Abstract
Asthma is a chronic inflammatory disease. Despite the fact that current therapies, such as the combination of inhaled corticosteroids and β2-agonists, can control the symptoms of asthma in most patients, there is still an urgent need for an alternative anti-inflammatory therapy for patients who suffer from severe asthma but lack acceptable response to conventional therapies. Many molecular factors are involved in the inflammatory process in asthma, and thus blocking the function of these factors could efficiently alleviate airway inflammation. RNA interference (RNAi) is often thought to be the answer in the search for more efficient and biocompatible treatments. However, difficulties of efficient delivery of small interference RNA (siRNA), the key factor in RNAi, to target cells and tissues has limited its clinical application. In this review, we summarize cytokines and chemokines, transcription factors, tyrosine kinases and costimulatory factors that have been reported as targets of siRNA mediated treatment in experimental asthma. Additionally, we conclude several targeted delivery systems of siRNA to specific cells such as T cells, macrophages and dendritic cells, which could potentially be applied in asthma therapy.
Keywords: Small interfering RNA (siRNA), asthma, targeted delivery, polymer
1. Introduction
Asthma is one of the major worldwide public health problems affecting 334 million people [1] and accounting for 250,000 annual deaths [2]. It is estimated that there would be additional 70 million asthmatic patients in 2025 [1] and that the global market for asthma and chronic obstructive pulmonary disease (COPD) drugs would increase to $47.1 billion in 2017 [3]. Asthma can be effectively controlled in most patients by current therapies including inhaled β2-adrenergic receptor agonists, inhaled corticosteroids and injected immunoglobulin E (IgE) antibodies [4]. Nevertheless, compliance of inhaled corticosteroids is poor due to the requirement of frequent inhalation and concerns of long-term side effect [5]. In contrast, common overuse of β2-adrenergic receptor agonists (CDC,2007) increases the risk of systemic side effects [6]. Moreover, there are 5-10% asthmatic patients who lack adequate response to current therapies of asthma. The patients within that group who also have common asthmatic comorbidity conditions, such as rhinosinusitis and chronic infections, experience worse symptoms of asthma and a higher risk of morbidity [7]. Thus, it is necessary to develop safer and more efficient additional treatments for asthma.
Asthma is a chronic inflammatory disorder and airway disease characterized by limitation of airflow, airway hyperresponsiveness (AHR), mucus hypersecretion and infiltration of inflammatory cells. In the pathogenesis of asthma, common environmental stimuli (e.g. dust, pollen) are recognized inappropriately by the immune system as antigens and consequently induce IgE-dependent inflammatory response. Asthmatic symptoms are majorly driven by inflammatory cells, for example, mast cells, eosinophils, dendritic cells, lymphocytes, macrophages and their cytokines [8, 9]. Dendritic cells, the most efficient antigen presenting cells (APCs), can present allergens to naïve T helper cells (Th0) and promote the differentiation of T helper 2 cells (Th2). Th2 cells can orchestrate the allergic response of asthma via secretion of Th2 interleukins (IL-4, IL-5 and IL-13) [8]. For instance, IL- 4 can promote differentiation and proliferation of Th2 cells and together with IL-13 stimulates B cells to proliferate and produce IgE. IL-5 can recruit and activate eosinophils as well as maintain their proliferation and survival. IL-13 is associated not only with IgE synthesis but also with chemoattraction of eosinophils, AHR, airway remodeling and mucus cell hyperplasia [8-12]. Other than Th2 cells, mast cells also contribute to asthmatic inflammation by secreting histamine, tryptase and prostaglandin D2 (PGD2) which can induce potent bronchoconstriction, airway edema and lung fibroblast proliferation as the result of IgE-dependent stimulation by allergens [13]. Another essential type of inflammatory cells are eosinophils which can produce lipid mediators and pro-inflammatory cytokines to directly damage epithelial cells, induce bronchoconstriction and amplify inflammatory responses [14].
New drugs for asthma in pre-clinic research or clinical trails include new bronchodilators and corticosteroids as well as inhibitors of novel therapeutic targets, for example, kinase inhibitors and cytokine/chemokine blocking agents. RNA interference (RNAi) is thought to be an efficient approach to block the functions of molecular targets. RNAi is an endogenous and sequence specific messenger RNA (mRNA) degradation process involving small interference RNA (siRNA). Delivery of synthetic siRNA, of 21-23 bp in length, into mammalian cells can induce cleavage of mRNA containing complementary sequence and consequently decrease the expression of the corresponding protein [15]. siRNA mediated therapies are post-transcriptional, transient and based on sequence-specific gene silencing, hence they are believed to offer a more potent and specific treatment with less side effects for different diseases [16, 17]. Pulmonary delivery of siRNA as non-invasive and local administration can reduce systemic side effects and prolong the lung retention period for the treatment of lung diseases like asthma [18]. Furthermore, rapid therapeutic effects and lower dose requirement can be achieved due to the physiological properties of the lung such as the large alveolar surface, absence of serum proteins and the presence of a thin epithelium [19, 20]. Despite of the advantages of pulmonary delivery of siRNA, the biggest obstacle of clinical application of siRNA in asthma is the lack of biocompatible vectors to efficiently deliver siRNA into target cells.
In this review, we will discuss the molecular targets for siRNA mediated therapies and the formulation of siRNA applied in experimental asthma and will summarize available siRNA delivery systems that are targeted to immune cells.
2. Therapeutic Molecular Targets of siRNA in Asthma
As described in the introduction, asthma is a very complex inflammatory disease implicating numerous cells and cellular factors. The common therapeutic molecular targets for RNAi therapy include cytokines/chemokines, transcription factors, tyrosine kinases/tyrosine kinase receptors, and costimulatory molecules. Additionally, new biological targets have been discovered, such as the transient receptor potential cation channel (TRPV1) [21], nerve growth factor [22], and the soluble membrane N-ethylmaleimide-sensitive factor attachment protein receptor protein (syntaxin4) [23].
