Abstract
Background
We sought to determine the prevalence of drug resistant TB among outpatients initiating TB treatment in Lilongwe, Malawi.
Methods
This was a prospective cohort study of patients 18 years and older initiating TB treatment at Martin Preuss Centre, the primary integrated HIV/TB clinic in Lilongwe, Malawi, from April 2011 to July 2012. Procedures included questionnaires, physical exam, chest x-ray, full blood count and sputum collection. Sputum samples underwent acid-fast bacilli (AFB) smear testing and culture by Lowenstein-Jensen (LJ) and liquid Mycobacteria Growth Indicator Tube (MGIT) methods. Drug sensitivity was investigated using the Hain GenoType MTBDRplus line probe assay.
Results
Of the 702 patients, 219 (31.2%) were female and 653 (93.0%) were presenting for first-time TB treatment. HIV co-infection was present in 420 (59.8%) cases, with 137 (32.6%) of those patients receiving antiretroviral therapy at presentation. TB was culture-confirmed in 375 (53.4%) patients, 349 of which were first time treatment and 26 retreatment. Ten cases of isoniazid-resistant TB (2.9% of culture confirmed cases of newly treated TB), one of rifampin-resistant TB (0.3% culture confirmed cases of newly treated TB) and one of multi-drug resistant TB (MDR-TB) (3.8% of culture confirmed cases of retreatment TB) were detected.
Conclusions
MDR-TB prevalence is low among outpatients initiating TB treatment in Lilongwe.
Keywords: Africa South of the Sahara, Epidemiology, Isoniazid, Multi-drug resistant, Rifampin, Tuberculosis
Introduction
Despite efforts to control the spread of TB in Africa, there are 2.3 million new cases that lead to a quarter million deaths annually.1 To further complicate this situation, Africa is currently experiencing a nearly 6% annual increase in cases of multi-drug resistant TB (MDR-TB), the fastest of any WHO region. Given that most countries in Sub-Saharan Africa have limited laboratory capacity and public health care infrastructure, resources which are already under significant strain from the longstanding HIV epidemic, the region has become a potentially fertile breeding ground for MDR-TB. Despite this concern, data on drug resistance in Sub-Saharan Africa is scarce outside of South Africa, which has a more advanced public health care system than its surrounding countries.
Malawi, a country of 14 million people, has been struck hard by both HIV and TB. Despite successful expansion of the antiretroviral therapy programme with marked reductions in HIV mortality and prevalence2 HIV prevalence remains high at 10.6%, and 68% of TB cases are HIV co-infected.3 Meanwhile, MDR-TB remains a concern for this vulnerable population. The WHO estimates that there were up to 990 cases of MDR-TB in Malawi in 2009, though only six of those cases were identified and enrolled in treatment.4
Previous studies of drug resistant TB in Malawi have focused on inpatients being treated for TB5 or only patients undergoing treatment for recurrent smear positive pulmonary TB.6 However, inpatients make up only a small portion of the total population of those with TB, and smear negative pulmonary TB is common among HIV co-infected patients.7 Given the significant numbers of TB patients who are treated as outpatients and the high burden of HIV/TB co-infection, a broader investigation of drug resistance in Malawian patients living with TB is needed. In this study we evaluate the prevalence of drug resistant TB in Lilongwe, Malawi, among outpatients with new and retreatment TB, as well as smear positive and smear negative TB.
Methods
Study setting
In Malawi all patients diagnosed with TB are registered for treatment at TB clinics. Martin Preuss Centre, located on the campus of Bwaila Hospital, serves as Lilongwe's primary integrated HIV/TB treatment clinic and contains Malawi's largest TB registry. Patients arrive by two different routes, one being referral from surrounding facilities for commencement of treatment once they have been diagnosed with TB and the other being self-referral for sputum submission for those who have experienced chronic cough, defined as cough lasting longer than 3 weeks.
