Abstract
Malignant triton tumor (MTT) is a malignant peripheral nerve sheath tumor with rhabdomyoblastic differentiation. The prognosis of patients is poor, and due to its rarity, large case studies are lacking. The aim of this study is to describe the clinical features and identify potential prognostic factors. Two patients with MTT in the head and neck treated at our department are reported. A literature search revealed another 198 published cases. All of these cases then went through a retrospective analysis. The ratio of male-to-female incidence was 1.5:1, and the median age at diagnosis was 29 years. In 41.7% of cases it occurred in patients with neurofibromatosis type 1. The five-year survival of MTT was found to be just 35%. Cox proportional hazards analysis revealed that complete resection (hazard ratio, 0.396; P=0.032) and metastases (hazard ratio, 3.188; P=0.004) were associated with mortality, indicating that complete resection may lead to a longer life span, and that the existence of metastasis suggested a worse prognosis for patients with MTT.
Keywords: malignant triton tumor, surgery, prognosis, survival analyses
Introduction
Malignant triton tumor (MTT) is a malignant peripheral nerve sheath tumor (MPNST) with rhabdomyoblastic differentiation (1,2). The name ‘triton’ derived from an experiment in which sciatic nerve tissue of the triton salamander was stimulated to grow a neoplasm with skeletal muscle components (3). It was first described as a disease in 1932 (4) and first reported as ‘malignant triton tumor’ in 1973 (5). Woodruff et al proposed three criteria for its diagnosis (5): i) arising along a peripheral nerve in ganglioneuroma or in a patient with neurofibromatosis type 1 (NF-1) or representing a metastasis from such a tumor; ii) having the growth characteristics of a Schwann cell tumor; and iii) rhabdomyoblasts arise within the body of the tumor. Daimaru et al considered that sporadic cases fulfilling the latter two criteria could be diagnosed (6). Clinically, the morbidity of MTT is less than 1 per 1,000,000 (7). As it is a rare tumor, only limited cases have been reported so far, and no large group studies exist. For further understanding of the clinical features of MTT, we share our experiences of the treatment of MTT in our hospital and conduct a literature analysis. Here, we review two cases of MTT occurring in the head and neck, with no evidence or symptoms of NF-1. Written informed consent was obtained from both patients and the study was approved by the Research Ethics Committee of Central South University (Changsha, China).
Case report
Case 1
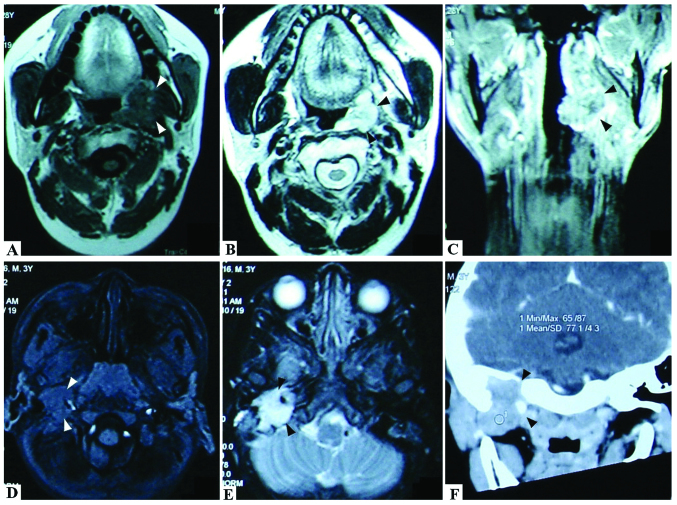
A 27-year-old male was admitted to Xiangya Hospital, Changsha, China, in March 2007, complaining of pain and a foreign body sensation in the left side of the pharynx. Physical examination revealed a cauliflower-like brown mass (30×20 mm) covered with grayish membrane at the left tonsillar fossa. It infiltrated into the surrounding tissues. The patient's mouth opened freely and no lymphadenopathy was observed. Magnetic resonance imaging (MRI) revealed isodense T1 and long T2 signals, and there was no overt submaxillary bony destruction (Fig. 1A-C). On April 5, 2007, the patient underwent ligation of the external carotid artery via a combined jaw-and-neck approach and tumor resection en bloc via a combined parapharyngeal space and intra-oral approach. The surrounding tissues were invaded and the tumor had adhered too tightly to be separated. The resected tissues included partial mucosa of the posterior pharyngeal wall and left fauces, partial medial and lateral pterygoid muscle and the left submandibular gland en bloc. The histological diagnosis was MTT with positive myogenin and S-100. The marginal mandibular branch was preserved. Following an uneventful postoperative recovery, the patient was discharged 7 days after surgery.