2.1. Cytokines and chemokines
It is known that cytokines/chemokines, particularly Th2 cytokines, play prominent roles in airway inflammatory response and remodeling. Thus, blockage of cytokines/chemokines and their corresponding receptors by using siRNA has been reported. Examples are the downregulation of suppressor of cytokine signaling 3 (SOCS3) [24], IL-4 [25] and IL-5. Given that the presence of eosinophils is implicated in airway hyperresponsiveness and remodeling and IL-5 is the most important cytokine involved in eosinophil growth, activation as well as recruitment, siRNA against IL-5 was applied to reduce inflammatory response in an ovalbumin (OVA)-induced murine asthma model. Huang et al. showed that intratracheal (i.t.) administration of a lentivirus containing a siRNA against IL-5 expressing cassette (SEC4) to asthmatic mice for three consecutive days reduced the expression of IL-5 in the lung by 50% quantified by real time PCR (RT-PCR). Reduced eosinophilia was observed in SEC4 treated animals confirmed by a lower percentage of eosinophils in the bronchoalveolar lavage fluid (BALF) cells and H&E staining slides as well as by reduced levels of eosinophil chemoattractive cytokine (eotaxin) in the BALF. Consequently, the AHR was significantly relieved according to non-invasive whole body plethysmography (PenH) and invasive techniques [26].
2.2. Transcription factors
Blockage of a single mediator or receptor cannot achieve great therapeutic effects due to the complexity of asthma. Therefore, the upstream molecules of the inflammatory process have become more attractive therapeutic targets. Transcription factors that control the production of cytokines/chemokines and are involved in the growth and differentiation of major inflammatory cells were reported as targets for siRNA mediated treatment, such as GATA-3, transducer and activator of transcription 6 (STAT6), and receptor interacting protein 2 (Rip2) [27]. As shown in Figure 1, GATA-3, a transcription factor highly expressed in Th2 cells, can determine the differentiation of Th2 cells and promote the production of Th2 interleukins [28-30]. Antisense therapeutic intervention of GATA-3 in experimental asthma has been achieved not only with siRNA. Finotto et al. reported that 200 μg intranasally (i.n.) administrated GATA-3 antisense oligonucleotides for four continuous days to mice with OVA-induced inflammation can achieve local anti-inflammatory effects which were comparable to the positive control group treated with the first-line drug dexamethasone [31]. This report of a therapeutic effect of blockage of GATA-3 in asthma is in line with the observation by Lee et al. who i.n. delivered GATA-3 small hairpin RNA with a lentiviral vector (2.2×106 IFU) to OVA-induced asthmatic mice [32]. The only asthma therapy study involving GATA-3 siRNA was published by Sel et al. To assess the efficacy of GATA-3 specific DNAzyme (Gd21) to prevent and treat experimental allergic asthma, they i.n. applied 200 μg Gd21 to mice with acute experimental asthma and compared to animals that were i.n. treated with 200 μg siRNA against GATA-3 or GATA-3 antisense oligonucleotides. These treatments were alternative antisense strategies in contrast to the DNAzyme. They concluded that Gd21 can more effectively reduce the inflammation than GATA-3 specific oligonucleotides and siRNA because these alternative antisense therapies showed a less efficient reduction of the number of eosinophils in the BAL cells and decreased the IL-5/IFN-γ ratio less successfully [33]. However, their results also showed that GATA-3 specific siRNA treatment can reduce the number of mucus-producing goblet cells and AHR comparably to the Gd21 treatment. In addition, the authors did not use any delivery system for siRNA, anti-sense nucleotides or DNAzyme administration, and “naked” nucleic acids were applied directly. The approach of delivering free nucleic acids has been reported to lead to less efficiency in silencing genes in vivo and in vitro compared with the delivery via any kind of vector. Therefore, the therapeutic effect of GATA-3 siRNA or even antisense oligonucleotides is worth to be further evaluated in vivo. The concept of therapeutically downregulating GATA-3, however, was recently efficiently tested in a human phase 3 clinical trial [34]. STAT-6, another promising target, is the transcription factor that controls the onset and maintenance of GATA-3 expression and is involved in T cells differentiation and IgE production [35]. The anti-inflammatory effect of i.n. administration of 100 μg siRNA against STAT-6 to asthmatic mice was described by Darcan-Nicolasien et al. They showed a decreased amount of infiltrated leukocytes in the BALF and reduced AHR in the STAT-6 siRNA treatment group compared with animals treated with siRNA against green fluorescent protein (GFP) which served as a negative control. Additionally, decreased levels of IL-4, IL-5, IL-13 and less histological alterations as well as less T cells infiltration were observed in lung tissue from the STAT-6 siRNA treatment group. However, the biodistribution of nanoparticles in the central and peripheral area of the lung was majorly in airway epithelial cells instead of lymphocytes, where the expression of STAT-6 is most important in asthma. This observation may lead to higher doses being necessary to obtain efficient therapeutic effects [36].
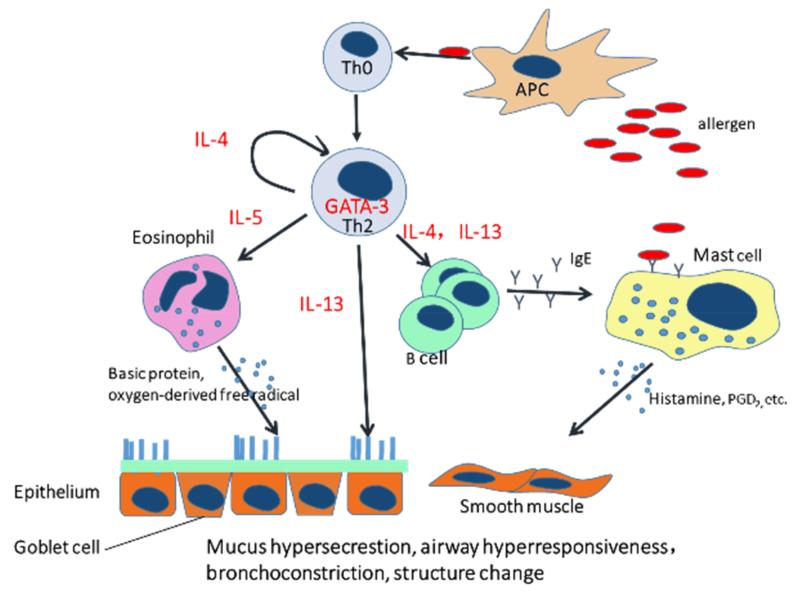
Figure 1.
