Abstract
Ischemia-reperfusion injury (IRI) following lung transplantation is associated with increased pulmonary inflammatory responses during reperfusion. Mesenchymal stem cells (MSCs) may be able to modulate inflammatory responses in IRI. The aim of the present study was to evaluate the beneficial effects of an intravenous infusion of bone marrow-derived MSCs (BMSCs) in a rat model of pulmonary IRI. IRI was induced in male Lewis rats by 1-h ischemia followed by 2-h reperfusion. The rats received phosphate-buffered saline (PBS) or BMSC infusion at the onset of reperfusion. Pulmonary injury was determined based on the mean blood oxygenation, lung edema and vascular permeability, and performing histopathological examination. Pulmonary inflammation was also evaluated through the examination of the levels of inflammatory cytokines. Compared with the PBS infusion, the BMSC infusion significantly preserved lung function, reduced lung edema and pulmonary microvascular permeability, and decreased the total injury score in rats with IRI. The mRNA levels of the pro-inflammatory cytokines, tumor necrosis factor-α (TNF-α), interleukin (IL)-1β and IL-6, were significantly reduced, while the expression of anti-inflammatory cytokine IL-10 was increased in the rats receiving BMSC infusion. The levels of cytokine-induced neutrophil chemoattractant-1, IL-1β, and TNF-α in bronchoalveolar lavage fluid were also markedly reduced following BMCS infusion. In conclusion, the present results suggested that BMSC infusion exerts protective effects against pulmonary IRI by alleviating IRI-induced inflammation. These findings provide experimental evidence for the treatment of pulmonary IRI using BMSC cell therapy.
Keywords: bone marrow mesenchymal stem cells, interleukin-10, inflammation, ischemia-reperfusion injury
Introduction
Primary graft failure resulting from ischemia-reperfusion injury (IRI) remains one of the most severe perioperative complications following lung transplantation. It accounts for ~30% of patient mortality within 30 days following lung transplantation (1). A growing body of evidence suggested that IRI is associated with enhanced inflammatory responses during reperfusion (2–4). Our previous study and other reports from the literature have demonstrated that an increased production of inflammatory mediators, including tumor necrosis factor-α (TNF-α), interleukin (IL)-1 and inducible nitric oxide synthase enzyme, which is triggered by nuclear factor-κB (NF-κB), play a critical role in IRI development (4–7). Therefore, a therapeutic strategy that directly inhibits inflammatory responses may alleviate IRI effectively.
Mesenchymal stem cells (MSCs) are emerging as a potential cell therapy for various inflammatory diseases. Recent studies have shown that transplantation of MSCs in animal models of IRI in the liver, kidney, intestine, heart, brain and lung promotes organ functional recovery and reduces tissue damages, indicating that MSC transplantation could be a promising approach to promote tissue repair or prevent IRI-induced tissue damage (8–15). MSCs have been found to possess immunomodulatory properties. It has been suggested that MSCs can mediate immunosuppression in vitro by suppressing the activation and proliferation of T lymphocytes, B lymphocytes and natural killer cells, inhibiting the differentiation of dendritic cells and promoting the formation of immature dendritic cells that carry inhibitory phenotype (16–18). Although it is indicated that the protective effects of MSC transplantation may be associated with MSC-mediated suppression of inflammation and apoptosis, the molecular mechanism underlying their beneficial effects remains unclear. In the present study, the role and underlying mechanism of MSCs in modulating pulmonary function and inflammation in a rat model of pulmonary IRI (5) was further investigated.
Materials and methods
Animals and study design
All animals were obtained from the Experimental Center in Zhongshan Hospital, Fudan University (Shanghai, China), and handling protocols were approved by the Institutional Animal Care and Use Committee of Zhongshan Hospital, Fudan University. Male Lewis rats (age, 8–10 weeks; body weight, 250–275 g) were housed in a room with a constant temperature of 24°C under a 12-h light/dark cycle. The rats had free access to tap water and food. A total of 54 rats were divided randomly into the following three groups (n=18 per group): Sham control, IRI + phosphate-buffered saline (PBS) and IRI + bone marrow-derived MSCs (BMSCs) groups. Rats in the sham control group received thoracotomy without IRI. Rats in the IRI + PBS group underwent 1-h ischemia followed by 2-h reperfusion and received an intravenous infusion of PBS at the onset of reperfusion. Finally, rats in the IRI + BMSC group underwent 1-h ischemia followed by 2-h reperfusion and received an infusion of BMSCs at the onset of reperfusion.
