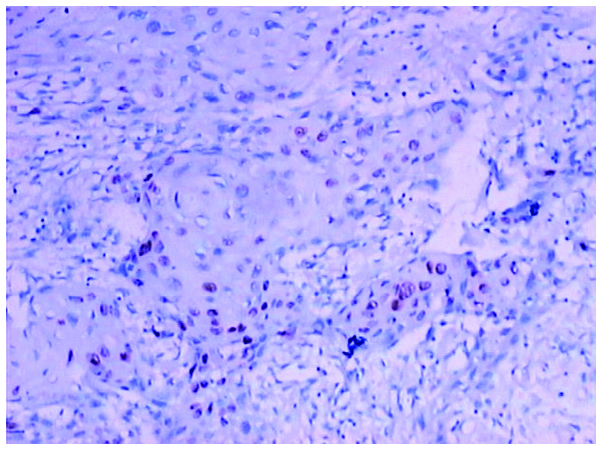
Weak expression of mutant p53 in oral squamous cell carcinoma (+) detected by immunohistochemical analysis (magnification, ×100).
Figure 3.
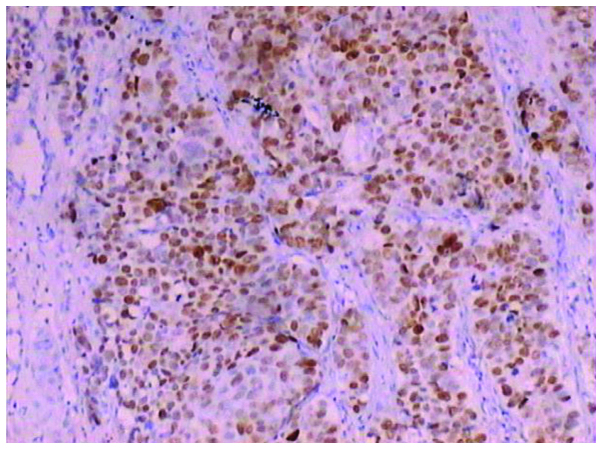
Strong positive expression of mutant p53 in oral squamous cell carcinoma (+++) detected by immunohistochemical analysis (magnification, ×100).
Table I.
Expression of mutant p53 and its association with the clinicopathological features of patients with oral squamous cell carcinoma.
| p53 expression | ||||
|---|---|---|---|---|
| Parameter | Cases, n | Negative | Positive | P-value |
| Age, years | >0.05 | |||
| ≥60 | 19 | 9 | 10 | |
| <60 | 32 | 18 | 14 | |
| Gender | >0.05 | |||
| Female | 14 | 8 | 6 | |
| Male | 37 | 19 | 18 | |
| Tumor differentiation | <0.05 | |||
| Well | 19 | 19 | 0 | |
| Moderate | 21 | 8 | 13 | |
| Poor | 11 | 0 | 11 | |
| Lymph node metastasis | <0.05 | |||
| No | 25 | 20 | 5 | |
| Yes | 26 | 7 | 19 | |
| Clinical stage | <0.05 | |||
| II | 18 | 15 | 3 | |
| III or IV | 33 | 12 | 21 | |
| Tumor site | >0.05 | |||
| Cheek | 15 | 7 | 8 | |
| Floor of mouth | 14 | 8 | 6 | |
| Tongue | 12 | 7 | 5 | |
| Palate | 5 | 3 | 2 | |
| Gum | 5 | 2 | 3 | |
| Therapeutic effectiveness | <0.05 | |||
| Responsive | 35 | 22 | 13 | |
| Non-responsive | 16 | 5 | 11 | |
χ2 test was performed for statistical analysis. P<0.05 was considered statistically significant.
Correlations between mutant p53 expression and the efficacy of intra-arterial chemotherapy
The mutant p53 positive rate prior to chemotherapy was 47% (24/51), which decreased to 18% (9/51) following chemotherapy (P<0.05; Table II). Expression of mutant p53 was decreased in all 13 (100%) chemotherapy-responsive patients, while only 5 (45%) chemotherapy non-responsive patients exhibited a reduced level of mutant p53 expression (P<0.05; Table III).