T helper 2 cells (Th2) orchestrate the pathogenesis of asthma. Antigen presenting cells (APC) present allergens to naïve T helper cells (Th0). Th0 will be activated and GATA-3 expression will be up-regulated which determines differentiation of Th2 and promotes production of Th2 interleukins. IL-13 can stimulate mucus production in goblet cells. IL-4 can switch B cells to produce IgE which can bind on the surface of mast cells and can directly interact with allergens to induce mast cell response. IL-5 can recruit eosinophils. Both eosinophils and mast cells response can affect epithelium and airway smooth muscle to induce airway hyperresponsiveness, bronchioconstriction and airway remodeling.
2.3. Tyrosine kinase / tyrosine kinase receptor
Other upstream targets are kinases involved in the inflammatory process such as spleen tyrosine kinase (Syk) and the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) [37]. Spleen tyrosine kinase (Syk) is widely expressed in immune cells and can initiate or mediate various cellular signaling pathways, including the development and maturation of B cells, proliferation of T cells, and production of inflammatory mediators from macrophages and mast cells [38]. It was described by Stenton et al. that three i.n. aerosolized doses of Syk antisense oligodexoynucleotides (ASO)/ liposome to OVA-induced asthmatic rats can reduce the infiltration of leukocytes found in the BALF and inhibit the antigen induced up-regulation of adhesion molecules in eosinophils, neutrophils and macrophages. However, there was no statistical difference of pulmonary inflammation between the Syk-ASO treatment and the saline treatment group according to the histological analysis of lung sections, and two doses of Syk-ASO treatments were not as sufficient to inhibit the airway inflammation as three treatments were. Therefore, Syk-ASO is not a very efficient anti-inflammatory therapy but Syk was proven to be a valuable target [39]. Inspired by this study, Huang et al. i.n. instilled different doses of siRNA against Syk (10, 1 and 0.1 μg) on three consecutive days before the induction of acute lung inflammation. They observed less infiltration of inflammatory cells in animals treated with 10 μg siRNA and 1 μg siRNA but not in the group treated with 0.1 μg siRNA, indicating Syk siRNA can effectively reduce the recruitment of inflammatory cells in experimental lung inflammation, and this effect was dose dependent [40]. Moreover, Excellair™ (ZaBeCor, Bala Cynwyd, PA, USA), a therapeutic siRNA against Syk, moved to Phase II clinical trails as a new drug for asthma in 2009 and confirmed the potential of Syk as a therapeutic target in asthma. A different potential target is the tyrosine kinase receptor C-kit of which the ligand is stem cell factor (SCF). Wu et al. reported that i.n. administration of methylation modified siRNA against C-kit (35 μg/day) for three days to mice with experimental allergic asthma can significantly reduce the levels of IL-4, IL-5 and SCF in the BALF. In addition, the treatment can inhibit the infiltration of inflammatory cells including eosinophils and the secretion of mucus. Significantly reduced expression of C-kit in lung tissue of C-kit siRNA treated animals was confirmed by PCR and Western blot compared with a scrambled siRNA treated group. Nevertheless, the therapeutic effect of C-kit siRNA needs further verification considering that a healthy control group was missing in this study. [41].
2.4. Costimulatory molecules
APCs can initiate and maintain the airway inflammatory response, particularly a Th2-mediated response. The interaction between APCs and T cells requires the facilitation by costimulatory molecules such as CD86 and CD40 [42]. Asai-Tajiri et al. reported that bone marrow derived dendritic cells (DCs) in vitro transfected with siRNA against CD86 can lose their ability to activate Th2 cells. In an OVA-induced murine asthma model, after i.n. administration of Texas Red labeled CD86 siRNA (12.5 μg), it was observed that Texas Red-siRNA was able to pass through the epithelial barrier and co-localized with DCs. With this biodistribution result in mind, they i.n. applied siRNA against CD86 (12.5 μg/ day) for three days and achieved reduction of Th2 interleukin levels, decreased eosinophil infiltration and reduced AHR. However, treatment of CD86 specific siRNA did not reduce the goblet cell-mediated hyperplasia, indicating that higher doses of CD86 specific siRNA may be required to sufficiently alleviate the symptoms of asthma [43].
3. Targeted Polymeric Delivery of siRNA for asthma therapy
As summarized in Table 1, pulmonary delivery of siRNA is the most common administration approach for the treatment of experimental asthma, and the majority of the formulations are either naked/modified siRNAs or use lentiviral vectors.
