Abstract
Extracorporeal membrane oxygenation (ECMO) is generally used as a last resort to provide cardiopulmonary support in patients whose advanced cardiac or respiratory failure does not respond to less invasive treatments. Lower-limb ischemia secondary to the large diameter of the arterial cannula is one of ECMO's major limitations: in patients who have small-caliber arteries, the cannulas can reduce native blood flow. The creation of a T-graft—a well-described technique to avoid limb ischemia—enables flow into the ECMO cannula without jeopardizing blood flow to the limb. However, leaving the graft exposed through an open groin wound can result in dislodgment, and it increases the risk of infection.
We describe our modification of a conventional T-graft in an 18-year-old woman who had systolic heart failure, acute respiratory distress syndrome, and small-caliber femoral vessels. We tunneled a polytetrafluoroethylene graft inside a Dacron graft, then ran the combined graft through a subcutaneous tunnel similar to that created for a peripheral bypass. Thus, the graft was protected from environmental exposure and the risk of infection. Our technique seems safer and more secure than the original T-graft technique, and we recommend its consideration during ECMO cannulation.
Keywords: Anastomosis, surgical; blood vessel prosthesis; extracorporeal membrane oxygenation/adverse effects/methods; femoral artery/surgery; ischemia/etiology/prevention & control; leg/blood supply; respiratory distress syndrome, adult/therapy; risk factors; treatment outcome; vascular surgical procedures/methods
Extracorporeal membrane oxygenation (ECMO) is a short-term mechanical circulatory support system for patients who are in advanced cardiac, pulmonary, or combined cardiopulmonary failure. Patients in cardiac failure are typically placed on venoarterial ECMO, and patients in respiratory failure are placed on venovenous ECMO. Large-diameter cannulas are needed to maintain high blood flow through the mechanical circulatory support system. However, upon insertion into blood vessels, these cannulas can reduce native blood flow, especially when vessel diameters are small. Acute limb ischemia can necessitate vascular reconstruction or amputation of the patient's extremities. We describe a modified T-graft technique that we used in order to avoid acute limb ischemia from ECMO in a young woman whose femoral arteries (FAs) were small.
Case Report
In April 2012, an 18-year-old woman presented with viral cardiomyopathy, systolic heart failure, and acute respiratory distress syndrome. She was intubated and was started on full mechanical ventilation. However, her pulmonary status continued to deteriorate, so we emergently placed her on ECMO. A 15F cannula was inserted in her right common FA, and a 19F cannula was inserted in her right common femoral vein. Several attempts to percutaneously place an antegrade distal-perfusion catheter in the right superficial FA failed because of small vessel size (diameter, 3–4 mm) and vasoconstriction. Critical limb ischemia subsequently developed in the patient's right leg. She remained hemodynamically unstable and would not have been able to tolerate a limb-revascularization procedure. During the next few days, her hemodynamic status improved; however, she became increasingly acidotic and sustained rhabdomyolysis and renal failure as the limb ischemia progressed. To prevent progression of the renal failure secondary to rhabdomyolysis, an emergency guillotine amputation of her right leg was performed, above the knee. At the same time, we decided to switch the ECMO to left femoral access by means of a modified T-graft technique, to avoid critical limb ischemia of her left leg.
The patient was taken to the operating room and was placed under general anesthesia. A vertical incision was made in her left groin to expose the femoral artery and vein. The common FA was approximately 6 mm in diameter and was not diseased. A 6-mm longitudinal arteriotomy was performed in that vessel. An 8-mm polytetrafluoroethylene (PTFE) graft was sewn end-to-side with use of a running 5-0 Prolene suture, in 4-quadrant fashion. This graft was then tunneled inside a 10-mm Dacron graft. We created a tunnel in the subcutaneous plane of the patient's thigh, similar to tunnels that are created for lower-extremity bypasses. Both grafts were then tunneled and brought through a separate stab wound distally. The PTFE graft was connected to the ECMO arterial cannula (Figs. 1 and 2). The distal stab incision was closed with use of nylon suture, in vertical mattress fashion. The left-groin wound was closed in 2 layers (Fig. 3); because of the poor condition of the overlying skin and the high risk of postoperative wound infection, vacuum-assisted closure was performed in the groin wound. After ECMO was reestablished in the left femoral system, the arterial cannula was removed from the right groin, and a bovine pericardial patch was used for right femoral patch angioplasty.
