Abstract
Cataractogenic stresses are associated with the induction of endoplasmic reticulum (ER) stress. However, little is known about oxygen (O2)-induced ER stress in the lens. Cataract research has focused on elevated levels of O2 in lens epithelial cells (LECs). Excessive levels or a lack of O2 are known to induce ER stress whereas chronic ER stress activates the unfolded protein response (UPR). The present study investigated the hypothesis that the fluctuation of O2 levels induces a UPR, and may be controlled by maintaining human LECs (hLECs) in a specific concentration of O2. Human LECs were cultured in different atmospheric levels of O2. Hypoxic conditions were determined by the level of hypoxia-inducible factor (HIF)-1α. 2′,7′-Dichlorodihydrofluorescein diacetate and ethidium homodimer-1 staining were conducted to detect reactive oxygen species (ROS) and cell death, respectively. Protein blot analyses were performed with antibodies specific to antioxidant and UPR-specific proteins. Reverse transcription-quantitatative polymerase chain reaction assays were performed to quantify the mRNA levels of activated NF-E2-related factor 2 (Nrf2) and kelch-like ECH-associated protein 1 (Keap1). The treatment of human LECs with 0 and 20% atmospheric O2 activated Nrf2/Keap1. The LECs shifted to 1% atmospheric O2 from 0, 4 or 20% for 24 h showed decreased levels of Keap1. By contrast, hLECs cultured in 1% atmospheric O2 for 24 h and then shifted to 0, 4 or 20% O2 exhibited a significant upregulation of Nrf2. These results suggest that oxidative stress proteins were not expressed in a 1% O2 environment. The O2 levels in the culture medium were equilibrated within 2 h in the cell culture plates. These results showed that an appropriate oxygen environment for the culture of LECs is ~1 % atmospheric O2. Either 0 or 20% of atmospheric O2 activated the UPR and the Nrf2/Keap1-mediated antioxidant system in LECs and chronic exposure to O2 fluctuation led to ROS production and cell death. This study revealed that O2 fluctuation-induced UPR/ER stress could be prevented by maintaining the cells in a 1% O2 environment.
Keywords: unfolded protein response, low oxygen, hypoxia inducible factor-1α, antioxidants, NF-E2-related factor 2
Introduction
Cataract, the foremost cause of visual impairment, is found notably in diabetic patients and causes health and economic problems predominantly in developing countries (1). Genetic and environmental stresses combined with age are considered as leading contributors to aggregation, crystalline modification and pathogenesis in lens oxidation. The ciliary body and blood vessels of the iris supply glucose and oxygen (O2) to the lens, where it is present in a hypoxic environment containing 0.5–2.3% O2 (2–4). The level of O2 is a very important factor in cataract, and various cataractogenic stressors such as hypoxia, hypoxic conditions along with a low glucose level (5) or high glucose level (6), homocysteine (7), and galactose (6) are found to induce stress in the endoplasmic reticulum (ER), thereby mediating the activation of the unfolded protein response (UPR) along with the production of reactive oxygen species (ROS), which is usually abnormally increased, and lens epithelial cell (LEC) death (3,8,9).
Osmotic stress, primarily caused by the accumulation of sugars, has been found to induce stress in the ER, which is a major site of protein synthesis, thereby leading to free radical generation. The stress is also found to result from fluctuations in glucose levels initiating an UPR that further generates ROS, and thus causes oxidative stress damage to lens fibers (10).
ER-induced UPR is reported to be activated by the phosphorylation of inositol-requiring enzyme-1 (IRE-1), PKR-like endoplasmic reticulum kinase (PERK) and eukaryotic translation initiation factor 2α (eIF2α) and this mechanism is also reported to be protective, although the cellular components are a stress response, whereas in prolonged UPR, apoptosis is induced by caspases (caspase-12) (11) and C/EBP homologous protein (CHOP) (12), death factors that are activated by activating transcription factor 4 (ATF4), which is a significant controller of mammalian lens development (13). Free radicals such as ROS are generated by the UPR in increased intensities (14) and in response, the upregulation of NF-E2-related factor 2 (Nrf2) and activation of the PERK-dependent antioxidant defense system occurs due to the UPR (15).