Preparation of BMSCs
BMSCs Isolation and expansion of were performed as previously described (19). Briefly, male Lewis rats were sacrificed, following an overdose of pentobarbital (100 mg/kg, intraperitoneally), and their bone marrow was harvested by flushing the cavity of the femurs and tibias with complete culture medium [Iscove's modified Dulbecco's medium with 10% fetal bovine serum and 1% pen-strep (Invitrogen Life Technologies, Carlsbad, CA, USA)]. The harvested cells were washed with complete media and centrifuged at 398.3 × g at 24°C for 5 min. The supernatant was removed, and the cell pellet was then re-suspended and cultured in complete media at 37°C with 5% CO2 and 90% humidity for 3 days. Culture media were replaced every 3 days. The BMSC population was enriched based on its ability to adhere to the tissue culture plate. BMSCs were collected at the third passage and detached using trypsin-free cell detachment buffer (Invitrogen, Shanghai, China). Cells were characterized by CD marker expression using flow cytometry (BD LSR II Flow Cytometer System; BD Biosciences, San Jose, USA) and immunofluorescence staining. Rat monoclonal CD73 (551123; 1:100), mouse and rat monoclonal CD29 (561796; 1:100) and rat monoclonal CD90 (554895; 1:100) were purchased from BD Biosciences, San Jose, CA, USA). In addition, human and rat monoclonal CD45 (sc-70696; 1:50), and human and rat monoclonal CD34 antibodies (sc-7324; 1:5) were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). For immunofluorescence staining, BMSCs were cultured on chamber slides. Following fixation, the cells were stained with phycoerythrin-conjugated CD90 and fluorescein isothiocyanate-conjugated CD29 (20). The fluorescent images were captured using an inverted fluorescence microscope (IX70, Olympus Corporation, Tokyo, Japan).
Induction of pulmonary IRI
An in vivo hilar clamp model of IRI was established as previously described (5). The rats were subjected to 1-h ischemia by left lung hilar occlusion followed by 2-h reperfusion. Briefly, rats were anesthetized using isoflurane as an inhalational anesthetic (Baxter, Deerfield, IL, USA) in a drop jar. Following an intramuscular injection of 0.04 mg atropine (American Reagent Inc., Shirley, NY, USA), the rats were endotracheally intubated with a 14-gauge angiocatheter (SURFLO; Terumo Medical Corporation, Tokyo, Japan) and connected to a pressure-controlled ventilator (Harvard Apparatus Co., South Natick, MA, USA). The mechanical ventilation was controlled at a standardized inspired oxygen flow of 0.2 l/min to achieve a rate of 75–85 breaths/min and a positive end-expiratory pressure of 2 cm H2O. The rats were positioned on their right side on a heating pad in order to maintain a body temperature of 37–38°C. Blood pressure was monitored with a XBP1000 non-invasive tail blood pressure system (Kent Scientific Corp., Torrington, CT, USA) following the initiation of anesthesia and continuously throughout the surgery. A left posterior lateral thoracotomy was then performed by cutting the fifth intercostal space to expose the left hilum. The left lung was mobilized atraumatically, and 50 units of heparin (dissolved in normal saline solution to obtain a total volume of 0.5 ml) were administered via the dorsal penile vein. At 5 min after the administration of heparin, the left hilum was exposed and a 6-0 prolene suture was placed around the hilum using a curved 22-gauge gavage needle. The two ends of the suture were then threaded through a 5 mm-long PE-50 tubing (American Health & Medical Supply International Corp. Co., Ltd., Scarsdale, NY, USA). Ischemia was initiated by pulling up on the suture and thus pushing the tube against the hilum and occluding it. The application of a small surgical clip to the suture on top of the tube followed, to maintain the tension of the tube against the hilum. The thoracotomy was then closed, the rats were extubated and the left lung was kept ischemic for 1 h. After 1 h of ischemia, the rats were re-anesthetized and re-intubated. Reperfusion was achieved through the removal of the clip and the tube/suture. Next, the lung was ventilated and reperfused, and the thoracotomy wound was closed. The rats were subsequently reperfused for 2 h prior to analysis (2). Sham animals only received thoracotomy without hilar occlusion, while the rats that underwent the induction of IRI received an intravenous infusion of PBS or BMSCs at the onset of reperfusion.
Intravenous BMSC infusion
Ex vivo-expanded BMSCs were intravenously injected into the rats via the dorsal penile vein (1×106 cells in 200 µl per rat) at the onset of reperfusion. Harvested BMSCs were first filtered through a 40-µm filter, to obtain single-cell suspensions, and kept in cold calcium and magnesium-free PBS to prevent cell aggregation until injection. The same amount of PBS was infused to the rats in the IRI + PBS group in a similar manner.
Assessment of pulmonary function
The pulmonary function was assessed by analyzing blood gases [arterial oxygen pressure (PaO2)/fraction of inspired oxygen (FiO2), mmHg] on a Stat Profile pHOx Plus L autoanalyzer (Nova Biomedical, Waltham, MA, USA). Blood samples from the left and right pulmonary veins were collected following reperfusion for blood gas analysis.
Estimation of lung edema
Lung edema was estimated by the lung wet-to-dry (W/D) weight ratio. After the blood was completely drained, the left lung was weighed immediately following harvesting, and then placed in a vacuum oven (at 58°C) to completely dry until a stable dry weight was achieved. The lung W/D weight ratio was then calculated.
