Abstract
CGA-N46 is a novel antifungal peptide derived from the N-terminus of human Chromogranin A, corresponding to the 31st to 76th amino acids. Further research on its activities and characteristics may be helpful for the application of CGA-N46 in medical or other situations. In the present study, the antifungal spectrum and physicochemical characteristics of CGA-N46 were investigated using an antifungal assay, its antiproliferative effects on cancer and normal cells were assessed using MTT assay and its combinatorial effect with other antibiotics was analyzed using checkerboard analysis. The results showed that CGA-N46 exhibited antifungal activity against the tested Candidas (C. glabrata, C. parapsilosis, C. krusei, C. tropicalis and C. albicans) at a concentration of <0.8 mM, but had no effect on the growth of filamentous fungi or other types of fungi (Cryptococcus neoformans, Aspergillus fumigatus, Aspergillus flavus, Aspergillus niger, Fusarium moniliforme, Microsporum canis, Microsporum gypseum, Trichophyton rubrum and Trichophyton mentagrophytes), even at a concentration of 3.2 mM. CGA-N46 had an inhibitory effect on the proliferation of lung cancer A549 cells and a reversible effect on the growth of normal primary chicken embryo fibroblast cells, but no hemolytic activity on human erythrocytes at the minimum inhibitory concentration of CGA-N46 against yeasts. The antifungal activity of CGA-N46 was stable at a temperature <40°C or within a broad pH range (pH 5.0–7.0). Its antifungal activity was enhanced when the peptide was used in combination with fluconazole and terbinafine. The present results indicate that CGA-N46 is a safe, physicochemically stable, antifungal peptide with anticancer cell activity that exhibits an additive effect with conventional antibiotics.
Keywords: Chromogranin A, CGA-N46, antifungal peptide, anti-proliferation, physicochemical stability, additive combination
Introduction
Fungal infections have emerged worldwide due to an increasing number of immunocompromised patients, including patients with cancer, acquired immune deficiency syndrome, solid-organ and hematopoietic stem cell transplant recipients, premature neonates and patients recovering from major surgery. Nosocomial bloodstream fungal infection remains serious among hospitalized patients (1–3). Invasive Candida are responsible for the highest mortality rates (1,4,5). Candida albicans is the major fungus causing mucosal and invasive infections in humans (3,6); however, an increasing number of infections caused by non-albicans Candida has also been reported (7–9). Azole drugs are commonly used to treat infections caused by Candidas; however, a number of reports have indicated that Candidas are exhibiting reduced susceptibility to azole drugs (1,3,10); certain Candida species are even intrinsically resistant to them (3,11). A search for novel antifungal agents has therefore been undertaken for candidiasis.
Antimicrobial peptides (AMPs) are important components of innate immunity for humans and several other forms of life. The most significant characteristic of AMPs is their broad-spectrum antimicrobial activity against bacteria, viruses and fungi, including multi-drug-resistant microbes (12–14). This antimicrobial activity is not associated with the rapid emergence of resistance (13,15,16), which has become a serious problem for conventional antibiotics. Furthermore, unlike traditional antibiotics that inhibit specific biosynthetic pathways, AMPs are multi-target agents. Studies into AMPs may provide an insight into the innate immunity of invertebrates and produce templates for designing novel, broad-spectrum antibiotics that would function in humans (13); therefore, AMPs have emerged as the most promising group of candidates for the development of a new class of antibiotics (13,15).
AMPs are currently the focus of extensive research in order to assess their possible use as a new class of antibiotics. Possible problems in the stability, delivery and pathogen-targeting may arise in the use of AMPs as antimicrobial agents. To improve the application of such peptides, combinations with antibiotics or other compounds are generally used to increase the in vivo activity of AMPs (17).
Chromogranin A (CGA) is a protein expressed in all types of neurons (18). A series of studies on the antimicrobial function of the CGA-derived peptides has been conducted (19–22). Catestatin (derived from the C-terminus of bovine CGA) is expressed in murine skin in response to injury and infection, and it has a potent antimicrobial activity against bacteria, fungi and yeast. Similar activity has also been reported for vasostatin-I (CGA1–76; corresponding to CGA residues 1–76), which can trigger the selective migration of human monocytes and eosinophils. CGA1–76 and catestatin have been described to be innate immunity components of mammals (19,21). A study by Lugardon et al (19) showed that the C-terminal moiety of bovine CGA1–76 exhibited a potent antifungal activity and that the disulfide-bridge loop Cys17-Cys38 was crucial for its antibacterial activity, but not for the antifungal activity. In order to investigate antifungal peptides derived from human CGA, we have studied several recombinant peptides derived from the N-terminus of human CGA: CGA18–76, CGA18–66 and CGA31–76 (corresponding to human CGA residues 18–76, 18–66 and 31–76, respectively). This ongoing research has shown that CGA-N46, a derived peptide containing amino acids 31–76 of the N-terminus of human CGA, has antagonistic activity against Candida albicans (23).