Figure 1.
Magnetic resonance imaging (MRI) and computed tomography (CT) scan results of the malignant triton tumor (MTT) cases in our hospital. (A) MRI results revealed that the mass had long or isodense T1 signals. (B, C) The mass demonstrated long T2 signals. In certain images, there are ring or linear forms, as a type of isolation strip, with isodense T2 signals, which are likely due to necrosis and hemorrhage in the mass. (D) MRI revealed isodense T1 signals in the tonsil MTT. (E) Gadolinium-diethylenetriamine pentaacetic acid-enhanced MRI indicated an enhancement of the mass with a distinct lobulated margin in the parapharyngeal space MTT. (F) Enhanced spiral CT scanning of the parapharyngeal space MTT.
Following a course of radiotherapy at a total dose of 52 Gy in May 2007, two courses of chemotherapy with combined pirarubicin, ifosfamide and carboplatin were administered. The patient underwent partial mandibulectomy for osteonecrosis in 2008 and was treated for granulocytopenia following chemotherapy. Follow-up was carried out until April 2014. There was no evidence of further disease.
Case 2
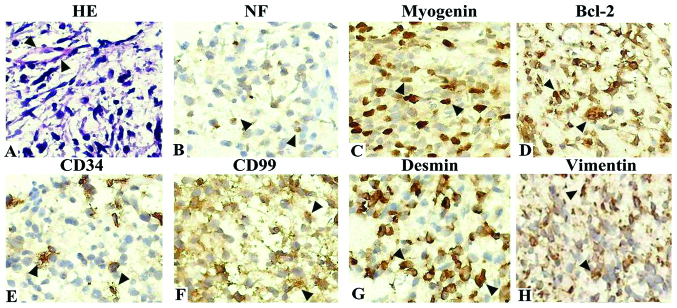
A 43-month-old boy presented with hoarseness for 10 days, failure to swallow saliva for 5 days and headache and vomiting for 2 days. Clinical and radiological examinations revealed a mass of ~2.2×2.8×2.5 cm in the right jugular foramen area with destruction of the skull base. MRI demonstrated isodense T1 and long T2 signals, while gadolinium-diethylenetriamine pentaacetic acid-enhanced MRI indicated enhancement of the mass with a distinct lobulated margin (Fig. 1D-F). Surgery revealed that the neoplasm was located behind the temporomandibular joint and had destroyed the temporal bone and skull base. With an invasion of the deep paroid, the neoplasm had a tight connection with the internal jugular vein. Rapid intraoperative pathological diagnosis suggested a spindle cell sarcoma with an inclination of MPNST. At the request of his parents, surgery was not performed and no treatment was offered. Hematoxylin and eosin staining and immunohistochemistry results suggested MTT with Bcl-2(+), CD34(+), CD57(−), CD99(+), desmin(+), HMB45(−), myogenin(++), NF(+), vimentin(+) and S-100(−) (Fig. 2). The child then survived only seven months.
Figure 2.
Images of hematoxylin and eosin (HE) staining and immunohistochemistry results of malignant triton tumor (MTT). (A) Images of HE staining. Large pleomorphic rhabdomyoblastic cells with abundant eosinophilic cytoplasm are embedded in a spindle cell tumor with a fine fibrillary matrix. (B-H) Immunohistochemistry results of MTT. The immunohistochemistry results were positive for NF(+), myogenin(++), Bcl-2(+), CD34(+), CD99(+), desmin(+) and vimentin(+). Magnification, ×400.
Literature review
Database analysis
Literature searches on PubMed were conducted with the terms ‘malignant triton tumor’ and ‘malignant peripheral nerve sheath tumors AND rhabdomyosarcoma’ for the period prior to April 2014. All MTT cases were extracted from the databases. The relevant information included standard demographic information (age, gender and year of diagnosis) as well as NF-1, onset location, surgical approach, type of adjuvant therapy (radiotherapy and chemotherapy), relapse and metastasis. Survival time and final status were also recorded and the follow-up period was designated to be 60 months.
