Abstract
Mesenchymal stem cells (MSCs), which exhibit the property of immune-modulation, have been shown to treat various diseases, including pulmonary hypertension. There is a functional similarity between the pulmonary circulation and the placenta, but it remains to be elucidated whether MSCs can be applied to treat endotoxin-induced hypertension during pregnancy; therefore, the aim of the present study was to investigate the therapeutic effect of a human umbilical cord-derived MSC infusion on endotoxin-induced hypertension during pregnancy. Rats were randomly divided into three groups (n=7 per group): Control, endotoxin-treated and endotoxin + MSCs. The model of preeclampsia (PE) was established via the intravenous injection of endotoxin. In the endotoxin + MSCs group, MSCs at 2×106 cells/rat were injected via the vena caudalis. The blood pressure, urine protein and number of white blood cells were measured. In addition, the protein expression levels of the pro-inflammatory cytokines interleukin (IL)-1β and tumor necrosis factor-α (TNF-α) and the anti-inflammatory cytokine IL-10 were examined by ELISA. The blood pressure, levels of urine protein and number of white blood cells in the endotoxin-treated group were significantly higher than those in the control group (P<0.05); however, this increase was significantly attenuated in the endotoxin + MSCs group (P<0.05). In addition, the application of MSCs significantly reduced the levels of pro-inflammatory TNF-α and IL-1β and increased the levels of anti-inflammatory IL-10 in the endotoxin-treated rats. In conclusion, umbilical cord-derived MSCs have a protective effect in an endotoxin-induced model of PE, and this effect is likely elicited through the suppression of inflammatory factors. Umbilical cord-derived MSC-based therapy may provide a potential therapeutic method for endotoxin-induced hypertension during pregnancy.
Keywords: umbilical cord-derived mesenchymal stem cells, hypertension, inflammation, tumor necrosis factor-α, interleukin-1β, interleukin-10, preeclampsia
Introduction
Preeclampsia (PE) is the most serious pregnancy-specific disease in the world (1). It is characterized by hypertension, edema and proteinuria. The exact etiology and pathogenic mechanism of this syndrome are far from being understood; however, it is widely accepted that PE is an autoimmune disease induced in pregnancy due to an immune imbalance at the maternal-fetal interface (2,3). Several studies have indicated that the T-helper 1 (Th1)-type cytokine tumor necrosis factor-α (TNF-α) is associated with PE (4–6). It has been reported that TNF-α can activate the autoantibody-mediated angiotensin receptor. The production of two critical etiological factors in PE, soluble fms-like tyrosine-1 (sFlt-1) and soluble endoglin, can be affected by both TNF-α and the autoantibody-mediated angiotensin receptor (7,8). It has therefore been proposed that TNF-α is involved in the pathogenesis of PE.
A number of approaches have been developed to target specific factors associated with the pathogenesis of PE. The application of vascular endothelial growth factor 121 to Sprague Dawley (SD) rats exhibiting elevated sFlt-1 levels has been shown to successfully ameliorate the main characteristics of PE, including hypertension, proteinuria and glomerular endotheliosis (9). Regarding the treatment of PE, no therapeutic intervention has been proven to be successful to date; however, we hypothesize that focusing on the immune disorder of PE may be a reasonable approach.
Mesenchymal stem cells (MSCs) were first identified by Friedenstein et al in 1966 in the bone marrow (BM) (10). MSCs, which are adult stem cells with self-renewal and multilineage differentiation potentials, have received increasing attention over the last decade. Human MSCs have been demonstrated to exert anti-inflammatory, immunoregulatory and repair effects in a variety of animal models, including models of cardiac disease, lung injury and pulmonary hypertension (11–13). BM is a major source of MSCs in most investigations; however, the collection of BM is invasive and the number of MSCs reduces with age, which significantly limits the clinical application of MSCs (14,15). In 2004, Wang et al (16) demonstrated that the human umbilical cord blood is rich in MSCs that have surface markers and are capable of induced differentiation, similar to MSCs from various tissues including the BM. Thus, umbilical cord blood-derived MSCs have been widely applied in various experimental models, including models of Parkinson's disease, spinal cord injury, lung injury and pulmonary hypertension (13,17–19). Furthermore, there is a functional similarity between the pulmonary circulation and the placenta system; however, whether MSCs can be applied to treat endotoxin-induced hypertension during pregnancy remains unknown. PE can be established in rats via the intravenous administration of endotoxin; this disease model exhibits a similar pathogenesis to that of human PE (20). In the present study, an endotoxin-induced rat model infused with umbilical cord blood-derived MSCs was used to investigate the therapeutic effect of umbilical cord blood-derived MSCs in PE, in order to provide experimental evidence for the future clinical application of stem cells.
