Abstract
Aberrant methylation of the breast cancer susceptibility gene 1 (BRCA1) promoter is a mechanism for its functional inactivation. It may potentially be used as a prognostic marker in studies for patients with breast cancer and plays an important role in tumorigenesis. Numerous studies have suggested that the methylation of the BRCA1 promoter is associated with the prognosis of breast cancer. However, the prognosis of BRCA1 promoter methylation in breast cancer patients of different ethnicities remains ambiguous. The present meta-analysis was performed to adjust and augment a previously published study, which estimated the correlations between promoter methylation of BRCA1 and the clinical outcomes of breast cancer patients. These results indicated that BRCA1 methylation was significantly correlated with a poor prognosis of breast cancer, particularly for Asian patients, but the correlation was over-estimated in the previous study. The combined hazard ratios (HRs) in the present study were 1.76 (1.15–2.68) and 1.97 (1.12–3.44) for univariate and multivariate analysis of overall survival, which were different from 2.02 (1.35–3.03) and 1.38 (1.04–1.84) in the previous study. For studies of disease-free survival, the univariate and multivariate analyses also have different pooled HRs: 2.89 (1.73–4.83) and 3.92 (1.49–10.32) in the previously published study and 1.28 (0.68–2.43) and 1.64 (0.64–4.19) in the present study. In addition, the BRCA1 promoter regions used to detect the hypermethylation were different. All the studies using the Baldwin's primer reported that breast cancer patients with BRCA1 promoter methylation had a better prognosis. There were also correlations between BRCA1 promoter methylation and receptor-negativity of the estrogen receptors, progesterone receptor, human epidermal growth factor receptor 2 and a triple-negative status. Patients with the estrogen, progesterone and epidermal growth factor-related receptor-negative status were more likely to be negative for the BRCA1 protein.
Keywords: breast cancer, BRCA1, methylation, prognosis, survival, estrogen receptors, progesterone receptor, human epidermal growth factor receptor 2
Introduction
Breast cancer is the most frequently diagnosed cancer in women worldwide (1,2). It has an increasing mortality and morbidity rate in women <45 years. Every year in China, ~1.6 million women are diagnosed and ~1.2 million people succumb to breast cancer. Breast cancer results from the accumulation of abnormal genetic and epigenetic changes in tumor-suppressor genes and proto-oncogenes (3). Genes, such as р53, АТМ and human epidermal growth factor receptor 2 (HER2), are involved in different types of tumors. Breast cancer susceptibility gene 1 (BRCA1) is another specific gene, which was identified as a genetic cause of hereditary breast cancer.
BRCA1 is located on chromosome 17q12-21 (2). It is an important tumor-suppressor gene associated with human breast cancer (4). The BRCA1 protein plays an important role in DNA repair of double-strand breaks (5), transcriptional regulation, ubiquitinylation, as well as other functions (6,7). The hypermethylation of the BRCA1 promoter has been considered as an inactivating mechanism of BRCA1 expression (8). This low expression or non-expression of BRCA1 may not be adequate for repairing DNA damage that further promotes the accumulation of mutations in cell growth and division. Certain results suggest that BRCA1 promoter hypermethylation is associated with poor clinical outcomes. In the present study, the source data used by the study of Wu et al (9) was adjusted and augmented to investigate the association between BRCA1 methylation and the outcome of breast cancer.
The therapeutic targets for breast cancer are the receptors. The progesterone receptor (PR) is a nuclear receptor located inside cells. PR is encoded by chromosome 11q22 in humans. Estrogen receptors (ER) are receptors that are activated by the hormone estrogen (10). HER2 is a member of the epidermal growth factor receptor (EGFR/ERBB) family. In recent years these proteins have been used as therapy targets in <30% of breast cancer patients (11). Triple-negative breast cancer is defined as the absence of ER, PR and HER2 (12). The present treatment for triple-negative breast cancer is a type of chemotherapy and often has a poor outcome. Therefore, it is essential to find new and alternative therapeutic strategies. In the present meta-analysis, the correlations between therapy target-related negative-receptors and BRCA1 promoter methylation were also studied. To ensure the quality of analysis, the Begg's test, χ2-based Q test, Egger's test, sensitivity analysis and publication bias analysis were used.