The onset location of MTT was mainly classified as trunk, head/neck or limbs, according to the original location. Where the tumor occurred in more than two places without a known original location, it was defined as ‘multiple locations’. The concept of complete resection refers to radical resection, amputation or en bloc resection with microscopically negative margins. The data on adjuvant radiotherapy and chemotherapy were recorded as dichotomous variables. Local recurrence was defined as any tumor with complete resection which redeveloped in the surgical excision bed. Metastasis was defined as any distant tumor spread which was confirmed via biopsy or diagnosed with clinical experience, such as positron emission tomography-computed tomography (PET-CT) or radionuclide bone imaging. One patient who succumbed during surgery was also excluded from our analysis. The data that was not available from the literature was classified as ‘Unknown’.
All the data were imported into SPSS version 18.0 software (SPSS Inc, Chicago, IL, USA). Standard descriptive statistics were computed for those selected variables. Kaplan-Meier survival analysis was performed for the whole cohort. The potential effects of clinical variables on survival were determined by the Cox proportional hazards model. All variables were entered simultaneously into a multivariate model. P<0.05 was considered to indicate a statistically significant difference.
Demographic and clinical data of MTT
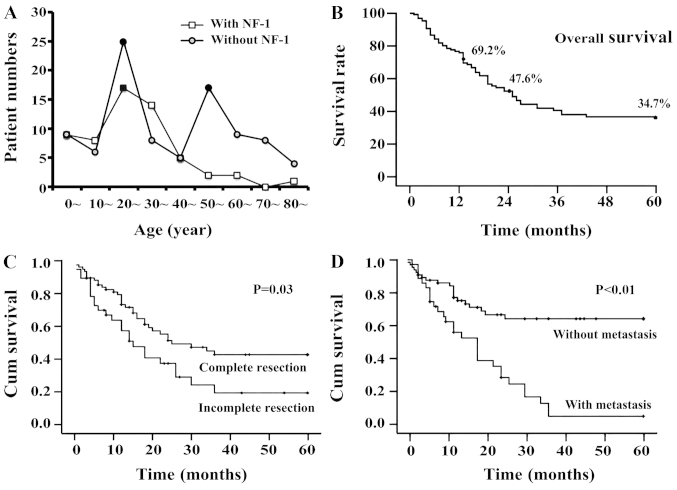
A total of 198 cases were retrieved from 135 studies related to MTT in the databases. Pooling the data of our cases, the data of 200 MTT cases were collected in total. The male-to-female ratio was 1.5 to 1. The median age at diagnosis was 29 years old. The age group distribution chart revealed that the patients with NF-1 were most common in the 20–39 age groups while patients without NF-1 had two peak age groups: the 20s and 50s (Fig. 3A). A total of 41.7% of patients had a history of NF-1 and the others were sporadic. Among all patients, the affected sites included the trunk (n=76, 38.0%), head and neck (n=73, 36.5%) and extremities (n=44, 22.0%; Table I). The clinical treatment data revealed that 68.7% of tumors were removed completely. Radiotherapy and chemotherapy were administered to 54.0% and 35.3% of patients, respectively. The rates of recurrence and metastasis were 42.6% and 34.4%, respectively.
Figure 3.
Age distribution and survival analysis of MTT patients. (A) Results revealed that patients with NF-1 were most common in the 20–39 age group while those without NF-1 had two peaks in the 20s and 50s. (B) Life span analysis revealed that the 1-, 2- and 5-year survival rates were 69%, 48% and 35%, respectively. (C and D) Kaplan-Meier survival analysis of the data for the 133 cases with follow-up revealed that complete resection (P=0.010) and metastasis (P<0.001) had a statistically significant effect on the survival curves.
Table I.
Onset location of malignant triton tumors.