Table 1. Summary of biological molecular targets for siRNA mediated asthma therapies.
| Administration | Formulation/vector | Target Molecule | Ref |
|---|---|---|---|
| Intratracheal | Naked/Modified siRNA | CD86 | [43] |
| Rip2 | [27] | ||
| Lentivirus | IL-5 | [26] | |
| Nerve growth factor | [22] | ||
| Atelocollagen | Syntaxin4 | [23] | |
| Intranasal | Lentivirus | GATA-3 | [32] |
| Naked/Modified siRNA | GATA-3 | [33] | |
| STAT-6 | [36] | ||
| Syk | [40] | ||
| c-kit | [41] | ||
| SOCS-3 | [24] | ||
| IL-4 | [25] | ||
| Intravenously | Plasmid | CD40 | [42] |
Considering the large size, instability and negative surface charge, siRNA is easily enzymatically degraded, phagocytically cleared, aggregates with serum proteins, and does not readily cross physiological membranes [23, 44]. Hence, viral or non-viral carriers are needed, even though there are many reports in the literature of pulmonary delivery of high doses of naked/modified siRNA [45]. Due to the safety issues of viral vectors such as immunogenesis and insertional mutagenesis, non-viral delivery vectors, particularly polymeric carriers, are more attractive and favorable approaches to deliver siRNA. With the flexible and modifiable chemical structure, polymeric carriers have the potential to overcome extracellular barriers including instability in the blood stream, cell/tissue targeted delivery and crossing biological membranes, as well as intracellular barriers like endosomal escape and cytoplasmic siRNA release. Additionally, polymeric vectors can maintain a high capacity of siRNA loading, biocompatibility and the ability to co-deliver small molecule drugs [46, 47].
There are many polymeric delivery carriers that have been prepared and optimized for pulmonary delivery of siRNA, such as polyethylenimine (PEI) and chitosan [45]. However, administration of polymeric nanoparticles encapsulating siRNA is limited in asthma therapy due to the potential side effects induced by non-specific cellular uptake and the difficulty to transfect differentiated immune cells like lymphocytes in vivo. Receptor-ligand mediated targeted delivery of siRNA could be a solution.
3.1. T cell targeted delivery systems
It is believed that Th2 cells can initiate and aggravate symptoms of asthma via the production of Th2 interleukins. For this reason, there are many studies that aim to block the effects of Th2 responses via RNAi. Currently, the most popular lymphocyte transfection method is electroporation/nucleofection which, however, is not suitable for in vivo application [48, 49]. Moreover, T cells generally resist the transfection of conventional polymeric or lipid-based vectors (e.g. lipofectamine). Therefore, the difficulty to efficiently transfect T lymphocytes hinders the clinical application of RNAi in asthma. Recently, a few polymeric siRNA delivery systems specific for T lymphocytes have been discovered. CD7 is a surface antigen of human T cells which rapidly internalizes upon binding of an antibody. To target and deliver nanoparticles encapsulating siRNA to T cells for the therapy of HIV infection, Kumar et al. conjugated a CD7 specific single-chain variable fragment with oligo-9-arginine peptide (scFvCD7-9R). Oligo-arginine is a positively charged cell-penetrating short peptide which has been reported to efficiently encapsulate siRNA and enhance the membrane permeability of nanoparticles [50]. scFvCD7-9R was accordingly able to transduce FITC-labeled siRNA selectively to primary human CD3+ T cells but not macrophages or B cells in vitro. The in vivo specificity of nanoparticles was tested in two kinds of humanized mouse models implanted with human peripheral mononuclear cells (Hu-PBL and Hu-HSC). The i.v. injection of 50 μg siRNA against CD4 with scFvCD7-9R on two consecutive days significantly reduced the expression of CD4 on activated CD3+ T cells in Hu-PBL mice as well as on the resting/naïve CD3+ T cells in Hu-HSC mice. Additionally, to test the therapeutic effect, the authors systemically administrated scFvCD7-9R encapsulating a mixture of anti-viral siRNAs including CCR5, Vif and Tat to Hu-PBL mice. Nanoparticles were shown to protect Hu-PBL mice implanted mit HIV-seronegative donor’s PBMCs from HIV-1 challenge and significantly inhibited the viral replication. Furthermore, treatment with antiviral-nanoparticles of the Hu-PBL mice implanted with HIV-seropositive donor’s PBMCs was able to protect the CD4+ cell loss compared with the animals treated with nanoparticles encapsulating siRNA against luciferase, which served as a negative control. When PBMCs were exposed to nanoparticles in vitro, negligible toxicity was confirmed by the percentage of Annexin V positive cells and production of IFN-γ. However, this scFvCD7-9R conjugate was optimized for i.v. administration for the therapy and prevention of HIV infection. It remains to be answered whether this conjugate can also efficiently and selectively deliver siRNA to T lymphocytes through pulmonary administration. This study should be highlighted, however, because it demonstrates the feasibility to selectively deliver siRNA to T lymphocytes in vitro and in vivo which could be potentially applied in different inflammatory diseases like asthma and arthritis [51]. Another scFvCD7 conjugate was described by Lee et al. who conjugated scFvCD7 with chitosan and measured the in vitro transfection efficiency in T cell lines (Jurkat and A3.01 cells). Chitosan is a biocompatible and biodegradable polymeric vector, and chitosan/modified chitosan have been widely studied for pulmonary delivery of siRNA. It is thus a promising candidate for delivering siRNA as asthma therapy. The scFvCD7-chitosan/siRNA nanoparticles were around 300 nm in size and had a slightly positive zeta-potential of +17 mV. There was significantly higher uptake of FITC-siRNA when it was encapsulated by scFvCD7-chitosan compared with unmodified chitosan in T cell lines and increased uptake was observed as the molar ratio of scFvCD7 per total chitosan increased. scFvCD7-chitosan nanoparticles prepared with siRNA against CD4 were tested for in vitro gene silencing, and the CD4 gene knockdown efficiency was similar to that of Lipofectamine 2000 but significantly higher than that of chitosan as measured by flow cytometry and RT-PCR. However, scFvCD7-chitosan condensed CD4 siRNA at a weight ratio of chitosan to siRNA of 40:1, indicating that the siRNA encapsulation efficiency of the conjugate was fairly low. Additionally, scFvCD7-chitosan did not show better transfection efficiency in T cells lines than Lipofectamine 2000, thus high doses of nanoparticles may be required to achieve efficient gene silencing. Furthermore, the authors did not perform any in vivo studies, and the pulmonary delivery efficiency of siRNA via scFvCD7-chitosan remains to be tested [52].