Materials and methods
Literature search
Two investigators independently conducted a literature search using PubMed, Embase and Google scholar (last search updated on September 14, 2014). The keywords used included: BRCA1, breast carcinoma, breast cancer, methylation, prognosis and survival. In addition, the PubMed additional function: Related citations; and the references of the selected studies were scrutinized to identify additional studies.
Eligibility criteria
Studies were included in the meta-analysis only if they had met the following criteria: i) Evaluated prognostic risk of patients with BRCA1 methylation; ii) provided overall survival (OS) or disease-free survival (DFS); iii) hazard ratios (HR) or odds ratios (OR) with its 95% confidence intervals (CIs); iv) published in English; and v) data from human subjects. In addition, studies were excluded if: i) Data was from reviews or animal studies; and ii) studies had the same population resources or overlapping datasets.
Data extraction
Following the exclusions, 9 studies met all the criteria. Two investigators independently extracted the following data from each study: First author's last name, year of publication, population, number of study subjects, effects on clinical outcomes (OS and DFS), and the number of methylated and unmethylated patients with a different status of ER, PR, HER2 and triple-negative receptors. OS is a term that denotes the chances of remaining alive for a group of individuals suffering from a type of cancer. At a basic level, the OS is representative of cure rates. DFS was defined as the chances of staying free of disease following a particular treatment for a group of individuals suffering from a type of cancer. It is an indication of how effective a particular treatment is.
Statistical analysis
Random effects and subgroup meta-analysis were performed according to the DerSimonian Laird method (13), due to the existence of heterogeneity between studies. The data were divided into two groups by population: European and Asian. The HRs were used to estimate the pooled effect of BRCA1 methylation on the prognosis of patients with breast cancer, and the ORs were pooled to estimate the strength of the association between BRCA1 hypermethylation and the risk of three negative statuses of receptors (ER, PR and HER2). When HRs (95% CIs) were shown only in the figure of survival curves, the authors were contacted for the exact value or the investigators estimated them according to the methods provided by Tierney et al (14). The Cochran Q (significant cut-off point: P=0.10) and I2 (I2>50%, strong heterogeneity) statistics (15,16) were used to assess heterogeneity between studies. The Galbraith plot (17) was used to detect the potential sources of heterogeneity from the meta-analysis. Publication bias was assessed by funnel plot and the test of Egger et al (18). Sensitivity analyses were performed by the trim-and-fill method (19). All the analyses and graphs were obtained using STATA 11.0 software (StataCorp LP, College Station, TX, USA).
Results
Characteristics of studies
Fig. 1 summarizes the process of identifying eligible studies. Following the screening by two investigators independently, according to the inclusion criteria there were 9 studies with 3,131 study subjects entered into the meta-analysis (20–28). The characteristics of these studies are listed in Tables I and II. There were 4 studies from Europe and 5 studies from Asia.
Figure 1.
Study selection flow chart for the meta-analysis.
Table I.
Characteristics of the included studies.
| OS | DFS | |||||||
|---|---|---|---|---|---|---|---|---|
| First author, year | Population | Patients, n | Primer | Univariate analysis HR (95% CI) | Multivariate analysis HR (95% CI) | Univariate analysis HR (95% CI) | Multivariate analysis HR (95% CI) | (Refs.) |
| Sharma, 2014 | European | 39 | Esteller | 3.37 (0.23–50.02) | 6.2 (2–19.4) | N/A | 3.5 (1.3–9.8) | (20) |
| Ignatov, 2013 | European | 65 | Baldwin | N/A | N/A | 0.325 (0.16–0.662) | 0.224 (0.092–0.546) | (21) |
| Krasteva, 2012 | European | 135 | Baldwin | 0.47 (0.14–1.54) | 0.91 (0.24–3.41) | N/A | N/A | (22) |
| Xu, 2009 | European | 851 | Others | 1.72 (1.06–2.79) | 1.67 (0.99–2.81) | N/A | N/A | (23) |
| Xu, 2013 | Asian | 1,163 | Esteller | 1.29 (0.96–1.73) | N/A | 1.34 (1.06–1.71) | N/A | (24) |
| Hsu, 2013 | Asian | 139 | Esteller | N/A | 16.38 (1.37–195.45) | N/A | 12.19 (2.29–64.75) | (25) |
| Sharma, 2009 | Asian | 101 | Esteller | 5.06 (1.58–16.22) | 2.12 (0.47–9.63) | 3.88 (2.05–7.34) | 2.03 (0.96–4.29) | (26) |
| Chen, 2009 | Asian | 536 | Esteller | 1.56 (1.02–2.37) | 1.27 (0.81–1.99) | 1.45 (1.01–2.09) | 1.23 (0.84–1.8) | (27) |
| Jing, 2008 | Asian | 102 | Esteller | 6.4 (2.0–20.5) | N/A | N/A | N/A | (28) |
OS, overall survival; DFS, disease-free survival; HR, CI hazard ratio; CI, confidence interval.
