Abstract
Early initiation of statin therapy in acute coronary syndrome patients has a favorable prognostic impact because of its anti-inflammatory and antithrombotic properties. In this study, we explored the effect of atorvastatin-loading, followed by intensive atorvastatin therapy, on clinical and biochemical outcomes in non-ST-segment-elevation acute coronary syndrome patients who were scheduled for percutaneous coronary intervention.
We prospectively enrolled 140 patients (mean age, 56 ± 9 years, 68% men). Once eligible, patients were randomly assigned to receive either a moderate 20-mg daily dose of atorvastatin (Group A) or a 160-mg loading dose followed by an intensified 80-mg daily dose (Group B). High-sensitivity C-reactive protein (hs-CRP) levels were recorded before and after intervention. Evaluation after 6 months included hs-CRP levels, left ventricular systolic function, and major adverse cardiac events.
We found no significant difference between the 2 groups in regard to the interventional data. However, blood sampling after coronary intervention, and again 6 months later, revealed a significant decline in mean hs-CRP level among Group B patients (P <0.001). Moreover, patients in Group B manifested a higher left ventricular ejection fraction than did patients in Group A (P <0.05). After 6 months, we found no significant difference between groups in the incidence of major adverse cardiac events.
We conclude that intensive atorvastatin therapy in non-ST-segment-elevation acute coronary syndrome patients is associated with lower hs-CRP levels and with higher left ventricular ejection fraction after 6 months, with no significant impact on adverse cardiac events.
Keywords: Acute coronary syndrome/drug therapy; atorvastatin/therapeutic use; C-reactive protein, high-sensitivity; coronary angioplasty; hydroxymethylgutaryl-CoA reductase inhibitors; lipoproteins, low-density; prospective studies; treatment outcome
In the current study, we explored the effect of atorvastatin-loading, followed by intensive long-term atorvastatin therapy, on clinical and biochemical outcomes in patients with non-ST-segment-elevation acute coronary syndrome (ACS) who have been scheduled for percutaneous coronary intervention (PCI). First, however, we present some background information.
Among cardiovascular diseases, ACS is a very complex disorder that encompasses tissue remodeling, necrosis, and thrombosis.1 Inflammatory processes are involved in almost all states of atherosclerotic disease progression and are especially important in the initiation of ACS.2 C-reactive protein (CRP) is an acute-phase reactant that is considered to be a biomarker of inflammation.3 It also plays an active role in the progression of atherosclerosis, through its direct pro-atherogenic effects on the vasculature.4 Serum CRP is a strong independent predictor of future cardiovascular events. High CRP levels predict an increase of risk that goes beyond classical risk factors.5 Similarly, serum CRP levels are elevated in patients with unstable coronary artery disease, as opposed to those with stable levels.6 This protein is produced chiefly by the liver, in response to proinflammatory cytokines, and is secreted into the systemic circulation.7 It has been reported that, in endothelial cells, CRP increases the expression of cell-adhesion molecules8 and decreases endothelial nitric oxide synthase expression and activity.9 Moreover, it promotes the production of reactive oxygen species.10
Statins, the competitive inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A reductase, are associated with further risk reduction in patients with coronary artery disease.11 In addition to their lipid-lowering properties, these agents exhibit pleiotropic properties, both in vivo and in vitro.12 These drugs decrease reactive oxygen species production, reduce vascular smooth-muscle-cell proliferation, exhibit antithrombotic and anti-inflammatory effects, increase the production of nitric oxide in endothelial cells by the up-regulation of endothelial nitric oxide synthase expression, and stabilize atherosclerotic plaques.13 Moreover, statins are able to decrease CRP levels in hyperlipidemic patients.14 Data from randomized controlled trials have indicated that intensive lipid-lowering by means of statins provides additional clinical benefits after ACS.15,16 However, data are scarce on the impact of long-term intensive statin treatment on left ventricular (LV) systolic function.
Patients and Methods
A total of 140 patients (out of 270 patients initially screened) who had non-ST-segment-elevation ACS (NSTE-ACS) were prospectively enrolled in this study. They were referred to our coronary care unit (CCU) from November 2012 through February 2014. All patients underwent coronary angiography and PCI within 72 hours of CCU admission.
Exclusions. Excluded patients were those scheduled for coronary artery bypass grafting (CABG) or intensified medical treatment after coronary angiography; pregnant patients; patients with a history of ACS, with prior PCI or CABG, with congenital heart disease or any myocardial disease apart from ischemia, with active or recent (within the past month) infections, with a history of inflammatory or connective-tissue disorders, with chronic or occasional (within the past 3 weeks) anti-inflammatory or corticosteroid treatment, and patients who had been given strong inhibitors of cytochrome P450 3A4 (for example, macrolides) within one month before randomization or who were likely to need such treatment during the study period (reason: atorvastatin is metabolized by this pathway). We also excluded patients with contraindications to aspirin or clopidogrel use, with short life expectancy because of coexistent disease (for example, malignancy), and with known skeletal-muscle disorders or chronic liver or kidney disease.
