Abstract
Several arenavirus pathogens, such as Lassa and Junin viruses, inhibit macrophage activation, the molecular mechanism of which is unclear. We show that lymphocytic choriomeningitis virus (LCMV) can also inhibit macrophage activation, in contrast to Pichinde and Tacaribe viruses, which are not known to naturally cause human diseases. Using a recombinant Pichinde virus system, we show that the LCMV Z N-terminal domain (NTD) mediates the inhibition of macrophage activation and immune functions.
TEXT
Arenaviruses include several hemorrhagic fever (HF)-causing agents, such as Lassa fever virus (LASV) and Junin virus (JUNV), with limited preventative or therapeutic measures (1, 2). Arenavirus pathogenesis is associated with high viremia and generalized immune suppression, the mechanism of which is poorly understood (3, 4). Macrophages and dendritic cells (DCs) are the early target cells of arenavirus infections. Activated macrophages and DCs play important roles in both innate and adaptive immunity (5, 6). Several studies have shown that LASV and JUNV, but not their nonpathogenic counterparts Mopeia virus (MOPV) and Tacaribe virus (TCRV), can inhibit human macrophage activation (7–10), the molecular mechanism of which is unknown. As all arenaviruses encode a conserved nucleoprotein (NP) RNase with activity to block the interferon (IFN) induction (11–18), it seems unlikely that NP mediates the differential inhibition of macrophages by various arenaviruses. We have recently found that the Z proteins from known arenavirus pathogens, including HF-causing LASV and JUNV as well as lymphocytic choriomeningitis virus (LCMV), that can cause neurologic diseases (19), but not those from others, such as TCRV and Pichinde virus (PICV), which have not been associated with significant human diseases (20, 21), can inhibit RIG-i/MDA5 and block the RIG-i-like-receptor (RLR)-dependent IFN production in macrophages and that the differential RLR inhibition is determined by the N-terminal domain (NTD) (22).
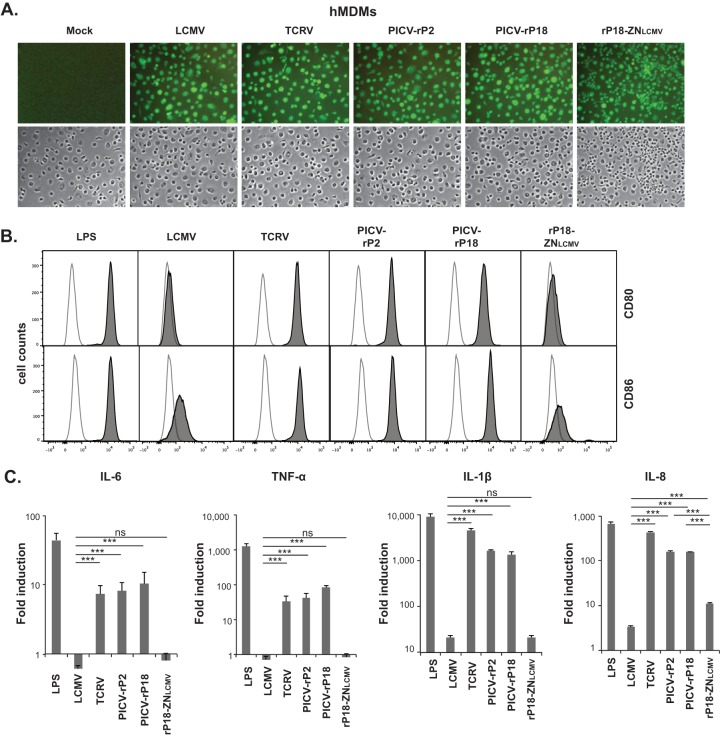
To determine whether this newly discovered Z NTD-mediated RLR inhibition contributes to the differential inhibition of human macrophages by distinct arenaviruses, we analyzed the macrophage activation upon infection with LCMV, TCRV, PICV (both the P2 and the P18 strains), or a recombinant PICV encoding a chimeric Z protein with the 31-residue NTD from LCMV (rP18-ZNLCMV) (22). Human monocyte-derived macrophages (hMDMs) were obtained as described previously (22) and treated with lipopolysaccharide (LPS) for 6 h or infected with the aforementioned arenaviruses at a multiplicity of infection (MOI) of 2 for 1 and 3 days. The infection rate was nearly 100% for each virus as demonstrated by the intracellular immunofluorescence staining of viral NP with anti-NP antibody (Fig. 1A). Macrophage activation was first assessed by flow cytometric analysis of the surface expression of CD80 and CD86, the two established macrophage activation markers and costimulatory molecules. LPS, a known strong inducer of macrophage activation, caused substantial increase in CD80 and CD86 surface expression levels as evidenced by the shifted peaks (Fig. 1B) and the increased median fluorescence intensity (MFI) (Table 1). Infection by TCRV and PICV (both the P2 and the P18 strains) also strongly upregulated CD80 and CD86 surface expression levels at both 1 and 3 days postinfection (dpi). In sharp contrast, infection by LCMV and rP18-ZNLCMV failed to increase the surface expression levels of CD80 or CD86 (Fig. 1B and Table 1). We also evaluated macrophage activation by quantifying cytokines (interleukin-6 [IL-6], tumor necrosis factor alpha [TNF-α], IL-1β, and IL-8) at the transcriptional level by quantitative reverse transcription-PCR (qRT-PCR). Macrophages infected with LCMV or rP18-ZNLCMV produced significantly lower levels of cytokines than those treated with LPS or infected with TCRV or PICV (Fig. 1C). It is worth noting that infections with rP18-ZNLCMV and LCMV produced similarly low levels of IL-6, TNF-α, and IL-1β but that rP18-ZNLCMV produced a higher level of IL-8 than LCMV, the reason for which is unknown and may possibly involve differences between PICV and LCMV. Taken together, our data on the expression of surface activation markers and cytokines strongly suggest that introduction of the LCMV Z NTD into the PICV genome leads to the inhibition of macrophage activation.
FIG 1.
Arenaviruses differentially activate hMDMs via the Z NTD. (A) All tested arenaviruses efficiently infected hMDMs. Mock-treated or virus-infected hMDMs at 24 hours postinfection (hpi) were fixed and detected with anti-NP rabbit antibody followed by AlexFluor488-conjugated anti-rabbit secondary antibody in an immunofluorescence assay. (B) Flow cytometric analysis of macrophage activation markers (CD80 and CD86) on hMDM surfaces. Cells at 1 dpi were stained with phycoerythrin (PE)-conjugated anti-human CD80 or PE-conjugated anti-human CD86 antibody (BD Biosciences). Representative histogram overlays are shown, with the mock-treated control represented by a nonfilled peak and other samples represented by a dark gray peak. (C) The mRNA levels of IL-6, TNF-α, IL-1β, and IL-8 in the LPS-treated (6 h) or virus-infected (3 dpi) hMDMs were measured by qRT-PCR and shown as 2−ΔΔCT values (fold induction) in log scale. Results shown are a representative of three independent experiments, each using PBMCs from a different donor. Statistical analysis was conducted using the Student t test. ***, P < 0.001; ns, not statistically significant.
TABLE 1.
Surface expression levels of CD80 and CD86 on hMDMs after LPS treatment or arenavirus infectiona
| Treatment or infection | Time point | CD80 |
CD86 |
||
|---|---|---|---|---|---|
| MFI | Fold induction | MFI | Fold induction | ||
| Mock | 474 | 1.0 | 613 | 1.0 | |
| LPS | 6 h | 15,672 | 33.1 | 14,250 | 23.2 |
| LCMV | 1 dpi | 600 | 1.3 | 1,515 | 2.5 |
| LCMV | 3 dpi | 521 | 1.1 | 1,067 | 1.7 |
| TCRV | 1 dpi | 9,951 | 21.0 | 12,964 | 21.1 |
| TCRV | 3 dpi | 8,913 | 18.8 | 12,296 | 20.1 |
| PICV-P2 | 1 dpi | 8,973 | 18.9 | 9,095 | 14.8 |
| PICV-P2 | 3 dpi | 11,146 | 23.5 | 10,846 | 17.7 |
| PICV-P18 | 1 dpi | 6,377 | 13.5 | 11,908 | 19.4 |
| PICV-P18 | 3 dpi | 6,911 | 14.6 | 10,364 | 16.9 |
| PICV-ZNLCMV | 1 dpi | 628 | 1.3 | 1,123 | 1.8 |
| PICV-ZNLCMV | 3 dpi | 565 | 1.2 | 982 | 1.6 |
Results shown are a representative of three independent experiments, each using PBMCs from a different donor.