Table II.
Distribution of the BRCA1 methylation status with different hormone and epidermal growth factor receptors.
| BRCA1 methylation, n (total) | BRCA1 non-methylation, n (total) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| First author, year | Population | ER negative | PR negative | HER2 negative | Triple-negative | ER negative | PR negative | HER2 negative | Triple-negative | (Refs.) |
| Ignatov, 2013 | European | NA | NA | NA | 43 (86) | NA | NA | NA | 22 (46) | (21) |
| Krasteva, 2012 | European | 11 (23) | 11 (23) | 14 (17) | NA | 48 (112) | 11 (23) | 37 (53) | NA | (22) |
| Xu, 2009 | European | 89 (372) | 135 (372) | NA | NA | 127 (320) | 135 (372) | NA | NA | (23) |
| Xu, 2013 | Asian | 109 (285) | 149 (282) | 220 (279) | 64 (282) | 295 (830) | 149 (282) | 654 (810) | 142 (817) | (24) |
| Hsu, 2013 | Asian | 30 (77) | 36 (77) | 55 (77) | 16 (77) | 21 (61) | 36 (78) | 91 (138) | 5 (61) | (25) |
| Sharma, 2009 | Asian | 22 (27) | 21 (27) | 18 (27) | 15 (27) | 35 (74) | 21 (27) | 60 (74) | 25 (74) | (26) |
| Chen, 2009 | Asian | 55 (138) | 80 (137) | 98 (135) | 31 (136) | 127 (383) | 80 (137) | 298 (378) | 64 (382) | (27) |
| Jing, 2008 | Asian | 21 (33) | 26 (33) | NA | NA | 127 (168) | 26 (33) | NA | NA | (28) |
Total refers to the total number of identified samples in BRCA1 methylation or non-methylation with the corresponding hormone receptor: ER, PR and HER2. Triple-negative, lacks expression of ER, PR and HER2 amplification. ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; BRCA1, breast cancer susceptibility gene 1.
As shown in Table II, there were 8 studies that met the inclusion criteria and were included in the present meta-analysis. The studies involved 337 BRCA1 promoter hypermethylations with an ER-negative status, 458 with a PR-negative status, 405 with an HER2-negative status, 169 with triple-negative receptors, and 780, 458, 1,140 and 258 controls without BRCA1 promoter methylation but with a negative status, correspondingly.
When the same investigators reported the results obtained from the same cohort of patients in several studies, only the largest series was included in the analysis. A cohort of patients was excluded due to duplicate studies.
Due to insufficient data, HRs on OS could be extracted from 7 studies for univariate analysis and 6 studies for multivariate analysis. According to DFS analysis, there were 4 studies with available data for univariate analysis and 5 studies with available data for multivariate analysis.