After enrollment, patients were randomly assigned in 1:1 fashion to receive either a moderate daily dose of atorvastatin (20 mg) (Group A) or an intensified daily dose of 80 mg (Group B) of atorvastatin, in addition to an equally divided loading dose (80 mg each) given 12 and 2 hours before coronary angiography. These assignments were in accordance with a computer-generated series of numbers randomized by blocks of 10 patients each. Physicians participating in PCI procedures were unaware of the block randomization. Before inclusion in the study, each patient gave informed written consent, and the study protocol was reviewed and approved by our local institutional human research committee; the protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki, as revised in 2008.
All patients received the proper antithrombotic therapy including antiplatelets (aspirin, 100 mg/d; and clopidogrel, 75 mg/d), in addition to a tailored anti-ischemic medical regimen consisting of β-blockers, angiotensin-converting enzyme inhibitors, calcium antagonists, and nitrates.
Hypertension was defined as a systolic blood pressure ≥140 mmHg or a diastolic blood pressure ≥90 mmHg, previously recorded by repeated noninvasive office measurements, which led to lifestyle modification, intake of antihypertensive drug therapy, or both.17 Diabetes mellitus was defined as a fasting plasma glucose level ≥126 mg/dL (7 mmol/L) or a 2-hour post-glucose load ≥200 mg/dL (≥11 mmol/L), or the intake of a specific antidiabetic drug.18 Dyslipidemia was defined as a low-density-lipoprotein cholesterol (LDL-C) level >100 mg/dL (>2.6 mmol/L), a serum triglyceride level >150 mg/dL (>1.7 mmol/L), or a high-density-lipoprotein cholesterol (HDL-C) level <40 mg/dL (<1 mmol/L).19 All of the included smokers had reported regular cigarette smoking in the last 6 months before enrollment. Figure 1 shows the enrollment flow chart.
Fig. 1.
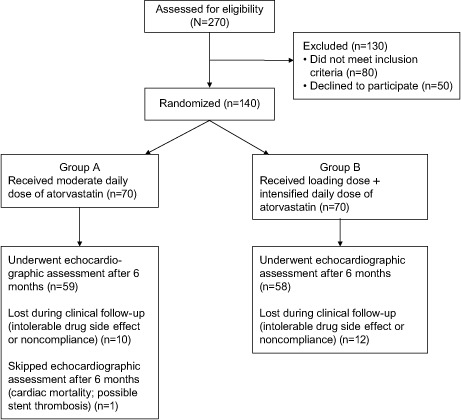
Flow chart shows the patient-enrollment process.
Echocardiographic Evaluation
Evaluation of regional and global LV systolic functions was performed in all patients by transthoracic echocardiography with use of a Vivid 7® cardiac ultrasonography system (GE VingMed Ultrasound AS; Horten, Norway) equipped with harmonic imaging capabilities. A 2.5-MHz phased-array probe was used to obtain standard 2-dimensional, M-mode, and Doppler images. Patients were examined in the left lateral recumbent position, in standard parasternal and apical views. For every patient, we echocardiographically recorded left ventricular ejection fraction (LVEF) by the modified Simpson method, and LV internal dimensions by M-mode and wall-motion abnormalities. Images were digitized in cine-loop format and saved for subsequent playback and analysis. Regional wall motion was evaluated in accordance with the standard 17-segment model, as recommended by the American Society of Echocardiography.20 Regional wall-motion abnormalities were visually evaluated for each individual segment, taking into consideration both endocardial excursion and systolic thickening. Each segment was graded according to the semiquantitative scoring system described by Knudsen and colleagues.21 Views were acquired and analyzed upon CCU admission, and again 6 months later, by a single echocardiographer, using the software program of the echocardiography machine and blinded to the study protocol.
Laboratory Studies
Venous sampling for cardiac troponin I was done upon CCU admission and again 12 hours after PCI. Patients with initially negative results also underwent serial measurements (every 12 hr) before PCI. The plasma concentration of cardiac troponin I was measured using the Troponin I Ultra assay on an ADVIA Centaur® CP Immunoassay System (Siemens Healthcare GmbH; Eschborn, Germany) with a detection limit of 6 pg/mL, a 99th percentile at 40 pg/mL, and a coefficient of variation of less than 10% at 30 pg/mL, as specified by the manufacturer.
High-sensitivity CRP (hs-CRP) serum samples were collected upon CCU admission, 12 hours after PCI, and 6 months later. Venous samples were stored at −20 °C to be processed (within 24 hours after collection) by automated microparticle immunoassay, with a cutoff value of 0.3 mg/L and an interassay imprecision of <5%.
Percutaneous Coronary Intervention and Medications
The vascular access site (femoral or radial artery) was recorded for every patient. All patients were given the same nonionic, low-osmolality contrast agent iopramide (Ultravist 370/100 mL), which was used at boli of 5 to 15 mL. The choice of periprocedural nitroglycerin or glycoprotein IIb/IIIa inhibitor was left to the interventional cardiologists.