Study design and population
This was a prospective observational cohort study including all adult (age 18 years and older) patients registering for TB treatment at Martin Preuss Centre from April 2011 to July 2012. Originally, only patients presenting for TB treatment for the first time were enrolled. However, in November 2011 the study protocol was amended to allow for the additional enrollment of patients who had previously defaulted (been lost to follow-up), experienced treatment failure or whose TB had relapsed. Exclusion criteria included being less than 18 years of age, being a prisoner, or already being on TB treatment initiated at another facility. Once registered, patients were treated according to the Malawi National Tuberculosis Control Programme manual,8 with first-treatment patients receiving isoniazid, rifampicin, pyrazinamide and ethambutol, and retreatment patients receiving streptomycin, isoniazid, rifampicin, pyrazinamide and ethambutol. Those with MDR-TB were admitted to the hospital and received capreomycin, levofloxacin, ethionamide, cycloserine and pyrazinamide for 6 months, followed by 18 months of levofloxacin, ethionamide, and cycloserine at home through a community based approach.
Enrollment procedures
Study personnel obtained written informed consent from each patient and administered a questionnaire that covered demographics, past medical history, symptoms, and diagnostic details (acid-fast bacilli [AFB] smear status and radiographic details). We collected blood for full blood count, sputum samples, and performed a chest x-ray on each patient. We offered rapid HIV testing and counseling to patients with an unknown HIV status at the time of enrollment. HIV infected individuals received a CD4 count and, if on antiretroviral therapy for greater than one year, a plasma HIV RNA test. Highly active antiretroviral therapy was initiated in those HIV infected individuals not yet on therapy, according to Malawi's national guidelines for the treatment of HIV.9
Laboratory procedures
All specimens were analyzed at the University of North Carolina Project laboratory, which has been given a four star ranking on the WHO Stepwise Laboratory Improvement Process Towards Accreditation (SLIPTA) Tier of Recognition by the African Society for Laboratory Medicine.
Sputum samples were visualized microscopically after auramine O staining and were considered to be smear positive if bacilli were observed. Additionally, they were cultured on Lowenstein-Jensen (LJ) and BACTEC™ Mycobacteria Growth Indicator Tube (MGIT; Becton, Dickinson and Company, Franklin Lakes, NJ, USA). For culture-positive samples, the presence of Mycobacterium tuberculosis complex (MTBC) was confirmed, and rifampicin and isoniazid resistance determined, using the GenoType MTBDRplus line probe assay (Hain Lifescience GmbH, Nehren, Baden-Württemberg, Germany). If the presence of MTBC was not confirmed using the GenoType MTBDRplus, samples were tested with the Genotype Mycobacterium CM (Hain Lifescience GmbH) to identify other strains of Mycobacterium. Drug susceptibility testing is not included as part of Malawi's standard of care.
Full blood count was performed using Beckman Coulter AcT 5diff Cap Pierce Hematology Analyzer, CD4 counts by BD FACSCount System, and HIVRNA levels by Roche AMPLICOR HIV-1 MONITOR Test version 1.5.
Statistical analysis
Data were double-entered into a Microsoft Access 2007 database (Microsoft Corp., Redmond, WA, USA) and statistically analyzed using Stata version 11.0 (Stata Corporation, College Station, TX, USA). Standard χ², Fisher's exact tests (two tailed) and t-tests (two tailed, unpaired) were used to determine associations between drug resistance and patient characteristics. A p<0.05 was considered statistically significant.
Ethical considerations
Study approval was obtained from the University of North Carolina at Chapel Hill institutional review board and the Malawi National Health Science Research Committee.