AMPs have a broad spectrum of activity and can act as antibacterial, antifungal, antiviral and sometimes even as anticancer peptides. Extensive research has been performed in the field of antibacterial peptides, describing their identification, characterization and mechanism of action (13,14,24,25). In order to further our ongoing search for novel antimicrobial agents that could be used in drug therapy, recombinant CGA-N46 was expressed and purified in the present study, and the activities of CGA-N46 against selected fungi, cancer cells, human erythrocytes and normal fibroblast cells, as well as the stability of CGA-N46 and its effect in combination with conventional antibiotics, were investigated.
Materials and methods
Reagents
MTT, Triton X-100, dimethylsulfoxide (DMSO), fluconazole and terbinafine were purchased from Sigma-Aldrich (Shanghai, China). All of the solvents used were of high-performance liquid chromatography grade. The water used for all experiments was supplied by a Milli-Q® water purification system from Millipore (Beijing, China).
Culture and suspensions of fungal strains
The fungal strains Candida glabrata, Candida parapsilosis, Candida krusei, Candida tropicalis, Candida albicans, Cryptococcus neoformans, Aspergillus fumigatus, Aspergillus flavus, Aspergillus niger, Fusarium moniliforme, Microsporum canis, Microsporum gypseum, Trichophyton rubrum and Trichophyton mentagrophytes were supplied by the China Academy of Chinese Medical Sciences (Beijing, China). For each experiment, strains were sub-cultured onto Sabouraud dextrose (SD) agar (Oxoid Ltd., Basingstoke, UK) at 35°C for between 48 h and 7 days, depending on the species. For yeasts, the inoculum suspension was prepared by selecting 5 colonies measuring ≥1 mm in diameter and suspending the material in 5 ml sterile 0.85% NaCl. The working suspension (1–5×103 CFU/ml) was established by a 1:10 dilution with SD broth (Oxoid Ltd.). For filamentous fungi, colonies were covered with ~1 ml sterile 0.85% saline, and the suspensions were established by gently probing the colonies with the tip of a Pasteur pipette. The resulting mixture of conidia or sporangiospores and hyphal fragments was withdrawn and transferred to a sterile tube. Heavy particles were allowed to settle for 3–5 min, and the upper homogeneous suspension was then collected and mixed. The density of the suspension was measured using a microscope. The suspension was adjusted to 0.8–10×104 CFU/ml by diluting with SD broth.
Cell line culture
A549 lung cancer cells (American Type Culture Collection, Manassas, VA, USA) were maintained at the Henan University of Technology (Zhengzhou, China) in RPMI-1640 medium (Gibco-BRL, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Hyclone, Rockford, IL, USA), with 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C in a humidified atmosphere containing 5% CO2. The normal primary chicken embryo fibroblasts (CEFs) from specific pathogen-free chickens (Experimental Animal Center, Jenan University of Technology, Zhengzhou, China) were isolated using a standard protocol described elsewhere (26). Fibroblasts were grown as a monolayer in Dulbecco's modified Eagle's medium (Gibco-BRL) containing 10% fetal bovine serum (Hyclone).
Antifungal assay
Minimum inhibitory concentrations (MICs) of CGA-N46 against the fungi were measured according to a modified version of the broth microdilution method of the Clinical and Laboratory Standards Institute (CLSI) (27). Briefly, CGA-N46 was serially diluted to concentrations of between 0.62 µM and 3.2 mM in 20 mM phosphate-buffered saline (PBS) (pH 6.0), and 100-µl samples were dispensed into the wells of a 96-well, U-shaped plate. Each sample was mixed with 100 µl 2X inoculum suspension (yeast, 1–5×103 CFU/ml; conidia or sporangiospores, 0.8–10×104 CFU/ml) of a log-phase fungal culture in SD broth. The cultures were incubated at 30°C without agitation: C. glabrata, C. parapsilosis, C. krusei, C. tropicalis and C. albicans were incubated for 48 h; C. neoformans, A. fumigatus, A. flavus, A. niger and F. moniliforme were incubated for 72 h; M. canis, M. gypseum, T. rubrum and T. mentagrophytes were incubated for 96 h. The MIC was defined as the lowest peptide concentration that completely inhibited fungal growth. Cultures without CGA-N46 or SD broth were employed as positive and negative controls, respectively. Three replicates were set for each experiment.