| Onset location | Onset location in detail | Cases, n (%) |
|---|---|---|
| Trunk | Pelvic cavity | 12 (6.0) |
| Mediastinum | 12 (6.0) | |
| Spine | 10 (5.0) | |
| Retroperitoneal space | 9 (4.5) | |
| Chest wall | 8 (4.0) | |
| Buttocks | 5 (2.5) | |
| Lung | 5 (2.5) | |
| Back | 3 (1.5) | |
| Other | 12 (6.0) | |
| Total | 76 (38.0) | |
| Head and neck | Nasal cavity and sinus | 20 (10.0) |
| Neck | 19 (9.5) | |
| Brain | 11 (5.5) | |
| Oral cavity and around | 9 (4.5) | |
| Parapharyngeal space | 4 (2.0) | |
| Occiput | 3 (1.5) | |
| Other | 7 (3.5) | |
| Total | 73 (36.5) | |
| Limbs | Legs | 35 (17.5) |
| Arm | 7 (3.5) | |
| Palm | 1 (0.5) | |
| Foot | 1 (0.5) | |
| Total | 44 (22.0) | |
| Multiple locations | Total | 3 (1.5) |
| Unknown | Total | 4 (2.0) |
| Overall total | 200 (100.0) |
Analysis of prognostic factors of MTT
A total of 133 cases with follow-up were selected for the prognosis analysis. Life span analysis revealed that the 1-, 2- and 5-year survival rates were 69%, 48% and 35%, respectively (Fig. 3B). The results of Kaplan-Meier survival analysis demonstrated that five factors had a statistically significant effect on the survival curves; namely, age (P=0.013), presence of NF-1 (P<0.001), complete resection (P=0.010, Fig. 3C), radiotherapy (P=0.002) and presence of metastasis (P<0.001, Fig. 3D). The factors of gender, onset site, chemotherapy and relapse had no significant effect on the survival curves (P>0.05, Table II).
Table II.
Kaplan-Meier analysis and Cox proportional hazards analyses of patients with malignant triton tumor.
| Kaplan-Meier analysis | Cox proportional hazards analyses | |||||
|---|---|---|---|---|---|---|
| Category | Numbera | Mean time to death, monthsb | P-value | HR | 95% CI for HR | P-value |
| Gender | 0.207 | 0.494 | ||||
| Male | 78 | 34.24 | ||||
| Female | 53 | 28.46 | 1.312 | 0.602–2.858 | ||
| Age, yearsc | ||||||
| ≤32 | 68 | 25.68 | 0.013d | 0.423 | ||
| >32 | 65 | 36.87 | 0.736 | 0.602–1.558 | ||
| Location | 0.638 | |||||
| Head and neck | 48 | 29.22 | ||||
| Trunk | 56 | 31.87 | 0.944 | 0.392–2.272 | 0.845 | |
| Limbs | 26 | 30.37 | 0.912 | 0.602–2.303 | 0.942 | |
| NF-1 | 0.000d | 0.440 | ||||
| Yes | 72 | 17.80 | ||||
| No | 40 | 36.59 | 1.312 | 0.628–2.914 | ||
| Complete resection | 0.010d | 0.032d | ||||
| Yes | 76 | 34.23 | ||||
| No | 39 | 22.50 | 0.396 | 0.170–0.925 | ||
| Radiotherapy | 0.002d | 0.131 | ||||
| Yes | 67 | 36.24 | ||||
| No | 54 | 22.99 | 0.572 | 0.277–1.181 | ||
| Chemotherapy | 0.430 | 0.244 | ||||
| Yes | 41 | 31.97 | ||||
| No | 80 | 29.15 | 0.594 | 0.248–1.426 | ||
| Recurrence | 0.129 | 0.511 | ||||
| Yes | 45 | 27.82 | ||||
| No | 66 | 35.96 | 1.278 | 0.615–2.655 | ||
| Metastasis | 0.000d | 0.004d | ||||
| Yes | 37 | 18.12 | ||||
| No | 71 | 39.80 | 3.188 | 1.450–7.008 | ||
Follow-up data was available for 133 patients, but the total number in every category varies due to lack of data for certain cases.
Estimation is limited to the longest survival time if censored.
The median age of the 133 patients with follow-up data was 32 years.
P<0.05. HR, hazard ratio; CI, confidence interval.
On this basis, all factors in the Kaplan-Meier survival analysis were entered simultaneously into the Cox proportional hazards model for analysis. The result revealed that the factors of complete resection and metastasis had a significant effect on survival (P<0.05, Table II). The hazard ratio (HR) for the former was 0.396. This indicated that if tumor excision was performed en bloc, there was a reduction in mortality risk. The HR of the latter was 3.188, implying that mortality risk increased more than three times.