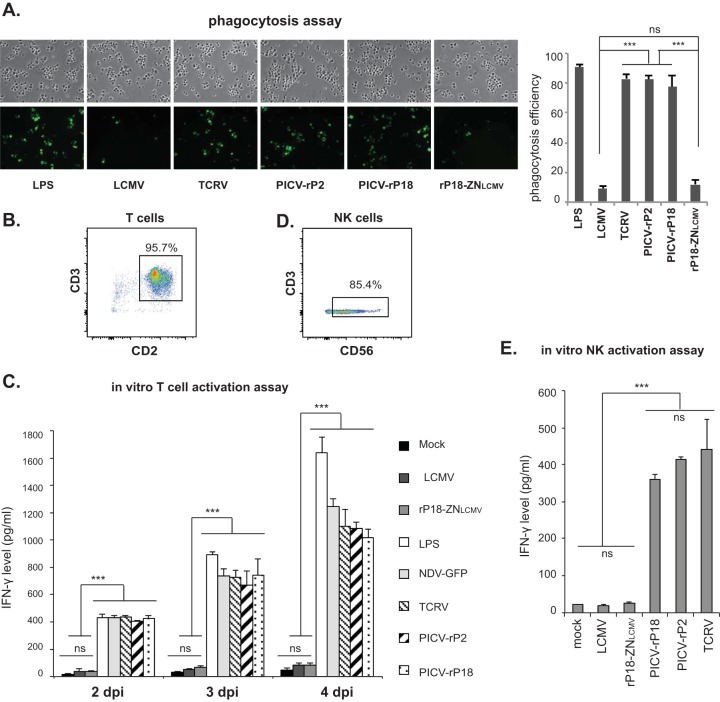
A major function of macrophage is its phagocytic activity. We measured the phagocytic activity of hMDMs (23) after phosphate-buffered-saline (PBS) (mock) or LPS treatment or viral infection at an MOI of 1. LPS treatment as well as infection with TCRV and PICV (both the P2 and the P18 strains) led to a significantly higher number of internalized particles than mock treatment or infection with LCMV or rP18-ZNLCMV (Fig. 2A), demonstrating that the LCMV Z NTD leads to inhibition of macrophage phagocytic activity during arenavirus infection.
FIG 2.
The Z NTD determines the differential inhibition of macrophage functions during arenavirus infections. (A) The LCMV Z NTD mediates the inhibition of macrophage phagocytosis activity. LPS-treated or arenavirus-infected hMDMs were incubated with Zymosan A BioParticles (Molecular Probes) at a ratio of 10 particles per cell for 1 h at 37°C. Engulfed particles were visualized via fluorescence microscopy. Representative images are shown on the left. The number of cells showing internalized particles per 100 cells was counted for each treatment, and results shown are averages for three independent experiments (right). (B) The purity of the T-cell population (CD2+ CD3+) was determined as >95% by flow cytometric analysis using PE-conjugated anti-CD2 and fluorescein isothiocyanate (FITC)-conjugated anti-CD3 antibodies. (C) The LCMV Z NTD inhibits macrophage-induced T-cell activation. Allogeneic T cells were cocultured, at a ratio of 1:1, with hMDMs that had previously been treated with PBS (mock) or LPS (0.5 μg/ml) or infected with NDV-GFP or the indicated arenaviruses for 2, 3, or 4 days (38). The levels of IFN-γ in the supernatants were quantified by using a human IFN-γ ELISA kit (Thermo Fisher Scientific, Inc., Rockford, IL). (D) The purity of the NK cell population (CD3− CD56+) was determined as >85% by flow cytometric analysis using PE-conjugated anti-CD56 and FITC-conjugated anti-CD3 antibodies. (E) The LCMV Z NTD inhibits macrophage-induced NK cell activation. Allogeneic NK cells were cocultured, at a ratio of 1:1, with hMDMs that had previously been treated with PBS (mock) or infected with the indicated arenaviruses for 2 days. The IFN-γ level was quantified by ELISA. Results shown are a representative of three independent experiments, each using PBMCs from a different donor. Statistical analysis was conducted using the Student t test to compare two groups and using one-way analysis of variance (ANOVA) to compare multiple treatment groups. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not statistically significant.