Fig. 1.

Photograph shows the end-to-side anastomosis between the common femoral artery and the polytetrafluoroethylene graft. This graft was tunneled inside the Dacron graft, and both were then tunneled underneath the subcutaneous plane of the thigh. The femoral venous cannula is visible on the medial side.
Fig. 2.
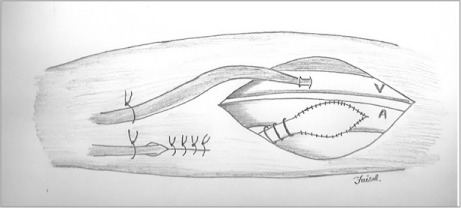
Illustration shows the end-to-side anastomosis between the common femoral artery and the polytetrafluoroethylene graft. The femoral venous cannula (V) is visible on the medial side.
A = femoral arterial cannula
Fig. 3.

Photograph shows the secured arterial and venous cannulas running parallel to each other. The groin wound is closed, and no graft is exposed.
The patient remained on this modified ECMO system for 7 days without further ischemic sequelae. Her overall condition improved, and she was gradually weaned from ECMO. During surgical ECMO decannulation, the prosthetic grafts were completely explanted, and the FA was patched with use of the ipsilateral saphenous vein. Afterwards, the patient had palpable pedal pulses and no signs of limb ischemia.
Discussion
Patients whose cardiopulmonary failure does not respond to conventional treatment can benefit from ECMO. The FA has become the preferred access site for venoarterial ECMO because of easy cannulation, percutaneous accessibility, less bleeding, and fewer neurologic sequelae.1,2 However, vasoconstriction, hemodynamic instability, pressor support, and small FA diameter can contribute to diminished blood flow and possible limb ischemia after large-diameter ECMO cannulas are placed. The prevalence of ischemic sequelae in such patients is 40% to 70%.2–5
In these instances, limb ischemia can be avoided in multiple ways. A small sheath can be placed in the superficial FA to maintain antegrade distal-limb perfusion. However, profound vasoconstriction and small FA size, as in young women and children, can make sheath placement extremely difficult. Several attempts to place the perfusion sheath in our patient failed, because of her small-caliber femoral vessels. She was not hemodynamically stable enough to undergo open surgical cutdown and placement of an antegrade cannula in the superficial FA. When the critical limb ischemia developed in her right leg and the transition of ECMO to her left leg became necessary, it was evident that direct femoral cannulation, even with use of a distal-perfusion sheath, would not be plausible.
Another technique to avoid limb ischemia is the T-graft. This involves an end-to-side anastomosis between the common FA and a prosthetic graft. The theoretical advantage of this technique is that it enables blood flow through the cannula without compromising blood flow through the native artery. The T-graft technique especially suits those patients who have small-diameter FAs.
Apparently, neither PTFE nor Dacron has been used often as a conduit for femoral venoarterial ECMO. A PTFE graft was used for TandemHeart® cannula insertion (CardiacAssist, Inc.; Pittsburgh, Pa).6 That patient's excessive postoperative bleeding necessitated another operation, during which the PTFE graft was tunneled inside a Dacron graft to stop the excessive bleeding and provide double-layer strength to the sidearm graft. Burkle and colleagues7 described chimney-graft cannulation with use of a single 6-mm Dacron graft, but without subcutaneous tunneling. After graft anastomosis, the chimney-graft was extended over the skin and was connected to the ECMO cannula.