Association of BRCA1 methylation with OS and DFS of patients with breast cancer
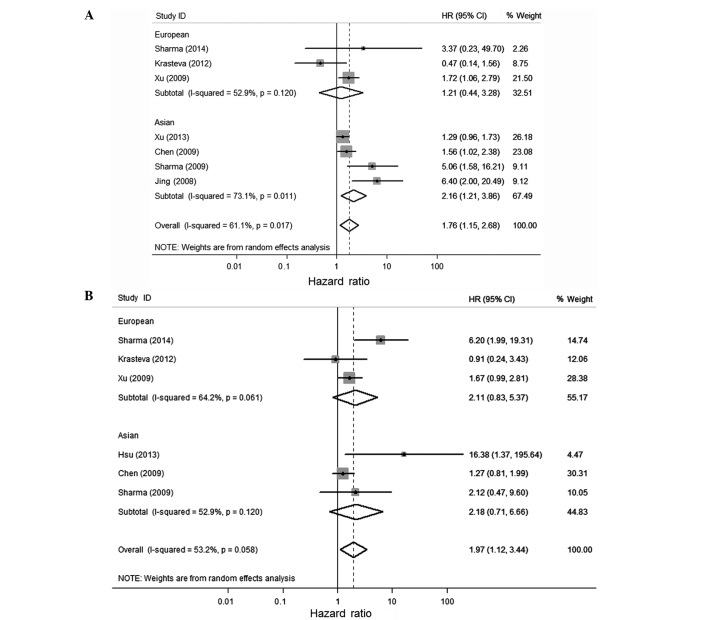
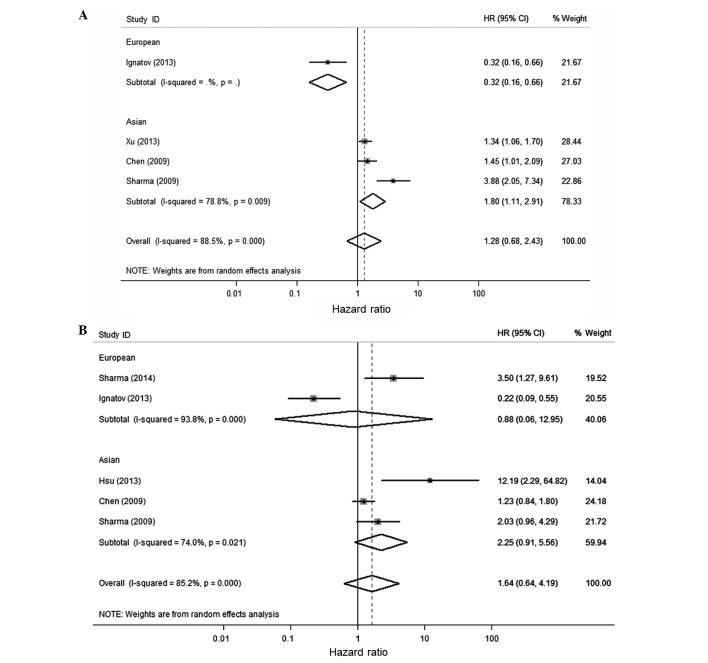
Considering the significant heterogeneity among studies (P=0.017, I2=61.1%), the random-effect model was used and a subgroup analysis was performed by considering different ethnicities or the population of the participants to estimate the combined effect of BRCA1 methylation. BRCA1 methylation was significantly associated with a poor OS and DFS of breast cancer in the univariate and multivariate analysis (Figs. 2 and 3 and Table III). The combined HR was 1.76 (1.15–2.68) and 1.97 (1.12–3.44) for univariate analysis and multivariate analysis of OS, respectively. For studies of DFS, the pooled HR was 1.28 (0.68–2.43) and 1.64 (0.64–4.19), respectively. The combined HRs (95% CIs) on OS by univariate analysis was 2.16 (1.21–3.86) for the Asian population, which was significantly higher than 1.21 (0.44–3.28) for the European population. All the combined HR scores in the present study are different from the study of Wu et al (9). They over-estimated the risk of fatality by using the OR instead of HR value from the Karray-Chouayekh study (29) and using the incorrect value of OS in the Xu et al study (23).
Figure 2.
Forest plot for the association between breast cancer susceptibility gene 1 (BRCA1) promoter methylation and the overall survival (OS) using univariate (A) and multivariate (B) analysis. The values of the last line in (A and B) are the combined hazard ratio (HR).
Figure 3.
Forest plot for the association between breast cancer susceptibility gene 1 (BRCA1) promoter methylation and disease-free survival (DFS) using univariate (A) and multivariate (B) analysis. The values of the last line in (A and B) are the combined hazard ratio (HR).
Table III.