This study aimed to determine whether hypoxic conditions or O2 fluctuation in the environment of LECs induces UPR, leading to ROS production and failure of the Nrf2-dependent antioxidant defense protection. To elucidate this, UPR was studied along with the production of free radicals and the levels of Nrf2 in human LECs (hLECs) that were treated with various O2 environments.
Materials and methods
hLEC culture
hLECs (Lonza Clonetics™, Basel, Switzerland) were cultured overnight in Dulbecco's modified Eagle's medium (DMEM; Invitrogen Life Technologies, Carlsbad, CA, USA) containing 25 mM glucose, along with 10% fetal calf serum (FCS) under 20% atmospheric O2 at 37°C. Prior to each experiment, the hLECs were precultured overnight in DMEM with 5 mM glucose and 4% atmospheric O2. The hLECs were then cultured under various conditions. Some were cultured in glucose-free (GF) DMEM supplemented with 2% FCS and were maintained at 37°C in 20% atmospheric O2. Some hLECs were maintained under anaerobic conditions (0% atmospheric O2) in an AnaeroGen vacuum bag (Sigma-Aldrich, St. Louis, MO, USA). Atmospheric O2 environments of 1 and 4% were maintained in an O2/CO2 incubator (SANYO MCO-19M CO2 incubator; Sanyo, Tokyo, Japan) attached to a liquid nitrogen gas tank. A normal tissue culture incubator (SANYO MCO-19M CO2 incubator; Sanyo) was used to maintain the 20% atmospheric O2 environment.
Cell viability/death and ROS staining
Ethidium homodimer-1 (EthD) and calcein AM (Viability/Cytotoxicity assay kits; Biotium Inc., Hayward, CA, USA) mixtures were used to stain the cultured hLECs and used in accordance with the manufacturer's recommendations. 2′,7′-Dichlorodihydrofluorescein diacetate (H2-DCFH-DA; Invitrogen Life Technologies, Grand island, NY, USA) in phosphate-buffered saline (PBS) at a concentration of 1 mg/ml was used to determine the cytosolic ROS level. The mixture was allowed to stand for 40 min at 20°C, then washed with PBS twice and subjected to microscopic imaging using a fluorescence microscope (Nikon TE2000-U; Nikon Corporation, Tokyo, Japan).
Evaluation of protein levels in hLECs
Protein levels were determined by western blotting. RIPA buffer (Cell Signaling Technology, Danvers, MA, USA) was used to lyse the cultured hLECs and the proteins were separated by SDS-PAGE. The gels were then blotted onto nitrocellulose membranes, which were blocked in PBS buffer (pH 8.0) containing 5% non-fat milk for 1 h. The primary antibodies were to the following proteins: Binding immunoglobulin protein (BiP; cat. no. sc-33757; rabbit polyclonal IgG, 1:500; Santa Cruz Biotechnology Inc., Dallas, TX, USA), ATF4 (cat. no. ab23760; rabbit polyclonal IgG, 1:500; Abcam, Cambridge, MA, USA), ATF6 (cat. no. ab37149; rabbit polyclonal IgG, 1:500; Abcam), CHOP (cat. no. MA1-250; mouse monoclonal IgG, 1:500; Invitrogen Life Technologies, Carlsbad, CA, USA), ER oxidoreductin 1-like (Ero1-L)α (cat. no. sc-100805; mouse polyclonal IgG, 1:500; Santa Cruz Biotechnology, Inc.), Ero1-Lβ (cat. no. sc-162776; goat