Evaluation of pulmonary microvascular permeability
Lung microvascular permeability, which was induced by IRI was determined using the Evans blue dye extravasation technique (2). Evans blue (30 mg/kg; Sigma-Aldrich, St. Louis, MO, USA) was injected into the tail vein 30 min prior to sacrifice. The pulmonary vasculature was then perfused for 15 min using the isolated and buffer-perfused lung system to remove extra dye that was lodged in the vasculature, and then the left lung was harvested. The lung tissues were homogenized in PBS to extract the Evans blue dye and then centrifuged at 5,000 × g for 30 min at 4°C. The absorbance of the supernatant was measured at 620 nm using a G Series Vis Light Spectrophotometer (Suzhou Taomsun Commerce and Trade Co., Ltd., Jiangsu, China). Absorbance was corrected for the presence of heme pigments as follows: A620 (corrected) = A620-(1.426 × A740 + 0.030).
Bronchoalveolar lavage (BAL) and cytokine measurement
The left lung was selectively instilled with 5 ml sterile saline via the endotracheal tube at the end of the experiment. A clamp was placed across the right hilum to achieve selective left lung lavage. The BAL fluid was immediately centrifuged at 1,500 × g for 10 min at 4°C. The levels of cytokine-induced neutrophil chemoattractant-1 (CINC-1), IL-1β and TNF-α in the BAL fluid were analyzed using ELISA (R&D Systems Inc., Minneapolis, MN, USA), as previously described (5).
Histopathological examination of lung tissues
Following the sacrifice of the rats, the heart and lungs were rapidly dissected, and the pulmonary circulation was perfused via the right ventricle with 20 ml normal saline. The left lung was then removed, and half of the lung tissue was cut into three equal sections (cranial to caudal end) and fixed in buffered formalin for histological analyses. Histopathological examination was performed on 4-µm paraffin-embedded and formalin-fixed lung tissue sections. Hematoxylin and eosin-stained tissue sections were used to determine the IRI score according to previously established criteria (21). The severity of acute pulmonary injury was scored between 0 and 4 according to comprehensive assessments of alveolar congestion, hemorrhage, edema and infiltration of inflammatory cells in the airspace or the vessel wall. The scoring system was as follows: Score 0, minimal damage; score 1, mild damage; score 2, moderate damage; score 3, severe damage; and score 4, maximal damage. Two pathologists, blinded to the tissue information, conducted histological assessments independently. The mean score from the two pathologists was used for each rat.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
The mRNA levels of TNF-α, IL-1β, IL-6 and IL-10 in the rat lung tissues were measured. Total RNA was extracted from the left lung using TRIzol® reagent (Invitrogen Life Technologies, Carlsbad, CA, USA), according to the manufacturer's instructions. Next, complementary DNA was synthesized using oligo (dT) primer and reverse transcriptase (Takara Bio Inc., Kusatsu, Japan). RT-qPCR reactions were performed using SYBR® Premix Ex Taq™ (Takara Bio Inc.) and 0.2 mmol/l of gene-specific primers with the following thermal cycler conditions: 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. β-actin was used as the internal control. The sequences for PCR primers are displayed in Table I. PCR products were analyzed using agarose gel electrophoresis and the relative mRNA level of the target gene to the housekeeping gene was calculated based on: 2(β-actin cycle threshold-target gene cycle threshold).
Table I.
Primer sequences for reverse transcription quantitative polymerase chain reaction.
| Primer | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| β-actin | AACCCTAAGGCCAACCGTGAAAA | CGACCAGAGGCATACAGGGACAA |
| TNF-α | GCTCTGTGAGGCGACTGGCG | CCGTAAGGAAGGCTGGGCGC |
| IL-1β | GCCTCGTGCTGTCTGACCCATG | AGGGGGCTCCCTAGCATGTCCT |
| IL-10 | GCCAGACCCACATGCTCCGAG | GCAGTCCAGTAGATGCCGGGTG |
| IL-6 | TCTGTCTCGAGCCCACCAGGA | AACGGAACTCCAGAAGACCAGAGCA |
TNF-α, tumor necrosis factor-α; IL, interleukin.
Statistical analysis
SPSS software, version 16.0 (SPSS, Inc., Chicago, IL, USA) was used for all statistical analyses. Data are presented as the mean ± standard error. The comparison among the data was performed using a one-way analysis of variance, or the Student's t-test for unpaired data using Bonferroni correction. P<0.05 was considered to indicate a statistically significant difference.