The first procedural step after gaining successful arterial access and coronary cannulation was the passage of a floppy, steerable guidewire through the target lesion. Direct stenting was left to the operator's discretion and was usually performed in patent vessels, with no or mild calcification. When necessary for stent delivery, balloon dilation was performed before stenting. Bare-metal stents were used in all patients. Intravenous unfractionated heparin (100 IU/kg) was administered on the operating table. Activated clotting time (200–300 s) was adjusted with use of a Hemochron® device. Morphologic features of the coronary lesions were evaluated before PCI. They were classified in accordance with the American College of Cardiology/American Heart Association Task Force classification system.22 In cases that required multilesion stenting, only the worst lesion types were recorded. Additional recorded data included the number of diseased coronary arteries that were amenable to PCI, the number of stents per patient, the final Thrombolysis in Myocardial Infarction (TIMI) flow grade, and procedural sequelae, if any.
Follow-Up Evaluation
Both groups were monitored (at the outpatient clinic and through telephone calls) for 6 months and were compared regarding the incidence of major adverse cardiac events (MACE), including stent thrombosis according to the Academic Research Consortium classification,23 cardiac death, nonfatal myocardial infarction, and target-lesion revascularization. Patients were seen for follow-up visits and received dietary counseling at 30 days, 3 months, and 6 months (final visit). Blood samples were obtained for quantification of liver enzymes (and of creatine kinase, if necessary) after 3 months and finally after 6 months. Lipid profile data (total cholesterol, triglycerides, and HDL-C) were recorded as well.
Statistical Analysis
The sample size was projected from the study of Chyrchel and colleagues,24 which evaluated the impact of pre-PCI high-dose statin therapy in NSTE-ACS patients. The power of the test was set at 80%, the confidence interval was set at 95%, and the acceptable margin of error was set at 5%. The sample size was 70 patients per group, which was found by estimating the incidence of MACE among both study groups during the follow-up period. All continuous variables were statistically described in terms of mean ± SD. Categorical variables were described with absolute and relative (percentage) frequencies. Comparison of continuous variables between the study groups was performed by means of the Student t test. For comparing categorical data, the Pearson χ2 and Fisher exact tests were performed. A P value <0.05 was considered statistically significant. The normality of data was judged and confirmed with use of the Kolmogorov-Smirnov test. A paired t test was used to analyze pre- and post-PCI results. The statistical significance level was set at α=0.05. All statistical calculations were performed with use of SPSS for Windows software version 15.0 (IBM Corporation; Armonk, NY).
Results
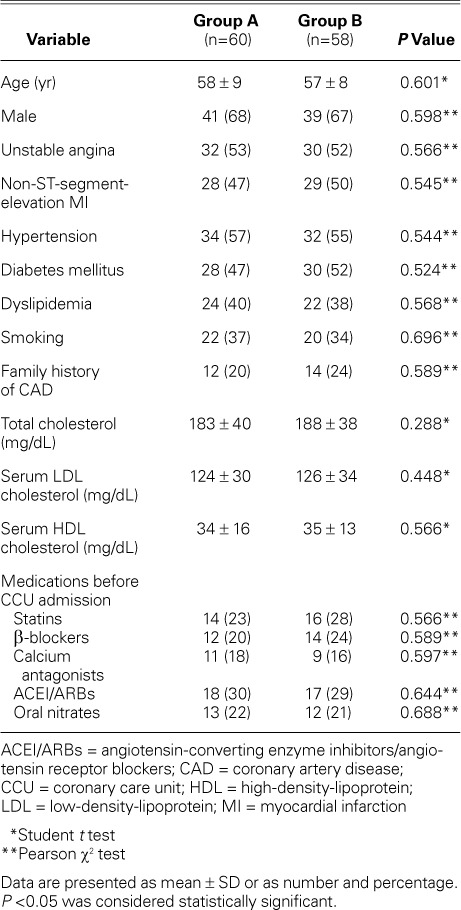
In total, 118 patients completed the study protocol after the withdrawal of 22 patients because of intolerable drug side effects or noncompliance. Sixty patients were in Group A and 58 patients in Group B. The mean age of the whole study cohort was 56 ± 9 years, and 80 (68%) were men. There was no recorded significant difference between the groups in regard to their baseline characteristics (Table I).
TABLE I.
Baseline Characteristics in the Study Groups
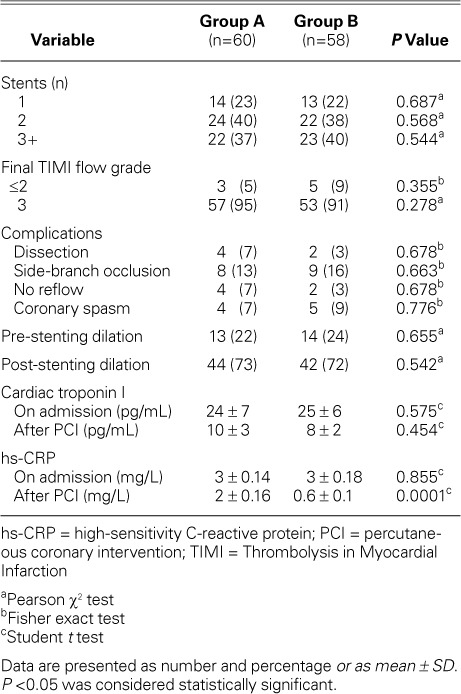
Data Related to Percutaneous Coronary Intervention
Femoral artery access was used for most of the patients in both groups. There were no recorded cases of stent loss or improper placement. There was no statistically significant difference between Groups A and B in regard to preliminary angiographic findings and procedural characteristics. Angiographic data were interpreted by 2 interventional cardiologists who were unaware of the study protocol. Analysis of interobserver variability revealed a close correlation between repeated interpretations, with a correlation coefficient of r=0.95. There was no significant difference between groups in regard to the hs-CRP levels at admission. However, post-PCI sampling revealed a significant decline in mean hs-CRP level among Group B patients (P=0.0001) (Fig. 2). Tables II and III show summarized PCI-related data.