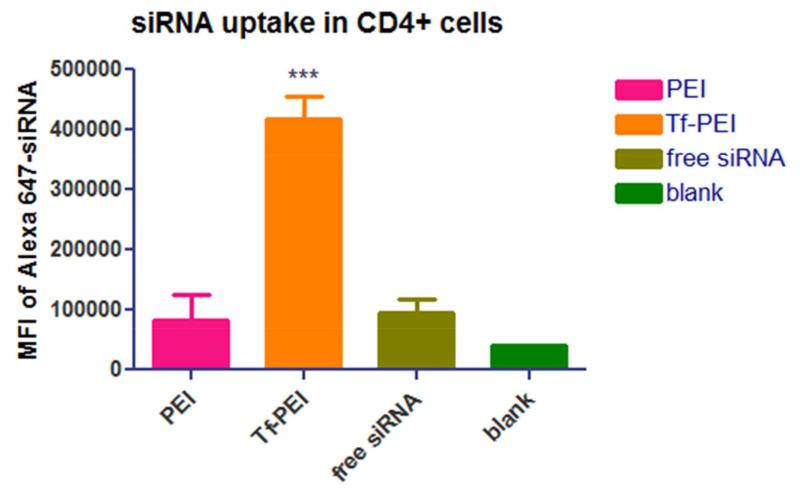
In asthma pathogenesis, activated T cells, particularly Th2 cells, play an essential role but not naïve T cells. However, CD7 is expressed on the majority of mature T cells resulting in the fact that a CD7 targeted siRNA delivery system will non-selectively deliver siRNA to both naïve and activated T cells. Therefore, a pulmonary siRNA delivery system that can selectively transport siRNA to activated T cells but not naïve T cells could achieve more efficient anti-inflammatory effect and reduce potential side effects. In 2013, our group described a more selective delivery system of siRNA for activated T cells in which the transferrin receptor (TfR) was targeted instead of CD7. TfR is an iron uptake membrane receptor universally expressed at low level on most cells/tissues. In contrast, it is overexpressed on highly proliferating and differentiating cells. It was reported that activated T lymphocytes highly express TfR to meet the iron requirement for further differentiation and growth while naïve T lymphocytes do not. For this reason, we linked transferrin (Tf), the native ligand which can trigger TfR internalization, with low molecular weight PEI (LMW-PEI). PEI is the most popular polycation for pulmonary delivery of siRNA, and applying low molecular weight PEI can dramatically reduce the toxicity induced by the high density of cations, which is the biggest obstacle limiting the clinical application of PEI. To determine the selective delivery efficiency of Tf-LMW-PEI in vitro, primary human activated T cells (ATC) or naïve T cells were transfected by Tf-LMW-PEI encapsulating Alexa Fluor488-labeled siRNA. Flow cytometry quantified cellular uptake of Tf-LMW-PEI/siRNA in ATCs was significantly higher than that of non-modified LMW-PEI as well as Lipofectamine 2000. Additionally, the uptake of Tf-LMW-PEI and LMW-PEI was minimal on naïve T cells compared with that on ATCs indicating that the conjugate can distinguish ATCs from naïve T cells [53]. In the meantime, our group also determined the biodistribution of Tf-LMW-PEI formulated with Alexa Fluor 647 labeled siRNA in OVA-induced asthmatic mice [54]. Significantly higher cellular uptake of Tf-LMW-PEI nanoparticles in CD4+ cells in asthmatic mice compared with that of LMW-PEI was observed as shown in Figure 2. In vitro and in vivo studies proved that Tf-LMW-PEI is another promising candidate for pulmonary delivery of siRNA for asthma treatment (data not shown).
Figure 2.
Flow cytometry results of Alexa647-labeled siRNA uptake in CD4+ cells in the lung mediated by PEI, Tf-PEI or after administration as free siRNA. (*** indicates P < 0.001 compared to free siRNA). Reproduced, by permission, from Xie et al. 2014 [54]. Copyright 2014 The Controlled Release Society.
3.2. Macrophage targeted delivery systems
Macrophages are the most abundant inflammatory cells in the lung and are potentially involved in airway remodeling and eosinophilic inflammation in asthma. However, macrophages can perform anti-inflammatory as well as pro-inflammatory effects, so the role of macrophage in asthma is not yet clear [55]. A delivery system of siRNA that targets macrophages can be not only a therapeutic delivery vector but also a tool to facilitate the understanding of the role of macrophages in asthma. Kim et al. described a siRNA delivery system targeting macrophages through a macrophage expressing nicotinic acetycholine receptor (AchR) that binds the peptide rabies virus glycoprotein (RVG). To target and deliver siRNA to macrophages in the central nervous system for the treatment of neuroinflammatory diseases, RVG was linked with nona-D-arginine residues (RVG-9R). The authors first confirmed that RVG-9R can selectively deliver FITC-labeled siRNA to primary splenic murine macrophages (CD11b+) in vitro. AchR mediated in vivo targeted delivery of RVG-9R/FITC-siRNA to peripheral macrophages, microglia, and resident macrophages in the central nervous system, was confirmed by the significantly higher median fluorescent intensity of CD11b+ macrophages from wild type mice compared to AchR knockout mice. The therapeutic effect of RVG-9R prepared with siRNA against TNF-α was tested in a lipopolysaccharide (LPS)-induced murine inflammatory model. I.v. administration of RVG-9R/ siTNF-α was reported to significantly inhibit the increased secretion of TNF-α in blood and brain compared with RVG-9R/luciferase siRNA and was shown to protect the neurons from LPS-induced apoptosis [56]. Another macrophage targeting system is mediated by folic acid as it has been discovered recently that folate receptors are upregulated on activated macrophage during the inflammatory process [57]. Inspired by this property, Yang et al. linked folic acid with chitosan (FA-chitosan) to target macrophages for anti-inflammatory effects. Up to 62% in vitro gene silencing was achieved with FA-chitosan nanoparticles prepared with siRNA against GAPDH on LPS activated RAW 264.7 cells. The biodistribution of i.v. injection of FA-chitosan encapsulating Cy5 labeled siRNA was monitored in a murine subcutaneous inflammation model using an IVIS® 200 imagine system. Accumulation of Cy5-siRNA/FA-chitosan nanoparticles was observed in the LPS induced lesion site while no clear fluorescent signal was detected in the Cy-5siRNA/chitosan group [58].