Results
Isolation and characterization of BMSCs
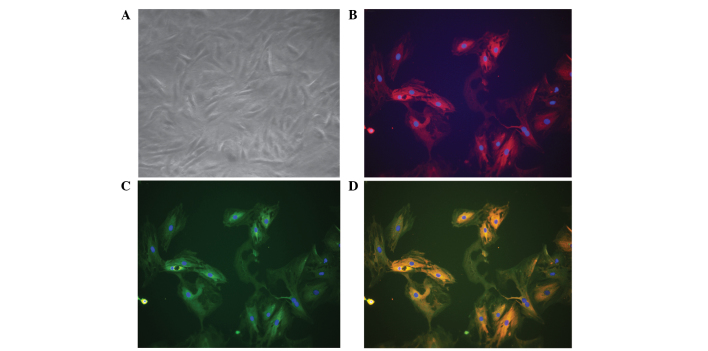
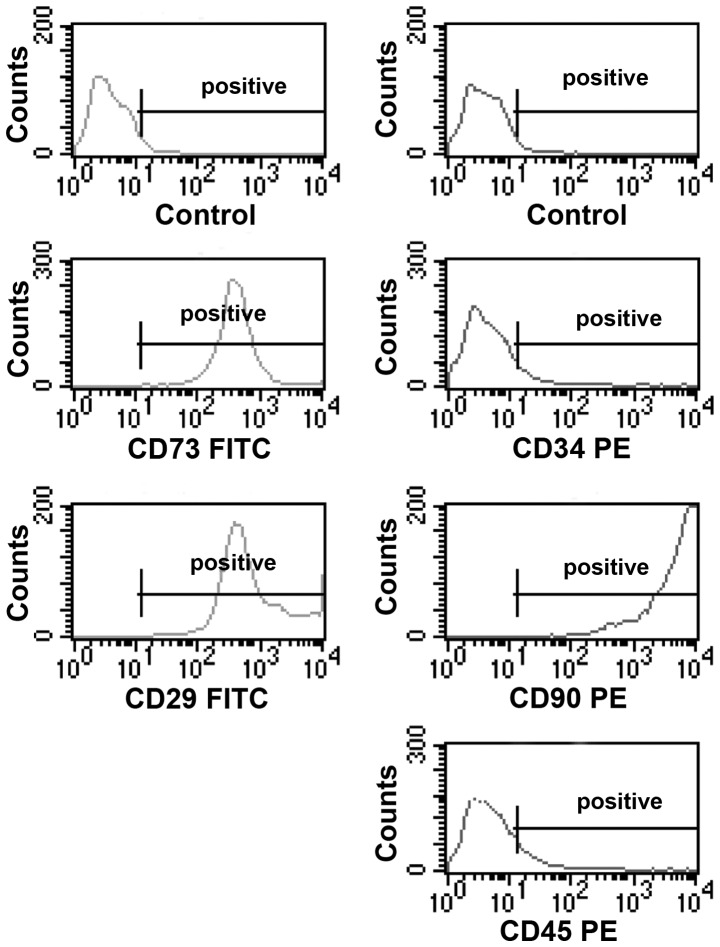
Cultured BMSCs isolated from the bone marrow of male rats appeared as fibroblast-like spindle shape on tissue culture plate (Fig. 1A). Immunofluorescence staining revealed that these cells were positive for CD90 (Fig. 1B and D) and CD29 (Fig. 1C and D). To further characterize these cells, the expression of a panel of markers, including CD29, CD34, CD45, CD73 and CD90, were examined by flow cytometry. The BMSCs were found to be positive for CD29, CD73 and CD90, and negative for CD34 and CD45 (Fig. 2).
Figure 1.
Morphology of BMSCs, and CD29 and CD90 expression. (A) BMSCs appeared as fibroblast-like spindle shape under phase-contrast microscopy. The image was captured using a ×20 objective lens. (B) BMSCs were positive for CD90 expression. BMSCs were cultured on chamber slides. Following fixation, the cells were stained with phycoerythrin-conjugated CD90 (dilution, 1:100). (C) BMSCs were positive for CD29 expression. Following fixation, the cells were stained with fluorescein isothiocyanate-conjugated CD29 (dilution, 1:100). (D) Merged image of parts (B) and (C). The fluorescent images were captured using a ×20 objective lens. Images are representative of 3–5 independent experiments. BMSCs, bone marrow-derived mesenchymal stem cells.
Figure 2.
Flow cytometric analyses of the expression of various BMSC markers. BMSCs were cultured for 3 passages and then the cells were lifted with trypsin-free cell detachment buffer. Following incubation with the fluorescence dye-conjugated antibodies specific for CD29, CD73, CD90, CD34 and CD45, the cells were analyzed by flow cytometry. Images are representative of 3–5 independent experiments. BMSCs, bone marrow-derived mesenchymal stem cells; PE, phycoerythrin; FITC, fluorescein isothiocyanate.
BMSC infusion preserves pulmonary function and reduces lung edema in rats with pulmonary IRI
The mean arterial blood oxygenation [PaO2/FiO2 ratio, mmHg] in rats from the IRI + PBS group decreased markedly 2 h after ischemia when compared with that in rats from the sham control group (P<0.05, Fig. 3A), suggesting that pulmonary function was severely impaired in rats with IRI. Notably, the mean arterial blood oxygenation in the IRI + BMSC group was significantly higher than that in the IRI + PBS group, indicating that BMSC infusion following ischemia preserved the pulmonary function in rats (P<0.05, Fig. 3A). In addition to preserving pulmonary function, BMSC infusion also attenuated lung edema in rats following ischemia. The W/D weight ratio of lung tissues was significantly reduced in rats from the IRI + BMSC group compared with that in rats from the IRI + PBS group (P<0.05, Fig. 3B). These results suggest that BMSC infusion may preserve pulmonary function in rats following ischemia.