Macrophages are known to stimulate the proliferation and activation of lymphocytes through antigen presentation and/or secreted cytokines. We examined the ability of arenavirus-infected macrophages to stimulate T cells in an in vitro T-cell activation assay. Human allogeneic T cells were isolated from peripheral blood mononuclear cells (PBMCs) to >95% purity (Fig. 2B) using MACS pan T-cell isolation kit II (Miltenyi Biotech, Inc.) and were cocultured with hMDMs previously treated with PBS (mock) or LPS or infected with the aforementioned viruses for 2, 3, or 4 days. The levels of gamma interferon (IFN-γ) secreted by the activated T cells were quantified by enzyme-linked immunosorbent assay (ELISA) (Fig. 2C). At all time points tested, significantly higher levels of IFN-γ were detected in cocultures with hMDMs that were previously treated with LPS, infected with a control virus (Newcastle disease virus expressing green fluorescent protein [NDV-GFP], which activates macrophages upon infection [24]), or infected with TCRV or PICV than in those that were mock treated or infected with LCMV or rP18-ZNLCMV. Therefore, the LCMV Z NTD inhibits the function of arenavirus-infected macrophages to activate T cells.
We next determined the ability of arenavirus-infected macrophages to stimulate NK cells by performing an in vitro NK cell activation assay. Human allogeneic NK cells were isolated from PBMCs to >85% purity (Fig. 2D) using the MACS NK cell isolation kit (Miltenyi Biotech, Inc.) and cocultured with mock-treated or arenavirus-infected hMDMs. As shown in Fig. 2E, NK cells produced significantly higher levels of IFN-γ when cocultured with TCRV- or PICV (P2 and P18)-infected hMDMs than when cocultured with mock-treated or LCMV- or rP18-ZNLCMV-infected hMDMs, demonstrating that the LCMV Z NTD inhibits the function of macrophages to activate NK cells. Taken together, our data demonstrate that the LCMV Z NTD mediates the inhibition of important immune functions (phagocytosis and lymphocyte stimulation) of macrophages during an authentic arenavirus infection.
Our results, together with previous studies (7–10), have shown that macrophage maturation and immune functions are selectively inhibited by multiple arenavirus pathogens such as LASV, JUNV, and LCMV but not by MOPV, TCRV, or PICV and that the differential inhibition is possibly mediated by the Z NTD. We have previously shown that the Z proteins of multiple arenavirus pathogens can strongly inhibit RLRs (22), which is consistent with a previous report on RIG-i inhibition by the Z proteins of New World (NW) pathogens (JUNV, Machupo virus [MACV], Guanarito virus [GTOV], and Sabia virus [SABV]) (25). There are, however, discrepancies between our studies and two previous reports on LCMV and LASV Z proteins (11, 25), possibly due to the different experimental systems used that result in the different levels of protein expression, differences in the sensitivity of detecting IFN inhibition, and/or different cell types used in these experiments. Using infectious arenaviruses and the human primary macrophage system in our previous (22) and current studies, we have demonstrated an important function of LCMV Z in blocking the IFN responses and macrophage activation process in macrophages.