Results
Enrollment
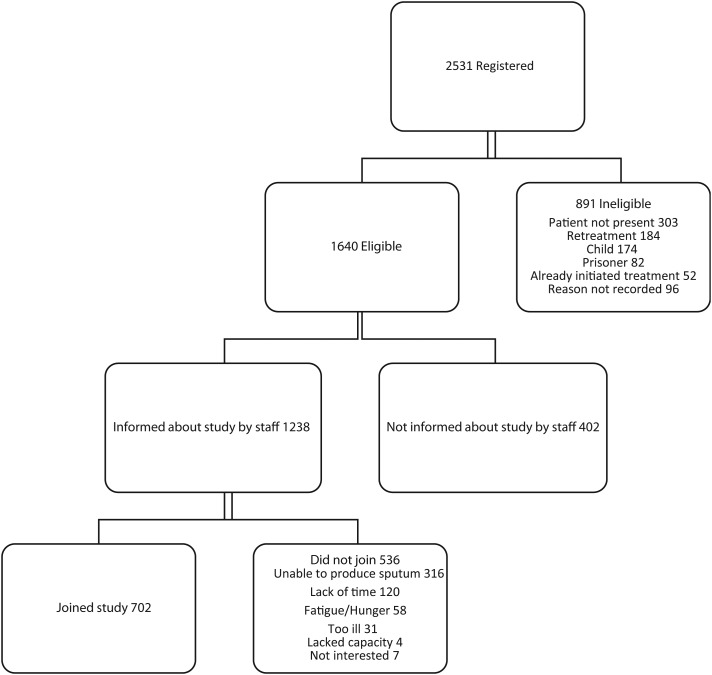
Between April 2011 and July 2012, 2531 patients were registered for TB treatment at Martin Preuss Centre. Of these, 1640 patients were eligible to enroll in the study and 702 completed the enrollment process. The most common reasons for ineligibility were the patient not being physically present at registration (303), the patient being retreated for TB (184) during the initial portion of the study when retreatment patients were excluded, and the patient being less than 18 years of age (174). Patients that were ineligible for our study due to not being present at time of registration were admitted at local hospitals and were therefore not part of the outpatient population under investigation. Of eligible patients, the most common reasons for not joining were not being asked to join the study due to staffing constraints (402), being unable to produce a sputum sample (316) and reported lack of time to participate in the study (120) (Figure 1).
Figure 1.
Study enrollment.
Patient characteristics
The median age of study patients was 35 years old (range 19–80), 219 (31.2%) were female, and 653 (93.0%) were presenting with TB for the first time (Table 1). Characteristics of both first time treatment patients and retreatment patients did not demonstrate statistically significant differences, except for smear positivity of sputum samples being more likely in retreatment patients (p=0.02). Pulmonary TB was the predominant form of TB, being diagnosed in 614 (87.5%) cases. Abnormal findings on chest x-ray were observed in 415 (59.1%) patients. HIV co-infection was present in 420 (59.8%) cases, but only 137 (32.6%) of those patients were receiving antiretroviral therapy at presentation, since most patients with HIV were diagnosed on the day of enrollment in the study. Among HIV infected patients, those in the retreatment group (p<0.001) and females (p=0.02) were more likely to be receiving antiretroviral therapy (Table 2). Of the 402 eligible patients that were not asked to join the study, 380 (94.5%) were receiving first time TB treatment, which was not statistically different from the percentage of enrolled patients receiving first time TB treatment (p=0.66).
Table 1.
Demographic and clinical characteristics of patients
| Characteristic | All patients (n=702) | First treatment (n=659) | Retreatment (n=43) | p-value |
|---|---|---|---|---|
| Median age with range (years) | 35 (19–80) | 34 (19–80) | 37 (20–71) | NS |
| Female | 219 (31.2%) | 203 (30.8%) | 16 (37%) | NS |
| Smear positive | 244 (34.8%) | 222 (33.7%) | 22 (51%) | 0.02 |
| Pulmonary TB | 614 (87.5%) | 579 (87.9%) | 35 (81%) | NS |
| Extrapulmonary TB | 88 (12.5%) | 80 (12.1%) | 8 (19%) | NS |
| Meningitis (% EPTB) | 1 (1%) | 1 (1%) | 0 | NS |
| Lymphadenitis (% EPTB) | 17 (19%) | 16 (20%) | 1 (13%) | NS |
| Pericarditis (% EPTB) | 10 (11%) | 8 (10%) | 2 (25%) | NS |
| Pleuritis (% EPTB) | 54 (61%) | 49 (61%) | 5 (63%) | NS |
| Arthritis (% EPTB) | 1 (1%) | 1 (1%) | 0 | NS |
| Spinal (% EPTB) | 2 (2%) | 2 (3%) | 0 | NS |
| Miliarya (% EPTB) | 8 (9%) | 8 (10%) | 0 | NS |
| Abnormal CXRb | 415 (59.1%) | 392 (59.5%) | 23 (53%) | NS |
| Unilateral infiltrate (% Abnormal CXR) | 156 (37.6%) | 151 (38.5%) | 5 (22%) | NS |