Hemolysis assay
The hemolytic activity of CGA-N46 was determined by measuring the amount of hemoglobin released by the lysis of human erythrocytes using a modified version of the method described by Huang et al (28). Briefly, CGA-N46 was serially diluted using PBS in 96-well plates to give 100 µl sample solution in each well. Human erythrocytes that had undergone anticoagulation using EDTAK2 (Sigma-Aldrich) were collected by centrifugation at 1,000 × g for 5 min at 4°C, washed twice with PBS and then diluted to a concentration of 2% in PBS. The erythrocytes (100 µl of 2% solution) were added to each well to achieve a final concentration of 1% human erythrocytes per well, and the reactions were incubated at 37°C for 30 min. Following incubation, the plates were centrifuged for 10 min and 100 µl supernatant was transferred to a new 96-well, U-shaped plate. Hemoglobin release was determined by measuring the absorbance of the supernatant at a wavelength of 570 nm. Peptide samples were diluted in a 2-fold series to determine the minimum concentration at which hemolysis occurred. Erythrocytes in PBS and 1% (v/v) Triton-X100 were used as the negative and positive controls, respectively. The hemolysis of erythrocytes was calculated using the following formula: Erythrocyte hemolysis = (Asample - Anegative)/(Apositive - Anegative) × 100%.
MTT assay
Cell viability was assessed using the MTT assay, based on the reduction of MTT into formazan dye by active mitochondria (24). Briefly, 50 µl cell suspension (1×105 CFU/ml) was seeded in 96-well microtiter plates. Following the attachment of the cells, various concentrations of CGA-N46 (5 µl) were added to each well to give final concentrations of between 0.1 and 1.6 mM for incubation at 37°C in 5% CO2 for 24, 48 and 72 h. A total of 10 µl MTT solution (5 mg/ml MTT in PBS) was then added to each well and incubated for an additional 4 h. After rinsing, 100 µl DMSO was added to dissolve the MTT formazan crystals. The absorbance was read using an ELISA reader (BioTek Instruments, Inc., Winooski, VT, USA) at 490 nm. Cultures without CGA-N46 or SD broth were appointed as the control and blank groups, respectively. The relative cellular activity was calculated according to the following equation: Cell survival (% of control) = (Atest - Ablank)/(Acontrol - Ablank) × 100. Each experiment was repeated in triplicate.
Physicochemical stability analysis
The physicochemical stability of CGA-N46 at different temperatures and pHs was assessed. CGA-N46 was dissolved in 20 mM PBS (pH 6.0) to form a solution (6.4 mM). The heat stability of CGA-N46 was assessed by incubating the CGA-N46 solution at different temperatures (40–100°C) for 30 min and then comparing the MICs of the heat-treated CGA-N46 solutions with the MIC of the control. To determine the pH-stability of CGA-N46, the pH of the CGA-N46 solutions was adjusted to 1.0–12.0 with 0.1 M HCl or 1 M NaOH, and the solutions were maintained at room temperature. After 60 min, the pH was adjusted back to 6.0 in order to assess the antifungal activity against C. krusei. The MIC of CGA-N46 was analyzed by the broth microdilution method of the CLSI (27). A freshly prepared CGA-N46 solution in 20 mM PBS (pH 6.0) was used as control.
Checkerboard analysis
The combination effect of CGA-N46 with conventional antibiotics was analyzed using the checkerboard method described by Su et al (29), with slight modification. Briefly, an inoculum of logarithmic-phase C. krusei cells (1×103 CFU/ml) was cultured at 30°C for 20 h in each well of a 96-well, U-shaped culture plate containing 100 µl SD broth with CGA-N46 (3.2 mM to 0.62 µM) or an antibiotic (10 µM to 0.1 nM) added, alone or in combination, at a specified concentration. The lowest concentration of each drug combination causing growth inhibition was plotted on an arithmetic scale and any synergy or additive effect was identified from the shape of the curve and the fractional inhibitory concentration (FIC) index. The FIC index was calculated as follows: FIC index = (A/MICA) + (B/MICB), where MICA and MICB are the MICs of drugs A and B alone, respectively, and A and B are the MICs of drugs A and B when used in combination, respectively (30). Drug interactions are usually classified as synergistic, additive or antagonistic on the basis of the FIC index and are defined as follows: Synergy, ≤0.5; additive effect, 0.5–1.0; indifference (or no effect), 1.0–2.0; antagonism, >2.0 (29).
Statistical analysis
Experiments were performed three times, and experimental data were analyzed using one-way analysis of variance followed by Least Significant Difference and Duncan's tests using PASW statistical software, version 18 (SPSS, Inc., Chicago, IL, USA). The results are presented as the mean ± standard error of the mean. P<0.01 was considered to indicate a statistically significant difference.