3.3. Targeted delivery systems for other cell types
Dendritic cells (DCs), the most efficient antigen presenting cells (APCs), can initiate and maintain the inflammatory response; hence they are another popular target cell type for asthma therapy. A strategy to target DCs was described by Zheng et al. who encapsulated siRNA in liposomes decorated with DC-specific antibody DEC-205 which are so-called immunoliposomes (siILs). The siILs prepared with siRNA against CD40 were shown to more efficiently silence the expression of CD40 in DCs derived from bone marrow (BMDCs) compared with commercially available GenePORTER® in vitro. The biodistribution of siILs encapsulating Cy3-labeled siRNA after i.v. injection was observed in tissue sections of liver, spleen and kidney. Accumulation of siILs/Cy3-siRNA in the spleen at 4 hours and even stronger accumulation at 8 hours after injection was described, but no detectable siILs were found in the kidney at any time point. In contrast, naked Cy3-siRNA was cleared through the kidneys soon after injection, suggesting siILs nanoparticles may selectively accumulate in the DCs-rich tissues like the spleen and have a long circulation time with slower clearance via the kidneys. The in vivo gene silencing of siILs prepared with CD40 siRNA was determined in mice with inflammation induced by keyhole limpet hemocyanin/complete Freund’s adjuvant. Significant CD40 knockdown in DCs was achieved in animals treated with siILs/CD40 siRNA or naked CD40 siRNA compared to mice administered with siILs/ negative control siRNA, IgG coupled non-targeted liposomes and PBS. However, silencing of CD40 expression induced by siILs/CD40 siRNA can last at least 12 days while that induced by naked CD40 siRNA did not last this long. Meanwhile, the authors also measured the expression of CD40 in B cells and concluded that the CD40 silencing of siILs nanoparticles selectively happened in DCs [59]. In contrast, another APC-targeted delivery system can transport siRNA to more than just DCs. TLR9 is an endosomal location toll like receptor (TLRs) which can bind with CpG oligonucleotides and facilitate the uptake of siRNA to the cytosol. This receptor is as well expressed on various APCs including DCs, B cells and macrophages. Uncontrollable growth of immune cells can result in blood cancer, for example, B cells lymphoma. Immunosuppression is a common obstacle in cancer therapy, and therefore, immune cells are important therapeutic targets in anti-cancer treatment. To target and deliver siRNA to human hematopoietic cells for anti-tumor therapy, siRNA against STAT3 was covalently linked to CpG oligonucleotides by Zhang et al. The selective cellular uptake of CpG-Cy3-siRNA in primary human DCs, monocytes and B cells but not in T cells and natural killer cells was observed via flow cytometry. After intratumoral injection of the CpG-siSTAT3 in murine subcutaneous tumor models (established by KMS-11, MonoMac6, MV4-11 or A20 tumor cells), the in vivo gene silencing in tumor cells and the inhibition of tumor growth were significantly higher than with the control CpG- luciferase specific siRNA [60].
Except inflammatory cells, lung structure cells are thought to be therapeutic targets for asthma therapy. Beta2-adrenergic receptor (β2-AR) is abundant on bronchial smooth muscle cells and is the target for β2-AR agonist bronchodilators. Therefore, Luo et al. conjugated β2-AR agonist salbutamol to guanidinylated chitosan (SGCS) as a pulmonary delivery carrier of siRNA to improve the efficacy of gene silence. SGCS formulated with siRNA against EGFP yielded nanoparticles with sizes of around 100 nm and zeta-potentials of 2-4 mV. Nanoparticles were nebulized and the aerosol was collected and was i.t. sprayed into the lung of EGFP transgenic mice on three consecutive days. The in vivo EGFP gene silencing of SGCS was significantly better than that of non-targeted GCS determined by confocal microscope and western blot [61].
4. Conclusion
In the last decades, many groups have tried to translate siRNA mediated RNAi into a biocompatible, efficient and long term therapy to treat airway inflammation in asthma. Lots of novel therapeutic targets have been discovered and numerous studies have been performed with the results of significant anti-inflammatory effects. However, asthma is such a complex inflammatory disorder which involves various factors, thus blockage of a single mediator or receptor cannot efficiently relieve the symptoms. Accordingly, the delivery of siRNA mixtures against multiple genes or against the upstream factors in the inflammatory process such as transcription factors, kinases or costimulatory molecules may be more effective. Furthermore, the lack of efficient and specific siRNA delivery systems results in low therapeutic efficiency and high potential of side effects. Although, there are several ligand-receptor mediated targeted delivery systems of siRNA that could potentially be applied in asthma, none of the current immune cell targeted delivery systems has been optimized for asthma disease except the Tf-LMW-PEI conjugate reported by our group. Therefore, further studies and tests are required to develop an efficient siRNA delivery system for asthma therapy.
Supplementary Material
Acknowledgements
We gratefully acknowledge the Start-Up Grant, FRAP Award, BOOST Award and ERC-2014-StG – 637830 awarded to Olivia Merkel.
References
- 1.World Health Organization . The Global Asthma Report. 2014. [Google Scholar]
- 2.World Health Organization . Global surveillance, prevention and control of chronic respiratory diseases: a comprehensive approach. 2007. [Google Scholar]
- 3.Global Markets for Asthma & COPD Drugs. 2012 RnRMarketResearch.com.