| Bilateral infiltrate (% Abnormal CXR) | 201 (48.4%) | 188 (48.0%) | 13 (57%) | NS |
| Adenopathy (% Abnormal CXR) | 69 (16.6%) | 68 (17.3%) | 1 (4%) | NS |
| Miliary (% Abnormal CXR) | 31 (7.5%) | 30 (7.7%) | 1 (4%) | NS |
| Cavitary lesion (% Abnormal CXR) | 96 (23.1%) | 92 (23.5%) | 4 (17%) | NS |
| Pleural effusion (% Abnormal CXR) | 68 (16.4%) | 61 (15.6%) | 7 (30%) | NS |
| Pericardial effusion (% Abnormal CXR) | 11 (2.7%) | 10 (2.6%) | 1 (4%) | NS |
| HIV positive | 420 (59.8%) | 393 (59.6%) | 27 (63%) | NS |
| On ART (% of HIV+) | 137 (32.6%) | 121 (30.8%) | 16 (59%) | <0.001 |
| Median CD4 Count among positives (IQR) | 167 (81–293) | 160 (76–292) | 185 (86–253) | NS |
| VL suppressed to <400 (% of HIV+on ART) (n=53 VLs) | 38 (72%) | 33 (75%) | 5 (56%) | NS |
ART: antiretroviral therapy; CXR: chest X-ray; EPTB: extrapulmonary TB; NS: not significant; VL: viral load.
a More than one Extrapulmonary category; b Total sums to >100% because patients often had more than one finding on CXR.
Table 2.
Tests for association between patient characteristics in patients with HIV (n=420) and ART usage
| On ART (n=137) | No ART (n=283) | p-value | |
|---|---|---|---|
| Mean age with range (years) | 37 (19–71) | 36 (19–76) | NS |
| Female | 42 (30.7%) | 30 (10.6%) | 0.02 |
| Smear positive | 28 (20.4%) | 27 (9.5%) | NS |
| Pulmonary TB | 82 (59.9%) | 86 (30.4%) | NS |
| Extrapulmonary TB | 18 (13.1%) | 14 (4.9%) | NS |
| Abnormal CXR | 63 (46.0%) | 63 (22.3%) | NS |
| Any resistance | 3 (2.2%) | 3 (1.1%) | NS |
ART: antiretroviral therapy; CXR: chest x-ray; NS: not significant.
Laboratory findings
Of the 702 sputum samples collected from patients for laboratory analysis, seven were inadequate in volume and one was lost, leaving a total of 694 samples that were cultured for mycobacteria. Of these 694 samples, AFB smear testing was positive in 244 (35.2%) patients. Three hundred and seventy nine samples (54.6%) were positive for mycobacteria by either LJ or MGIT culture. For MGIT culture, four samples were discarded due to contamination and three due to technical failure, leaving 687 MGIT cultured samples. Of these, 368 samples were culture positive (53.6%). For LJ culture, 20 samples were discarded due to contamination and 25 were unculturable due to LJ medium being out of stock, leaving 649 LJ cultured samples. Of these, 333 samples were culture positive (51.3%).
Of the 379 samples that were either LJ or MGIT positive for mycobacteria, testing by the Genotype Mycobacterium CM demonstrated that three samples were positive for Mycobacterium intracellulare and one for Mycobacterium avium. The remaining 375 culture positive samples were confirmed to be MTBC using the GenoType MTBDRplus.
Both AFB and culture positivity differed according to HIV status. AFB smear was positive in 116 of 420 (27.6%) HIV positive patients versus 128 of 282 (45.4%) for HIV negative patients (p<0.01). LJ or MGIT culture was positive for MBTC in 204 of 420 (48.6%) patients with HIV versus 171 of 282 (60.6%) of patients without HIV (p<0.01) (Table 3). Of the 204 HIV positive patients with culture confirmed mycobacteria, 89 (43.6%) had sputum samples that were AFB smear negative.