Our findings provide important insights into mechanisms of arenavirus-induced immune suppression and pathogenesis. Arenavirus-caused human diseases differ greatly in degree of disease severity and clinical manifestations (reviewed in reference 2). The Z NTD-mediated RLR inhibition in macrophages seems to be a common theme underlying the diverse arenavirus pathogens, including the mild pathogen LCMV and the highly virulent pathogens LASV and JUNV. By targeting the RLR pathway, a key pathogen detection pathway, the Z proteins of pathogenic arenaviruses may increase the virulence potential of an arenavirus infection by (i) inhibiting the type I IFN responses to benefit viral replication in its early target cells (i.e., macrophages) (22) and (ii) blocking the macrophage activation process and inhibiting the functions of macrophages in phagocytosis, antigen presentation, and lymphocyte stimulation (6). The net effect is the impairment of both innate and adaptive arms of immunity. Sequence changes in the Z NTD have been noticed among LCMV strains and among different LASV isolates; whether these changes may partially account for the disease heterogeneity observed after LCMV and LASV infections remains to be examined. It is noteworthy that the Z-dependent RLR inhibition alone is not sufficient to cause diseases but instead may help establish a potential for virus to replicate and spread in vivo. Whether this potential leads to self-limiting infections or severe diseases with diverse clinical symptoms (neurological diseases or full-blown HFs) depends on multiple other factors such as the virus strains, viral inoculum dose, infection route, host immune status, preexisting immunity, and/or genetic variations. Furthermore, even though arenavirus pathogens encode both NP and Z as strong IFN antagonists (11, 12, 14–17, 22, 25), the roles of IFNs in arenavirus-caused diseases remain controversial. LASV infection is associated with a low level of IFN responses (26, 27), and type I IFNs have been shown to control LASV, LCMV, and PICV replication (18, 28–32). However, NW arenaviruses such as MACV and JUNV have been shown to induce high levels of IFNs in A549 cells and human DCs (33, 34) but not in human macrophages and monocytes (10). High levels of IFN-α have been detected in patient serum and correlate with disease severity for JUNV-caused Argentine hemorrhagic fever (4), though the source cells have not been identified in vivo. We speculate that the IFN level during arenavirus infection is regulated at multiple levels, such as host species, cell type, viral strain, and/or stage of infection. How type I IFNs are regulated and whether they play an antiviral or pathological role or both (35–37) in arenavirus infections and disease pathogenesis need to be carefully evaluated for individual arenavirus pathogens, using experimental settings that would closely mimic human infections.
ACKNOWLEDGMENTS
We thank J. Aronson (UTMB) for Pichinde virus (P2 and P18 strains), B. Gowen (Utah State University) for Tacaribe virus, and the staff in A. Fernandez-Sesma's laboratory (Icahn School of Medicine at Mount Sinai) and in J. Miller's laboratory (University of Minnesota) for advice on isolating and culturing human primary macrophage and NK cells.
This work was supported in parts by the NIH grants R01 AI093580 (to H.L.) and R01 AI083409 (to Y.L.).
REFERENCES
- 1.McLay L, Ansari A, Liang Y, Ly H. 2013. Targeting virulence mechanisms for the prevention and therapy of arenaviral hemorrhagic fever. Antiviral Res 97:81–92. doi: 10.1016/j.antiviral.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McLay L, Liang Y, Ly H. 2014. Comparative analysis of disease pathogenesis and molecular mechanisms of New World and Old World arenavirus infections. J Gen Virol 95:1–15. doi: 10.1099/vir.0.057000-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yun NE, Walker DH. 2012. Pathogenesis of Lassa fever. Viruses 4:2031–2048. doi: 10.3390/v4102031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grant A, Seregin A, Huang C, Kolokoltsova O, Brasier A, Peters C, Paessler S. 2012. Junin virus pathogenesis and virus replication. Viruses 4:2317–2339. doi: 10.3390/v4102317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joffre O, Nolte MA, Sporri R, Reis e Sousa C. 2009. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol Rev 227:234–247. doi: 10.1111/j.1600-065X.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- 6.Mosser DM, Edwards JP. 2008. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baize S, Kaplon J, Faure C, Pannetier D, Georges-Courbot MC, Deubel V. 2004. Lassa virus infection of human dendritic cells and macrophages is productive but fails to activate cells. J Immunol 172:2861–2869. doi: 10.4049/jimmunol.172.5.2861. [DOI] [PubMed] [Google Scholar]