Fig. 2.
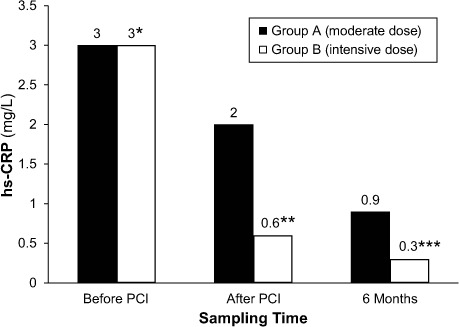
Graph shows the magnitude of change of mean high-sensitivity C-reactive protein (hs-CRP) levels before and after percutaneous coronary intervention (PCI).
*P>0.05
**P<0.001
***P<0.05
P<0.05 was considered statistically significant.
TABLE II.
Preliminary Angiographic Characteristics in the Groups
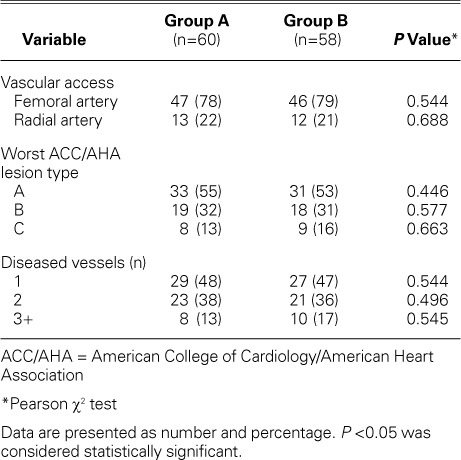
TABLE III.
Procedural Characteristics and Complications in the Groups
Follow-Up Data
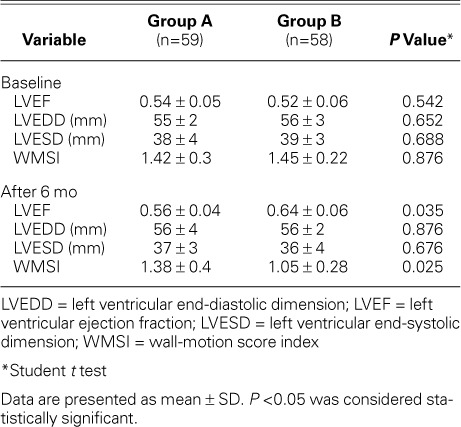
After 6 months, recorded transthoracic echocardiographic data revealed a statistically significant higher mean LVEF among Group B patients (P=0.035), when compared to the baseline value (P=0.024) (Fig. 3). Group A did not show such improvement (P=0.545). Analysis of intraobserver variability revealed a close correlation between repeated evaluations of LV systolic function by a single operator, with a correlation coefficient of r=0.97. All patients underwent follow-up echocardiography, except for one Group A patient, who was recorded (after 170 d) as a case of cardiac death, from possible stent thrombosis. Three other patients (Group A, 2 patients; Group B, 1 patient) developed definite late (>30 d after PCI) stent thrombosis with subsequent percutaneous revascularization. There was no statistically significant difference between the groups regarding the incidence of MACE. Group B patients showed a significantly lower mean hs-CRP level after 6 months of intensive atorvastatin therapy (0.3 ± 0.02 vs 0.9 ± 0.13 mg/L, P=0.035). The mean LDL-C level recorded after 6 months was significantly lower in Group B patients (1.6 ± 0.4 vs 2.5 ± 0.7 mmol/L, P=0.0003). The recorded mean HDL-C level was significantly higher in the same group (1.1 ± 0.2 vs 0.8 ± 0.3 mmol/L, P=0.046). Tables IV and V show summarized clinical and echocardiographic outcomes, respectively.
Fig. 3.
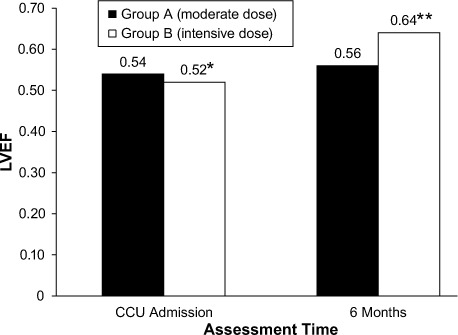
Graph shows the magnitude of change in left ventricular ejection fraction (LVEF) upon coronary care unit (CCU) admission and after 6 months of atorvastatin therapy.
