ABSTRACT
Transmission of chronic wasting disease (CWD) between cervids is influenced by the primary structure of the host cellular prion protein (PrPC). In white-tailed deer, PRNP alleles encode the polymorphisms Q95 G96 (wild type [wt]), Q95 S96 (referred to as the S96 allele), and H95 G96 (referred to as the H95 allele), which differentially impact CWD progression. We hypothesize that the transmission of CWD prions between deer expressing different allotypes of PrPC modifies the contagious agent affecting disease spread. To evaluate the transmission properties of CWD prions derived experimentally from deer of four PRNP genotypes (wt/wt, S96/wt, H95/wt, or H95/S96), transgenic (tg) mice expressing the wt allele (tg33) or S96 allele (tg60) were challenged with these prion agents. Passage of deer CWD prions into tg33 mice resulted in 100% attack rates, with the CWD H95/S96 prions having significantly longer incubation periods. The disease signs and neuropathological and protease-resistant prion protein (PrP-res) profiles in infected tg33 mice were similar between groups, indicating that a prion strain (Wisc-1) common to all CWD inocula was amplified. In contrast, tg60 mice developed prion disease only when inoculated with the H95/wt and H95/S96 CWD allotypes. Serial passage in tg60 mice resulted in adaptation of a novel CWD strain (H95+) with distinct biological properties. Transmission of first-passage tg60CWD-H95+ isolates into tg33 mice, however, elicited two prion disease presentations consistent with a mixture of strains associated with different PrP-res glycotypes. Our data indicate that H95-PRNP heterozygous deer accumulated two CWD strains whose emergence was dictated by the PrPC primary structure of the recipient host. These findings suggest that CWD transmission between cervids expressing distinct PrPC molecules results in the generation of novel CWD strains.
IMPORTANCE CWD prions are contagious among wild and captive cervids in North America and in South Korea. We present data linking the amino acid variant Q95H in white-tailed deer cellular prion protein (PrPC) to the emergence of a novel CWD strain (H95+). We show that, upon infection, deer expressing H95-PrPC molecules accumulated a mixture of CWD strains that selectively propagated depending on the PRNP genotype of the host in which they were passaged. Our study also demonstrates that mice expressing the deer S96-PRNP allele, previously shown to be resistant to various cervid prions, are susceptible to H95+ CWD prions. The potential for the generation of novel strains raises the possibility of an expanded host range for CWD.
INTRODUCTION
Chronic wasting disease (CWD) is an emerging prion disease or transmissible spongiform encephalopathy (TSE) of cervids, affecting free-ranging white-tailed deer (Odocoileus virginianus), mule deer (Odocoileus hemionus), elk (Cervus elaphus canadensis), and moose (Alces americanus) (1, 2). CWD occurs in captive herds of these species in North America and in red deer (Cervus elaphus) and sika deer (Cervus nippon) in South Korea (1, 3, 4). Reindeer (Rangifer tarandus), also known as caribou, are susceptible to experimental infection (5).
TSEs are slowly progressive, fatal neurodegenerative disorders for which no effective treatment or vaccine is available. Neuropathological changes include prion protein deposits, spongiform degeneration, neuronal loss, and astrogliosis. These hallmarks are diagnostic for CWD in cervids, scrapie in sheep and goats, bovine spongiform encephalopathy (BSE), as well as kuru, iatrogenic Creutzfeldt-Jakob disease (iCJD), and variant Creutzfeldt-Jakob disease (vCJD) in humans (6–10).
The pathogenesis of TSEs is associated with misfolded prion protein (PrPSc; or PrPCWD for cervid infections), whose ability to propagate, persist, and trigger neuropathology requires the expression of host PRNP-encoded cellular prion protein (PrPC). Prion propagation involves the posttranslational misfolding of normal cellular PrP molecules into pathognomonic, transmissible, generally protease-resistant prion protein (PrP-res) conformers that progressively accumulate in brain and other tissues (11–14). The primary structure of PrPC influences host susceptibility to infection, its disease progression, and its neuropathological and biochemical profiles (15–23). Knockout (Prnp) mice are refractory to experimental infection with mouse-adapted scrapie (24).
The difficulty of prion transmission from one species to another is defined as the species barrier, and that between individuals of the same species with different PRNP genotypes is defined as the transmission barrier and is influenced by the primary structure of the recipient's PrPC (15, 17, 19, 20, 25, 26). This barrier does not necessarily render the host refractory to infection and is impacted by the invading prion strain (20, 21, 26, 27). Prions can exhibit strain diversity. Strains are distinguished on the basis of their host range, clinical presentation, disease progression, and neuropathological and PrP biochemical profiles (28–31). The propagation of prion strains is dependent on both the PRNP genotype of the recipient and the properties of the invading agent (27, 32). For example, sheep expressing the V136-R154-Q171 PrPC (GenBank accession number AJ567988) are most susceptible to classical scrapie, while sheep with distinct PRNP genotypes have reduced susceptibility (33–35). The strain of the agent also plays a role, as sheep expressing A136-R154-R171 PrPC (GenBank accession number AJ567985) or A136-H154-Q171 PrPC (GenBank accession number AJ567983) are susceptible to atypical scrapie, although they are relatively resistant to classical scrapie (31, 36). Similarly, the PrPC primary structure and the invading agent modulate human susceptibility to prion infection; polymorphisms at codon 129 affect susceptibility to vCJD, kuru, and iCJD (20, 21, 26, 27, 37–40), while the G127V mutation renders carriers resistant against kuru (41).
In regions of North America where CWD is enzootic, transmission occurs between cervids expressing heterologous PrPC molecules (PrPC allotypes [18]). Analysis of PRNP allelic frequencies in wild and captive white-tailed deer identified two PrPC polymorphisms, Q95H and G96S, that impact susceptibility to CWD (42–44) (GenBank accession numbers AF156185, AF156184, and AY275711). Homozygous wild-type (wt; Q95 G96) deer are most susceptible to CWD and have relatively short incubation periods. In contrast, deer heterozygous for the S96/wt, H95/wt, and H95/S96 alleles had extended incubation periods, suggesting that S96-PrPC and H95-PrPC impact CWD prion propagation (45). Miller et al. (46) reported similar observations in experimentally challenged S96/wt and S96/S96 deer when the incubation periods for those deer were compared to those for wt/wt white-tailed deer and mule deer. To further explore the diversity of CWD strains and the consequences of propagation in deer expressing different PrPC primary structures, brain homogenates from CWD-infected white-tailed deer of different PRNP genotypes (wt/wt, S96/wt, H95/wt, or H95/S96 [45]) were inoculated into transgenic (tg) mice expressing the deer wt or S96 allele (47, 48). Our data show that CWD prions passaged in deer expressing H95-PrPC have altered transmission properties. The H95/wt and H95/S96 CWD allotypes efficiently triggered prion disease in tg mice with S96-PRNP genotypes, leading to the identification and adaptation of a novel CWD strain. Transmission of first-passage tg60CWD-H95+ prions into tg33 mice resulted in two distinct prion disease phenotypes which resembled those observed after primary passage of H95-PrP heterozygous deer CWD in both tg lines.
MATERIALS AND METHODS
Deer CWD inocula.
Four CWD agents consisting of 10% or 1% (wt/vol) brain homogenates (Bh) in phosphate-buffered saline were used for transmission studies (45). The inocula were designated on the basis of their specific PRNP genotypes. CWD brain homogenates were obtained from orally infected white-tailed deer expressing different PrPC molecules: homozygous Q95 G96 (wt/wt), heterozygous Q95 S96/wt (S96/wt), heterozygous H95 G96/wt (H95/wt), and H95 G96/Q95 S96 (H95/S96) (45). Brain homogenate from an uninfected white-tailed deer was used as a negative control. Frozen sagittal brain halves were homogenized (blended) to 20% (wt/vol) in cold phosphate buffer (1.3 M NaCl, 70 mM Na2HPO4·2H2O, 30 mM NaH2PO4·2H2O, pH 7.4), aliquoted, and stored at −80°C. Subsequently, aliquots were homogenized in a 50-ml syringe by passage through needles of different sizes (18 gauge to 21 gauge).
Transmission studies in transgenic mice.
Animal studies were conducted in accordance with the Canadian Council on Animal Care Guidelines and Policies with approval from the Health Sciences Animal Care and Use Committee of the University of Alberta Animal Care and Use Committee. Bioassays were performed with transgenic mouse lines expressing the deer wt allele (tg33+/+ and tg33+/− mice) or the S96-PRNP allele (tg60 mice, which express 30% less PrPC than tg33+/+ mice). (47, 48). Weanling pups were inoculated intracerebrally with 30 μl of deer CWD brain homogenates. Animals were monitored for the appearance of clinical signs and disease progression. Individual incubation periods are expressed as the number of days postinoculation (dpi) and were calculated from the time that the mice were inoculated until the time that clinical disease was established. The distribution of incubation periods between groups of tg33 mice was compared using the Kruskal-Wallis test with Dunn's multiple-comparison posttest (P < 0.05). Survival times postinoculation between tg60 mice inoculated with the H95/wt deer CWD allotype and the H95/S96 deer CWD allotype were compared using the Mann-Whitney test (P < 0.05). The statistical analysis of transmission experiments was performed with GraphPad Prism (version 5.04) software.
