Abstract
Left ventricular assist devices improve survival prospects in patients with end-stage heart failure; however, infection complicates up to 59% of implantation cases. How many of these infections are caused by multidrug-resistant organisms is unknown. We sought to identify the incidence, risk factors, and outcomes of multidrug-resistant organism infection in patients who have left ventricular assist devices.
We retrospectively evaluated the incidence of multidrug-resistant organisms and the independent risk factors associated with them in 57 patients who had permanent left ventricular assist devices implanted at our institution from May 2007 through October 2011. Outcomes included death, transplantation, device explantation, number of subsequent hospital admissions, and number of subsequent admissions related to infection. Infections were categorized in accordance with criteria from the Infectious Diseases Council of the International Society for Heart and Lung Transplantation.
Multidrug-resistant organism infections developed in 18 of 57 patients (31.6%)—a high incidence. We found 3 independent risk factors: therapeutic goal (destination therapy vs bridging), P=0.01; body mass index, P=0.04; and exposed velour at driveline exit sites, P=0.004. We found no significant differences in mortality, transplantation, or device explantation rates; however, there was a statistically significant increase in postimplantation hospital admissions in patients with multidrug-resistant organism infection. To our knowledge, this is the first report in the medical literature concerning multidrug-resistant organism infection in patients who have permanent left ventricular assist devices.
Keywords: Cross-infection/etiology/prevention & control; drug resistance, multiple/bacterial; heart failure/therapy; heart-assist devices/adverse effects/microbiology; postoperative complications; prosthesis-related infections/diagnosis/microbiology; retrospective studies; risk factors; ventricular dysfunction, left/therapy
Heart failure is a chronic disease state with substantial morbidity and mortality rates.1 End-stage heart failure is characterized by worsening symptoms despite optimal medical management, and heart transplantation is the definitive treatment. However, rates of heart failure are increasing, whereas the number of organs available for transplantation is static.2 Therefore, mechanical support for the failing heart, especially ventricular assist devices (VADs), has attracted strong interest. Left VAD (LVAD) implantation imparts higher survival rates in end-stage heart failure than does optimal medical management.3–6 Although LVADs are potentially life-saving, implantation sequelae include right ventricular failure, thromboembolism, bleeding, and infection. Investigators have reported infection rates of 18% to 59% in patients with LVADs, and mortality rates estimated at 70% for infections such as VAD-related mediastinitis and endocarditis.3,7–9 Depending on the site of the infection, treatment can range from simple wound care to device explantation.7,10 Multidrug-resistant organisms (MDROs) are increasingly prevalent and are associated with substantial morbidity and mortality rates.11–13
Patients with LVADs are at greater risk for MDRO infection because of their exposure during medical procedures and their generally longer lifespans after implantation. To our knowledge, no data have been published about the incidence of MDRO infections in patients who have LVADs. Our primary objective in this study was to determine that incidence, and our secondary objective was to evaluate outcomes and risk factors in MDRO infection.
Patients and Methods
Our retrospective cohort study included patients whose permanent LVADs had been implanted at Emory University Hospital from May 2007 through October 2011. The Emory institutional review board approved the study. Baseline data collected from the VAD database included demographic details; device type—either the HeartMate® II Left Ventricular Assist System (Thoratec Corporation; Pleasanton, Calif) or the HeartWare® Ventricular Assist System (HeartWare Inc.; Framingham, Mass); indication for implantation; burial or exposure of the velour at driveline exit sites; hospital and intensive care unit days before implantation and before the development of an MDRO; class, number, and duration of antibiotics administered before implantation; the presence or absence of preimplantation diabetes mellitus; and preimplantation albumin and prealbumin values. Infection-related data included infection type (VAD-specific, VAD-related, or non-VAD-related, and MDRO or non-MDRO), organism type, and each infection's antibiotic susceptibility. All infections were recorded; however, organisms were counted only once per patient. Patients were considered to be uninfected if they had never had an infection or if they had had an infection before but not after implantation.
The primary endpoint was the incidence of MDRO infections in VAD patients, defined as the new occurrence of an MDRO infection after LVAD implantation. Secondary endpoints were outcomes (death, transplantation, device removal, number of subsequent hospital admissions, and number of subsequent hospital admissions related to infection) and risk factors for the development of MDRO infection.