*P>0.05
**P<0.05
P<0.05 was considered statistically significant.
TABLE IV.
Clinical Events after 6 Months
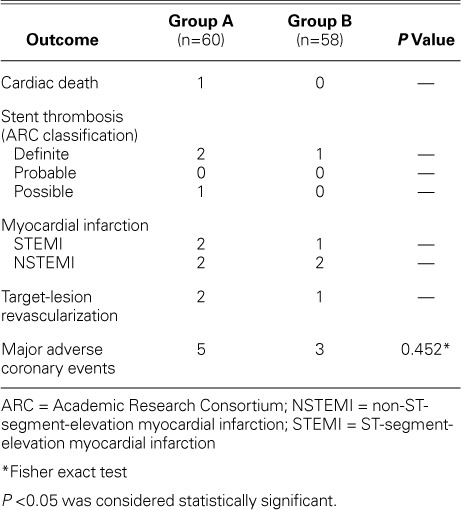
TABLE V.
Echocardiographic Data on Left Ventricular Function
Tolerability and Safety
The rates of discontinuation of therapy because of an intolerable adverse event or noncompliance (leading to exclusion from the study) were 14% (10 patients) in Group A and 17% (12 patients) in Group B (P=0.54). During therapy, the dose was halved among 6.6% (4 patients) of those in Group A and 8.6% (5 patients) of those in the intensive-dose Group B (P=0.67), consequential to adverse effects, liver enzymes, or creatine kinase abnormalities.
Discussion
The current study presents the favorable effect of pre-PCI atorvastatin-loading, followed by intensive atorvastatin therapy in NSTE-ACS patients. The dosing regimen adopted in this study was associated with better biochemical outcome in terms of lower hs-CRP (after PCI, and 6 months later) and LDL-C levels (after 6 months). Moreover, intensive atorvastatin therapy was associated with improvement of LV systolic function, with no significant decline in the incidence of MACE.
It cannot be determined whether this intermediate-term benefit was due to intensive lipid-lowering or due to an early benefit—the stabilization of vulnerable plaques and the attenuation of inflammatory response. These effects can be ascribed to the beneficial pleiotropic effects of statins, regardless of a statin's ability to lower the level of serum lipids. The ability of statins to decrease the plasma level of hs-CRP has already been proved.14 However, the mechanisms involved in this process are not entirely clear. It has been reported that statins reduce interleukin-6–induced CRP production and gene expression in human hepatocytes by decreasing the activation of the transcription factor STAT3.7 Also, statins prevent the transactivation of nuclear factor kappa B, a transcription factor involved in immune and inflammatory responses in endothelial cells, which is stimulated in response to interleukin-1 or tumor necrosis factor-α.25 Because of the essential role of inflammation in the pathogenesis of atherosclerosis, the most promising hopes for the improvement in outcomes of ACS treatment are placed on the anti-inflammatory activity of statins.26 This activity was manifest in the current study as an anti-hs-CRP effect, which was magnified upon intensifying the statin dose.
Statins have inhibitory actions on platelet functions, coagulation factors, and rheology.27 In addition, they are thought to stabilize atherosclerotic plaques by decreasing lipid oxidation, inflammation, matrix metalloproteinase-2, and cell death; and further, by increasing the content of tissue inhibitor of metalloproteinase-1 and collagen. These effects might reduce the incidence of unsatisfactory TIMI flow after PCI.28 However, in the current study, intensive atorvastatin-loading was not associated with better final TIMI flow grade. We hypothesize that atorvastatin effect, in this regard, seems not to be dose-dependent.
Statins are expected to reverse subclinical LV dysfunction after acute ACS through lipid-dependent mechanisms that retard coronary atherosclerosis and non-lipid-dependent mechanisms. These last (pleiotropic) mechanisms include the improvement of endothelial function, the inhibition of neurohormonal activation (by inhibiting the renin-angiotensin system, for example), the prevention of ventricular remodeling (by altering matrix metalloproteinase activity and by reducing fibrosis, for example), the reduction of myocardial necrosis, and the promotion of neoangiogenesis.29 These effects seemed to be pronounced in the present study, as shown by the significant improvement of LV systolic function in patients who were given intensive atorvastatin therapy. However, this improvement was not associated with a decreased incidence of MACE. This might be because we included patients with relatively favorable risk profiles (no prior PCI or CABG and a fair baseline LVEF) or because the follow-up period was relatively short. It is important to note that our safety and efficacy results were obtained in a carefully selected and monitored study population (we excluded, for example, patients who had concomitantly received strong inhibitors of cytochrome P4503A4, because cytochrome inhibition interferes with atorvastatin's route of metabolism).