polyclonal IgG, 1:500; Santa Cruz Biotechnology, Inc.), hypoxia-inducible factor (HIF)-1α (cat. no. ab16066; mouse monoclonal IgG, 1:500; Santa Cruz Biotechnology, Inc.), kelch-like ECH-associated protein 1 (Keap1; cat. no. sc-15246; goat polyclonal IgG, 1:500; Santa Cruz Biotechnology, Inc.), Nrf2 (cat. no. sc-722; rabbit polyclonal IgG, 1:500; Santa Cruz Biotechnology, Inc.), protein disulfide isomerase (PDI; cat. no. sc-20132; rabbit polyclonal IgG, 1:500; Santa Cruz Biotechnology, Inc.), phospho (p)-eIF2α (cat. no. sc-101670; rabbit polyclonal IgG, 1:500; Santa Cruz Biotechnology, Inc.), p-IRE1α (cat. no. PA1-16927; rabbit polyclonal IgG, 1:500; Invitrogen Life Technologies), p-PERK (cat. no. sc-32577; rabbit polyclonal IgG, 1:500; Santa Cruz Biotechnology, Inc.) and GAPDH (cat. no. sc-25778; rabbit polyclonal IgG, 1:500; Santa Cruz Biotechnology Inc.). Anti-caspase-4 antibodies (cat. no. sc-56056; mouse monoclonal IgG; 1:500; Santa Cruz Biotechnology Inc.) were used in western blot analysis. The membranes were incubated with primary antibody at 4°C overnight and then with the secondary antibodies (cat. no. sc-56056; goat anti-rabbit IgG-horseradish peroxidase goat polyclonal, 1:5000; Santa Cruz Biotechnology Inc.) for 1 h at room temperature. The intensity of each band was normalized to that of GAPDH, and the data are presented as relative intensities, which were determined using ImageJ analysis software (National Institutes of Health, Bethesda,. MD, USA).
Evaluation of mRNA levels in hLECs
Total RNA was extracted from the hLECs exposed to various O2 environments using TRIzol reagent (Invitrogen Life Technologies) according to the manufacturer's instructions. The purified total RNA was reverse transcribed using iScript™ Reverse Transcription Supermix for real-time PCR (Bio-Rad T100; Bio-Rad, Hercules, CA, USA) following the manufacturer's instructions. The reverse transcribed RNA was analyzed by quantitative polymerase chain reaction using SsoFast™ EvaGreen® Supermix (Bio-Rad). The primer sequences for Nrf2, Keap1, and β-actin as described by Elanchezhian et al (5) were used for mRNA detection. The primer sequences for Nrf2, Keap1, and β-actin were: Nrf2 sense, 5′-ACACGGTCCACAGCTCATC-3′ and antisense, 5′-TGCCTCCAAAGTATGTCAATCA-3′: Keap1 sense, 5′-GGGTCCCCTACAGCCAAG-3′ and antisense, 5′-TGGGGTTCCAGAAGATAAGC-3′; and β-actin sense 5′-CCAACCGCGAGAAGATGA-3′ and 5′-CCAGAGGCGTACAGGGATAG-3′ antisense. Each reaction was carried out in triplicate and three independent experiments were run. A standard curve was prepared, relative copy numbers were obtained from the standard curve and the relative expression levels were normalized to the values obtained for β-actin.
Statistical analysis
Results are presented as the mean ± standard deviation from three individual experiments. P-values were determined by Student's t-tests and analyzed using SPSS software, (version 16.0; SPSS Inc., Chicago, IL, USA).P<0.05 was considered to indicate a statistically significant result.