Figure 3.
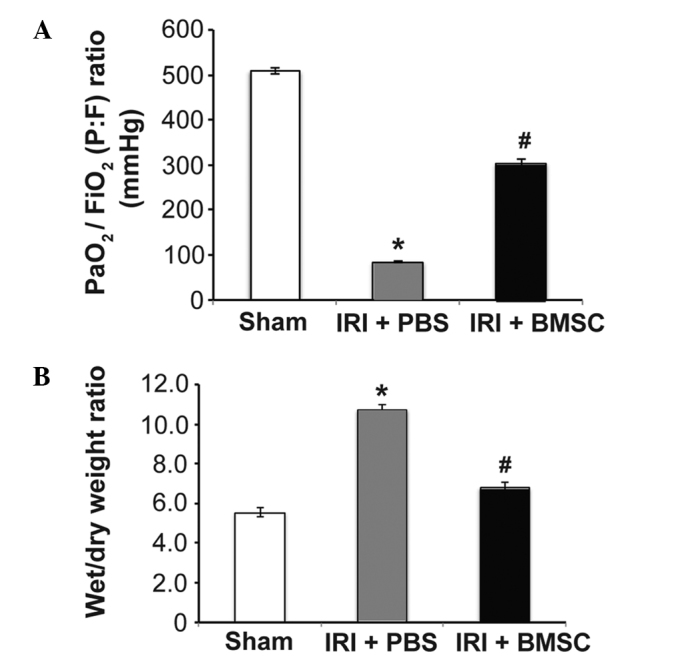
BMSC infusion (A) preserves pulmonary function and (B) significantly reduces lung edema. Pulmonary blood gases were analyzed, using blood samples from the left pulmonary vein that were collected following reperfusion (n=18). Lung edema was measured using lung wet-to-dry weight ratio following reperfusion. Data are presented as the mean ± standard error (n=6). *P<0.05, vs. sham group; #P<0.05, vs. IRI + PBS group. BMSCs, bone marrow-derived mesenchymal stem cells; IRI, ischemia-reperfusion injury; PBS, phosphate-buffered saline.
BMSC infusion alleviates pulmonary tissue damage induced by IRI
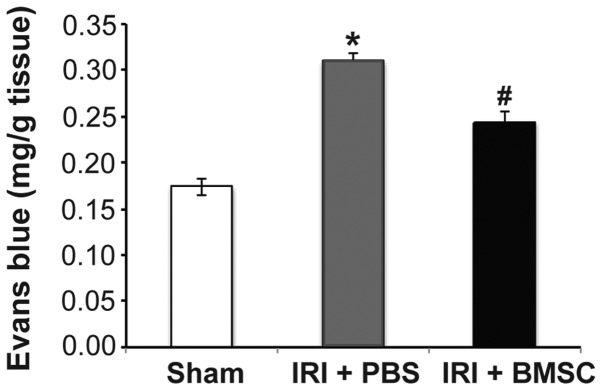
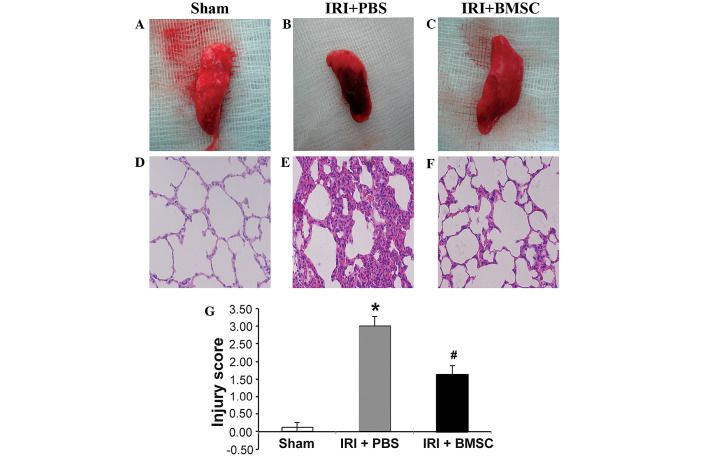
Based on the beneficial effects of BMSC infusion on pulmonary function, we hypothesized that the pulmonary tissue damage caused by IRI may be alleviated by BMSC infusion. Indeed, the Evans blue assay results demonstrated that pulmonary microvascular leakage was significantly reduced in rats from the IRI + BMSC group compared with that in rats from the IRI + PBS group (P<0.05; Fig. 4). To further verify that BMSC infusion reduces pulmonary tissue damage, the total injury score of the rats from each group was assessed. A representative image of a left lung from each group is illustrated in Fig. 5. Compared with the lung tissue from the sham control group (Fig. 5A), the lung tissue from the IRI + PBS group was severely damaged (Fig. 5B). H&E staining revealed that the alveolar structure was intact in the lung from the sham control group (Fig. 5D), while the alveoli in the lung from the IRI + PBS group were severely injured (Fig. 5E). Although the alveoli in the lung from the IRI + BMSC group exhibited mild damage compared with that in the sham control group, the alveolar structure was maintained (Fig. 5F). In addition, the lung tissue from the IRI + BMSC group showed milder damage than the lung tissue from the IRI + PBS group (Fig. 5C vs. 5B, respectively). The average pulmonary injury score of the IRI + BMSC group was significantly lower compared with that of the IRI + PBS group (P<0.05, Fig. 5G), therefore indicating that BMSC infusion can alleviate IRI-induced pulmonary tissue damage.