Although the T-graft technique is well described, most relevant case reports describe open groin wounds with prosthetic tubes emerging therefrom. Prosthetic grafts exposed to the atmosphere are associated with a high risk of infection, are inherently insecure, and can contribute to the inadvertent dislodgment of the large cannulas. In view of these factors, we decided to create a subcutaneous tunnel in our patient's thigh, fashioned as vascular surgeons might create extremity bypasses. We reasoned that this technique would enable groin closure and avoid atmospheric exposure, thus preventing graft infection. In addition, the secure attachment for the ECMO circuit would be less susceptible to accidental dislodgment. When this conduit was removed a week later, there were no signs of infection, and the tunneled part of the prosthetic tube was well incorporated in the surrounding tissues.
In summary, our modified T-graft technique achieved 3 goals:
1) The completely covered anastomosis between the graft and native FA reduced the risks of anastomotic dehiscence, arterial infection, and inadvertent dislodgment of the prosthesis. 2) Tunneling the prosthetic tube underneath the skin tissue prevented graft infection and lowered the risk of dislodgment. 3) Tunneling the PTFE graft inside the Dacron graft reduced bleeding (and “sweating”) from the PTFE graft.
This modified T-graft technique seems safe, viable, and not inferior to the original technique. We think that it should be considered by surgeons who perform routine arterial cannulations for ECMO catheters.
Footnotes
From: Section of Vascular Surgery, Department of Surgery (Drs. Aziz, Calderon, and Reed) and Heart and Vascular Critical Care Unit (Drs. El-Banayosy and Koerner), Heart and Vascular Institute, Pennsylvania State University College of Medicine, Hershey, Pennsylvania 17033
Presented at the Eastern Vascular Society Annual Meeting, White Sulphur Springs, WV; 21 September 2013.
Drs. El-Banayosy and Koerner are now at Integris Baptist Medical Center, Oklahoma City, Oklahoma.
References
- 1.Jackson KW, Timpa J, McIlwain RB, O'Meara C, Kirklin JK, Borasino S, Alten JA. Side-arm grafts for femoral extracorporeal membrane oxygenation cannulation. Ann Thorac Surg. 2012;94(5):e111–2. doi: 10.1016/j.athoracsur.2012.05.064. [DOI] [PubMed] [Google Scholar]
- 2.Bisdas T, Beutel G, Warnecke G, Hoeper MM, Kuehn C, Haverich A, Teebken OE. Vascular complications in patients undergoing femoral cannulation for extracorporeal membrane oxygenation support. Ann Thorac Surg. 2011;92(2):626–31. doi: 10.1016/j.athoracsur.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 3.Aziz F, Brehm CE, El-Banayosy A, Han DC, Atnip RG, Reed AB. Arterial complications in patients undergoing extracorporeal membrane oxygenation via femoral cannulation. Ann Vasc Surg. 2014;28(1):178–83. doi: 10.1016/j.avsg.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Zimpfer D, Heinisch B, Czerny M, Hoelzenbein T, Taghavi S, Wolner E, Grimm M. Late vascular complications after extracorporeal membrane oxygenation support. Ann Thorac Surg. 2006;81(3):892–5. doi: 10.1016/j.athoracsur.2005.09.066. [DOI] [PubMed] [Google Scholar]
- 5.Foley PJ, Morris RJ, Woo EY, Acker MA, Wang GJ, Fairman RM, Jackson BM. Limb ischemia during femoral cannulation for cardiopulmonary support. J Vasc Surg. 2010;52(4):850–3. doi: 10.1016/j.jvs.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Busch J, Torre-Amione G, Noon G, Loebe M. TandemHeart insertion via a femoral arterial GORE-TEX graft conduit in a high-risk patient. Tex Heart Inst J. 2008;35(4):462–5. [PMC free article] [PubMed] [Google Scholar]
- 7.Burkle MA, Sodian R, Kaczmarek I, Weig T, Frey L, Irlbeck M, Dolch ME. Arterial chimney graft cannulation for interventional lung assist. Ann Thorac Surg. 2012;94(4):1335–7. doi: 10.1016/j.athoracsur.2012.02.031. [DOI] [PubMed] [Google Scholar]