Results
ROS production and cell death
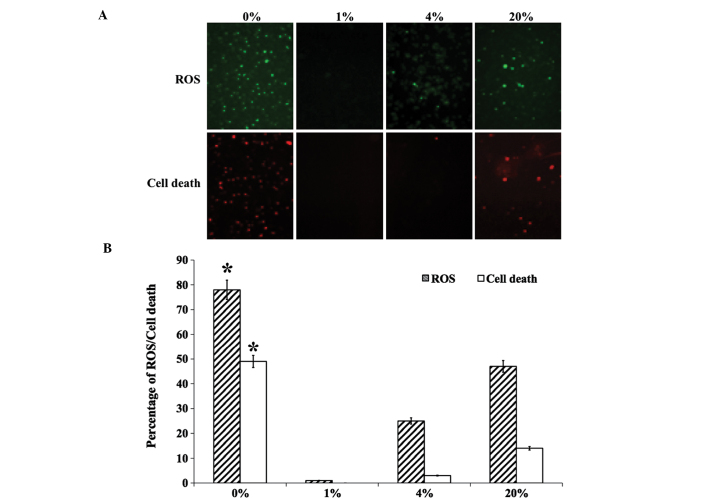
hLECs that were cultured in different levels of O2 were stained for the evaluation of ROS production and cell death (Fig. 1A). The production of ROS was found to be significantly increased in 0 and 20% O2 than in 1 and 4% O2 (Fig. 1B). A similar pattern was observed for dead cell staining, with no cell death observed in 1 and 4% O2 (Fig. 1). The levels of ROS- (Fig. 2A) and apoptosis-related ER stress proteins (Fig. 2B) were then investigated. The ROS-related ER stress proteins that were investigated were Ero1-Lα, Ero1-Lβ and PDI. An increased level of Ero1-Lα was detected in cells cultured with 0, 4 and 20% O2. However, Ero1-Lβ and PDI were detected at greater levels in hLECs cultured in 1% O2 than in those cultured in other percentages of O2 (Fig. 2A). The apoptosis-related ER stress proteins that were investigated were ATF4, CHOP and caspase-4. Similar to the ROS-related protein Ero1-Lα, apoptotic proteins were detected in greater quantities in hLECs cultured in 0% O2 than in those cultured in other percentages of O2 (Fig. 2B). Notably, cells cultured in 1% O2 revealed a protective effect when compared with the cells cultured in other percentages of O2.
Figure 1.
(A) Fluorescent staining of ROS and cell death in hLECs cultured for 24 h with 0, 1, 4 and 20% oxygen (magnification, ×100). (B) Graphical representation of percentage of ROS and cell death obtained by quantifying the staining intensity of the cells. * P<0.05 vs. 20% oxygen. ROS, reactive oxygen species; hLECs, human lens epithelial cells.
Figure 2.
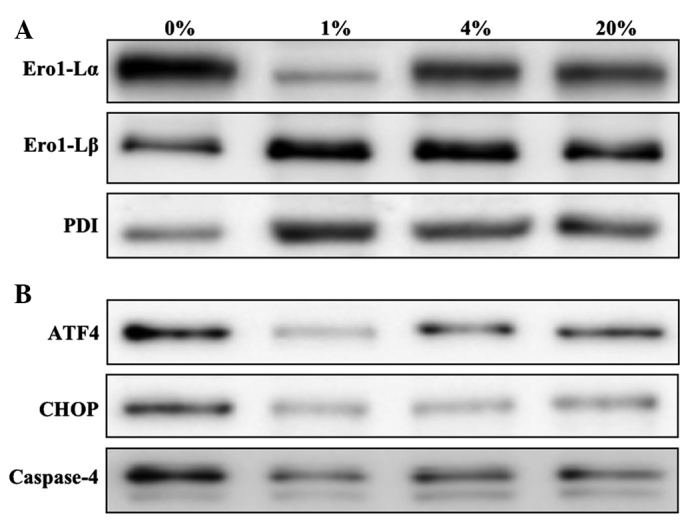
Western blot analysis of ROS- and apoptosis-related UPR proteins. (A) ROS-related and (B) apoptosis-related UPR proteins in the hLECs cultured for 24 h with 0, 1, 4 and 20% oxygen. ROS, reactive oxygen species; UPR, unfolded protein response; Ero1-L, endoplasmic reticulum oxidoreductin 1-like; PDI, protein disulfide isomerase; ATF4, activating transcription factor 4; CHOP, C/EBP homologous protein.
Detection of O2 deprivation with different percentages of O2
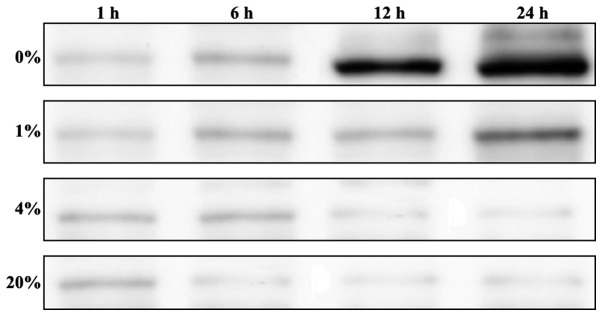
hLECs were cultured in 0, 1, 4 and 20% O2 and cells were collected at various time intervals (1, 6, 12 and 24 h) for the investigation of HIF-1α levels. Increased levels of HIF-1α were detected in the cells cultured in 0% O2 from 6 h, and the HIF-1α levels were significantly increased following 24 h of culture (Fig. 3). However, the cells cultured in 1% O2 showed minimum levels of HIF-1α when compared with those cultured in 0% O2. The other O2 percentages, 4 and 20%, showed a minimal HIF-1α protein levels (Fig. 3).
