Summary
Abdominal compartment syndrome (ACS) occurs when increasing intra abdominal-pressure (IAP) reduces blood flow to abdominal organs. This results in impairment of pulmonary, cardiovascular, renal, hepatic, central nervous system and gastro-intestinal (gi) function, causing multiple organ dysfunction syndrome and death. The significant prognostic value of elevated intra-abdominal pressure has prompted many intensive care units to adopt measurement of this physiologic parameter as a routine vital sign in patients at risk. ACS generally occurs in patients who are critically ill due to any of a wide variety of medical and surgical conditions. it has been recently described as a rare complication of burn injury. it is fundamental to: 1) recognize IAP and ACS; 2) resuscitate effectively; and 3) prevent the development IAP-induced end-organ dysfunction and failure. We present our recent experience with one patient suffering from ACS secondary to burn injury and the physiologic results of abdominal wall escharotomy.
Keywords: abdominal compartment syndrome, burn
Abstract
Le syndrome du compartiment abdominal (SCA) se produit lorsque l’augmente de la pression intra-abdominale (PIA) réduit le flux sanguin vers les organes abdominaux. Il en résulte une dépréciation de pulmonaires, cardiovasculaires, rénales, hépatiques, système nerveux central et la fonction (GI) gastro-intestinale, causant le syndrome de défaillance multiviscérale et la mort. La valeur pronostique de la pression intra-abdominale élevée a incité de nombreuses unités de soins intensifs à adopter la mesure de ce paramètre physiologique comme un signe vital de routine chez les patients à risque. Le SCA se produit généralement chez des patients gravement malades en raison d’une grande variété de conditions médicales et chirurgicales. Il a récemment été décrit comme une complication rare associée aux brûlures. Il est fondamental de: 1) reconnaître la PIA et le SCA; 2) ressusciter efficacement; et 3) prévenir le développement de la dysfonction et la défaillance des organes cibles induites par la PIA. Nous présentons notre expérience récente d’un patient souffrant du SCA suite aux brûlures et les résultats physiologiques d’une escarrotomie de la paroi abdominale.
Case report
A 48-year-old patient was admitted to the intensive Care Unit with severe burn injuries. He suffered from inhalation injury and full-thickness burns affecting more than 55% of his total body surface area (TBSA), involving the face, neck, trunk and torso (particularly the anterior and lateral surface of the abdomen) and upper extremities (Figs. 1, 2, 3 and 4).
Fig. 1. patient upon submission.
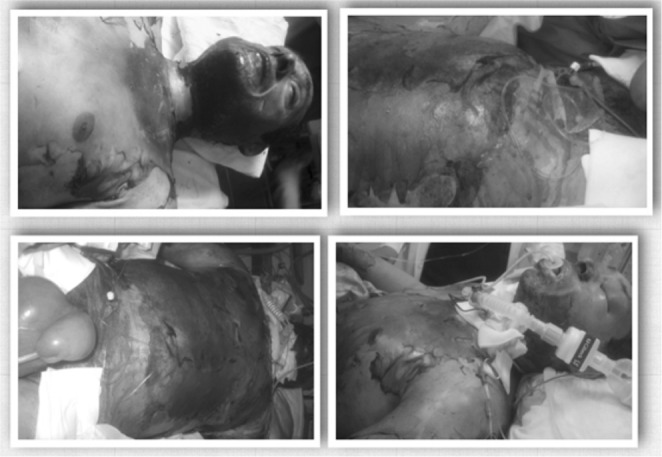
Fig. 2. Escharotomies.
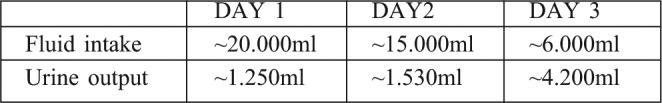
Upon admission, the patient was haemodynamically stable, tachypneic, anxious but oriented. He was intubated and mechanically ventilated and resuscitated with intravenous fluids by a central venous line (femoral vein). The protocol used for the resuscitation was the parkland formula, using lactated ringer’s solution. During the first day post-burn he received 20l of the solution and his respiratory, cardiovascular and renal functions were closely monitored. His urine output was 1250ml, which slightly exceeds the upper limit of the recommended 0.5 to 1ml/kg/h range. Measurement of bladder pressure, as a reflection of IAP, via the urinary catheter, and calculation of arterial perfusion pressure (App), permitted monitoring of intra-abdominal hypertension (iAH) and ACS. Fluid resuscitation and urine output during the first three days following injury are summarized below (Table I).