Figure 4.
BMSC infusion reduces pulmonary microvascular permeability, which was analyzed using the Evans blue dye extravasation technique. Evans blue dye (30 mg/kg) was injected into the tail vein 30 min before sacrifice. Following the removal of extra dye by 15 min perfusion of pulmonary vasculature, the left lung was harvested. Evans blue dye in the lung was extracted and the absorption of the buffer containing the dye was measured at 620 nm and corrected for the presence of heme pigments (n=6). *P<0.05, vs. sham group; #P<0.05, vs. IRI + PBS group. BMSCs, bone marrow-derived mesenchymal stem cells; IRI, ischemia-reperfusion injury; PBS, phosphate-buffered saline.
Figure 5.
BMSC infusion decreases IRI-induced pulmonary tissue damage. Images of left lung tissue from the (A) sham control, (B) IRI + PBS and (C) IRI + BMSC groups. Images of the H&E staining in the (D) sham, (E) IRI + PBS and (F) IRI + BMSC groups. The lungs were harvested following reperfusion. Photographs of the lungs were captured and then the lung tissues were fixed and sectioned. The pulmonary tissue sections were stained with H&E (n=6). (G) BMSC infusion significantly reduced the pulmonary injury severity score, which was assessed according to the histopathological analysis of the lung tissues. The evaluation was conducted by two independent pathologists, blinded to the tissue information. Data are presented as the mean ± standard error (n=6). *P<0.05, vs. sham group; #P<0.05, vs. IRI + PBS group. BMSCs, bone marrow-derived mesenchymal stem cells; IRI, ischemia-reperfusion injury; PBS, phosphate-buffered saline; H&E, hematoxylin and eosin.
BMCS infusion suppresses pulmonary inflammation
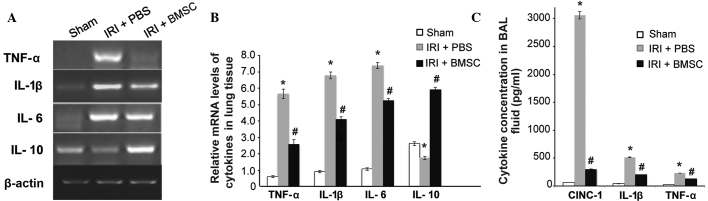
The mechanism underlying the beneficial effects of BMSC infusion was subsequently investigated. Based on previous studies on BMSC transplantation in other diseases (13,16), we hypothesized that BMSCs alleviates IRI-induced pulmonary tissue damage in rats by suppressing inflammation. The results from the RT-qPCR demonstrated that the mRNA expression levels of the pro-inflammatory cytokines TNF-α, IL-1β and IL-6 were significantly increased, while the expression of the anti-inflammatory cytokine IL-10 was significantly reduced in the lung tissue from the IRI + PBS group, compared with that from the sham control group (P<0.05; Fig. 6A and B). Furthermore, BMSC infusion significantly reduced the IRI-mediated overexpression of the pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 (P<0.05, Fig. 6A and B). Notably, BMSC infusion significantly stimulated the IL-10 expression (P<0.05; Fig. 6A and B). Consistent with the RT-qPCR results, the ELISA results revealed that the pro-inflammatory cytokines CINC-1, IL-1β and TNF-α in BAL fluid reached their highest expression levels in the IRI + PBS group, and that BMSC infusion significantly reduced the IRI-mediated secretion of these cytokines (P<0.05, Fig. 6C). These findings clearly suggested that BMSC infusion reduced IRI-mediated inflammation.
Figure 6.
BMSC infusion significantly reduced the pro-inflammatory cytokine levels and stimulated the anti-inflammatory cytokine levels. (A) Image of agarose electrophoresis of the reverse transcription-quantitative polymerase chain reaction product. (B) Quantitative analysis of the mRNA levels of TNF-α, IL-1β, IL-6, and IL-10 and β-actin in lung tissues. Data are presented as the mean ± standard error (n=6). (C) BMSC infusion significantly reduced IRI-induced secretion of pro-inflammatory cytokines. The levels of CINC-1, IL-1β, and TNF-α in BAL fluid were measured using ELISA. Data are presented as the mean ± standard error (n=10). *P<0.05, vs. sham group; #P<0.05, vs. IRI + PBS group. BMSCs, bone marrow-derived mesenchymal stem cells; IRI, ischemia-reperfusion injury; PBS, phosphate-buffered saline; TNF-α, tumor necrosis factor-α, IL, interleukin; BAL, bronchoalveolar lavage; CINC-1, cytokine-induced neutrophil chemoattractant-1.