Outcome Definitions
Infections were identified by means of culture-positive documentation in the electronic medical records. We defined infections as MDRO if they were resistant to 2 or more classes of antibiotics. Definitions of infection type were obtained from the Infectious Diseases Council of the International Society for Heart and Lung Transplantation.8 We defined VAD-specific infections as infection of the VAD hardware, driveline, or pocket tissue. We defined VAD-related infections as those related to the presence of the VAD but not directly involving the hardware or the containing body tissue; examples included endocarditis, catheter-associated bloodstream infection, and mediastinitis. We defined non-VAD-related infections as those occurring independently of the VAD, such as pneumonia, urinary tract infections, and Clostridium difficile. Urinary tract infections were not included as infections unless they had specifically been treated with antibiotics. We defined infection-related hospital admissions as those admissions for which infection had been recorded as the primary indication for hospitalization.
Statistical Analysis
For descriptive analysis, all patients were classified after LVAD implantation according to whether they had a non-MDRO infection, an MDRO infection, or neither. To compare the distributions of predictors across these groups, 2-sample t tests and χ2 tests were used for numerical and categorical variables, respectively. Variables that were significant at the α=0.20 level were considered for further multivariable regression analysis, a conservative approach to determining which predictors were independently associated with MDRO. Because 4 of the 57 patients underwent more than one LVAD implantation, a repeated-measures generalized linear model was constructed to account for the correlation of responses. An iterative backwards-elimination strategy was used, whereby the least significant of the candidate variables were sequentially removed but were later tested for the confounding effect. The final regression model contained all variables that were significant at the α=0.05 level and any confounders that were identified. All analyses were performed with use of SAS version 9.3 (SAS Institute Inc.; Cary, NC). Appropriate tests were selected on the basis of normality. No adjustments for multiple tests were performed. A P value <0.05 was considered statistically significant.
Results
Table I shows the baseline characteristics of the patients. In total, 61 LVADs were implanted in 57 patients (4 of whom underwent reimplantation). Their mean age at implantation was 49.5 ± 13.5 years; 70.5% of recipients were men. HeartMate II devices were more often implanted than HeartWare (in 52 patients; 85.2%), and destination therapy was the chief indication for LVAD implantation (26 patients; 42.6%). Significant differences for development of an MDRO were as follows: therapeutic goal (destination therapy vs bridging), P=0.001; body mass index (BMI), P=0.04; and velour exposure at driveline exit sites, P=0.004.
TABLE I.
Baseline Characteristics of the Study Patients
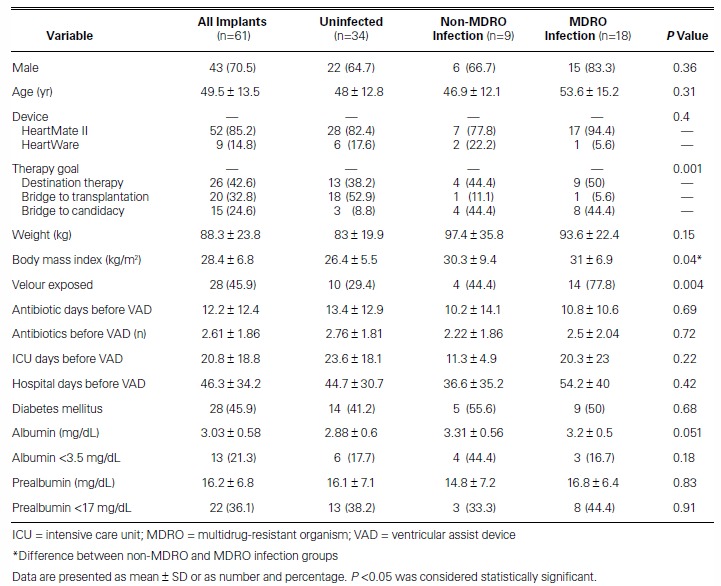
Overall, 27 patients (47.4%) developed an infection. Eighteen of the 57 patients (31.6%) had an MDRO infection. Table II shows descriptions of infections by group. In patients who had MDROs, more infections were VAD-specific (10 MDRO vs 6 non-MDRO, P=0.001) or VAD-related (7 MDRO vs 3 non-MDRO, P=0.003) than in patients who had no MDRO. Table III shows all the organisms found in the study population; the most prevalent MDRO was Staphylococcus aureus.
TABLE II.
Incidence of MDROs by Infection Type
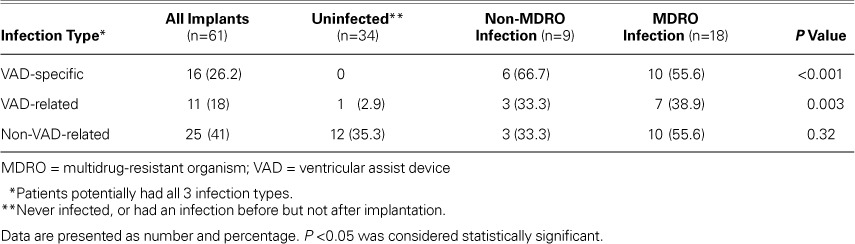
TABLE III.