Table I. Overview of fluid resuscitation and urine output for Days 1-3 post-burn.
Three days after initial clinical improvement, the patient’s cardiovascular, respiratory and urinary status deteriorated. Despite the aggressive and apparently adequate initial resuscitation and fluid volume replacement, he became heamodynamically unstable and developed hypotension, tachycardia, elevated jugular venous pressure, peripheral oedema, and lactic acidosis. Urine output decreased below 0,5ml/h/kg body weight.
Despite chemical paralysis with succinylcholine, ventilation was difficult (peak pressure = 60cm H20) and advanced ventilation methods were required. Serious abdominal tenderness was also noted. The burn eschar affig fecting both chest and abdomen was parchment, rigid and inelastic and the abdomen was tense. Urinary bladder pressure was 30mmHg; combined with a net decrease in App to 55mmHg, it indicated ACS and critical decrease in perfusion of intra-abdominal organs.
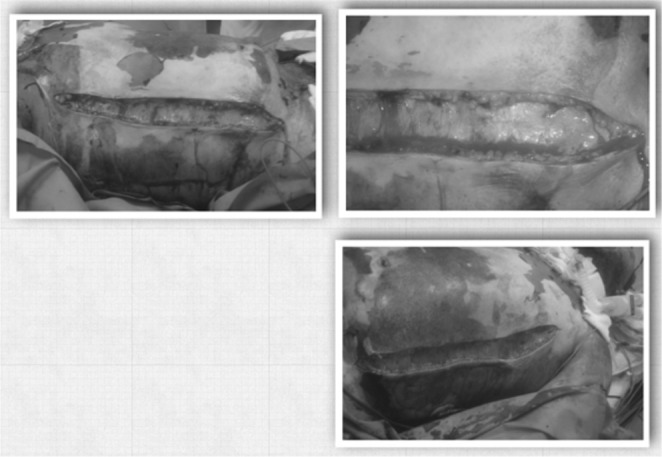
Medical management of the patient included hemodynamic support (inotropes), increased ventilatory positive pressures, and fluid infusion of approximately 4l of crystalloids per day. The plastic surgery service was then consulted for surgical management of ACS. Urgent abdominal escharotomy-decompression at the bedside was recommended and was performed under controlled conditions. postoperatively, the patient’s condition temporarily ameliorated with improvement of all the parameters measured. IAP dropped adequately to almost normal levels; therefore complete release of the abdominal wall was not indicated.
Three days later the patient started to be unstable again and required excessive fluid loads and inotropic support. no pressure measurement data is available, consequently his deterioration may not be related to ACS but it could be due to sepsis. He finally developed septic shock (septic fever, positive blood cultures) and multi-organ failure and succumbed a week later.
Discussion
The abdominal cavity has a certain tolerance to accommodate increasing intra-abdominal volume without any noticeable increase in IAP. in the case of massive overload in a severely burned patient, splanchnic edema increases intra abdominal volume. As a consequence, the abdominal wall distends to such a point where more distention is no more possible. At this point IAP increases sharply and iAH and ACS result.1
Regular calculation of the ivy index (cumulative ml/kg: 24h volumes exceeding 250 ml/kg) will identify patients at risk of developing ACS. However, in the eventuality of a thick abdominal eschar, abdominal distention is restricted, thus the critical point of increased IAP is reached with lesser increase in intra abdominal volume and iAH and ACS may occur with lesser fluid resuscitation volumes.4-6
Increasing IAP causes progressive hypoperfusion and ischemia of the intestines and other peritoneal and retroperitoneal structures. The effects of iAH are not limited just to the intra-abdominal organs, but rather have an impact either directly or indirectly on every organ system in the body (dysfunction of pulmonary, cardiovascular, renal, hepatic, central nervous and gastro-intestinal system).7
IAP may be measured directly by means of an intraperitoneal catheter or indirectly by measuring rectal, gastric, inferior vena cava, or urinary bladder pressures. The most accepted and practical method is recording the urinary bladder pressure as a reflection of IAP. The perfusion pressure (APP) of intra-abdominal organs is the difference between the mean arterial pressure and the intraabdominal pressure (APP = MAP-IAP). Higher systemic blood pressure may maintain abdominal organ perfusion even when the intra-abdominal pressure may be increased. patients with an IAP ≤ 10 mmHg are not at risk of developing ACS, while patients with an IAP ≥ 25 mmHg usually develop ACS.1,4,8-11
Severe burns represent a devastating injury that results in massive fluid loss and induces profound systemic inflammation requiring large volumes of resuscitative fluids. There is no perfect resuscitation protocol and studies have demonstrated that patients frequently receive larger amounts of fluids than required precipitating a condition recently recognized as “fluid creep”. Fluid creep is an iatrogenic phenomenon resulting from misuse of the originally described approaches to crystalloid resuscitation. it is associated with massive edema and compartment syndromes (orbital, abdominal, and extremity compartment syndrome).11,13,14
Groups of burn patients that have been identified in whom resuscitation requirements are usually greater than the parkland Formula predictions include patients with inhalation injuries, electrical burns, those with additional injuries, patients with high alcohol or drug intake, and those in whom resuscitation was delayed. To avoid “fluid creep”, the resuscitation formulas have to be used only as indicators for the initial fluid resuscitation rate. This rate must be adjusted according to several parameters, the most important and most frequently used being urine output. This parameter should not be allowed to exceed the recommended hourly urine output range of 0.5 to 1 ml/kg/h.