HRs and 95% CI for the association between BRCA1 methylation and OS or DFS.
| Analysis | Population | Survival | Pooled HR (95% CI), P-value | Heterogeneity test P-value | Eggers test P-value | Beggs test P-value |
|---|---|---|---|---|---|---|
| Univariate | European | OS | 1.206 (0.444–3.276), 0.713 | 0.120 | 0.797 | 0.602 |
| DFS | – | – | – | – | ||
| Asian | OS | 2.164 (1.212–3.863), 0.009 | 0.011 | 0.004 | 0.174 | |
| DFS | 1.795 (1.109–2.907) | 0.009 | 0.236 | 0.117 | ||
| Overall | OS | 1.758 (1.154–2.679), 0.009 | 0.017 | <0.001 | 0.393 | |
| DFS | 1.284 (0.679–2.430), 0.442 | <0.001 | 1.000 | 0.885 | ||
| Multivariate | European | OS | 2.107 (0.827–5.368), 0.118 | 0.061 | 0.830 | 0.602 |
| DFS | 0.876 (0.059–12.953), 0.923 | <0.001 | – | 0.317 | ||
| Asian | OS | 2.177 (0.711–6.663), 0.173 | 0.120 | 0.256 | 0.117 | |
| DFS | 2.250 (0.911–5.562), 0.079 | 0.021 | 0.074 | 0.117 | ||
| Overall | OS | 1.966 (1.124–3.438), 0.018 | 0.058 | 0.019 | 0.460 | |
| DFS | 1.635 (0.638–4.191), 0.306 | <0.001 | <0.001 | 0.064 |
HR, hazard ratio; CI, confidence interval; BRCA1, breast cancer susceptibility gene 1; OS, overall survival; DFS, disease-free survival.
Primer for identifying BRCA1 promoter methylation
The majority of the studies identified that BRCA1 promoter methylation is correlated with poor survival, as shown in the present meta-analysis. However, an opposing opinion remains as BRCA1 promoter methylation is a protective factor in 2 studies [Krasteva et al (22) and Ignatov et al (21)]. To explore this difference, we identified that they used different primers. In the Krasteva et al (22) and Ignatov et al (21) studies, which found a better clinical prognosis in patients with BRCA1 promoter methylation, they used Baldwin's primer (30) while others used Esteller's primer (31). The two primer sequences were blasted to the human genome in the NCBI database to find the amplified regions. The two primers are for different regions where there was a different concentration of GC-rich regions. The Baldwin primer exhibited a larger amplification that contained more GC-rich regions and included the transcriptional start point (Fig. 4). Therefore, the difference of BRCA1 promoter methylation in the region of chr17: 43, 125, 429-43, 125, 541 (GRCh38 assembly) may affect the different prognosis. However, all of these require more testing by molecular-biological experiments to confirm.
Figure 4.
Schematic illustration of the breast cancer susceptibility gene 1 (BRCA1) promoter region of methylation-specific polymerase chain reaction (PCR) by two different primers. The view of the genomic context was adapted from UCSC Genome Browsers. The black bar shows the predicted GC-rich region in the promoter region. The amplification region for Esteller and Baldwin primers are chr17: 43125355–43125429, chr17: 43125360–43125541, respectively (assembly version: GRCh38/hg38).
Hormone receptor-negativity correlation analysis
The number of patients who carried BRCA1 methylation and one of the negative statuses of ER, PR, HER2 or triple-negative were extracted for the cases, as well as the number of patients without promoter methylation of BRCA1 correspondingly for the controls. The correlations between BRCA1 methylation and the negative status of different breast cancer-related receptors were meta-analyzed separately. The results of the association between hormone receptors that were negative, BRCA1 promoter methylation and the heterogeneity test are shown in Table IV. The overall results suggested that, particularly for the Asian populations, all the receptors negativity listed in Table II are associated with the BRCA1 promoter methylation. Patients with those estrogen, progesterone and epidermal growth factor-related negative receptors were more likely to be negative for the BRCA1 protein (OR>1.247, P<0.014). However, further molecular-biological experiments are required to determine whether the correlation is spurious, as the receptor and BRCA1 promoter methylation are associated with breast cancer.
Table IV.