Isolates derived from tg60 mice infected with the H95/wt or H95/S96 deer CWD allotype (tg60CWD-H95/wt and tg60CWD-H95/S96 isolates, respectively) were used for syngeneic and allogeneic passages. One tg60CWD-H95/wt isolate (10%, wt/vol) was transmitted in tg33 and tg60 mice. A tg60CWD-H95/S96 isolate (10%, 1%, 0.0001%, wt/vol) was also passaged into both tg lines.
Histopathological analysis.
Brain tissues from at least 5 (range, 5 to 11) tg mice per inoculum group were formalin fixed and paraffin embedded for histopathological analysis. Sagittal brain sections were obtained from 2 to 4 mice in each group of animals receiving each inoculum, and coronal brain sections were obtained from 3 to 7 mice in each group. Six consecutive slides of both sagittal and coronal brain sections (4 coronal sections from each brain) were examined as follows: 2 slides (4 to 6 μm thick) were stained with hematoxylin and eosin (H&E) to evaluate the sections for spongiform degeneration, and the other 4 slides were immunostained for PrPCWD deposition and glial fibrillary acidic protein (GFAP)-positive astroglia. The sagittal paramedian brain sections were 0.36 to 0.60 mm lateral from the brain midline. All immunostaining experiments included CWD-positive tissue and negative mock-infected control sections. Differences in PrPCWD deposition patterns could exist in areas of the central nervous system that were not examined. For the purpose of comparison, identification of the structures in H&E-stained slides was performed according to the mouse brain atlas (49).
Lesion profile analysis was performed using coronal brain sections as described previously (50). The lesion profile scores for the first passage in tg33 mice were obtained from 3 to 7 mice per inoculum group. For the CWD-affected tg60 mice, lesion profiles were obtained by scoring 4 mice per inoculum group. The density of spongiform lesions in nine gray matter areas from the brains of prion disease-affected mice were scored by three independent observers in a blind manner. The scores are reported as the mean ± standard deviation.
PrPCWD deposits were visualized by immunostaining using anti-PrP monoclonal antibody BAR224 or 8G8 (0.2 μg/ml diluted 1:2,000 or 1:100, respectively; Bertin Pharma, formerly Spi-Bio). Briefly, brain slides were pretreated with high-pressure autoclaving (2.1 × 105 Pa) for 30 min in citric acid (10 mM), pH 6.0, at 121°C, followed by treatment with 98% formic acid for 30 min and 4 M guanidine thiocyanate for 2 h at room temperature. Astrogliosis was evaluated by immunostaining of glial fibrillary acidic protein using an anti-GFAP antibody (0.5 mg/ml diluted 1:1,000; BD Biosciences) after hydrated autoclaving for epitope exposure. Immunohistochemical detection was achieved with biotinylated secondary antibodies according to the manufacturer's instructions (ARK animal research kit; Dako). Tissue sections were scanned with a NanoZoomer 2.0RS scanner (Hamamatsu Photonics) and analyzed using NanoZoomer digital pathology software (Hamamatsu Photonics).
Immunoblot analysis.
Brain tissues from tg mice were homogenized to 10% (wt/vol) in sterile water using disposable syringes and needles of decreasing diameters (18 gauge to 21 gauge), aliquoted, and stored at −80°C. The brain homogenate protein content was determined using a micro-bicinchoninic acid assay kit (Life Technologies). For the proteinase digestion reactions, 50 to 70 μg total protein (final sample protein concentration, 1 to 1.4 mg/ml) was treated with 150 μg/ml of proteinase K (Life Technologies) for 45 min at 37°C. Reactions were terminated by boiling the samples in 2.5× Laemmli buffer (150 mM Tris-HCl, pH 6.8, 0.5% bromophenol blue, 25% glycerol, 5% [wt/vol] SDS, 12.5% β-mercaptoethanol) at 95°C for 10 min. Samples (10 to 15 μg) were resolved on 12% NuPAGE bis-Tris gels (Life Technologies) and transferred onto polyvinylidene difluoride Immobilon-P membranes (Millipore). The membranes were blocked for 1 h at room temperature with 5% (wt/vol) nonfat dry milk in Tris-buffered saline containing 0.1% (vol/vol) Tween 20 (TBST). Detection was performed using primary monoclonal antibody BAR224 (0.2 μg/ml diluted 1:10,000 in 5% [wt/vol] nonfat dry milk in TBST; Bertin Pharma) or 8G8 (0.2 μg/ml diluted 1:2,000; Bertin Pharma), secondary horseradish peroxidase-conjugated goat anti-mouse IgG antibody, and chemiluminescent substrate (diluted 1:10,000; Life Technologies). Images were acquired on X-ray film (Super Rx; Fujifilm). PrP-res glycoform ratios were determined using three animals per inoculum group; samples were resolved by Western blotting and detected with X-ray film. Quantification of PrP-res ratios was performed using ImageJ software (NIH). Independent triplicate measurements from each sample group were averaged, and the values were compared using GraphPad Prism (version 5.04) software.
RESULTS
Transmission of experimental CWD into tg-deer-PRNP mice.
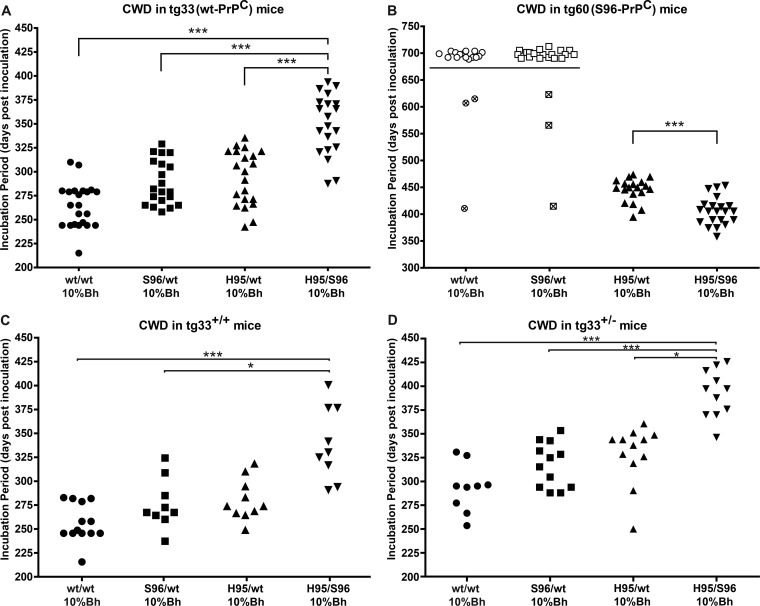
To evaluate the transmission properties of CWD prions derived from experimentally infected white-tailed deer of different PRNP genotypes (45), tg33 mice (expressing deer wt-PrPC) or tg60 mice (expressing deer S96-PrPC) (47, 48) were inoculated intracerebrally with deer CWD brain homogenates of 10% or 1% (wt/vol). All CWD inocula (wt/wt, S96/wt, H95/wt, H95/S96) resulted in clinical prion disease in mice expressing deer wt-PrPC (tg33+/+ and tg33+/− mice) (Fig. 1 and Table 1). Mice presented with similar disease signs, including hyperactivity, kyphosis, ataxia, and myoclonus. Clinical signs variably progressed into a general weakening, at which time the animals were euthanized. tg33 mice inoculated with the H95/S96 CWD agent had significantly longer incubation periods than mice receiving the other CWD inocula (the Kruskal-Wallis test with Dunn's multiple-comparison posttest P < 0.05) (Fig. 1A, C, and D). No significant differences in the incubation periods were observed between tg33 mice inoculated with the S96/wt, H95/wt, or wt/wt CWD inoculum (the Kruskal-Wallis test with Dunn's multiple-comparison posttest, P > 0.05) (Fig. 1).
FIG 1.
Transmission of CWD allotypes into transgenic mice expressing deer wt or S96-PrPC. (A) Susceptibility of tg33 (wt-PrPC) mice to infection with 10% (wt/vol) Bh from deer with CWD. (B) S96-PrPC (tg60) mice developed clinical prion disease only when inoculated with CWD prions derived from deer expressing H95-PrPC. Mice inoculated with wt/wt (open circles) or S96/wt (open squares) CWD prions did not show clinical signs. Symbols with crosses represent animals euthanized due to intercurrent disease. (C and D) Comparison of incubation periods in tg33+/+ and tg33+/− mice. ***, significant differences between groups (the Kruskal-Wallis test with Dunn's multiple-comparison posttest, P < 0.05). The Mann-Whitney test (P < 0.05) was used to compare the distribution of incubation periods in tg60 mice.