Discussion
Although the mechanism of MTT remains elusive, its association with nerves or nervous lesions should be noted. The literature revealed that a number of nerves could be involved, including the cervical plexus (8), brachial plexus (9), optic nerve (10), sciatic nerve (11), cervical sympathetic nerve (12) and spinal nerve root (13). There was no conclusive evidence that the masses in our cases came from the nerves; however, the onset locations were observed to be in or around the parapharyngeal space which contains the nerves. A total of 50–70% of cases were observed to arise in patients with NF-1 (1,7), and this rate was ~40% in our own data. Radiotherapy and repeated surgery on the benign nerve fibroma may increase the risk of rhabdomyosarcoma differentiation (11,14). At the sub-cellular level, aberrations in chromosomes 1, 6, 7, 8, 9, 16, 17, 19, 20 and 22 were correlated with the genesis and recurrence of MTT (15). The missense mutation and a loss of heterozygosity were observed in the majority of MTT cases (16,17). Nucleotide deletion in P53, amplification of C-myc and aberrations in the hedgehog-patched pathway were also noted in MTT (18–20).
Clinically, the age at diagnosis age ranged from newborn to over 80 years old, with a median age of 29–35 (1,21,22). More than 60% of MTT cases with NF-1 were diagnosed in patients between 20 and 50 years old, when the benign masses were suspected to undergo a malignant transformation (23). MTT was reported mainly in the head, neck and trunk, and less frequently in the extremities (1); our data yielded similar results. In the early stage, patients often had no self-reported symptoms, and only sought medical attention when there were such symptoms as pain or dysfunction caused by the involvement with adjacent tissues. The size of the abdominal mass increased due to the lack of space constraints, and the maximal diameter was reported at 82 cm (24).
MRI has been the most common method of identifying the location, scale and shape of lesions. In most cases, the masses demonstrated short or mixed isodensity T1 and long T2 signals. In certain images, ring or linear forms were observed as a type of isolation strip; these are likely to be due to necrosis and hemorrhage in the mass as a result of a quick growth (25). Enhanced spiral CT scanning is capable of visualizing bone erosion around the mass and providing indications of its malignancy. 18FDG-PET-CT was also considered to be a more effective method of estimating the prognosis and metastasis (9). All of the above methods are used for auxiliary examination only, and the final diagnosis should be based upon pathology. Microscopically, MTT always contains shuttle-like malignant peripheral nerve cells, additional rhabdomyoblastic differentiation and zonal cytoplasm (7). Immunohistochemical results facilitated our diagnosis, particularly with positive desmin and myoglobin (13). Here, additional oncogene proteins including Bcl-2, CD34, CD99, HHF35, NF and vimentin were also noted to be positive, which confirmed the malignant features of this neoplasm.
Literature revealed that the 5-year survival rates of patients with MPNST and MTT were 34–44% and 10–20%, respectively (3,4). Our data revealed 1-, 2- and 5-year survival rates of 69%, 48% and 35%, respectively. Theoretically, the actual survival rates may be lower, since our censored proportion accounted for almost 20%. Our analysis also revealed no survival difference among MTT of the trunk, head and neck, or extremities. Terzic et al observed that the 1-, 2- and 5-year survival rates of head and neck MTT were 76%, 61% and 49%, respectively, and that paranasal sinus MTT had an even better prognosis (22). The poor survival rate associated with distant metastasis was in keeping with the findings made in previous studies (1,13).
In ~60% of the cases in the literature, tumors were completely resected. Wide local excision, whole organ excision and amputation were performed (7,22). In our analysis, Cox proportional hazards analysis demonstrated that complete resection is an independent prognostic factor, and decreased the mortality risk. Thus, radical mass excision is key to prolonging postoperative survival. The effectiveness of radiotherapy remains controversial. The single factor analysis of radiotherapy revealed a statistically significant difference; however, multi-factorial analysis revealed no difference, which may be related to the limited number of cases. Given our findings and experience, radiotherapy should be considered as a routine for all MTT patients, whether the residual mass remains or not. Ifosfamide, dactinomycin, carboplatin and fluorouracil are the main drugs used in chemotherapy (4,22). One study described a tumor expressing retinoic acid receptors-a and b, and the treatment of isotretinoin and interferon-α was offered to control the disease (26). Kaplan-Meier analysis revealed no significant difference for the patients with chemotherapy, which might be attributed to the reason that there was no standard or guidelines. Simultaneously, the side effects of radiotherapy and chemotherapy should be recorded and managed promptly. Our patient (case 1) suffered osteonecrosis and granulocytopenia following adjunctive therapies. Thus, prolonging the survival time is not the only target for patients: improving their quality of life is also considered to be a priority.