Results
Antifungal activity
The antifungal activity spectrum of CGA-N46 was indicated using the MICs of CGA-N46 against the tested fungi and yeasts. As shown in Table I, CGA-N46 was active against yeasts (MICs of 0.1–0.8 mM), but had no effect on the growth of filamentous fungi, even with peptide concentrations up to 3.2 mM. The highest activity was found against C. krusei, with an MIC of 0.1 mM.
Table I.
Antifungal spectrum and MICs of CGA-N46.
| Strains | MICs (mM) |
|---|---|
| Candida glabrata | 0.8 |
| Candida parapsilosis | 0.8 |
| Candida krusei | 0.1 |
| Candida tropicalis | 0.2 |
| Candida albicans | 0.2 |
| Cryptococcus neoformans | ND |
| Aspergillus fumigatus | ND |
| Aspergillus flavus | ND |
| Aspergillus niger | ND |
| Fusarium moniliforme | ND |
| Microsporum canis | ND |
| Microsporum gypseum | ND |
| Trichophyton rubrum | ND |
| Trichophyton mentagrophytes | ND |
ND indicates that no inhibitory activity was detected, even at a concentration of 3.2 mM. MIC, minimum inhibitory concentration.
Hemolytic activity of CGA-N46
The hemolytic activity of CGA-N46 against the highly sensitive human erythrocytes was determined as a measure of its toxicity to mammalian cells. The release of hemoglobin was monitored by measuring the absorbance at 570 nm. As negative and positive controls, erythrocytes in PBS without CGA-N46 and 0.1% (v/v) Triton X-100 in PBS were employed, respectively. CGA-N46 only showed weak hemolytic activity at the concentration of 1.6 mM (Fig. 1).
Figure 1.
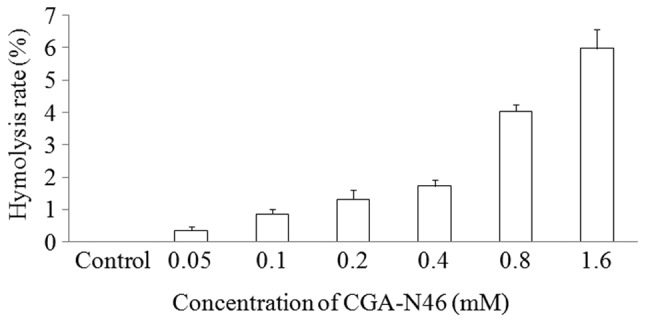
Hemolytic activity of CGA-N46. The minimal hemolytic concentration was determined against human red blood cells. Values are presented as the mean ± standard error of at least three independent experiments.
Effect of CGA-N46 on the growth of primary CEF cells
The antiproliferative activity of CGA-N46 against normal cells was assessed by investigating the effect on normal primary CEF cells using the MTT colorimetric assay. As shown in Fig. 2, CGA-N46 promoted the growth of the cells up to 24 h of incubation, and the promoting effect decreased as the concentration increased >0.2 mM. When the incubation time was extended to 48 h and beyond, CGA-N46 showed an inhibitory activity on cell growth, and the inhibitory activity decreased with the increasing concentration. Compared with the control, the effect of CGA-N46 on normal primary CEF cells was reversible at concentrations <1.6 mM, and the growth promotion and inhibition were not observed at the concentration of 1.6 mM.
Figure 2.
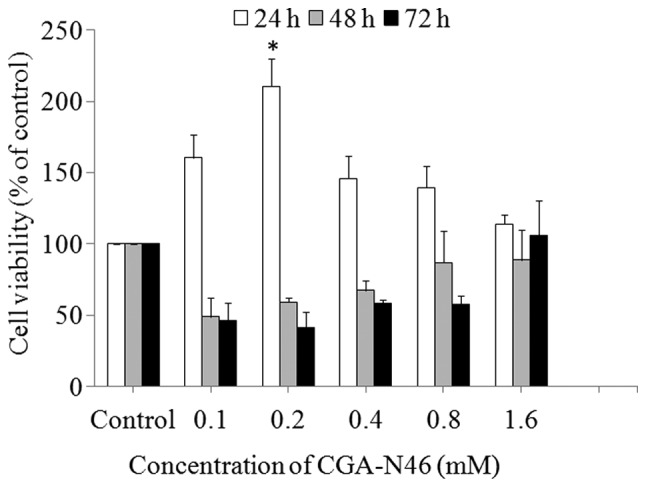
Effect of CGA-N46 on the growth of normal primary CEF cells. The proliferative activity of primary CEF cells was assessed using MTT assay. Values are presented as the mean ± standard error of at least three independent experiments. *P<0.01 compared with control (one-way analysis of variance and subsequent Least Significant Difference and Duncan's tests). CEF, chicken embryo fibroblast.