Recently, the U.S. Army institute of Surgical research proposed a simplified guideline called “the rule of 10” for the calculation of the initial resuscitation fluid rate minimizing the risk of overload:
Estimate burn size to the nearest 10
%TBSA × 10 = initial fluid rate in ml/h (for adult patients weighing 40–80kg)
For every 10kg above 80kg, increase the rate by 100ml/h15-17
Oda et al.4 retrospectively reviewed burn patients (> 40% total body surface area) who received either isotonic or hypertonic crystalloid resuscitation. Hypertonic resuscitation was associated with a significantly decreased fluid requirement and significantly higher App levels. On the other hand, isotonic resuscitation was associated with a 3.5-fold increased risk for developing iAH (defined as IAP> 30cm H2O). Oda et al. concluded that the decreased fluid load afforded by hypertonic crystalloid resuscitation could reduce the risk of secondary ACS, however hypertonic resuscitation may be associated with complications of its own as well as with higher mortality.
Some authors add colloids to their resuscitation regimen within the first 24h to reduce the total resuscitation volumes. However, this remains a debatable issue even though there is growing evidence of its usefulness. Despite some reservation concerning the use of albumin in the early phases of burn resuscitation, recent work demonstrated a decreased mortality rate. There is also growing evidence that vitamin C supplementation, in the early post-burn period, seems to decrease the needed fluid volumes. Tanaka et al. found that adjuvant high dose ascorbic acid (66 mg/kg/h for 24h), administered during the first 24h after thermal injury, significantly decreased the amount of fluid given compared to the control.18-20
Close monitoring of intra-abdominal pressure is recommended in any burn patient who has received 0.25ml/kg or more of fluid resuscitation than estimated. A resuscitation volume greater than 237cc/kg over the course of 12h (or 16l during a 12 hour period in a 70kg man) appears to be the threshold for the development of Abdominal Compartment Syndrome.21
It is currently unknown whether the syndrome is an iatrogenic consequence of excessive fluid resuscitation or an unavoidable sequelae of the primary injury. A recent systematic review of severely burned patients concluded that the fluid resuscitation volume was directly responsible for the development of ACS. it exacerbates splanchnic oedema leading to an increase in gut permeability, bacterial translocation, and increased intra-abdominal pressure. resuscitation-related ACS is associated with a mortality of 97% when burn size is greater than 60% TBSA.22-26
Capillary leak and third spacing are universal in major burns. in patients with burns of more than 60% of their body surface area and without abdominal pathology, the pathogenesis for increased iAp is most likely due to massive fluid resuscitation with third spacing and secondary extrinsic compression by burn eschars. “Capillary leak” following shock, with ischemia-reperfusion injury and the release of vasoactive substances and oxygen-derived free radicals increases extracellular volume. Especially when it occurs with associated inhalational injury, delayed resuscitation, and abdominal wall injuries.26-28
Despite a number of noninvasive management strategies (sedation, chemical paralysis, diuresis), interventions such as decompressive escharotomy, percutaneous peritoneal drainage or even laparotomy are often required once the ACS is established. it is unclear from the available literature what level of iAp requires surgical intervention (decompression), or what length of time intra-abdominal hypertension can be tolerated before significant end-organ damage occurs.29
in the patient we are presenting, urine output, exceeded by far on the second and third post injury days the recommended hourly rate. Had this been timely addressed properly, probably the patient’s outcome might have been different. Was abdominal escharotomy delayed? Was a laparotomy indicated? These are difficult questions to answer with our current state of knowledge. What is definite is that urine output of burn patients must not be allowed to remain high for a prolonged period without radical reduction in perfused fluid volumes.