Table 3.
Acid-fast bacilli smear and Lowenstein-Jensen culture positivity in HIV positive and negative patients
| HIV positive (n=420) | HIV negative (n=282) | p-value | |
|---|---|---|---|
| AFB smear positive | 116 (27.6%) | 128 (45.4%) | <0.01 |
| LJ or MGIT culture positive | 201 (47.9%) | 171 (60.6%) | <0.01 |
AFB: acid-fast bacilli; LJ: Lowenstein-Jensen; MGIT: Mycobacteria Growth Indicator Tube.
The 375 MTBC culture positive samples were assessed for drug resistance using the GenoType MTBDRplus line probe assay. Ten cases (2.6%) of isoniazid mono-resistant TB were detected, all from patients being treated for TB for the first time. All ten of these cases were also positive by LJ and MGIT culture; however, three (30%) were AFB smear negative. Three hundred and forty nine of MTBC culture positive patients were being treated for TB for the first time, while 26 were receiving re-treatment. This resulted in a calculated prevalence of 2.9% (10/349) for isoniazid mono-resistance in newly treated and culture confirmed cases of MTBC. One case of rifampicin mono-resistant TB was detected, from a patient being treated for TB for the first time. This case was AFB smear positive and also positive by LJ and MGIT cultures, resulting in a prevalence of 0.3% (1/349) in newly treated and culture confirmed cases of MTBC. One case of MDR-TB (resistant to both isoniazid and rifampicin) was detected, originating from a patient being retreated for TB. It was detected by LJ and MGIT cultures, but was AFB smear negative, resulting in a prevalence of 3.8% (1/26) in retreatment culture confirmed cases of MTBC.
Drug resistance was not associated with age, gender, TB treatment status, TB site, AFB smear result, chest X-ray results or HIV status (Table 4).
Table 4.
Tests for association between patient characteristics and any type of drug resistance
| Pansensitive (n=363) | Any drug resistancea (n=12) | p-value | |
|---|---|---|---|
| Mean age (years) | 34 | 36 | NS |
| Female | 106 (29.2%) | 3 (25%) | NS |
| First treatment | 335 (92.3%) | 11 (92%) | NS |
| Retreatment | 25 (6.9%) | 1 (8%) | NS |
| Abnormal X-ray (n=155 and n=7 x-rays) | 147 (94.8%) | 7 (100%) | NS |
| Pulmonary TB | 332 (91.5%) | 11 (92%) | NS |
| Extrapulmonary TB | 28 (7.7%) | 1 (8%) | NS |
| Smear positive | 234 (64.5%) | 8 (67%) | NS |
| HIV positive | 195 (53.7%) | 6 (50%) | NS |
| Not on ART (% of HIV+) | 130 (66.7%) | 3 (50%) | NS |
| Mean CD4 count among HIV+ | 203 | 148 | NS |
| Mean absolute neutrophil count among HIV+ | 3689 | 3117 | NS |
ART: antiretroviral therapy; NS: not significant.
a Includes 10 samples with isoniazid mono-resistance, one sample with rifampin mono-resistance and one sample with multi-drug resistance (both isoniazid and rifampin).
Discussion
Though others have reported low rates of MDR-TB in Malawi,5,6 our study is one of the first to demonstrate that the presence of MDR-TB in Malawi is very low among both first time and retreatment outpatient cases, as well as among those who are sputum positive or negative. This latter point is important as our study confirmed a high rate of smear negative, LJ culture confirmed TB (44%) among HIV co-infected patients, who represented 60% of our study population. Because TB is the leading cause of death among HIV-infected persons,10 it is especially important to understand the prevalence of MDR-TB in this population. Our results are in line with a recently published nationally representative cross sectional survey assessing drug resistant TB in Malawi conducted from February 2010 to March 201111 that also demonstrated low prevalence of MDR-TB (0.4% in new cases and 4.8% retreatment cases), among patients with sputum smear positive TB.