The early initiation of statins after ACS is still an area of intense debate. Our study did not show a significant decrease in the incidence of MACE among NSTE-ACS patients who received early initiation and continuation of intensive statin therapy. In concordance with the present study, Choudhry and colleagues30 showed the non-superiority of intensive-dose statin therapy in decreasing the incidence of MACE after a long-term follow-up period in elderly ACS patients. In addition, a meta-analysis of randomized controlled trials failed to show a reduction, after 1 and 4 months, in the composite primary endpoint (death, myocardial infarction, or stroke) in ACS patients treated early with statins.31 On the basis of the available evidence (18 studies), the initiation of statin therapy within 14 days after ACS does not reduce death, myocardial infarction, or stroke up to 4 months, but does reduce the occurrence of unstable angina at 4 months after ACS.32 Results of the PROVE IT–TIMI 22 trial showed that intensive statin therapy after ACS was associated with better protection against MACE after a follow-up period of 24 months; however, 2 different statins were compared in that study.15 The same results were recorded in elderly patients after a longer follow-up period (5 yr).33 Chyrchel and colleagues24 reported the same advantage after administering 80 mg of atorvastatin (vs placebo) before PCI in NSTE-ACS patients. Chyrchel's 2 groups of patients received the same dose of maintenance therapy. Our current study reports different results in the presence of a higher-maintenance dose in one group. Another point of view was presented by Wong and associates,34 who reported that in-hospital revascularization and the prescription of statins at hospital discharge independently improved outcome over a follow-up period of 2 to 5 years. There was no prognostic interaction detected between these 2 beneficial therapies. The authors thought that different conclusions evolved from different dosing regimens and various time points of drug initiation adopted in various studies.34
Data from the literature have indicated that the favorable impact of statins on clinical outcome is augmented by more declines in hs-CRP levels. This was reported in terms of primary prevention through the JUPITER trial,35 and through secondary prevention36 as well. In the current study, both groups showed a comparable MACE incidence after exposure to different doses of atorvastatin, although one of those groups experienced a significant decline in hs-CRP levels after 6 months. Most probably, this was related to a relatively short follow-up period.
Of note in our study, a follow-up period of 6 months showed that intensive atorvastatin therapy was associated with better LV systolic function. In another study, cerivastatin was shown to improve LV function after myocardial infarction in rats.37 This effect was associated with an attenuated LV expression of fetal myosin heavy-chain isoenzymes and collagen I, which suggests an occurring benefit through the prevention of adverse ventricular remodeling. However, in human beings, intensive statin therapy (80 mg of atorvastatin) after primary PCI was not associated with improved LV systolic function after a follow-up period of 4 months.38
Limitations of the Study
The data presented in our study apply only to patients defined by the inclusion and exclusion criteria. Moreover, this is a single-center study, with a relatively small sample size and with results that apply only to atorvastatin-treated patients during a follow-up period limited to 6 months. A larger sample size monitored for a longer period of time—perhaps with the use of drug-eluting stents and different classes of statins—is warranted for confirmation of the current study results. Withdrawal of patients during the follow-up period undermined our ability to maintain the assumed number of patients estimated for sample sizing and rendered the study underpowered. Perhaps a meta-analysis of clinical-trial data—pooling all available studies—would overcome these power short-falls. It is of paramount importance that all randomized evidential results be published and made available to research scientists, regardless of their statistical power or study outcomes. Our use of echocardiographic values reported by a single experienced operator might be seen as a double-edged sword: we aimed at avoiding the confounding interpretive factors that might arise in the presence of multiple echocardiographers, but our use of a single echocardiographer might be considered a weakness, for lack of interobserver variability. Finally, we categorized the patients into drug-intensity categories on the basis of their initial and maintenance statin dosages and assumed that they continued this treatment until they had an outcome. In actual practice, patients often discontinue treatment or change drugs and doses; such patients probably differ in important ways from patients who continue their initially prescribed treatment. To avoid confounding results as much as possible, we monitored altered doses and excluded (after initial enrollment) patients who discontinued treatment.
Conclusions
Intensive atorvastatin therapy in NSTE-ACS patients undergoing PCI was associated with lower hs-CRP levels (maintained after 6 mo) and with higher LVEFs. However, there was no significant impact on the incidence of MACE.
Acknowledgments
We are grateful to the medical, technical, and nursing staffs of the cardiac catheterization and echocardiography laboratories in the Cardiology Department, Faculty of Medicine, Ain Shams University, for their cooperation in this work.