Materials and methods
Isolation and culture of human MSCs
Human umbilical cords were collected from local maternity hospitals, following the provision of written informed consent by all donors. The human tissue collection for research was approved by the institutional review board of the Chinese Academy of Medical Science and Peking Union Medical College (Beijing, China). Each umbilical cord was cut into 1- to 3-mm pieces, which were transferred to Dulbecco's modified Eagle's medium (DMEM)/F12 (Gibco-BRL, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 µg/ml streptomycin (Invitrogen Life Technologies, Carlsbad, CA, USA). The tissue was placed in a mixture of 5 U/ml hyaluronidase, 125 U/ml collagenase and 50 U/ml dispase (Sigma-Aldrich, St. Louis, MO, USA) at 37°C for 30 min with gentle agitation. The cells were subsequently pelleted through low-speed centrifugation (2,000 × g for 5 min), suspended in fresh medium and transferred to T-75 cm2 cell plates containing DMEM/F12 along with 20% FBS in a humidified environment with 5% CO2 at saturated humidity. When the cell confluence reached ~80%, the cells were digested with 0.25% trypsin and passaging was performed at a ratio of 1:3. Cells of the fifth to eighth generation were subjected to flow cytometry for identification and used for differentiation induction and transplantation.
Flow cytometry
In the fluorescence-activated cell sorting (FACS) analysis, the following human monoclonal antibodies were used: Human anti-cluster of differentiation (CD) 90 (cat. no. 555596), anti-CD105 (cat. no. 560839), anti-CD73 (cat. no. 550257), anti-CD19 (cat. no. 555412), anti-CD45 (cat. no. 555483), anti-human leukocyte antigen (HLA)-DR (cat. no. 555812), anti-CD11b (cat. no. 555388) and anti-CD34 (cat. no. 555821) (all BD Biosciences, San Jose, CA, USA). The antibodies were either purified or directly conjugated with phycoerythrin or fluorescein isothiocyanate. Data from 10,000 single cell events were collected using a standard FACSCalibur™ flow cytometer (Immunocytometry Systems; Becton Dickinson, Franklin Lakes, NJ, USA) for the fluorescence measurements. Analysis of the obtained data was performed using CellQuest™ software (Becton Dickinson).
Osteogenic and adipogenic differentiation
Osteogenic differentiation was induced using an induction medium of DMEM-F12 supplemented with 10% FBS, 3.06 mg/ml β-glycerophosphate, 100 nmol/l dexamethasone, 10 nmol/l 1,25-dihydroxyvitamin D3 and 0.15 mmol/l ascorbic acid-2-phosphate (all from Sigma-Aldrich). To induce adipogenic differentiation, DMEM supplemented with 10% FBS, 1 µmol/l dexamethasone, 5 µg/ml insulin, 0.5 mmol/l isobutylmethylxanthine and 60 µmol/l indomethacin (all from Sigma-Aldrich) was used. A control was established by maintaining cells in regular growth medium. After 3–4 weeks of osteogenic or adipogenic induction, the presence of calcium deposition in osteocytes or neutral lipid vacuoles in adipocytes was assessed by staining the cells with alizarin red or oil red solution, respectively.
Preparation of the rat PE model and cell transplantation
The rat model of PE was established according to the protocol described by Faas et al (21). All research involving animals was carried out strictly in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals approved by China Medical University (Shenyang, China). Specific pathogen-free 7–8-week-old SD rats (Institute of Laboratory Animal Science, CAMS & PUMC, Beijing, China) were kept under specific pathogen-free conditions in a room with a 12-h light/dark cycle and a temperature maintained at 18–22°C. Standard laboratory pelleted formula and tap water were provided. Separate rack-mounted wire cages, all on the same shelf, were used to house each group of rats The experiments were conducted in accordance with the institutional animal ethics guidelines (China Medical University), and all efforts were made to minimize the suffering of the animals.