Table II.
Changes in mutant p53 expression in OSCC tissues following intra-arterial chemotherapy.
| Mutant p53 expression | |||||||
|---|---|---|---|---|---|---|---|
| Intra-arterial chemotherapy | Cases, n | - | + | ++ | +++ | P-value | |
| Prior to | 51 | 27 | 7 | 8 | 9 | <0.05 | |
| Following | 51 | 42 | 3 | 0 | 6 | ||
χ2 test was performed for statistical analysis. P<0.05 was considered statistically significant.
Table III.
Correlation between mutant p53 expression and the efficacy of intra-arterial chemotherapy.
| Expression of mutant p53 | ||||
|---|---|---|---|---|
| Efficacy | p53 positive cases, n | No reduction | Reduction | P-value |
| Responsive | 13 | 0 | 13 | <0.005 |
| Non-responsive | 11 | 6 | 5 | |
χ2 test was performed for statistical analysis. P<0.05 was considered statistically significant. Comparisons were made between the mutant p53 positive rate in chemotherapy responsive patients and that of the chemotherapy non-responsive patients.
Discussion
In the present study, the expression of mutant p53 was observed in OSCC tissues, suggesting that p53 mutation was present in OSCC. Mutated p53 loses its ability to suppress the function of oncogenes. Furthermore, mutant p53 may function as an oncogene to stimulate cell division and promote the growth of tumor cells (25). The results of the present study indicated that with the increase of OSCC pathological grading, mutant p53 positive rate was also increased, which was consistent with the results of previous studies (26–32), suggesting that the mutation of p53 may be significant in the pathological differentiation of OSCC. The negative correlation detected between the mutant p53 expression and the therapeutic efficacy indicated that mutant p53 may be used to predict the sensitivity of OSCC to 5-Fu and carboplatin chemotherapy. For example, positive expression of mutant p53 in OSCC tissues would indicate poor efficacy of chemotherapy with 5-Fu and carboplatin, and therefore an alternative chemotherapy scheme should be used. The results also indicated that the mutant p53 positive rate was higher in OSCC patients with lymph node metastasis than those without metastasis (33), suggesting that neck dissection should be performed for OSCC patients with expression of mutant p53. Mutant p53 positive rate in later clinical phases is higher than that in the early clinical phase (34), suggesting that mutant p53 promotes the development of OSCC. Overall, expression of mutant p53 is associated with the occurrence and development of OSCC, and contributes to the effective design of OSCC treatment programs.
Regional arterial infusion of carboplatin and 5-Fu was used to treat OSCC. 5-Fu is a pyrimidine antagonist, and may be transformed into floxuridine to inhibit thymidine synthase and interfere with the DNA synthesis (35). Carboplatin is a cytotoxic drug. Binding of platinum with guanine residues results in inter- and intra-DNA chain crosslinking, which prevents DNA synthesis and induces cell toxicity (36). The results demonstrated that intra-arterial chemotherapy with carboplatin and 5-Fu inhibited the expression of mutant p53 in OSCC. Gao et al (37) demonstrated that 5-Fu upregulates the expression of p53 through the p38-MAPK pathway in HepG2 cells, leading to apoptosis. The antitumor effects of 5-Fu may be achieved by reducing mutant p53 expression in OSCC. The results of the present study indicate that all chemotherapy-responsive patients exhibited reduced levels of mutant p53 expression, indicating that carboplatin and 5-Fu achieve their therapeutic effect on OSCC through reduction of mutant p53 expression. These results also suggest that the reduction of mutant p53 expression may represent an effective treatment strategy for OSCC. Therefore, the use of intra-arterial chemotherapy with 5-Fu and carboplatin was suggested for the treatment of OSCC.
Figure 2.