TABLE 1.
Prion disease in tg-deer-PRNP mice inoculated with white-tailed deer and tg mouse-passaged CWD prions
| Inoculum | tg-deer -PRNP mouse line | Bh dose (%) | No. of mice positivea/total no. of mice tested | Incubation period range (dpi) | PrP-res type | PrPCWD distribution pattern |
|---|---|---|---|---|---|---|
| Deer CWD wt/wt | tg33 | 10 | 22/22 | 215–310 | High MMb | Widespread |
| 1 | 8/8 | 256–345 | High MM | Widespread | ||
| tg60 | 10 | 0/18 | >700 | Negative | Not determined | |
| 1 | 0/10 | >600 | Negative | Not determined | ||
| Deer CWD S96/wt | tg33 | 10 | 20/20 | 258–329 | High MM | Widespread |
| 1 | 8/8 | 225–357 | High MM | Widespread | ||
| tg60 | 10 | 0/21 | >700 | Negative | Not determined | |
| 1 | 0/10 | >600 | Negative | Not determined | ||
| Deer CWD H95/wt | tg33 | 10 | 21/21 | 242–335 | High MM | Widespread |
| 1 | 10/10 | 316–356 | High MM | Widespread | ||
| tg60 | 10 | 19/19 | 394–473 | Low MM | Localized | |
| 1 | 10/10 | 465–608 | Low MM | Localized | ||
| Deer CWD H95/S96 | tg33 | 10 | 20/20 | 288–394 | High MM | Widespread |
| 1 | 9/9 | 323–433 | High MM | Widespread | ||
| tg60 | 10 | 21/21 | 359–454 | Low MM | Localized | |
| 1 | 16/16 | 359–554 | Low MM | Localized | ||
| tg60CWD-H95/wt | tg33 | 10 | 15/15 | 340–383 | High MM | Widespread |
| tg60 | 10 | 16/16 | 310–380 | Low MM | Localized | |
| tg60CWD-H95/S96 | tg33 | 10 | 7/7 | 373–409 | High or low MM | Localized or widespread |
| tg33 | 1 | 8/8 | 397–448 | High or low MM | Localized or widespread | |
| tg33 | 0.0001 | 8/8 | 329–490 | High or low MM | Localized or widespread | |
| tg60 | 10 | 18/18 | 331–369 | Low MM | Localized | |
| Uninfected deer wt/wt | tg33 | 10 | 0/6 | >560 | Negative | Negative |
| tg60 | 10 | 0/5 | >560 | Negative | Negative |
Clinically positive animals.
MM, molecular mass.
In contrast to the susceptibility of the tg33 mouse line, mice expressing deer S96-PrPC (tg60) developed clinical disease only when inoculated with CWD agents derived from deer expressing the H95-PRNP allele (Fig. 1B). Mice inoculated with the H95/S96 and H95/wt CWD allotypes were clinically positive for prion disease between 359 and 473 dpi. Affected mice became lethargic with myoclonus, kyphosis, labored breathing, and ataxic gait characterized by limb weakness. Incubation periods were significantly different between tg60 mice inoculated with the H95/S96 CWD agent and tg60 mice challenged with the H95/wt CWD agent (Mann-Whitney test, P < 0.05). S96-PrPC mice inoculated with the wt/wt and S96/wt CWD agents did not develop prion disease at >700 days postinoculation.
Neuropathology of tg-deer-PRNP mice infected with CWD agents.
To define the neuropathological hallmarks and assess differences between groups of mice inoculated with the different CWD inocula, sagittal and coronal brain sections were examined histologically for spongiform changes and immunohistochemically for PrPCWD aggregates and GFAP-positive astroglia (Fig. 2).
FIG 2.
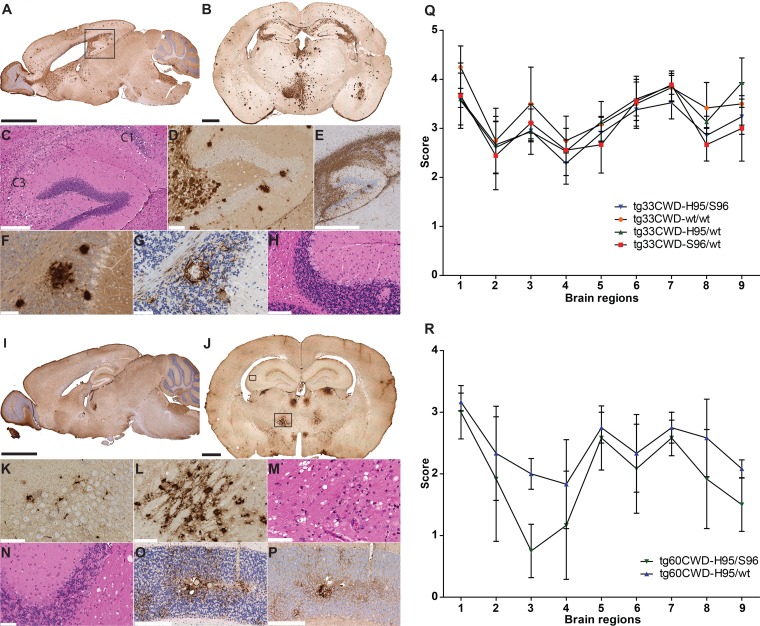
Neuropathology of tg-deer-PRNP mice following the first passage of white-tailed deer CWD allotypes. (A and B) Accumulation of wt-PrPCWD aggregates in tg33 mice. The regional distribution of wt-PrPCWD aggregates was similar in mice receiving different CWD inocula (Fig. 3A to G). (C to E) Hippocampal degeneration (box in panel A) was characterized by spongiform change and a loss of pyramidal neurons of the Ammon's horn (C1 and C3), accompanied by the extensive accumulation of PrPCWD aggregates and abundant astrocytosis (GFAP). (F and G) Cerebellum pathology involved the loss of granular neurons and the presence of prion protein deposits flanked by astrocytes (as seen in sequential tissue sections). (H) Vacuolation was observed in Purkinje neurons and cerebellar white matter. (I and J) Detection of S96-PrPCWD aggregates in tg60 mice infected with the H95/wt and H95/S96 CWD allotypes. The distribution of PrPCWD aggregates was similar between animals receiving the H95+ deer CWD agent (Fig. 3J to K). (K) S96-PrPCWD aggregates in the hippocampus were noticeable at a higher magnification of the small box in panel J. (L and M) Abnormal prion protein deposits and spongiosis in thalamic nuclei shown by a higher magnification of the large box in panel J. (N to P) Cerebellar pathology included white matter vacuolation (N) and astrocytosis (O) that colocalized with diffuse and punctate protein aggregates (P). (Q) Lesion profile of tg33 mice infected with deer CWD allotypes. (R) Lesion profile of tg60 mice infected with the H95+ deer CWD agent. Brain regions are as follows: 1, medulla; 2, cerebellum; 3, superior colliculus; 4, hypothalamus; 5, thalamus; 6, hippocampus; 7, septum; 8, posterior cortex; 9, anterior cortex. Bars, 2.5 mm (A and I), 1 mm (B and J), 850 μm (E), 300 μm (C, O and P), 125 μm (D, L and H), and 60 μm (F, G, K, M, and N). PrPCWD detection was achieved with anti-PrP monoclonal antibody BAR224. (A) Brain section from a tg33 mouse infected with S96/wt CWD prions at 270 dpi; (B) brain section from a tg33 mouse infected with H95/S96 CWD prions at 387 dpi, (I) brain section from a tg60 mouse infected with H95/S96 CWD prions at 375 dpi; (J) brain section from a tg60 mouse infected with H95/S96 CWD prions at 414 dpi.
CWD-infected tg33 (wt-PrPC) mice presented with extensive pathology in various brain regions and were characterized by neuronal loss, spongiform change, the widespread accumulation of PrPCWD aggregates, and astrogliosis (Fig. 2A to H). The distribution of pathological changes in the brain (i.e., PrPCWD distribution) was similar between mice inoculated with the four CWD inocula (Fig. 3A to G). The average spongiform change scores of various brain structures were similar among the infected tg33 mice (Fig. 2Q). In general, the lesions (vacuolation and PrPCWD accumulation) observed in the forebrain and cerebellum agree with previous results obtained with this transgenic mouse line after infection with CWD prions from other sources (47). Additionally, the granular layer of the cerebellum had areas of neuronal loss, where dense PrPCWD aggregates surrounded by GFAP-positive astrocytes were revealed in consecutive tissue sections (Fig. 2F to G). The spongiform changes and cell death in the cerebellum of tg33 mice were less conspicuous in the molecular layer and more abundant in the white matter and the Purkinje cell layers (Fig. 2H). Infection of tg33 mice resulted in more prominent PrPCWD accumulation in the corpus callosum (Fig. 2A and B) than that described in other studies (47).
FIG 3.