Pregnancy was achieved by housing two female SD rats on the night of proestrus with a fertile male for 1 night The next day, when spermatozoa were detected in the smear, was considered as day 0 of pregnancy, and the plugged females were removed from the breeding cages. The pregnant rats were randomly divided into three groups (control, endotoxin-treated and endotoxin + MSCs), with 7 rats in each group. The pregnant rats subsequently received an infusion of either 2 ml endotoxin solution (Sigma-Aldrich; 1.0 µg endotoxin/kg body weight; endotoxin-treated and endotoxin + MSCs groups) or 2 ml saline (control group) for 1 h via the vena caudalis. In the endotoxin + MSCs group, the 7 pregnant rats were injected with MSCs suspended in 100 µl phosphate-buffered saline (2×106 cells/100 µl) on day 14.
Systolic blood pressure was measured in calm, warmed and restrained rats using the tail-cuff method on gestation days (DGs) 8, 15 and 19. Urine samples were analyzed for proteinuria using a pyrogallol phenol red kit (Sigma-Aldrich) in an automatic biochemical analyzer (Hitachi 7600-020; Hitachi, Tokyo, Japan) on DGs 9, 16 and 20. In addition, 20 µl blood was collected and used to determine the total white blood cell count, which was measured with a microcell counter (Sysmex F800; Toa Medical Electronics Co., Ltd., Kobe, Japan).
ELISA
ELISA kits (R&D Systems, Minneapolis, MN, USA) were used to detect the levels of interleukin (IL)-1β, IL-10 and TNF-α in the serum according to manufacturer's instructions.
Statistical analysis
The statistical analysis was performed using SPSS version 14.0 statistical software (SPSS, Inc., Chicago, IL, USA). Data are expressed as the mean ± standard deviation. Comparisons were conducted with one-way analysis of variance, and P<0.05 was considered to indicate a statistically significant difference.
Results
Culture and identification of MSCs derived from human umbilical cords
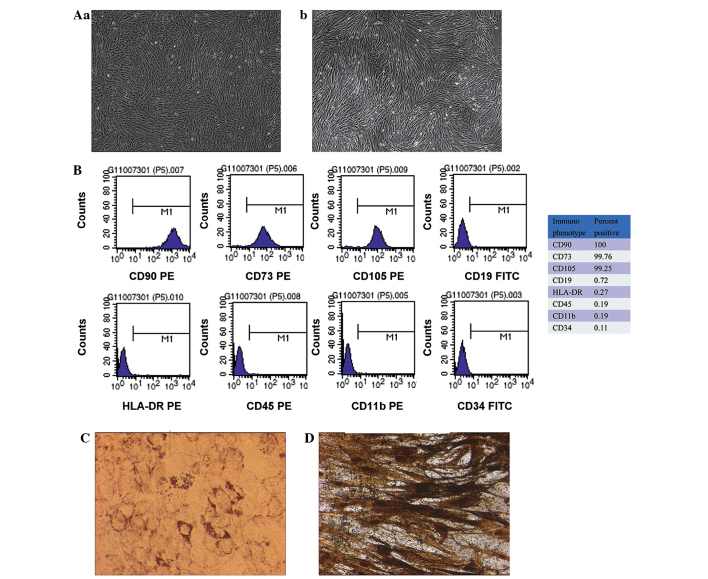
The MSCs formed a monolayer of long, spindle-shaped cells within 1 week and exhibited potent proliferation activity (Fig. 1A). The flow cytometry showed that the umbilical cord-derived MSCs were symmetric with phenotypic surface antigens. The cells were positive for CD105, CD73 and CD90 and negative for CD19, CD45, CD11b, HLA-DR and CD34 (Fig. 1B). In addition, the differentiation induction experiment showed that the MSCs had the capacity for adipogenesis (Fig. 1C) and osteogenesis (Fig. 1D), which was detected using oil red O staining and by the calcification of the alizarin red staining, respectively. These findings suggested that the cells were MSCs.
Figure 1.