Acknowledgements
Grants were obtained by the National Natural Science Foundation of China (nos. 81372426, 81372906, 81202128 and 81172558), the Research Fund for the Doctoral Program of Higher Education of China (no. 20120162120049), the Graduate Student Research Innovation Project of Hunan Province (no. CX2013B108), the Freedom Explore Program of Central South University (no. 2012QNZT099), and the Open-End Fund for the Valuable and Precision Instruments of Central South University (no. CSUZC2014048).
The authors are grateful for the statistical guidance from Deng Jing at the Department of Statistics, School of Public Health, Central South University, and Zhu Lei at the Library of Medicine, Central South University, who offered assistance in the literature searches.
References
- 1.McConnell YJ, Giacomantonio CA. Malignant triton tumors - complete surgical resection and adjuvant radiotherapy associated with improved survival. J Surg Oncol. 2012;106:51–56. doi: 10.1002/jso.23042. [DOI] [PubMed] [Google Scholar]
- 2.Friesenbichler J, Leithner A, Maurer-Ertl W, Szkandera J, Sadoghi P, Frings A, Maier A, Andreou D, Windhager R, Tunn PU. Surgical therapy of primary malignant bone tumours and soft tissue sarcomas of the chest wall: a two-institutional experience. Int Orthop. 2014;38:1235–1240. doi: 10.1007/s00264-014-2304-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Locatelli P. C. R. Assoc. des Anatomistes. 20e reunion Turin: 1925. Formation de Membres Surnumeraires; pp. 279–282. [Google Scholar]
- 4.Masson P. Libman Anniversary. Vol. 2. New York, NY: International Press; 1932. Recklinghausen's neurofibromatosis, sensory neuromas and motor neuromas; pp. 793–802. [Google Scholar]
- 5.Woodruff JM, Chernik NL, Smith MC, Millett WB, Foote FW., Jr Peripheral nerve tumors with rhabdomyosarcomatous differentiation (malignant ‘Triton’ tumors) Cancer. 1973;32:426–439. doi: 10.1002/1097-0142(197308)32:2<426::AID-CNCR2820320221>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 6.Daimaru Y, Hashimoto H, Enjoji M. Malignant ‘triton’ tumors: a clinicopathologic and immunohistochemical study of nine cases. Hum Pathol. 1984;15:768–778. doi: 10.1016/S0046-8177(84)80169-0. [DOI] [PubMed] [Google Scholar]
- 7.John M. Anderson's Pathology. Philadelphia: The C. V. Mosby Company; 1990. pp. 1894–1896. [Google Scholar]
- 8.Yang BB, Jiang H, Chang HY. Malignant triton tumour of the parapharyngeal space: a case arising from the cervical sympathetic nerve. J Laryngol Otol. 2008;122:531–534. doi: 10.1017/S0022215107008134. [DOI] [PubMed] [Google Scholar]
- 9.Dartnell J, Pilling J, Ferner R, Cane P, Lang-Lazdunski L. Malignant triton tumor of the brachial plexus invading the left thoracic inlet: a rare differential diagnosis of pancoast tumor. J Thorac Oncol. 2009;4:135–137. doi: 10.1097/JTO.0b013e31819151ab. [DOI] [PubMed] [Google Scholar]
- 10.Han DH, Kim DG, Chi JG, Park SH, Jung HW, Kim YG. Malignant triton tumor of the acoustic nerve. Case report. J Neurosurg. 1992;76:874–877. doi: 10.3171/jns.1992.76.5.0874. [DOI] [PubMed] [Google Scholar]
- 11.Tripathy K, Mallik R, Mishra A, Misra D, Rout N, Nayak P, Samantray S, Rath J. A rare malignant triton tumor. Case Rep Neurol. 2010;2:69–73. doi: 10.1159/000315621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cano JR, Algar FJ, Alvarez A, Salvatierra A. Triton tumor of the left sympathetic nerve. Interact Cardiovasc Thorac Surg. 2006;5:790–791. doi: 10.1510/icvts.2006.136994. [DOI] [PubMed] [Google Scholar]