Frequency of the Infectious Organisms
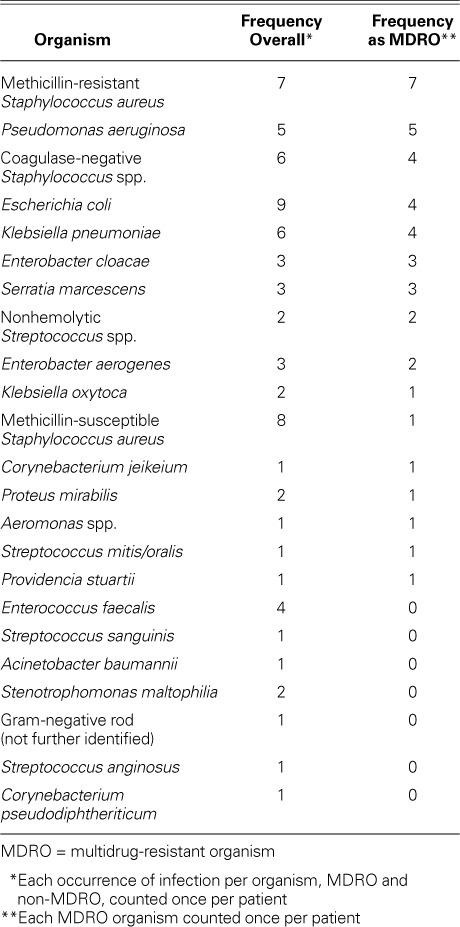
Figure 1 shows the time to development of an MDRO infection. In the Kaplan-Meier analysis, patients were censored upon development of the first MDRO; however, subsequent MDROs were recorded and included in the final analyses. The mean time to development of a new MDRO was 174 ± 37 days.
Fig. 1.
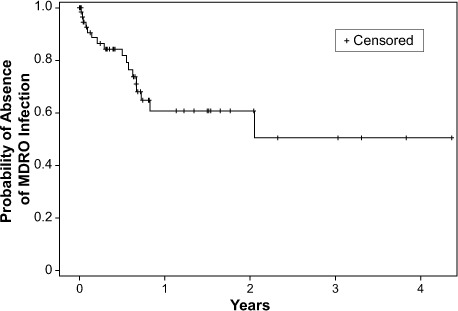
Kaplan-Meier curve shows time to development of a multidrug-resistant organism (MDRO). Patients were censored upon development of their first MDRO.
Outcomes
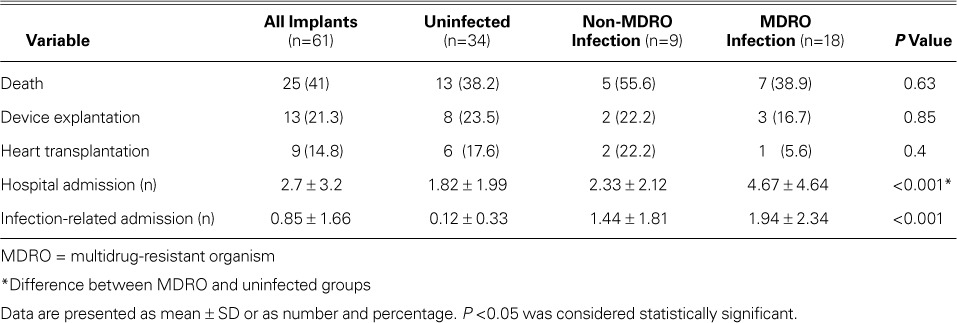
Table IV shows the patients' outcomes. Hospital admissions, including those related to infection, were significantly more numerous in patients with infections (P <0.001). No other variable was statistically significant.
TABLE IV.