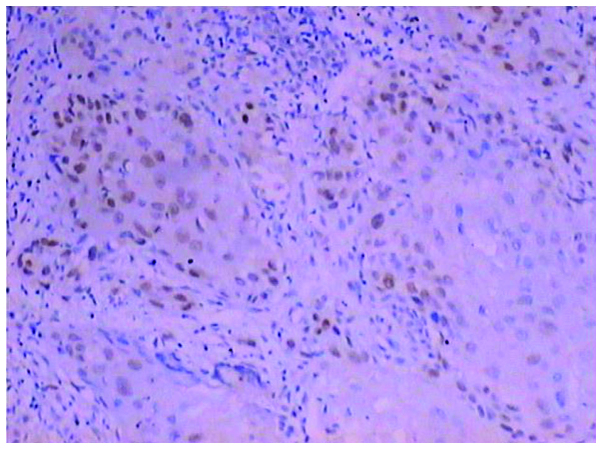
Moderately positive expression of mutant p53 in oral squamous cell carcinoma (++) detected by immunohistochemical analysis (magnification, ×100).
Acknowledgements
The present study was supported in part by the Science and Technology Research Project of Chongqing Municipal Commission of Education (grant nos. J130318 and 14SKD07), the Natural Science Foundation Project of CQ CSTC (grant no. cstc2012jjA10039) and the Chongqing Municipal Health Bureau (grant no. 2011-2-013).
References
- 1.Agrawal U, Rai H, Jain AK. Morphological and ultrastructural characteristics of extracellular matrix changes in oral squamous cell carcinoma. Indian J Dent Res. 2011;22:16–21. doi: 10.4103/0970-9290.79968. [DOI] [PubMed] [Google Scholar]
- 2.Li-Ting C, Chung-Ho C, Yi-Hsin Y, Pei-Shan H. The development and validation of oral cancer staging using administrative health data. BMC Cancer. 2014;14:380. doi: 10.1186/1471-2407-14-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y, Zhang J, Yang K. Evaluation of the efficacy of a novel radical neck dissection preserving the external jugular vein, greater auricular nerve and deep branches of the cervical nerve. Onco Targets Ther. 2013;6:361–367. doi: 10.2147/OTT.S43073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurokawa H, Miura K, Yamashita Y, Tokudome S, Murata T, Kajiyama M. Evaluation of neo-adjuvant chemotherapy for oral squamous cell carcinoma. Gan To Kagaku Ryoho. 1997;24:1273–1278. (In Japanese) [PubMed] [Google Scholar]
- 5.Kawashiri S, Noguchi N, Tanaka A, Nakaya H, Kato K, Yamamoto E. Inhibitory effect of neoadjuvant chemotherapy on metastasis of oral squamous cell carcinoma in a mouse model. Oral Oncol. 2009;45:794–797. doi: 10.1016/j.oraloncology.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Korogi Y, Hirai T, Nishimura R, Hamatake S, Sakamoto Y, Murakami R, Baba Y, Arakawa A, Takahashi M, Uji Y, et al. Superselective intraarterial infusion of cisplatin for squamous cell carcinoma of the mouth: Preliminary clinical experience. AJR Am J Roentgenol. 1995;165:1269–1272. doi: 10.2214/ajr.165.5.7572516. [DOI] [PubMed] [Google Scholar]
- 7.Eckardt A, Kelber A, Pytlik C. Palliative intra-arterial (i.a.) chemotherapy with carboplatin (CBDCA) and 5-FU in unresectable advanced (stage III and IV) head and neck cancer using implantable port-systems. Eur J Surg Oncol. 1995;21:486–489. doi: 10.1016/S0748-7983(95)96824-5. [DOI] [PubMed] [Google Scholar]
- 8.Brockstein BE, Vokes EE. Oral chemotherapy in head and neck cancer. Drugs. 1999;58(Suppl 3):S91–S97. doi: 10.2165/00003495-199958003-00013. [DOI] [PubMed] [Google Scholar]
- 9.Fuwa N, Kodaira T, Furutani K, Tachibana H, Nakamura T, Daimon T. Chemoradiation therapy using radiotherapy, systemic chemotherapy with 5-fluorouracil and nedaplatin and intra-arterial infusion using carboplatin for locally advanced head and neck cancer - Phase II study. Oral Oncol. 2007;43:1014–1020. doi: 10.1016/j.oraloncology.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Yang K, Ji P, Zhang F, Chen R, Deng D. Regional arterial infusion chemotherapy for the treatment of oral squamous cell carcinoma(OSCC) Shanxi Med J. 2006;35:529–530. (In Chinese) [Google Scholar]