Isolation and characterization of MSCs derived from human umbilical cord tissue. (A) Morphology of passage (a) 3 and (b) 8 umbilical cord-derived MSCs: The cells exhibited uniform spindle-shape morphology and were ranked compactly in a parallel or vortex form (magnification, ×40). (B) Flow cytometric characterization of isolated umbilical cord-derived MSCs during passage. Expression of the surface antigens CD90, CD73, CD105, CD19, HLA-DR, CD45, CD11b and CD34 was detected using flow cytometry. The positive percentage of each marker is shown on the right. Data are representative of three independent experiments. (C) Detection of adipogenic differentiation using oil red O staining. Adipogenic differentiation was evidenced by the formation of lipid vacuoles by oil-red O staining in umbilical cord-derived MSCs following adipogenic induction (magnification, ×200). (D) Osteogenic differentiation was assayed using alizarin red staining (magnification, ×200). Osteogenic differentiation was evidenced by the formation of mineralized matrix in the umbilical cord-derived MSCs following osteogenic induction. MSC, mesenchymal stem cell; CD, cluster of differentiation; HLA, human leukocyte antigen; PE, phycoerythrin; FITC, fluorescein isothiocyanate.
MSC-based therapy ameliorates the symptoms of PE
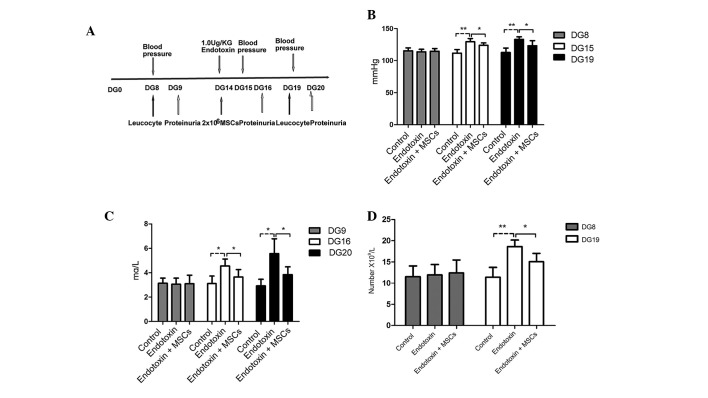
Following the positive identification of the MSCs, PE model rats were established and treated with a suspension of MSCs in order to investigate the potential therapeutic effects of the MSCs in PE (Fig. 2A). The characteristic features of increased blood pressure (Fig. 2B), proteinuria (Fig. 2C) and an increased white blood cell count (Fig. 2D) indicated that the rat model of PE had been successfully established. Notably, MSC infusion was found to ameliorate the symptoms exhibited by the PE model rats, eliciting a decrease in the blood pressure and proteinuria (Fig. 2B–D).
Figure 2.
MSC-based therapy ameliorates endotoxin-induced PE in mice. (A) Schematic for the experimental course of the PE model induced by endotoxin and the MSC-based therapy. (B) Blood pressure was detected on DG8, DG15 and DG19. (C) Proteinuria was detected on DG9, DG16 and DG20. (D) The number of white blood cells was detected on DG8 and DG19. All data are representative of three independent experiments. Values are presented as the mean ± standard deviation. *P<0.05 and **P<0.01. MSC, mesenchymal stem cell; PE, preeclampsia; DG, gestation day.
Therapeutic effect of MSCs is mainly dependent on the serum levels of inflammatory cytokines
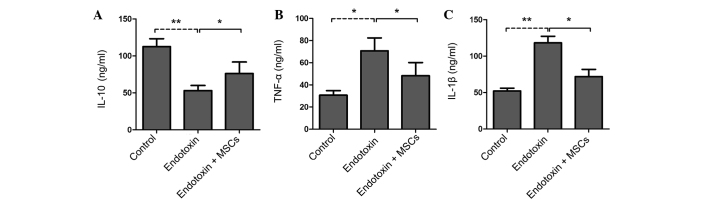
Compared with the control rats, the endotoxin-induced PE rat group showed increased expression levels of IL-1β and TNF-α but decreased levels of IL-10 in the serum (Fig. 3A–C). Of note, the infusion of MSCs significantly attenuated the increases in IL-1β and TNF-α expression and increased the IL-10 levels in the serum (Fig. 3A–C). These findings suggest that MSCs may ameliorate endotoxin-induced PE mainly via the suppression of the levels of inflammatory factors.