- 4.Barnes PJ. Nat Rev Drug Discov. 2004;3:831–844. doi: 10.1038/nrd1524. [DOI] [PubMed] [Google Scholar]
- 5.Barnes PJ. Trends in pharmacological sciences. 2010;31:335–43. doi: 10.1016/j.tips.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Cockcroft DW. Clinic Rev Allerg Immunol. 2006;31:197–207. [Google Scholar]
- 7.Boulet LP. The European respiratory journal. 2009;33:897–906. doi: 10.1183/09031936.00121308. [DOI] [PubMed] [Google Scholar]
- 8.Barnes PJ. Brit J Clin Pharmaco. 1996;42:3–10. doi: 10.1046/j.1365-2125.1996.03721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pelaia G, Vatrella A, Maselli R. Nat Rev Drug Discov. 2012;11:958–72. doi: 10.1038/nrd3792. [DOI] [PubMed] [Google Scholar]
- 10.Gould HJ, Sutton BJ. Nature reviews. Immunology. 2008;8:205–17. doi: 10.1038/nri2273. [DOI] [PubMed] [Google Scholar]
- 11.Lloyd CM, Hessel EM. Nature reviews. Immunology. 2010;10:838–48. doi: 10.1038/nri2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corrigan CJ, Kay AB. Immunology Today. 1992;13:501–507. doi: 10.1016/0167-5699(92)90026-4. [DOI] [PubMed] [Google Scholar]
- 13.Barnes PJ. British journal of clinical pharmacology. 1996;42:3–10. doi: 10.1046/j.1365-2125.1996.03721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humbert ABKM. European Respiratory Society Journals. 2003:126–137. [Google Scholar]
- 15.Agrawal N, Dasaradhi PV, Mohmmed A, Malhotra P, Bhatnagar RK, Mukherjee SK. Microbiol Mol Biol Rev. 2003;67:657–85. doi: 10.1128/MMBR.67.4.657-685.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rettig GR, Behlke MA. Molecular therapy. 2012;20:483–512. doi: 10.1038/mt.2011.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng Y, Wang CC, Choy KW, Du Q, Chen J, Wang Q, Li L, Chung TK, Tang T. Gene. 2014;538:217–27. doi: 10.1016/j.gene.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 18.Feldmann DP, Merkel OM. Ther Deliv. 2015;6:407–9. doi: 10.4155/tde.15.8. [DOI] [PubMed] [Google Scholar]
- 19.Beck-Broichsitter M, Merkel OM, Kissel T. Journal of Controlled Release. 2012;161:214–224. doi: 10.1016/j.jconrel.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Merkel OM, Rubinstein I, Kissel T. Advanced Drug Delivery Reviews. 2014;75:112–128. doi: 10.1016/j.addr.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rehman R, Bhat YA, Panda L, Mabalirajan U. International Immunopharmacology. 2013;15:597–605. doi: 10.1016/j.intimp.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Chen YL, Huang HY, Lee CC, Chiang BL. Molecular therapy. Nucleic acids. 2014;3:e158. doi: 10.1038/mtna.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu S, Nugroho AE, Shudou M, Maeyama K. Immunology and cell biology. 2012;90:337–45. doi: 10.1038/icb.2011.41. [DOI] [PubMed] [Google Scholar]
- 24.Zafra MP, Mazzeo C, Gamez C, Rodriguez Marco A, de Zulueta A, Sanz V, Bilbao I, Ruiz-Cabello J, Zubeldia JM, del Pozo V. PloS one. 2014;9:e91996. doi: 10.1371/journal.pone.0091996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khaitov MR, Shilovskiy IP, Nikonova AA, Shershakova NN, Kamyshnikov OY, Babakhin AA, Zverev VV, Johnston SL, Khaitov RM. Human gene therapy. 2014;25:642–50. doi: 10.1089/hum.2013.142. [DOI] [PubMed] [Google Scholar]
- 26.Huang HY, Lee CC, Chiang BL. Gene Ther. 2008;15:660–7. doi: 10.1038/gt.2008.15. [DOI] [PubMed] [Google Scholar]
- 27.Goh FY, Cook KL, Upton N, Tao L, Lah LC, Leung BP, Wong WS. Journal of immunolog. 2013;191:2691–9. doi: 10.4049/jimmunol.1202416. [DOI] [PubMed] [Google Scholar]
- 28.Zhu J, Yamane H, Cote-Sierra J, Guo L, Paul WE. Cell research. 2006;16:3–10. doi: 10.1038/sj.cr.7310002. [DOI] [PubMed] [Google Scholar]
- 29.Yagi R, Zhu J, Paul WE. International immunology. 2011;23:415–20. doi: 10.1093/intimm/dxr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng W, Flavell RA. Cell. 1997;89:587–96. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 31.Finotto S, De Sanctis GT, Lehr HA, Herz U, Buerke M, Schipp M, Bartsch B, Atreya R, Schmitt E, Galle PR, Renz H, Neurath MF. The Journal of experimental medicine. 2001;193:1247–60. doi: 10.1084/jem.193.11.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee CC, Huang HY, Chiang BL. Molecular therapy. 2008;16:60–5. doi: 10.1038/sj.mt.6300309. [DOI] [PubMed] [Google Scholar]
- 33.Sel S, Wegmann M, Dicke T, Sel S, Henke W, Yildirim AO, Renz H, Garn H. The Journal of allergy and clinical immunology. 2008;121:910–916. e5. doi: 10.1016/j.jaci.2007.12.1175. [DOI] [PubMed] [Google Scholar]
- 34.Krug N, Hohlfeld JM, Kirsten A-M, Kornmann O, Beeh KM, Kappeler D, Korn S, Ignatenko S, Timmer W, Rogon C, Zeitvogel J, Zhang N, Bille J, Homburg U, Turowska A, Bachert C, Werfel T, Buhl R, Renz J, Garn H, Renz H. New England Journal of Medicine. 2015;372:1987–1995. doi: 10.1056/NEJMoa1411776. [DOI] [PubMed] [Google Scholar]