Malawi currently appears to be faring better than some of its neighboring countries in the MDR-TB epidemic, such as Swaziland (prevalence of 0.9% of new cases and 9.1% of retreatment cases), South Africa (prevalence of 1.8% of new cases and 6.7% of retreatment cases) and Mozambique (prevalence of 3.5% of new cases and 11.2% of retreatment cases).4 This can be partially attributed to the country's well-managed national TB control programme, which has used the directly observed treatment short-course (DOTS) strategy since 1964.12 Treatment success rates have continually improved, rising from 65% in 1995 to 88% in 2010,1 putting the country's performance above the WHO's target of 85%. Though the importance of future evaluation of prevalence in Malawi cannot be understated, our study demonstrates that prevention of MDR-TB is possible, even in a low resource country, through proper planning, implementation, regular monitoring and evaluation of treatment administration.
The predominance of isoniazid mono-resistance in our study is important due to the recent advent of the Xpert MTB/RIF assay, which allows for the detection of both MTB and rifampicin resistance simultaneously in less than two hours.13,14 The assay was endorsed by the WHO in December 2010 and has since been specifically recommended as the initial diagnostic test in patients with suspected drug resistant TB or HIV associated TB. As a result, since June 2012, two-thirds of countries struggling with a high prevalence of TB have included the assay in their national TB control programmes. However, due to the predominance of isoniazid mono-resistance in our study results, the Xpert assay will underestimate overall TB drug resistance since it only detects rifampicin resistance. Given the low prevalence of rifampicin resistance in Malawi, the positive predictive value of the Xpert MTB/RIF assay is low. Therefore cases of rifampicin resistance detected by this assay should also be confirmed using drug susceptibility testing.15 The 3% prevalence of isoniazid resistance in patients being newly treated for TB indicates a need for rapid detection of this mono-resistance so that treatment regimens can be modified quickly to prevent development of additional rifampicin resistance and subsequent MDR-TB.
Prior studies have found associations between previous treatment for TB, treatment not directly observed by a healthcare worker and exposure to a known case of MDR-TB and the development of MDR-TB.16,17 Associations have also been observed between HIV co-infection and the development of MDR-TB; however, a recent meta-analysis of studies from Sub-Saharan Africa revealed no association.16 We found no statistical associations between patient characteristics and TB drug resistance. However, our study was handicapped to show a statistical difference, given that there were so few drug resistant cases. Other limitations of this study include the initial exclusion of patients seeking retreatment for TB, which likely contributed to underestimation of MDR-TB prevalence since these individuals have the highest risk for MDR-TB, and the sizable number of potential patients enrolling for TB treatment that were not approached by our study staff due to staffing shortages. Among those not approached, there was no difference in the proportion that were registering for first time TB treatment or retreatment, but we do not have access to other information such as age, gender and HIV status for further comparison. Regardless, we feel a systematic bias is unlikely. Prisoners were also excluded from our study due to concerns about their ability to give consent. Given that these individuals live in an environment known to have a higher prevalence of MDR-TB than the general population, it is possible that their exclusion contributed to underestimation of MDR-TB prevalence.
Conclusions
MDR-TB prevalence is low among outpatients seeking treatment in Lilongwe, emphasizing the effectiveness of Malawi's national TB control programme. However, future evaluation will be necessary to identify cases of isoniazid and rifampicin resistance in order to monitor TB control programme performance and prevent establishment of MDR-TB strains in the country.
Acknowledgments
Authors' contributions: BB was involved in study design, oversaw patient enrollment and study implementation, analyzed data and wrote the manuscript. RHG was involved in study design, gained institutional study approval and oversaw enrollment. CK, TC and RK carried out laboratory analyses of specimens and interpreted data. MB enrolled patients and collected/interpreted data. CM and FS interpreted laboratory/radiographic results. SP, IFH and MCH assisted with study design, study implementation, data interpretation and manuscript preparation. All authors read and approved the final paper. BB is the guarantor of the paper.
Funding: This work was funded by the University of North Carolina Center for AIDS Research [P30 AI50410], the International Union Against Tuberculosis and Lung Disease, and the National Institutes of Health (NIH) Fogarty International Center Clinical Research Scholars and Fellows Program at Vanderbilt University [R24 TW007988, 3 D43 TW001039-11S1]. BB and RG received an NIH-Fogarty-Fulbright fellowship (2011–2012) and an NIH-Fogarty International Clinical Research fellowship (2010–2011), respectively.