Figure 3.
Western blot analysis of the protein levels of HIF)-1α hypoxia-inducible factor in hLECs cultured for 1, 6, 12 and 24 h with 0, 1, 4 and 20% oxygen. HIF, hypoxia-inducible factor; hLECs, human lens epithelial cells.
Evaluation of ER stress proteins
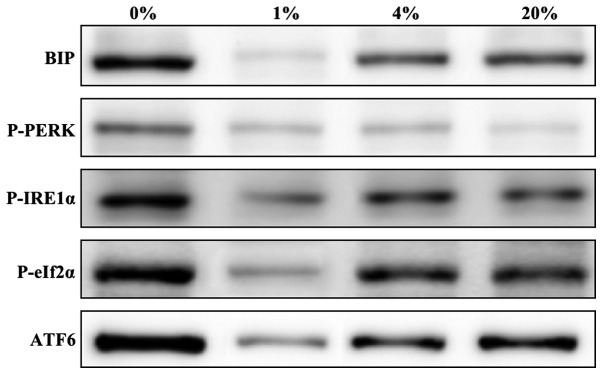
To elucidate the hypothesis concerning the induction of ER stress by O2 fluctuation, the levels of ER stress proteins in cells cultured for 24 h with 0, 1, 4 and 20% O2 were investigated. The ER stress marker protein BiP and the UPR-associated proteins p-PERK, p-IRE1α, p-eIF2α and ATF6 were investigated. Increased levels of ER stress proteins were found in cells cultured with 0 and 20% O2 when compared with those cultured with 1 and 4% O2 (Fig. 4). However, very minimal or negligible amounts of ER stress proteins were detected in cells cultured in 1% O2, which clearly indicates that this is a protective environment for the growth of hLECs.
Figure 4.
Representative western blots of ER stress-related UPR proteins in hLECs cultured for 24 h with 0, 1, 4 and 20% oxygen. ER, endoplasmic reticulum; UPR, unfolded protein response; BiP, binding immunoglobulin protein; p, phospho; PERK, PKR-like endoplasmic reticulum kinase; IRE1α, inositol-requiring enzyme 1α; eIf2α, eukaryotic translation initiation factor 2α; ATF6, activating transcription factor 6.
Evaluation of key antioxidant proteins
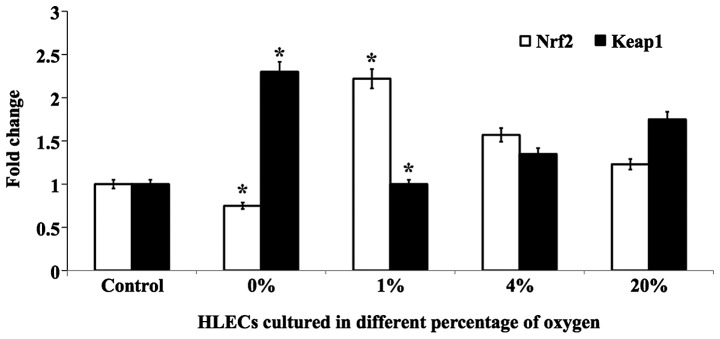
The effect of different percentages of O2 on the levels of the lens antioxidant proteins Nrf2 and Keap1 were investigated. The effects were evaluated in cells cultured for 24 h in 0, 1, 4 and 20% O2 by quantifying Nrf2 and Keap1 at the protein and mRNA levels. Notably, cells cultured in 1% O2 showed a protective effect and the level of Nrf2 protein was increased to near normal levels (Fig. 5). However, cells cultured in 0, 4 and 20% O2 showed decreased levels of this protein. The inverse effect was observed for Keap1 protein (Fig. 5). The mRNA levels of Nrf2 and Keap1 followed similar trends (Fig. 6). Altogether, it is clear that 1% O2 was found to be protective for cultured hLECs when compared with other percentages of O2. In addition, a complete lack of O2 (0%) resulted in severe damage to lens proteins due to the induction of ER stress, where chronic exposure led to the production of ROS and cell death.