Effect of CGA-N46 on cancer cell proliferation
The antiproliferative activity of CGA-N46 against cancer cells was investigated using an A549 human lung cancer cell line. A549 cells were treated with different concentrations of CGA-N46 (0.62 µM to 3.2 mM) for 72 h, and 20 mM PBS (pH 6.0) was used as a negative control. MTT was used to assess the effect of CGA-N46 on A549 cell viability. As seen in Fig. 3, the number of metabolically active cells decreased significantly as the concentration of CGA-N46 increased. The proliferation of A549 cells was inhibited by CGA-N46 in a dose-dependent manner.
Figure 3.
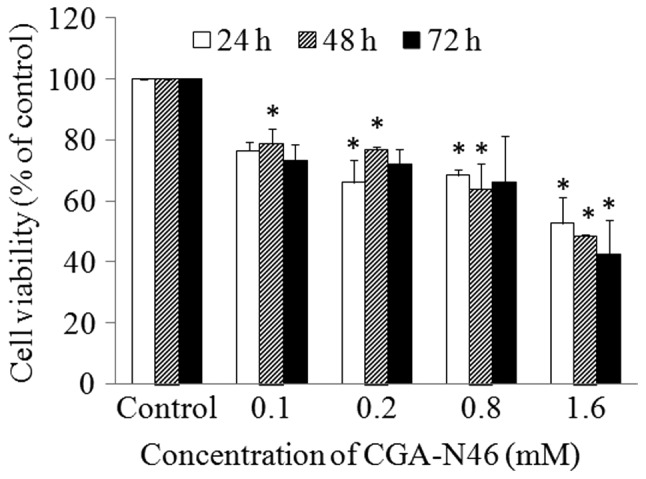
Effect of CGA-N46 on the growth of lung cancer cells. The proliferative activity of A549 cells was assessed using MTT assay. Values are presented as the mean ± standard error of at least three independent experiments. *P<0.01 compared with control (one-way analysis of variance and subsequent Least Significant Difference and Duncan's tests).
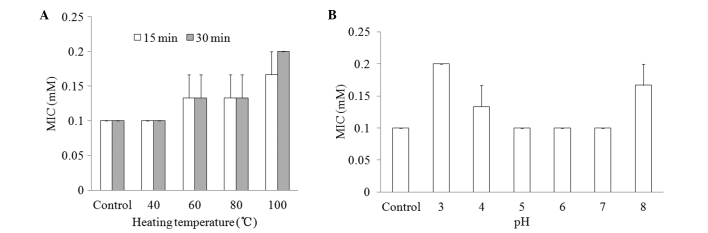
Physicochemical properties
To determine the effect of the physicochemical factors heat and pH on the antifungal activity of CGA-N46, the MIC values of CGA-N46 against C. krusei were examined and compared following the treatment of the CGA-N46 with different temperatures and pHs. As shown in Fig. 4, the MICs remained at 0.1 mM at a temperature <40°C or within a broad pH range (5.0–7.0).
Figure 4.
Effect of physicochemical factors on the stability of the antifungal activity of CGA-N46 against C. krusei. (A) The heat stability of CGA-N46 was assessed by comparing the MICs of CGA-N46 following treatment at different temperatures for 30 min. (B) The pH stability of CGA-N46 was indicated by the MICs of CGA-N46 following treatment in different pH environments. MIC, minimum inhibitory concentration.
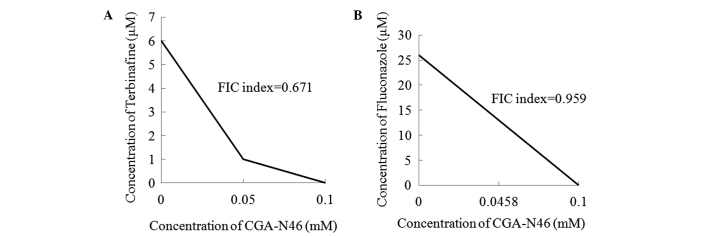
Combination effect of CGA-N46 and antibiotics
The combination effects of CGA-N46 with terbinafine and fluconazole against C. krusei are shown in Fig. 5. The MICs of terbinafine, fluconazole and CGA-N46 alone were 6 µM, 26 µM and 0.1 mM, respectively. When terbinafine was combined with CGA-N46, the MICs were 1 µM and 0.05 mM, respectively. When fluconazole and CGA-N46 were used in combination, the MICs were 13 µM and 0.0458 mM, respectively. The antifungal activities of the conventional antibiotics and CGA-N46 were increased when the agents were applied in combination. The FIC indices of CGA-N46 in combination with terbinafine and fluconazole, respectively, were 0.671 and 0.959, showing that the antifungal activities of the combinations against C. krusei were additive (FIC index 0.5–1.0).