Competing interests: None declared.
Ethical approval: Study approval was obtained from the University of North Carolina at Chapel Hill institutional review board and the Malawi National Health Science Research Committee.
References
- 1.WHO. Global Tuberculosis Control: WHO Report 2011. Geneva: World Health Organization; 2011. [Google Scholar]
- 2.Government of Malawi Ministry of Health. 2012 Global AIDS Response Progress Report: Malawi Country Report for 2010 and 2011. Lilongwe, Malawi: Ministry of Health; 2012. [Google Scholar]
- 3.WHO. Malawi Country Office 2010 Annual Report. Lilongwe, Malawi: World Health Organization; 2010. [Google Scholar]
- 4.WHO. Towards Universal Access to Diagnosis and Treatment of Multi-Drug Resistant and Extensively Drug-Resistant Tuberculosis by 2015. Geneva: World Health Organization; 2011. [Google Scholar]
- 5.Vorkas C, Kayira D, van der Horst C et al. . Tuberculosis drug resistance and outcomes among tuberculosis inpatients in Lilongwe, Malawi. Malawi Med J 2012;24:21–4. [PMC free article] [PubMed] [Google Scholar]
- 6.Salaniponi FM, Nyirenda TE, Kemp JR et al. . Characteristics, management and outcome of patients with recurrent tuberculosis under routine programme conditions in Malawi. Int J Tuberc Lung Dis 2003;7:948–52. [PubMed] [Google Scholar]
- 7.Palmieri F, Girardi E, Pellicelli AM et al. . Pulmonary tuberculosis in HIV-infected patients presenting with normal chest radiograph and negative sputum smear. Infection 2002;30:68–74. [DOI] [PubMed] [Google Scholar]
- 8.Government of Malawi Ministry of Health. National Tuberculosis Control Programme Manual: Seventh Edition. Lilongwe, Malawi: Ministry of Health; 2012. [Google Scholar]
- 9.Government of Malawi Ministry of Health. Clinical management of HIV in children and adults. Lilongwe, Malawi: Ministry of Health; 2011. [Google Scholar]
- 10.Laserson KF, Wells CD. Reaching the targets for tuberculosis control: the impact of HIV. Bull World Health Organ 2007;85:377–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abouyannis M, Dacombe R, Dambe I et al. . Drug resistance of Mycobacterium tuberculosis in Malawi: a cross sectional survey. Bull World Health Organ 2014;92:798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simwaka BN, Bello G, Banda H et al. . The Malawi National Tuberculosis Programme: an equity analysis. Int J Equity Health 2007;6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO. Automated Real-Time Nucleic Acid Amplification Technology for Rapid and Simultaneous Detection of Tuberculosis and Rifampicin Resistance: Xpert MTB/RIF Assay for the Diagnosis of Pulmonary and Extrapulmonary TB in Adults and Children. Policy Update Geneva: World Health Organization; 2013. [PubMed] [Google Scholar]
- 14.Lawn SD, Mwaba P, Bates M et al. . Advances in tuberculosis diagnostics: the Xpert MTB/RIF assay and future prospects for a point-of-care test. Lancet Infect Dis 2013;13:349–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO. Policy Statement. Automated Realtime Nucleic Acid Amplification Technology for Rapid and Simultaneous Detection of Tuberculosis and Rifampicin Resistance: Xpert MTB/RIF system. Geneva: World Health Organization; 2011. [PubMed] [Google Scholar]
- 16.Berhan A, Berhan Y, Yizengaw D. A meta-analysis of drug resistant tuberculosis in Sub-Saharan Africa: How strongly associated with previous treatment and HIV Co-infection? Ethiop J Health Sci 2013;23:271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biadglegne F, Sack U, Rodloff AC. Multidrug-resistant tuberculosis in Ethiopia: efforts to expand diagnostic services, treatment and care. Antimicrob Resist Infect Control 2014;3:31. [DOI] [PMC free article] [PubMed] [Google Scholar]