Conclusion
Development of ACS in burn patients is associated with high mortality. prevention, early detection and proper management may avoid this usually fatal complication. intra-abdominal hypertension is a not an infrequent complication in severe burn patients requiring massive fluid resuscitation. Over-resuscitation and fluid creep is a well recognised causative factor for ACS. Though fluid resuscitation to correct hypovolemia and avoid organ failure remains a cornerstone for critical care management, in severely burned patients judicious fluid resuscitation needs to be established. Circumferential abdominal burn eschars might also lead to ACS by producing a tourniquet effect. At bedside, urgent decompressive escharotomy of the abdominal wall is a safe surgical procedure that provides rapid relief of intra-abdominal pressure. it improves ventilation, hemodynamic parameters, and oxygen metabolism and can decrease morbidity and mortality. Further decompression with laparotomy also needs to be considered when simple escharatomy or abdominal skin releasing incisions do not result in a net decrease of IAP.
References
- 1.Walker J, Criddle lM. Pathophysiology and management of abdominal compartment syndrome. Am J Crit Care. 2003;12:367–71. [PubMed] [Google Scholar]
- 2.Burd A, et al. Burns. 2006;32:284–92. doi: 10.1016/j.burns.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 3.Markell K, Renz EM, et al. Abdominal complications after severe burns. J Am Coll Surg. 2009;208:940–9. doi: 10.1016/j.jamcollsurg.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 4.Oda J, Ueyama M, Yamashita K, Effects of escharotomy as abdominal decompression on cardiopulmonary function and visceral perfusion in abdominal compartment syndrome with burn patients. Department of Trauma and Critical Care Medicine, Social insurance Chukyo Hospital, nagoya, Aichi, Japan; [DOI] [PubMed] [Google Scholar]
- 5.Kirkpatrick AW, Ball Cg, Nickerson D, D’Amours SK. Intra-abdominal hypertension and the abdominal compartment syndrome in burn patients. World J Surg. 2009;33:1142–9. doi: 10.1007/s00268-009-9995-4. [DOI] [PubMed] [Google Scholar]
- 6.Abu-Sittah GS, Sarhane KA, et al. Cardiovascular dysfunction in burns: review of the literature. Ann Burns Fire Disasters. 2012;25:26–37. [PMC free article] [PubMed] [Google Scholar]
- 7.Burd A, Frederick V, et al. Decompression not escharotomy in acute burns. Division of plastic and reconstructive Surgery, Department of Surgery, The Chinese University of Hong Kong, prince of Wales Hospital. Burns. 2006;32:284–92. doi: 10.1016/j.burns.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 8.Tsoutsos D, Rodopoulou S, Keramidas E, Lagios M, Stamatopoulos K, Ioannovich J. Early escharotomy as a measure to reduce intraabdominal hypertension in full-thickness burns of the thoracic and abdominal area. World Journal of Surgery. 2003;27:1323–8. doi: 10.1007/s00268-003-6962-3. [DOI] [PubMed] [Google Scholar]
- 9.Cheatham ML. Abdominal compartment syndrome: pathophysiology and definitions. Scandinavian Journal of Trauma, resuscitation and Emergency Medicine. 2009 doi: 10.1186/1757-7241-17-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hershberger RC, Hunt JL, Arnoldo BD, Purdue GF. Abdominal compartment syndrome in the severely burned patient. J Burn Care Res. 2007;28:708–14. doi: 10.1097/BCR.0b013E318148C988. [DOI] [PubMed] [Google Scholar]
- 11.Tuggle D, Skinner S, Garza J, Vandijck D, Blot S. The abdominal compartment syndrome in patients with burn injury. Acta Clin Belg Suppl. 2007;1:136–40. [PubMed] [Google Scholar]