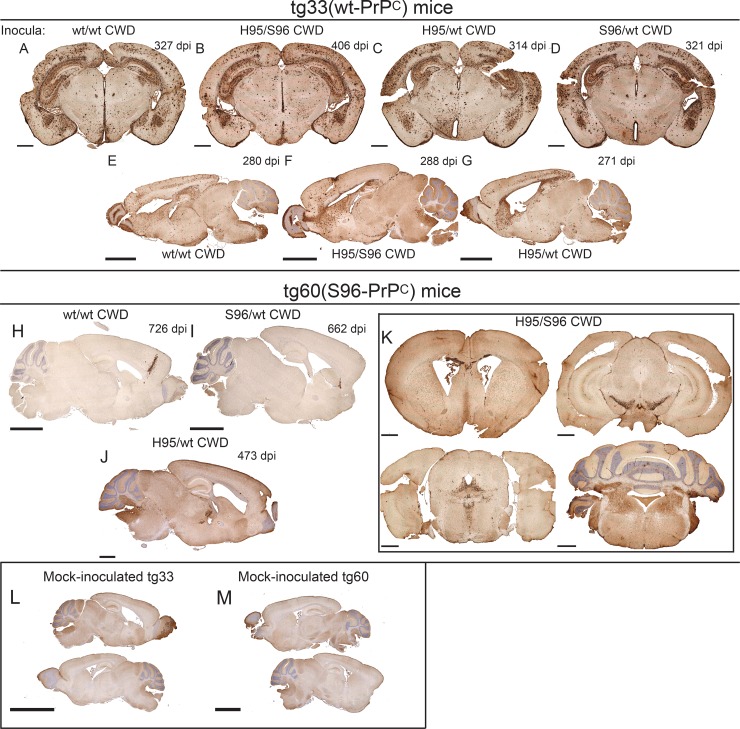
Distribution of PrPCWD aggregates in the brains of tg-deer-PRNP mice inoculated with different white-tailed deer CWD allotypes. (A to G) PrPCWD aggregates in the brains of tg33 mice inoculated with different white-tailed deer CWD allotypes. (H and I) Abnormal PrP aggregates were detected after >700 dpi in the brains of tg60 mice without clinical signs inoculated with the wt/wt or S96/wt CWD allotypes. (J and K) Only tg60 mice inoculated with H95+ CWD allotypes had clinical signs and were consistently positive for PrPCWD aggregates. (K) Coronal brain sections from a clinically ill tg60 mouse infected with H95+ CWD prions (414 dpi). (L and M) Brain sections of mock-infected tg33 and tg60 mice. Bars, 1 mm (A to D, J, and K), 2.5 mm (E to I and M), and 5 mm (L). Tissue sections were stained with anti-PrP monoclonal antibody BAR224.
The susceptibility of S96-PrPC (tg60) mice to CWD agent infection was strongly influenced by the invading CWD allotype. All tg60 mice exposed to the H95/wt or H95/S96 CWD agent developed clinical prion disease with similar neuropathologies (Fig. 2I to P and R and 3J to K). The distribution and severity of the neuropathological changes observed in diseased tg60 mice infected with H95+ deer CWD allotypes followed a consistent lesion pattern (Fig. 2I to P and R and 3J to K). Spongiform degeneration and abnormal S96-PrPCWD aggregates were localized in the caudoputamen and the corpus callosum and extended down the septum to the diagonal band nucleus (Fig. 3K). Both vacuolation and PrPCWD deposition were of milder intensity in the cerebral cortex and hippocampus than in the other brain areas; however, immunohistochemical staining revealed the presence of small, punctate S96-PrPCWD aggregates at higher magnification (Fig. 2K). Pathological changes were more severe in various regions of the thalamus, including the medial-dorsal, ventral-medial, and ventral anterior-lateral thalamic nuclei (Fig. 2I to J, L, and M and 3J) and also involved the zona incerta, cerebral peduncle, and subthalamic and hypothalamic nuclei (Fig. 2I to J and 3J). In the midbrain, lesions were localized in the substantia nigra adjacent to the ventral tegmental area and extended to periaqueductal gray and adjacent structures, including the raphe nucleus, mesencephalic reticular formation, and superior cerebellar peduncle (Fig. 2I and 3K). Pathology was also observed in the hindbrain and affected various regions, including the median raphe nucleus and pontine reticular nucleus (Fig. 3K). In the cerebellum, the spongiform change was the most prominent in the white matter; however, small vacuoles were also observed in the molecular, Purkinje, and granular layers, with the granular layer showing loss of granular neurons (Fig. 2N). PrPCWD staining revealed either diffuse deposits (lightly stained) or larger confluent aggregates in the cerebellar nuclei and the granular layer (Fig. 2I and P and 3K).
A few tg60 (S96-PrPC) mice that did not have clinical signs and that were challenged with the wt/wt or S96/wt CWD agents (3/28 and 3/31 mice, respectively) had detectable prion aggregates at >700 days postinoculation (Fig. 3H to I), highlighting the low efficacy of these CWD agents for establishing infection in this transgenic line. The accumulation of PrPCWD aggregates in these particular mice did not follow the PrPCWD distribution patterns described in the other mice.
PrP-res glycotypes in transgenic mice expressing deer PrPC.
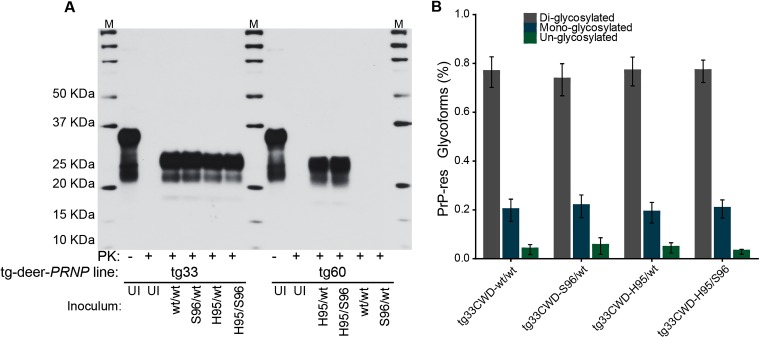
Distinct PrP-res isoforms have been associated with different prion strains (28, 51, 52). PrP-res can vary in their molecular masses, glycoform ratios, and other biochemical properties related to the structural stability of the abnormal PrP conformers (53). These properties have been interpreted to be conformational differences in the structures of the misfolded PrP molecules that carry the information that defines different prion strains (28, 51–53). To compare the PrP-res in mice infected with the different CWD inocula, brain homogenates were digested with proteinase K and analyzed by Western blotting using anti-PrP monoclonal antibodies 8G8 (which recognizes deer PrP amino acid residues 98 to 113) or Bar224 (which epitope comprises deer PrP residues 144 to 154). All clinically affected tg33 mice were PrP-res positive; no differences with respect to molecular masses and glycoform patterns were observed (Fig. 4). Although the electrophoretic profile of PrP-res from clinically affected tg60 mice was similar between mice inoculated with the H95/wt or H95/S96 CWD allotypes, this PrP-res type was distinct from that observed in tg33 mice. The gel migration of the proteinase K cleavage products indicated that S96-PrP-res has a lower molecular mass than wt-PrP-res (Fig. 4 and 5). PrP-res was not detected at 700 dpi in brain homogenates from tg60 mice inoculated with the wt/wt or S96/wt CWD allotypes.
FIG 4.
Disease-associated PrP-res in tg-deer-PRNP mice inoculated with different CWD allotypes. (A) PrP-res from brains of prion-affected tg33 (wt-PrPC) and tg60 (S96-PrPC) mice. Brain homogenates were digested with proteinase K (PK) and analyzed by SDS-PAGE and Western blotting. Lanes M, molecular size markers. PrP-res from tg33 mice had similar molecular masses after enzymatic cleavage (A) and equivalent glycoform ratios (B). S96-PrP-res has a lower molecular mass and was detectable only in brain homogenates derived from tg60 mice infected with H95+ CWD allotypes. UI, homogenates from tg mice inoculated with uninfected deer brain homogenate. PrP-res detection was achieved with anti-PrP monoclonal antibody BAR224.
FIG 5.
Serial passage of tg60 (S96-PrPC) mouse-passaged CWD prions. (A) Syngeneic transmission of tg60CWD-H95+ isolates into tg60 mice led to reduction in the incubation period following intracerebral inoculation of 10% (wt/vol) brain homogenates. (B) S96-PrP-res properties were maintained following secondary passage in tg60 mice. S96-PrP-res has a lower molecular mass than wt-PrP-res derived from tg33 mice. Lanes M, molecular size markers. (C and D) Distribution of S96-PrPCWD aggregates in the brains of tg60 mice infected with tg60CWD-H95+ prions. Immunohistochemical comparison revealed a similar distribution of abnormal PrPCWD aggregates, as observed in tg60 mice from the first passage of H95+ deer CWD agent (Fig. 2I to J and 3J and K). Detection of abnormal PrP (B to D) was performed with antibody BAR224. Bars, 2.5 mm.