Discussion
In the present study, the intravenous injection of BMSCs was found to preserve the pulmonary function and significantly reduce IRI-induced pulmonary tissue damage in rats, possibly by attenuating the IRI-mediated pulmonary inflammation. To the best of our knowledge, the present study is the first to show that BMSC infusion markedly stimulates the expression of anti-inflammatory cytokine IL-10 in the lung tissue of rats with pulmonary IRI. The beneficial effects of MSC transplantation have been observed in animal models of IRI in the heart, brain, kidney, liver and intestine (8–13). The application of MSC cell therapy has also been investigated in animal models of pulmonary IRI. Chen et al (14) found that ischemic postconditioning treatment may improve the survival rate of MSC engraftment and subsequently enhance the protective effects of MSC engraftment on the lung in a rat model of IRI. It has also been reported that autologous transplantation of adipose-derived MSCs can markedly alleviate IRI in a rat model by suppressing oxidative stress and inflammatory reactions (15). The present findings are consistent with the results of the aforementioned studies.
The precise molecular mechanism underlying the protective effects of MSC transplantation against pulmonary IRI remains unclear; however, the immunomodulatory properties of MSCs have been suggested to contribute to these protective effects. The current study demonstrated that BMSC infusion markedly increased the expression of the anti-inflammatory cytokine IL-10 and simultaneously reduced the IRI-mediated upregulation of the pro-inflammatory cytokines TNF-α, IL-1β and IL-6. In addition, the IRI-induced release of the pro-inflammatory cytokines CINC-1, TNF-α and IL-1β was significantly reduced by BMSC infusion. The essential role of IL-10 in mediating the beneficial effects of MSC transplantation has been previously demonstrated. For instance, Németh et al (16) found that BMSCs, which can be activated by TNF-α, may reprogram macrophage or monocytes to increase IL-10 production in the septic lung in a mouse model of sepsis, leading to the reduction of mortality and improvement of organ function. Furthermore, it was observed that the beneficial effects of BMSCs may be inhibited by macrophage depletion or pretreatment with functional blocking antibodies for IL-10 or IL-10 receptor (16), indicating the key role of IL-10 in mediating the beneficial effects of MSC transplantation.
IL-10 may mediate the beneficial effects of BMSC infusion in rats with pulmonary IRI by inhibiting the function of neutrophils. Yang et al (2) and Sharma et al (3) found that the accumulation of CD4+ T lymphocytes and neutrophils during reperfusion contributes to pulmonary IRI. Neutrophils are considered to play a critical role in the development of pulmonary microvascular leakage during pulmonary IRI (21). Ajuebor et al (22) reported that IL-10 can block transepithelial migration of neutrophils. In addition, Mei et al (23) demonstrated that decreased levels of TNF-α and elevated levels of IL-10 in the BAL fluid and plasma are associated with the MSCs-mediated improvement of survival and attenuation of pulmonary injury in a mouse model of intrabronchial E. coli endotoxin-induced acute pulmonary injury. Similar to the results of the aforementioned studies, the present results further support the role of IL-10 in mediating the protective effects of BMSCs.
In the present study, BMSC infusion initiated at the onset of reperfusion and had the same duration as reperfusion; therefore, BMSCs were administered during the course of pulmonary injury development, indicating that this administrating route may be therapeutic. However, the long-term effects of BMSC infusion on pulmonary IRI require further investigation.
In conclusion, the present study demonstrated that the intravenous infusion of BMSCs significantly preserved the pulmonary function in rats with pulmonary IRI, as well as substantially reduced IRI-induced pulmonary tissue damage. The mechanism underlying the beneficial effects of BMSC infusion may be associated with a reduction in pro-inflammatory cytokines and a stimulation of anti-inflammatory cytokines. The present results indicate that BMSC transplantation may be a promising cell therapy for patients with pulmonary IRI.
Acknowledgements
This study was supported by grants from the National Natural Science Fund of China (no. 81100225) and the Science and Technology Project of Xiamen (no. 3502Z20134013).