- 13.Rekhi B, Jambhekar NA, Puri A, Agrawal M, Chinoy RF. Clinicomorphologic features of a series of 10 cases of malignant triton tumors diagnosed over 10 years at a tertiary cancer hospital in Mumbai, India. Ann Diagn Pathol. 2008;12:90–97. doi: 10.1016/j.anndiagpath.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Ozer E, Erkilic S, Bayazit YA, Mumbuç S, Aydin A, Kanlikama M. Malignant triton tumor of the supraclavicular region arising after radiotherapy. Auris Nasus Larynx. 2002;29:405–407. doi: 10.1016/S0385-8146(02)00026-3. [DOI] [PubMed] [Google Scholar]
- 15.Koutsimpelas D, Brieger J, Heinrich U, Torzewski M, Sommer C, Mann WJ. Cytogenetic analysis of a malignant triton tumour by comparative genomic hybridization (CGH) and review of the literature. Eur Arch Otorhinolaryngol. 2011;268:1391–1396. doi: 10.1007/s00405-011-1658-z. [DOI] [PubMed] [Google Scholar]
- 16.Strauss BL, Gutmann DH, Dehner LP, Hirbe A, Zhu X, Marley EF, Liapis H. Molecular analysis of malignant triton tumors. Hum Pathol. 1999;30:984–988. doi: 10.1016/S0046-8177(99)90255-1. [DOI] [PubMed] [Google Scholar]
- 17.Ferrari A, Bisogno G, Macaluso A, Casanova M, D'Angelo P, Pierani P, Zanetti I, Alaggio R, Cecchetto G, Carli M. Soft-tissue sarcomas in children and adolescents with neurofibromatosis type 1. Cancer. 2007;109:1406–1412. doi: 10.1002/cncr.22533. [DOI] [PubMed] [Google Scholar]
- 18.Chao MM, Levine JE, Ruiz RE, Kohlmann WK, Bower MA, Petty EM, Mody RJ. Malignant triton tumor in a patient with Li-Fraumeni syndrome and a novel TP53 mutation. Pediatr Blood Cancer. 2007;49:1000–1004. doi: 10.1002/pbc.20700. [DOI] [PubMed] [Google Scholar]
- 19.Haddadin MH, Hawkins AL, Long P, Morsberger LA, Depew D, Epstein JI, Griffin CA. Cytogenetic study of malignant triton tumor: A case report. Cancer Genet Cytogenet. 2003;144:100–105. doi: 10.1016/S0165-4608(02)00935-4. [DOI] [PubMed] [Google Scholar]
- 20.Bura M, Musani V, Cretnik M, Botica I, Levanat S. Hedgehog-Patched pathway aberrations in a malignant triton tumor case study. Oncol Rep. 2008;20:347–352. [PubMed] [Google Scholar]
- 21.Sönmez K, Türkyilmaz Z, Karabulut R, Kapisiz A, Eser EP, Memiş L, Başaklar AC. A Triton tumor mimicking sacrococcygeal teratoma. J Pediatr Surg. 2009;44:e5–e8. doi: 10.1016/j.jpedsurg.2009.07.042. [DOI] [PubMed] [Google Scholar]
- 22.Terzic A, Bode B, Gratz KW, Stoeckli SJ. Prognostic factors for the malignant triton tumor of the head and neck. Head Neck. 2009;31:679–688. doi: 10.1002/hed.21051. [DOI] [PubMed] [Google Scholar]
- 23.Duat-Rodríguez A, Carceller Lechón F, Pino López MÁ, Rodríguez Fernández C, González-Gutiérrez-Solana L. Neurofibromatosis type 1 associated with moyamoya syndrome in children. Pediatr Neurol. 2014;50:96–98. doi: 10.1016/j.pediatrneurol.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Murtaza B, Gondal ZI, Mehmood A, Shah SS, Abbasi MH, Tamimy MS, Kazmi ST. A huge malignant triton tumour. J Coll Physicians Surg Pak. 2005;15:728–730. [PubMed] [Google Scholar]
- 25.Ghosh A, Sastri SB, Srinivas D, Mahadevan A, Anandappa CB, Shankar SK. Malignant triton tumor of cervical spine with hemorrhage. J Clin Neurosci. 2011;18:721–723. doi: 10.1016/j.jocn.2010.07.148. [DOI] [PubMed] [Google Scholar]
- 26.Köstler WJ, Amann G, Grunt TW, Singer CF, Schneider SM, Brodowicz T, Tomek S, Zielinski CC. Recurrent malignant Triton tumour: First report on a long time survivor. Oncol Rep. 2003;10:533–535. [PubMed] [Google Scholar]