Serial transmission of passage 1 tg60CWD-H95+ isolates into tg-deer-PRNP mice.
To evaluate the transmission properties of tg60 (S96-PrPC) mouse-passaged CWD prions, we inoculated these isolates into both the tg33 and tg60 mouse lines. Serial transmission of first-passage tg60CWD-H95+ isolates back into tg60 mice (syngeneic passage) resulted in a reduction of the incubation periods (Fig. 5A and Table 1). The disease signs, biochemical PrP-res glycotype, and neuropathology resembled those after first passage (Fig. 5B to D).
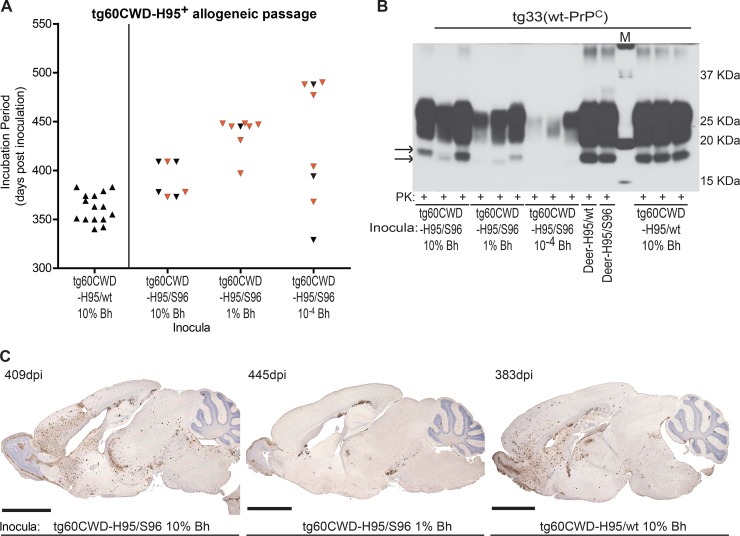
Passage of tg60CWD-H95+ isolates into tg33 mice (allogeneic passage) resulted in two different prion disease presentations. After exposure to 10% (wt/vol) tg60CWD-H95/wt brain homogenate, the mice had extended incubation periods compared to those of tg33 mice infected with the H95/wt deer CWD allotype (Fig. 6A and Table 1). Disease signs and pathological hallmarks were similar to those described during the first passage of deer CWD prions in tg33 mice, characterized by hyperactivity, a widespread distribution of aggregates in the brain, and high-molecular-mass PrP-res (Fig. 6B and C). Transmission of the tg60CWD-H95/S96 isolate into tg33 mice resulted in divergent prion disease phenotypes. Inoculation of 10% brain homogenates resulted in extended incubation periods, with some mice developing disease signs and pathology characteristic of tg33 mice infected with the deer CWD agents, while others developed disease signs and neuropathology that resembled the disease phenotype described for tg60 mice (Fig. 6). Evaluation of proteinase K-resistant PrP in brain homogenates from affected mice revealed PrP-res glycotypes of distinct molecular masses (Fig. 6B). Passage of 1% and 0.0001% (wt/vol) brain homogenates resulted in further extension of the incubation period and increased the abundance of mice presenting with lethargy (like tg60 mice), accompanied by the accumulation of low-molecular-mass PrP-res and localized deposition of PrP aggregates in brain (Fig. 6).
FIG 6.
Allogeneic transmission of tg60 (S96-PrPC) mouse-passaged CWD prions into tg33 mice. (A) Incubation periods of tg33 mice upon challenge with passage 1 tg60CWD-H95+ isolates. Passage of tg60CWD-H95/S96 brain homogenates gave rise to different clinical presentations (hyperactivity versus lethargy) resembling the disease phenotypes described for both tg-deer-PRNP lines during the first passage of deer CWD prions. Black symbols, tg33 animals with hyperactive disease presentation, high-molecular-mass PrP-res, and a widespread distribution of brain PrPCWD aggregates; orange symbols, tg33 mice with a lethargic presentation, low-molecular-mass PrP-res, and a localized distribution of PrPCWD aggregates. (B) PrP-res glycotypes in brains of tg33 mice inoculated with different tg60CWD-H95+ isolates. Infected tg33 mice accumulated different proteinase K-resistant PrP types resembling those observed after the first passage of deer CWD prions. Lane M, molecular size markers. (C) Divergent histological phenotypes in tg33 mice infected with tg60CWD-H95/S96 or tg60CWD-H95/wt brain homogenates. Bars, 2.5 mm. Detection of abnormal PrP was performed with anti-PrP monoclonal BAR224.
DISCUSSION
To explore the transmission properties of CWD prions derived from white-tailed deer of four different PRNP genotypes (45), we inoculated transgenic mice expressing deer prion proteins associated with susceptibility (tg33 mice expressing deer wt-PrPC) or resistance (tg60 mice expressing deer S96-PrPC) to CWD prions (47, 48). Transmission of the deer H95/wt and H95/S96 CWD allotypes resulted in the emergence of a distinct CWD strain (H95+). This novel prion agent was identified when brain homogenates from deer containing H95-PrP molecules were transmitted into tg60 mice. Passage of these deer brain homogenates into tg33 mice, however, resulted in a prion disease phenotype indistinguishable from that observed following infection with the wt/wt or S96/wt CWD agents. The ability of H95+ CWD agent to cause clinical prion disease in tg60 mice, which have been shown to be resistant to other CWD isolates, indicates that a new strain has emerged (45, 47, 48). Our data show that the passage of CWD (wt/wt pool) through deer with the H95/wt and H95/S96 allotypes resulted in a mixture of at least two CWD strains, distinguishable on the basis of the tg-deer-PRNP genotype in which they were propagated.
Upon first passage into tg33 mice, all deer CWD agents resulted in similar disease signs, PrP-res glycotypes, and neuropathological features, suggesting that expression of wt-PrPC favored the propagation of a CWD strain (prion conformer) common to all inocula. We refer to this agent as “Wisc-1.” Our results suggest that Wisc-1 is similar to strain CWD-1 described by Angers et al. (54). The white-tailed deer sample analyzed in the study of Angers et al. (54) was a wt/wt CWD isolate from Wisconsin. Whether Wisc-1 and CWD-1 are identical is difficult to ascertain, as the white-tailed deer agents were passaged in different transgenic mice. The H95+ CWD strain differs from the Wisc-1, CWD-1, and CWD-2 strains (54, 55).
We found that inoculation of the H95/S96 CWD agent into tg33 mice resulted in incubation periods significantly different from those obtained by inoculation of CWD prions of the other allotypes. The absence of wt-PrPCWD in this inoculum and, thus, the lack of homologous prion conversion likely contributed to the prolonged incubation period. The presence of more than one prion conformer within this inoculum may result in competition between agents, leading to propagation interference and extension of the incubation periods (56–59).
Incubation periods were not significantly different between tg33 mice infected with the wt/wt, H95/wt or S96/wt CWD agents. Additionally, all tg33 mice presented the same prion disease phenotype irrespective of the CWD inoculum that they received. One possible interpretation for the phenotypic similarities observed between tg33 mice is that the Wisc-1 conformers have an adaptive advantage in hosts (either in deer or in tg mice) expressing wt-PrPC. The differences in incubation periods between the H95/wt CWD allotype- and H95/S96 CWD allotype-infected tg33 mice suggest that the PrPC sequence in these deer impacted the proportion of accumulated CWD strains. It has previously been demonstrated in hamster coinfection experiments that the ratio of the strains in a prion mixture influences the emergence of the fastest-replicating or dominant strain (56, 57, 59).
The differential susceptibility to prion infection is modulated by PrPC amino acid sequence variability and the invading prion strain (15, 17, 20, 21, 25, 26, 60). Both natural and experimental infections support the association of S96-PrPC with reduced susceptibility and the slower progression of CWD (3, 42–48, 55). tg60 (S96-PrPC) mice were previously shown to be resistant to CWD isolates from different cervid species (47, 48). In our study, tg60 mice inoculated with the wt/wt or S96/wt CWD agents did not present with clinical disease after >700 dpi; however, mice receiving the H95/wt and H95/S96 CWD allotypes developed disease signs and presented consistent neuropathology and PrP-res glycotypes. A second passage of the tg60CWD-H95+ isolates into tg60 mice resulted in a reduction of the incubation periods and similar phenotypic characteristics.