References
- 1.Trulock EP, Edwards LB, Taylor DO, Boucek MM, Keck BM, Hertz MI. International Society for Heart and Lung Transplantation: Registry of the international society for heart and lung transplantation: Twenty-third official adult lung and heart-lung transplantation report-2006. J Heart Lung Transplant. 2006;25:880–892. doi: 10.1016/j.healun.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Yang Z, Sharma AK, Linden J, Kron IL, Laubach VE. CD4+ T lymphocytes mediate acute pulmonary ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2009;137:695–702. doi: 10.1016/j.jtcvs.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma AK, Laubach VE, Ramos SI, Zhao Y, Stukenborg G, Linden J, Kron IL, Yang Z. Adenosine A2A receptor activation on CD4+ T lymphocytes and neutrophils attenuates lung ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2010;139:474–482. doi: 10.1016/j.jtcvs.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anvari F, Sharma AK, Fernandez LG, Hranjec T, Ravid K, Kron IL, Laubach VE. Tissue-derived proinflammatory effect of adenosine A2B receptor in lung ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2010;140:871–877. doi: 10.1016/j.jtcvs.2010.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naidu BV, Woolley SM, Farivar AS, Thomas R, Fraga CH, Goss CH, Mulligan MS. Early tumor necrosis factor-alpha release from the pulmonary macrophage in lung ischemia reperfusion injury. J Thorac Cardiovasc Surg. 2004;127:1502–1508. doi: 10.1016/j.jtcvs.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 6.Maxey TS, Enelow RI, Gaston B, Kron IL, Laubach VE, Doctor A. Tumor necrosis factor-alpha from resident lung cells is a key initiating factor in pulmonary ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2004;127:541–547. doi: 10.1016/j.jtcvs.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Ding JY, Tang L, Xu ST, Wang Q. Influence of pyrrolidine dithiocarbamate pretreatment on function of the transplanted rat lung. Zhong Guo Lin Chuang Yi Xue. 2010;17:795–798. (In Chinese) [Google Scholar]
- 8.Kanazawa H, Fujimoto Y, Teratani T, Iwasaki J, Kasahara N, Negishi K, Tsuruyama T, Uemoto S, Kobayashi E. Bone marrow-derived mesenchymal stem cells ameliorate hepatic ischemia reperfusion injury in a rat model. PLoS One. 2011;6:e19195. doi: 10.1371/journal.pone.0019195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu H, Liu S, Li Y, Wang X, Xue W, Ge G, Luo X. The Role of SDF-1-CXCR4/CXCR7 Axis in the therapeutic effects of hypoxia-preconditioned mesenchymal stem cells for renal ischemia/reperfusion injury. PLoS ONE. 2012;7:e34608. doi: 10.1371/journal.pone.0034608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang H, Qu L, Li Y, Gu L, Shi Y, Zhang J, Zhu W, Li J. Bone marrow mesenchymal stem cells reduce intestinal ischemia/reperfusion injuries in rats. J Surg Res. 2011;168:127–134. doi: 10.1016/j.jss.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 11.Wang M, Tsai BM, Crisostomo PR, Meldrum DR. Pretreatment with adult progenitor cells improves recovery and decreases native myocardial proinflammatory signaling after ischemia. Shock. 2006;25:454–459. doi: 10.1097/01.shk.0000209536.68682.90. [DOI] [PubMed] [Google Scholar]
- 12.Dharmasaroja P. Bone marrow-derived mesenchymal stem cells for the treatment of ischemic stroke. J Clin Neurosci. 2009;16:12–20. doi: 10.1016/j.jocn.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–F42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 14.Chen S, Chen L, Wu X, Lin J, Fang J, Chen X, Wei S, Xu J, Gao Q, Kang M. Ischemia postconditioning and mesenchymal stem cells engraftment synergistically attenuate ischemia reperfusion-induced lung injury in rats. J Surg Res. 2012;178:81–91. doi: 10.1016/j.jss.2012.01.039. [DOI] [PubMed] [Google Scholar]
- 15.Sun CK, Yen CH, Lin YC, Tsai TH, Chang LT, Kao YH, Chua S, Fu M, Ko SF, Leu S, Yip HK. Autologous transplantation of adipose-derived mesenchymal stem cells markedly reduced acute ischemia-reperfusion lung injury in a rodent model. J Transl Med. 2011;9:118. doi: 10.1186/1479-5876-9-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 18.Rasmusson I. Immune modulation by mesenchymal stem cells. Exp Cell Res. 2006;312:2169–2179. doi: 10.1016/j.yexcr.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 19.Ishikane S, Ohnishi S, Yamahara K, Sada M, Harada K, Mishima K, Iwasaki K, Fujiwara M, Kitamura S, Nagaya N, Ikeda T. Allogeneic injection of fetal membrane-derived mesenchymal stem cells induces therapeutic angiogenesis in a rat model of hind limb ischemia. Stem Cells. 2008;26:2625–2633. doi: 10.1634/stemcells.2008-0236. [DOI] [PubMed] [Google Scholar]
- 20.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 21.Kabay B, Aytekin FO, Aydin C, Ozer A, Kabay N, Tekin K, Sungurtekin U, Erdem E, Ozden A. Interleukin-10 gene therapy attenuates pulmonary tissue injury caused by mesenteric ischemia-reperfusion in a mouse model. Tohoku J Exp Med. 2005;207:133–142. doi: 10.1620/tjem.207.133. [DOI] [PubMed] [Google Scholar]
- 22.Ajuebor MN, Das AM, Virág L, Flower RJ, Szabó C, Perretti M. Role of resident peritoneal macrophages and mast cells in chemokine production and neutrophil migration in acute inflammation: Evidence for an inhibitory loop involving endogenous IL-10. J Immunol. 1999;162:1685–1691. [PubMed] [Google Scholar]
- 23.Mei SH, Haitsma JJ, Dos Santos CC, Deng Y, Lai PF, Slutsky AS, Liles WC, Stewart DJ. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med. 2010;182:1047–1057. doi: 10.1164/rccm.201001-0010OC. [DOI] [PubMed] [Google Scholar]