Figure 5.
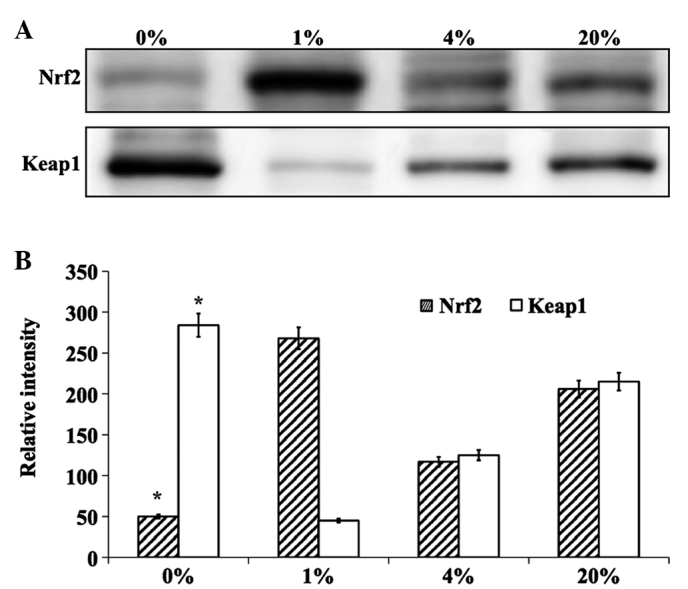
(A) Representative western blot analysis of antioxidant proteins in hLECs cultured for 24 h with 0, 1, 4 and 20% oxygen. (B) Graphical representation of relative intensity of corresponding antioxidant blots quantified by image analysis. * P<0.05 vs. 20% oxygen. hLECs, human lens epithelial cells; Nrf2, NF-E2-related factor 2; Keap1, kelch-like ECH-associated protein 1.
Figure 6.
Graphical representation of mRNA quantification of antioxidants Nrf2 and Keap1 in hLECs cultured for 24 h with 0, 1, 4 and 20% oxygen. *P<0.05 vs. 20% oxygen. hLECs, human lens epithelial cells; Nrf2, NF-E2-related factor 2; Keap1, kelch-like ECH-associated protein 1.
Discussion
Lens researchers generally consider that age-related cataracts (ARCs) are strongly associated with lens oxidation and aging (16–18). Although a direct association between cataract formation and hypoxia has not been clearly demonstrated, there are many adverse conditions that lead to hypoxic disorders in the human lens. In the present study, exposure of hLECs to certain O2 environments was found to activate a protective UPR, whereas prolonged exposure to severe hypoxia induced ROS production and apoptotic UPR. This response was observed as LEC death and lens oxidation. The obtained results are in line with previous studies, in which cataracts under diabetic conditions have been found to be strongly associated with age-dependent circulatory disorders and also dependent on diastolic blood pressure, the duration of diabetes, elevated glycosylated hemoglobin levels and lower intraocular pressure (19,20).
In addition, metabolic diseases and extreme fasting conditions have been found to induce hypoglycemia; a recent study showed that a 30-min exposure to very low glucose was adequate to induce an UPR in the germinative zone of the lens in rodents (5) and hyperoxia and hyperglycemia are been widely reported to induce oxidation in the lens and cataract formation (21,22). The present study has revealed that physiological concentrations of O2 concentrations also induce lens oxidation and activate the UPR, indicating that hypoxia and hyperoxia are potentially contributing factors to oxidation in lenses.