Allogeneic transmission of the first-passage tg60CWD-H95+ isolates into tg33 mice resulted in the development of prion disease with two distinct phenotypes resembling those caused by the Wisc-1 and H95+ prion strains. While some animals presented with hyperactivity and displayed a widespread accumulation of disease-associated PrP in the brain as well as high-molecular-mass PrP-res, others were lethargic with localized PrPCWD deposits and a distinct PrP-res glycotype. Transmission of a diluted tg60CWD-H95+ inoculum resulted in more mice presenting the tg60-like phenotype. This suggests that the tg60 donor mouse, which preferentially amplified the H95+ strain, contained a persistent Wisc-1 fraction that was amplified upon passage at a high dose (10% Bh) in tg33 mice. Transmission of lower doses of the inoculum likely altered the proportion of the two prion conformers, favoring the propagation of the H95+ strain. A similar outcome was observed when dilutions of the transmissible mink encephalopathy agent were passaged in hamsters, resulting in the isolation of the Hyper and Drowsy strains (56). Transmission of tg60CWD-H95+ isolates into tg33 mice indicates that individual tg60 mice accumulated mixtures of CWD agents. Although prion transmission experiments in tg mice do not always recapitulate what is observed in the wild (i.e., tg60 mice are resistant to a number of different CWD strains, whereas 96S homozygous deer are naturally infected), natural scrapie and CWD isolates have been shown to contain strain mixtures that can be differentiated by serial passage in mouse models or by histopathological and biochemical analyses (54, 61–63).
Deer with S96-PRNP alleles can be infected with the CWD agent but have extended preclinical periods, suggesting that they could be infectious over longer periods of time than wt homozygous deer (45, 46, 48). Additionally, in areas where CWD is endemic, white-tailed deer with S96-PRNP alleles likely have a fitness advantage over deer with the more susceptible genotypes, and as a result, the resistance allele may become more abundant in the population (64). An increase in the S96-PRNP allele frequency could also affect the potential for the selection of CWD strains able to infect deer with resistant genotypes. Likewise, other PRNP alleles associated with extension of the CWD preclinical phase, such as H95-PRNP, could also be subjected to a disease-driven increase in white-tailed deer populations. Our transmission data show that deer expressing H95-PrP accumulate a CWD strain capable of infecting deer with S96-PRNP genotypes, unlike other CWD agents. An increase in the frequency of H95-PRNP would also increase the likelihood of the emergence of H95+ CWD prions. Our data suggest that white-tailed deer expressing different PrPC allotypes can accumulate and transmit CWD strain mixtures.
CWD epizootics involve multiple factors, including the contagious nature of the agent, host-pathogen interactions, agent strains, and cervid population genetics. Our data indicate that CWD strain emergence is modulated by amino acid polymorphisms in the cervid PrP. CWD transmission between hosts with different PRNP genotypes (65) has the potential to generate and select novel prion conformations. Deer expressing H95-PrPC accumulate CWD prions with different transmission properties, as exemplified by its ability to infect resistant S96-PRNP mice. Finally, our study highlights the importance of characterizing the diversity of CWD strains and their potential for interspecies transmission, as various mammalian species are susceptible to experimental CWD infection (66–69). Although several lines of evidence suggest that humans are resistant to CWD prions (70–73), not all CWD strains have been tested for their zoonotic potential. Our results demonstrating that H95+ deer CWD prions have transmission properties different from those of CWD prions composed of wt-PrP or S96-PrP suggest the need for evaluation of the transmissibility of CWD allotypes.
ACKNOWLEDGMENTS
We thank Bruce Chesebro and Brent Race for the tg33 and tg60 mouse lines. We also thank the animal care staff (Jing Yang, Jennifer Grams, Karla Bergen, Lesley Down, Hyena Jong, and Kelan Hirtle) and histology technician (Hristina Gapeshina) of the Center for Prions and Protein Folding Diseases (CPPFD). We extend our gratitude to all members of the D. McKenzie and J. Aiken laboratories and the CPPFD for their helpful discussions.
REFERENCES
- 1.Williams ES, Young S. 1980. Chronic wasting disease of captive mule deer: a spongiform encephalopathy. J Wildl Dis 16:89–98. doi: 10.7589/0090-3558-16.1.89. [DOI] [PubMed] [Google Scholar]
- 2.Baeten LA, Powers BE, Jewell JE, Spraker TR, Miller MW. 2007. A natural case of chronic wasting disease in a free-ranging moose (Alces alces shirasi). J Wildl Dis 43:309–314. doi: 10.7589/0090-3558-43.2.309. [DOI] [PubMed] [Google Scholar]
- 3.Thomsen BV, Schneider DA, O'Rourke KI, Gidlewski T, McLane J, Allen RW, McIsaac AA, Mitchell GB, Keane DP, Spraker TR, Balachandran A. 2012. Diagnostic accuracy of rectal mucosa biopsy testing for chronic wasting disease within white-tailed deer (Odocoileus virginianus) herds in North America: effects of age, sex, polymorphism at PRNP codon 96, and disease progression. J Vet Diagn Invest 24:878–887. doi: 10.1177/1040638712453582. [DOI] [PubMed] [Google Scholar]
- 4.Lee YH, Sohn HJ, Kim MJ, Kim HJ, Park KJ, Lee WY, Yun EI, Tark DS, Choi YP, Cho IS, Balachandran A. 2013. Experimental chronic wasting disease in wild type VM mice. J Vet Med Sci 75:1107–1110. doi: 10.1292/jvms.13-0018. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell GB, Sigurdson CJ, O'Rourke KI, Algire J, Harrington NP, Walther I, Spraker TR, Balachandran A. 2012. Experimental oral transmission of chronic wasting disease to reindeer (Rangifer tarandus tarandus). PLoS One 7:e39055. doi: 10.1371/journal.pone.0039055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hadlow WJ, Kennedy RC, Race RE, Eklund CM. 1980. Virologic and neurohistologic findings in dairy goats affected with natural scrapie. Vet Pathol 17:187–199. [DOI] [PubMed] [Google Scholar]
- 7.Gajdusek DC, Zigas V. 1959. Kuru; clinical, pathological and epidemiological study of an acute progressive degenerative disease of the central nervous system among natives of the Eastern Highlands of New Guinea. Am J Med 26:442–469. doi: 10.1016/0002-9343(59)90251-7. [DOI] [PubMed] [Google Scholar]
- 8.Wells GA, Scott AC, Johnson CT, Gunning RF, Hancock RD, Jeffrey M, Dawson M, Bradley R. 1987. A novel progressive spongiform encephalopathy in cattle. Vet Rec 121:419–420. doi: 10.1136/vr.121.18.419. [DOI] [PubMed] [Google Scholar]
- 9.Duffy P, Wolf J, Collins G, DeVoe AG, Streeten B, Cowen D. 1974. Letter: possible person-to-person transmission of Creutzfeldt-Jakob disease. N Engl J Med 290:692–693. [PubMed] [Google Scholar]
- 10.Will RG, Ironside JW, Zeidler M, Cousens SN, Estibeiro K, Alperovitch A, Poser S, Pocchiari M, Hofman A, Smith PG. 1996. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet 347:921–925. doi: 10.1016/S0140-6736(96)91412-9. [DOI] [PubMed] [Google Scholar]
- 11.Prusiner SB. 1982. Novel proteinaceous infectious particles cause scrapie. Science 216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 12.Bolton DC, McKinley MP, Prusiner SB. 1982. Identification of a protein that purifies with the scrapie prion. Science 218:1309–1311. doi: 10.1126/science.6815801. [DOI] [PubMed] [Google Scholar]
- 13.Basler K, Oesch B, Scott M, Westaway D, Walchli M, Groth DF, McKinley MP, Prusiner SB, Weissmann C. 1986. Scrapie and cellular PrP isoforms are encoded by the same chromosomal gene. Cell 46:417–428. doi: 10.1016/0092-8674(86)90662-8. [DOI] [PubMed] [Google Scholar]
- 14.Angers RC, Browning SR, Seward TS, Sigurdson CJ, Miller MW, Hoover EA, Telling GC. 2006. Prions in skeletal muscles of deer with chronic wasting disease. Science 311:1117. doi: 10.1126/science.1122864. [DOI] [PubMed] [Google Scholar]
- 15.Dickinson AG, Meikle VM, Fraser H. 1968. Identification of a gene which controls the incubation period of some strains of scrapie agent in mice. J Comp Pathol 78:293–299. doi: 10.1016/0021-9975(68)90005-4. [DOI] [PubMed] [Google Scholar]