Odds ratios and 95% CI for BRCA1 promoter methylation and breast cancer subtype.
| Heterogeneity test | |||||
|---|---|---|---|---|---|
| Factors | Pooled OR (95% CI) | P-valuea | χ2 | P-value | I2 (%) |
| ER negative | |||||
| Asian | 1.329 (1.084–1.630) | 0.006 | 9.41 | 0.052 | 57.5 |
| European | 1.040 (0.736–1.471) | 0.824 | 0.14 | 0.704 | 0.0 |
| Overall | 1.247 (1.045–1.487) | 0.014 | 10.83 | 0.094 | 44.6 |
| PR negative | |||||
| Asian | 1.459 (1.195–1.782) | <0.001 | 15.62 | 0.004 | 74.4 |
| European | 1.010 (0.740–1.379) | 0.948 | 0.28 | 0.599 | 0.0 |
| Overall | 1.311 (1.108–1.550) | 0.002 | 19.30 | 0.004 | 68.9 |
| HER2 negative | |||||
| Asian | 2.834 (2.277–3.528) | <0.001 | 2.98 | 0.394 | 0.0 |
| European | 2.881 (2.322–3.574) | <0.001 | 3.65 | 0.456 | 0.0 |
| Triple-negative | |||||
| Asian | 1.557 (1.210–2.002) | 0.001 | 2.81 | 0.422 | 0.0 |
| European | 1.494 (1.178–1.896) | 0.001 | 3.61 | 0.461 | 0.0 |
P-value for the association from the meta-analysis. CI, confidence interval; OR, odds ratio; ER, estrogen receptor; BRCA1, breast cancer susceptibility gene 1; HER2, human epidermal growth factor receptor 2.
Sensitivity analysis and publication bias
The Patsopoulos method (32) was used to test if an individual study affected the heterogeneity in the OS and DFS analysis. As a result, if the Jing et al (28) and Sharma et al (26) studies were removed the heterogeneity disappeared (I2=17.3%, P=0.30). Funnel plot and Begg's test were performed to check the publication bias, and they suggest the absence of publication bias. The Begg's test for OS and DFS were P=0.187 and P=0.625, respectively.
Discussion
The present meta-analysis suggested that patients with BRCA1 promoter methylation had a more significant OS and DFS disadvantage than those without the methylated status, similar to the previous meta-analysis by Wu et al (9), which showed that breast cancer patients with hypermethylation in the promoter of BRCA1 exhibited poor survival. However, three problems were identified in the Wu et al (9) study, which were adjusted in the present study: i) A meta-analysis was performed for the 5 adjusted HR scores of DFS, but one was an OR (20.7) score instead of HR, [from the Karray-Chouayekh study (29)]and was much larger than the others; ii) in the meta-analysis for the 5 adjusted HR scores of OS, the value observed for all-cause mortality instead of fatality from breast cancer in the Xu et al (23) study was used; iii) 3 of the 9 HRs from the Sharma et al (20,26,33) studies published in 2009 and 2010 for meta-analysis were from the same subjects. Following adjustment for these problems and the addition of a study, the present meta-analysis suggested that the previous study had over-estimated the risk of fatality from breast cancer. The pooled HRs in the present study and the Wu et al (9) study were 1.28 vs. 2.89 and 1.64 vs. 3.92 for univariate and multivariate analysis of DFS, respectively.
The present meta-analysis additionally assessed the associations between the promoter methylation of BRCA1 and breast cancer-related receptors and the results showed that there are significant correlations between them. This suggested that potential interactions may exist between the hypermethylation of the BRCA1 gene and the negative status of ER, PR and HER2 receptors through complex regulation pathways on tumor progression. However, further studies are also required to explore the correlations in order to assist in finding a therapeutic target for breast cancer.
The consistency of two studies that reported the protective effect for patients with hypermethylation in the promoter of BRCA1 was found to be due to the different primers used. Therefore, further studies are required to test whether the different regions of the promoter methylation of BRCA1 exhibit different clinical outcomes.
However, certain limitations of the study should be considered. First, the number of studies contained in the present meta-analysis is relatively small, particularly in non-African populations, and the results should be confirmed in large samples. Second, although the Egger's test did not have statistical significance, the publication bias may still exist and influence the results. Asymmetrical appearance of the funnel plot could be caused by heterogeneity, smaller studies and other factors. Considering the limitations of the study, the associations among BRCA1 promoter methylation, prognosis of patients and the negative status of the breast cancer-related therapeutic target receptors should be further investigated.