Figure 5.
Checkerboard analysis of the activity of CGA-N46 plus antibiotics against C. krusei. The lowest concentration of each drug combination with growth inhibitory activity (minimum inhibitory concentration) is shown for (A) CGA-N46 combined with terbinafine and (B) CGA-N46 combined with fluconazole. An FIC index of >0.5 and ≤1 denotes an additive combination. FIC, fractional inhibitory concentration.
Discussion
The majority of AMPs are alkaline cationic peptides (25,31). It has been postulated that their mechanism of action against microbes involves compromising the membrane of the target organism (14,31). It is generally accepted that the positive charge allows the electrostatic attraction of AMPs with the phospholipid membranes of microbes; therefore, environmental pH has an effect on the charges of the alkaline cationic peptides. The acidic metabolites produced by fungi in the local microenvironment of the human body may neutralize the alkaline cationic peptides, and thus impede the electrostatic attraction of the AMPs with fungal outer phospholipid membranes (32). CGA-N46 has almost zero charge at physiological pH due to its calculated pI of 7.38; therefore, it has a broad spectrum of pH stability. CGA-N46 may be a good model for researching the mechanisms of non-cationic AMPs.
CGA-N46 exerted an antiproliferative effect on Candida spp. and A549 cancer cells in a concentration- and time-dependent manner. It had no hemolytic activity at the MIC of CGA-N46 against yeasts. Furthermore, the results showed that CGA-N46 had no significant effect on normal primary CEF cells at a concentration of 1.6 mM and a reversible effect at concentrations <1.6 mM. This observation was consistent with that for Magainin II (33). CGA-N46 therefore represents a member of a novel, non-cationic, α-helical peptide family with antimicrobial and anticancer activities but without toxicity to erythrocytes, indicating the possibility for use in treatment for infection and cancer.
AMPs have different mechanisms of action from conventional antibiotics (14,15). Previous studies have indicated that the limitations of AMPs, which are long amino acid sequences, are poor bioavailability and susceptibility to protease degradation and pathogen targeting (14,17). To overcome these limitations, combination with conventional antibiotics or other compounds is generally used. Combined with minocycline or azole antifungal agents, lactoferricin has synergistic effects against an antibiotic-resistant strain of Staphylococcus aureus (34). The bactericidal effect was enhanced when human β-defensin was used in combination with the antimicrobial agents (17). Fluconazole and terbinafine are two antibiotics to which Candidas are prone to be resistant (35). In the present study, CGA-N46 was combined with these two drugs. In the checkerboard assay, the combination of CGA-N46 with each of the drugs exhibited enhanced antifungal activity. The combinations of CGA-N46 with terbinafine and fluconazole demonstrated additive effects against C. krusei. The results of this study may prove in the clinical application of AMPs in combination with other compounds.
Acknowledgements
This study was supported by the National Science Foundation of China (grant nos. 31071922, 31271948 and 21306040) and Henan University of Technology (no. 11JCYJ10). The authors would like to express their appreciation to Dr Jin Huang of Henan University of Technology for performing the SPSS analysis.
References
- 1.Bassetti M, Taramasso L, Nicco E, Molinari MP, Mussap M, Viscoli C. Epidemiology, species distribution, antifungal susceptibility and outcome of nosocomial candidemia in a tertiary care hospital in Italy. PLoS One. 2011;6:e24198. doi: 10.1371/journal.pone.0024198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ortega M, Marco F, Soriano A, Almela M, Martínez JA, López J, Pitart C, Mensa J. Candida species bloodstream infection: Epidemiology and outcome in a single institution from 1991 to 2008. J Hosp Infect. 2011;77:157–161. doi: 10.1016/j.jhin.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 3.Scorzoni L, de Lucas MP, Mesa-Arango AC, Fusco-Almeida AM, Lozano E, Cuenca-Estrella M, Mendes-Giannini MJ, Zaragoza O. Antifungal efficacy during Candida krusei infection in non-conventional models correlates with the yeast in vitro susceptibility profile. PLoS One. 2013;8:e60047. doi: 10.1371/journal.pone.0060047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gudlaugsson O, Gillespie S, Lee K, Van de Berg J, Hu J, Messer S, Herwaldt L, Pfaller M, Diekema D. Attributable mortality of nosocomial candidemia, revisited. Clin Infect Dis. 2003;37:1172–1177. doi: 10.1086/378745. [DOI] [PubMed] [Google Scholar]