Figure 3.
Serum cytokine protein levels in the normal control, endotoxin and endotoxin + MSCs groups. (A-C) Serum levels of (A) IL-10, (B) TNF-α and (C) IL-1β. All data are representative of three independent experiments. Values are presented as the mean ± standard deviation. *P<0.05 and **P<0.01. MSC, mesenchymal stem cell; IL, interleukin; TNF-α, tumor necrosis factor-α.
Discussion
PE is specific to pregnancy, and is the most common serious complication of pregnancy in the world (1). Although the exact etiology and pathogenesis of PE remain unclear, there is now consensus that an activated inflammatory response is involved (22). It has been confirmed that MSCs exert anti-inflammatory and immunoregulatory effects in animal models, including models of arthritis, colitis and autoimmune encephalomyelitis (23–25); however, the therapeutic efficacy of human umbilical cord-derived MSCs in a rat model of PE and a detailed understanding of any associated cellular and molecular mechanisms have yet to be established. In the present study, the question of whether human umbilical cord-derived MSCs could be applied for the treatment of PE was addressed.
To date, MSCs have been successfully cultivated from a range of tissues, including trabecular bone, adipose tissue, placenta, pancreas and umbilical cord blood (26–32). In the present study, fibroblast-like cells were isolated from the umbilical cord tissue of newborns by a modified enzymatic digestion procedure and cultured (21,33). The human umbilical cord-derived MSCs adhered to the cell culture plates and showed a high expression of CD105, CD73 and CD90, the characteristic MSC surface markers; by contrast, neither CD34 nor CD45 expression was observed. The umbilical cord-derived MSCs were induced to differentiate into either bone or adipose tissue, demonstrating that the MSCs isolated from newborn umbilical cord tissue met the criteria used to identify MSCs (34).
It has been suggested that PE is a pregnancy-induced autoimmune disease. Compared with non-pregnant women, the circulating levels of TNF-α and IL-6 are further raised in patients with PE (35). It was indicated that Th1 cytokines, which are believed to be the main cause of PE, could directly damage organs (36). Consistent with previous findings that TNF-α is a key player in the etiology of PE (37), it was found in the present study that the levels of IL-1β and TNF-α were increased in the blood of the pregnant endotoxin-treated rats, suggesting that increased levels of Th1 pro-inflammatory cytokines, including IL-1β and TNF-α, play a crucial role in control of PE.
An important characteristic of MSCs is their immunosuppressive properties (38). Several studies have indicated that MSCs can suppress the expression of pro-inflammatory cytokines, such as IL-1β and TNF-α, in vitro and in vivo (39–41). A reduction in the level of TNF-α has been observed following the co-culture of MSCs with anti-CD3/CD28-activated peripheral blood mononuclear cells (39). Since MSCs primarily mediate a downregulation of Th1 pro-inflammatory cytokines, we hypothesized that an MSC infusion could be a suitable strategy to promote immune balance and effectively control PE. Notably, the results of the present study showed that the infusion of MSCs significantly reduced the IL-1β and TNF-α expression and increased the IL-10 levels in the serum. These findings suggest that MSCs may ameliorate the symptoms of endotoxin-induced PE rats mainly via the suppression of pro-inflammatory factors.
In conclusion, the present study demonstrated that human umbilical cord-derived MSC-based therapy significantly ameliorated the symptoms of an endotoxin-induced PE rat model, as evidenced by decreased blood pressure and proteinuria. Furthermore, the therapy reversed abnormal IL-1β and TNF-α expression in the serum. These data suggest that MSCs may play an important role in the immune balance at the maternal-fetal interface and that human umbilical cord-derived MSC-based therapy may provide a potential therapeutic strategy for PE.
Acknowledgements
The authors are grateful for the support of the Natural Science Foundation of China (grant no. 30872618).