- 11.Li Y, Yang K, Zhang J, Zhang F, Chen R, Deng D. Clinical study of chron-chemotherapy together with arterial chemotherapy for oral and maxillofacial squamous cell carcinoma. Kouqiang Yi Xue Yan Jiu. 2007;23:668–670. (In Chinese) [Google Scholar]
- 12.Muller PA, Vousden KH. Mutant p53 in cancer: New functions and therapeutic opportunities. Cancer Cell. 2014;25:304–317. doi: 10.1016/j.ccr.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu Y, Liu X, Yang Y, Zhao X, Xue J, Zhang W, Yang A. Effect of FHIT loss and p53 mutation on HPV-infected lung carcinoma development. Oncol Lett. 2015;10:392–398. doi: 10.3892/ol.2015.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Z, Sun Y, Chen X, et al. p53 mutation directs AURKA overexpression via miR-25 and FBXW7 in prostatic small cell neuroendocrine carcinoma. Mol Cancer Res. 2015;13:584–591. doi: 10.1158/1541-7786.MCR-14-0277-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L, Xiong H, Wu F, et al. Hexokinase 2-mediated Warburg effect is required for PTEN- and p53-deficiency-driven prostate cancer growth. Cell Rep. 2014;8:1461–1474. doi: 10.1016/j.celrep.2014.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin L, Grigoryan A, Wang D, et al. Identification and characterization of small molecules that inhibit nonsense-mediated RNA decay and suppress nonsense p53 mutations. Cancer Res. 2014;74:3104–3113. doi: 10.1158/0008-5472.CAN-13-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibbons DL, Byers LA, Kurie JM. Smoking, p53 mutation, and lung cancer. Mol Cancer Res. 2014;12:3–13. doi: 10.1158/1541-7786.MCR-13-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrington EA, Bruce JL, Harlow E, Dyson N. pRB plays an essential role in cell cycle arrest induced by DNA damage. Proc Natl Acad Sci USA. 1998;95:11945–11950. doi: 10.1073/pnas.95.20.11945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lam AK, Chan SS, Leung M. Synchronous colorectal cancer: Clinical, pathological and molecular implications. World J Gastroenterol. 2014;20:6815–6820. doi: 10.3748/wjg.v20.i22.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng T, Yin D, Lu Z, et al. Nutlin-3 overcomes arsenic trioxide resistance and tumor metastasis mediated by mutant p53 in Hepatocellular Carcinoma. Mol Cancer. 2014;13:133. doi: 10.1186/1476-4598-13-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bykov VJ, Wiman KG. Mutant p53 reactivation by small molecules makes its way to the clinic. FEBS Lett. 2014;588:2622–2627. doi: 10.1016/j.febslet.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 22.Mazumdar M, Smith A, Schwartz LH. A statistical simulation study finds discordance between WHO criteria and RECIST guideline. J Clin Epidemiol. 2004;57:358–365. doi: 10.1016/j.jclinepi.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Pindborg JJ, Reichart PA, Smith CJ, van der Waal I, editors. Histological Typing of Cancer and Precancer of the Oral Mucosa. 2nd. Springer-Verlag; Berlin: 1997. World Health Organisation Histological Classification of Tumours. [DOI] [Google Scholar]
- 24.Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM Classification of Malignant Tumours. 7th. Wiley-Blackwell; Chichester: 2009. [Google Scholar]