Footnotes
From: Department of Cardiology, Faculty of Medicine, Ain Shams University, 11774 Cairo, Egypt
References
- 1.Balasubramaniam K, Viswanathan GN, Marshall SM, Zaman AG. Increased atherothrombotic burden in patients with diabetes mellitus and acute coronary syndrome: a review of antiplatelet therapy. Cardiol Res Pract. 2012;2012:909154. doi: 10.1155/2012/909154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340(2):115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 3.Yeh ET, Willerson JT. Coming of age of C-reactive protein: using inflammation markers in cardiology. Circulation. 2003;107(3):370–1. doi: 10.1161/01.cir.0000053731.05365.5a. [DOI] [PubMed] [Google Scholar]
- 4.Scirica BM, Morrow DA. Is C-reactive protein an innocent bystander or proatherogenic culprit? The verdict is still out. Circulation. 2006;113(17):2128–34. doi: 10.1161/CIRCULATIONAHA.105.611350. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation. 2001;103(13):1813–8. doi: 10.1161/01.cir.103.13.1813. [DOI] [PubMed] [Google Scholar]
- 6.Berk BC, Weintraub WS, Alexander RW. Elevation of C-reactive protein in “active” coronary artery disease. Am J Cardiol. 1990;65(3):168–72. doi: 10.1016/0002-9149(90)90079-g. [DOI] [PubMed] [Google Scholar]
- 7.Arnaud C, Burger F, Steffens S, Veillard NR, Nguyen TH, Trono D, Mach F. Statins reduce interleukin-6-induced C-reactive protein in human hepatocytes: new evidence for direct antiinflammatory effects of statins. Arterioscler Thromb Vasc Biol. 2005;25(6):1231–6. doi: 10.1161/01.ATV.0000163840.63685.0c. [DOI] [PubMed] [Google Scholar]
- 8.Zhong Y, Li SH, Liu SM, Szmitko PE, He XQ, Fedak PW, Verma S. C-Reactive protein upregulates receptor for advanced glycation end products expression in human endothelial cells. Hypertension. 2006;48(3):504–11. doi: 10.1161/01.HYP.0000234904.43861.f7. [DOI] [PubMed] [Google Scholar]
- 9.Venugopal SK, Devaraj S, Yuhanna I, Shaul P, Jialal I. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation. 2002;106(12):1439–41. doi: 10.1161/01.cir.0000033116.22237.f9. [DOI] [PubMed] [Google Scholar]
- 10.Huang X, Zhang J, Liu J, Sun L, Zhao H, Lu Y et al. C-reactive protein promotes adhesion of monocytes to endothelial cells via NADPH oxidase-mediated oxidative stress. J Cell Biochem. 2012;113(3):857–67. doi: 10.1002/jcb.23415. [DOI] [PubMed] [Google Scholar]
- 11.Levine GN, Keaney JF, Jr, Vita JA. Cholesterol reduction in cardiovascular disease. Clinical benefits and possible mechanisms. N Engl J Med. 1995;332(8):512–21. doi: 10.1056/NEJM199502233320807. [DOI] [PubMed] [Google Scholar]
- 12.Calkin AC, Giunti S, Sheehy KJ, Chew C, Boolell V, Rajaram YS et al. The HMG-CoA reductase inhibitor rosuvastatin and the angiotensin receptor antagonist candesartan attenuate atherosclerosis in an apolipoprotein E-deficient mouse model of diabetes via effects on advanced glycation, oxidative stress and inflammation. Diabetologia. 2008;51(9):1731–40. doi: 10.1007/s00125-008-1060-6. [DOI] [PubMed] [Google Scholar]
- 13.Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation. 2004;109(23 Suppl 1):III39–43. doi: 10.1161/01.CIR.0000131517.20177.5a. [DOI] [PubMed] [Google Scholar]
- 14.Jialal I, Stein D, Balis D, Grundy SM, Adams-Huet B, Devaraj S. Effect of hydroxymethyl glutaryl coenzyme A reductase inhibitor therapy on high sensitive C-reactive protein levels. Circulation. 2001;103(15):1933–5. doi: 10.1161/01.cir.103.15.1933. [DOI] [PubMed] [Google Scholar]
- 15.Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes [published erratum appears in N Engl J Med 2006;354(7):778] N Engl J Med. 2004;350(15):1495–504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 16.de Lemos JA, Blazing MA, Wiviott SD, Lewis EF, Fox KA, White HD et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292(11):1307–16. doi: 10.1001/jama.292.11.1307. [DOI] [PubMed] [Google Scholar]
- 17.ESH/ESC Task Force for the Management of Arterial Hypertension. 2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens. 2013;31(10):1925–38. doi: 10.1097/HJH.0b013e328364ca4c. [DOI] [PubMed] [Google Scholar]
- 18.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(Suppl 1):S5–20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 19.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 20.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105(4):539–42. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 21.Knudsen AS, Darwish AZ, Norgaard A, Gotzsche O, Thygesen K. Time course of myocardial viability after acute myocardial infarction: an echocardiographic study. Am Heart J. 1998;135(1):51–7. doi: 10.1016/s0002-8703(98)70342-4. [DOI] [PubMed] [Google Scholar]
- 22.Smith SC, Jr, Dove JT, Jacobs AK, Kennedy JW, Kereiakes D, Kern MJ et al. ACC/AHA guidelines for percutaneous coronary intervention (revision of the 1993 PTCA guidelines)-executive summary: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (committee to revise the 1993 guidelines for percutaneous transluminal coronary angioplasty) endorsed by the Society for Cardiac Angiography and Interventions. Circulation. 2001;103(24):3019–41. doi: 10.1161/01.cir.103.24.3019. [DOI] [PubMed] [Google Scholar]