- 5.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 6.Pushpanathan M, Rajendhran J, Jayashree S, Sundarakrishnan B, Jayachandran S, Gunasekaran P. Direct cell penetration of the antifungal peptide, MMGP1, in Candida albicans. J Pept Sci. 2012;18:657–660. doi: 10.1002/psc.2445. [DOI] [PubMed] [Google Scholar]
- 7.Trick WE, Fridkin WE, Edwards SK, Hajjeh JR, Gaynes RA, National RP. Nosocomial Infections Surveillance System Hospitals: Secular trend of hospital-acquired candidemia among intensive care unit patients in the United States during 1989–1999. Clin Infect Dis. 2002;35:627–630. doi: 10.1086/342300. [DOI] [PubMed] [Google Scholar]
- 8.Arendrup MC. Epidemiology of invasive candidiasis. Curr Opin Crit Care. 2010;16:445–452. doi: 10.1097/MCC.0b013e32833e84d2. [DOI] [PubMed] [Google Scholar]
- 9.Pemán J, Cantón E, Quindós G, Eraso E, Alcoba J, Guinea J, Merino P, Ruiz-Pérez-de-Pipaon MT, Pérez-del-Molino L, Linares-Sicilia MJ, et al. FUNGEMYCA Study Group; Epidemiology, species distribution and in vitro antifungal susceptibility of fungaemia in a Spanish multicentre prospective survey. J Antimicrob Chemother. 2012;67:1181–1187. doi: 10.1093/jac/dks019. [DOI] [PubMed] [Google Scholar]
- 10.Leroy O, Gangneux JP, Montravers P, Mira JP, Gouin F, Sollet JP, Carlet J, Reynes J, Rosenheim M, Regnier B, Lortholary O. AmarCand Study Group: Epidemiology, management and risk factors for death of invasive Candida infections in critical care: A multicenter, prospective, observational study in France (2005–2006) Crit Care Med. 2009;37:1612–1618. doi: 10.1097/CCM.0b013e31819efac0. [DOI] [PubMed] [Google Scholar]
- 11.Muñoz P, Sánchez-Somolinos M, Alcalá L, Rodríguez-Créixems M, Peláez T, Bouza E. Candida krusei fungaemia: Antifungal susceptibility and clinical presentation of an uncommon entity during 15 years in a single general hospital. J Antimicrob Chemother. 2005;55:188–193. doi: 10.1093/jac/dkh532. [DOI] [PubMed] [Google Scholar]
- 12.Lata S, Mishra N, Raghava G. AntiBP2: Improved version of antibacterial peptide prediction. BMC Bioinformatics. 2010;11(Suppl 1):S19. doi: 10.1186/1471-2105-11-S1-S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu J, Chen ZW. Isolation, characterization and anti-cancer activity of SK84, a novel glycine-rich antimicrobial peptide from Drosophila virilis. Peptides. 2010;31:44–50. doi: 10.1016/j.peptides.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 14.Güell I, Micaló L, Cano L, Badosa E, Ferre R, Montesinos E, Bardají E, Feliu L, Planas M. Peptidotriazoles with antimicrobial activity against bacterial and fungal plant pathogens. Peptides. 2012;33:9–17. doi: 10.1016/j.peptides.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Hancock RE. Peptide antibiotics. Lancet. 1997;349:418–422. doi: 10.1016/S0140-6736(97)80051-7. [DOI] [PubMed] [Google Scholar]
- 16.Foubister V. Superpeptide to treat Candida albicans. Drug Discov Today. 2003;8:380–381. doi: 10.1016/S1359-6446(03)02684-9. [DOI] [PubMed] [Google Scholar]
- 17.Maisetta G, Batoni G, Esin S, Luperini F, Pardini M, Bottai D, Florio W, Giuca MR, Gabriele M, Campa M. Activity of human beta-defensin 3 alone or combined with other antimicrobial agents against oral bacteria. Antimicrob Agents Chemother. 2003;47:3349–3351. doi: 10.1128/AAC.47.10.3349-3351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eiden LE. Is chromogranin a prohormone? Nature. 1987;325:301. doi: 10.1038/325301a0. [DOI] [PubMed] [Google Scholar]
- 19.Lugardon K, Raffner R, Goumon Y, Corti A, Delmas A, Bulet P, Aunis D, Metz-Boutigue MH. Antibacterial and antifungal activities of vasostatin-1, the N-terminal fragment of chromogranin A. J Biol Chem. 2000;275:10745–10753. doi: 10.1074/jbc.275.15.10745. [DOI] [PubMed] [Google Scholar]