References
- 1.Chesley LC. Hypertensive disorders in pregnancy. J Nurse Midwifery. 1985;30:99–104. doi: 10.1016/0091-2182(85)90116-8. [DOI] [PubMed] [Google Scholar]
- 2.Kanasaki K, Kalluri R. The biology of preeclampsia. Kidney Int. 2009;76:831–837. doi: 10.1038/ki.2009.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldman-Wohl DS, Yagel S. Examination of distinct fetal and maternal molecular pathways suggests a mechanism for the development of preeclampsia. J Reprod Immunol. 2007;76:54–60. doi: 10.1016/j.jri.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Conrad KP, Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol. 1997;37:240–249. doi: 10.1111/j.1600-0897.1997.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 5.Gulati R. Raised serum TNF-alpha, blood sugar and uric acid in preeclampsia in third trimester of pregnancy. JNMA J Nepal Med Assoc. 2005;44:36–38. [PubMed] [Google Scholar]
- 6.Peraçoli MT, Bannwart CF, Cristofalo R, et al. Increased reactive oxygen species and tumor necrosis factor-alpha production by monocytes are associated with elevated levels of uric acid in pre-eclamptic women. Am J Reprod Immunol. 2011;66:460–467. doi: 10.1111/j.1600-0897.2011.01016.x. [DOI] [PubMed] [Google Scholar]
- 7.Irani RA, Zhang Y, Zhou CC, et al. Autoantibody-mediated angiotensin receptor activation contributes to preeclampsia through tumor necrosis factor-alpha signaling. Hypertension. 2010;55:1246–1253. doi: 10.1161/HYPERTENSIONAHA.110.150540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parrish MR, Murphy SR, Rutland S, et al. The effect of immune factors, tumor necrosis factor-alpha, and agonistic autoantibodies to the angiotensin II type I receptor on soluble fms-like tyrosine-1 and soluble endoglin production in response to hypertension during pregnancy. Am J Hypertens. 2010;23:911–916. doi: 10.1038/ajh.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Z, Zhang Y, Ying Ma J, et al. Recombinant vascular endothelial growth factor 121 attenuates hypertension and improves kidney damage in a rat model of preeclampsia. Hypertension. 2007;50:686–692. doi: 10.1161/HYPERTENSIONAHA.107.092098. [DOI] [PubMed] [Google Scholar]
- 10.Friedenstein AJ, Piatetzky-Shapiro II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381–390. [PubMed] [Google Scholar]
- 11.Williams AR, Hare JM. Mesenchymal stem cells: Biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109:923–940. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kode JA, Mukherjee S, Joglekar MV, Hardikar AA. Mesenchymal stem cells: Immunobiology and role in immunomodulation and tissue regeneration. Cytotherapy. 2009;11:377–391. doi: 10.1080/14653240903080367. [DOI] [PubMed] [Google Scholar]
- 13.Pullamsetti SS, Schermuly R, Ghofrani A, et al. Novel and emerging therapies for pulmonary hypertension. Am J Respir Crit Care Med. 2014;189:394–400. doi: 10.1164/rccm.201308-1543PP. [DOI] [PubMed] [Google Scholar]
- 14.Nishida S, Endo N, Yamagiwa H, et al. Number of osteoprogenitor cells in human bone marrow markedly decreases after skeletal maturation. J Bone Miner Metab. 1999;17:171–177. doi: 10.1007/s007740050081. [DOI] [PubMed] [Google Scholar]
- 15.Mueller SM, Glowacki J. Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. J Cell Biochem. 2001;82:583–590. doi: 10.1002/jcb.1174. [DOI] [PubMed] [Google Scholar]
- 16.Wang HS, Hung SC, Peng ST, et al. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells. 2004;22:1330–1337. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- 17.Fu YS, Cheng YC, Lin MY, et al. Conversion of human umbilical cord mesenchymal stem cells in Wharton's jelly to dopaminergic neurons in vitro: Potential therapeutic application for Parkinsonism. Stem Cells. 2006;24:115–124. doi: 10.1634/stemcells.2005-0053. [DOI] [PubMed] [Google Scholar]
- 18.Yang CC, Shih YH, Ko MH, et al. Transplantation of human umbilical mesenchymal stem cells from Wharton's jelly after complete transection of the rat spinal cord. PLoS One. 2008;3:e3336. doi: 10.1371/journal.pone.0003336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moodley Y, Atienza D, Manuelpillai U, et al. Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. Am J Pathol. 2009;175:303–313. doi: 10.2353/ajpath.2009.080629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faas MM, Schuiling GA, Baller JF, et al. A new animal model for human preeclampsia: Ultra-low-dose endotoxin infusion in pregnant rats. Am J Obstet Gynecol. 1994;171:158–164. doi: 10.1016/0002-9378(94)90463-4. [DOI] [PubMed] [Google Scholar]