- 16.Carlson GA, Westaway D, DeArmond SJ, Peterson-Torchia M, Prusiner SB. 1989. Primary structure of prion protein may modify scrapie isolate properties. Proc Natl Acad Sci U S A 86:7475–7479. doi: 10.1073/pnas.86.19.7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruce ME, McConnell I, Fraser H, Dickinson AG. 1991. The disease characteristics of different strains of scrapie in Sinc congenic mouse lines: implications for the nature of the agent and host control of pathogenesis. J Gen Virol 72(Pt 3):595–603. [DOI] [PubMed] [Google Scholar]
- 18.Carlson GA, Ebeling C, Yang SL, Telling G, Torchia M, Groth D, Westaway D, DeArmond SJ, Prusiner SB. 1994. Prion isolate specified allotypic interactions between the cellular and scrapie prion proteins in congenic and transgenic mice. Proc Natl Acad Sci U S A 91:5690–5694. doi: 10.1073/pnas.91.12.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruce ME. 2003. TSE strain variation. Br Med Bull 66:99–108. doi: 10.1093/bmb/66.1.99. [DOI] [PubMed] [Google Scholar]
- 20.Mead S, Whitfield J, Poulter M, Shah P, Uphill J, Beck J, Campbell T, Al-Dujaily H, Hummerich H, Alpers MP, Collinge J. 2008. Genetic susceptibility, evolution and the kuru epidemic. Philos Trans R Soc Lond B Biol Sci 363:3741–3746. doi: 10.1098/rstb.2008.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown P, Brandel JP, Sato T, Nakamura Y, MacKenzie J, Will RG, Ladogana A, Pocchiari M, Leschek EW, Schonberger LB. 2012. Iatrogenic Creutzfeldt-Jakob disease, final assessment. Emerg Infect Dis 18:901–907. doi: 10.3201/eid1806.120116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez L, Pitarch JL, Martin S, Thurston L, Simmons H, Acin C, Jeffrey M. 2014. Influence of polymorphisms in the prion protein gene on the pathogenesis and neuropathological phenotype of sheep scrapie after oral infection. J Comp Pathol 150:57–70. doi: 10.1016/j.jcpa.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Wolfe LL, Fox KA, Miller MW. 2014. “Atypical” chronic wasting disease in PRNP genotype 225FF mule deer. J Wildl Dis 50:660–665. doi: 10.7589/2013-10-274. [DOI] [PubMed] [Google Scholar]
- 24.Bueler H, Aguzzi A, Sailer A, Greiner RA, Autenried P, Aguet M, Weissmann C. 1993. Mice devoid of PrP are resistant to scrapie. Cell 73:1339–1347. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- 25.Prusiner SB, Scott M, Foster D, Pan KM, Groth D, Mirenda C, Torchia M, Yang SL, Serban D, Carlson GA, Hoppe PC, Westaway D, DeArmond SJ. 1990. Transgenetic studies implicate interactions between homologous PrP isoforms in scrapie prion replication. Cell 63:673–686. doi: 10.1016/0092-8674(90)90134-Z. [DOI] [PubMed] [Google Scholar]
- 26.Lee HS, Brown P, Cervenakova L, Garruto RM, Alpers MP, Gajdusek DC, Goldfarb LG. 2001. Increased susceptibility to kuru of carriers of the PRNP 129 methionine/methionine genotype. J Infect Dis 183:192–196. doi: 10.1086/317935. [DOI] [PubMed] [Google Scholar]
- 27.Wadsworth JD, Asante EA, Desbruslais M, Linehan JM, Joiner S, Gowland I, Welch J, Stone L, Lloyd SE, Hill AF, Brandner S, Collinge J. 2004. Human prion protein with valine 129 prevents expression of variant CJD phenotype. Science 306:1793–1796. doi: 10.1126/science.1103932. [DOI] [PubMed] [Google Scholar]
- 28.Bruce ME, Dickinson AG. 1987. Biological evidence that scrapie agent has an independent genome. J Gen Virol 68(Pt 1):79–89. doi: 10.1099/0022-1317-68-1-79. [DOI] [PubMed] [Google Scholar]
- 29.Bessen RA, Marsh RF. 1992. Biochemical and physical properties of the prion protein from two strains of the transmissible mink encephalopathy agent. J Virol 66:2096–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Capobianco R, Casalone C, Suardi S, Mangieri M, Miccolo C, Limido L, Catania M, Rossi G, Di Fede G, Giaccone G, Bruzzone MG, Minati L, Corona C, Acutis P, Gelmetti D, Lombardi G, Groschup MH, Buschmann A, Zanusso G, Monaco S, Caramelli M, Tagliavini F. 2007. Conversion of the BASE prion strain into the BSE strain: the origin of BSE? PLoS Pathog 3:e31. doi: 10.1371/journal.ppat.0030031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Griffiths PC, Spiropoulos J, Lockey R, Tout AC, Jayasena D, Plater JM, Chave A, Green RB, Simonini S, Thorne L, Dexter I, Balkema-Buschmann A, Groschup MH, Beringue V, Le Dur A, Laude H, Hope J. 2010. Characterization of atypical scrapie cases from Great Britain in transgenic ovine PrP mice. J Gen Virol 91:2132–2138. doi: 10.1099/vir.0.018986-0. [DOI] [PubMed] [Google Scholar]
- 32.Dickinson AG, Fraser H. 1979. An assessment of the genetics of scrapie in sheep and mice, p 367–385. In Prusiner SB, Hadlow WJ (ed), Slow transmissible diseases of the nervous system, vol 1 Academic Press, New York, NY. [Google Scholar]
- 33.Le Dur A, Beringue V, Andreoletti O, Reine F, Lai TL, Baron T, Bratberg B, Vilotte JL, Sarradin P, Benestad SL, Laude H. 2005. A newly identified type of scrapie agent can naturally infect sheep with resistant PrP genotypes. Proc Natl Acad Sci U S A 102:16031–16036. doi: 10.1073/pnas.0502296102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moum T, Olsaker I, Hopp P, Moldal T, Valheim M, Moum T, Benestad SL. 2005. Polymorphisms at codons 141 and 154 in the ovine prion protein gene are associated with scrapie Nor98 cases. J Gen Virol 86:231–235. doi: 10.1099/vir.0.80437-0. [DOI] [PubMed] [Google Scholar]
- 35.Saunders GC, Cawthraw S, Mountjoy SJ, Hope J, Windl O. 2006. PrP genotypes of atypical scrapie cases in Great Britain. J Gen Virol 87:3141–3149. doi: 10.1099/vir.0.81779-0. [DOI] [PubMed] [Google Scholar]
- 36.Gotte DR, Benestad SL, Laude H, Zurbriggen A, Oevermann A, Seuberlich T. 2011. Atypical scrapie isolates involve a uniform prion species with a complex molecular signature. PLoS One 6:e27510. doi: 10.1371/journal.pone.0027510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruce ME, Will RG, Ironside JW, McConnell I, Drummond D, Suttie A, McCardle L, Chree A, Hope J, Birkett C, Cousens S, Fraser H, Bostock CJ. 1997. Transmissions to mice indicate that ‘new variant’ CJD is caused by the BSE agent. Nature 389:498–501. doi: 10.1038/39057. [DOI] [PubMed] [Google Scholar]
- 38.Ironside JW, Bishop MT, Connolly K, Hegazy D, Lowrie S, Le Grice M, Ritchie DL, McCardle LM, Hilton DA. 2006. Variant Creutzfeldt-Jakob disease: prion protein genotype analysis of positive appendix tissue samples from a retrospective prevalence study. BMJ 332:1186–1188. doi: 10.1136/bmj.38804.511644.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mead S, Joiner S, Desbruslais M, Beck JA, O'Donoghue M, Lantos P, Wadsworth JD, Collinge J. 2007. Creutzfeldt-Jakob disease, prion protein gene codon 129VV, and a novel PrPSc type in a young British woman. Arch Neurol 64:1780–1784. doi: 10.1001/archneur.64.12.1780. [DOI] [PubMed] [Google Scholar]
- 40.Kaski D, Mead S, Hyare H, Cooper S, Jampana R, Overell J, Knight R, Collinge J, Rudge P. 2009. Variant CJD in an individual heterozygous for PRNP codon 129. Lancet 374:2128. doi: 10.1016/S0140-6736(09)61568-3. [DOI] [PubMed] [Google Scholar]
- 41.Asante EA, Smidak M, Grimshaw A, Houghton R, Tomlinson A, Jeelani A, Jakubcova T, Hamdan S, Richard-Londt A, Linehan JM, Brandner S, Alpers M, Whitfield J, Mead S, Wadsworth JD, Collinge J. 2015. A naturally occurring variant of the human prion protein completely prevents prion disease. Nature 522:478–481. doi: 10.1038/nature14510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson C, Johnson J, Clayton M, McKenzie D, Aiken J. 2003. Prion protein gene heterogeneity in free-ranging white-tailed deer within the chronic wasting disease affected region of Wisconsin. J Wildl Dis 39:576–581. doi: 10.7589/0090-3558-39.3.576. [DOI] [PubMed] [Google Scholar]
- 43.O'Rourke KI, Spraker TR, Hamburg LK, Besser TE, Brayton KA, Knowles DP. 2004. Polymorphisms in the prion precursor functional gene but not the pseudogene are associated with susceptibility to chronic wasting disease in white-tailed deer. J Gen Virol 85:1339–1346. doi: 10.1099/vir.0.79785-0. [DOI] [PubMed] [Google Scholar]