In conclusion, the results revealed that breast cancer patients with BRCA1 promoter methylation had lower OS and DFS and had significant correlations with the negative status of the ER, PR and HER2 receptors. Hypermethylation in different regions of the BRCA1 promoter may exhibit a different clinical performance.
Acknowledgements
The present study was financially supported by the National Science Foundation of China (grant no. 81101547), the Planned Science and Technology Project of Yunnan Province (grant nos. 2009CA012 and 2011DH011), and the Fund of State Key Laboratory of Genetics Resources and Evolution (grant no. GREKF10-07).
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Russo J, Yang X, Hu YF, Bove BA, Huang Y, Silva ID, Tahin Q, Wu Y, Higgy N, Zekri A, et al. Biological and molecular basis of human breast cancer. Front Biosci. 1998;3:D944–D960. doi: 10.2741/a335. [DOI] [PubMed] [Google Scholar]
- 4.Miki Y, Swensen J, ShattuckEidens D, Futreal PA, Harshman K, Tavtigian S, Liu Q, Cochran C, Bennett LM, Ding W, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 5.Friedenson B. The BRCA1/2 pathway prevents hematologic cancers in addition to breast and ovarian cancers. BMC Cancer. 2007;7:152. doi: 10.1186/1471-2407-7-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ralhan R, Kaur J, Kreienberg R, Wiesmüller L. Links between DNA double strand break repair and breast cancer: Accumulating evidence from both familial and nonfamilial cases. Cancer Lett. 2007;248:1–17. doi: 10.1016/j.canlet.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Hall JM, Lee MK, Newman B, Morrow JE, Anderson LA, Huey B, King MC. Linkage of early-onset familial breast cancer to chromosome 17q21. Science. 1990;250:1684–1689. doi: 10.1126/science.2270482. [DOI] [PubMed] [Google Scholar]
- 8.Birgisdottir V, Stefansson OA, Bodvarsdottir SK, Hilmarsdottir H, Jonasson JG, Eyfjord JE. Epigenetic silencing and deletion of the BRCA1 gene in sporadic breast cancer. Breast Cancer Res. 2006;8:R38. doi: 10.1186/bcr1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu L, Wang F, Xu R, Zhang S, Peng X, Feng Y, Wang J, Lu C. Promoter methylation of BRCA1 in the prognosis of breast cancer: A meta-analysis. Breast Cancer Res Treat. 2013;142:619–627. doi: 10.1007/s10549-013-2774-9. [DOI] [PubMed] [Google Scholar]
- 10.DahlmanWright K, Cavailles V, Fuqua SA, Jordan VC, Katzenellenbogen JA, Korach KS, Maggi A, Muramatsu M, Parker MG, Gustafsson JA. International Union of Pharmacology. LXIV. Estrogen receptors. Pharmacol Rev. 2006;58:773–781. doi: 10.1124/pr.58.4.8. [DOI] [PubMed] [Google Scholar]
- 11.Mitri Z, Constantine T, O'Regan R. The HER2 receptor in breast cancer: Pathophysiology, clinical use, and new advances in therapy. Chemother Res Pract. 2012;2012:743193. doi: 10.1155/2012/743193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bosch A, Eroles P, Zaragoza R, Viña JR, Lluch A. Triple-negative breast cancer: Molecular features, pathogenesis, treatment and current lines of research. Cancer Treat Rev. 2010;36:206–215. doi: 10.1016/j.ctrv.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Fleiss JL. The statistical basis of meta-analysis. Stat Methods Med Res. 1993;2:121–145. doi: 10.1177/096228029300200202. [DOI] [PubMed] [Google Scholar]
- 14.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DerSimonian R. Meta-analysis in the design and monitoring of clinical trials. Stat Med. 1996;15:1237–1252. doi: 10.1002/(SICI)1097-0258(19960630)15:12<1237::AID-SIM301>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bax L, Ikeda N, Fukui N, Yaju Y, Tsuruta H, Moons KG. More than numbers: The power of graphs in meta-analysis. Am J Epidemiol. 2009;169:249–255. doi: 10.1093/aje/kwn340. [DOI] [PubMed] [Google Scholar]