- 20.Lugardon K, Chasserot-Golaz S, Kieffer AE, Maget-Dana R, Nullans G, Kieffer B, Aunis D, Metz-Boutigue MH. Structural and biological characterization of chromofungin, the antifungal chromogranin A-(47–66)-derived peptide. J Biol Chem. 2001;276:35875–35882. doi: 10.1074/jbc.M104670200. [DOI] [PubMed] [Google Scholar]
- 21.Briolat J, Wu SD, Mahata SK, Gonthier B, Bagnard D, Chasserot-Golaz S, Helle KB, Aunis D, Metz-Boutigue MH. New antimicrobial activity for the catecholamine release-inhibitory peptide from chromogranin A. Cell Mol Life Sci. 2005;62:377–385. doi: 10.1007/s00018-004-4461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radek KA, Lopez-Garcia B, Hupe M, Niesman IR, Elias PM, Taupenot L, Mahata SK, O'Connor DT, Gallo RL. The neuroendocrine peptide catestatin is a cutaneous antimicrobial and induced in the skin after injury. J Invest Dermatol. 2008;128:1525–1534. doi: 10.1038/sj.jid.5701225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li R, Zhang T, Luo J, Wang F, Gu Q, Gan J, Xiao F. Antifungal activity fragments of N domain of chromogranin A. Zhong Shan Da Xue Xue Bao. 2006;45:64–67. (In Chinese) [Google Scholar]
- 24.Aleinein RA, Hamoud R, Schäfer H, Wink M. Molecular cloning and expression of ranalexin, a bioactive antimicrobial peptide from Rana catesbeiana in Escherichia coli and assessments of its biological activities. Appl Microbiol Biotechnol. 2013;97:3535–3543. doi: 10.1007/s00253-012-4441-1. [DOI] [PubMed] [Google Scholar]
- 25.Mochon AB, Liu H. The antimicrobial peptide histatin-5 causes a spatially restricted disruption on the Candida albicans surface, allowing rapid entry of the peptide into the cytoplasm. PLoS Pathog. 2008;4:e1000190. doi: 10.1371/journal.ppat.1000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kong BW, Lee J, Bottje WG, Lassiter K, Lee J, Gentles LE, Chandra YG, Foster DN. Microarray analysis of early and late passage chicken embryo fibroblast cells. Poult Sci. 2013;92:770–781. doi: 10.3382/ps.2012-02540. [DOI] [PubMed] [Google Scholar]
- 27.National Committee for Clinical Laboratory Standards (NCCLS): Reference method for broth dilution antifungal susceptibility testing of yeasts: Approved standard. 2nd. Wayne, USA: NCCLS; 1997. pp. 1–13. [Google Scholar]
- 28.Huang J, Hao D, Chen Y, Xu Y, Tan J, Huang Y, Li F, Chen Y. Inhibitory effects and mechanisms of physiological conditions on the activity of enantiomeric forms of an α-helical antibacterial peptide against bacteria. Peptides. 2011;32:1488–1495. doi: 10.1016/j.peptides.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 29.Su Y, Ma L, Wen Y, Wang H, Zhang S. Studies of the in vitro antibacterial activities of several polyphenols against clinical isolates of methicillin-resistant Staphylococcus aureus. Molecules. 2014;19:12630–12639. doi: 10.3390/molecules190812630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eliopoulos GM, Moellering RC. Antimicrobial combinations. In: Lorian V, editor. Antibiotics in Laboratory Medicine. Baltimore, MD: Williams and Wilkins; 1991. pp. 432–492. [Google Scholar]
- 31.Hancock RE, Diamond G. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 2000;8:402–410. doi: 10.1016/S0966-842X(00)01823-0. [DOI] [PubMed] [Google Scholar]
- 32.Tlaskalová-Hogenová H, Stepánková R, Hudcovic T, Tucková L, Cukrowska B, Lodinová-Zádníková R, Kozáková H, Rossmann P, Bártová J, Sokol D, et al. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunol Lett. 2004;93:97–108. doi: 10.1016/j.imlet.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Lehmann J, Retz M, Sidhu SS, Suttmann H, Sell M, Paulsen F, Harder J, Unteregger G, Stöckle M. Antitumor activity of the antimicrobial peptide magainin II against bladder cancer cell lines. Eur Urol. 2006;50:141–147. doi: 10.1016/j.eururo.2005.12.043. [DOI] [PubMed] [Google Scholar]
- 34.Wakabayashi H, Teraguchi S, Tamura Y. Increased Staphylococcus-killing activity of an antimicrobial peptide, lactoferricin B, with minocycline and monoacylglycerol. Biosci Biotechnol Biochem. 2002;66:2161–2167. doi: 10.1271/bbb.66.2161. [DOI] [PubMed] [Google Scholar]
- 35.Olafsson JH, Sigurgeirsson B, Baran R. Combination therapy for onychomycosis. Br J Dermatol. 2003;149:15–18. doi: 10.1046/j.1365-2133.149.s65.2.x. [DOI] [PubMed] [Google Scholar]