- 44.Johnson C, Johnson J, Vanderloo JP, Keane D, Aiken JM, McKenzie D. 2006. Prion protein polymorphisms in white-tailed deer influence susceptibility to chronic wasting disease. J Gen Virol 87:2109–2114. doi: 10.1099/vir.0.81615-0. [DOI] [PubMed] [Google Scholar]
- 45.Johnson CJ, Herbst A, Duque-Velasquez C, Vanderloo JP, Bochsler P, Chappell R, McKenzie D. 2011. Prion protein polymorphisms affect chronic wasting disease progression. PLoS One 6:e17450. doi: 10.1371/journal.pone.0017450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller MW, Wolfe LL, Sirochman TM, Sirochman MA, Jewell JE, Williams ES. 2012. Survival patterns in white-tailed and mule deer after oral inoculation with a standardized, conspecific prion dose. J Wildl Dis 48:526–529. doi: 10.7589/0090-3558-48.2.526. [DOI] [PubMed] [Google Scholar]
- 47.Meade-White K, Race B, Trifilo M, Bossers A, Favara C, Lacasse R, Miller M, Williams E, Oldstone M, Race R, Chesebro B. 2007. Resistance to chronic wasting disease in transgenic mice expressing a naturally occurring allelic variant of deer prion protein. J Virol 81:4533–4539. doi: 10.1128/JVI.02762-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Race B, Meade-White K, Miller MW, Fox KA, Chesebro B. 2011. In vivo comparison of chronic wasting disease infectivity from deer with variation at prion protein residue 96. J Virol 85:9235–9238. doi: 10.1128/JVI.00790-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Franklin K, Paxinos G. 2007. The mouse brain in stereotaxic coordinates, 3rd ed. Academic Press, New York, NY. [Google Scholar]
- 50.Fraser H, Dickinson AG. 1968. The sequential development of the brain lesion of scrapie in three strains of mice. J Comp Pathol 78:301–311. doi: 10.1016/0021-9975(68)90006-6. [DOI] [PubMed] [Google Scholar]
- 51.Parchi P, Capellari S, Chen SG, Petersen RB, Gambetti P, Kopp N, Brown P, Kitamoto T, Tateishi J, Giese A, Kretzschmar H. 1997. Typing prion isoforms. Nature 386:232–234. doi: 10.1038/386232a0. [DOI] [PubMed] [Google Scholar]
- 52.Hill AF, Joiner S, Wadsworth JD, Sidle KC, Bell JE, Budka H, Ironside JW, Collinge J. 2003. Molecular classification of sporadic Creutzfeldt-Jakob disease. Brain 126:1333–1346. doi: 10.1093/brain/awg125. [DOI] [PubMed] [Google Scholar]
- 53.Safar J, Wille H, Itri V, Groth D, Serban H, Torchia M, Cohen FE, Prusiner SB. 1998. Eight prion strains have PrP(Sc) molecules with different conformations. Nat Med 4:1157–1165. doi: 10.1038/2654. [DOI] [PubMed] [Google Scholar]
- 54.Angers RC, Kang HE, Napier D, Browning S, Seward T, Mathiason C, Balachandran A, McKenzie D, Castilla J, Soto C, Jewell J, Graham C, Hoover EA, Telling GC. 2010. Prion strain mutation determined by prion protein conformational compatibility and primary structure. Science 328:1154–1158. doi: 10.1126/science.1187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Angers R, Christiansen J, Nalls AV, Kang HE, Hunter N, Hoover E, Mathiason CK, Sheetz M, Telling GC. 2014. Structural effects of PrP polymorphisms on intra- and interspecies prion transmission. Proc Natl Acad Sci U S A 111:11169–11174. doi: 10.1073/pnas.1404739111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bartz JC, Bessen RA, McKenzie D, Marsh RF, Aiken JM. 2000. Adaptation and selection of prion protein strain conformations following interspecies transmission of transmissible mink encephalopathy. J Virol 74:5542–5547. doi: 10.1128/JVI.74.12.5542-5547.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bartz JC, Aiken JM, Bessen RA. 2004. Delay in onset of prion disease for the HY strain of transmissible mink encephalopathy as a result of prior peripheral inoculation with the replication-deficient DY strain. J Gen Virol 85:265–273. doi: 10.1099/vir.0.19394-0. [DOI] [PubMed] [Google Scholar]
- 58.Bartz JC, Kramer ML, Sheehan MH, Hutter JA, Ayers JI, Bessen RA, Kincaid AE. 2007. Prion interference is due to a reduction in strain-specific PrPSc levels. J Virol 81:689–697. doi: 10.1128/JVI.01751-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shikiya RA, Ayers JI, Schutt CR, Kincaid AE, Bartz JC. 2010. Coinfecting prion strains compete for a limiting cellular resource. J Virol 84:5706–5714. doi: 10.1128/JVI.00243-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Westaway D, Goodman PA, Mirenda CA, McKinley MP, Carlson GA, Prusiner SB. 1987. Distinct prion proteins in short and long scrapie incubation period mice. Cell 51:651–662. doi: 10.1016/0092-8674(87)90134-6. [DOI] [PubMed] [Google Scholar]
- 61.Thackray AM, Hopkins L, Lockey R, Spiropoulos J, Bujdoso R. 2011. Emergence of multiple prion strains from single isolates of ovine scrapie. J Gen Virol 92:1482–1491. doi: 10.1099/vir.0.028886-0. [DOI] [PubMed] [Google Scholar]
- 62.Thackray AM, Lockey R, Beck KE, Spiropoulos J, Bujdoso R. 2012. Evidence for co-infection of ovine prion strains in classical scrapie isolates. J Comp Pathol 147:316–329. doi: 10.1016/j.jcpa.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 63.Mazza M, Iulini B, Vaccari G, Acutis PL, Martucci F, Esposito E, Peletto S, Barocci S, Chiappini B, Corona C, Barbieri I, Caramelli M, Agrimi U, Casalone C, Nonno R. 2010. Co-existence of classical scrapie and Nor98 in a sheep from an Italian outbreak. Res Vet Sci 88:478–485. doi: 10.1016/j.rvsc.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 64.Robinson SJ, Samuel MD, Johnson CJ, Adams M, McKenzie DI. 2012. Emerging prion disease drives host selection in a wildlife population. Ecol Appl 22:1050–1059. doi: 10.1890/11-0907.1. [DOI] [PubMed] [Google Scholar]
- 65.Robinson SJ, Samuel MD, O'Rourke KI, Johnson CJ. 2012. The role of genetics in chronic wasting disease of North American cervids. Prion 6:153–162. doi: 10.4161/pri.19640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hamir AN, Kunkle RA, Miller JM, Bartz JC, Richt JA. 2006. First and second cattle passage of transmissible mink encephalopathy by intracerebral inoculation. Vet Pathol 43:118–126. doi: 10.1354/vp.43-2-118. [DOI] [PubMed] [Google Scholar]
- 67.Hamir AN, Kunkle RA, Miller JM, Greenlee JJ, Richt JA. 2006. Experimental second passage of chronic wasting disease (CWD(mule deer)) agent to cattle. J Comp Pathol 134:63–69. doi: 10.1016/j.jcpa.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 68.Heisey DM, Mickelsen NA, Schneider JR, Johnson CJ, Johnson CJ, Langenberg JA, Bochsler PN, Keane DP, Barr DJ. 2010. Chronic wasting disease (CWD) susceptibility of several North American rodents that are sympatric with cervid CWD epidemics. J Virol 84:210–215. doi: 10.1128/JVI.00560-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mathiason CK, Nalls AV, Seelig DM, Kraft SL, Carnes K, Anderson KR, Hayes-Klug J, Hoover EA. 2013. Susceptibility of domestic cats to chronic wasting disease. J Virol 87:1947–1956. doi: 10.1128/JVI.02592-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kong Q, Huang S, Zou W, Vanegas D, Wang M, Wu D, Yuan J, Zheng M, Bai H, Deng H, Chen K, Jenny AL, O'Rourke K, Belay ED, Schonberger LB, Petersen RB, Sy MS, Chen SG, Gambetti P. 2005. Chronic wasting disease of elk: transmissibility to humans examined by transgenic mouse models. J Neurosci 25:7944–7949. doi: 10.1523/JNEUROSCI.2467-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marsh RF, Kincaid AE, Bessen RA, Bartz JC. 2005. Interspecies transmission of chronic wasting disease prions to squirrel monkeys (Saimiri sciureus). J Virol 79:13794–13796. doi: 10.1128/JVI.79.21.13794-13796.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Race B, Meade-White KD, Miller MW, Barbian KD, Rubenstein R, LaFauci G, Cervenakova L, Favara C, Gardner D, Long D, Parnell M, Striebel J, Priola SA, Ward A, Williams ES, Race R, Chesebro B. 2009. Susceptibilities of nonhuman primates to chronic wasting disease. Emerg Infect Dis 15:1366–1376. doi: 10.3201/eid1509.090253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Race B, Meade-White KD, Phillips K, Striebel J, Race R, Chesebro B. 2014. Chronic wasting disease agents in nonhuman primates. Emerg Infect Dis 20:833–837. doi: 10.3201/eid2005.130778. [DOI] [PMC free article] [PubMed] [Google Scholar]