- 18.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 20.Sharma P, Stecklein SR, Kimler BF, Sethi G, Petroff BK, Phillips TA, Tawfik OW, Godwin AK, Jensen RA. The prognostic value of BRCA1 promoter methylation in early stage triple negative breast cancer. J Cancer Ther Res. 2014;3:1–11. doi: 10.7243/2049-7962-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ignatov T, Poehlmann A, Ignatov A, Schinlauer A, Costa SD, Roessner A, Kalinski T, Bischoff J. BRCA1 promoter methylation is a marker of better response to anthracycline-based therapy in sporadic TNBC. Breast Cancer Res Treat. 2013;141:205–212. doi: 10.1007/s10549-013-2693-9. [DOI] [PubMed] [Google Scholar]
- 22.Krasteva ME, Bozhanov SS, Antov GG, Gospodinova ZI, Angelov SG. Breast cancer patients with hypermethylation in the promoter of BRCA1 gene exhibit favorable clinical status. Neoplasma. 2012;59:85–91. doi: 10.4149/neo_2012_011. [DOI] [PubMed] [Google Scholar]
- 23.Xu X, Gammon MD, Zhang Y, Bestor TH, Zeisel SH, Wetmur JG, Wallenstein S, Bradshaw PT, Garbowski G, Teitelbaum SL, et al. BRCA1 promoter methylation is associated with increased mortality among women with breast cancer. Breast Cancer Res Treat. 2009;115:397–404. doi: 10.1007/s10549-008-0075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Y, Diao L, Chen Y, Liu Y, Wang C, Ouyang T, Li J, Wang T, Fan Z, Fan T, et al. Promoter methylation of BRCA1 in triple-negative breast cancer predicts sensitivity to adjuvant chemotherapy. Ann Oncol. 2013;24:1498–1505. doi: 10.1093/annonc/mdt011. [DOI] [PubMed] [Google Scholar]
- 25.Hsu NC, Huang YF, Yokoyama KK, Chu PY, Chen FM, Hou MF. Methylation of BRCA1 promoter region is associated with unfavorable prognosis in women with early-stage breast cancer. PLoS One. 2013;8:e56256. doi: 10.1371/journal.pone.0056256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma G, Mirza S, Yang YH, Parshad R, Hazrah P, Datta Gupta S, Ralhan R. Prognostic relevance of promoter hypermethylation of multiple genes in breast cancer patients. Cell Oncol. 2009;31:487–500. doi: 10.3233/CLO-2009-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y, Zhou J, Xu Y, Li Z, Wen X, Yao L, Xie Y, Deng D. BRCA1 promoter methylation associated with poor survival in Chinese patients with sporadic breast cancer. Cancer Sci. 2009;100:1663–1667. doi: 10.1111/j.1349-7006.2009.01225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jing F, Jun L, Yong Z, Wang Y, Fei X, Zhang J, Hu L. Multigene methylation in serum of sporadic Chinese female breast cancer patients as a prognostic biomarker. Oncology. 2008;75:60–66. doi: 10.1159/000155145. [DOI] [PubMed] [Google Scholar]
- 29.KarrayChouayekh S, Trifa F, Khabir A, Boujelbane N, SellamiBoudawara T, et al. Clinical significance of epigenetic inactivation of hMLH1 and BRCA1 in Tunisian patients with invasive breast carcinoma. J Biomed Biotechnol. 2009;2009:369129. doi: 10.1155/2009/369129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baldwin RL, Nemeth E, Tran H, Shvartsman H, Cass I, et al. BRCA1 promoter region hypermethylation in ovarian carcinoma: a population-based study. Cancer Res. 2000;60:5329–5333. [PubMed] [Google Scholar]
- 31.Esteller M, Silva JM, Dominguez G, Bonilla F, MatiasGuiu X, et al. Promoter hypermethylation and BRCA1 inactivation in sporadic breast and ovarian tumors. J Natl Cancer Inst. 2000;92:564–569. doi: 10.1093/jnci/92.7.564. [DOI] [PubMed] [Google Scholar]
- 32.Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. 2008;37:1148–1157. doi: 10.1093/ije/dyn065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma G, Mirza S, Parshad R, et al. Clinical significance of promoter hypermethylation of DNA repair genes in tumor and serum DNA in invasive ductal breast carcinoma patients. Life Sci. 2010;87:83–91. doi: 10.1016/j.lfs.2010.05.001. [DOI] [PubMed] [Google Scholar]