- 12.Tricklebank S. Modern trends in fluid therapy for burns. Burns. 2009;35:757–67. doi: 10.1016/j.burns.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Saffle JI. The phenomenon of “fluid creep” in acute burn resuscitation. J Burn Care Res. 2007;28:382–95. doi: 10.1097/BCR.0B013E318053D3A1. [DOI] [PubMed] [Google Scholar]
- 14.Atiyeh BS, Dibo SA, Ibrahim AE, Zgheib ER. Acute burn resuscitation and fluid creep: it is time for colloid rehabilitation. Ann Burns Fire Disasters. 2012;25:59–65. [PMC free article] [PubMed] [Google Scholar]
- 15.Pham TN, Cancio LC, Gibran NS. American Burn Association: American Burn Association practice guidelines burn shock resuscitation. J Burn Care Res. 2008;29:257–66. doi: 10.1097/BCR.0b013e31815f3876. [DOI] [PubMed] [Google Scholar]
- 16.Chung KK, Salinas J, Renz EM, Alvarade RA, King BT, Barillo DJ, et al. Simple derivation of the initial fluid rate for the resuscitation of severely burned adult combat casualties: in Silico validation of the rule of ten. J Trauma. 2010;69:49–54. doi: 10.1097/TA.0b013e3181e425f1. [DOI] [PubMed] [Google Scholar]
- 17.Ferdinand K, Bacomo , Chung KK. A primer on burn resuscitation. J Emerg Trauma Shock. 2011;4:109–13. doi: 10.4103/0974-2700.76845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung KK, Wolf SE, Cancio LC, et al. Resuscitation of severely burned military casualties: Fluid begets more fluid. J Trauma Crit Care. 2009;67:231–7. doi: 10.1097/TA.0b013e3181ac68cf. [DOI] [PubMed] [Google Scholar]
- 19.Cartotto R, Zhou A. Fluid creep: The pendulum hasn’t swung back yet! J Burn Care Res. 2010;31:551–8. doi: 10.1097/BCR.0b013e3181e4d732. [DOI] [PubMed] [Google Scholar]
- 20.Alvarado R, Chung KK, Cancio LC, Wolf SE. Burns resuscitation. Burns. 2009;35:4–14. doi: 10.1016/j.burns.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Ivy ME, Atweh NA, Palmer J, Possenti PP, Pineau M, D’Aiuto M. Intra-abdominal hypertension and abdominal compartment syndrome in burn patients. J Trauma. 2000;49:391–7. doi: 10.1097/00005373-200009000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Markell KW, Renz EM, White CE, Albrecht ME, Blackbourne LH, Park MS, Barillo DA, Chung KK, Kozar RA, Minei JP, Cohn SM, Herndon DN, Cancio LC, Holcomb JB, Wolf SE. Abdominal complications after severe burns. J Am Coll Surg. 2009;208:940–9. doi: 10.1016/j.jamcollsurg.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 23.Hayek S, Ibrahim A, Abu Sittah G, Atiyeh BS. Burn resuscitation: is it straightforward or a challenge? Ann Burns Fire Disasters. 2011;24:17–21. [PMC free article] [PubMed] [Google Scholar]
- 24.Pruitt BA. Protection from excessive resuscitation: “pushing the pendulum back”. J Trauma. 2000;49:567–8. doi: 10.1097/00005373-200009000-00030. [DOI] [PubMed] [Google Scholar]
- 25.Matsuda T, Tanaka H, Shimazaki S, et al. High-dose vitamin C therapy for extensive second-degree burns. Burns. 1991;2:127–31. doi: 10.1016/0305-4179(92)90009-j. [DOI] [PubMed] [Google Scholar]
- 26.Kremer T, Harenberg P, Hernekamp F, Riedel K, Gebhardt MM, Germann G, Heitmann C, Walther A. High-dose vitamin C treatment reduces capillary leakage after burn plasma transfer in rats. J Burn Care Res. 2010;31:470–9. doi: 10.1097/BCR.0b013e3181db5199. [DOI] [PubMed] [Google Scholar]
- 27.Bakomo FK, Chung KK. A primer on burn resuscitation. J Emerg Trauma Shock. 2001;4:109–13. doi: 10.4103/0974-2700.76845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demling RH. The burn edema process: Current concepts. J Burn Care Rehab. 2005;26:207–27. [PubMed] [Google Scholar]
- 29.Edgar DW, Fish JS, Gomez M, et al. Local and systemic treatments for acute edema after burn injury: A systemic review of the literature. J Burn Care Res. 2011;32:334–47. doi: 10.1097/BCR.0b013e31820ab019. [DOI] [PubMed] [Google Scholar]