- 21.Faas MM, Broekema M, Moes H, et al. Altered monocyte function in experimental preeclampsia in the rat. Am J Obstet Gynecol. 2004;191:1192–1198. doi: 10.1016/j.ajog.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 22.Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 1998;179:80–86. doi: 10.1016/S0002-9378(98)70254-6. [DOI] [PubMed] [Google Scholar]
- 23.González MA, Gonzalez-Rey E, Rico L, et al. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum. 2009;60:1006–1019. doi: 10.1002/art.24405. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez-Rey E, Anderson P, González MA, et al. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929–939. doi: 10.1136/gut.2008.168534. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Li Y, Chen J, et al. Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Exp Neurol. 2005;195:16–26. doi: 10.1016/j.expneurol.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 26.Tuli R, Tuli S, Nandi S, et al. Characterization of multipotential mesenchymal progenitor cells derived from human trabecular bone. Stem Cells. 2003;21:681–693. doi: 10.1634/stemcells.21-6-681. [DOI] [PubMed] [Google Scholar]
- 27.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 28.Fukuchi Y, Nakajima H, Sugiyama D, et al. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells. 2004;22:649–658. doi: 10.1634/stemcells.22-5-649. [DOI] [PubMed] [Google Scholar]
- 29.In't Anker PS, Scherjon SA, der Keur Kleijburg-van C, et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22:1338–1345. doi: 10.1634/stemcells.2004-0058. [DOI] [PubMed] [Google Scholar]
- 30.Hu Y, Liao L, Wang Q, et al. Isolation and identification of mesenchymal stem cells from human fetal pancreas. J Lab Clin Med. 2003;141:342–349. doi: 10.1016/S0022-2143(03)00022-2. [DOI] [PubMed] [Google Scholar]
- 31.Bieback K, Kern S, Klüter H, Eichler H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells. 2004;22:625–634. doi: 10.1634/stemcells.22-4-625. [DOI] [PubMed] [Google Scholar]
- 32.Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103:1669–1675. doi: 10.1182/blood-2003-05-1670. [DOI] [PubMed] [Google Scholar]
- 33.Han YF, Tao R, Sun TJ, et al. Optimization of human umbilical cord mesenchymal stem cell isolation and culture methods. Cytotechnology. 2013;65:819–827. doi: 10.1007/s10616-012-9528-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 35.Conrad KP, Miles TM, Benyo DF. Circulating levels of immunoreactive cytokines in women with preeclampsia. Am J Reprod Immunol. 1998;40:102–111. doi: 10.1111/j.1600-0897.1998.tb00398.x. [DOI] [PubMed] [Google Scholar]
- 36.Zenclussen AC, Fest S, Joachim R, et al. Introducing a mouse model for pre-eclampsia: Adoptive transfer of activated Th1 cells leads to pre-eclampsia-like symptoms exclusively in pregnant mice. Eur J Immunol. 2004;34:377–387. doi: 10.1002/eji.200324469. [DOI] [PubMed] [Google Scholar]
- 37.Redman CWG, Sacks GP, Sargent IL. Preeclampsia: An excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180:499–506. doi: 10.1016/S0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- 38.Shi Y, Hu G, Su J, et al. Mesenchymal stem cells: A new strategy for immunosuppression and tissue repair. Cell Res. 2010;20:510–518. doi: 10.1038/cr.2010.44. [DOI] [PubMed] [Google Scholar]
- 39.Carter D, Tyrell A, Bubnic S, et al. Characterization of MSC potential to treat GVHD using molecular markers linked to MSC-mediated immunosuppression in vitro. Blood. 2005;106:160b. [Google Scholar]
- 40.Gupta N, Su X, Popov B, et al. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- 41.Rafei M, Campeau PM, Aguilar-Mahecha A, et al. Mesenchymal stromal cells ameliorate experimental autoimmune encephalomyelitis by inhibiting CD4 Th17 T cells in a CC chemokine ligand 2-dependent manner. J Immunol. 2009;182:5994–6002. doi: 10.4049/jimmunol.0803962. [DOI] [PubMed] [Google Scholar]