- 35.Pernis AB, Rothman PB. The Journal of Clinical Investigation. 2002;109:1279–1283. doi: 10.1172/JCI15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Darcan-Nicolaisen Y, Meinicke H, Fels G, Hegend O, Haberland A, Kühl A, Loddenkemper C, Witzenrath M, Kube S, Henke W, Hamelmann E. The Journal of Immunology. 2009;182:7501–7508. doi: 10.4049/jimmunol.0713433. [DOI] [PubMed] [Google Scholar]
- 37.Edwards MR, Bartlett NW, Clarke D, Birrell M, Belvisi M, Johnston SL. Pharmacology & therapeutics. 2009;121:1–13. doi: 10.1016/j.pharmthera.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riccaboni M, Bianchi I, Petrillo P. Drug discovery today. 2010;15:517–30. doi: 10.1016/j.drudis.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Stenton GR, Ulanova M, Déry RE, Merani S, Kim M-K, Gilchrist M, Puttagunta L, Musat-Marcu S, James D, Schreiber AD, Befus AD. The Journal of Immunology. 2002;169:1028–1036. doi: 10.4049/jimmunol.169.2.1028. [DOI] [PubMed] [Google Scholar]
- 40.Huang ZY, Kim MK, Kim-Han TH, Indik ZK, Schreiber AD. Molecular immunology. 2013;53:52–9. doi: 10.1016/j.molimm.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 41.Wu W, Chen H, Li YM, Wang SY, Diao X, Liu KG. International journal of clinical and experimental pathology. 2014;7:5505–14. [PMC free article] [PubMed] [Google Scholar]
- 42.Suzuki M, Zheng X, Zhang X, Ichim TE, Sun H, Kubo N, Beduhn M, Shunnar A, Garcia B, Min WP. Allergy. 2009;64:387–97. doi: 10.1111/j.1398-9995.2008.01839.x. [DOI] [PubMed] [Google Scholar]
- 43.Asai-Tajiri Y, Matsumoto K, Fukuyama S, Kan-o K, Nakano T, Tonai K, Ohno T, Azuma M, Inoue H, Nakanishi Y. Respiratory Research. 2014;15:132. doi: 10.1186/s12931-014-0132-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whitehead KA, Langer R, Anderson DG. Nat Rev Drug Discov. 2009;8:129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Merkel OM, Rubinstein I, Kissel T. Adv Drug Deliv Rev. 2014;75C:112–128. doi: 10.1016/j.addr.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas CE, Ehrhardt A, Kay MA. Nat Rev Genet. 2003;4:346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 47.Pack DW, Hoffman AS, Pun S, Stayton PS. Nat Rev Drug Discov. 2005;4:581–93. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 48.Schoenborn J, Sekimata M, Weaver W, Wilson C. Nature Protocol Exchange. 2007 ISSN 2043-0116. [Google Scholar]
- 49.Zhao Y, Zheng Z, Cohen CJ, Gattinoni L, Palmer DC, Restifo NP, Rosenberg SA, Morgan RA. Molecular therapy. 2006;13:151–159. doi: 10.1016/j.ymthe.2005.07.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kumar P, Wu H, McBride JL, Jung K-E, Hee Kim M, Davidson BL, Kyung Lee S, Shankar P, Manjunath N. Nature. 2007;448:39–43. doi: 10.1038/nature05901. [DOI] [PubMed] [Google Scholar]
- 51.Kumar P, Ban HS, Kim SS, Wu H, Pearson T, Greiner DL, Laouar A, Yao J, Haridas V, Habiro K, Yang YG, Jeong JH, Lee KY, Kim YH, Kim SW, Peipp M, Fey GH, Manjunath N, Shultz LD, Lee SK, Shankar P. Cell. 2008;134:577–86. doi: 10.1016/j.cell.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee J, Yun KS, Choi CS, Shin SH, Ban HS, Rhim T, Lee SK, Lee KY. Bioconjugate chemistry. 2012;23:1174–80. doi: 10.1021/bc2006219. [DOI] [PubMed] [Google Scholar]
- 53.Kim NH, Nadithe V, Elsayed M, Merkel OM. Journal of drug delivery science and technology. 2013;23:17–21. doi: 10.1016/s1773-2247(13)50002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xie Y, Kim NH, Nadithe V, Thakur A, Lum LG, Bassett DJ, Merkel OM. CRS Newsletter. 2014;31:18–19. [Google Scholar]
- 55.Balhara J, Gounni AS. Mucosal Immunol. 2012;5:605–609. doi: 10.1038/mi.2012.74. [DOI] [PubMed] [Google Scholar]
- 56.Kim S-S, Ye C, Kumar P, Chiu I, Subramanya S, Wu H, Shankar P, Manjunath N. Molecular therapy. 2010;18:993–1001. doi: 10.1038/mt.2010.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paulos CM, Turk MJ, Breur GJ, Low PS. Advanced Drug Delivery Reviews. 2004;56:1205–1217. doi: 10.1016/j.addr.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 58.Yang C, Gao S, Kjems J. Journal of Materials Chemistry B. 2014;2:8608–8615. doi: 10.1039/c4tb01374c. [DOI] [PubMed] [Google Scholar]
- 59.Zheng X, Vladau C, Zhang X, Suzuki M, Ichim TE, Zhang ZX, Li M, Carrier E, Garcia B, Jevnikar AM, Min WP. Blood. 2009;113:2646–54. doi: 10.1182/blood-2008-04-151191. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Q, Hossain DMS, Nechaev S, Kozlowska A, Zhang W, Liu Y, Kowolik CM, Swiderski P, Rossi JJ, Forman S, Pal S, Bhatia R, Raubitschek A, Yu H, Kortylewski M. Blood. 2013;121:1304–1315. doi: 10.1182/blood-2012-07-442590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luo Y, Zhai X, Ma C, Sun P, Fu Z, Liu W, Xu J. Journal of controlled release. 2012;162:28–36. doi: 10.1016/j.jconrel.2012.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.