Kiviluoto et al (23) reported that the activation of ER stress proteins deplete the level of Ca2+ in the ER-; the ER-Ca2+ level along with ER localized oxidative machinery is vital for appropriate protein folding. The results of the present study suggest that the culture of hLECs under severe hypoxia (0% O2) or hyperoxia (20% O2) induces apoptotic UPR leading to ROS production; this may also lead to the release of Ca2+ from the ER, consequently increasing cytosolic Ca2+ and thus inducing apoptosis and causing severe impairment in the hLECs. It may be hypothesized that aged individuals develop cortical or nuclear cataracts due to the induction of UPR and also due to ROS production in LECs.
A study by Elanchezhian et al (5) demonstrated that ROS increased in LECs in the germinative zone that differentiate into cortical fiber cells, where new fiber cells are generated over old lens fiber cells. These lens fiber cells were suggested to contain less Nrf2-dependent antioxidant protection, and the changes result in crystallin aggregation and oxidation in the posterior and cortical regions. Moreover, earlier findings have reported that diabetic cataracts are intensely associated with diabetic exposure time (19,20), where longer exposure results in an increased density of the cortical lens fiber cell layer.
Thus, the present study has revealed that 1% O2 is a protective environment for the healthy growth of hLECs. However, 0 and 20% O2 may activate the UPR, and prolonged exposure leads to the production of ROS, oxidation of the lens and ultimately leads to cell death. Maintaining cells in 1% O2 can attenuate O2-fluctuation induced ER stress. The results of this study suggest that 1% O2 provides a protective environment for the healthy cell culture and experimental use of hLECs.
Acknowledgements
The present study was supported by grants from the National Science Foundation of China (no. 81202021), the National Science Foundation of Zhejiang Province, China (no. LQ13H120001) and the Key Laboratory of Diagnosis and Treatment of Neonatal Diseases of Zhejiang Province.
References
- 1.Tabin G, Chen M, Espandar L. Cataract surgery for the developing world. Curr Opin Ophthalmol. 2008;19:55–59. doi: 10.1097/ICU.0b013e3282f154bd. [DOI] [PubMed] [Google Scholar]
- 2.Barbazetto IA, Liang J, Chang S, Zheng L, Spector A, Dillon JP. Oxygen tension in the rabbit lens and vitreous before and after vitrectomy. Exp Eye Res. 2004;78:917–924. doi: 10.1016/j.exer.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 3.McNulty R, Wang H, Mathias RT, Ortwerth BJ, Truscott RJ, Bassnett S. Regulation of tissue oxygen levels in the mammalian lens. J Physiol. 2004;559:883–898. doi: 10.1113/jphysiol.2004.068619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shui YB, Fu JJ, Garcia C, Dattilo LK, Rajagopal R, McMillan S, Mak G, Holekamp NM, Lewis A, Beebe DC. Oxygen distribution in the rabbit eye and oxygen consumption by the lens. Invest Ophthalmol Vis Sci. 2006;47:1571–1580. doi: 10.1167/iovs.05-1475. [DOI] [PubMed] [Google Scholar]
- 5.Elanchezhian R, Palsamy P, Madson CJ, Mulhern ML, Lynch DW, Troia AM, Usukura J, Shinohara T. Low glucose under hypoxic conditions induces unfolded protein response and produces reactive oxygen species in lens epithelial cells. Cell Death Dis. 2012;3:e301. doi: 10.1038/cddis.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikesugi K, Yamamoto R, Mulhern ML, Shinohara T. Role of the unfolded protein response (UPR) in cataract formation. Exp Eye Res. 2006;83:508–516. doi: 10.1016/j.exer.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 7.Elanchezhian R, Palsamy P, Madson CJ, Lynch DW, Shinohara T. Age-related cataracts: Homocysteine coupled endoplasmic reticulum stress and suppression of Nrf2-dependent antioxidant protection. Chem Biol Interact. 2012;200:1–10. doi: 10.1016/j.cbi.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helbig H, Hinz JP, Kellner U, Foerster MH. Oxygen in the anterior chamber of the human eye. Ger J Ophthalmol. 1993;2:161–164. [PubMed] [Google Scholar]