Outcomes in Patients with Left Ventricular Assist Devices
Discussion
In this retrospective, single-center study, we found an MDRO infection rate of 31.6% in patients who had undergone LVAD implantation. To our knowledge, these are the first such data reported in the medical literature. We found an overall incidence of infection of 47%, similar to that reported in previous studies.3,7,9,14,15
Left ventricular assist device implantations are increasing in number because of increasing rates of heart failure and the shortage of donor hearts for transplantation. As LVAD technology and medical therapy improve, patients with LVADs are living longer and are at greater risk of developing infection.16
The baseline characteristics of our patients are similar to those of LVAD patients in other retrospective reviews, with the exception of the indication for LVAD implantation.13,17 Destination therapy was the indication in 42.6% of our patients—more than the 34% of patients in the INTERMACS database who were given an LVAD for that indication.16 Previous investigators have also reported lower percentages of destination-therapy patients than we did.9 Destination therapy is typically reserved for patients with contraindications to heart transplantation,3,18 such as age >70 years, cancer with an elevated risk of recurrence, BMI >30 kg/m2, diabetes mellitus with end-organ damage, and active tobacco use or substance abuse.19 Therefore, it is possible that patients in destination-therapy cohorts are in poorer overall health than are patients who are bridged.18
The infective MDROs that we identified most frequently were methicillin-resistant S. aureus and Pseudomonas aeruginosa. In type and frequency, the organisms in our study population were similar to those reported in other LVAD-infection studies.9,16
We found an independent increase in MDRO incidence in patients whose drivelines were tunneled in such a way that some of the velour covering protruded from the exit site, rather than being entirely inside the patient with only silicone-elastic exposed at the exit site. Exposed velour has been associated with an increased risk of infection.20 At our institution, velour was left exposed until April 2010—after which time it was buried, to decrease risk of infection. Several risk factors contributing to death and morbidity in LVAD patients have been proposed, including obesity, poor nutritional status, and comorbid diabetes mellitus.13,21 However, of these, only BMI was a statistically significant predictor of MDRO infection in our study, specifically in our uninfected and infected groups.
As might be expected, we found a significant increase in hospital admissions in MDRO-infected patients. Although poor outcomes have been associated with infections in patients with VAD implants, we did not find a statistically significant increase in deaths, transplantation, device explantation, or overall hospital admissions in relation to the occurrence of an MDRO infection.9,22 Our study might have been underpowered to detect these differences.
Limitations of the Study
Our study has several limitations. We had a relatively small patient population, and the patients had a greater likelihood of undergoing implantation for destination therapy than did LVAD-implant patients overall. Destination therapy typically signifies poorer health, a possible confounding factor. In this retrospective chart analysis, infection was defined as culture-positive documentation; clinical indicators of infection were not considered. In addition, care that patients might have received at other institutions was not part of our data. However, most patients received most of their care at Emory University Hospital after their implantation and were closely monitored in the Emory VAD clinic. Records of care that was delivered in other institutions were typically forwarded to the Emory VAD clinic and were screened for infection-related events.
Conclusion
We found a 31.6% incidence of MDRO infection in patients who had undergone LVAD implantation—to our knowledge, the first such data to be reported. Therapeutic goal, BMI, and exposed velour were independent risk factors for the development of MDRO infection. Our MDRO patients had more hospital admissions caused by infection and therefore more total hospital admissions. A larger study, perhaps prospective, is warranted to evaluate additional MDRO risk factors and outcomes.
Footnotes
From: Department of Pharmaceutical Services (Drs. Donahey and Paciullo) and Center for Heart Failure Therapy (Ms Pekarek and Ms Wittersheim), Emory University Hospital, Atlanta, Georgia 30322; Department of Pharmaceutical Services (Dr. Polly), Emory University Hospital Midtown, Atlanta, Georgia 30308; Departments of Surgery (Drs. Nguyen and Vega) and Medicine (Drs. Butler and Lyon), Emory University School of Medicine, Atlanta, Georgia 30322; and Department of Biostatistics and Bioinformatics (Mr. Kilgo), Rollins School of Public Health, Atlanta, Georgia 30322
Dr. Donahey is now at the Department of Pharmacy, Loyola University Hospital, Maywood, Illinois.