- 23.Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115(17):2344–51. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 24.Chyrchel M, Rakowski T, Rzeszutko L, Legutko J, Dziewierz A, Dubiel JS, Dudek D. Effects of high-dose statin administered prior to coronary angioplasty on the incidence of cardiac events in patients with acute coronary syndrome [in Polish] Kardiol Pol. 2006;64(12):1357–63. [PubMed] [Google Scholar]
- 25.Holschermann H, Schuster D, Parviz B, Haberbosch W, Tillmanns H, Muth H. Statins prevent NF-kappaB transactivation independently of the IKK-pathway in human endothelial cells. Atherosclerosis. 2006;185(2):240–5. doi: 10.1016/j.atherosclerosis.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 26.Schonbeck U, Libby P. Inflammation, immunity, and HMG-CoA reductase inhibitors: statins as antiinflammatory agents? Circulation. 2004;109(21 Suppl 1):II18–26. doi: 10.1161/01.CIR.0000129505.34151.23. [DOI] [PubMed] [Google Scholar]
- 27.Koh KK, Son JW, Ahn JY, Jin DK, Kim HS, Choi YM et al. Comparative effects of diet and statin on NO bioactivity and matrix metalloproteinases in hypercholesterolemic patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2002;22(9):e19–23. doi: 10.1161/01.atv.0000030997.02059.bb. [DOI] [PubMed] [Google Scholar]
- 28.Crisby M, Nordin-Fredriksson G, Shah PK, Yano J, Zhu J, Nilsson J. Pravastatin treatment increases collagen content and decreases lipid content, inflammation, metalloproteinases, and cell death in human carotid plaques: implications for plaque stabilization. Circulation. 2001;103(7):926–33. doi: 10.1161/01.cir.103.7.926. [DOI] [PubMed] [Google Scholar]
- 29.Kumar A, Cannon CP. The role of statins in the prevention of heart failure after acute coronary syndrome. Heart Fail Clin. 2008;4(2):129–39. doi: 10.1016/j.hfc.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 30.Choudhry NK, Levin R, Winkelmayer WC. Statins in elderly patients with acute coronary syndrome: an analysis of dose and class effects in typical practice. Heart. 2007;93(8):945–51. doi: 10.1136/hrt.2006.110197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Briel M, Schwartz GG, Thompson PL, de Lemos JA, Blazing MA, van Es GA et al. Effects of early treatment with statins on short-term clinical outcomes in acute coronary syndromes: a meta-analysis of randomized controlled trials. JAMA. 2006;295(17):2046–56. doi: 10.1001/jama.295.17.2046. [DOI] [PubMed] [Google Scholar]
- 32.Vale N, Nordmann AJ, Schwartz GG, de Lemos J, Colivicchi F, den Hartog F et al. Statins for acute coronary syndrome. Cochrane Database Syst Rev. 2011;(6):CD006870. doi: 10.1002/14651858.CD006870.pub2. [DOI] [PubMed] [Google Scholar]
- 33.Ko DT, Wijeysundera HC, Jackevicius CA, Yousef A, Wang J, Tu JV. Diabetes mellitus and cardiovascular events in older patients with myocardial infarction prescribed intensive-dose and moderate-dose statins. Circ Cardiovasc Qual Outcomes. 2013;6(3):315–22. doi: 10.1161/CIRCOUTCOMES.111.000015. [DOI] [PubMed] [Google Scholar]
- 34.Wong CK, Tang EW, Herbison P. Prognostic interactions between statins and in-hospital revascularisation on the outcome of acute coronary syndrome. Heart Lung Circ. 2009;18(4):262–5. doi: 10.1016/j.hlc.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Mora S, Glynn RJ, Boekholdt SM, Nordestgaard BG, Kastelein JJ, Ridker PM. On-treatment non-high-density lipoprotein cholesterol, apolipoprotein B, triglycerides, and lipid ratios in relation to residual vascular risk after treatment with potent statin therapy: JUPITER (justification for the use of statins in prevention: an intervention trial evaluating rosuvastatin) J Am Coll Cardiol. 2012;59(17):1521–8. doi: 10.1016/j.jacc.2011.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muhlestein JB, Anderson JL, Horne BD, Carlquist JF, Bair TL, Bunch TJ et al. Early effects of statins in patients with coronary artery disease and high C-reactive protein. Am J Cardiol. 2004;94(9):1107–12. doi: 10.1016/j.amjcard.2004.07.074. [DOI] [PubMed] [Google Scholar]
- 37.Bauersachs J, Galuppo P, Fraccarollo D, Christ M, Ertl G. Improvement of left ventricular remodeling and function by hydroxymethylglutaryl coenzyme A reductase inhibition with cerivastatin in rats with heart failure after myocardial infarction. Circulation. 2001;104(9):982–5. doi: 10.1161/hc3401.095946. [DOI] [PubMed] [Google Scholar]
- 38.Leone AM, Rutella S, Giannico MB, Perfetti M, Zaccone V, Brugaletta S et al. Effect of intensive vs standard statin therapy on endothelial progenitor cells and left ventricular function in patients with acute myocardial infarction: statins for regeneration after acute myocardial infarction and PCI (STRAP) trial. Int J Cardiol. 2008;130(3):457–62. doi: 10.1016/j.ijcard.2008.05.036. [DOI] [PubMed] [Google Scholar]