- 8.Lukashevich IS, Maryankova R, Vladyko AS, Nashkevich N, Koleda S, Djavani M, Horejsh D, Voitenok NN, Salvato MS. 1999. Lassa and Mopeia virus replication in human monocytes/macrophages and in endothelial cells: different effects on IL-8 and TNF-alpha gene expression. J Med Virol 59:552–560. [PMC free article] [PubMed] [Google Scholar]
- 9.Pannetier D, Faure C, Georges-Courbot MC, Deubel V, Baize S. 2004. Human macrophages, but not dendritic cells, are activated and produce alpha/beta interferons in response to Mopeia virus infection. J Virol 78:10516–10524. doi: 10.1128/JVI.78.19.10516-10524.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groseth A, Hoenen T, Weber M, Wolff S, Herwig A, Kaufmann A, Becker S. 2011. Tacaribe virus but not junin virus infection induces cytokine release from primary human monocytes and macrophages. PLoS Negl Trop Dis 5:e1137. doi: 10.1371/journal.pntd.0001137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez-Sobrido L, Zuniga EI, Rosario D, Garcia-Sastre A, de la Torre JC. 2006. Inhibition of the type I interferon response by the nucleoprotein of the prototypic arenavirus lymphocytic choriomeningitis virus. J Virol 80:9192–9199. doi: 10.1128/JVI.00555-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qi X, Lan S, Wang W, Schelde LM, Dong H, Wallat GD, Ly H, Liang Y, Dong C. 2010. Cap binding and immune evasion revealed by Lassa nucleoprotein structure. Nature 468:779–783. doi: 10.1038/nature09605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez-Sobrido L, Giannakas P, Cubitt B, Garcia-Sastre A, de la Torre JC. 2007. Differential inhibition of type I interferon induction by arenavirus nucleoproteins. J Virol 81:12696–12703. doi: 10.1128/JVI.00882-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hastie KM, Liu T, Li S, King LB, Ngo N, Zandonatti MA, Woods VL Jr, de la Torre JC, Saphire EO. 2011. Crystal structure of the Lassa virus nucleoprotein-RNA complex reveals a gating mechanism for RNA binding. Proc Natl Acad Sci U S A 108:19365–19370. doi: 10.1073/pnas.1108515108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang X, Huang Q, Wang W, Dong H, Ly H, Liang Y, Dong C. 2013. Structures of arenaviral nucleoproteins with triphosphate dsRNA reveal a unique mechanism of immune suppression. J Biol Chem 288:16949–16959. doi: 10.1074/jbc.M112.420521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hastie KM, King LB, Zandonatti MA, Saphire EO. 2012. Structural basis for the dsRNA specificity of the Lassa virus NP exonuclease. PLoS One 7:e44211. doi: 10.1371/journal.pone.0044211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hastie KM, Kimberlin CR, Zandonatti MA, Macrae IJ, Saphire EO. 2011. Structure of the Lassa virus nucleoprotein reveals a dsRNA-specific 3′ to 5′ exonuclease activity essential for immune suppression. Proc Natl Acad Sci U S A 108:2396–2401. doi: 10.1073/pnas.1016404108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang Q, Shao J, Lan S, Zhou Y, Xing J, Dong C, Liang Y, Ly H. 2015. In vitro and in vivo characterizations of Pichinde viral nucleoprotein exoribonuclease functions. J Virol 89:6595–6607. doi: 10.1128/JVI.00009-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonthius DJ. 2012. Lymphocytic choriomeningitis virus: an underrecognized cause of neurologic disease in the fetus, child, and adult. Semin Pediatr Neurol 19:89–95. doi: 10.1016/j.spen.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aronson JF, Herzog NK, Jerrells TR. 1994. Pathological and virological features of arenavirus disease in guinea pigs. Comparison of two Pichinde virus strains. Am J Pathol 145:228–235. [PMC free article] [PubMed] [Google Scholar]