- 25.Rybanska I, Ishaq O, Chou J, et al. PARP1 and DNA-PKcs synergize to suppress p53 mutation and telomere fusions during T-lineage lymphomagenesis. Oncogene. 2013;32:1761–1771. doi: 10.1038/onc.2012.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Decastel M, Ossondo M, Andrea AM, et al. Colorectal cancer in patients seen at the teaching hospitals of Guadeloupe and Martinique: Discrepancies, similarities in clinicopathological features, and p53 status. BMC Clin Pathol. 2014;14:12. doi: 10.1186/1472-6890-14-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imamura H, Ohishi Y, Aman M, et al. Ovarian high-grade serous carcinoma with a noninvasive growth pattern simulating a serous borderline tumor. ((Epub ahead of print)).Hum Pathol. 2015 Jun 16; doi: 10.1016/j.humpath.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Jasar Dz, Smichkoska S, Kubelka K, et al. Expression of p53 protein product in triple negative breast cancers and relation with clinical and histopathological parameters. Prilozi. 2015;36:69–79. doi: 10.1515/prilozi-2015-0031. [DOI] [PubMed] [Google Scholar]
- 29.Tan PH, Jayabaskar T, Yip G, et al. p53 and c-kit (CD117) protein expression as prognostic indicators in breast phyllodes tumors: A tissue microarray study. Mod Pathol. 2005;18:1527–1534. doi: 10.1038/modpathol.3800488. [DOI] [PubMed] [Google Scholar]
- 30.Reiher F, Ozer O, Pins M, et al. p53 and microvessel density in primary resection specimens of superficial bladder cancer. J Urol. 2002;167:1469–1474. doi: 10.1016/S0022-5347(05)65347-5. [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Bao W, Jiang F, et al. Mutant p53 (p53-R248Q) functions as an oncogene in promoting endometrial cancer by up-regulating REGγ. Cancer Lett. 2015;360:269–279. doi: 10.1016/j.canlet.2015.02.028. [DOI] [PubMed] [Google Scholar]
- 32.Huang K, Chen L, Zhang J, et al. Elevated p53 expression levels correlate with tumor progression and poor prognosis in patients exhibiting esophageal squamous cell carcinoma. Oncol Lett. 2014;8:1441–1446. doi: 10.3892/ol.2014.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Y, Zhen T, Zhang F, et al. p53 and hepatoma-derived growth factor expression and their clinicopathological association with Ewing family tumour. J Clin Pathol. 2014;67:235–242. doi: 10.1136/jclinpath-2013-201705. [DOI] [PubMed] [Google Scholar]
- 34.Karim S. Clinicopathological and p53 gene alteration comparison between young and older patients with gastric cancer. Asian Pac J Cancer Prev. 2014;15:1375–1379. doi: 10.7314/APJCP.2014.15.3.1375. [DOI] [PubMed] [Google Scholar]
- 35.Wu Z, Han X, Qin S, et al. Interleukin 1 receptor antagonist reduces lethality and intestinal toxicity of 5-Fluorouracil in a mouse mucositis model. Biomed Pharmacother. 2011;65:339–344. doi: 10.1016/j.biopha.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 36.Wu J, Xu H, Zhang L, Zhang X. Radix astragali and tanshinone help Carboplatin inhibit B16 tumor cell growth. ((Epub ahead of print)).Technol Cancer Res Treat 2015. 2015 Jun 3; doi: 10.1177/1533034615588682. [DOI] [PubMed] [Google Scholar]
- 37.Gao J, Gao J, Qian L, et al. Activation of p38-MAPK by CXCL4/CXCR3 axis contributes to p53-dependent intestinal apoptosis initiated by 5-fluorouracil. Cancer Biol Ther. 2014;15:982–991. doi: 10.4161/cbt.29114. [DOI] [PMC free article] [PubMed] [Google Scholar]