- 9.Bassnett S, McNulty R. The effect of elevated intraocular oxygen on organelle degradation in the embryonic chicken lens. J Exp Biol. 2003;206:4353–4361. doi: 10.1242/jeb.00670. [DOI] [PubMed] [Google Scholar]
- 10.Mulhern ML, Madson CJ, Danford A, Ikesugi K, Kador PF, Shinohara T. The unfolded protein response in lens epithelial cells from galactosemic rat lenses. Invest Ophthalmol Vis Sci. 2006;47:3951–3959. doi: 10.1167/iovs.06-0193. [DOI] [PubMed] [Google Scholar]
- 11.Xie Q, Khaoustov VI, Chung CC, Sohn J, Krishnan B, Lewis DE, Yoffe B. Effect of tauroursodeoxycholic acid on endoplasmic reticulum stress-induced caspase-12 activation. Hepatology. 2002;36:592–601. doi: 10.1053/jhep.2002.35441. [DOI] [PubMed] [Google Scholar]
- 12.Tinhofer I, Anether G, Senfter M, Pfaller K, Bernhard D, Hara M, Greil R. Stressful death of T-ALL tumor cells after treatment with the anti-tumor agent Tetrocarcin-A. FASEB J. 2002;16:1295–1297. doi: 10.1096/fj.02-0020fje. [DOI] [PubMed] [Google Scholar]
- 13.Hettmann T, Barton K, Leiden JM. Microphthalmia due to p53-mediated apoptosis of anterior lens epithelial cells in mice lacking the CREB-2 transcription factor. Dev Biol. 2000;222:110–123. doi: 10.1006/dbio.2000.9699. [DOI] [PubMed] [Google Scholar]
- 14.Tu BP, Weissman JS. The FAD- and O2-dependent reaction cycle of Ero1-mediated oxidative protein folding in the endoplasmic reticulum. Mol Cell. 2002;10:983–994. doi: 10.1016/S1097-2765(02)00696-2. [DOI] [PubMed] [Google Scholar]
- 15.Pagani M, Fabbri M, Benedetti C, Fassio A, Pilati S, Bulleid NJ, Cabibbo A, Sitia R. Endoplasmic reticulum oxidoreductin 1-Lbeta (ERO1-Lbeta), a human gene induced in the course of the unfolded protein response. J Biol Chem. 2000;275:23685–23692. doi: 10.1074/jbc.M003061200. [DOI] [PubMed] [Google Scholar]
- 16.Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: A new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Brennan LA, Kantorow M. Mitochondrial function and redox control in the aging eye: Role of MsrA and other repair systems in cataract and macular degenerations. Exp Eye Res. 2009;88:195–203. doi: 10.1016/j.exer.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lou MF. Redox regulation in the lens. Prog Retin Eye Res. 2003;22:657–682. doi: 10.1016/S1350-9462(03)00050-8. [DOI] [PubMed] [Google Scholar]
- 19.Harding JJ. Recent studies of risk factors and protective factors for cataract. Curr Opin Ophthalmol. 1997;8:46–49. doi: 10.1097/00055735-199702000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Negahban K, Chern K. Cataracts associated with systemic disorders and syndromes. Curr Opin Ophthalmol. 2002;13:419–422. doi: 10.1097/00055735-200212000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Simpanya MF, Ansari RR, Suh KI, Leverenz VR, Giblin FJ. Aggregation of lens crystallins in an in vivo hyperbaric oxygen guinea pig model of nuclear cataract: Dynamic light-scattering and HPLC analysis. Invest Ophthalmol Vis Sci. 2005;46:4641–4651. doi: 10.1167/iovs.05-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simpanya MF, Ansari RR, Leverenz V, Giblin FJ. Measurement of lens protein aggregation in vivo using dynamic light scattering in a guinea pig/UVA model for nuclear cataract. Photochem Photobiol. 2008;84:1589–1595. doi: 10.1111/j.1751-1097.2008.00390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiviluoto S, Vervliet T, Ivanova H, Decuypere JP, De Smedt H, Missiaen L, Bultynck G, Parys JB. Regulation of inositol 1,4,5-trisphosphate receptors during endoplasmic reticulum stress. Biochim Biophys Acta. 2013;1833:1612–1624. doi: 10.1016/j.bbamcr.2013.01.026. [DOI] [PubMed] [Google Scholar]