References
- 1.Writing Group Members. Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association [published erratum appears in Circulation 2011;124 (16):e425] Circulation. 2010;121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Stehlik J, Edwards LB, Kucheryavaya AY, Aurora P, Christie JD, Kirk R et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult heart transplant report--2010. J Heart Lung Transplant. 2010;29(10):1089–103. doi: 10.1016/j.healun.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345(20):1435–43. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 4.Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357(9):885–96. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 5.Slaughter MS, Pagani FD, Rogers JG, Miller LW, Sun B, Russell SD et al. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant. 2010;29(4 Suppl):S1–39. doi: 10.1016/j.healun.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Wieselthaler GM, O'Driscoll G, Jansz P, Khaghani A, Strueber M, HVAD Clinical Investigators Initial clinical experience with a novel left ventricular assist device with a magnetically levitated rotor in a multi-institutional trial. J Heart Lung Transplant. 2010;29(11):1218–25. doi: 10.1016/j.healun.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 7.Gordon RJ, Quagliarello B, Lowy FD. Ventricular assist device-related infections. Lancet Infect Dis. 2006;6(7):426–37. doi: 10.1016/S1473-3099(06)70522-9. [DOI] [PubMed] [Google Scholar]
- 8.Hannan MM, Husain S, Mattner F, Danziger-Isakov L, Drew RJ, Corey GR et al. Working formulation for the standardization of definitions of infections in patients using ventricular assist devices. J Heart Lung Transplant. 2011;30(4):375–84. doi: 10.1016/j.healun.2011.01.717. [DOI] [PubMed] [Google Scholar]
- 9.Topkara VK, Kondareddy S, Malik F, Wang IW, Mann DL, Ewald GA, Moazami N. Infectious complications in patients with left ventricular assist device: etiology and outcomes in the continuous-flow era. Ann Thorac Surg. 2010;90(4):1270–7. doi: 10.1016/j.athoracsur.2010.04.093. [DOI] [PubMed] [Google Scholar]
- 10.Ensor CR, Paciullo CA, Cahoon WD, Jr, Nolan PE., Jr. Pharmacotherapy for mechanical circulatory support: a comprehensive review. Ann Pharmacother. 2011;45(1):60–77. doi: 10.1345/aph.1P459. [DOI] [PubMed] [Google Scholar]
- 11.Paterson DL, Rogers BA. How soon is now? The urgent need for randomized, controlled trials evaluating treatment of multidrug-resistant bacterial infection. Clin Infect Dis. 2010;51(11):1245–7. doi: 10.1086/657243. [DOI] [PubMed] [Google Scholar]
- 12.Barrett SP. Control of the spread of multi-resistant gram-positive organisms. Curr Anaesth Crit Care. 1999;10(1):27–31. [Google Scholar]
- 13.Butler J, Howser R, Portner PM, Pierson RN., 3rd. Body mass index and outcomes after left ventricular assist device placement. Ann Thorac Surg. 2005;79(1):66–73. doi: 10.1016/j.athoracsur.2004.06.047. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein DJ, Naftel D, Holman W, Bellumkonda L, Pamboukian SV, Pagani FD, Kirklin J. Continuous-flow devices and percutaneous site infections: clinical outcomes. J Heart Lung Transplant. 2012;31(11):1151–7. doi: 10.1016/j.healun.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Nienaber JJ, Kusne S, Riaz T, Walker RC, Baddour LM, Wright AJ et al. Clinical manifestations and management of left ventricular assist device-associated infections. Clin Infect Dis. 2013;57(10):1438–48. doi: 10.1093/cid/cit536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA et al. The Fourth INTERMACS Annual Report: 4,000 implants and counting. J Heart Lung Transplant. 2012;31(2):117–26. doi: 10.1016/j.healun.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Schaffer JM, Allen JG, Weiss ES, Arnaoutakis GJ, Patel ND, Russell SD et al. Infectious complications after pulsatile-flow and continuous-flow left ventricular assist device implantation. J Heart Lung Transplant. 2011;30(2):164–74. doi: 10.1016/j.healun.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Rose EA, Moskowitz AJ, Packer M, Sollano JA, Williams DL, Tierney AR et al. The REMATCH trial: rationale, design, and end points. Randomized evaluation of mechanical assistance for the treatment of congestive heart failure. Ann Thorac Surg. 1999;67(3):723–30. doi: 10.1016/s0003-4975(99)00042-9. [DOI] [PubMed] [Google Scholar]
- 19.Mehra MR, Kobashigawa J, Starling R, Russell S, Uber PA, Parameshwar J et al. Listing criteria for heart transplantation: International Society for Heart and Lung Transplantation guidelines for the care of cardiac transplant candidates--2006. J Heart Lung Transplant. 2006;25(9):1024–42. doi: 10.1016/j.healun.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 20.Ledford ID, Miller DV, Mason NO, Alharethi RA, Rasmusson BY, Budge D et al. 8 differential infection rates between velour versus silicone interface at the HeartMate II driveline exit site: structural and ultrastructural insight into possible causes. J Heart Lung Transplant. 2011;30(4 Suppl):S10–S11. [Google Scholar]
- 21.Dang NC, Topkara VK, Kim BT, Lee BJ, Remoli R, Naka Y. Nutritional status in patients on left ventricular assist device support. J Thorac Cardiovasc Surg. 2005;130(5):e3–4. doi: 10.1016/j.jtcvs.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 22.Holman WL, Kirklin JK, Naftel DC, Kormos RL, Desvign-Nickens P, Camacho MT, Ascheim DD. Infection after implantation of pulsatile mechanical circulatory support devices. J Thorac Cardiovasc Surg. 2010;139(6):1632–6.e2. doi: 10.1016/j.jtcvs.2010.01.014. [DOI] [PubMed] [Google Scholar]