- 21.Lan S, McLay Schelde L, Wang J, Kumar N, Ly H, Liang Y. 2009. Development of infectious clones for virulent and avirulent pichinde viruses: a model virus to study arenavirus-induced hemorrhagic fevers. J Virol 83:6357–6362. doi: 10.1128/JVI.00019-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xing J, Ly H, Liang Y. 2015. The Z Proteins of pathogenic but not nonpathogenic arenaviruses inhibit RIG-i-like receptor-dependent interferon production. J Virol 89:2944–2955. doi: 10.1128/JVI.03349-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yokoyama K, Kaji H, He J, Tanaka C, Hazama R, Kamigaki T, Ku Y, Tohyama K, Tohyama Y. 2011. Rab27a negatively regulates phagocytosis by prolongation of the actin-coating stage around phagosomes. J Biol Chem 286:5375–5382. doi: 10.1074/jbc.M110.171702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamburg SI, Manejias RE, Rabinovitch M. 1978. Macrophage activation: increased ingestion of IgG-coated erythrocytes after administration of interferon inducers to mice. J Exp Med 147:593–598. doi: 10.1084/jem.147.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan L, Briese T, Lipkin WI. 2010. Z proteins of New World arenaviruses bind RIG-I and interfere with type I interferon induction. J Virol 84:1785–1791. doi: 10.1128/JVI.01362-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muller S, Geffers R, Gunther S. 2007. Analysis of gene expression in Lassa virus-infected HuH-7 cells. J Gen Virol 88:1568–1575. doi: 10.1099/vir.0.82529-0. [DOI] [PubMed] [Google Scholar]
- 27.Mahanty S, Bausch DG, Thomas RL, Goba A, Bah A, Peters CJ, Rollin PE. 2001. Low levels of interleukin-8 and interferon-inducible protein-10 in serum are associated with fatal infections in acute Lassa fever. J Infect Dis 183:1713–1721. doi: 10.1086/320722. [DOI] [PubMed] [Google Scholar]
- 28.Yun NE, Poussard AL, Seregin AV, Walker AG, Smith JK, Aronson JF, Smith JN, Soong L, Paessler S. 2012. Functional interferon system is required for clearance of Lassa virus. J Virol 86:3389–3392. doi: 10.1128/JVI.06284-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asper M, Sternsdorf T, Hass M, Drosten C, Rhode A, Schmitz H, Gunther S. 2004. Inhibition of different Lassa virus strains by alpha and gamma interferons and comparison with a less pathogenic arenavirus. J Virol 78:3162–3169. doi: 10.1128/JVI.78.6.3162-3169.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baize S, Marianneau P, Loth P, Reynard S, Journeaux A, Chevallier M, Tordo N, Deubel V, Contamin H. 2009. Early and strong immune responses are associated with control of viral replication and recovery in Lassa virus-infected cynomolgus monkeys. J Virol 83:5890–5903. doi: 10.1128/JVI.01948-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baize S, Pannetier D, Faure C, Marianneau P, Marendat I, Georges-Courbot MC, Deubel V. 2006. Role of interferons in the control of Lassa virus replication in human dendritic cells and macrophages. Microbes Infect 8:1194–1202. doi: 10.1016/j.micinf.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Rieger T, Merkler D, Gunther S. 2013. Infection of type I interferon receptor-deficient mice with various old world arenaviruses: a model for studying virulence and host species barriers. PLoS One 8:e72290. doi: 10.1371/journal.pone.0072290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang C, Kolokoltsova OA, Yun NE, Seregin AV, Poussard AL, Walker AG, Brasier AR, Zhao Y, Tian B, de la Torre JC, Paessler S. 2012. Junin virus infection activates the type I interferon pathway in a RIG-I-dependent manner. PLoS Negl Trop Dis 6:e1659. doi: 10.1371/journal.pntd.0001659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang C, Kolokoltsova OA, Yun NE, Seregin AV, Ronca S, Koma T, Paessler S. 2015. Highly pathogenic New World and Old World human arenaviruses induce distinct interferon responses in human cells. J Virol 89:7079–7088. doi: 10.1128/JVI.00526-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trinchieri G. 2010. Type I interferon: friend or foe? J Exp Med 207:2053–2063. doi: 10.1084/jem.20101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teijaro JR, Ng C, Lee AM, Sullivan BM, Sheehan KC, Welch M, Schreiber RD, de la Torre JC, Oldstone MB. 2013. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science 340:207–211. doi: 10.1126/science.1235214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson EB, Yamada DH, Elsaesser H, Herskovitz J, Deng J, Cheng G, Aronow BJ, Karp CL, Brooks DG. 2013. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science 340:202–207. doi: 10.1126/science.1235208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez-Madoz JR, Bernal-Rubio D, Kaminski D, Boyd K, Fernandez-Sesma A. 2010. Dengue virus inhibits the production of type I interferon in primary human dendritic cells. J Virol 84:4845–4850. doi: 10.1128/JVI.02514-09. [DOI] [PMC free article] [PubMed] [Google Scholar]